Abstract
Management of large acetabular bone defects is challenging. The Masquelet technique has successfully reconstructed segmental defects in long bones arising from trauma, tumors, or infection but not been described for large acetabular defects. We present 3 cases of large acetabular bone defects arising from chronic prosthetic joint infection, treated via a novel induced membrane bone grafting technique, drawn from the Masquelet technique. All cases showed satisfactory clinical and radiological outcomes at midterm follow-up. This technique holds promise and can be an alternative means when treating large acetabular bone defects.
Keywords: Acetabular bone defect, Bone grafting, Induced membrane, Clinical outcomes
Introduction
The management of large bone defects, secondary to either trauma or infection, has been traditionally challenging. The Masquelet technique was initially described in 1986, as a 2-stage procedure to facilitate reconstruction of large bony defects of up to 25 cm [[1], [2], [3]]. The first stage involves thorough debridement of devitalized tissue, followed by soft-tissue reconstruction and insertion of a polymethylmethacrylate (PMMA) cement spacer at the site of the bone defect. This technique allows the creation of a self-induced membrane around the space, which provides mechanical and biological advantages. The hypervascularized nature promotes osteoinduction and corticalization while reducing the risk of resorption [4]. After a PMMA-induced membrane is achieved around 6-8 weeks later, the spacer is removed, and a bone-graft procedure is carried out to fill the bone defects. The PMMA spacer has been replaced with other superior alternatives such as calcium sulfate [5] as a localized antibiotic delivery vehicle and bone substitute, but the techniques and concepts remain largely similar and applicable in today’s context.
A recent systematic review in 2016 collated data of available studies and reported a union rate of up to 89.7% and also noted that infections rectified in 91.1% of cases [6]. In the same recent systematic review, it was demonstrated that the most common area operated on was the tibia (67.2%), followed by the fibula (12.9%) and femur (19.4%). There has, however, never been use of such technique in the acetabulum or hip joint as it is traditionally used to treat diaphyseal and metaphyseal bone defects of the lower extremities. It is carried out to mitigate bone loss with the eventual aim of restoring limb function [7]. Authors of preceding literature have found the method especially useful in femoral defects and bilateral tibial defects [8].
Contraindications to this technique [9] include nonresolving infection, osteomyelitis, inadequate soft-tissue coverage in the region of a bone defect, or osteoporosis.
Our study is the first known case series that aims to describe the use of the induced membrane technique, drawn from the Masquelet technique, for management of large acetabular bone defects, due to osteomyelitis.
Case Histories
This series describes 3 cases of hip arthroplasty prosthetic joint infection (PJI) complicated by large acetabular bone defects, treated with our institution’s novel induced membrane bone-grafting technique from 2015 to 2017. Operations were performed by a single surgeon in a high-volume institution. Informed consent was obtained from all patients. Deidentified patient-specific information was used.
The inclusion criteria were chronic PJI with acetabular bony loss classified as either Paprosky IIC or IIIA [10].
All patients underwent 2-stage revision total hip arthroplasty (THA) using our induced membrane bone-grafting technique. A modified Hardinge lateral approach was used for all cases. The first-stage revision entailed removal of all infected implants and foreign material, followed by implantation of an antibiotic cement spacer. Synovasure Alpha Defensin lateral flow test (Zimmer Biomet, Warsaw, IN) was used to aid in diagnosis of PJI. The femoral canal was packed with either a dowel rod-coiled wire or Steinmann pin coated with 40 g of Simplex Bone Cement (Stryker, Kalamazoo, MI) and colored with methylene blue. The antibiotic mixture in the cement is composed of tobramycin and 2 g of vancomycin for each of the 3 cases. After coating the dowel rod, we packed cancellous bone chips into the acetabular defect and used a tamp to compact the bone chips. We then finger-packed the remaining cement into the acetabulum over the bone chips (Fig. 1). It is important to ensure that the cement is well packed into the acetabulum so it does not dislodge when the patient is up and moving around.
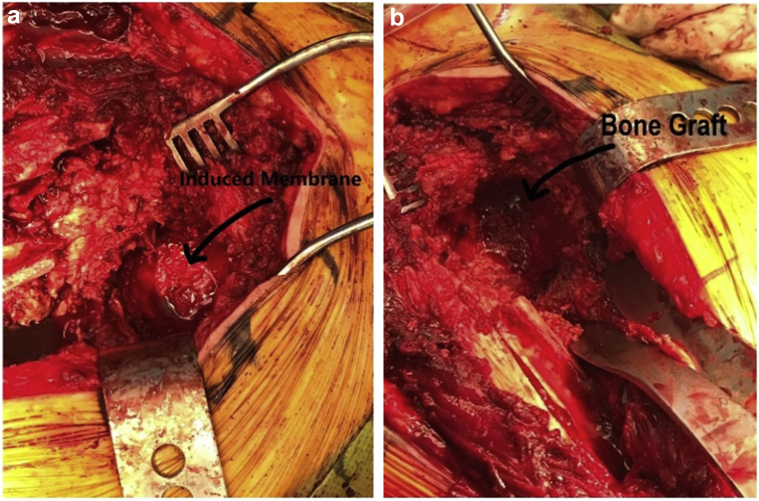
Figure 1.
Intraoperative image showing (a) pseudomembrane formation and (b) allogenic bone graft.
After the first-stage revision, the patient was kept on non–weight bearing status on the operated side until reimplantation. Patients were commenced on 6 weeks of intensive culture-specific intravenous antibiotic therapy, after which a 2-week antibiotic holiday was observed and repeat test for C-reactive protein (CRP) performed. We proceeded with the second-stage revision only when this reading had normalized.
In the second stage of the revision surgery, there was no difficulty encountered in removing the cement spacer as a pseudomembrane had formed between the cement spacer and the graft. It is important not to disturb this pseudomembrane for graft integration. Trabecular Metal Acetabular Revision System by Zimmer Biomet was then used. All patients received highly porous hemispherical shells without the need for augment as the bony defects were downsized with bone grafting. We ensured that at least 3 screws were inserted around the periphery of the cup. Burring was also performed to create screw tracks for additional screws. We used monoblock tapered titanium stems for the femur. Weight-bearing status gradually progressed from partial to full based on individual tolerance levels.
For data collection, patient demographic data, including age, gender, body mass index (BMI), smoking status, and comorbidities, were noted. Patient records and postoperative follow-up notes were reviewed to determine the following: presence of infection, spacer time, time from the initiation of the procedure to the commencement of full-weight bearing. They were also screened for the presence of secondary grafting procedures or complications. Functional outcome measures were conducted preoperatively and postoperatively at 2 years. They include Oxford Hip Scores, Western Ontario and McMaster Universities Osteoarthritis Index, and a 36-Item Short Form Survey. Preoperative, immediate postoperative, and final follow-up radiographs of the hip were used to assess progression of defect size and to determine time for radiographic consolidation.
All patients were followed up clinically and radiologically, with the mean follow-up duration exceeding 2 years.
Baseline characteristics were recorded as demonstrated in Table 1. The mean age was 77 years, and the mean BMI was 25.3 kg/m2.
Table 1.
Patient demographics and operative details.
| Age | Sex | BMI (kg/m2) | Stage 1 revision | Stage 2 revision | Date of the last follow-up | Duration of follow-up (months) |
|---|---|---|---|---|---|---|
| 75 | Female | 22 | May 2014 | June 2014 | September 2016 | 27 |
| 71 | Female | 31.9 | November 2014 | January 2015 | February 2017 | 25 |
| 86 | Female | 22 | May 2015 | July 2015 | September 2017 | 24 |
Case 1
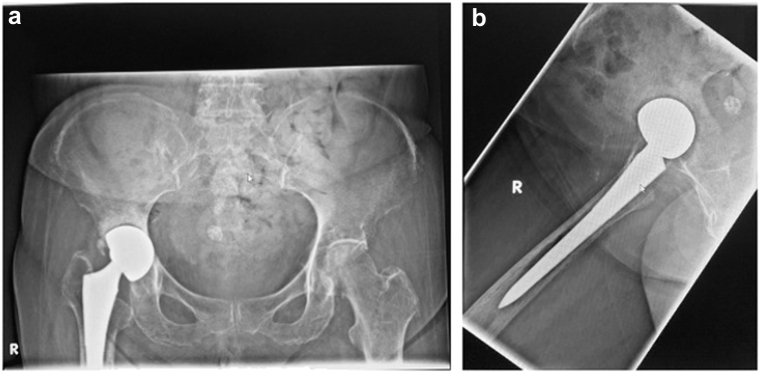
A 75-year-old female, with a BMI of 22.0, with underlying diabetes, hypertension, hyperlipidemia, and hypothyroidism on levothyroxine replacement and a history of the right THA in 1993 presented with worsening right hip pain over 1 year. Radiographs showed wear of the polyethylene liner, pedestal formation, and periarticular calcifications (Fig. 2).
Figure 2.
Preoperative pelvic radiographs: (a) the anteroposterior view; (b) the lateral view demonstrating wear of the polyethylene liner, pedestal formation, and periarticular calcifications.
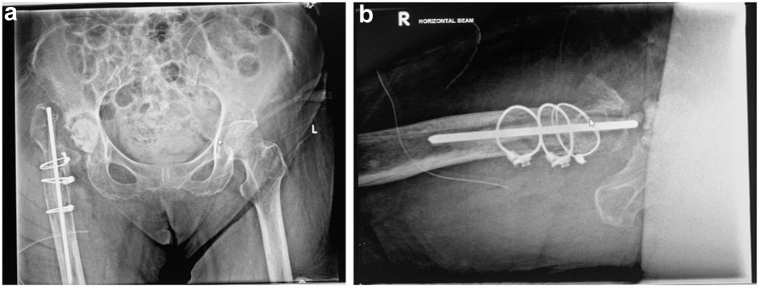
The patient underwent stage-1 revision THA as demonstrated in Figure 3. Large amount of caseous fluid was noted on breaching the joint capsule, tracking from the medial to lateral aspect of the femoral stem. Synovasure Alpha Defensin Lateral Flow Test (Zimmer Biomet, Warsaw, IN) was positive. Intraoperative findings included right-hip PJI with osteolysis and wear, as well as heterotropic ossification (Brooker 3). Medial wall protrusion and defect was noted, consistent with a grading of Paprosky 2C. A 15-cm extended transfemoral osteotomy was performed to remove the femoral stem. The acetabular cup and liner were removed. The femoral canal was prepared as per the process described previously. The patient was placed on non–weight bearing status on the operated side after the surgery until reimplantation. Multiple intraoperative cultures were negative, but she was treated presumptively for gram-positive PJI with 6 weeks of intravenous (IV) cefazolin. We proceeded further with stage 2 when CRP had normalized after a 2-week antibiotic holiday.
Figure 3.
Pelvic radiographs after first revision operation, showing insertion of a temporary femoral head prosthesis, as well as a bone graft packed into the medial floor in (a) anteroposterior view and (b) cross table true lateral hip view.
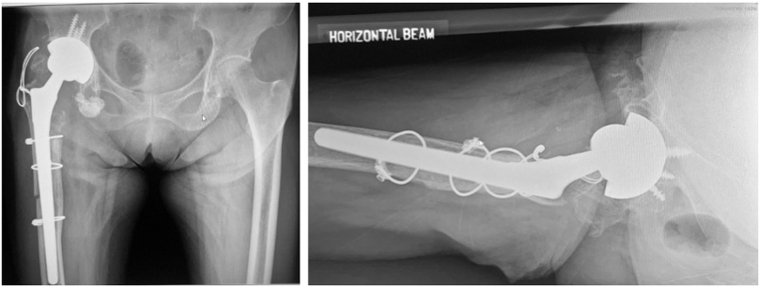
Stage 2 of revision was completed uneventfully. A monoblock tapered titanium stem used for the femur is shown in Figure 4. The patient sustained a greater trochanteric fracture during dislocation of the hip. This was fixed with cerclage wires. Partial weight-bearing instructions were given to the patient, and she was also informed to strictly avoid any abduction or adduction exercises for the next 6 weeks.
Figure 4.
Pelvic radiographs after second-stage revision operation, stable well-fixed new implants (highly porous hemispherical shell for the acetabulum and monoblock tapered titanium stems for the femur).
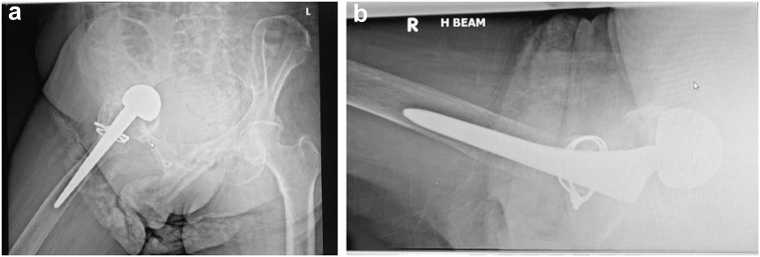
Radiographs at 2 years postoperatively (Fig. 5) showed stable implants and radiological consolidation.
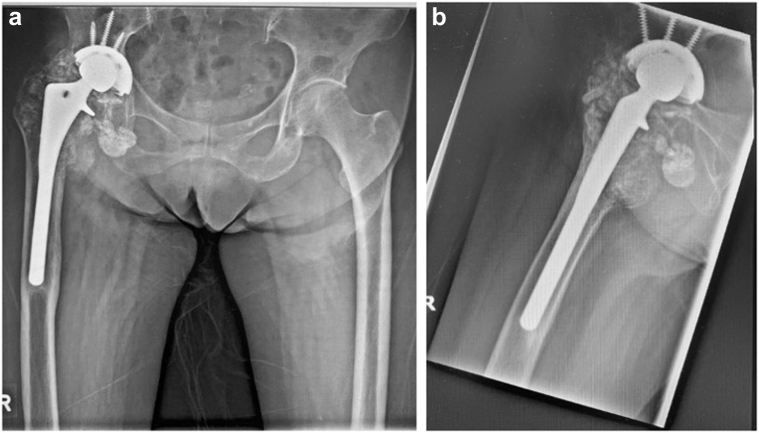
Figure 5.
Pelvic radiographs at 2 y postoperatively showing that the implant is stable, intact, and unchanged in position. Mild right acetabular protrusio is noted.
Case 2
A 71-year-old female with a BMI of 31.9 presented to the outpatient orthopaedic clinic with 3 days of right hip pain and inability to bear weight after an unwitnessed fall. This was on a background of previous right-hip bipolar hemiarthroplasty performed 5 years prior for a femoral neck fracture. Other relevant medical history included diabetes, hypertension, hyperlipidemia, mild congestive cardiac failure, stable peripheral vascular disease, and a history of pulmonary tuberculosis in remission.
Preoperative radiographs performed in the outpatient clinic revealed a right proximal femur periprosthetic fracture, associated with protrusio acetabuli (Fig. 6). Inpatient admission was arranged, and this was further confirmed on a computed tomography and magnetic resonance imaging of the pelvis (Fig. 7). Findings from these additional imaging did not reveal additional signs of loosening or infection.
Figure 6.
Preoperative radiograph of the pelvis in (a) judet and (b) lateral views, demonstrating Vancouver B2 periprosthetic fracture of the right-sided bipolar hemiarthroplasty, and protrusio acetabuli.
Figure 7.
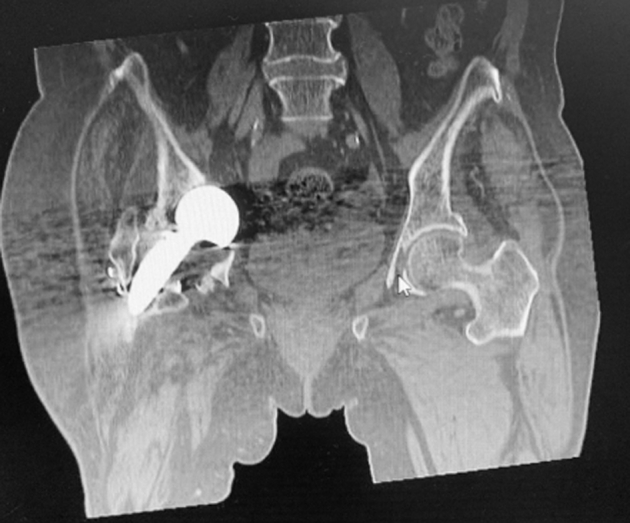
Computed tomography (coronal view) of the pelvis further demonstrates right prosthetic hip protrusio acetabuli, and a Vancouver B2 periprosthetic fracture.
She underwent right-hip aspiration under fluoroscopic guidance, and multiple studies including fluid cultures, cytology, gram stains, and tuberculosis testing did not reveal any signs of infection. However, elevated white cell count (13 × 10ˆ9 L), erythrocyte sedimentation rate (77 mm/h), and CRP (30 mg/L) were noted. Considering she had an episode of pulmonary tuberculosis 2-3 years ago, and protrusio acetabuli appeared chronic, a 2-stage revision hip arthroplasty was decided on in view of concerns of PJI.
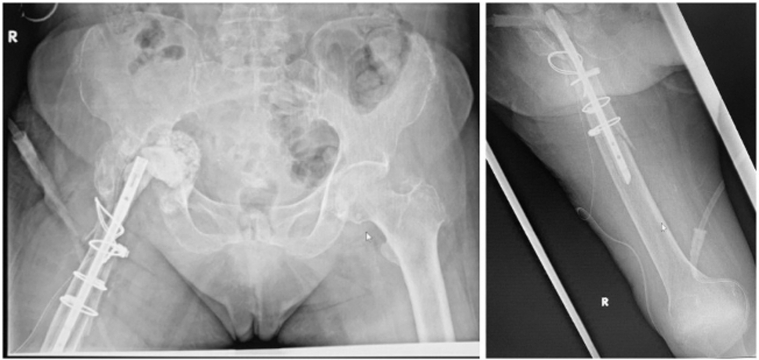
Postoperative radiographs after stage 1 of revision THA are shown in Figure 8. Intraoperatively, there were no signs of infection but significant amounts of fibrotic scar tissue around the implant and within the acetabulum. An extended trochanteric osteotomy was performed to expose the stem and gain access to the acetabulum. A Paprosky 2C acetabular defect was noted. The superior and anterior edges of acetabulum had to be osteotomized as they were impeding removal of the bipolar head. The femoral canal was prepared as per the surgical technique described previously.
Figure 8.
Radiographs showing an antibiotic cement spacer, with prosthesis for concurrent fixation of right proximal femoral fracture.
The patient was placed on non–weight bearing status on the operated side after the surgery until reimplantation. Tissue cultures grew Citrobacter koseri, and she was started on IV aztreonam for 6 weeks per our local infectious diseases department’s recommendations.
Similarly, a 2-week antibiotic holiday was adopted, and we proceeded with stage-2 revision when CRP was normalized after this period.
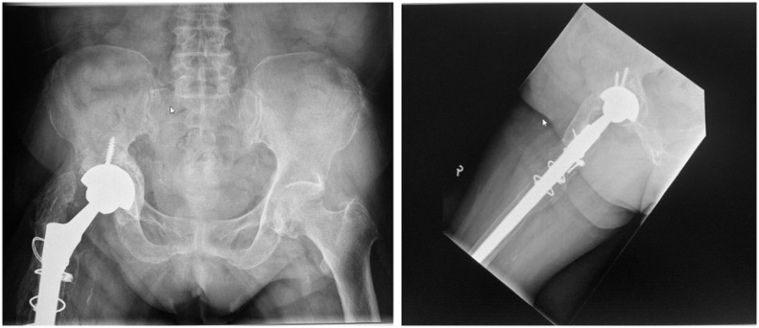
The patient was discharged to a community hospital for further rehabilitation shortly after and performed well. Postoperative radiographs at 2 years showed no radiological complications such as periprosthetic loosening (Fig. 9).
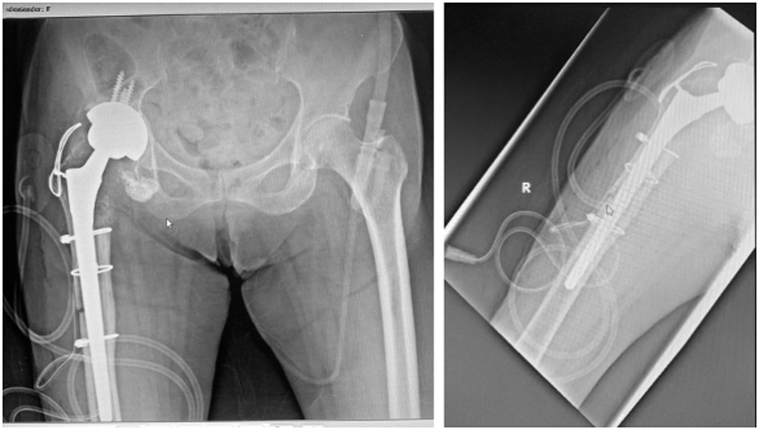
Figure 9.
Postoperative radiographs at 2 y showing good radiological graft integration with no periprosthetic loosening.
Case 3
An 86-year-old female, with a BMI of 22.0 with underlying diabetes, hypertension, hyperlipidemia, and a history of right bipolar hemiarthroplasty performed in 2011 for a right neck of femur fracture, presented to the outpatient clinic with a 3-month history of right hip pain.
Radiographs showed sclerosis of the right acetabulum, periprosthetic loosening, and early protrusion (Fig. 10). A technetium bone scan was also performed, showing no scintigraphy evidence of periprosthetic infection around the right-hip prosthesis.
Figure 10.
Pelvic radiographs: (a) anteroposterior and (b) lateral showing sclerosis of the right acetabulum, with early protrusion.
She underwent right stage-1 revision THA (Fig. 11), and a modified Hardinge lateral approach was adopted. Intraoperatively, chronic PJI with accelerated osteolysis and osteomyelitis was found. There was exuberant synovitis and florid pus. Synovasure Alpha Defensin Lateral Flow Test (Zimmer Biomet, Warsaw, IN) was positive. There was protrusion, with loss of the medial wall consistent with a Paprosky 2C defect. There was also proximal femoral loss.
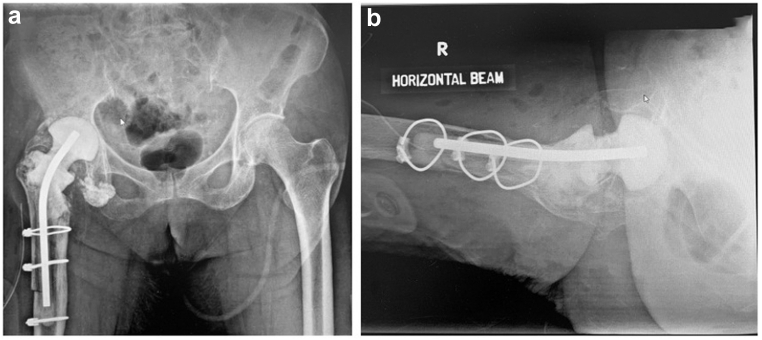
Figure 11.
Pelvic radiographs: (a) anteroposterior and (b) lateral after stage-1 revision THA, showing removal of right total hip arthroplasty, with a dowel rod in the femoral shaft, and a density within the right hip joint representing the antibiotic cement spacer.
As most of the pus was noted to accumulate posteriorly, the decision was made to convert to a posterior approach. The femoral canal was packed with a Steinmann pin coated with 40 g Simplex tobramycin (Stryker, Kalamazoo, MI) and colored with methylene blue. After coating, we packed cancellous bone chips into the acetabular defect and used a temp to compact the bone chips before finger-packing the remaining cement into the acetabulum over the bone chips.
The patient was placed on non–weight bearing status on the operated side after the surgery until reimplantation. Intraoperative cultures showed Candida albicans, and the patient was treated for fungal PJI. She was discharged to the community hospital and placed on IV fluconazole for 6 weeks via a peripherally inserted central catheter. She developed a reinfection, and repeat stage 1 was performed. A culture-directed antibiotic-impregnated (amphotericin and fluconazole) cement spacer block was used.
After a 2-week antibiotic holiday, CRP had normalized, and we proceeded with stage-2 revision THA, implanting a highly porous hemispherical shell and monoblock tapered titanium stem for the femur. She was discharged to a community hospital for further rehabilitation shortly after and performed well. Postoperative radiographs showed no radiological complications.
Discussion
In this study, we have successfully shown the viability of our induced membrane allogenic bone-grafting technique in treating infected hip hemiarthroplasties complicated by large acetabular bone defects.
Radiographic consolidation was achieved in all cases, and there was satisfactory downsizing of the bone defect in all cases. There were no reinfections, and bony union was achieved in all cases. There was improvement in mean patient-reported outcome measures during midterm follow-up as shown in Table 2.
Table 2.
Tabulation of preoperative and postoperative patient-reported outcome measures.
| Scores | Preoperative mean | 2-y Postoperative mean |
|---|---|---|
| WOMAC scores | ||
| Pain | 76 | 100 |
| Stiffness | 65 | 95 |
| Physical function | 46 | 91 |
| SF-36 scores | ||
| Physical functioning (PF) | 45 | 85 |
| Role physical (RP) | 0 | 50 |
| Bodily pain (BP) | 41 | 84 |
| General health (GH) | 72 | 97 |
| Vitality (VT) | 55 | 90 |
| Social function (SF) | 25 | 100 |
| Role emotional (RE) | 67 | 100 |
| Mental health (MH) | 67 | 100 |
| Oxford Hip Score | ||
| Score | 34 | 14 |
WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; SF-36, 36-Item Short Form Survey.
All cases achieved good bone union, which is in line with previous studies reporting bone union rates of 82%-90% [[11], [12], [13]]. Although there was a high degree of variability in time to weight bearing and consolidation observed in our study group, it is worthwhile to note that all patients eventually progressed to pain-free weight bearing.
The Masquelet technique was initially designed for the treatment of bone defects caused by infection. It hinges on the concept of a membrane-induced technique that aids in bone reconstruction promotion by preventing the bone-graft resorption and also playing an important role in revascularization and consolidation. The use of a spacer also helps to maintain the defect and inhibit fibrous ingrowth [3]. Owing to its success, its applications have widely expanded, and there is abundance of literature that describes how it is successfully used to treat post-traumatic bone loss in long bones of the lower extremities [4,12,14], tumors [15], osteomyelitis, infected nonunions, and tibia pseudarthrosis [16]. There has also been increasing expansion of usage, including but not limited to, the hand [17] and feet [18].
To date, our study is the only study, albeit a low-volume case series, that evaluates the outcomes of a similar technique drawn from the Masquelet technique, on the hip, specifically large acetabular bone defects. Our induced membrane technique differs from the true Masquelet technique in that the secondary bone is formed in the central acetabulum before removal of the antibiotic spacer. The temporary cement spacer inserted in stage-1 revision served as a template for secondary ossification around the central acetabular defect.
There are a few key advantages to this technique that provides feasibility and versatility in treatment. Bone union is not directly related to the defect size, and the technique can be combined with a variety of implants, allowing surgeons to choose the best implant, considering location of and size of the defect, as well as severity of infection [13].
Regarding operative considerations, the graft source may be autologous, homologous, or mixed and can be supplemented with growth factors. We decided to use an allograft rather than autologous bone graft in managing massive bone defects as autologous grafts have demonstrated an increased risk of resorption and suboptimal healing when the defect is over 4 to 5 cm [19].
The benefit of this technique over an articulating spacer is that large acetabular defects often are unable to accommodate an articulating spacer because of acetabular bone loss. In addition to local antibiotic delivery from cement, formation of the pseudomembrane also makes revision surgical dissection easier, especially when trying to identify pelvic and acetabular landmarks. Bone grafting also downsized the amount of bone loss so that hemispherical cups may be used, making subsequent reimplantation surgery easier.
Alternatives that may be considered in treating these defects include antiprotrusio cages and custom triflange acetabular prostheses. For supplementation of the acetabular bed, modular metallic augments, structural allograft, and morselized impacted allograft are all plausible options [20].
Our case series was limited by its retrospective nature with low patient volume. There was variability in time to weight bearing and radiographic consolidation, but all patients were ultimately able to progress to full-weight bearing. Further prospective large-scale randomized and comparative studies are required to validate and support the efficacy of our proposed technique.
Summary
Our induced membrane bone-grafting technique has shown preliminary success in treating large bone defects arising from previous hip hemiarthroplasty PJI. All cases showed satisfactory clinical and radiological outcomes at midterm follow-up. This technique holds promise and can be an alternative means when treating large acetabular bone defects.
Conflict of interest
The authors declare there are no conflicts of interest.
Appendix A. Supplementary data
References
- 1.Masquelet A.C., Fitoussi F., Begue T., Muller G.P. [Reconstruction of the long bones by the induced membrane and spongy autograft] Ann Chir Plast Esthet. 2000;45:346. [PubMed] [Google Scholar]
- 2.Masquelet A.C. Muscle reconstruction in reconstructive surgery: soft tissue repair and long bone reconstruction. Langenbecks Arch Surg. 2003;388:344. doi: 10.1007/s00423-003-0379-1. [DOI] [PubMed] [Google Scholar]
- 3.Masquelet A.C., Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am. 2010;41:27. doi: 10.1016/j.ocl.2009.07.011. table of contents. [DOI] [PubMed] [Google Scholar]
- 4.Apard T., Bigorre N., Cronier P., Duteille F., Bizot P., Massin P. Two-stage reconstruction of post-traumatic segmental tibia bone loss with nailing. Orthop Traumatol Surg Res. 2010;96:549. doi: 10.1016/j.otsr.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Jiang N., Qin C.-H., Ma Y.-F., Wang L., Yu B. Possibility of one-stage surgery to reconstruct bone defects using the modified Masquelet technique with degradable calcium sulfate as a cement spacer: a case report and hypothesis. Biomed Rep. 2016;4:374. doi: 10.3892/br.2016.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morelli I., Drago L., George D.A., Gallazzi E., Scarponi S., Romanò C.L. Masquelet technique: myth or reality? A systematic review and meta-analysis. Injury. 2016;47:S68. doi: 10.1016/S0020-1383(16)30842-7. [DOI] [PubMed] [Google Scholar]
- 7.Giannoudis P.V., Faour O., Goff T., Kanakaris N., Dimitriou R. Masquelet technique for the treatment of bone defects: tips-tricks and future directions. Injury. 2011;42:591. doi: 10.1016/j.injury.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 8.Olesen U.K., Eckardt H., Bosemark P., Paulsen A.W., Dahl B., Hede A. The Masquelet technique of induced membrane for healing of bone defects. A review of 8 cases. Injury. 2015;46(Suppl 8):S44. doi: 10.1016/S0020-1383(15)30054-1. [DOI] [PubMed] [Google Scholar]
- 9.Krappinger D., Lindtner R.A., Zegg M., Dal Pont A., Huber B. [Masquelet technique for the treatment of large dia- and metaphyseal bone defects] Oper Orthop Traumatol. 2015;27:357. doi: 10.1007/s00064-014-0300-9. [DOI] [PubMed] [Google Scholar]
- 10.Paprosky W.G., Perona P.G., Lawrence J.M. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6-year follow-up evaluation. J Arthroplasty. 1994;9:33. doi: 10.1016/0883-5403(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 11.Taylor B.C., Hancock J., Zitzke R., Castaneda J. Treatment of bone loss with the induced membrane technique: techniques and outcomes. J Orthop Trauma. 2015;29:554. doi: 10.1097/BOT.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 12.Karger C., Kishi T., Schneider L., Fitoussi F., Masquelet A.-C., French Society of Orthopaedic Surgery and Traumatology (SoFCOT) Treatment of posttraumatic bone defects by the induced membrane technique. Orthop Traumatol Surg Res. 2012;98:97. doi: 10.1016/j.otsr.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Stafford P.R., Norris B.L. Reamer-irrigator-aspirator bone graft and bi Masquelet technique for segmental bone defect nonunions: a review of 25 cases. Injury. 2010;41(Suppl 2):S72. doi: 10.1016/S0020-1383(10)70014-0. [DOI] [PubMed] [Google Scholar]
- 14.Wong T.M., Lau T.W., Li X., Fang C., Yeung K., Leung F. Masquelet technique for treatment of posttraumatic bone defects. ScientificWorldJournal. 2014;2014:710302. doi: 10.1155/2014/710302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villemagne T., Bonnard C., Accadbled F., L’kaissi M., de Billy B., Sales de Gauzy J. Intercalary segmental reconstruction of long bones after malignant bone tumor resection using primary methyl methacrylate cement spacer interposition and secondary bone grafting: the induced membrane technique. J Pediatr Orthop. 2011;31:570. doi: 10.1097/BPO.0b013e31821ffa82. [DOI] [PubMed] [Google Scholar]
- 16.Gouron R., Deroussen F., Juvet M., Ursu C., Plancq M.C., Collet L.M. Early resection of congenital pseudarthrosis of the tibia and successful reconstruction using the Masquelet technique. J Bone Joint Surg Br. 2011;93:552. doi: 10.1302/0301-620X.93B4.25826. [DOI] [PubMed] [Google Scholar]
- 17.Flamans B., Pauchot J., Petite H. [Use of the induced membrane technique for the treatment of bone defects in the hand or wrist, in emergency] Chir Main. 2010;29:307. doi: 10.1016/j.main.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Makridis K.G., Theocharakis S., Fragkakis E.M., Giannoudis P.V. Reconstruction of an extensive soft tissue and bone defect of the first metatarsal with the use of Masquelet technique: a case report. Foot Ankle Surg. 2014;20:e19. doi: 10.1016/j.fas.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Weiland A.J., Phillips T.W., Randolph M.A. Bone grafts: a radiologic, histologic, and biomechanical model comparing autografts, allografts, and free vascularized bone grafts. Plast Reconstr Surg. 1984;74:368. [PubMed] [Google Scholar]
- 20.Taylor E.D., Browne J.A. Reconstruction options for acetabular revision. World J Orthop. 2012;3:95. doi: 10.5312/wjo.v3.i7.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.