Introduction
According to the National Institutes of Health, bladder cancer is the sixth most common cancer in the United States; 90% of those cases are urothelial carcinoma (UC). Typical metastatic sites of UC include bone, liver, and lung.1 Although cutaneous metastasis rates among primary internal malignancies are estimated to be between 2.9% and 5.8%,2,3 the cutaneous metastasis rate of UC is only 0.84%.2 UC is one of a small group of malignancies that is CK7+/CK20+; therefore, pathologic analysis of tissue specimens using immunohistochemistry can be helpful in differentiating UC from adenocarcinomas, which more commonly metastasize to the skin. Cutaneous metastases of UC indicate advanced disease and poor prognosis.
Livedo reticularis is a physical sign of the skin that presents as a net-like pattern consisting of interconnecting macular, violaceous rings. In most instances, livedo reticularis is a benign finding related to cold exposure, but prolonged or worsening livedo reticularis can indicate underlying vascular occlusion in the dermis or subcutis.4 Livedo racemosa is a pathologic form of livedo reticularis that is often associated with vaso-occlusive disorders such as Sneddon syndrome, antiphospholipid syndrome, and lymphocytic thrombophilic arteritis.5 Livedo racemosa presents as a violaceous, net-like pattern comprising rings that are comparatively more irregular, discontinuous, or branching than that of livedo reticularis. Both livedo reticularis and livedo racemosa arise secondary to dilation of the skin's venous plexus, which may be caused by vasospasm, vasculopathy, or coagulopathy.4 Reports of livedo reticularis are relatively common, and livedo racemosa has been reported in up to 75% of patients with antiphospholipid syndrome secondary to systemic lupus erythematosus. However, few cases of livedo reticularis or racemosa related to malignancy have been reported in the literature. These cases include livedo patterns developing on the skin overlying inflammatory breast cancer6 and in patients with acute lymphocytic leukemia7 and lymphoma.8 In each of these cases, vascular obstruction was the suggested cause of the livedo pattern; however, none specifically describes the vascular obstruction as resulting from tumor embolism.
Case report
Our patient is an 82-year-old man with cutaneous metastases and vaso-occlusion secondary to metastatic UC. He initially presented to the dermatology clinic for evaluation of painful, bleeding papules and nodules in the groin of 1-month duration. He noticed a rash in his groin that extended to his right thigh 4 to 5 months prior to eruption of groin lesions.
Medical history was significant for UC diagnosed 18 months before his initial presentation in the dermatology department as well as hypothyroidism and hypertension. Initial diagnosis of UC was confirmed through biopsy of the posterior bladder wall, which showed reactive, focally denuded urothelial epithelium with marked interstitial chronic and focal giant cell inflammation. The specimen did not show invasive carcinoma. His bladder cancer was resistant to initial therapy with intravesicular Bacillus Calmette-Guerin resulting in metastasis to regional lymph nodes, and he subsequently began chemotherapy with gemcitabine and carboplatin 13 months after initial diagnosis. He completed 5 cycles of gemcitabine/carboplatin treatment over the course of 5 months before presentation at the dermatology clinic.
On physical examination, the patient was found to have multiple erythematous, ulcerated papules and nodules in the right groin and erythematous-to-violaceous patches in an irregular, branching or broken reticular pattern on the lower abdomen, right groin, and right thigh. A shave biopsy, Specimen A (Fig 1) was obtained from the groin, and 0.4-cm punch biopsies, specimens B and C, were obtained from the thigh (Fig 2).
Fig 1.
Cutaneous metastasis of UC of right groin. Site of collection of specimen A noted.
Fig 2.
Livedo racemosa on right thigh. Locations of specimens B and C noted.
Microscopic examination of specimen A (Fig 3, A) confirmed a dermal malignancy comprised of large atypical cells with large, pleomorphic nuclei and ample pale, eosinophilic cytoplasm. The cells were arranged as nests and cords throughout the thickness of the reticular dermis. Numerous atypical mitotic figures were identified. The neoplastic cells showed strong immunoreactivity for pancytokeratin, CK7 (Fig 3, B), and CK20 (Fig 3, C).
Fig 3.
A, Photomicrograph of nodular groin lesion shows large atypical cells arranged in nests and cords throughout the reticular dermis consistent with dermal malignancy. Neoplastic cells showed strong immunoreactivity to B, CK7 stain and C, CK20 stain, consistent with UC.
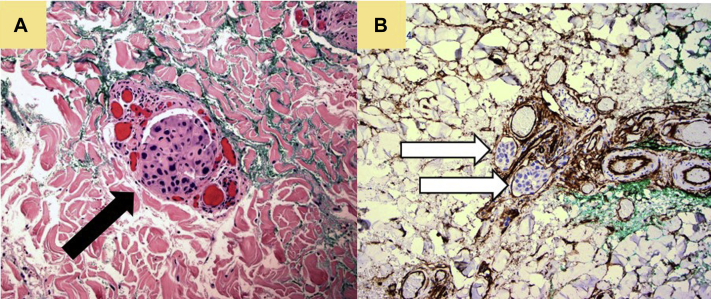
Immunohistochemical staining of specimens B and C (Fig 4, A) showed small, cohesive clusters of cells morphologically identical to those in specimen A, positioned within the superficial and deep reticular dermis. Notably, intravascular emboli comprised of cohesive tumor cells were identified in the papillary and deep reticular dermis as evidenced using endothelial cell markers CD31 (not shown) and CD34 (Fig 4, B) which delineate the vessel lumens.
Fig 4.
Photomicrograph of punch biopsy taken from livedoid rash on patient's thigh. A, Tissue stained with hematoxylin-eosin stain with cells morphologically identical to those found in the nodular groin lesion. Neoplastic cells are present within lumen of vessel (black arrow). B, Tissue stained with endothelial cell marker CD34 shows neoplastic cells present within vessel lumen (white arrows).
Discussion
Cutaneous metastasis of UC is rare. The skin is an uncommon location for metastasis among all visceral cancers, but it is exceptionally uncommon in UC, presenting in less than 1% of cases. Diagnosis of cutaneous metastasis can be aided by immunohistochemical testing and with increased clinical suspicion in the context of a recent history of malignancy. Few cancers are CK7+/CK20+ including UC, pancreatic adenocarcinoma, ovarian mucinous carcinoma, bladder adenocarcinoma, gastric adenocarcinoma, and cholangiocarcinoma.9 Eighty-nine percent of UCs are CK7+/CD20+.10 Tumors that more commonly metastasize to the skin, such as breast and lung (CK7+/CK20-) or colorectal (CK7-/CK20+) adenocarcinoma, may be ruled out based on the typical immunohistochemical results of metastatic UC biopsies.
Livedo reticularis and livedo racemosa in the setting of malignancy are rarely reported. Malignancy may produce a hypercoagulable state because of tumor cell activation of the coagulation cascade through a variety of pathways.11 It is estimated that between 10% and 15% of people with cancer experience venothromboembolism,12 and it is known that thrombosis is the second leading cause of death in cancer patients.13 Livedo reticularis and racemosa are reported to be caused by thromboemboli composed of platelets, cholesterol, eosinophils, and oxalate crystals.4 Vascular tumor embolism has not been documented in the literature as a cause of livedo reticularis or livedo racemosa. Although vascular tumor embolism is not common, it has important clinical implications, as traditional anticoagulants are not an appropriate treatment.
There is debate over the differentiating criteria between livedo reticularis and livedo racemosa. Based on the broken rings in the livedoid pattern, it was determined that our patient exhibited livedo racemosa.
We present a rare case of cutaneous metastasis of UC further complicated by a novel case of livedo racemosa secondary to vascular tumor embolism. Skin metastases have variable presentations, warranting further investigation of unusual lesions even in the absence of a clinical history of stage IV cancer.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
Presented at the Western Medical Research Conference. January 24-26, 2020. Carmel, California.
References
- 1.National Cancer Institute [Internet] Metastatic cancer. 2017. 2019. Accessed May 20, 2020. https://www.cancer.gov/types/metastatic-cancer Available at.
- 2.Mueller T.J., Wu H., Greenberg R.E. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63(6):1021–1026. doi: 10.1016/j.urology.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Krathen R.A., Orengo I.F., Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96(2):164–167. doi: 10.1097/01.SMJ.0000053676.73249.E5. [DOI] [PubMed] [Google Scholar]
- 4.Sajjan V.V., Lunge S., Swamy M.B., Pandit A.M. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6(5):315–321. doi: 10.4103/2229-5178.164493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolognia J.L., Schaffer J.V., Cerroni L. 4th ed. Elsevier; Philadephia, PA: 2017. Dermatology. [Google Scholar]
- 6.Spiers E.M., Fakharzadeh S.S. Livedo reticularis and inflammatory carcinoma of the breast. J Am Acad Dermatol. 1994;31:689–690. doi: 10.1016/s0190-9622(08)81748-3. [DOI] [PubMed] [Google Scholar]
- 7.Popkin G.L., Brodie S.J. Livedo reticularis in association with acute lymphocytic leukemia: report of one case. Arch Dermatol. 1965;92:160–161. [PubMed] [Google Scholar]
- 8.Fleischer A.B., Resnick S.D. Livedo reticularis. Dermatol Clin. 1990;8:347–354. [PubMed] [Google Scholar]
- 9.Selves J., Long-Mira E., Mathieu M.C., Rochaix P., Ilie M. Immunohistochemistry for diagnosis of metastatic carcinomas of unknown primary site. Cancers (Basel) 2018;10(4):108. doi: 10.3390/cancers10040108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang N.P., Zee S., Zarbo R.J., Bacchi C.E., Gown A.M. Coordinate expression of cytokeratins 7 and 20 defines unique subsets of carcinomas. Appl Immunhistochem. 1995;3:99–107. [Google Scholar]
- 11.Hisada Y., Mackman N. Cancer-associated pathways and biomarkers of venous thrombosis. Blood. 2017;130(13):1499–1506. doi: 10.1182/blood-2017-03-743211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caine G.J., Stonelake P.S., Lip G.Y.H., Kehoe S.T. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia. 2002;4(6):465–473. doi: 10.1038/sj.neo.7900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khorana A.A. Venous thromboembolism and prognosis in cancer. Thromb Res. 2010;125(6):490–493. doi: 10.1016/j.thromres.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]