Abstract
The novel coronavirus disease 2019 (COVID-19) is a highly infectious and rapidly spreading disease. There are limited published data on the epidemiology and outcomes of COVID-19 infection among organ transplant recipients. After initial flulike symptoms, progression to an inflammatory phase may occur, characterized by cytokine release rapidly leading to respiratory and multiorgan failure. We report the clinical course and management of a liver transplant recipient on hemodialysis, who presented with COVID-19 pneumonia, and despite completing a 5-day course of hydroxychloroquine, later developed marked inflammatory manifestations with rapid improvement after administration of off-label, single-dose tocilizumab. We also highlight the role of lung ultrasonography in early diagnosis of the inflammatory phase of COVID-19. Future investigation of the effects of immunomodulators among transplant recipients with COVID-19 infection will be important.
KEYWORDS: clinical research/practice, immunosuppressant - calcineurin inhibitor: tacrolimus, immunosuppression/immune modulation, infection and infectious agents - viral, infectious disease, liver transplantation/hepatology
Abbreviations: COVID-19, coronavirus disease 2019; CRS, cytokine release syndrome; CT, computed tomography; ESRD, end-stage renal disease; HCC, hepatocellular carcinoma; HCQ, hydroxychloroquine; HCV, hepatitis C; IL-6, interleukin-6; RT-PCR, reverse transcriptase-polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2
1. BACKGROUND
Coronavirus disease 2019 (COVID-19) is a highly infectious viral disease caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). There is evolving understanding of the epidemiology and outcomes of COVID-19, with a disease spectrum ranging from asymptomatic infection to critical illness.1, 2 Although advanced age and underlying medical comorbidities have been associated with risk for severe illness,2 there are limited data on the impact of COVID-19 infection on solid organ transplant recipients.3, 4, 5, 6 It has been theorized that the inflammatory response driven by the cytokine storm in response to COVID-19 is responsible for the clinical deterioration sometimes seen late in the illness. While transplant immunosuppression might impair control of viral infection, discontinuation of immunosuppression could be associated with an exacerbation of inflammatory responses to viral infection.5 We report the clinical course and management of a liver transplant recipient with confirmed COVID-19 infection who had a marked response to off-label tocilizumab, an anti-interleukin-6 (IL-6) receptor antibody.
2. CASE PRESENTATION
A 63-year-old African-American man received a deceased donor liver transplant 10 years previously for hepatitis C (HCV) cirrhosis with hepatocellular carcinoma (HCC), followed by retransplantation 6 months later for chronic rejection. He had received 12 weeks of ledipasvir/sofosbuvir for HCV with sustained viral response 5 years previously. Due to recurrent HCC, he received microwave ablation 7 weeks prior to presentation. Immunosuppression was maintained with low-dose tacrolimus 1.5 mg twice daily for a trough goal of 2-4 ng/mL. Other history included end-stage renal disease on hemodialysis, type 2 diabetes, hypertension, peripheral vascular disease, heart failure with preserved ejection fraction, and smoking.
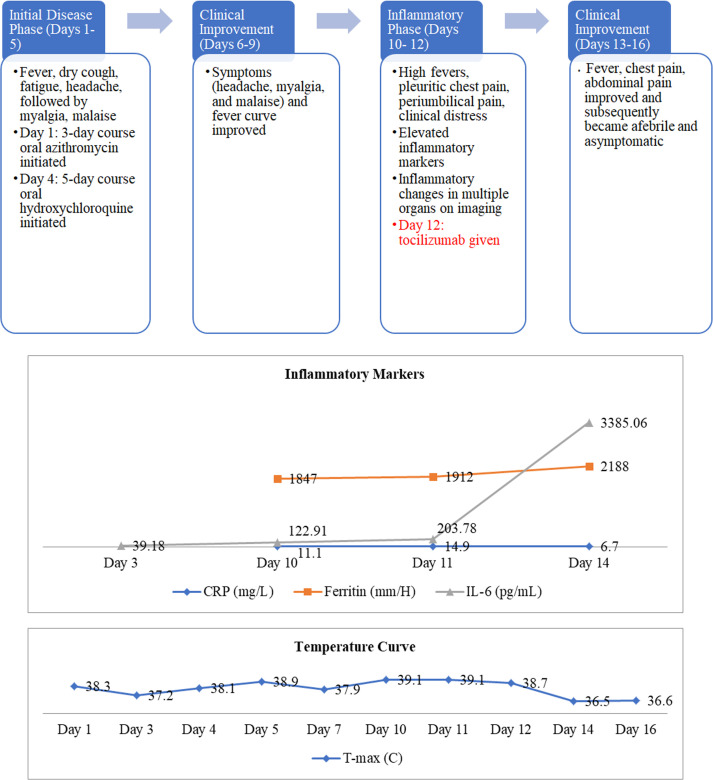
The patient was admitted with a 1-day history of fever, dry cough, fatigue, and headache. Physical exam revealed a temperature of 38.2°C, blood pressure 138/67 mm Hg, heart rate 71 beats/min, respiratory rate 11 breaths/min, and oxygen saturation 97% on room air. The exam was otherwise unremarkable, including lungs that were clear to auscultation. Notable laboratory results are presented in Table 1. Rapid nucleic acid amplification testing was negative on nasopharyngeal swab for influenza A and B, respiratory syncytial virus, parainfluenza 1-4, rhino/enterovirus, metapneumovirus, adenovirus, and Mycoplasma pneumoniae, but SARS-CoV-2 testing by reverse transcriptase-polymerase chain reaction (RT-PCR) assay was positive. Chest computed tomography (CT) revealed peripheral consolidation with surrounding ground-glass opacification in the posteromedial right lower lobe. He was empirically started on intravenous ceftriaxone 1 g daily and oral azithromycin 500 mg daily for the possibility of secondary bacterial pneumonia Figure 1.
TABLE 1.
Selected clinical laboratory results and daily maximum temperature
| Measure | Reference range | Day 1 (presentation) | Day 3 | Day 4 | Day 5 | Day 7 | Day 10 | Day 11 | Day 12a | Day 14 | Day 16 (discharge) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC (×109/L) | 4.5-11 | 4.1 | 3.18 | 4.17 | 2.86 | 3.07 | 4.3 | 4.31 | 5.39 | 4.85 | 4.39 |
| Neut % | 40-70 | 50.5 | 44.6 | 68.4 | 51.1 | 49.6 | 65 | 66.1 | 65.3 | 68.7 | 54.2 |
| Lymph % | 24-44 | 18.8 | 31.8 | 15.6 | 29.4 | 30.9 | 18.1 | 17.2 | 18 | 13.8 | 22.8 |
| Mono % | 2-11 | 28.5 | 18.9 | 13.2 | 17.1 | 15.6 | 15.1 | 14.4 | 12.8 | 10.5 | 15 |
| Abs lymph | 1.1-4.8 | 0.77 | 1.01 | 0.65 | 0.84 | 0.95 | 0.78 | 0.74 | 0.97 | 0.67 | 1.0 |
| Hgb (g/dL) | 13.9-16.3 | 10.4 | 10.9 | 10.5 | 10.3 | 9.8 | 10.2 | 9.1 | 8.8 | 8.2 | 8.6 |
| Plt (×109/L) | 150-350 | 71 | 54 | 53 | 49 | 61 | 110 | 121 | 134 | 143 | 157 |
| Alb (g/dL) | 3.5-5.3 | 3.7 | 3.8 | 3.5 | 3.5 | 3.3 | 3.4 | 3.3 | 3.2 | 3.4 | 3.4 |
| ALP (U/L) | 30-120 | 194 | 191 | 194 | 184 | 189 | 225 | 205 | 205 | 198 | 250 |
| AST (U/L) | 0-37 | 21 | 22 | 14 | 24 | 31 | 46 | 45 | 47 | 43 | 61 |
| ALT (U/L) | 0-40 | 17 | 16 | 14 | 17 | 15 | 18 | 19 | 18 | 20 | 27 |
| CRP (mg/L) | <0.5 | 11.1 | 14.9 | 6.7 | |||||||
| ESR (mm/H) | 1-20 | >130 | >130 | ||||||||
| Ferritin (mm/H) | 30-400 | 1847 | 1912 | 2188 | |||||||
| LDH (U/L) | 118-273 | 296 | |||||||||
| D-Dimer (mg/L) | 0-0.49 | 1.42 | 1.88 | ||||||||
| IL-6 (pg/mL) | <5 | 39.18 | 122.91 | 203.78 | 3385.06 | ||||||
| T-max (C) | 38.3 | 37.2 | 38.1 | 38.9 | 37.9 | 39.1 | 39.1 | 38.7 | 36.5 | 36.6 |
Abbreviations: Abs Lymph, absolute lymphocytes; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hgb, hemoglobin; IL-6, interleukin 6; LDH, lactate dehydrogenase; Lymph, lymphocytes; Mono, monocytes; Neut, neutrophils; Plt, platelet count; T-max, maximum temperature; WBC, white blood cell count.
Tocilizumab was administered on day 12.
FIGURE 1.
Hospital course summary
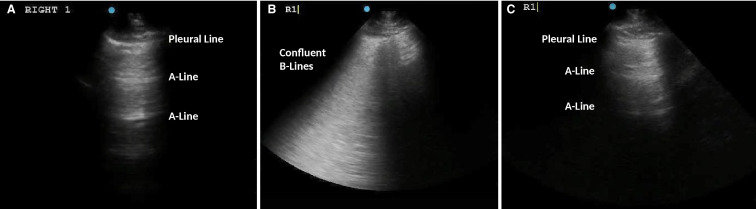
On day 3, IL-6 was 39.18 pg/mL (Table 1). Urine Legionella and pneumococcal antigen tests were negative, as were blood and sputum cultures. Lung ultrasound showed A-lines (normal lung artifacts) in 11 of the 12 lung zones, except for the right posterior lung zone with subpleural consolidation ( Figure 2A).
FIGURE 2.
A-C, Representative lung ultrasound on day 3, day 10, and day 16. A, This lung ultrasound showed A-lines, which are normal lung artifacts, on the right upper anterior lung zone on day 3. Eleven out of 12 lung zones revealed A-lines. B, On day 10 at the peak of the inflammatory symptoms, 8 out of 12 lung zones demonstrated confluent B-lines as shown in this figure. This image was obtained on the right upper anterior lung zone. C, On day 16, which was 5 days after tocilizumab, only 3 out of 12 lung zones had B-lines. The B-lines on the right upper anterior lung zone were resolved (images are courtesy of Dr Liu)
On day 4, he continued with fevers, headache, myalgias, and malaise. Azithromycin was discontinued, and a 5-day course of oral hydroxychloroquine (HCQ) was initiated, 400 mg twice daily on day 1, then 400 mg daily on days 2-5, with monitoring of the QTc on electrocardiogram (464 ms at baseline, 477 ms after HCQ).
On day 5, antibiotics were broadened to intravenous cefepime and vancomycin for a fever of 38.9°C. A repeat blood culture showed no growth.
Between days 6 and 9, his symptoms and fever curve improved, although he continued to have intermittent low-grade fevers (37.8 −38.1°C).
On day 10, he re-developed high fevers to 39.1°C with chills, sharp right pleuritic chest pain, severe periumbilical pain, and looked in clinical distress. Inflammatory markers were elevated (Table 1). A repeat chest CT revealed worsening bilateral subpleural and peri-broncho-vascular ground-glass and consolidative opacities. An abdomen and pelvis CT revealed wall thickening and stranding surrounding the gastric antrum, proximal duodenum, pancreatic head, and mesentery, with prominent mesenteric and retroperitoneal lymph nodes. Lung ultrasound demonstrated thick discrete and confluent B lines, which correspond to ground-glass opacities on chest CT,7 in 8 out of 12 zones (Figure 2B). IL-6 rose to 122.91 pg/mL (Table 1).
On day 11, he had persistently high fevers and further increase in inflammatory markers (Table 1), prompting concerns that he was progressing into the cytokine-release phase of the illness, although he had not yet developed hypoxemia. Based on this concern, on day 12, he received a single dose of intravenous tocilizumab 800 mg (8 mg/kg rounded to nearest vial size available), which was 9 mg/kg based on actual body weight.
On day 13, within 24 hours of receiving tocilizumab, his fevers, chest pain, and abdominal pain improved. An echocardiogram revealed normal left ventricular systolic function and abnormal diastolic function with pericardial effusion.
On day 14, he reported feeling well and antibacterials were discontinued. Laboratory results showed more than a 10-fold increase in IL-6 (3385.06 pg/mL); other inflammatory markers showed a mixed response (Table 1). Repeat SARS-CoV-2 RT-PCR assay remained positive.
He subsequently remained afebrile and asymptomatic and was discharged home on day 16. Notably, he never developed respiratory distress or hypoxemia, and maintained stable liver graft function throughout. He maintained a trough tacrolimus goal of 4-6 ng/mL. Laboratory results at the time of discharge are shown in Table 1. His lung ultrasound revealed only 3 out of 12 lung zones with B-lines, compared to 8 zones just prior to his tocilizumab (Figure 2C).
In follow-up testing at his dialysis center, he remained clinically well, but was still positive for SARS-CoV-2 by nasal swab RT-PCR on day 19. On day 25 and day 27, this assay turned negative.
3. DISCUSSION
Our patient’s presenting symptoms, as well as chest CT findings, were similar to previous descriptions of COVID-19.2, 3, 4, 5, 6 His symptoms and fever initially improved during a 5-day course of HCQ, but later flared with marked inflammatory symptoms on day 10. The area of therapeutics for COVID-19 infection is rapidly evolving. Although it is widely used, the benefits of off-label HCQ are still debated. A nonrandomized study by Gautret et al of 36 patients who did or did not receive HCQ suggested earlier virologic clearance in the HCQ group.8 A subsequent study by Molina et al of 11 patients treated with HCQ and azithromycin did not show virologic or clinical benefit.9 Nonetheless, the US Food and Drug Administration issued an Emergency Use Authorization to endorse use of HCQ for treating COVID-19 in hospitalized patients who cannot participate in clinical trials.10
Initial clinical improvement in our patient was followed by an inflammatory phase with high fevers, elevated inflammatory markers, and inflammatory changes in multiple organs on imaging. Since our patient was not eligible for any clinical trials, he received off-label, single-dose tocilizumab, after which he had rapid improvement in his fevers and inflammatory symptoms, and never progressed to respiratory failure. Serum levels of inflammatory mediators in COVID-19 often parallel the severity of the disease.11 IL-6 is produced in response to infections and tissue injury, and contributes to host defense by stimulating acute phase responses, hematopoiesis, and immune reactions.12 A progressive rise in IL-6 may be an indicator of COVID-19 disease severity.11 , 13 Use of tocilizumab for treatment of 21 patients with severe COVID-19 disease was reported by Xu et al, who described rapid improvements in fever, oxygenation, and chest CT, decrease in C-reactive protein, and normalization of lymphocyte counts.14 However, this was a single-arm trial with some patients receiving other therapies, thus, further data are awaited regarding the efficacy of tocilizumab. With regard to another recognized use of tocilizumab for cytokine release syndrome (CRS) associated with the administration of chimeric antigen-receptor T cells for leukemia, Gardner et al reported that early preemptive administration of tocilizumab might abrogate progression from mild CRS to severe CRS.15 Whether this is the case regarding the optimal timing of tocilizumab for COVID-19 remains to be established, but notably our patient improved rapidly after receiving tocilizumab, which was given shortly after he developed inflammatory manifestations, without waiting for progressive hypoxemia to occur. Of note, the extreme elevation of IL-6 levels in the aftermath of tocilizumab administration has been described, due to increased availability of IL-6 resulting from less binding to the IL-6 receptor.16
At our center, COVID-19 patients who are suspected of having CRS (regardless of transplant status) are considered for tocilizumab if a clinical trial is not available, with priority given to patients ≥18 years old with suspected, evolving CRS, clinical signs of severe disease (fever ≥ 38.3 C, hypotension, progressive hypoxemia, or sustained respiratory rate > 30 breaths/min), and elevated inflammatory markers (IL-6 > 100 pg/mL or 5-fold increase from a prior level, or D-dimer > 1 µg/mL, CRP ≥ 10 mg/mL, and ferritin > 750 ng/mL). The dose suggested is 8 mg/kg intravenously for 1 dose (with maximum dose not exceeding 800 mg).1 Although there are no formalized guidelines as yet for post-tocilizumab infection surveillance in SOT recipients with COVID-19, we have added acyclovir or valacyclovir prophylaxis for herpes simplex virus/varicella zoster virus for 3 months, cytomegalovirus PCR monitoring for 3 months, and baseline and follow-up fungal biomarkers (serum beta-d-glucan and galactomannan).
The optimal management of transplant immunosuppression in the setting of COVID-19 is also the subject of ongoing discussions. Analogous to other viral infections, reduction or discontinuation of mycophenolate mofetil (MMF) has been advocated. A 2012 study suggested that replication of human coronaviruses relies on intact immunophilin pathways and may be inhibited by tacrolimus.17 Our multidisciplinary team continued the patient’s tacrolimus, both to protect graft function and also to prevent an exacerbation of inflammatory response to viral infection.
Our patient also illustrates that duration of COVID-19 viral shedding can be protracted. In previous studies, patients with prolonged shedding for up to 25 days have been described, with a tendency for those with mild COVID-19 infection to show more rapid viral clearance.1 , 18 However, Wölfel et al described prolonged viral shedding in sputum in patients with mild COVID-19 infection without significant underlying disease.19 While our patient did not progress to severe disease, his prolonged viral shedding might be explained by his immunocompromised status. Further studies of time to virologic clearance in transplant recipients will be valuable.
Given the contagiousness of SARS-CoV-2, bedside lung ultrasound has become an alternative to chest CT in evaluating COVID-19 patients with the advantages of real-time interpretation, ease of serial evaluations, minimal radiation, better infection control, and reduction of exposure.7 The thickened pleural lines, confluent B-lines, and subpleural consolidations on lung ultrasound correspond to findings on chest CT.7 Although lung ultrasound cannot detect lesions deep within the lungs,7 ultrasound performed in patients with peripheral lung involvement may help track the evolution of disease and offer additional relevant data to help establish clinical trajectory.
In conclusion, we report a case of a hemodialysis-dependent liver transplant recipient with liver cancer, with COVID-19 pneumonia, who successfully recovered while continuing maintenance immunosuppression with tacrolimus. While the value of off-label use of tocilizumab in COVID-19 remains to be confirmed, the timing of tocilizumab as immunomodulatory therapy shortly after the onset of the inflammatory phase, rather than after progression to full respiratory failure, is noteworthy. Future studies of tocilizumab and other immunomodulators should note at which stage of COVID-19 illness these medications were administered. There is an urgent need for future randomized controlled clinical trials to validate and optimize treatment approaches for immunosuppressed transplant recipients with COVID-19.
Acknowledgments
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Tania Jain MBBS: Consultancy for Takeda Oncology, Advisory board for CareDx. Derek M. Fine MD: Data and Safety Monitoring Board GlaxoSmithKline. The other authors have no conflict of interests to disclose.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
ENDNOTE
1 COVID-19 Treatment Guidance Writing Group of Johns Hopkins University: Auwaerter PA (Chair), Hoffman CJ (Lead author), Jain T (Co-author), Avdic E, Avery RK, Ambinder R, Cameron, AM, Chang LW, Chida NM, D’Alessio FR, Garibaldi BT, Ignatius E, Karaba A, Marr K, Shah PD, Stephens RS, Sullivan DJ, Weld ED. Clinical Recommendations for Available Pharmacologic Therapies for COVID-19. Johns Hopkins Medical Institutions internal document, 4/20/20.
REFERENCES
- 1.Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19 [published online ahead of print 2020]. Lancet Infect Dis. 10.1016/s1473-3099(20)30232-2 [DOI] [PMC free article] [PubMed]
- 2.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michaels MG, La Hoz RM, Danziger-Isakov L, et al. Coronavirus disease 2019: implications of emerging infections for transplantation. Am J Transplant. 2020;209:1–5. doi: 10.1111/ajt.15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu L, Xu X, Ma KE, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant [published online ahead of print 2020]. 2020;1-5. 10.1111/ajt.15869 [DOI] [PMC free article] [PubMed]
- 5.Gandolfini I, Delsante M, Fiaccadori E, et al. COVID-19 in kidney transplant recipients [published online ahead of print 2020]. Am J Transplant. 2020;1-8. 10.1016/j.kint.2020.03.018 [DOI] [PMC free article] [PubMed]
- 6.Fishman JA, Grossi PA. Novel coronavirus-19 (COVID-19) in the the immunocompromised transplant recipient: #Flatteningthecurve [published online ahead of print 2020]. Am J Transplant. 2020;1-7. 10.1111/ajt.15890 [DOI] [PMC free article] [PubMed]
- 7.Peng QY, Wang X, Zhang LN. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med. 2020;46:849–850. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;20 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Molina JM, Delaugerre C, Goff JL, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020. 10.1016/j.medmal.2020.03.006 [DOI] [PMC free article] [PubMed]
- 10.Hinton DM. Food and Drug Administration. FDA Emergency use authorization (EUA) of chloroquine and hydroxychloroquine. 28 Mar 2020. https://www-fda-gov.proxy1.library.jhu.edu/media/136534/download. Accessed April 3 2020.
- 11.Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, Han M, Li T, et al. Effective treatment of severe COVID 19 patients with tocilizumab [published online ahead of print 2020]. Proc Natl Acad Sci USA. 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed]
- 15.Gardner RA, Ceppi F, Rivers J, et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood. 2019;134:2149–2158. doi: 10.1182/blood.2019001463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimoto N, Terao K, Mima T, et al. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112:3959–3964. doi: 10.1182/blood-2008-05-155846. [DOI] [PubMed] [Google Scholar]
- 17.Carbajo-Lozoya J, Müller MA, Kallies S, et al. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165(1):112–117. doi: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020. 10.1038/s41586-020-2196-x. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.