Abstract
Telomerase consists of the catalytic subunit Telomerase Reverse Transcriptase (TERT) and the Telomerase RNA Component. Its canonical function is the prevention of telomere erosion. Over the last years it became evident that TERT is also present in tissues with low replicative potential. Important non-canonical functions of TERT are protection against apoptosis and maintenance of the cellular redox homeostasis in cancer as well as in somatic tissues. Intriguingly, TERT and reactive oxygen species (ROS) are interdependent on each other, with TERT being regulated by changes in the redox balance and itself controlling ROS levels in the cytosol and in the mitochondria. The latter is achieved because TERT is present in the mitochondria, where it protects mitochondrial DNA and maintains levels of anti-oxidative enzymes. Since numerous diseases are associated with oxidative stress, increasing the mitochondrial TERT level could be of therapeutic value.
Keywords: Telomerase Reverse Transcriptase, Reactive oxygen species, Apoptosis, Mitochondria
Abbreviations: GSH, Glutathione; mtDNA, mitochondrial DNA; ROS, reactive oxygen species; SOD, Superoxide Dismutase; TERC, Telomerase RNA Component; TERT, Telomerase Reverse Transcriptase
Highlights
-
•
Telomerase Reverse Transcriptase has non-canonical functions.
-
•
Telomerase Reverse Transcriptase is also present in mitochondria.
-
•
Localization of Telomerase Reverse Transcriptase depends on phosphorylation.
-
•
Telomerase Reverse Transcriptase and reactive oxygen species are interdependent.
1. Introduction
Telomeres are located at the ends of each chromosome and consist of repetitive hexanucleotide sequences – in vertebrates 5′-TTAGGG-3' – with a single-stranded overhang. Together with a number of associated proteins they form a unique structure in which the single-stranded region invades the adjacent DNA duplex (for review see Ref. [1]. This prevents recognition of free double-stranded DNA ends at the termini of the chromosomes as damaged DNA, which could lead to chromosomal rearrangements. Thus, telomeres are critical for the preservation of the integrity and stability of chromosomes. The length of human telomeres has been estimated to be approximately 11 kilobases around birth [2] and decreases with age [3]. Due to the end-replication problem, telomeres shorten with each DNA replication cycle and a critical limit serves as a trigger for senescence induction [4,5].
The inevitable shortening of telomeres over generations, the so-called telomere erosion is counteracted by the enzyme telomerase. Telomerase is a ribonucleoprotein consisting of a catalytic subunit, Telomerase Reverse Transcriptase (TERT) and the Telomerase RNA Component (TERC). These two molecules cooperate to execute the canonical function of telomerase. After TERC binds to the single-stranded overhang at the telomere, it is used as template for reverse transcription by TERT resulting in the addition of a hexameric repeat. Recurrent relocation of TERT and TERC to the 3′-end of the chromosome and reverse transcription allows for telomere elongation. A DNA-dependent polymerase can then synthesize the opposite strand except for the extreme end, again leaving a single-stranded overhang consisting of the telomeric repeats. TERT and TERC are associated with accessory proteins required for assembly, activity and telomere recognition by the holoenzyme complex (for review see Ref. [6]. Although TERT and TERC are both essential for telomere maintenance, TERC, but not TERT seems to be the limiting component for telomerase activity [7].
Typically, TERT is expressed and, thus, telomerase activity is detectable in cells with a high replicative capacity such as stem cells, germ cells and tumors, thereby preventing telomere erosion. However, over the last years it became evident that TERT is also present in tissues with low replicative potential such as the vasculature [[8], [9]], the heart [[10], [11]] and the brain [[12], [13]].
2. Regulation of TERT
On the transcriptional level, regulation of TERT varies from cell type to cell type and is still not completely understood. Myc was the first transcription factor described to directly activate transcription of the TERT gene in primary epithelial cells and fibroblasts via E-boxes, canonical binding sites for Myc/Max heterodimers. Importantly, this transcriptional upregulation was independent of the proliferation state of the cells and of de novo protein synthesis, suggesting a direct role of Myc in the regulation of TERT transcription [14]. However, the mechanistic details of how Myc activates the TERT promoter have not been elucidated completely [15]. Besides Myc, E2F has been shown to be involved in transcriptional activation of TERT in normal somatic cells [16].
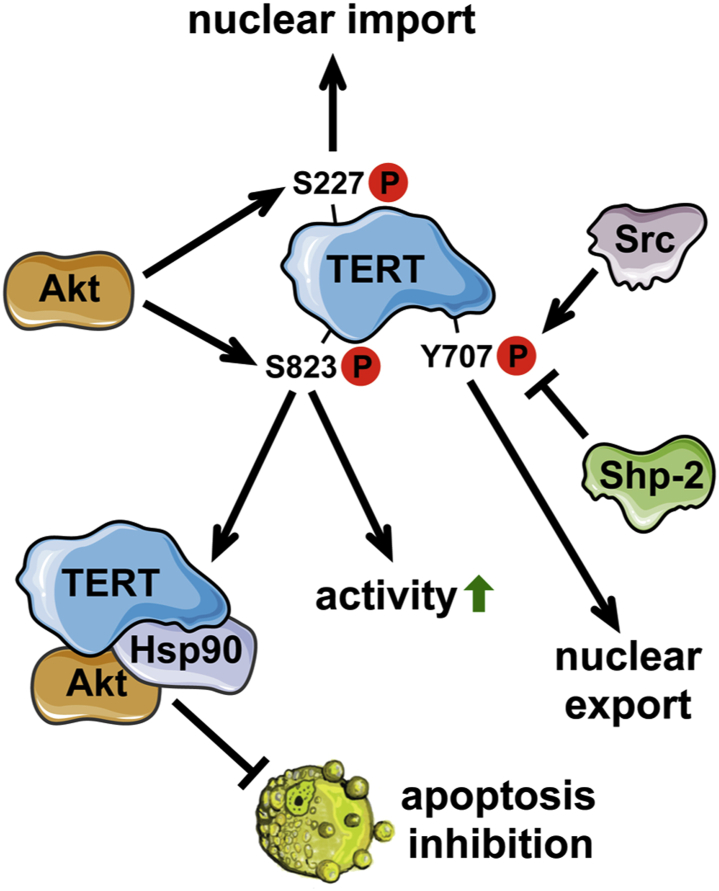
On the post-translational level, TERT is regulated by several kinases like protein kinase B (Akt), protein kinase C, and the Src kinase family. A dominant role in regulating telomerase activity has been described for Akt. Upon phosphorylation on serine 823, human TERT is activated [17,18]. The activation of TERT by Akt is accompanied by complex formation of TERT, Akt and the heat shock protein 90 (Hsp90), which is required for stabilization of this complex [18]. Moreover, phosphorylation of TERT by Akt is also required for nuclear import of TERT, since a dominant-negative mutant of Akt or treatment with an Akt inhibitor attenuated nuclear localization of TERT. This process, however, depends on phosphorylation on serine 227 [19]. In contrast, phosphorylation of TERT on tyrosine 707 by the Src kinase family leads to nuclear export of TERT, which is completely inhibited in Src-, Fyn-, Yes-deficient fibroblasts and counteracted by the tyrosine phosphatase Shp-2 [[20], [21], [22]]. The different phosphorylation events on TERT and their consequences are summarized in Fig. 1.
Fig. 1.
Regulation of TERT by phosphorylation and functional consequences.
TERT is not only regulated by phosphorylation, but also by changes in the intracellular redox homeostasis. Redox homeostasis describes the balance between intracellular oxidants and antioxidants. The most prominent producers of reactive oxygen species (ROS) are NADPH oxidases and mitochondria. On the other hand, the dominant antioxidants are Superoxide Dismutases (SODs), Catalase as well as the Thioredoxin and Glutathione systems. An imbalance in the redox homeostasis - either due to an increase in oxidants or a decrease in the anti-oxidant capacity - results in intracellular oxidative stress. Oxidative stress plays a key role in the development of various diseases, including atherosclerosis, diabetes, cancer, neurodegeneration, but also in aging. ROS also serve as signaling molecules and thereby regulate several cellular signaling pathways leading to e.g. proliferation and survival [23]. However, the influence of ROS on cellular processes and the communication between ROS and other signaling molecules are not entirely understood. With respect to TERT, we demonstrated that oxidative stress induces nuclear export of TERT by phosphorylation of tyrosine 707 by the Src kinase family in a time dependent manner [20]. In line with our findings, the Saretzki group showed that TERT is exported from the nucleus upon hydrogen peroxide (H2O2) treatment [24]. Interestingly, upon export of TERT from the nucleus, no degradation of the protein is observed, but rather a concomitant increase within the mitochondria [20,24]. In depth analysis of the TERT protein revealed that it contains nuclear localization signals as well as a mitochondrial targeting sequence. Furthermore, under physiological conditions reflecting an intact redox homeostasis, about 10–20% of the TERT protein is present in the mitochondria [25,26]. Within this organelle, TERT is localized in the mitochondrial matrix and associated with mitochondrial DNA (mtDNA) as well as mitochondrial tRNA [26,27].
3. Non-canonical functions of TERT
The majority of tumors is characterized by an upregulation of TERT and, thus, telomerase activity. Intuitively, this would imply a protection against telomere erosion in tumor cells. However, it was shown that telomerase also affects another hallmark of cancer, namely apoptosis resistance. This was demonstrated by studies showing that downregulation of TERT induces apoptosis or increases sensitivity towards apoptotic stimuli in cancer cells ex vivo and in vivo [[28], [29], [30]], independent of its telomeric function [[31], [32], [33]]. This goes along with activation of Bax and caspases [30,31,33]. Moreover, overexpression of TERT or a catalytically inactive mutant protected various tumor cell lines against apoptosis, clearly showing that the observed phenomena were independent of the canonical function of TERT [34]. Later, this anti-apoptotic effect of TERT was linked to effects on the cellular redox status. TERT overexpression in tumor cell lines reduced basal cellular ROS levels as well as intracellular ROS generation in response to different stimuli and inhibited apoptosis. Conversely, downregulation of TERT potentiated the increase in cellular ROS. These anti-oxidative effects of TERT are linked to an increase in reduced Glutathione (GSH) and nonoxidized peroxiredoxin [35]. An interdependence between TERT and ROS was demonstrated by treatment of tumor cell lines of different origin with sulphoraphane, an isothiocyanate found in cruciferous vegetables, known to possess anti-cancer and anti-inflammatory activities, which led to increased ROS and inhibition of TERT expression as well as telomerase activity [36].
This made TERT an interesting target in tumor therapy. However, as mentioned above, TERT is present in somatic cells, where it also plays a role in apoptosis protection. Already in 2000, it was shown that suppression of TERT expression rendered neurons more susceptible to apoptosis [37]. In addition, TERT also protects normal human fibroblasts against stress-induced apoptosis [38]. We demonstrated an anti-apoptotic activity of TERT in primary human endothelial cells, which was dependent on complex formation between TERT, Akt and Hsp90 [18]. Similar to tumor cells, TERT exerts anti-oxidative properties in non-transformed cells. Differentiated cells derived from TERT-overexpressing embryonic stem cells showed reduced intracellular ROS levels suggesting increased resistance to oxidative stress [39]. Moreover, TERT knockdown in embryonic kidney cells led to an increase in ROS and the opposite effects were observed upon TERT overexpression [26]. The mutual connection between TERT and ROS is evident from the fact that an increase in ROS entails a loss of TERT, which can be inhibited by antioxidant treatment [8,40].
Mitochondrial functions of TERT have been discussed controversially. As the circular mtDNA does not contain telomeres, the enzyme has to have non-telomeric functions within the mitochondria. Initially, Santos et al. showed aggravated mtDNA damage and apoptosis in TERT-overexpressing fibroblast cell lines upon oxidative stress [41,42]. This was later challenged by the finding - also in fibroblast cell lines - that TERT protects mtDNA, reduces mitochondrial ROS production, improves mitochondrial membrane potential [24] and increases manganese SOD protein levels [43]. Supporting this latter view, we demonstrated for the first time in primary cells that TERT protects mtDNA against UV-induced damage and that TERT-deficient cardiac mitochondria have reduced state 3 respiration, clearly indicating a protective role for TERT in mitochondria [26]. This was further supported by the notion that expression of a TERT variant that is excluded from the mitochondria increased mitochondrial ROS, reduced manganese SOD levels and ATP [44,45], while expression of mitochondrially-targeted TERT in lung fibroblasts from TERT-deficient animals, reduced mitochondrial ROS [46]. This could potentially be explained by the balance between the electron transport chain subunits, which are encoded in the nuclear and mitochondrial DNA. Thus, damage to mtDNA would alter the stoichiometry of respiratory chain complexes leading to increased ROS production. Therefore, one could assume that mitochondrial TERT - by protecting mtDNA - is essential for proper respiratory complex assembly and maintenance of the cellular redox homeostasis.
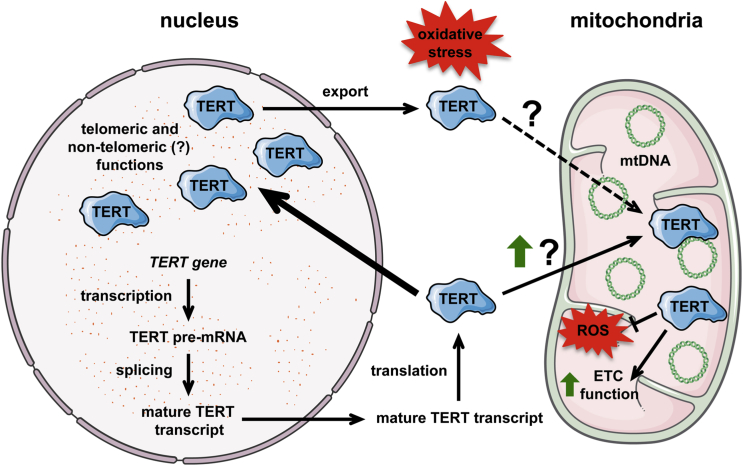
Interestingly, it has been shown that upon short-term oxidative stress, TERT is exported from the nucleus and increased in the mitochondria [20,24] (Fig. 2). This leads to the hypothesis that one of the primary responses of cells to oxidative stress is the protection of mitochondria by TERT. Currently, it is not clear, whether TERT is directly shuttled from the nucleus to the mitochondria or if newly synthesized molecules are directly funneled into these organelles. Sustained oxidative stress, however, finally leads to a reduction in mitochondrial TERT levels [47]. This downregulation is - like nuclear export - mediated by the Src kinase, which is also present in these organelles and activated upon persistent oxidative stress [47].
Fig. 2.
Intracellular redistribution of TERT upon oxidative stress. Under physiological conditions, TERT is distributed between the nucleus and the mitochondria with the majority being imported into the nucleus. In the nucleus, TERT is required for telomere maintenance and could, in addition, have non-telomeric functions. Mitochondrial TERT binds to and protects mitochondrial DNA (mtDNA), which might help to maintain proper electron transport chain (ETC) function; concomitantly, levels of mitochondrial reactive oxygen species (ROS) decrease. Upon short-term oxidative stress, TERT is exported from the nucleus with a concomitant increase in the mitochondria, which might serve as a mechanism to protect these organelles. Currently it is unclear, whether this is due to direct nuclear-mitochondrial shuttling or increased import of newly synthesized TERT molecules into the mitochondria.
Nowadays it is clear that mitochondria play a key role in oncogenesis, although glycolysis has long been viewed as the major metabolic process providing cancer cells with energy [48]. Indeed, TERT is also localized in mitochondria of cancer cells, where it improves mitochondrial function, inhibits endogenous ROS production [35] and apoptosis [49].
Recent investigations in microvascular arterioles revealed an in vivo role for mitochondrial TERT in the regulation of vasodilation related to its function in controlling mitochondrial ROS production. Inhibition of telomerase with BIBR-1532 in these vessels led to a switch from nitric oxide as physiological dilation mediator to mitochondrial H2O2 [44]. Moreover, this switch was already in operation in arterioles from patients with coronary artery disease, which had reduced cardiac and vascular TERT levels, but normal telomere length. Interestingly, this switch could be reverted with a telomerase activator. The authors concluded that mitochondrial TERT regulates mitochondrial ROS production with direct physiological consequences for the vessels [50].
In a therapeutic setting, the telomerase activator TA-65 was used in patients with metabolic syndrome, which is characterized by oxidative stress as one of its hallmarks [51]. TA-65 is small-molecule purified from the root of Astragalus membranaceus. It has been previously shown that TA-65 given over a 5-year period and an estimated 7000 person-years of use had no adverse events and reduced fasting blood sugar, insulin, total cholesterol and blood pressure [52], all considered positive changes of health state indicating that this treatment regimen could be effective in metabolic syndrome patients. Indeed, a recent crossover study showed not only an increase in HDL-cholesterol, a reduction in body mass index, plasma tumor necrosis factor α and C-reactive protein, but also in the ratio between total antioxidant capacity and 8-isoprostane levels, a marker for oxidative lipid damage during the TA-65 treatment period. These data demonstrate that telomerase activation may also affect the redox homeostasis in humans. It has to be noted that the effects of TA-65 on telomerase and the redox balance could be independent of each other. Nevertheless, one might speculate that some observations made after TA-65 treatment could be ascribed to mitochondrial TERT, as no changes in mean telomere length were observed [53,54]. However, the shortest telomeres seem to be stabilized by slightly elevated telomerase activity after treatment with TA-65 [53]. Thus, TA-65 could affect TERT in the nucleus and the mitochondria.
Although this substance has been used for nearly a decade and shows promising effects in humans, studies aimed at elucidating the molecular and biochemical mechanisms as to how TA-65 affects TERT are still missing. Studies in this direction are urgently needed to close this gap of knowledge and to exclude potential side effects.
4. Conclusion
Contrary to textbook knowledge, Telomerase Reverse Transcriptase (TERT), the catalytic subunit of telomerase, is not only present in tissues and cells with high proliferative capacity, like germ and stem cells as well as most all tumors, but also in slowly or non-proliferating tissues and cells. There, it has mostly non-canonical functions by protecting against apoptosis and maintaining the cellular redox homeostasis (Table 1). Moreover, nowadays it is undeniable that TERT is also present in mitochondria. Based on the recent evidence, one could speculate that most of the redox-dependent functions as well as the regulation of the cellular redox balance can be assigned to mitochondrial TERT. Therefore, it seems feasible to design therapeutic strategies aimed at increasing mitochondrial TERT levels, one of which could be treatment with TA-65. However this would require detailed analyses of the molecular and biochemical mechanisms underlying the TA-65 effects.
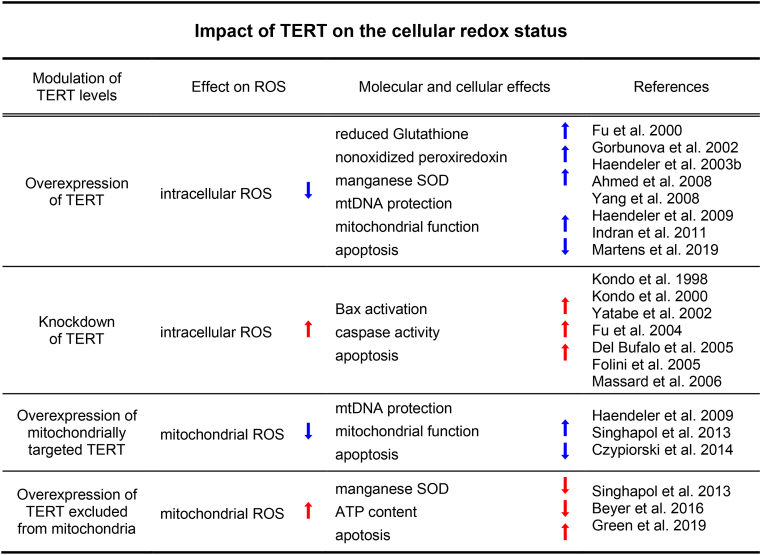
Table 1.
Impact of TERT on the cellular redox status. The table summmarizes the evidence how the modulation of endogenous TERT levels or the expression of TERT variants restricted to specific intracellular compartments affects the cellular redox status. The corresponding references are given in chronological order.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was in part supported by a grant of the Deutsche Forschungsgemeinschaft (SFB1116-2 A04) to J.A. and J.H. and a grant of the Corona Foundation (S199/10060/2014) to J.H.; J.R. is a scholarship holder of the IRTG1902. Single elements for figures were taken from Servier Medical Art (https://smart.servier.com/), which is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/)
Contributor Information
Joachim Altschmied, Email: joalt001@hhu.de.
Judith Haendeler, Email: juhae001@hhu.de.
References
- 1.Nandakumar J., Cech T.R. Finding the end: recruitment of telomerase to telomeres. Nat. Rev. Mol. Cell Biol. 2013;14:69–82. doi: 10.1038/nrm3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okuda K., Bardeguez A., Gardner J.P., Rodriguez P., Ganesh V., Kimura M., Skurnick J., Awad G., Aviv A. Telomere length in the newborn. Pediatr. Res. 2002;52:377–381. doi: 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Lindsey J., McGill N.I., Lindsey L.A., Green D.K., Cooke H.J. In vivo loss of telomeric repeats with age in humans. Mutat. Res. 1991;256:45–48. doi: 10.1016/0921-8734(91)90032-7. [DOI] [PubMed] [Google Scholar]
- 4.d'Adda di Fagagna F., Reaper P.M., Clay-Farrace L., Fiegler H., Carr P., Von Zglinicki T., Saretzki G., Carter N.P., Jackson S.P. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 5.Herbig U., Jobling W.A., Chen B.P., Chen D.J., Sedivy J.M. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a) Mol. Cell. 2004;14:501–513. doi: 10.1016/s1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- 6.Lewis K.A., Wuttke D.S. Telomerase and telomere-associated proteins: structural insights into mechanism and evolution. Structure. 2012;20:28–39. doi: 10.1016/j.str.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang Y.J., Hemann M.T., Hathcock K.S., Tessarollo L., Feigenbaum L., Hahn W.C., Hodes R.J. Expression of telomerase RNA template, but not telomerase reverse transcriptase, is limiting for telomere length maintenance in vivo. Mol. Cell Biol. 2004;24:7024–7031. doi: 10.1128/MCB.24.16.7024-7031.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haendeler J., Hoffmann J., Diehl J.F., Vasa M., Spyridopoulos I., Zeiher A.M., Dimmeler S. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ. Res. 2004;94:768–775. doi: 10.1161/01.RES.0000121104.05977.F3. [DOI] [PubMed] [Google Scholar]
- 9.Minamino T., Kourembanas S. Mechanisms of telomerase induction during vascular smooth muscle cell proliferation. Circ. Res. 2001;89:237–243. doi: 10.1161/hh1501.094267. [DOI] [PubMed] [Google Scholar]
- 10.Richardson G.D., Breault D., Horrocks G., Cormack S., Hole N., Owens W.A. Telomerase expression in the mammalian heart. Faseb. J. 2012;26:4832–4840. doi: 10.1096/fj.12-208843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zurek M., Altschmied J., Kohlgrüber S., Ale-Agha N., Haendeler J. Role of Telomerase in the Cardiovascular System. Genes. 2016;7:29. doi: 10.3390/genes7060029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iannilli F., Zalfa F., Gartner A., Bagni C., Dotti C.G. Cytoplasmic TERT associates to RNA granules in fully mature neurons: role in the translational control of the cell cycle inhibitor p15INK4B. PloS One. 2013;8 doi: 10.1371/journal.pone.0066602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spilsbury A., Miwa S., Attems J., Saretzki G. The role of telomerase protein TERT in Alzheimer's disease and in tau-related pathology in vitro. J. Neurosci. 2015;35:1659–1674. doi: 10.1523/JNEUROSCI.2925-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu K.J., Grandori C., Amacker M., Simon-Vermot N., Polack A., Lingner J., Dalla-Favera R. Direct activation of TERT transcription by c-MYC. Nat. Genet. 1999;21:220–224. doi: 10.1038/6010. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y., Cheng D., Wang S., Zhu J. Dual roles of c-Myc in the regulation of hTERT gene. Nucleic Acids Res. 2014;42:10385–10398. doi: 10.1093/nar/gku721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Won J., Yim J., Kim T.K. Opposing regulatory roles of E2F in human telomerase reverse transcriptase (hTERT) gene expression in human tumor and normal somatic cells. Faseb. J. 2002;16:1943–1945. doi: 10.1096/fj.02-0311fje. [DOI] [PubMed] [Google Scholar]
- 17.Breitschopf K., Zeiher A.M., Dimmeler S. Pro-atherogenic factors induce telomerase inactivation in endothelial cells through an Akt-dependent mechanism. FEBS Lett. 2001;493:21–25. doi: 10.1016/s0014-5793(01)02272-4. [DOI] [PubMed] [Google Scholar]
- 18.Haendeler J., Hoffmann J., Rahman S., Zeiher A.M., Dimmeler S. Regulation of telomerase activity and anti-apoptotic function by protein-protein interaction and phosphorylation. FEBS Lett. 2003;536:180–186. doi: 10.1016/s0014-5793(03)00058-9. [DOI] [PubMed] [Google Scholar]
- 19.Chung J., Khadka P., Chung I.K. Nuclear import of hTERT requires a bipartite nuclear localization signal and Akt-mediated phosphorylation. J. Cell Sci. 2012;125:2684–2697. doi: 10.1242/jcs.099267. [DOI] [PubMed] [Google Scholar]
- 20.Haendeler J., Hoffmann J., Brandes R.P., Zeiher A.M., Dimmeler S. Hydrogen peroxide triggers nuclear export of telomerase reverse transcriptase via Src kinase family-dependent phosphorylation of tyrosine 707. Mol. Cell Biol. 2003;23:4598–4610. doi: 10.1128/MCB.23.13.4598-4610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakob S., Altschmied J., Haendeler J. Shping 2" different cellular localizations - a potential new player in aging processes. Aging. 2009;1:664–668. doi: 10.18632/aging.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakob S., Schroeder P., Lukosz M., Büchner N., Spyridopoulos I., Altschmied J., Haendeler J. Nuclear protein tyrosine phosphatase Shp-2 is one important negative regulator of nuclear export of telomerase reverse transcriptase. J. Biol. Chem. 2008;283:33155–33161. doi: 10.1074/jbc.M805138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray P.D., Huang B.W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed S., Passos J.F., Birket M.J., Beckmann T., Brings S., Peters H., Birch-Machin M.A., von Zglinicki T., Saretzki G. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J. Cell Sci. 2008;121:1046–1053. doi: 10.1242/jcs.019372. [DOI] [PubMed] [Google Scholar]
- 25.Gordon D.M., Santos J.H. The emerging role of telomerase reverse transcriptase in mitochondrial DNA metabolism. J. Nucleic Acids. 2010;2020 doi: 10.4061/2010/390791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haendeler J., Dröse S., Büchner N., Jakob S., Altschmied J., Goy C., Spyridopoulos I., Zeiher A.M., Brandt U., Dimmeler S. Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage. Arterioscler. Thromb. Vasc. Biol. 2009;29:929–935. doi: 10.1161/ATVBAHA.109.185546. [DOI] [PubMed] [Google Scholar]
- 27.Sharma N.K., Reyes A., Green P., Caron M.J., Bonini M.G., Gordon D.M., Holt I.J., Santos J.H. Human telomerase acts as a hTR-independent reverse transcriptase in mitochondria. Nucleic Acids Res. 2012;40:712–725. doi: 10.1093/nar/gkr758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondo S., Tanaka Y., Kondo Y., Hitomi M., Barnett G.H., Ishizaka Y., Liu J., Haqqi T., Nishiyama A., Villeponteau B. Antisense telomerase treatment: induction of two distinct pathways, apoptosis and differentiation. Faseb. J. 1998;12:801–811. doi: 10.1096/fasebj.12.10.801. [DOI] [PubMed] [Google Scholar]
- 29.Kondo Y., Koga S., Komata T., Kondo S. Treatment of prostate cancer in vitro and in vivo with 2-5A-anti-telomerase RNA component. Oncogene. 2000;19:2205–2211. doi: 10.1038/sj.onc.1203538. [DOI] [PubMed] [Google Scholar]
- 30.Massard C., Zermati Y., Pauleau A.L., Larochette N., Metivier D., Sabatier L., Kroemer G., Soria J.C. hTERT: a novel endogenous inhibitor of the mitochondrial cell death pathway. Oncogene. 2006;25:4505–4514. doi: 10.1038/sj.onc.1209487. [DOI] [PubMed] [Google Scholar]
- 31.Del Bufalo D., Rizzo A., Trisciuoglio D., Cardinali G., Torrisi M.R., Zangemeister-Wittke U., Zupi G., Biroccio A. Involvement of hTERT in apoptosis induced by interference with Bcl-2 expression and function. Cell Death Differ. 2005;12:1429–1438. doi: 10.1038/sj.cdd.4401670. [DOI] [PubMed] [Google Scholar]
- 32.Folini M., Brambilla C., Villa R., Gandellini P., Vignati S., Paduano F., Daidone M.G., Zaffaroni N. Antisense oligonucleotide-mediated inhibition of hTERT, but not hTERC, induces rapid cell growth decline and apoptosis in the absence of telomere shortening in human prostate cancer cells. Eur. J. Canc. 2005;41:624–634. doi: 10.1016/j.ejca.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Yatabe N., Kyo S., Kondo S., Kanaya T., Wang Z., Maida Y., Takakura M., Nakamura M., Tanaka M., Inoue M. 2-5A antisense therapy directed against human telomerase RNA inhibits telomerase activity and induces apoptosis without telomere impairment in cervical cancer cells. Canc. Gene Ther. 2002;9:624–630. doi: 10.1038/sj.cgt.7700479. [DOI] [PubMed] [Google Scholar]
- 34.Rahman R., Latonen L., Wiman K.G. hTERT antagonizes p53-induced apoptosis independently of telomerase activity. Oncogene. 2005;24:1320–1327. doi: 10.1038/sj.onc.1208232. [DOI] [PubMed] [Google Scholar]
- 35.Indran I.R., Hande M.P., Pervaiz S. hTERT overexpression alleviates intracellular ROS production, improves mitochondrial function, and inhibits ROS-mediated apoptosis in cancer cells. Canc. Res. 2011;71:266–276. doi: 10.1158/0008-5472.CAN-10-1588. [DOI] [PubMed] [Google Scholar]
- 36.Moon D.O., Kang S.H., Kim K.C., Kim M.O., Choi Y.H., Kim G.Y. Sulforaphane decreases viability and telomerase activity in hepatocellular carcinoma Hep3B cells through the reactive oxygen species-dependent pathway. Canc. Lett. 2010;295:260–266. doi: 10.1016/j.canlet.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Fu W., Killen M., Culmsee C., Dhar S., Pandita T.K., Mattson M.P. The catalytic subunit of telomerase is expressed in developing brain neurons and serves a cell survival-promoting function. J. Mol. Neurosci. 2000;14:3–15. doi: 10.1385/JMN:14:1-2:003. [DOI] [PubMed] [Google Scholar]
- 38.Gorbunova V., Seluanov A., Pereira-Smith O.M. Expression of human telomerase (hTERT) does not prevent stress-induced senescence in normal human fibroblasts but protects the cells from stress-induced apoptosis and necrosis. J. Biol. Chem. 2002;277:38540–38549. doi: 10.1074/jbc.M202671200. [DOI] [PubMed] [Google Scholar]
- 39.Yang C., Przyborski S., Cooke M.J., Zhang X., Stewart R., Anyfantis G., Atkinson S.P., Saretzki G., Armstrong L., Lako M. A key role for telomerase reverse transcriptase unit in modulating human embryonic stem cell proliferation, cell cycle dynamics, and in vitro differentiation. Stem Cell. 2008;26:850–863. doi: 10.1634/stemcells.2007-0677. [DOI] [PubMed] [Google Scholar]
- 40.Borras C., Esteve J.M., Vina J.R., Sastre J., Vina J., Pallardo F.V. Glutathione regulates telomerase activity in 3T3 fibroblasts. J. Biol. Chem. 2004;279:34332–34335. doi: 10.1074/jbc.M402425200. [DOI] [PubMed] [Google Scholar]
- 41.Santos J.H., Meyer J.N., Skorvaga M., Annab L.A., Van Houten B. Mitochondrial hTERT exacerbates free-radical-mediated mtDNA damage. Aging Cell. 2004;3:399–411. doi: 10.1111/j.1474-9728.2004.00124.x. [DOI] [PubMed] [Google Scholar]
- 42.Santos J.H., Meyer J.N., Van Houten B. Mitochondrial localization of telomerase as a determinant for hydrogen peroxide-induced mitochondrial DNA damage and apoptosis. Hum. Mol. Genet. 2006;15:1757–1768. doi: 10.1093/hmg/ddl098. [DOI] [PubMed] [Google Scholar]
- 43.Martens A., Schmid B., Akintola O., Saretzki G. Telomerase does not improve DNA repair in mitochondria upon stress but increases MnSOD protein under serum-free conditions. Int. J. Mol. Sci. 2019;21 doi: 10.3390/ijms21010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beyer A.M., Freed J.K., Durand M.J., Riedel M., Ait-Aissa K., Green P., Hockenberry J.C., Morgan R.G., Donato A.J., Peleg R. Critical role for telomerase in the mechanism of flow-mediated dilation in the human microcirculation. Circ. Res. 2016;118:856–866. doi: 10.1161/CIRCRESAHA.115.307918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green P.D., Sharma N.K., Santos J.H. Telomerase impinges on the cellular response to oxidative stress through mitochondrial ROS-mediated regulation of autophagy. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20061509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Czypiorski P., Altschmied J., Rabanter L.L., Goy C., Jakob S., Haendeler J. Outfielders playing in the infield: functions of aging-associated "nuclear" proteins in the mitochondria. Curr. Mol. Med. 2014;14:1247–1251. doi: 10.2174/1566524014666141202125935. [DOI] [PubMed] [Google Scholar]
- 47.Büchner N., Zschauer T.C., Lukosz M., Altschmied J., Haendeler J. Downregulation of mitochondrial telomerase reverse transcriptase induced by H2O2 is Src kinase dependent. Exp. Gerontol. 2010;45:558–562. doi: 10.1016/j.exger.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Porporato P.E., Filigheddu N., Pedro J.M.B., Kroemer G., Galluzzi L. Mitochondrial metabolism and cancer. Cell Res. 2018;28:265–280. doi: 10.1038/cr.2017.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singhapol C., Pal D., Czapiewski R., Porika M., Nelson G., Saretzki G.C. Mitochondrial telomerase protects cancer cells from nuclear DNA damage and apoptosis. PloS One. 2013;8 doi: 10.1371/journal.pone.0052989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Durand M.J., Zinkevich N.S., Riedel M., Gutterman D.D., Nasci V.L., Salato V.K., Hijjawi J.B., Reuben C.F., North P.E., Beyer A.M. Vascular actions of angiotensin 1-7 in the human microcirculation: Novel role for telomerase. Arterioscler. Thromb. Vasc. Biol. 2016;36:1254–1262. doi: 10.1161/ATVBAHA.116.307518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hutcheson R., Rocic P. The metabolic syndrome, oxidative stress, environment, and cardiovascular disease: the great exploration. Exp. Diabetes Res. 2012 doi: 10.1155/2012/271028. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harley C.B., Liu W., Flom P.L., Raffaele J.M. A natural product telomerase activator as part of a health maintenance program: metabolic and cardiovascular response. Rejuvenation Res. 2013;16:386–395. doi: 10.1089/rej.2013.1430. [DOI] [PubMed] [Google Scholar]
- 53.Bernardes de Jesus B., Schneeberger K., Vera E., Tejera A., Harley C.B., Blasco M.A. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell. 2011;10:604–621. doi: 10.1111/j.1474-9726.2011.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernandez M.L., Thomas M.S., Lemos B.S., DiMarco D.M., Missimer A., Melough M., Chun O.K., Murillo A.G., Alyousef H., Medina-Vera I. TA-65, A telomerase activator, improves cardiovascular markers in patients with metabolic syndrome. Curr. Pharmaceut. Des. 2018;24:1905–1911. doi: 10.2174/1381612824666180316114832. [DOI] [PubMed] [Google Scholar]