Abstract
Retinal photoreceptor degeneration occurs frequently in several neurodegenerative retinal diseases such as age-related macular degeneration, retinitis pigmentosa, or genetic retinal diseases related to the photoreceptors. Despite the impact on daily life and the social and economic consequences, there is no cure for these diseases. Considering this, cell-based therapy may be an optimal therapeutic option. This study evaluated the neuroprotective in vitro potential of a secretome of human bone marrow mesenchymal stem cells (MSCs) for retinal photoreceptors in vitro. We analyzed the photoreceptor morphologic changes and the paracrine factors secreted by human bone marrow MSCs in a physically separated co-culture with degenerated neuroretinas, using organotypic neuroretinal cultures. The results showed that the secretome of human bone marrow MSCs had a neuroprotective effect over the neuroretinal general organization and neuropreserved the photoreceptors from degeneration probably by secretion of neuroprotective proteins. The study of the expression of 1,000 proteins showed increased paracrine factors secreted by MSCs that could be crucial in the neuroprotective effect of the stem cell secretome over in vitro retinal degeneration. The current results reinforce the hypothesis that the paracrine effect of the human bone marrow MSCs may slow photoreceptor neurodegeneration and be a therapeutic option in retinal photoreceptor degenerative diseases.
Keywords: neuroretina, mesenchymal stem cells, photoreceptors, advanced therapies, cell therapy, neuroprotection, neurodegeneration, secretome
Graphical Abstract

Usategui-Martín and colleagues evaluated the neuroprotective effect of the mesenchymal stem cell (MSC) secretome over in vitro retinal photoreceptor degeneration, and they identify the neuroprotective factors secreted by MSCs as a potential therapy for neurodegenerative retinal diseases. This is the first study analyzing the MSC secretome under experimental neuroretina degeneration.
Introduction
Retinal neurodegeneration, the main pathogenic mechanism involved in multiple eye diseases that are the most frequent causes of incurable low vision and blindness worldwide, includes retinal deterioration resulting from different causes that leads to progressive degeneration and cellular death. This neuronal death usually is accompanied by a glial cell response that replaces the visual neurons. Retinal photoreceptor degeneration occurs frequently in several neurodegenerative retinal diseases, e.g., age-related macular degeneration, diabetic retinopathies, retinitis pigmentosa, and genetic retinal diseases related to the photoreceptors.1 Reportedly, 240 million patients have photoreceptor-related degeneration worldwide.2
Despite the impact on daily life and the social and economic consequences, there is no cure for most retinal diseases in which photoreceptor atrophy and death are the main causes of impaired vision. Advanced therapies may be treatment options, of which cell-based therapy may be one of the most viable alternatives. Current research in stem cell therapy for retinal neurodegeneration is based on two main therapeutic approaches: replacement of damaged cells and neuroprotection via the paracrine stem cell properties.1,3,4 Cell-based therapy via the paracrine neuroprotective effects may be the most promising alternative. Although it has been reported that stem cell-derived photoreceptors may integrate and/or transfer material to the retinal cells,5, 6, 7 other studies have suggested that the retina does not provide a permissive environment in which stem cells can migrate, integrate, and function.6,8,9 However, several proteins secreted by stem cells are associated with a slowdown of the retina neurodegenerative process.1,10,11 Thus, advanced investigations to determine the composition of the stem cell neuroprotective secretome are crucial and will be the basis for future production of cell-free “secretome cocktails” with effective neuroprotective capacity over retinal degenerative diseases.
The cells that are studied most in cell-based therapy are mesenchymal stem cells (MSCs) because they are rapidly expandable, easily isolated, and are not associated with ethical issues.12,13 MSCs also are hypoimmunogenic or immunoprivileged because the major histocompatibility complex of class II (MHC-II) or the co-stimulatory molecules (CD40, CD80, and CD86) involved in transplant rejection are not expressed on their surface.14 However, some studies have suggested that cell-mediated and humoral immune responses to MHC mismatched with MSCs occur and the authors thereby defined the MSCs as immune evasive.15,16 Therefore, allogeneic transplantation of these cells has attractive advantages because they do not require host immunosuppression.14
In our previous studies, we have reported that the intravitreal injection of human MSCs is safe, well tolerated, and bio-available in the vitreous cavity from 2 to 6 weeks.17 This study was requested by the Spanish Agency for Drugs and Medical Devices before the authorization to use MSCs in a phase I/II clinical trial to evaluate the safety of intravitreal injection of human MSCs in patients with non-arteritic acute ischemic optic neuropathy (ClinicalTrials.gov: NCT03173638). Also, we have established that human MSCs improve the survival and maintenance of retinal ganglion cells (RGCs) via the paracrine neuroprotective potential of the MSCs.11 Our hypothesis is based on the idea that the paracrine effect of the human bone marrow MSCs also may produce a slow photoreceptor neurodegeneration. Thus, the current study evaluated in vitro the potential neuroprotection effect of human bone marrow MSCs in a porcine retinal photoreceptor degeneration model and identified some of the paracrine factors secreted in co-cultures of neuroretinas with human stem cells.
Results
Neuroretinal General Morphology, Morphometry, and Nuclei Counts
The results of the analyses of the neuroretinal general morphology, morphometry, and nuclei counts are summarized in Figures 1 and 2. Fresh porcine neuroretinal samples had a clearly defined layered retinal structure and adequate cellular preservation before culturing, with perfectly defined photoreceptor outer segments (OSs) and inner segments (ISs) (Figure 1A). The mean total neuroretinal thickness was 177.24 ± 1.65 μm (Figure 2A) and the mean number of nuclei/μm2 was 124.33 ± 1.53 (Figure 2B).
Figure 1.
General Morphology of Neuroretinas
(A–D) Neuroretinas general morphology before in vitro culture (A), after 3 days of culture (B), after co-culture with mesenchymal stem cells from Valladolid (MSCVs) for 3 days (C), and after co-culture with HEK293T cells for 3 days (D). Scale bar, 25 μm.
Figure 2.
Neuroretinal Morphometry
(A and B) Comparison of neuroretinal morphometry (A) and nuclei counts (B) in fresh neuroretinas, neuroretinas cultured for 3 days, neuroretinas co-cultured with MSCVs for 3 days, and neuroretinas co-cultured with HEK293T cells for 3 days. NR, neuroretina. ∗p < 0.05.
The general layered structure of the neuroretinas was preserved after 72 h of culture but with extensive photoreceptor degeneration. The OS photoreceptors were primarily absent, and those in the ISs were shorted, compacted, and edematous (Figure 1B) compared with the fresh controls. The thicknesses of the outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), and ganglion cell layer (GCL) were decreased significantly compared with the fresh neuroretinas (Figure 2A). The mean total number of nuclei/μm2 (88.66 ± 4.04) and the number of nuclei/μm2 in the ONL were also lower than in the fresh neuroretina (Figure 2B).
The neuroretinas co-cultured with MSCs from Valladolid (MSCVs) for 72 h were better preserved with shortened rod OSs and edematous cone ISs (Figure 1C). Compared with the fresh neuroretinas, there were no significant differences in the total thickness (183.06 ± 10.89 μm) and the retinal layer thickness (Figure 2A) or in the total number of nuclei/μm2 (125 ± 1) and the number of nuclei/μm2/layer (Figure 2B).
In the neuroretinas co-cultured with HEK293T cells for 72 h, the degenerative neuroretinal modifications were similar to those observed in the neuroretinas after 72 h in the untreated retinal culture. The OS and IS photoreceptors were not differentiated and nuclear layer disorganization was observed (Figure 1C). The ONL, OPL, INL, and GCL were significantly thinner than the fresh neuroretinas and the neuroretinas co-cultured with MSCVs for 72 h (Figure 2A). There were fewer total numbers of nuclei/μm2 (88.66 ± 4.04) and nuclei/μm2 in the ONL compared with the fresh neuroretinas and the neuroretinas co-cultured with MSCVs for 72 h (Figure 2B).
Immunochemical Characterization
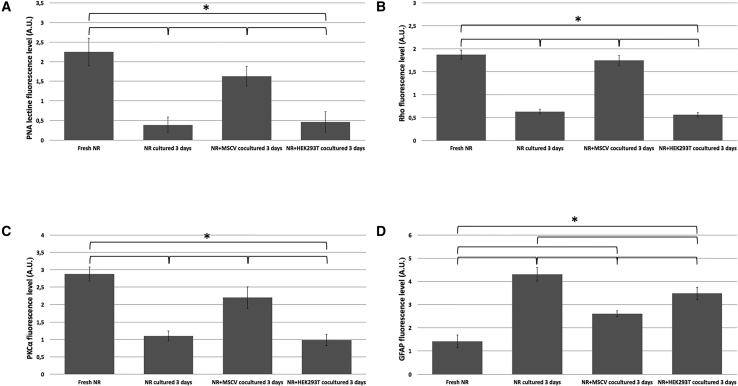
The peanut agglutinin (PNA) lectin marker was used to identify and evaluate the cones (Figures 3A–3D). PNA is specific for galactosyl-(β-1,3)-N-acetylgalactosamine; therefore, PNA lectin binds to the extracellular matrix around the cone OSs.22 The immunoexpression of PNA lectin in fresh neuroretinas was uniformly distributed in the cone OSs (Figure 3A). After 72 h of culture, the neuroretinas showed minute expression of PNA lectin with a heterogeneous distribution in the remaining compacted OSs (Figure 3B). The neuroretinas co-cultured with MSCVs showed shortened cone OSs expressing PNA and slight displacement around the IS region (Figure 3C). There were no significant (p = 0.872) differences in the PNA lectin immunoexpression between the fresh neuroretinas and those co-cultured with MSCVs (Figure 4A). Regarding the neuroretinas co-cultured with HEK293T cells, the PNA lectin immunoexpression was similar to the neuroretinas cultured for 72 h corresponding to degenerated cones (Figure 3D). The results showed significantly (p < 0.001 for both comparisons) higher PNA lectin immunoexpression when the neuroretinas co-cultured with MSCVs and neuroretinas cultured for 72 h were compared and when the neuroretinas co-cultured with MSCVs and those co-cultured with HEK293T cells were compared (Figure 4A). To identify and analyze the rods, we studied the immunoexpression of rhodopsin (Rho) protein (Figures 3E–3H). Rho is synthesized in the rough endoplasmic reticulum, packaged in the Golgi apparatus, transported through the IS cytoplasm to the connecting cilium, and inserted into newly forming membrane discs at the base of the OSs; therefore, Rho immunoexpression is located in the rod outer discs.18 The immunoexpression of Rho protein in fresh neuroretinas was distributed homogeneously in the rod OSs (Figure 3E). Almost no Rho protein immunoexpression was seen in the neuroretinas cultured for 72 h, and the distribution was limited to the remaining degenerated rods (Figure 3F). The results showed maintenance of the Rho immunoexpression in the shortened rods in the neuroretina OSs co-cultured with MSCVs (Figure 3G). There were no significant (p = 0.697) differences in the Rho protein immunoexpression between fresh neuroretinas and neuroretinas co-cultured with MSCVs (Figure 4B). The Rho protein immunoexpression in the neuroretinas co-cultured with HEK293T cells was similar to the neuroretinas cultured for 72 h (Figure 3H). The results showed significantly (p < 0.001 for both comparisons) higher Rho protein immunoexpression when the neuroretinas co-cultured with MSCVs and neuroretinas after 72 h of culture were compared and when neuroretinas co-cultured with MSCVs and neuroretinas co-cultured with HEK293T cells were compared (Figure 4B).
Figure 3.
Immunochemical Characterization
(A–D) Immunoexpression of the peanut agglutinin (PNA) lectin protein in fresh neuroretinas (A), neuroretinas cultured for 3 days (B), neuroretinas co-cultured with MSCVs for 3 days (C), and neuroretinas co-cultured with HEK293T cells for 3 days (D). (E–H) Immunoexpression of the rhodopsin (Rho) protein in fresh neuroretinas (E), neuroretinas cultured for 3 days (F), neuroretinas co-cultured with MSCVs for 3 days (G), and neuroretinas co-cultured with HEK293T cells for 3 days (H). (I–L) Immunoexpression of protein kinase Cα (PKCα) in fresh neuroretinas (I), neuroretinas cultured for 3 days (J), neuroretinas co-cultured with MSCVs for 3 days (K), and neuroretinas co-cultured with HEK293T cells for 3 days (L). (M–P) Immunoexpression of the of glial fibrillary acidic protein (GFAP) in fresh neuroretinas (M), neuroretinas cultured for 3 days (N), neuroretinas co-cultured with MSCVs for 3 days (O), and neuroretinas co-cultured with HEK293T cells for 3 days (P). Scale bars, 25 μm.
Figure 4.
Semiquantitative Immunohistochemical Analysis
(A–D) Immunoexpression of the PNA lectin protein (A), Rho protein (B), PKCα (C), and GFAP (D). ∗p < 0.05. A.U., arbitrary units; NR, neuroretina.
To analyze the morphology of the rod bipolar cells, the immunoexpression of protein kinase Cα (PKCα) protein was determined (Figures 3I–3L). PKCα modulates rod bipolar cell function by accelerating the glutamate-driven signal transduction and termination.19 The PKCα immunoexpression in the fresh neuroretinas was uniformly distributed along with the cytoplasm of the bipolar cells through the INL and inner plexiform layer (IPL), and the immunofluorescence was more intense in their terminal buttons (Figure 3I). Reduced PKCα protein immunoexpression with heterogeneous distribution in the rod bipolar cells (Figure 3J) was seen in the neuroretinas mono-cultured for 72 h; in the neuroretinas co-cultured with MSCVs for 72 h, the PKCα protein showed similar immunoexpression to that in fresh neuroretinas but with reduced fluorescence intensity at their terminal buttons (Figure 3K). No significant (p = 0.762) differences were seen in the PKCα protein immunoexpression between the fresh neuroretinas and neuroretinas co-cultured with MSCVs (Figure 4C). In the neuroretinas co-cultured with the HEK293T cells, the PKCα immunoexpression was close to that of the neuroretinas cultured for 72 h (Figure 3L). The results showed significantly (p = 0.003 and p = 0.002, respectively) and higher PKCα protein immunoexpression between the neuroretinas co-cultured with MSCVs and neuroretinas after 72 h of culture and between the neuroretinas co-cultured with MSCVs and neuroretinas co-cultured with HEK293T cells (Figure 4C).
To analyze the gliosis and retinal degeneration, the immunoexpression of glial fibrillary acidic protein (GFAP) was studied (Figures 3M–3P). Gliosis is an indicator of glial cell activation that is analyzed by GFAP immunoreaction. GFAP is expressed in glial cells in response to retinal degeneration.20 In the fresh neuroretinas, the GFAP immunoexpression was restricted to the retinal ganglion layer (RGL) and nerve fiber layer (NFL) (Figure 3M). The GFAP immunoexpression in the mono-cultured neuroretinas showed invasion of the cytoplasm of the glial cells to the INL and even the ONL (Figure 3N). GFAP immunoexpression distributed throughout the RGL and in the IPL (Figure 3O) was seen in neuroretinas co-cultured with MSCVs. In the neuroretinas co-cultured with HEK293T cells, the immunoexpression of GFAP extended to the outermost INL regions without reaching the ONL (Figure 3P). All of the comparisons reached significance (Figure 4D).
MSC Surface Marker Evaluation
MSC viability was greater than 95% in all experimental conditions. MSC cultures were confluent before co-culturing with the porcine neuroretinal explants. The results of the surface marker evaluation showed that mono-cultured and co-cultured MSCVs were positive for CD44, CD90, STRO1, and CD146 and negative for the CD14 and CD16 cell surface markers (Figure 5).
Figure 5.
Mesenchymal Stem Cell (MSC) Surface Marker Evaluation
(A–L) MSC surface markers after 3 days of culture (A, CD14; B, CD19; C, CD44; D, CD90; E, CD146; F, STRO-1) and after co-culture with neuroretina explants for 3 days (G, CD14; H, CD19; I, CD44; J, CD90; K, CD146; L, STRO-1). Scale bar, 25 μm.
Analysis of the Protein Profile
To identify possible paracrine factors secreted by MSCVs that may be implicated in slowing retinal neurodegeneration, the differential profiles of secreted proteins by MSCVs in the presence and absence of retinas in neurodegeneration were analyzed. The results showed statistically significant differences in the relative protein abundance profile of 653 proteins, of which 152 proteins were higher in the mono-cultured MSCVs than in the MSCVs co-cultured with neuroretinas, and 501 proteins were higher in MSCVs co-cultured with neuroretinas than those cultured alone (Table 1; Table S1). These proteins were involved in inflammation, immunity, death, cellular survival, stress, cellular oxidation, collagen metabolism, lipid metabolism, calcium metabolism, retinol metabolism, and angiogenesis processes. Table 2 summarizes the more relevant proteins with expression levels that were significantly higher in the MSCVs co-cultured with neuroretinas than in the mono-cultured MSCVs.
Table 1.
Primary Antibodies Used and Their Experimental Conditions
| Molecular Marker | Origin | Source | Sample Processing | Dilution | Time (h) | Temp. |
|---|---|---|---|---|---|---|
| Human Bone Marrow Mesenchymal Stem Cells | ||||||
| Cluster of differentiation present on B lymphocytes (CD19) | monoclonal mouse | Millipore, #SCR067 | methanol fixed | 1:500 | 12 | 4°C |
| Cluster of differentiation present on leukocytes (CD14) | monoclonal mouse | Millipore, #SCR067 | methanol fixed | 1:500 | 12 | 4°C |
| Homing cell adhesion molecule (H-CAM or CD44) | monoclonal mouse | Millipore, #SCR067 | methanol fixed | 1:500 | 12 | 4°C |
| Melanoma cell adhesion molecule (M-CAM or CD146) | monoclonal mouse | Millipore, #SCR067 | methanol fixed | 1:500 | 12 | 4°C |
| Stromal precursor antigen-1 (STRO-1) | monoclonal mouse | Millipore, #SCR067 | methanol fixed | 1:500 | 12 | 4°C |
| Thy-1 cell surface antigen (THY-1 or CD90) | monoclonal mouse | Millipore, #SCR067 | methanol fixed | 1:500 | 12 | 4°C |
| Neuroretinal Explants | ||||||
| Glial fibrillary acidic protein (GFAP) | polyclonal rabbit | Dako, #n1506 | paraffin | 1:250 | 1 | RT |
| Peanut agglutinin (PNA) lectin | arachis hypogaea | Molecular Probes, #L-21458 | cryosectioned | 1:100 | 1 | RT |
| Protein kinase Cα (PKCα) isoform | polyclonal rabbit | Santa Cruz Biotechnology, #SC-108000 |
paraffin | 1:100 | 12 | RT |
| Rhodopsin (Rho) | polyclonal rabbit | Chemicon-Millipore, #AB9279 | paraffin | 1:100 | 12 | 4°C |
Temp., temperature; RT, room temperature.
Table 2.
Relevant Proteins with Significantly Higher Expression in Human Bone Marrow MSCs Co-cultured with Neuroretinas Compared with Mono-Cultured Bone Marrow MSCs
| Inflammation/Immunity | |||||||
|---|---|---|---|---|---|---|---|
| IL-1F5 | IL-1F7 | IL-1F8 | IL-1R4 | IL-1ra | IL-2Rα | IL-2Rγ | IL-3 |
| IL-3Rα | IL-4R | IL-5 | IL-6R | IL-7 | IL-8 | IL-10 | IL-10Rα |
| IL-10Rβ | IL-11 | IL-12 p40 | IL-12 p70 | IL-12Rβ2 | IL-13 | IL-15Rα | IL-16 |
| IL-17B | IL-17BR | IL-17C | IL-17D | IL-17E | IL-18 BPa | IL-18Rα | IL-18Rβ |
| IL-19 | IL-20 | IL-20Rα | IL-20Rβ | IL-21 | IL-22 | IL-22 BP | IL-23R |
| IL-24 | IL-26 | IL-27 | IL-29 | IL-31 | haptoglobin | CXCR6 | TNFRSF17 |
| CD14 | CD30L | CD40L | IL-8RB | CXCR3 | CXCR4 (fusin) | DcR3 | HCR |
| CD46 | CD55 | CD61 | CD71 | CD74 | CD79α | CD90 | CD97 |
| CD200 | IGF-II | ICAM-2 | ICAM-3 | ICAM-5 | IFN-α | IFN-β | GLO-1 |
| HVEM | I-TAC | LECT2 | MCP-1 | MCP-2 | MCP-3 | L-selectin | TNFRSF3 |
| MIP-1b | MIP 2 | MIP-3β | MICA | RANTES | OX40L | PARC | pentraxin 3 |
| PF4 | P-selectin | TNFRSF19L | TNFRSF13B | thymopoietin | TLR2 | TLR3 | TLR4 |
| TRAIL | TRAIL R1 | TRAILR4 | ADAMTS-1 | ADAMTS-19 | ADAMTS-4 | AMICA | BLAME |
| CFHR2 | CHI3L1 | chymase | DPPIV | FAP | Fc RIIB/C | fibrinopeptide A | ficolin 3 |
| FOXP3 | furin | GATA-3 | IL-33 | IL-34 | IL36RN | Itk | LAG-3 |
| legumain | LOX-1 | LTF | MATK | MBL | MICB | midkine | Notch-1 |
| OX40 | pappalysin-1 | PD-1 | PYK2 | Tec | TIM-1 | adiponectin | hepassocin |
| TIMP-1 | ALCAM | EpCAM | OSM | IGF-I | Csk | Smad4 | Ckβ8-1 |
| ghrelin | COX2 | leptin R | THFSF3 | TCCR/WSX-1 | C3a | ||
| Death and Cell Survival | |||||||
| Pro-apoptosis | Cell Survival | Pro-apoptosis/Cell Survival | Autophagy | ||||
| PPP2R5C | RIP1 | HSP10 | clusterin | CCR4 | CD27/TNFRSF7 | FOXO1 | |
| FAM3B | SMAC | HSP20 | MINA | HGF | mer | PI3K p85β | |
| BAX | TOPORS | HSP27 | NAIP | NOV/CCN3 | Livin | ||
| BIK | TGF-β1 | HSP60 | NELL2 | SCFR/CD117 | IFN-α | ||
| caspase-3 | TGF-β5 | HSP70 | PAK7 | WISP-1/CCN4 | IFN-β | ||
| caspase-8 | FAK | HSP90 | PIM2 | BNIP2 | LRP-1 | ||
| IGFBP-3 | FRK | HSPA8 | PTN | survivin | lipocalin-2 | ||
| protein p65 | galectin-1 | ROS | |||||
| Cellular/Oxidative Stress | Retinol Metabolism | ||||||
| haptoglobin | APEX-1 | VDUP-1 | RBP4 | ||||
| Collagen Metabolism | |||||||
| MMP-8 | MMP-11 | NCAM-1 | MMP-1 | MMP-2 | MMP-7 | MMP-9 | MMP-10 |
| MMP-12 | MMP-13 | MMP-14 | MMP-15 | MMP-16 | MMP-19 | pro-MMP-7 | pro-MMP-9 |
| pro-MMP-13 | |||||||
| Lipid Metabolism | |||||||
| ApoA1 | ApoA2 | SERTAD2 | BMPR-II | BMPR-IA | ApoA4 | ApoC2 | ApoD |
| ApoE | ApoE3 | FABP1 | FABP4 | resistin | vitronectin | LRP-1 | |
| Angiogenesis | |||||||
| angiostatin | endothelin | VEGFR2 | angiopoietin-2 | angiopoietin-4 | angiopoietin-like 2 | NF1 | angiopoietin-like 1 |
| FGFR2 | |||||||
| Neuroprotection | |||||||
| DLL4 | nestin | NPTX1 | NPTXR | PEDF | BDNF | AR (amphiregulin) | CRIM1 |
| GFRα-4 | glypican 3 | glypican 5 | NGF | NrCAM | neuritin | thrombospondin 4 | TMEFF2 |
| CNTN1 | IGF2BP1 | ITM2B | Pro-BDNF | RECK | ROR1 | TPA | chordin-like1 |
| PDGF-AA | PDGF-C | PDGF-D | erythropoietin | NT-3 | FGF | ||
| Calcium Metabolism | |||||||
| ApoA1 | SERTAD2 | ALK-3 | ApoC1 | ApoD | ApoE3 | FABP4 | vitronectin |
| ApoA2 | BMPR-II | ApoA4 | ApoC2 | ApoE | FABP1 | resistin | |
Discussion
Many neurodegenerative retinal diseases are characterized by photoreceptor degeneration.1 Despite the impact on daily life and social and economic consequences, there is no cure for these diseases, and cell therapy based on the paracrine properties of stem cells may be a therapeutic option.1,3,4 Our previous experiments showed the biocompatibility of intravitreal injection of human MSCs and that they can improve the survival and maintenance of RGCs through paracrine neuroprotective potential.11,17We hypothesized that the paracrine effect of the human bone marrow stem cells may slow photoreceptor neurodegeneration. Therefore, the current study evaluated in vitro the neuroprotective potential of the MSC secretome over retinal photoreceptors using an ex vivo model of spontaneous neuroretinal degeneration. This is the first study analyzing the MSC secretome under experimental neuroretina degeneration, as this condition seems crucial since the extracellular environment would influence the secretome composition and, thus, induce its neuroprotective effect.
Organ retinal explant cultures are useful for studying neurodegeneration and neuroprotection processes because they bridge the gap between cell cultures and animal models. Organ retinal explant cultures also replicate, with in vitro limitations, the cellular changes that occur in the retina in vivo. The principal limitations of organ retinal explant cultures are the axotomy of the RGCs and the absence of a blood supply and retinal pigment epithelium. Despite these limitations, organ retinal explant cultures are an excellent resource to study retinal neurodegeneration.21,23 In the current study, neuroretinal explants were cultured over porous cell culture membranes that prevented the cells from migrating and integrating into the retinal tissue. We performed the study using stem cells and non-stem cells (HEK293T cells) as a negative control of neuroprotection with the objective to demonstrate that only stem cells (through their paracrine properties) show a neuroprotective effect over degenerating neuroretina.
Neuroretinas co-cultured with MSCVs for 72 h had better preserved structural organization than did mono-cultured neuroretinas. The glial response showed that GFAP expression was lower in neuroretinas co-cultured with MSCVs than in mono-cultured neuroretinas. GFAP is expressed in the astrocytes and Müller cells and is associated with glial activation as a consequence of the neurodegenerative process.20,24 Our findings suggested that MSCV paracrine properties have a neuroprotective effect in neuroretina against degeneration, results that are in complete agreement with previous reports.11,25,26 Those studies focused on the evaluation of RGCs or the neuroprotective properties of the human neural progenitor cells (hNPCs); therefore, to the best of our knowledge, this is the first study to evaluate the neuroprotective potential of human bone marrow MSCs over the retinal photoreceptor layer. Our results showed that the immunoexpression levels of PNA lectin, Rho, and PKCα proteins were similar in the neuroretinas co-cultured with MSCVs for 72 h and in the fresh neuroretinas, which may have resulted from the MSCV neuroprotective effect over cones, rods, and rod bipolar cells. These results agreed with those reported previously in organ retinal explant cultures, which determined that hNPCs slow the spontaneous photoreceptor degenerative process.25,26 hNPCs are a type of embryonic stem cell associated with ethical and political controversies. Human MSCs could be the best option for cell therapy strategies because their use does not present ethical problems and they are immunoprivileged14 or immune evasive.15,16 Thus, allogeneic transplantation of human bone marrow MSCs has the critical advantage of not requiring host immunosuppression,14 and retinal neuroprotection via MSC paracrine effects is currently viable.11,12
Stem cell therapy for retinal neurodegeneration could be based on paracrine stem cell properties.1,3,4 Our results showed that retinal neurodegeneration did not modify the MSC phenotype;27 therefore, during the 72 h of the experiment, the MSCVs could have been secreting paracrine factors that induce a neuroprotective effect over the photoreceptor cells. In this scenario, we performed the analysis of the bone marrow MSC protein profile on the basis that the secretome may be different depending on the extracellular environment. Therefore, we evaluated the protein profile secreted by MSCs in a “neurodegenerative condition” (neuroretinal explants cocultured with MSCs), and we also studied the secretome of MSC cultures alone, which allowed us to compare the protein profile secreted by the stem cells “per se” and under neuroretinal degeneration. The complete analysis of the secretome showed that 501 proteins display a higher expression levels in the MSCVs co-cultured with neuroretinas than in the mono-cultured MSCVs. These presented proteins are coming from two possible sources, i.e., secretion by the degenerating neuroretinas or by the MSCVs cultured with the neuroretinas. An inflammatory response, oxidative stress, and activation of apoptotic pathways are common features in the retinal neurodegenerative processes;1 therefore, the origin of the proteins implicated in these pathways could be retinal neurodegeneration. However, the secreted proteins by MSCVs may be involved in retinal neuroprotection. Previously, it has been described that several proteins such as glial-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), platelet-derived growth factor (PDGF), and ciliary neurotrophic factor (CNTF) were implicated in retinal neuroprotection by the paracrine effects of human bone marrow MSC,11,28 which are confirmed with the current results. Other proteins such as erythropoietin, Dll4, insulin growth factor (IGF), nerve growth factor (NGF), fibroblast growth factor (FGF), or pigment epithelium-derived factor (PEDF) also have been associated with retinal neuroprotection;10,29, 30, 31 however, to the best of our knowledge, this is the first report of photoreceptor neuroprotection mediated by these paracrine factors of human bone marrow MSCs. Our results also showed higher expression levels of neuroprotective proteins such as Crim1, Glupican3, Cntn1, or Tmeff2; thus, this is the first time that they were associated with the retinal neuroprotection mediated by human bone marrow MSCs. Retinal degeneration leads to protein misfolding, so understanding the mechanisms that maintain and re-establish retinal protein homeostasis is crucial for developing new therapeutic approaches.18 The neuroretinal cells have evolved many mechanisms to cope with misfolded proteins, including the heat shock response, the unfolded protein response, and autophagy. These mechanisms, referred to as proteostasis (protein homeostasis), generate and maintain correctly folded proteins and remove misfolded proteins to maintain the normal cellular function.18,32 Our results showed higher levels of Hsp10, Hsp20, Hsp27, Hsp60, Hsp70, clusterin, Kctd10, and Pyk2 proteins in the neuroretinas co-cultured with MSCVs, which are implicated in proteostasis via heat shock protein and unfolded protein responses.33,34 Thus, the co-culturing of neuroretinas with MSCVs may activate the mechanisms of protein homeostasis to fight the neurodegenerative process. Finally, the results also showed the expression of proteins with antioxidant activity (haptoglobin35), anti-apoptotic role (Apex1),36 and with anti-inflammatory activity (transforming growth factor-β and interleukins 10, 11, 13, and 4),37 which may slow retinal neurodegeneration. Several potential risks are associated with stem cell-based therapies, such as engraftment at an ectopic location, inappropriate differentiation, or aggregate formation.38 Therefore, knowing the MSC secretome with retinal neuroprotective properties may be crucial for developing cell-free compositions that allow use of a safe alternative to stem cell transplantation and avoid the potential risks. Besides, handling and storage of compositions based in the MSC secretome present important advantages over living cells in clinical practice.39
In summary, this study showed that human bone marrow MSCs favor preservation of the neuroretinal general structure and organization, reduce reactive gliosis, and preserve retinal neurons, mainly photoreceptors, from degeneration via secretion of proteins involved in the neuroprotective processes. These results are in line with those reported previously by our group; we determined that MSCs can improve the survival and maintenance of RGCs through the paracrine neuroprotective potential of the MSCs.11 The current results reinforced the hypothesis that the paracrine effect of the human bone marrow MSCs preserves the general retinal structure and organization by the secretion of proteins involved in neuroprotective processes. Therefore, in vitro evidence showed that a MSC secretome may be a therapeutic option in the treatment of retinal degenerative diseases.
Materials and Methods
Cell Culture and Culture Conditions
Human bone marrow MSCs from different healthy adult donors were provided by Citospin (MSCVs, Valladolid, Spain) after characterization, as reported previously.40, 41, 42 In brief, MSCVs are positive for the mesenchymal stem cell antigens (CD105, CD90, CD73, and CD166), established by the International Society for Cell Therapy (ISCT), and negative for specific markers of hematopoietic cells (CD14, CD34, CD45, and histocompatibility leukocyte antigen [HLA]-DR). MSCVs are produced under good manufacturing practice (GMP) regulations and have been approved by the Spanish Drug Agency (AEMPS) for several clinical trials of ophthalmology (European Union Drug Regulating Authorities Clinical Trials Database [EudraCT]: 2011-005321-51 and 2016-003029-40). Fresh MSCVs were provided in a vial containing 1 × 10 cells/mL. HEK293T cells, generously donated by Prof. González-Sarmiento from the University of Salamanca (Spain), were used as negative control of neuroprotection.28 A trypan blue assay (Sigma-Aldrich, St. Louis, MO, USA) was used to determine cell viability and cell counts (TC20 automated cell counter; Bio-Rad, Hercules, CA, USA). The cells were seeded on the bottom of Transwell 24-mm-diameter culture plates (Corning Life Sciences, Corning, NY) in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (Gibco, Invitrogen, Paisley, UK), 1% antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) (Gibco, Invitrogen), and 1% l-glutamine (Sigma-Aldrich, St. Louis, MO, USA). We seeded 30,000 cells/well of MSCs, as previously reported by our group,11,21 and 10,000 cells/well of HEK923T cells. The cells were cultured at 37°C in a 5% CO2 atmosphere for 72 h until confluence, for subsequent co-culturing with neuroretinal explants.
Central Neuroretinal Explant Preparation and Culture
Nine fresh porcine eyes from animals aged 6–8 months were obtained from a local slaughterhouse. Neuroretinal explants (n = 32) were obtained less than 2 h after enucleation, as previously described.11,21 Briefly, the eyes were dissected and the porcine area centralis (cone-enriched visual streak without blood vessels) was identified. Four adjacent explants from the area centralis were obtained from each eye. The neuroretinal explants were laid over Transwell membranes 24 mm in diameter with a 0.4-μm pore polycarbonate membrane insert (Corning Life Sciences) with the photoreceptor layer facing the membrane. The explants were cultured alone or with MSCVs or with HEK293T cells in the same culture well but physically separated by the Transwell porous membrane, which prevented cellular migration and integration into the neuroretinal tissue. The co-cultures were maintained in DMEM/Neurobasal A medium (1:1) (Gibco) supplemented with 10% fetal bovine serum, 1% antibiotics, 2% B-27, and 1% l-glutamine under standard culture conditions for 72 h. Contact between the culture medium (1.5 mL, as suggested by the manufacturer) and the support membrane beneath the explants was maintained by changing the culture medium with freshly prepared medium. The medium was entirely changed daily and stored at −80°C for secretome analysis. Furthermore, neuroretinal explants were obtained and processed in parallel before culturing (fresh neuroretinas).
Five experimental conditions were evaluated for a total of 45 experiments as follows: mono-cultured MSCVs (n = 9), mono-cultured neuroretinal explants (n = 9), neuroretinal explants co-cultured with MSCVs (n = 9), neuroretinal explants co-cultured with HEK293T cells (n = 9), and fresh neuroretinas (n = 9).
Neuroretinal Histologic and Immunochemical Characterization
Fresh or cultured neuroretinal explants were fixed with 4% paraformaldehyde (Panreac Quimica, Barcelona, Spain) in phosphate-buffered saline (PBS) for 2 h at 4°C, and each explant was cut in half. Half of the samples were embedded in paraffin (Paraplast Plus, Leica Biosystems, Nussloch, Germany) using an automatic tissue processor (ASP300, Leica Microsystems, Wetzlar, Germany), and 4-μm sections were obtained with a rotatory microtome (RM2145, Leica Microsystems). The other halves of the samples were subjected to sucrose (Panreac Quimica cryoprotection, embedded in Tissue-Tek OCT compound (Sakura Finetek Europe, Alphen, the Netherlands), and cut into 12-μm sections on a cryostat (CM1900, Leica Microsystems).
Paraffin-embedded sections were deparaffinized in xylene (Sigma-Aldrich) and rehydrated in decreasing ethanol concentrations. The sections then were stained with hematoxylin and eosin (Sigma-Aldrich) or processed for immunochemistry by incubating with 0.01% trypsin for 1 h and blocking in PBS with 5% goat serum for 2 h at room temperature. Frozen neuroretinal sections processed for immunochemistry were thawed, washed in water, and blocked in PBS with 5% goat serum and 0.1% Triton X-100 (Sigma-Aldrich) for 2 h at room temperature. The primary antibodies used and their conditions are summarized in Table 1. The corresponding species-specific secondary antibodies conjugated to Alexa Fluor 568 (red) (1:200, Molecular Probes) were then applied. The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (10 μg/mL, Molecular Probes). Finally, the samples were mounted in fluorescent mounting medium (Dako, Glostrup, Denmark) and coverslipped. Fluorescence images were captured with a Leica TCS SP5 DMI-6000B confocal microscope (Leica Microsystems) and analyzed with Leica LAS AF software. The final processing and composition of the figures were performed with Pixelmator 3.8.2 Phoenix (Pixelmator Team, Vilnius, Lithuania).
Semiquantitative immunohistochemical analysis was performed using ImageJ software (version 1.49, National Institutes of Health, Bethesda, MD, USA) on ×20 fluorescence images from non-serial neuroretinal sections (n = 5 sections per sample). Comparative analyses of images acquired at the same levels of exposure, intensity, and gain were performed. Two-channel micrographs were analyzed as previously described,43 and the micrographs were then split into different channels and each channel thresholder. The background in the red channel (corresponding to the protein of interest) was subtracted by using a threshold value obtained from negative controls. The same value was used for all images from a single experiment analyzed in a single session. The threshold for the blue channel, corresponding to the area of nuclei, was set according to the area stained. Mean gray value was measured by redirecting the measurement to the corresponding channel. The results, in arbitrary units (a.u.), were the ratio of the mean gray value for the red channel.
Neuroretinal Morphometry and Cell Counts
Neuroretinal parenchyma was analyzed by measuring the total thickness between the outer limiting membrane and the inner limiting membrane. The thicknesses of the ONL, OPL, INL, IPL, and GCL, including the NFL, were also measured. Measurements were performed using ImageJ software (version 1.49, National Institutes of Health, Bethesda, MD, USA) on ×20 images from non-serial hematoxylin-stained neuroretinal sections (n = 5 sections/sample). DAPI-stained nuclei were quantified automatically by the ImageJ software and the plugin RetFM-J.IS.44 A masked researcher performed all neuroretinal thickness measurements and nucleus quantifications in triplicate.
MSC Immunochemical Characterization
The MSCVs were fixed on the bottom of the Transwell culture plates with ice-cold methanol (Panreac Quimica) for 15 min at 4°C and then immunostained with a human MSC characterization kit (Millipore, Billerica, MA, USA). Primary antibodies (Table 1) were applied overnight in a ratio of 1:500 at 4°C. The corresponding species-specific secondary antibody conjugated to Alexa Fluor 488 (green, 1:200; Molecular Probes, Eugene, OR, USA) was applied for 2 h at room temperature. The nuclei were stained with DAPI (10 μg/mL). Finally, samples were mounted in fluorescent mounting medium (Dako, Demark) and coverslipped. Fluorescence images were captured with a Leica TCS SP5 DMI-6000B confocal microscope and were analyzed with Leica LAS AF software. The final processing and composition of the figures were performed with Pixelmator 3.8.2 Phoenix.
Protein Microarray Assay
For the protein profile analysis, collected culture media from stem cells cultured alone and from stem cells co-cultured with neuroretina were pooled to study the overall protein content over the entire experimental period. A human antibody array (RayBiotech, Peachtree Corners, GA, USA) was used according to the manufacturer’s guidelines to simultaneously detect the relative expression of 1,000 human proteins in cell culture supernatants. The protein concentration of each supernatant was determined in the range of 7–8 μg/mL using a BCA (bicinchoninic acid) protein assay (Pierce BCA protein assay kit, catalog no. 23227, Sigma-Aldrich, St. Louis, MO, USA). All samples were dialyzed against 1× PBS (pH 8.0) (as a dialysis buffer). Ten micrograms of each sample was then biotinylated according to the manufacturer’s instructions. The biotinylation was evaluated in all samples using a Pierce biotin quantitation kit to ensure equal conditions for all samples. The antibody arrays were blocked at 4°C with RayBiotech blocking buffer 1×. All biotinylated samples were the incubated in the same comparable conditions as previously described by Díez et al.45 After incubation, all of the arrays were incubated with RayBiotech horseradish peroxidase (HRP)-streptavidin 1×. The array images were acquired using a ChemiDoc MD (Bio-Rad, CA, USA) as a conventional western blot procedure at the optimized exposition. Semiquantitative data analysis was performed according to the RayBio analysis tool guidelines.46, 47, 48 All experimental conditions were performed in triplicate.
Data Acquisition and Statistical Analysis
All data were collected in an Excel database (Microsoft Office Excel, 2016, Microsoft, Redmond, WA, USA). SPSS (version 24.0, SPSS, Chicago, IL, USA) was used for statistical analyses. After confirming the data homogeneity of variance and normal distribution, we performed analysis of variance followed by pairwise comparisons (Bonferroni test). For non-parametric variables, the group means were compared using the Mann-Whitney U test (two groups) or the Kruskal-Wallis test (more than two groups). Differences were considered significant at p < 0.05.
Author Contributions
Conception and Design: R.U.M., J.C.P., and I.F.-B.; Experimental Work: R.U.M, K.P.-N, M.-T.G.-G., and M.F; Analysis and Interpretation of Data: R.U.M., K.P.-N., and I.F.-B; Drafting the Article: R.U.M and I.F.-B.; Revising the Article: all authors; Study Supervision: I.F.-B.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
The authors are grateful to the staff of the Justino Gutierrez S.L. Slaughterhouse (Valladolid, Spain) for providing the porcine eye globes used in this work and to Citospin S.L. (Valladolid, Spain) for providing the MSCs used in this work. We also thank Lynda Charters from Medical International (Framingham, MA, USA) for his assistance in the final editing and preparation of this manuscript. This work was supported by grants from Fondo Europeo de Desarrollo Regional (FEDER) and Consejería de Educación from Junta de Castilla y León, Spain (grant VA077P17), and from the Spanish Health Institute Carlos III (ISCIII) (grants PI17/01930 and CB16/12/00400). The Proteomics Unit belongs to ProteoRed (PRB3-ISCIII) supported by grant PT17/0019/0023, of the PEI+D+I 2017-2020, funded by ISCIII and FEDER. R.U.-M. was supported by FEDER and Consejería de Educación from Junta de Castilla y León, Spain (grant VA077P17); K.P.-N. was supported by Fundación Carolina, Madrid, Spain; and I.F.-B. was supported by Centro en Red de Medicina Regenerativa y Terapia Celular, Junta de Castilla y León, Spain.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2020.05.003.
Supplemental Information
References
- 1.Cuenca N., Fernández-Sánchez L., Campello L., Maneu V., De la Villa P., Lax P., Pinilla I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Retin. Eye Res. 2014;43:17–75. doi: 10.1016/j.preteyeres.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Gagliardi G., Ben M’Barek K., Goureau O. Photoreceptor cell replacement in macular degeneration and retinitis pigmentosa: a pluripotent stem cell-based approach. Prog. Retin. Eye Res. 2019;71:1–25. doi: 10.1016/j.preteyeres.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Tang Z., Zhang Y., Wang Y., Zhang D., Shen B., Luo M., Gu P. Progress of stem/progenitor cell-based therapy for retinal degeneration. J. Transl. Med. 2017;15:99. doi: 10.1186/s12967-017-1183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya S., Gangaraju R., Chaum E. Recent advances in retinal stem cell therapy. Curr. Mol. Biol. Rep. 2017;3:172–182. doi: 10.1007/s40610-017-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacLaren R.E., Pearson R.A., MacNeil A., Douglas R.H., Salt T.E., Akimoto M., Swaroop A., Sowden J.C., Ali R.R. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 6.Mellough C.B., Cui Q., Harvey A.R. Treatment of adult neural progenitor cells prior to transplantation affects graft survival and integration in a neonatal and adult rat model of selective retinal ganglion cell depletion. Restor. Neurol. Neurosci. 2007;25:177–190. [PubMed] [Google Scholar]
- 7.Waldron P.V., Di Marco F., Kruczek K., Ribeiro J., Graca A.B., Hippert C., Aghaizu N.D., Kalargyrou A.A., Barber A.C., Grimaldi G. Transplanted donor- or stem cell-derived cone photoreceptors can both integrate and undergo material transfer in an environment-dependent manner. Stem Cell Reports. 2018;10:406–421. doi: 10.1016/j.stemcr.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson T.V., Bull N.D., Martin K.R. Transplantation prospects for the inner retina. Eye (Lond.) 2009;23:1980–1984. doi: 10.1038/eye.2008.376. [DOI] [PubMed] [Google Scholar]
- 9.Hill A.J., Zwart I., Tam H.H., Chan J., Navarrete C., Jen L.S., Navarrete R. Human umbilical cord blood-derived mesenchymal stem cells do not differentiate into neural cell types or integrate into the retina after intravitreal grafting in neonatal rats. Stem Cells Dev. 2009;18:399–409. doi: 10.1089/scd.2008.0084. [DOI] [PubMed] [Google Scholar]
- 10.Kolomeyer A.M., Zarbin M.A. Trophic factors in the pathogenesis and therapy for retinal degenerative diseases. Surv. Ophthalmol. 2014;59:134–165. doi: 10.1016/j.survophthal.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Labrador-Velandia S., Alonso-Alonso M.L., Di Lauro S., García-Gutierrez M.T., Srivastava G.K., Pastor J.C., Fernandez-Bueno I. Mesenchymal stem cells provide paracrine neuroprotective resources that delay degeneration of co-cultured organotypic neuroretinal cultures. Exp. Eye Res. 2019;185:107671. doi: 10.1016/j.exer.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Xu W., Xu G.-X. Mesenchymal stem cells for retinal diseases. Int. J. Ophthalmol. 2011;4:413–421. doi: 10.3980/j.issn.2222-3959.2011.04.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salehi H., Amirpour N., Razavi S., Esfandiari E., Zavar R. Overview of retinal differentiation potential of mesenchymal stem cells: a promising approach for retinal cell therapy. Ann. Anat. 2017;210:52–63. doi: 10.1016/j.aanat.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Nauta A.J., Fibbe W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 15.Ankrum J.A., Ong J.F., Karp J.M. Mesenchymal stem cells: immune evasive, not immune privileged. Nat. Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berglund A.K., Fortier L.A., Antczak D.F., Schnabel L.V. Immunoprivileged no more: measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res. Ther. 2017;8:288. doi: 10.1186/s13287-017-0742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labrador Velandia S., Di Lauro S., Alonso-Alonso M.L., Tabera Bartolomé S., Srivastava G.K., Pastor J.C., Fernandez-Bueno I. Biocompatibility of intravitreal injection of human mesenchymal stem cells in immunocompetent rabbits. Graefes Arch. Clin. Exp. Ophthalmol. 2018;256:125–134. doi: 10.1007/s00417-017-3842-3. [DOI] [PubMed] [Google Scholar]
- 18.Athanasiou D., Aguilà M., Bevilacqua D., Novoselov S.S., Parfitt D.A., Cheetham M.E. The cell stress machinery and retinal degeneration. FEBS Lett. 2013;587:2008–2017. doi: 10.1016/j.febslet.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruether K., Feigenspan A., Pirngruber J., Leitges M., Baehr W., Strauss O. PKCα is essential for the proper activation and termination of rod bipolar cell response. Invest. Ophthalmol. Vis. Sci. 2010;51:6051–6058. doi: 10.1167/iovs.09-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vecino E., Rodriguez F.D., Ruzafa N., Pereiro X., Sharma S.C. Glia-neuron interactions in the mammalian retina. Prog. Retin. Eye Res. 2016;51:1–40. doi: 10.1016/j.preteyeres.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Di Lauro S., Rodriguez-Crespo D., Gayoso M.J., Garcia-Gutierrez M.T., Pastor J.C., Srivastava G.K., Fernandez-Bueno I. A novel coculture model of porcine central neuroretina explants and retinal pigment epithelium cells. Mol. Vis. 2016;22:243–253. [PMC free article] [PubMed] [Google Scholar]
- 22.Garlipp M.A., Nowak K.R., Gonzalez-Fernandez F. Cone outer segment extracellular matrix as binding domain for interphotoreceptor retinoid-binding protein. J. Comp. Neurol. 2012;520:756–769. doi: 10.1002/cne.22773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez-Bueno I., Fernández-Sánchez L., Gayoso M.J., García-Gutierrez M.T., Pastor J.C., Cuenca N. Time course modifications in organotypic culture of human neuroretina. Exp. Eye Res. 2012;104:26–38. doi: 10.1016/j.exer.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Bringmann A., Wiedemann P. Müller glial cells in retinal disease. Ophthalmologica. 2012;227:1–19. doi: 10.1159/000328979. [DOI] [PubMed] [Google Scholar]
- 25.Mollick T., Mohlin C., Johansson K. Human neural progenitor cells decrease photoreceptor degeneration, normalize opsin distribution and support synapse structure in cultured porcine retina. Brain Res. 2016;1646:522–534. doi: 10.1016/j.brainres.2016.06.039. [DOI] [PubMed] [Google Scholar]
- 26.Jones M.K., Lu B., Chen D.Z., Spivia W.R., Mercado A.T., Ljubimov A.V., Svendsen C.N., Van Eyk J.E., Wang S. In vitro and in vivo proteomic comparison of human neural progenitor cell-induced photoreceptor survival. Proteomics. 2019;19:e1800213. doi: 10.1002/pmic.201800213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delorme B., Ringe J., Gallay N., Le Vern Y., Kerboeuf D., Jorgensen C., Rosset P., Sensebé L., Layrolle P., Häupl T., Charbord P. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood. 2008;111:2631–2635. doi: 10.1182/blood-2007-07-099622. [DOI] [PubMed] [Google Scholar]
- 28.Johnson T.V., DeKorver N.W., Levasseur V.A., Osborne A., Tassoni A., Lorber B., Heller J.P., Villasmil R., Bull N.D., Martin K.R., Tomarev S.I. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain. 2014;137:503–519. doi: 10.1093/brain/awt292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo H., Jin K., Xie Z., Qiu F., Li S., Zou M., Cai L., Hozumi K., Shima D.T., Xiang M. Forkhead box N4 (Foxn4) activates Dll4-Notch signaling to suppress photoreceptor cell fates of early retinal progenitors. Proc. Natl. Acad. Sci. USA. 2012;109:E553–E562. doi: 10.1073/pnas.1115767109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding S.L.S., Koh A.E., Kumar S., Ali Khan M.S., Alzahrani B., Mok P.L. Genetically-modified human mesenchymal stem cells to express erythropoietin enhances differentiation into retinal photoreceptors: an in-vitro study. J. Photochem. Photobiol. B. 2019;195:33–38. doi: 10.1016/j.jphotobiol.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Vigneswara V., Ahmed Z. Pigment epithelium-derived factor mediates retinal ganglion cell neuroprotection by suppression of caspase-2. Cell Death Dis. 2019;10:102. doi: 10.1038/s41419-019-1379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balch W.E., Morimoto R.I., Dillin A., Kelly J.W. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 33.Vembar S.S., Brodsky J.L. One step at a time: endoplasmic reticulum-associated degradation. Nat. Rev. Mol. Cell Biol. 2008;9:944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vabulas R.M., Raychaudhuri S., Hayer-Hartl M., Hartl F.U. Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb. Perspect. Biol. 2010;2:a004390. doi: 10.1101/cshperspect.a004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tseng C.F., Lin C.C., Huang H.Y., Liu H.C., Mao S.J.T. Antioxidant role of human haptoglobin. Proteomics. 2004;4:2221–2228. doi: 10.1002/pmic.200300787. [DOI] [PubMed] [Google Scholar]
- 36.Dyballa-Rukes N., Jakobs P., Eckers A., Ale-Agha N., Serbulea V., Aufenvenne K., Zschauer T.C., Rabanter L.L., Jakob S., von Ameln F. The anti-apoptotic properties of APEX1 in the endothelium require the first 20 amino acids and converge on thioredoxin-1. Antioxid. Redox Signal. 2017;26:616–629. doi: 10.1089/ars.2016.6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Herberts C.A., Kwa M.S.G., Hermsen H.P.H. Risk factors in the development of stem cell therapy. J. Transl. Med. 2011;9:29. doi: 10.1186/1479-5876-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vizoso F.J., Eiro N., Cid S., Schneider J., Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci. 2017;18:E1852. doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orozco L., Soler R., Morera C., Alberca M., Sánchez A., García-Sancho J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011;92:822–828. doi: 10.1097/TP.0b013e3182298a15. [DOI] [PubMed] [Google Scholar]
- 41.Orozco L., Munar A., Soler R., Alberca M., Soler F., Huguet M., Sentís J., Sánchez A., García-Sancho J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation. 2013;95:1535–1541. doi: 10.1097/TP.0b013e318291a2da. [DOI] [PubMed] [Google Scholar]
- 42.Vega A., Martín-Ferrero M.A., Del Canto F., Alberca M., García V., Munar A., Orozco L., Soler R., Fuertes J.J., Huguet M. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99:1681–1690. doi: 10.1097/TP.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 43.Soriano-Romaní L., Contreras-Ruiz L., García-Posadas L., López-García A., Masli S., Diebold Y. Inflammatory cytokine-mediated regulation of thrombospondin-1 and CD36 in conjunctival cells. j. ocul. pharmacol. ther. 2015;31:419–428. doi: 10.1089/jop.2015.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.hedberg-Buenz A., Christopher M.A., Lewis C.J., Meyer K.J., Rudd D.S., Dutca L.M., Wang K., Garvin M.K., Scheetz T.E., Abràmoff M.D. RetFM-J, an ImageJ-based module for automated counting and quantifying features of nuclei in retinal whole-mounts. Exp. Eye Res. 2016;146:386–392. doi: 10.1016/j.exer.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Díez P., Lorenzo S., Dégano R.M., Ibarrola N., González-González M., Nieto W., Almeida J., González M., Orfao A., Fuentes M. Multipronged functional proteomics approaches for global identification of altered cell signalling pathways in B-cell chronic lymphocytic leukaemia. Proteomics. 2016;16:1193–1203. doi: 10.1002/pmic.201500372. [DOI] [PubMed] [Google Scholar]
- 46.Díez P., Dasilva N., González-González M., Matarraz S., Casado-Vela J., Orfao A., Fuentes M. Data analysis strategies for protein microarrays. Microarrays (Basel) 2012;1:64–83. doi: 10.3390/microarrays1020064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.González-González M., Bartolome R., Jara-Acevedo R., Casado-Vela J., Dasilva N., Matarraz S., García J., Alcazar J.A., Sayagues J.M., Orfao A., Fuentes M. Evaluation of homo- and hetero-functionally activated glass surfaces for optimized antibody arrays. Anal. Biochem. 2014;450:37–45. doi: 10.1016/j.ab.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 48.León I.E., Díez P., Etcheverry S.B., Fuentes M. Deciphering the effect of an oxovanadium(iv) complex with the flavonoid chrysin (VOChrys) on intracellular cell signalling pathways in an osteosarcoma cell line. Metallomics. 2016;8:739–749. doi: 10.1039/c6mt00045b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.