Graphical abstract

Keywords: Air pollution, Cardiovascular disease, India, Particulate matter, Black carbon, Personal exposure
Abbreviations: AIx, augmentation index; APCAPS, Andhra Pradesh Children and Parent Study; BC, black carbon; cf-PWV, carotid-femoral pulse wave velocity; CIMT, carotid intima-media thickness; CVD, cardiovascular diseases; LMIC, low-and-middle income countries; NIN, National Institute of Nutrition; PM, particulate matter; PM2.5, particulate matter with an aerodynamic diameter of 2.5 µm or less; SLI, Standard Living Index
Highlights
-
•
We evaluated personal exposure to PM2.5 and BC in adults from peri-urban India.
-
•
PM2.5 was associated with vascular damage by three cardiovascular markers in men.
-
•
PM2.5 was nonlinearly associated with increased augmentation index in men.
-
•
Both PM2.5 and BC were positively associated with augmentation index AIx in women.
Abstract
Objective
Air pollution is a leading preventable risk factor for cardiovascular diseases. Previous studies mostly relied on concentrations at residence, which might not represent personal exposure. Personal air pollution exposure has a greater variability compared with levels of ambient air pollution, facilitating evaluation of exposure-response functions and vascular pathophysiology. We aimed to evaluate the association between predicted annual personal exposure to PM2.5 and black carbon (BC) and three vascular damage markers in peri-urban South India.
Methods
We analyzed the third wave of the APCAPS cohort (2010–2012), which recruited participants from 28 villages. We used predicted personal exposure to PM2.5 and BC derived from 610 participant-days of 24 h average gravimetric PM2.5 and BC measurements and predictors related to usual time-activity. Outcomes included carotid intima-media thickness (CIMT), carotid-femoral pulse wave velocity (cf-PWV) and augmentation index (AIx). We fit linear mixed models, adjusting for potential confounders and accounting for the clustered data structure. We evaluated nonlinear associations using generalized additive mixed models.
Results
Of the 3017 participants (mean age 38 years), 1453 (48%) were women. The average PM2.5 exposure was 51 µg/m3 (range 13–85) for men, and 61 µg/m3 (range 40–120) for women, while the average BC was 4 µg/m3 (range 3–7) for men and 8 µg/m3 (range 3–22) for women. A 10 μg/m3 increase of PM2.5 was positively associated with CIMT (0.026 mm, 95% CI 0.014, 0.037), cf-PWV (0.069 m/s, 95% CI 0.008, 0.131) and AIx (0.8%, 95% CI 0.3, 1.3) among men. The exposure-response function for PM2.5 and AIx among men showed non-linearity, particularly within the exposure range dominated by tobacco smoking and occupational exposures. Both PM2.5 and BC were positively associated with AIx among women (0.6%, 95% CI 0.2, 1.0, per 10 μg/m3 PM2.5; 0.5%, 95% CI 0.1, 0.8, per 2 μg/m3 BC).
Conclusions
Personal exposure to particulate matter was associated with vascular damage in a peri-urban population in South India. Personal exposure to particulate matter appears to have gender-specific effects on the type of vascular damage, potentially reflecting differences in sources of personal exposure by gender.
1. Introduction
Particulate air pollution is one of the leading preventable risk factors for premature mortality, especially through its effect on cardiovascular diseases (CVD) (Abajobir et al., 2017, Brook et al., 2010). Considerable evidence links short- and long-term exposure to particles with fatal and non-fatal events such as acute myocardial infarction (Abajobir et al., 2017, Brook et al., 2010, Hoek et al., 2013). Additionally, particle exposure contributes to the development of subclinical cardiovascular pathologies, strongly associated with CVDs occurrence, such as atherosclerosis, arterial stiffness and other vascular alterations (Brook et al., 2010, Miller et al., 2017, Provost et al., 2015, Zanoli et al., 2017).
Despite the now relatively large body of evidence linking particulate matter of ≤2.5 µm in aerodynamic diameter (PM2.5) and CVD (Brook et al., 2010), this evidence has important gaps. The majority of studies are from high-income countries, where the main source of PM is traffic, with low-to-moderate PM2.5 concentrations (Hadley et al., 2018, Tonne, 2017), and recently from China, with high PM2.5 concentrations (Newell et al., 2017). Therefore, the available evidence might not be applicable to (1) settings where PM is dominated by non-traffic sources due to differences in the biological action of different PM components, and (2) populations exposed to high levels of PM such as in many low-and-middle income countries (LMICs) (Hadley et al., 2018, Tonne, 2017). Additionally, the majority of studies linking PM to vascular damage estimate an individual’s exposure based on spatial contrasts at the city, neighborhood, or home address level (Brook et al., 2010, Ljungman et al., 2018, Perez et al., 2015, Provost et al., 2015). Because individuals have different characteristics and behaviors (e.g., physical activity, mobility, work-related exposure), their actual PM exposure is likely to differ from estimated exposure at a given location (Steinle et al., 2013). Personal exposure assessment is a means to move beyond the limitations of exposure assignment based only on location, better reflecting the contribution of individual characteristics and behaviors. Personal exposure also has the potential to provide insights on the health effects of PM from sources not well captured in models of outdoor PM (e.g. land use regression, emissions-dispersion models) (Steinle et al., 2013).
Few studies have related measured personal PM exposure to intermediate cardiovascular outcomes (Baumgartner et al., 2018, Provost et al., 2016, Zhao et al., 2014) and evidence is mixed for markers of atherosclerosis and arterial stiffness (Baumgartner et al., 2018, Provost et al., 2016, Zanoli et al., 2017, Zhao et al., 2014). Moreover, previous studies are limited by their small sample size, and focus on specific subgroups, such as occupationally exposed individuals (Fang et al., 2008) and women (Baumgartner et al., 2018, Provost et al., 2016). We aimed to quantify the exposure-response function between predicted personal exposure to PM2.5 and black carbon (BC) and three cardiovascular markers in a sample of the general population residing in peri-urban South India. We hypothesized that increased exposure to personal predicted PM2.5 and BC would be associated with vascular damage, and a potential non-linear exposure-response function considering the expected wide exposure range in the same population.
2. Methods
2.1. Study design and population
We conducted a cross-sectional analysis using the third follow-up (2010–2012) of the Andhra Pradesh Children and Parent Study (APCAPS) cohort (Kinra et al., 2014). Participants resided in 2386 households, situated in 28 villages in a peri-urban area south of the city of Hyderabad. Data collection during the third follow-up of APCAPS was conducted primarily at clinics established within the villages. We collected information about demographic, socioeconomic status (education, occupation, Standard Living Index-SLI) (Kinra et al., 2014), health behaviors (smoking, environmental tobacco smoke, alcohol intake, diet, and physical activity) (Kinra et al., 2014), medical history, and household characteristics during participants’ first clinic visit via standardized questionnaires. Anthropometric measurements and arterial blood pressure were assessed by standardized physical examination, and fasting blood samples were collected following standard procedures (Kinra et al., 2014). Definitions for cardiometabolic risk factors are presented in the supplementary material. Further definitions are provided elsewhere (Kinra et al., 2014). All participants were invited to attend a second clinic visit at the National Institute of Nutrition (NIN) in Hyderabad and APCAPS provided transport from each village to attend the clinic at NIN.
APCAPS was approved by the London School of Hygiene & Tropical Medicine (London, UK) and the National Institute of Nutrition (NIN) (Hyderabad, India). CHAI was approved by the Ethics Committees of Parc de Salut MAR (Barcelona, Spain), the Indian Institute of Public Health (Hyderabad, India), and the NIN. Signed consent forms were obtained from all participants.
2.2. Outcomes assessment
All cardiovascular markers were measured by a trained physician during the second clinic visit at the NIN. Measurements were made using standard operating procedures, quality control and following recommended clinical guidelines (Kinra et al., 2014, Touboul et al., 2012, Townsend et al., 2015). Participants were invited to attend NIN as soon as possible after the first visit (median 9 days [3–20]).
We evaluated three cardiovascular markers that reflect three different domains of vascular damage and that are well-known risk factors for CVD events and mortality (Künzli et al., 2011, The Reference Values for Arterial Stiffness, 2010, Touboul et al., 2012, Townsend et al., 2015, Wolf et al., 2011): (1) carotid-intima media thickness (CIMT), a marker of atherosclerosis, (2) carotid-femoral pulse wave velocity (cf-PWV), a marker of arterial stiffness and 3) augmentation index (AIx), a marker of global vascular injury (e.g., wave reflection).
CIMT was measured using a B-mode ultrasound scanner (Ethiroli Tiny-16a, Surabhi Biomedical Instrumentation, India), at the right common carotid, close to the bulb. CIMT was analyzed using a semi-automated software which read a 10 mm segment from the near (ie, the artery wall close to the ultrasound probe) and far walls. We analyzed mean CIMT, calculated as the mean CIMT of available measurements. cf-PWV and AIx were measured using a validated device (Vicorder, Skidmore Medical, UK), in supine position and after 10 min resting. For the cf-PWV, the distance between carotid and femoral arteries was estimated by taking the carotid-to-suprasternal notch and suprasternal-notch-to-thigh distances. For the AIx, the distance was estimated between the top of the brachial cuff to the top of the femoral cuff. cf-PWV and AIx were recorded three times for each participant, with high reliability (intraclass correlation coefficient among the three measurements was 0.92 for cf-PWV and 0.91 for AIx). We used the mean of the two closest measures (Baker et al., 2015). We also recorded concurrent heart rate and brachial blood pressure upon cf-PWV and AIx measurement.
2.3. Exposure assessment
The personal exposure assessment was conducted as part of the Cardiovascular Health effects of Air pollution in Telangana, India (CHAI) project. The measurements and modelling approach have been detailed elsewhere (Sanchez et al., 2019, Tonne et al., 2017). Briefly, we measured 24 h personal exposure to PM2.5 and BC, in two sessions, on randomly selected participants of the APCAPS cohort, between September 2015 to April 2016. We used 610 participant-days measurements to derive a model to predict personal exposure to each pollutant. We used a two-stage model approach to estimate the personal exposure: first, we fit a linear mixed model having the 24 h personal exposure as outcome and ambient PM2.5 and temperature as covariates; subsequently, we used the marginal residuals of the first model as outcome in a linear mixed model, representing the ambient-adjusted personal exposure. The final model of the second stage was selected by forward stepwise mixed model selection and validated based on 10-fold cross-validation and 10-fold cross holdout validation. We fit separate exposure prediction models for men and women, given previous evidence of differences in mobility patterns and sources influencing personal exposure (Milà et al., 2018, Salmon et al., 2018, Sanchez et al., 2017). Selected predictors of PM2.5 for men were current smoking status (active, passive, not exposed), occupation and time spent cycling. For women, predictors were head of household occupation, main source of cooking fuel, and time spent near biomass stove. Predictors of BC for men were main source of cooking fuel, occupation, and ambient PM2.5; for women they were main source of cooking fuel, time spent near biomass stove, and ownership of motorized vehicle. The proportion of the between-participant variability explained (R2between) for PM2.5 was 53% for men and 38% for women. The proportion of the between-participant variability explained (R2between) for BC was 20% for men and 57% for women (Sanchez et al., 2019). We used these models to predict the personal exposure levels of PM2.5 and BC for each participant.
2.4. Statistical analysis
We evaluated the association of PM2.5 and BC on CIMT, cf-PWV and AIx fitting a nested linear-mixed model (nested random intercept, with households nested within villages). Our analysis strategy was specified prior to data analysis. We selected potential confounders according to a directed acyclic graph based on the literature. We did not adjust for variables selected as predictors of each personal exposure (Additional file 1, Fig. S1). We stratified our analysis by sex because of the differences between men and women in the population regarding exposure pattern (Milà et al., 2018, Salmon et al., 2018, Sanchez et al., 2017). We evaluated whether each exposure might have a nonlinear effect on each outcome using thin-plate splines in generalized additive mixed models. The smoothness selection was done via the algorithm implemented in the gamm function.
We performed sequential adjustment for potential confounders:
Model 1- exposure and random intercepts
Model 2- Model 1 + age (natural spline, df = 3, for CIMT and linear term for cf-PWV and AIx)
Model 3- Model 2 + body-mass index, alcohol, fruit and vegetable consumption, and physical activity
Model 4- Model 3 + education (main model).
For cf-PWV, we additionally adjusted for blood pressure (measured upon cf-PWV assessment), which is a main determinant of cf-PWV (The Reference Values for Arterial Stiffness, 2010, Townsend et al., 2015), but also is in the causal pathway between air pollution and cf-PWV; and for AIx, we additionally adjusted for height, which can explain part of the between-individual AIx variability (Baumgartner et al., 2018, Reeve et al., 2014). All models for AIx were adjusted for heart rate (measured upon AIx assessment) (Baumgartner et al., 2018, Townsend et al., 2015).
We applied inverse probability weighting to deal with potential selection bias, because not all participants attended the second clinic visit during which the cardiovascular outcomes were measured (Seaman et al., 2012). We used multiple imputation for missing covariate data and pooled results using Rubin’s rules (Seaman et al., 2012). Further description of inverse probability weighting and multiple imputation are included in the supplementary material (Supplementary material).
We conducted several additional analyses to assess the robustness of our findings and explore potential effect modification. First, we added to model 4 another construct of socioeconomic status (SLI) as potential confounder. We did not include SLI in the main analysis because cook stove characteristics, an important predictor of personal exposure, were included in the SLI. Second, we fit our models on multiple imputed data without selection bias correction, and on complete-case data with and without selection bias correction. Third, to explore potential effect modification and potential reverse causality (i.e., participants with high cardio-metabolic profile had changed their behavior and therefore their personal exposure), we fit model 4 (main model) in subgroups of participants by age (in participants aged <40 years and ≥40 years) (Ranzani et al., 2020), metabolic syndrome, hypertension, diabetes and obesity. Fourth, we fit the models using the average of two 24 h PM2.5 measurements in place of predicted personal exposure for those participants with outcome variables available (n = 104 for men and n = 83 for women).
All analyses were conducted with R-3.4.2 (R Core Team, 2016), with the packages tidyverse, mice, miceadds, lme4, mgcv, and ggplot2.
3. Results
3.1. Participants selection and characteristics
Of the 6944 participants enrolled in the third follow-up of APCAPS, we included adult (age ≥ 18 years) men and non-pregnant women. Among those eligible for inclusion (n = 6229), 3445 (55%) attended the second clinic visit for the cardiovascular risk measures; participants who attended the NIN clinic visit had a higher prevalence of known risk factors for CVD compared to those who did not attend (data not shown). Of the 3445 participants, we excluded 51 (1.5%) because of missing village, 262 (7.6%) because of missing cf-PWV/AIx measurements, and 115 (3.3%) because of missing CIMT measurements, resulting in 3017 participants included in the analysis.
Table 1 describes the participants. Participants had a bimodal distribution of age (mean age = 38 years, median age = 40 years), had low education level and mostly manual occupations. About 20% of participants met criteria for metabolic syndrome. Regarding health behaviors, 488 (31%) men were current smokers (average 10 cigarettes per day), while half of women were exposed to environmental tobacco smoke. The prevalence of low physical activity was high. About 60% of participants had biomass as main source of cooking fuel.
Table 1.
Participant characteristics stratified by gender.
| Variable | Category | Men (n = 1564) | Women (n = 1453) |
|---|---|---|---|
| Age (years) | Mean ± SD | 38 ± 16 | 38 ± 12 |
| Age (categories) | 18.0–29.9 | 761 (48.7%) | 452 (31.1%) |
| 30.0–39.9 | 99 (6.3%) | 186 (12.8%) | |
| 40.0–49.9 | 226 (14.5%) | 591 (40.7%) | |
| 50.0–59.9 | 332 (21.2%) | 201 (13.8%) | |
| 60.0 – | 146 (9.3%) | 23 (1.6%) | |
| Educationa | No formal education | 630 (40.3%) | 1032 (71.0%) |
| Primary (1–4 years) | 253 (16.2%) | 131 (9.0%) | |
| Secondary (5–12 years) | 542 (34.7%) | 238 (16.4%) | |
| Beyond secondary (>12 years) | 138 (8.8%) | 52 (3.6%) | |
| Occupationa | Unemployed | 302 (19.3%) | 421 (29.0%) |
| Unskilled manual | 696 (44.5%) | 863 (59.4%) | |
| Skilled manual | 469 (30.0%) | 145 (10.0%) | |
| Non-manual | 96 (6.1%) | 24 (1.7%) | |
| Standard living index (points)a | Mean ± SD | 29 ± 8 | 28 ± 8 |
| Comorbidities | |||
| Body-mass index (kg/m2)a | Underweight (<18.5) | 501 (32.1%) | 394 (27.1%) |
| Normal weight (18.5–22.9) | 715 (45.8%) | 624 (42.9%) | |
| Overweight (23.0–24.9) | 170 (10.9%) | 206 (14.2%) | |
| Obese (25.0 –) | 176 (11.3%) | 229 (15.8%) | |
| Central obesity | Waist circumference ≥ 80 cm for women and ≥ 90 cm for men | 122 (7.8%) | 272 (18.7%) |
| Hypertension | SBP ≥ 140 mmHg or DBP ≥ 90 mmHg or anti-hypertensive intake | 398 (25.5%) | 254 (17.5%) |
| Taking anti-hypertensive drugs | 64/398 (16.1%) | 51/254 (20.1%) | |
| Glucose intolerance | Impaired fasting glucose | 363 (23.2%) | 324 (22.3%) |
| Diabetes | 96 (6.1%) | 66 (4.5%) | |
| Taking anti-diabetic drugs | 30/96 (31.3%) | 22/66 (33.3%) | |
| Lipid profilea | Total cholesterol ≥ 200 mg/dL | 243 (15.7%) | 245 (17.4%) |
| HDL cholesterol < 50 mg/dL for female and < 40 mg/dL for male | 753 (48.5%) | 998 (70.8%) | |
| Non-HDL cholesterol ≥ 130 mg/dL | 553 (35.6%) | 524 (37.2%) | |
| Triglycerides ≥ 150 md/dL | 437 (28.4%) | 270 (19.2%) | |
| Metabolic syndrome | ≥3 criteria | 282 (18.0%) | 297 (20.4%) |
| Health behaviors | |||
| Smoking statusa | Never | 1045 (66.9%) | 1449 (99.7%) |
| Former | 30 (1.9%) | – | |
| Current | 488 (31.2%) | 4 (0.3%) | |
| Package-years | 14.8 ± 15 | – | |
| Environmental tobacco smoke | Yes | 400 (25.6%) | 613 (42.2%) |
| Alcohol usea | Most of the days | 671 (42.9%) | 264 (18.2%) |
| Physical activity (METs)a | Sedentary or light active (<1.70) | 1068 (69.9%) | 805 (56.7%) |
| Active or moderately active (1.70–1.99) | 379 (24.8%) | 513 (36.2%) | |
| Vigorously active (>2) | 81 (5.3%) | 101 (7.1%) | |
| Fuel use | |||
| Main source of cooking fuela | Biomass | 885 (57.2%) | 900 (62.5%) |
| Stove ventilation | Not vented to the outside | 407 (26.0%) | 364 (25.1%) |
| Main source of lighting fuela | Non-electricity | 15 (0.9%) | 21 (1.4%) |
Missing values were 1 (<0.1%) for occupation, education, smoking status, alcohol use, and lighting fuel use, 2 (<0.1%) for hypertension and body-mass index, 3 (<0.1%) for abdominal obesity, 28 (0.9%) for standard living index, 31 (1.0%) for main source of cooking fuel, 55 (1.8%) for cholesterol, 70 (2.3%) for physical activity, 72 (2.4%) for triglycerides. Data are mean ± SD or n (%). DBP = diastolic blood pressure, HDL = high density lipoprotein, METs = metabolic equivalents; SBP = systolic blood pressure.
3.2. Personal exposure distributions
Mean PM2.5 was 50.6 µg/m3 (IQR = 9.4) for men and 60.9 µg/m3 (IQR = 12.9) for women (Table 2); mean BC was 4.4 µg/m3 (IQR = 0.9) for men and 7.6 µg/m3 (IQR = 3.5) for women (Table 2). There were notable differences in the distribution of each pollutant by sex (Fig. 1), particularly for BC.
Table 2.
Summary of personal air pollution exposure and vascular damage markers from 3017 participants.
| Exposure/Outcome | Mean | SD | Median | IQR |
|---|---|---|---|---|
| Men (n = 1564) | ||||
| PM2.5 (µg/m3) | 50.61 | 8.95 | 49.84 | 9.38 |
| Black carbon (µg/m3) | 4.35 | 0.72 | 4.33 | 0.93 |
| CIMT (mm) | 0.80 | 0.26 | 0.78 | 0.38 |
| cf-PWV (m/s) | 7.02 | 1.39 | 6.8 | 1.80 |
| AIx (%) | 21.9 | 10.9 | 21.0 | 16.0 |
| Women (n = 1453) | ||||
| PM2.5 (µg/m3) | 60.93 | 11.73 | 61.70 | 12.86 |
| Black carbon (µg/m3) | 7.63 | 2.68 | 7.94 | 3.48 |
| CIMT (mm) | 0.87 | 0.24 | 0.87 | 0.32 |
| cf-PWV (m/s) | 6.99 | 1.31 | 6.90 | 1.70 |
| AIx (%) | 24.6 | 11.5 | 24.5 | 16.0 |
AIx = augmentation index; cf-PWV = carotid-femoral pulse wave velocity; CIMT = carotid intima-media thickness; PM2.5 = particulate matter with an aerodynamic diameter of 2.5 µm or less.
Fig. 1.
Distribution of predicted personal PM2.5 and BC stratified by gender. The probability densities were estimated using kernel smoothing methods. PM2.5 = particulate matter with an aerodynamic diameter of 2.5 µm or less.
3.3. Association between personal exposure to PM and cardiovascular markers
Overall, there were good linear correlations between the three cardiovascular markers, particularly for men (Fig. S2). We also observed expected correlations between cf-PWV and blood pressure and AIx and heart rate (Fig. S3).
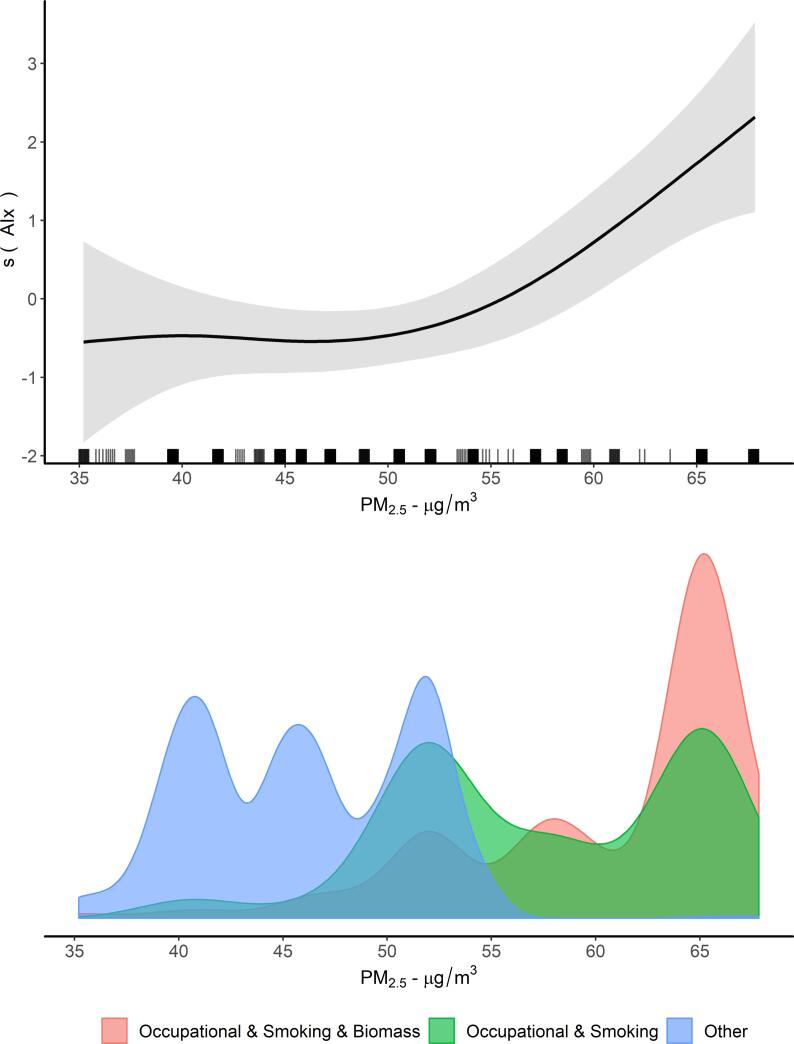
Mean CIMT was 0.802 mm (SD = 0.26) for men and 0.868 mm (SD = 0.24) for women. We observed a positive crude association between PM2.5 and CIMT for men, which remained after full adjustment (Model 4: +0.026 mm, 95% CI 0.014–0.037, per 10 µg/m3 increase in PM2.5) (Fig. 2, Table S1). For women, the crude association was positive (Model 1: +0.026 mm, 95% CI 0.016–0.037, per 10 µg/m3 increase in PM2.5), although it was null after full-adjustment (Model 4: +0.003 mm, 95% CI −0.006–0.013, per 10 µg/m3 increase in PM2.5) (Fig. 2, Table S1). Mean cf-PWV was 7.02 m/s (SD = 1.4) for men and 6.99 m/s (SD = 1.3) for women. There was a positive association between PM2.5 and cf-PWV for men throughout the sequential adjustment models; however the confidence intervals included the null after adjustment for blood pressure. We observed no association between PM2.5 and cf-PWV for women. Mean AIx was 21.9% (SD = 11) for men and 24.6% (SD = 12) for women. We observed strong evidence for a positive association between PM2.5 and AIx for both men and women (Fig. 2, Table S1). We observed a nonlinear exposure-response function for PM2.5 and AIx among men, as shown in Fig. 3 and Fig. S4.
Fig. 2.
Crude and adjusted associations between personal predicted PM2.5 and three cardiovascular markers stratified by gender. Analysis conducted in 10 multiple imputed datasets, using linear mixed models with households nested to villages as random intercepts and correction for selection bias through inverse probability weighting. Exposure PM2.5 was modelled as 10 μg/m3 increase. Model 1: PM2.5 and random effects for households nested to villages; Model 2: Model 1 + age (natural spline, df = 3 for CIMT and linear term for cf-PWV and AIx); Model 3: Model 2 + body-mass index, alcohol consumption, fruit and vegetable consumption, and physical activity; Model 4: Model 3 + education (main model); Model 5: Model 4 + blood pressure (for cf-PWV) and height (for AIx). All models for AIx were adjusted for heart rate. The point estimates are represented by boxes and their 95% confidence intervals as whiskers. AIx: aortic augmentation index; cfPWV: carotid-femoral pulse wave velocity; CIMT = carotid intima-media thickness; PM 2.5 = particulate matter with an aerodynamic diameter of 2.5 µm or less.
Fig. 3.
Exposure-response function between personal predicted exposure to PM2.5 and Augmentation index (AIx) among men and exposure distribution according to predictors. Additive mixed effects model with nested random intercepts (household within village) and a smooth term (thin-plate spline basis) on PM2.5. Adjustment as Model 4: age, body-mass index, alcohol consumption, fruit and vegetable consumption, physical activity, education and heart rate. Grey shade areas represent 95% credible intervals. At the bottom, the probability densities were estimated using kernel smoothing methods. The men exposed to manual job, active tobacco smoking and biomass fuel use and those exposed to manual job and active tobacco smoking correspond to 506 (32.4%) participants. The lowest and highest 1% PM2.5 levels have been trimmed for visualization (original values in Fig. S4). AIx: aortic augmentation index; PM 2.5 = particulate matter with an aerodynamic diameter of 2.5 µm or less.
We observed no evidence for the association between BC and CIMT, cf-PWV and AIx for men. For women, we observed a positive association for CIMT, which was attenuated after full-adjustment, no associations for cf-PWV, and a positive association between BC and AIx that was consistent across adjusted models (Model 4: +0.5%, 95% CI 0.1–0.8, per 2 µg/m3 increase in BC, Fig. S5, Table S2).
We observed small departures from the estimates in the main analysis in the sensitivity analyses for PM2.5 and CIMT (Table S1 and Fig. S6), cf-PWV (Table S1 and Fig. S7), AIx (Table S1 and Fig. S8) and for BC and CIMT (Table S2 and Fig. S9), cf-PWV (Table S2 and Fig. S10) and AIx (Table S2 and Fig. S11). We observed similar positive associations between PM2.5 and CIMT, cf-PWV and AIx for those participants with or without cardiometabolic risk factors among men (Fig. S12); and between PM2.5 and BC and AIx among women (Figs. S12 and S13). Point estimates for associations between PM2.5 and CIMT, cf-PWV and AIx were larger for men aged ≥40 years compared to younger men and among those with metabolic syndrome, hypertension, diabetes and obesity (Fig. S12). In contrast, point estimates for the association between PM2.5 and BC and AIx were larger for women aged <40 years compared to older women (Figs. S12 and S13). We observed small point estimates and wide confidence intervals when using measured rather than predicted PM2.5 for CIMT and cf-PWV. Estimates for AIx were smaller in magnitude compared to estimates using predicted personal exposure, but were in the opposite direction (Fig. S14).
4. Discussion
In a sample of the general population in peri-urban South India, we observed a positive association between personal PM2.5 exposure and cardiovascular markers of subclinical atherosclerosis, arterial stiffness and wave reflection for men. For women, we observed consistent associations between PM2.5 and BC and wave reflection. There was evidence for a nonlinear exposure-response function for PM2.5 and wave reflection among men.
Few studies have assessed the effect of personal exposure to PM2.5 and BC on cardiovascular markers. In a rural area of China, Baumgartner et al. measured 48 h personal exposure to air pollutants in 205 women over summer and winter, where exposure was largely influenced by household cooking and heating. Despite a consistent positive effect on blood pressure, there was no association between air pollutants and cf-PWV, and weak evidence for a positive association with AIx (1.1%, 95% CI −0.2, 2.4, per 1-ln [μg/m3] increase in PM2.5; and 0.3%, 95% CI −0.6, 1.2, per 1-ln [μg/m3] increase in BC) (Baumgartner et al., 2018). In a panel study in Beijing, Brook et al. reported similar findings as Baumgartner et al. (2018), evaluating personal exposure to BC in 65 non-smoker participants with metabolic syndrome (Brook et al., 2016). Other small panel studies reported positive associations between personal exposure to air pollutants and blood pressure (Louwies et al., 2015, Norris et al., 2016, Zhao et al., 2014). Only one study observed positive associations between short-term exposure to BC and arterial stiffness in 54 healthy participants (Provost et al., 2016), and another between PM2.5 and AIx, among 26 male welders (Fang et al., 2008). Direct comparisons of our results are difficult because of differences in population characteristics, exposure averaging times (short vs long-term), and sources driving personal exposure (e.g., smoking, occupation and biomass use). Nonetheless, taken together, these studies suggest that personal exposure to particulate matter, from different sources, contribute to vascular injury (Baumgartner et al., 2018, Brook et al., 2016, Brook et al., 2010).
Previous studies based on personal exposure had relatively small sample sizes, likely reflecting the high logistical complexity and resources required for personal monitoring of particles. Our approach takes advantage of a large number of personal exposure measurements to develop prediction models to estimate personal exposure in the full cohort, allowing for epidemiological analyses on several thousand participants (rather than tens or hundreds in previous studies). By using predictors based on usual activities (rather than activities the day of the monitoring), these estimates are likely to be a better reflection of average exposure over the course of a year compared to 24 h average measurements. Predicted personal exposures based on group-level exposure (rather than short-term personal measurements) are also likely to have the advantage of a greater proportion of Berkson-like error structure, which would affect the precision of our exposure-response estimates but not bias the estimates towards the null (Carroll et al., 2006). Our previous work in this cohort indicates that 33% of daily personal exposure occurs outside of the home for men and 11% for women (Milà et al., 2018). Detailed analysis based on time-resolved PM2.5 highlight the strong influence of cooking with biomass, smoking, and occupation on exposure in this population, all factors that could not be captured by estimates of ambient levels at residence (Milà et al., 2018). Our exposure assessment allowed us to account for these important sources of exposure in epidemiological analyses. We observed large within-participant variability in repeated measurements of personal exposure (very low ICC) (Sanchez et al., 2019). The goal of the prediction model was to focus on the between-participant variability, which although a small part of the total variability in personal exposure, was the relevant component of exposure given our study design. As expected, associations based on measured personal exposure, which was dominated by day-to-day variability in exposure, resulted in point estimates close to the null and large confidence intervals for CIMT and cf-PWV. For AIx, point estimates based on measured personal exposure were smaller, but in the opposite direction of predicted personal, possibly reflecting the influence of high values of measured PM2.5 (Sanchez et al., 2019) and selection factors (participants included in the analyses with measured PM2.5 were older and had lower level of education compared with participants included in the predicted exposure).
We analyzed three markers of vascular alterations. CIMT is an anatomical marker, influenced by cumulative exposure, and less suitable to short-term changes (Künzli et al., 2011). Our analysis is one of the first to evaluate personal exposure and CIMT. The positive association between predicted personal exposure to PM2.5 and CIMT among men agrees with results from a recent meta-analysis showing a strong evidence for a positive association between ambient PM2.5 and CIMT (Provost et al., 2015). Nevertheless, the comparability of our effect estimate with the meta-analysis is hampered because of different exposures domains: personal versus ambient. cf-PWV and AIx are physiological markers, representing a chronic process but vulnerable to acute changes, particularly blood pressure for cf-PWV and heart rate for AIx (Baumgartner et al., 2018, Künzli et al., 2011, The Reference Values for Arterial Stiffness, 2010). For cf-PWV, our findings were similar to those for CIMT: a positive association between predicted personal PM2.5 and cf-PWV among men. This association was attenuated after adjusting for blood pressure, suggesting the effect of air pollution on stiffness was mediated by increasing blood pressure. We observed consistent, positive associations between predicted personal exposure for both men and women for the wave reflection marker – AIx – which is a composite of several physiological alterations, including arterial stiffness and peripheral vascular pathologies (Sakurai et al., 2007, Townsend et al., 2015). Additionally, it has been reported that AIx alterations occur earlier in life compared to cf-PWV (McEniery et al., 2005); thus our ability to observe associations between air pollutants and cf-PWV (Zanoli et al., 2017) may have been limited by the large proportion of young adults in the study sample (50% of participants aged <40 years).
Multiple studies support the mechanistic link between particulate matter and vascular injury (Brook et al., 2010, De Brito et al., 2014, Delfino et al., 2005, Miller et al., 2017, Sun et al., 2005). Evidence indicates that inhaled particles cross from the lungs to the circulatory system, where particles or particle-induced inflammatory markers can affect several organs (Brook et al., 2010). The effect on the arterial vascular bed is pronounced; the combination of acute stresses (e.g., increased vascular tone) and chronic processes (e.g., continuous pro-inflammatory stimulation), results in accelerated vascular injury and atherosclerosis (Brook et al., 2010, De Brito et al., 2014, Delfino et al., 2005, Miller et al., 2017, Sun et al., 2005). Indeed, we evaluated three cardiovascular markers that are correlated but capture different aspects of vascular alterations. Furthermore, AIx depends on several functional and structural factors compared with cf-PWV (Townsend et al., 2015), and potentially could be more sensitive to PM exposure. For instance, long-term exposure to household air pollution has been associated with detrimental effects on cardiac function, which is a main determinant of AIx together with vascular alterations (Agarwal et al., 2018). Greater sensitivity of AIx compared to the other two markers may explain our findings of positive associations for women as well as men for AIx. Further studies in controlled scenarios could evaluate if one cardiovascular marker is more sensitive to particulate matter effects (Künzli et al., 2011). There were differences in the dominant sources contributing to personal exposure to PM2.5 between men and women, which could explain observed differences in the shape of the exposure-response function for AIx by gender and differences in the age-related vulnerability to particles.
Strengths of our study include a large cohort, sampled from the general population. Our analysis moves beyond most previous studies by examining personal exposure in a relatively large population. Estimates of personal exposure were based on extensive measurements. This exposure assessment approach captures the contribution of sources other than traffic that are highly prevalent in this and other LMIC settings, and which would not have been captured by approaches focusing on ambient concentrations. We evaluated multiple intermediate cardiovascular outcomes that reflect different aspects of vascular damage in a population with high cardiometabolic risk profile. About 80% of hypertensive and 70% of diabetic participants were not taking medication; which although an important public health issue in its own right, allowed us to analyze the association of particulate matter and vascular injury close to the natural history of this exposure-outcome association (Brook et al., 2010, Sun et al., 2005).
Our study is cross-sectional by design, and reverse causality is a possibility: participants with high cardio-metabolic risk or symptomatic cardiovascular disease could change their behavior, reducing (e.g. avoiding exposure sources) or increasing (e.g. spending more time at home, increasing exposure to household sources) their personal exposure. We believe the potential of reverse causality cannot totally explain our results based on predicted personal exposure due to the following reasons: (1) if individuals with higher levels of vascular damage were to change their behavior to reduce air pollution exposure, this would not explain the largely positive associations we observed, even for those aged below 40 years and without cardiometabolic risk factors, (2) we did not observe differences between personal exposure to PM2.5 and BC and the presence of impaired self-reported quality of life (mobility, self-care, activities, pain, anxiety and overall quality of life scale; data not shown), (3) the population is representative of the general population, so we expect very few participants with clinically diagnosed cardiovascular disease (1.2%, n = 37, participants self-reported coronary heart disease and stroke), (4) results among those without metabolic syndrome, a classical marker of high cardio-metabolic risk profile, were comparable to our main analyses. Nevertheless, we were unable to rule out or identify the likely direction of potential bias due to reverse causality due to the intrinsic limitations of our study design.
Limitations of our study include the following. First, our study is a cross-sectional analysis because of data availability of cardiovascular markers. We were therefore unable to evaluate the effect of exposure on longitudinal changes in cardiovascular outcomes. Second, we estimated personal exposure based on participants routine daily activities, which likely represents personal exposure on the order of a year, but not cumulative personal exposure, which is likely to be more relevant biologically for vascular injury. Because men were active smokers for a long period (average duration of 24 years), the main change over time in exposure predictors is likely to be cooking fuel, as some households had shifted to cleaner liquid petroleum gas from biomass. However, we did not have accurate information on the timing and intensity of the transition from biomass to cleaner cooking fuel, which may have led to some exposure measurement error. Third, predicting personal exposure is challenging, particularly in this setting where most variability in measured personal exposure was within, rather than between, person. However, the prediction models were able to explain a reasonable amount of between-person variability, which is most relevant for our study design. The exposure models did not perform well for BC in men, perhaps owing to the large variation in occupation-related tasks from day to day which were not well captured via questionnaire, likely explaining the null, imprecise estimates in the epidemiological analyses for BC in men. Additionally, the exposure measurement was done in 2015–2016 and the outcome measurement in 2010–2012, but we accounted for that using the personal exposure predictors from 2010 to 2012 and assumed that the association between usual activities and personal exposure was comparable between 2010 and 2015. Fourth, about half of APCAPS adult participants did not attend NIN for the vascular damage measurements. However, we observed very little sensitivity in our estimates between models that included inverse probability weighting and those that did not, suggesting that the influence of selection on our results was likely to be small (Ranzani et al., 2020). Finally, some of the predicted personal exposure predictors are surrogates of low socioeconomic position. Therefore, we cannot rule out residual confounding by other pathways than air pollution. However, in our sensitivity analyses including the SLI, a socioeconomic index, the estimates for the association between predicted personal exposure and vascular damage remained similar.
Air pollution is responsible for a significant proportion of cardiovascular events worldwide. Our results provide new evidence that personal exposures across a wide range of PM2.5 and BC are associated with intermediate cardiovascular markers in a peri-urban area of South India.
5. Contributors
OTR and CT conceived the study. SB, BK, KB, SS, SK, JM, CT were responsible for data collection. OTR conducted the analysis and drafted the report. All authors revised the report and provided intellectual input. All authors approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
6. Ethics approval
APCAPS was approved by the London School of Hygiene & Tropical Medicine (London, UK) and the National Institute of Nutrition (NIN) (Hyderabad, India). CHAI was approved by the Ethics Committees of Parc de Salut MAR (Barcelona, Spain), the Indian Institute of Public Health (Hyderabad, India), and the NIN. Signed consent forms were obtained from all participants.
7. Role of the funding sources
The funders had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; in the preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication. All authors were responsible for the decision to submit for publication.
8. Data sharing
Data related to the APCAPS cohort is available to researchers through a brief application to the cohort's Steering Group (form available from APCAPS website, http://apcaps.lshtm.ac.uk, and submitted to email: apcaps@iiphh.org).
CRediT authorship contribution statement
Otavio T. Ranzani: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing. Carles Milà: Writing - review & editing. Margaux Sanchez: Writing - review & editing. Santhi Bhogadi: Data curation, Writing - review & editing. Bharati Kulkarni: Data curation, Writing - review & editing. Kalpana Balakrishnan: Data curation. Sankar Sambandam: Data curation, Writing - review & editing. Jordi Sunyer: Writing - review & editing. Julian D Marshall: Data curation, Writing - review & editing. Sanjay Kinra: Funding acquisition, Writing - review & editing. Cathryn Tonne: Conceptualization, Data curation, Funding acquisition, Project administration, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
Funding acknowledgement: The research leading to these results received funding from the European Research Council under ERC Grant Agreement number 336167 for the CHAI Project. The third wave of data collection and village socio-demographic surveys for the APCAPS study were funded by the Wellcome Trust (Grant: 084674/Z). CT was funded through a Ramón y Cajal fellowship (RYC-2015-17402) awarded by the Spanish Ministry of Economy and Competitiveness. We acknowledge support from the Spanish Ministry of Science and Innovation through the “Centro de Excelencia Severo Ochoa 2019-2023” Program (CEX2018-000806-S), and support from the Generalitat de Catalunya through the CERCA Program.
General acknowledgement: We thank all participants of the APCAPS and CHAI studies as well as the study teams who made the research possible.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2020.105734.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abajobir A.A., Abbafati C., Abbas K.M., Abd-Allah F., Abera S.F., Aboyans V., Adetokunboh O., Afshin A., Agrawal A., Ahmadi A., Ahmed M.B., Aichour A.N., Aichour M.T.E., Aichour I., Aiyar S., Alahdab F., Al-Aly Z., Alam K., Alam N., Alam T., Alene K.A., Al-Eyadhy A., Ali S.D., Alizadeh-Navaei R., Alkaabi J.M., Alkerwi A., Alla F., Allebeck P., Allen C., Al-Raddadi R., Alsharif U., Altirkawi K.A., Alvis-Guzman N., Amare A.T., Amini E., Ammar W., Amoako Y.A., Anber N., Andersen H.H., Andrei C.L., Androudi S., Ansari H., Antonio C.A.T., Anwari P., Ärnlöv J., Arora M., Artaman A., Aryal K.K., Asayesh H., Asgedom S.W., Atey T.M., Avila-Burgos L., Avokpaho E.F.G., Awasthi A., Babalola T.K., Bacha U., Balakrishnan K., Barac A., Barboza M.A., Barker-Collo S.L., Barquera S., Barregard L., Barrero L.H., Baune B.T., Bedi N., Beghi E., Béjot Y., Bekele B.B., Bell M.L., Bennett J.R., Bensenor I.M., Berhane A., Bernabé E., Betsu B.D., Beuran M., Bhatt S., Biadgilign S., Bienhoff K., Bikbov B., Bisanzio D., Bourne R.R.A., Breitborde N.J.K., Bulto L.N.B., Bumgarner B.R., Butt Z.A., Cahuana-Hurtado L., Cameron E., Campuzano J.C., Car J., Cárdenas R., Carrero J.J., Carter A., Casey D.C., Castañeda-Orjuela C.A., Catalá-López F., Charlson F.J., Chibueze C.E., Chimed-Ochir O., Chisumpa V.H., Chitheer A.A., Christopher D.J., Ciobanu L.G., Cirillo M., Cohen A.J., Colombara D., Cooper C., Cowie B.C., Criqui M.H., Dandona L., Dandona R., Dargan P.I., Das Neves J., Davitoiu D.V., Davletov de Courten K.B., Defo B.K., Degenhardt L., Deiparine S., Deribe K., Deribew A., Dey S., Dicker D., Ding E.L., Djalalinia S., Do H.P., Doku D.T., Douwes-Schultz D., Driscoll T.R., Dubey M., Duncan B.B., Echko M., El-Khatib Z.Z., Ellingsen C.L., Enayati A., Erskine H.E., Eskandarieh S., Esteghamati A., Estep K., Farinha C.S.E.S., Faro A., Farzadfar F., Feigin V.L., Fereshtehnejad S.M., Fernandes J.C., Ferrari A.J., Feyissa T.R., Filip I., Finegold S., Fischer F., Fitzmaurice C., Flaxman A.D., Foigt N., Frank T., Fraser M., Fullman N., Fürst T., Furtado J.M., Gakidou E., Garcia-Basteiro A.L., Gebre T., Gebregergs G.B., Gebrehiwot T.T., Gebremichael D.Y., Geleijnse J.M., Genova-Maleras R., Gesesew H.A., Gething P.W., Gillum R.F., Giroud M., Giussani G., Godwin W.W., Gold A.L., Goldberg E.M., Gona P.N., Gopalani S.V., Gouda H.N., Goulart A.C., Griswold M., Gupta R., Gupta T., Gupta V., Haagsma J.A., Hafezi-Nejad N., Hailu A.D., Hailu G.B., Hamadeh R.R., Hambisa M.T., Hamidi S., Hammami M., Hancock J., Handal A.J., Hankey G.J., Hao Y., Harb H.L., Hareri H.A., Hassanvand M.S., Havmoeller R., Hay S.I., He F., Hedayati M.T., Henry N.J., Heredia-Pi I.B., Herteliu C., Hoek H.W., Horino M., Horita N., Hosgood H.D., Hostiuc S., Hotez P.J., Hoy D.G., Huynh C., Iburg K.M., Ikeda C., Ileanu B.V., Irenso A.A., Irvine C.M.S., Islam S.M.S., Jacobsen K.H., Jahanmehr N., Jakovljevic M.B., Javanbakht M., Jayaraman S.P., Jeemon P., Jha V., John D., Johnson C.O., Johnson S.C., Jonas J.B., Jürisson M., Kabir Z., Kadel R., Kahsay A., Kamal R., Karch A., Karimi S.M., Karimkhani C., Kasaeian A., Kassaw N.A., Kassebaum N.J., Katikireddi S.V., Kawakami N., Keiyoro P.N., Kemmer L., Kesavachandran C.N., Khader Y.S., Khan E.A., Khang Y.H., Khoja A.T.A., Khosravi M.H., Khosravi A., Khubchandani J., Kiadaliri A.A., Kieling C., Kievlan D., Kim Y.J., Kim D., Kimokoti R.W., Kinfu Y., Kissoon N., Kivimaki M., Knudsen A.K., Kopec J.A., Kosen S., Koul P.A., Koyanagi A., Kulikoff X.R., Kumar G.A., Kumar P., Kutz M., Kyu H.H., Lal D.K., Lalloo R., Lambert T.L.N., Lan Q., Lansingh V.C., Larsson A., Lee P.H., Leigh J., Leung J., Levi M., Li Y., Li Kappe D., Liang X., Liben M.L., Lim S.S., Liu P.Y., Liu A., Liu Y., Lodha R., Logroscino G., Lopez A.D., Lorkowski S., Lotufo P.A., Lozano R., Lucas T.C.D., Ma S., Macarayan E.R.K., Maddison E.R., Magdy Abd El Razek M., Majdan M., Majdzadeh R., Majeed A., Malekzadeh R., Malhotra R., Malta D.C., Manguerra H., Manyazewal T., Mapoma C.C., Marczak L.B., Markos D., Martinez-Raga J., Martins-Melo F.R., Martopullo I., McAlinden C., McGaughey M., McGrath J.J., Mehata S., Meier T., Meles K.G., Memiah P., Memish Z.A., Mengesha M.M., Mengistu D.T., Menota B.G., Mensah G.A., Meretoja T.J., Meretoja A., Millear A., Miller T.R., Minnig S., Mirarefin M., Mirrakhimov E.M., Misganaw A., Mishra S.R., Mohamed I.A., Mohammad K.A., Mohammadi A., Mohammed S., Mokdad A.H., Mola G.L.D., Mollenkopf S.K., Molokhia M., Monasta L., Montañez J.C., Montico M., Mooney M.D., Moradi-Lakeh M., Moraga P., Morawska L., Morozoff C., Morrison S.D., Mountjoy-Venning C., Mruts K.B., Muller K., Murray C.J.L., Murthy G.V.S., Musa K.I., Nachega J.B., Naghavi M., Naheed A., Naldi L., Nangia V., Nascimento B.R., Nasher J.T., Natarajan G., Negoi I., Ngunjiri J.W., Nguyen C.T., Nguyen Q Le, Nguyen T.H., Nguyen G., Nguyen M., Nichols E., Ningrum D.N.A., Nong V.M., Noubiap J.J.N., Ogbo F.A., Oh I.H., Okoro A., Olagunju A.T., Olsen H.E., Olusanya B.O., Olusanya J.O., Ong K., Opio J.N., Oren E., Ortiz A., Osman M., Ota E., Pa M., Pacella R.E., Pakhale S., Pana A., Panda B.K., Panda-Jonas S., Papachristou C., Park E.K., Patten S.B., Patton G.C., Paudel D., Paulson K., Pereira D.M., Perez-Ruiz F., Perico N., Pervaiz A., Petzold M., Phillips M.R., Pigott D.M., Pinho C., Plass D., Pletcher M.A., Polinder S., Postma M.J., Pourmalek F., Purcell C., Qorbani M., Quintanilla B.P.A., Radfar A., Rahimi-Movaghar V., Rahman M.H.U., Rahman M., Rai R.K., Ranabhat C.L., Rankin Z., Rao P.C., Rath G.K., Rawaf S., Ray S.E., Rehm J., Reiner R.C., Reitsma M.B., Remuzzi G., Rezaei S., Rezai M.S., Rokni M.B., Ronfani L., Roshandel G., Roth G.A., Rothenbacher D., Ruhago G.M., Sa R., Saadat S., Sachdev P.S., Sadat N., Safdarian M., Safi S., Safiri S., Sagar R., Sahathevan R., Salama J., Salamati P., Salomon J.A., Samy A.M., Sanabria J.R., Sanchez-Niño M.D., Santomauro D., Santos I.S., Santric Milicevic M.M., Sartorius B., Satpathy M., Schmidt M.I., Schneider I.J.C., Schulhofer-Wohl S., Schutte A.E., Schwebel D.C., Schwendicke F., Sepanlou S.G., Servan-Mori E.E., Shackelford K.A., Shahraz S., Shaikh M.A., Shamsipour M., Shamsizadeh M., Sharma J., Sharma R., She J., Sheikhbahaei S., Shey M., Shields C., Shigematsu M., Shiri R., Shirude S., Shiue I., Shoman H., Shrime M.G., Sigfusdottir I.D., Silpakit N., Silva J.P., Singh J.A., Singh A., Skiadaresi E., Sligar A., Smith D.L., Smith A., Smith M., Sobaih B.H.A., Soneji S., Sorensen R.J.D., Soriano J.B., Sreeramareddy C.T., Srinivasan V., Stanaway J.D., Stathopoulou V., Steel N., Stein D.J., Steiner C., Steinke S., Stokes M.A., Strong M., Strub B., Subart M., Sufiyan M.B., Sunguya B.F., Sur P.J., Swaminathan S., Sykes B.L., Tabarés-Seisdedos R., Tadakamadla S.K., Takahashi K., Takala J.S., Talongwa R.T., Tarawneh M.R., Tavakkoli M., Taveira N., Tegegne T.K., Tehrani-Banihashemi A., Temsah M.H., Terkawi A.S., Thakur J.S., Thamsuwan O., Thankappan K.R., Thomas K.E., Thompson A.H., Thomson A.J., Thrift A.G., Tobe-Gai R., Topor-Madry R., Torre A., Tortajada M., Towbin J.A., Tran B.X., Troeger C., Truelsen T., Tsoi D., Tyrovolas S., Ukwaja K.N., Undurraga E.A., Updike R., Uthman O.A., Uzochukwu B.S.C., van Boven J.F.M., Vasankari T., Venketasubramanian N., Violante F.S., Vlassov V.V., Vollset S.E., Vos T., Wakayo T., Wallin M.T., Wang Y.P., Weiderpass E., Weintraub R.G., Weiss D.J., Werdecker A., Westerman R., Whetter B., Whiteford H.A., Wijeratne T., Wiysonge C.S., Woldeyes B.G., Wolfe C.D.A., Woodbrook R., Workicho A., Xavier D., Xiao Q., Xu G., Yaghoubi M., Yakob B., Yano Y., Yaseri M., Yimam H.H., Yonemoto N., Yoon S.J., Yotebieng M., Younis M.Z., Zaidi Z., Zaki M.E.S., Zegeye E.A., Zenebe Z.M., Zerfu T.A., Zhang A.L., Zhang X., Zipkin B., Zodpey S., Giref A.Z., Rafay A., Tuzcu E.M., Ermakov S.P., Shi P., Gupta P.C. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A., Kirwa K., Eliot M.N., Alenezi F., Menya D., Mitter S.S., Velazquez E.J., Vedanthan R., Wellenius G.A., Bloomfield G.S. Household air pollution is associated with altered cardiac function among women in Kenya. Am. J. Respir. Crit. Care Med. 2018;197:958–961. doi: 10.1164/rccm.201704-0832LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C.P., Kulkarni B., Radhakrishna K.V., Charyulu M.S., Gregson J., Matsuzaki M., Taylor A.E., Prabhakaran D., Mamidi R.S., Wells J., Wilkinson I., McEniery C., Yasmin, Davey Smith G., Ben-Shlomo Y., Kuper H., Kinra S. Is the association between vitamin D and cardiovascular disease risk confounded by obesity? Evidence from the Andhra Pradesh Children and Parents Study (APCAPS) PLoS One. 2015;10 doi: 10.1371/journal.pone.0129468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner J., Carter E., Schauer J.J., Ezzati M., Daskalopoulou S.S., Valois M.F., Shan M., Yang X. Household air pollution and measures of blood pressure, arterial stiffness and central haemodynamics. Heart. 2018;104:1515–1521. doi: 10.1136/heartjnl-2017-312595. [DOI] [PubMed] [Google Scholar]
- Brook, R.D., Rajagopalan, S., Pope, C.A., Brook, J.R., Bhatnagar, A., Diez-Roux, A. V, Holguin, F., Hong, Y.L., Luepker, R. V, Mittleman, M.A., Peters, A., Siscovick, D., Smith, S.C., Whitsel, L., Kaufman, J.D., Amer Heart Assoc Council, E., Council Kidney Cardiovasc, D., Council Nutr Phys Activity, M., 2010. Particulate matter air pollution and cardiovascular disease an update to the scientific statement from the American Heart Association. Circulation 121, 2331–2378. 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed]
- Brook R.D., Sun Z., Brook J.R., Zhao X., Ruan Y., Yan J., Mukherjee B., Rao X., Duan F., Sun L., Liang R., Lian H., Zhang S., Fang Q., Gu D., Sun Q., Fan Z., Rajagopalan S. Extreme air pollution conditions adversely affect blood pressure and insulin resistance: the air pollution and cardiometabolic disease study. Hypertension. 2016;67:77–85. doi: 10.1161/HYPERTENSIONAHA.115.06237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R.J., Ruppert D., Stefanski L.A., Crainiceanu C.M. Chapman; 2006. Measurement Error in Nonlinear Models: A Modern Perspective. https://doi.org/10.1080/00401706.1997.10485096. [Google Scholar]
- De Brito J.M., Macchione M., Yoshizaki K., Toledo-Arruda A.C., Saraiva-Romanholo B.M., De Fátima Andrade M., Mauad T., Rivero D.H.R.F., Saldiva P.H.N. Acute cardiopulmonary effects induced by the inhalation of concentrated ambient particles during seasonal variation in the city of São Paulo. J. Appl. Physiol. 2014;117:492–499. doi: 10.1152/japplphysiol.00156.2014. [DOI] [PubMed] [Google Scholar]
- Delfino R.J., Sioutas C., Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environ. Health Perspect. 2005 doi: 10.1289/ehp.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S.C., Eisen E.A., Cavallari J.M., Mittleman M.A., Christiani D.C. Acute changes in vascular function among welders exposed to metal-rich particulate matter. Epidemiology. 2008;19:217–225. doi: 10.1097/EDE.0b013e31816334dc. [DOI] [PubMed] [Google Scholar]
- Hadley M.B., Baumgartner J., Vedanthan R. Developing a clinical approach to air pollution and cardiovascular health. Circulation. 2018;137:725–742. doi: 10.1161/CIRCULATIONAHA.117.030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek G., Krishnan R.M., Beelen R., Peters A., Ostro B., Brunekreef B., Kaufman J.D. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ. Heal. 2013;12:43. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinra S., Radha Krishna K.V., Kuper H., Rameshwar Sarma K.V., Prabhakaran P., Gupta V., Walia G.K., Bhogadi S., Kulkarni B., Kumar A., Aggarwal A., Gupta R., Prabhakaran D., Srinath Reddy K., Smith G.D., Ben-Shlomo Y., Ebrahim S. Cohort profile: Andhra Pradesh children and parents study (APCAPS) Int. J. Epidemiol. 2014;43:1417–1424. doi: 10.1093/ije/dyt128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzli N., Perez L., von Klot S., Baldassarre D., Bauer M., Basagana X., Breton C., Dratva J., Elosua R., de Faire U., Fuks K., de Groot E., Marrugat J., Penell J., Seissler J., Peters A., Hoffmann B. Investigating air pollution and atherosclerosis in humans: concepts and outlook. Prog. Cardiovasc. Dis. 2011;53:334–343. doi: 10.1016/j.pcad.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Ljungman P.L.S., Li W., Rice M.B., Wilker E.H., Schwartz J., Gold D.R., Koutrakis P., Benjamin E.J., Vasan R.S., Mitchell G.F., Hamburg N.M., Mittleman M.A. Long- and short-term air pollution exposure and measures of arterial stiffness in the Framingham Heart Study. Environ. Int. 2018;121:139–147. doi: 10.1016/j.envint.2018.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louwies T., Nawrot T., Cox B., Dons E., Penders J., Provost E., Panis L.I., De Boever P. Blood pressure changes in association with black carbon exposure in a panel of healthy adults are independent of retinal microcirculation. Environ. Int. 2015;75:81–86. doi: 10.1016/j.envint.2014.11.006. [DOI] [PubMed] [Google Scholar]
- McEniery C.M., Yasmin, Hall I.R., Qasem A., Wilkinson I.B., Cockcroft J.R. Normal vascular aging: Differential effects on wave reflection and aortic pulse wave velocity - The Anglo-Cardiff Collaborative Trial (ACCT) J. Am. Coll. Cardiol. 2005;46:1753–1760. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- Milà C., Salmon M., Sanchez M., Ambrós A., Bhogadi S., Sreekanth V., Nieuwenhuijsen M., Kinra S., Marshall J.D., Tonne C. When, where, and what? Characterizing personal PM2.5 exposure in periurban India by integrating GPS, wearable camera, and ambient and personal monitoring data. Environ. Sci. Technol. 2018;52:13481–13490. doi: 10.1021/acs.est.8b03075. [DOI] [PubMed] [Google Scholar]
- Miller M.R., Raftis J.B., Langrish J.P., McLean S.G., Samutrtai P., Connell S.P., Wilson S., Vesey A.T., Fokkens P.H.B., Boere A.J.F., Krystek P., Campbell C.J., Hadoke P.W.F., Donaldson K., Cassee F.R., Newby D.E., Duffin R., Mills N.L. Inhaled nanoparticles accumulate at sites of vascular disease. ACS Nano. 2017;11:4542–4552. doi: 10.1021/acsnano.6b08551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell K., Kartsonaki C., Lam K.B.H., Kurmi O.P. Cardiorespiratory health effects of particulate ambient air pollution exposure in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Planet. Heal. 2017;1:e368–e380. doi: 10.1016/S2542-5196(17)30166-3. [DOI] [PubMed] [Google Scholar]
- Norris C., Goldberg M.S., Marshall J.D., Valois M.-F., Pradeep T., Narayanswamy M., Jain G., Sethuraman K., Baumgartner J. A panel study of the acute effects of personal exposure to household air pollution on ambulatory blood pressure in rural Indian women. Environ. Res. 2016;147:331–342. doi: 10.1016/j.envres.2016.02.024. [DOI] [PubMed] [Google Scholar]
- Perez L., Wolf K., Hennig F., Penell J., Basagaña X., Foraster M., Aguilera I., Agis D., Beelen R., Brunekreef B., Cyrys J., Fuks K.B., Adam M., Baldassarre D., Cirach M., Elosua R., Dratva J., Hampel R., Koenig W., Marrugat J., de Faire U., Pershagen G., Probst-Hensch N.M., de Nazelle A., Nieuwenhuijsen M.J., Rathmann W., Rivera M., Seissler J., Schindler C., Thiery J., Hoffmann B., Peters A., Künzli N. Air pollution and atherosclerosis: a cross-sectional analysis of four European cohort studies in the ESCAPE study. Environ. Health Perspect. 2015;123:597–605. doi: 10.1289/ehp.1307711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost E.B., Louwies T., Cox B., op ’t Roodt J., Solmi F., Dons E., Int Panis L., De Boever P., Nawrot T.S. Short-term fluctuations in personal black carbon exposure are associated with rapid changes in carotid arterial stiffening. Environ. Int. 2016;88:228–234. doi: 10.1016/j.envint.2015.12.023. [DOI] [PubMed] [Google Scholar]
- Provost E.B., Madhloum N., Panis L.I., De Boever P., Nawrot T.S., Int Panis L., De Boever P., Nawrot T.S. Carotid intima-media thickness, a marker of subclinical atherosclerosis, and particulate air pollution exposure: the meta-analytical evidence. PLoS One. 2015;10 doi: 10.1371/journal.pone.0127014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2016. R Core Team (2016). R: A language and environment for statistical computing. R Found. Stat. Comput. Vienna, Austria. URL http://www.R-project.org/. R Foundation for Statistical Computing.
- Ranzani O.T., Milà C., Kulkarni B., Kinra S., Tonne C. Association of ambient and household air pollution with bone mineral content among adults in peri-urban south India. JAMA Netw. Open. 2020 doi: 10.1001/jamanetworkopen.2019.18504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzani O.T., Milà C., Sanchez M., Bhogadi S., Kulkarni B., Balakrishnan K., Sambandam S., Sunyer J., Marshall J.D., Kinra S., Tonne C. Association between ambient and household air pollution with carotid intima-media thickness in peri-urban South India: CHAI-Project. Int. J. Epidemiol. 2020;49(1):69–79. doi: 10.1093/ije/dyz208. 31605119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J.C., Abhayaratna W.P., Davies J.E., Sharman J.E. Central hemodynamics could explain the inverse association between height and cardiovascular mortality. Am. J. Hypertens. 2014;27:392–400. doi: 10.1093/ajh/hpt222. [DOI] [PubMed] [Google Scholar]
- Sakurai M., Yamakado T., Kurachi H., Kato T., Kuroda K., Ishisu R., Okamoto S., Isaka N., Nakano T., Ito M. The relationship between aortic augmentation index and pulse wave velocity: an invasive study. J. Hypertens. 2007;25:391–397. doi: 10.1097/HJH.0b013e3280115b7c. [DOI] [PubMed] [Google Scholar]
- Salmon M., Milà C., Bhogadi S., Addanki S., Madhira P., Muddepaka N., Mora A., Sanchez M., Kinra S., Sreekanth V., Doherty A., Marshall J.D., Tonne C. Wearable camera-derived microenvironments in relation to personal exposure to PM 2.5. Environ. Int. 2018;117:300–307. doi: 10.1016/j.envint.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M., Ambros A., Salmon M., Bhogadi S., Wilson R.T., Kinra S., Marshall J.D., Tonne C. Predictors of daily mobility of adults in peri-urban south India. Int. J. Environ. Res. Public Health. 2017;14:783. doi: 10.3390/ijerph14070783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M., Milà C., Sreekanth V., Balakrishnan K., Sambandam S., Nieuwenhuijsen M., Kinra S., Marshall J.D., Tonne C. Personal exposure to particulate matter in peri-urban India: predictors and association with ambient concentration at residence. J. Expo. Sci. Environ. Epidemiol. 2019 doi: 10.1038/s41370-019-0150-5. [DOI] [PubMed] [Google Scholar]
- Seaman S.R., White I.R., Copas A.J., Li L. Combining multiple imputation and inverse-probability weighting. Biometrics. 2012;68:129–137. doi: 10.1111/j.1541-0420.2011.01666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinle S., Reis S., Sabel C.E. Quantifying human exposure to air pollution-Moving from static monitoring to spatio-temporally resolved personal exposure assessment. Sci. Total Environ. 2013 doi: 10.1016/j.scitotenv.2012.10.098. [DOI] [PubMed] [Google Scholar]
- Sun Q., Wang A., Jin X., Natanzon A., Duquaine D., Brook R.D., Aguinaldo J.G.S., Fayad Z.A., Fuster V., Lippmann M., Chen L.C., Rajagopalan S. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. J. Am. Med. Assoc. 2005;294:3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- The Reference Values for Arterial Stiffness, C., 2010. Determinants of pulse wave-velocity in healthy people and in the presence of cardiovascular risk factors: “establishing normal reference values.” Eur Hear. J 31, 2338–2350. https://doi.org/ehq165[pii]\r10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed]
- Tonne C. A call for epidemiology where the air pollution is. Lancet Planet. Heal. 2017;1:e360–e367. doi: 10.1016/S2542-5196(17)30163-8. [DOI] [PubMed] [Google Scholar]
- Tonne C., Salmon M., Sanchez M., Sreekanth V., Bhogadi S., Sambandam S., Balakrishnan K., Kinra S., Marshall J.D. Integrated assessment of exposure to PM2.5 in South India and its relation with cardiovascular risk: design of the CHAI observational cohort study. Int. J. Hyg. Environ. Health. 2017;220:1081–1088. doi: 10.1016/j.ijheh.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Touboul P.J., Hennerici M.G., Meairs S., Adams H., Amarenco P., Bornstein N., Csiba L., Desvarieux M., Ebrahim S., Hernandez Hernandez R., Jaff M., Kownator S., Naqvi T., Prati P., Rundek T., Sitzer M., Schminke U., Tardif J.C., Taylor A., Vicaut E., Woo K.S. Mannheim carotid intima-media thickness and plaque consensus (2004–2006-2011) Cerebrovasc. Dis. 2012 doi: 10.1159/000343145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend R.R., Wilkinson I.B., Schiffrin E.L., Avolio A.P., Chirinos J.A., Cockcroft J.R., Heffernan K.S., Lakatta E.G., McEniery C.M., Mitchell G.F., Najjar S.S., Nichols W.W., Urbina E.M., Weber T. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf P.A., Agostino R.B.D., Ph D. Carotid intima-media thickness and cardiovascular events. N. Engl. J. Med. 2011;365:1640–1642. doi: 10.1056/NEJMc1109714. [DOI] [PubMed] [Google Scholar]
- Zanoli L., Lentini P., Granata A., Gaudio A., Fatuzzo P., Serafino L., Rastelli S., Fiore V., D’Anca A., Signorelli S.S., Castellino P. A systematic review of arterial stiffness, wave reflection and air pollution. Mol. Med. Rep. 2017;15:3425–3429. doi: 10.3892/mmr.2017.6392. [DOI] [PubMed] [Google Scholar]
- Zhao X., Sun Z., Ruan Y., Yan J., Mukherjee B., Yang F., Duan F., Sun L., Liang R., Lian H., Zhang S., Fang Q., Gu D., Brook J.R., Sun Q., Brook R.D., Rajagopalan S., Fan Z. Personal black carbon exposure influences ambulatory blood pressure: air pollution and cardiometabolic disease (AIRCMD-China) study. Hypertension. 2014;63:871–877. doi: 10.1161/HYPERTENSIONAHA.113.02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.