Abstract
Widespread indications for use of molecular diagnostics in various aspects of clinical medicine have driven proliferation of testing. The rapid adoption and continuous technological evolution of molecular diagnostics have often strained the development and maintenance of a functional underlying framework of coding, coverage, and reimbursement policies, thereby presenting challenges to various stakeholders, including molecular professionals, payers, and patients. A multidisciplinary working group convened by the Association for Molecular Pathology Economic Affairs Committee was tasked to describe the complex landscape of molecular pathology economics and highlight opportunities for member engagement. In this article, on the basis of review and synthesis of government regulations and procedures, published payer policy documents, peer-reviewed literature, and expert consensus, the Working Group navigates the ecosystem of molecular pathology economics in terms of stakeholders, coding systems and processes, coverage policy determination, and pricing mechanisms. The composition and interrelatedness of various working groups and committees are emphasized to highlight the functional underpinnings of the system. Molecular professionals must be conversant in the language and complex inner workings of molecular pathology economics to lead successful, viable laboratories and advocate effectively for policy development on their behalf. This overview is provided to be a resource to molecular professionals as they navigate the reimbursement landscape.
The economic landscape of molecular pathology testing can be overwhelming as it is an increasingly complex world of stakeholders, regulations, processes, and acronyms. This overview of molecular pathology economics has helped to prepare new members of the Association for Molecular Pathology (AMP) Economic Affairs Committee for service: it is also intended to serve as an educational resource for residents, molecular pathologists, and clinical molecular geneticists, as well as laboratory directors and hospital/laboratory administrators who seek to understand the complex landscape of molecular pathology economics. This overview starts by defining the major stakeholders and then takes the reader through the areas of coding, coverage, and reimbursement, as applied to molecular pathology diagnostics. An understanding of the complexities and challenges facing the molecular pathology community from an economic perspective is crucial for molecular diagnostic laboratories to be successful, as well as for practitioners to participate in effective advocacy and policy development.
The Major Stakeholders in Molecular Pathology Economics
Health care economics is characterized by a complex ecosystem comprising numerous stakeholders; molecular pathology is no exception. These stakeholders fall into one of three categories: health care providers, payers, and patients. The success of the system—as measured in terms of delivering accessible, high-quality, value-informed care—is predicated on buy-in and coordination between these diverse participants. Challenges arise when there is misalignment of incentives and underlying interests of the various participants; therefore, understanding and cooperation between the major stakeholders are crucial for effective problem resolution and optimal patient care.
Providers
In molecular diagnostic testing, individual providers are a broad group of specialty-trained laboratory medicine professionals, including molecular pathologists (typically board-certified M.D. pathologists), clinical molecular geneticists (typically board-certified Ph.D. laboratorians), and medical technologists, collectively referred to in this primer as molecular professionals. Often, these providers are represented nationally and in policy discussions by professional organizations. The involvement of providers in the molecular pathology economic issues, however, is not limited to professional organizations. Individual molecular professionals engage in economic discussions directly through involvement in a wide spectrum of committees and may provide written or public comments to coverage and pricing determinations of both public and private payers.
The AMP was established in 1995 to provide structure and a voice to the then-emerging field of molecular diagnostics/molecular pathology. AMP membership includes molecular professionals from academic and community medical centers, government, and industry, including pathologist and doctoral scientist laboratory directors, basic and translational scientists, technologists, and trainees. The College of American Pathologists (CAP) is an organization of board-certified pathologists from all subspecialties of anatomic pathology and clinical pathology. The American Society for Clinical Pathology is an organization consisting of both pathologists and laboratory professionals. The American College of Medical Genetics and Genomics is an organization that represents the interests of medical genetics professionals, including clinical geneticists, clinical laboratory geneticists, and genetic counselors. The American Clinical Laboratory Association (ACLA) is a trade organization that largely represents commercial laboratories. The American Association for Clinical Chemistry is an organization that represents clinical laboratory science and its application to health care. These organizations and many others are actively involved in shaping and influencing the economic landscape of molecular pathology through involvement in coding, coverage, and reimbursement discussions at the local and national level.
The American Medical Association (AMA) is the largest association of physicians and medical students in the United States. In addition, the AMA administers and licenses its proprietary Current Procedural Terminology (CPT) codes, making it an integral player in the coding portion of health care economics. Advisory committees, such as the Pathology Coding Caucus (PCC) and the Molecular Pathology Advisory Group (MPAG), are the primary mechanisms through which trade and professional organizations, like AMP, individual providers, and others actively participate in the AMA CPT process for molecular pathology codes.
Payers
An integral part of the economics of medicine, third-party payers can be classified as governmental (public) or commercial (private). The two major public payers are the nationwide Medicare program and state Medicaid programs [Centers for Medicare & Medicaid Services (CMS), https://www.cms.gov/About-CMS/Agency-Information/History/index.html, last accessed February 25, 2018]. Managed Medicaid and Medicare Advantage plans, which are administered by commercial payers, are two variations on government plans. Among commercial payers, there are numerous private health insurers, which offer a variety of plans with an assortment of coverage options. Increasingly, there is a blending of provider and payer networks, resulting in integrated delivery networks that are changing historical paradigms. In addition, large corporations and businesses often choose to self-insure to provide health insurance to their employees, presumably at a cost savings.
Medicare was generated in 1965 by expanding the Social Security Act to provide health coverage for elderly adults and disabled individuals. Medicare is managed by the CMS, which is a part of the Department of Health and Human Services within the federal government. Medicare is the largest payer of clinical laboratory services in the United States (The Medicare Payment Advisory Commission, http://medpac.gov/docs/default-source/payment-basics/medpac_briefs_payment_basics_17_clinical_lab_finalf2a211adfa9c665e80adff00009edf9c.pdf?sfvrsn=0, last accessed July 19, 2019).
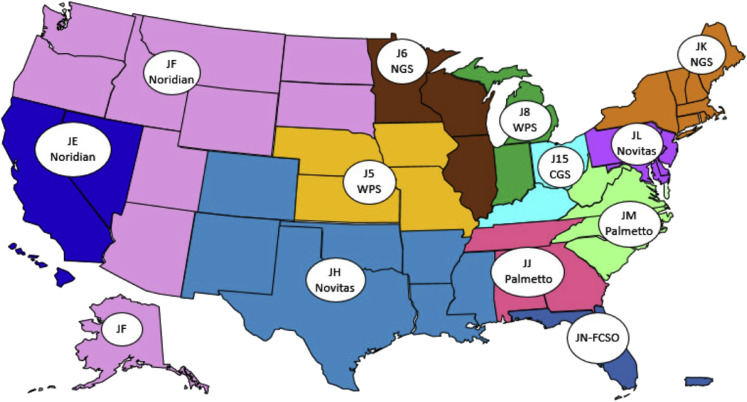
The Medicare program comprises four parts (Parts A, B, C, and D). Part A covers most inpatient hospital services, and Part B covers a portion of outpatient physician's visits, home health care, and outpatient procedures. Beneficiaries are automatically enrolled in Medicare Part A. Medicare Part B enrollment is optional for Medicare beneficiaries and generally covers 80% of approved nonhospital expenses. Medicare Parts A and B claims, including laboratory services, are processed through private companies that contract with Medicare and are called Medicare administrative contractors (MACs). CMS currently defines 12 MAC jurisdictions, which are groups of contiguous states. However, a single company can manage more than one MAC jurisdiction; currently, four contractors control eight of the available jurisdictions (Figure 1 ). Several changes have been implemented in the Medicare program over the past decades, including expanded eligibility of some special patient categories, as well as the generation of Medicare Parts C and D. Medicare Part C (alias Medicare Advantage) is offered through private insurance companies under contract with Medicare. By law, these plans must cover and pay at least what Medicare does as a baseline. Medicare Part D was generated by the Medicare Modernization Act of 2003 and provides outpatient drug benefits.
Figure 1.
A/B Medicare administrative contractor (MAC) jurisdictions as of October 2017. From “Map of A/B MAC Jurisdictions as of June 2019,” by the Centers for Medicare & Medicaid Services (https://www.cms.gov/Medicare/Medicare-Contracting/Medicare-Administrative-Contractors/Downloads/AB-MAC-Jurisdiction-Map-Oct-2017.pdf, last accessed June 1, 2019). In the public domain. CGS, CGS Administrators, LLC; J5, Jurisdiction 5; J6, Jurisdiction 6; J8, Jurisdiction 8; J15, Jurisdiction 15; JE, Jurisdiction E; JF, Jurisdiction F; JH, Jurisdiction H; JJ, Jurisdiction J; JK, Jurisdiction K; JL, Jurisdiction L; JM, Jurisdiction M; JN-FCSO, Jurisdiction N–First Coast Service Options, Inc; NGS, National Government Services, Inc.; WPS, Wisconsin Physicians Service Government Health Administrators.
Laboratory services accounted for approximately 2% of all Medicare Part B payments in 2016 (Department of Health and Human Services, https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMS-Statistics-Reference-Booklet/Downloads/2016_CMS_Stats.pdf, last accessed February 7, 2019). Medicare provides payments for approximately 1300 laboratory tests at a value of approximately $8 billion annually (Department of Health and Human Services, https://oig.hhs.gov/oei/reports/oei-09-16-00100.pdf, last accessed October 13, 2018). As discussed in more detail in Pricing, most laboratory tests are paid under a system called the Clinical Laboratory Fee Schedule (CLFS), whereas those tests that are always interpreted by physicians (eg, biopsies and immunohistochemical studies) are paid under a Physician Fee Schedule (PFS). The impact of Medicare coverage and payment policies extends well beyond the program itself because these policies exert significant influence on the practices of both state Medicaid programs and commercial carriers.
Medicaid provides health care to low-income people and some special groups, such as pregnant women (Centers for Medicare & Medicaid Services, https://www.medicaid.gov/about-us/program-history/index.html, last accessed February 25, 2018). The federal government establishes parameters for Medicaid, but each state administers its own Medicaid program. To variable degrees, states may contract Medicaid program administration to commercial carriers, which is called Medicaid Managed Care. The Children's Health Insurance Program is a separately administered program that ensures coverage for qualifying children (Centers for Medicare & Medicaid Services, https://www.medicaid.gov/chip/index.html, last accessed August 21, 2018). The Affordable Care Act of 2010 standardized the rules for determining eligibility and providing benefits through Medicaid and the Children's Health Insurance Program, in addition to giving states the authority to expand eligibility criteria (Centers for Medicare & Medicaid Services, Medicaid Program History). However, the future of the Affordable Care Act and its component parts is subject to ongoing congressional debates.
As of 2017, there are >59 million Medicare beneficiary enrollees, but most Americans (approximately 140 million) derive health coverage from commercial insurance carriers (Henry J. Kaiser Family Foundation, https://www.kff.org/medicare/issue-brief/an-overview-of-medicare, last accessed April 28, 2018; and CDC, https://www.cdc.gov/nchs/data/nhis/earlyrelease/insur201805.pdf, last accessed October 13, 2018). As of 2018, Medicaid and the Children's Health Insurance Program cover 74 million Americans (Centers for Medicaid & Medicare Services, https://www.medicaid.gov/medicaid/program-information/medicaid-and-chip-enrollment-data/report-highlights/index.html, last accessed April 28, 2018). In 2017, the census reported that 28.5 million Americans were uninsured (US Department of Commerce, https://www.census.gov/content/dam/Census/library/publications/2018/demo/p60-264.pdf, last accessed April 10, 2020). From 2010 to 2016, the number of uninsured trended lower, and it was estimated that 20 million adults gained coverage via the Affordable Care Act through 2016, through Medicaid expansion or subsidized insurance (US Department of Health and Human Services, https://aspe.hhs.gov/system/files/pdf/187551/ACA2010-2016.pdf, last accessed April 14, 2017).
Commercial insurance carriers collectively provide health coverage for most Americans (approximately 60%; US Department of Commerce, Health Insurance Coverage in the United States: 2017). The plans are sold by private carriers to individuals or as a group plan through employers (Henry J. Kaiser Family Foundation, https://www.kff.org/state-category/health-coverage-uninsured/health-insurance-status, last accessed September 4, 2018). A Kaiser Family Foundation survey of data for 2016 reported private insurance covered 173.9 million nonelderly adults and 42.3 million children. A total of 56% of adults (48% of children) were covered by employer-based group insurance; 8% of adults (5.6% of children) were covered by nongroup private insurance; 22% of adults (39.0% of children) were covered by Medicaid; 4.0% of adults (2.5% of children) were covered by other public; and 10.0% of adults (5% of children) were uninsured (Henry J. Kaiser Family Foundation, State Health Facts–Health Insurance Status). Commercial carriers also administer Medicare Advantage and Managed Medicaid plans and may serve as secondary insurance policies for those whose primary insurer is Medicare.
Private insurance covers more lives than does Medicare; however, medical care rates generally increase with age, so the Medicare population accounts for a larger proportion of medical costs. Depending on the type of molecular testing (ie, oncology, germline, or infectious disease), coverage for and payment policies of commercial insurers may vary more or less drastically compared with Medicare. Although inherited disease (germline) testing occurs more often in the non-Medicare population (eg, pediatric or pregnant patients), inherited disorders are also relevant considerations in older patients (eg, those with unexplained heart failure, patients with hereditary breast and ovarian cancer syndrome, or those with renal disease). In contrast, molecular testing in oncology (somatic testing) is most prominent in the Medicare population but by no means limited to that group. Although molecular testing for infectious diseases and human leukocyte antigens is common, and many of the policies and processes discussed in this work apply to them, discussion in this primer has been limited to germline and somatic testing.
Patients
Patients are the ultimate beneficiaries of access to molecular pathology services. Patients across the spectrum of medicine can and do benefit from molecular services; however, most patients are unaware of this distinct medical subspecialty. Concert Genetics reported that as of March 2019 the total number of genetic tests on the market was >70,000 and includes single-gene tests, multigene panels, and whole exome sequencing tests (Concert Genetics, http://www.concertgenetics.com/wp-content/uploads/2018/04/12_ConcertGenetics_CurrentLandscapeOfGeneticTesting2018.pdf, last accessed November 20, 2019). Utilization of molecular pathology services varies based on disease type and several other factors, and the proportion of patients who benefit from molecular pathology services continues to grow (Personalized Medicine Coalition, http://www.personalizedmedicinecoalition.org/Userfiles/PMC-Corporate/file/The_PM_Report.pdf, last accessed October 24, 2019). Advances in identifying clinically useful genetic markers, diagnostic methods, and related pharmaceutical interventions continue to increase, particularly in the oncology space, and patients are becoming more aware than ever of the value of molecular genetic testing.
Patient advocacy organizations are increasingly recognizing the role of molecular pathology and the need to engage in and respond to reimbursement policies that affect patient access to these procedures. Patient advocates, including individuals or an organization, work to improve the care and life of patients, including patient rights, support, education, and care. Many patient advocacy organizations exist as disease-centric nonprofit organizations (eg, American Cancer Society and American Diabetes Association) and work diligently to monitor both Medicare and private payer policies that may affect patient access to services, including molecular testing. For example, both Facing Our Risk of Cancer Empowered (a hereditary cancer organization) and the Ovarian Cancer Research Alliance have played large roles in engaging with CMS regarding both Medicare and Medicaid coverage policies that would affect patient access to appropriate molecular testing. Facing Our Risk of Cancer Empowered has engaged with CMS regarding Medicaid policies for molecular testing and as lead advocate in responding to recommendation statements on BRCA-related cancer released by the US Preventive Task Force (Facing Our Risk of Cancer Empowered, https://www.facingourrisk.org/advocacy/advocacy-category.php?id=4, last accessed February 20, 2019). In addition, the National Organization for Rare Disorders supports the development and access to diagnostics to speed early diagnosis and supports updates to diagnostic reimbursement to better reflect their importance.
Patient advocacy organizations work independently to promote coverage of molecular testing within their specific disease focus, but also work together to form coalitions to streamline engagement with the larger stakeholder community to advance health care policy, including reimbursement for molecular testing. A great example of these is evident in the work that LUNGevity, the largest national lung cancer–focused nonprofit, is doing. With its Take Aim Initiative, it is working with several aligned and engaged stakeholders to ensure that patients have access to testing to help guide their treatment decision in a timely way (LUNGevity, https://lungevity.org/public-policy/access-to-biomarker-testing, last accessed November 20, 2019). The growing patient voice has become crucial in crafting and responding to reimbursement policy, particularly in discussions of coverage and the standards of evidence needed to justify coverage of medical services.
Coding: The Language of Laboratory Economics
Several different coding systems that are used in US health care and most relevant to molecular genetic testing are explained in brief in this section. The coding system most immediately applicable to molecular genetic testing is the AMA CPT system, which is a level 1 Healthcare Common Procedure Coding System (HCPCS) code; others include HCPCS level 2 codes, including G codes, code modifiers, and Z codes. Finally, International Classification of Diseases, Tenth Revision (ICD-10) codes are discussed, which categorize diseases and conditions, rather than items or services.
History of CPT
CPT, copyrighted by the AMA, is the systemized language through which providers communicate with each other, their health care systems, and third-party payers. The CPT system was first developed as a series of four-digit codes and published by the AMA in 1966 mainly to describe surgical procedures.1 In 1970, the code set expanded in a second edition, which added codes for medical and therapeutic procedures and was the first five-digit code set, the code length that is still used today.
In 1983, the Health Care Finance Administration (now CMS) established the HCPCS and mandated use of HCPCS codes to report all services billed to Medicare. CPT was also identified by the Health Care Finance Administration as the main procedure code set and designated as level 1 HCPCS codes. HCPCS coding was adopted by Medicaid in 1986, effectively making CPT the language of Medicare and Medicaid. A short time later, in 1987, the Omnibus Budget Reconciliation Act mandated the use of HCPCS codes (including level 1 or CPT codes) for all outpatient procedures. Today, CPT is used by all federal programs and commercial payers, as a de facto standard for coding across the health care system, established by regulation at 45 CFR § 162.1002 (2014).
The Three Categories of CPT Codes
There are three main categories of CPT codes: category I (Healthcare Common Procedural Codes), category II (performance measurement tracking codes), and category III (emerging technology). (Do not confuse category I, II, and III CPT codes with the subset of molecular pathology CPT category I codes, which are called tier 1 and tier 2 codes, discussed later).
CPT category I codes consist of the set of frequently used codes that describe medical procedures or services (Medical Billing & Coding Certification, https://www.medicalbillingandcoding.org/intro-to-cpt, last accessed October 17, 2018). Category I CPT codes have one or both of two components, referred to as the physician work or professional component (eg, performing a biopsy, interpreting a radiologic image, or making a microscopic diagnosis) and the technical component (TC; eg, generating an X-ray image or a microscope tissue slide). Many physician services include both a professional component and a TC and are paid from the Medicare PFS using a relative value scale. Most clinical laboratory category I CPT codes only have a TC and are paid from the Medicare CLFS. Most surgical pathology category I CPT codes consist of both a professional component and TC and are paid from the PFS.
Category II codes are five-digit alphanumeric codes (4 digits followed by letter F) utilized along with the category I codes to track nationally established performance criteria for good patient care. Category II codes facilitate quality data collection; they are not associated with any relative value and are billed with a $0.00 billable charge amount. For instance, code 3155F refers to cytogenetic testing performed on bone marrow at time of diagnosis or prior to initiating treatment (American Medical Association, https://www.ama-assn.org/practice-management/category-ii-codes, last accessed August 21, 2018; and eMDs, http://www.e-mds.com/what-are-cpt-ii-codes-and-how-are-they-used-medical-billing, last accessed August 21, 2018). Performance measures are now moving away from claims-based reporting, making these codes less and less utilized.
Category I and II codes are published yearly in the CPT manual and are effective each year on January 1. In contrast, category III codes were developed in 2001 to provide temporary and more rapidly released codes for emerging services. They comprise four digits followed by the letter T and are released biannually. Payment for these codes is payer dependent. There are a few cases where laboratory services are represented by category III codes, such as code 0500T describing human papillomavirus genotyping of greater than five high-risk subtypes. Category III codes are typically generated for a 5-year period. After 5 years, the category III code is deleted, unless a need for its renewal is established or it is revised as a category I code.
Systems Outside the Category I to III CPT Codes
PLA Codes
AMA CPT developed the CPT Proprietary Laboratory Analysis (PLA) code set to accommodate requirements established by Section 216 of the Protecting Access to Medicare Act (PAMA) and subsequent final rule [45 CFR § 162.1002 (2014)]. The CPT PLA code set allows laboratories or manufacturers to specifically identify and track their test. New PLA codes are reviewed and voted on quarterly by the AMA PLA Technical Advisory Group and then subsequently are reviewed by the CPT Editorial Panel. Following approval by the panel, PLA codes become effective the quarter immediately following their publication online by AMA. In addition, the CPT Editorial Panel approved in 2019 and made effective January 1, 2020, the addition of a new symbol (↑↓) to indicate a PLA code that has been approved for category I status. This symbol also indicates that although this code has achieved category I status, the code will remain in the PLA code section of the CPT code book (American Medical Association, https://www.ama-assn.org/system/files/2019-08/may-2019-summary-panel-actions.pdf, last accessed April 1, 2020).
The requirements for PLA codes are less stringent than those for category I/III codes: the test must be performed on human specimens and requested by the clinical laboratory or manufacturer. Each PLA code has a CPT descriptor, and a subset of PLA codes are advanced diagnostic laboratory tests (ADLTs), a special category of tests subject to different policy and payment generated under PAMA, which was passed into law in 2014 and is explained in more detail below. An ADLT is defined as a test (ie, offered and furnished only by a single laboratory) that meets one of the following criteria: the test is an analysis of multiple biomarkers of DNA, RNA, or proteins that, when combined with a unique algorithm, yields a single patient-specific result, or it is a sole-source test cleared or approved by the US Food and Drug Administration (FDA; American Medical Association, https://www.ama-assn.org/practice-management/cpt/cpt-pla-codes, last accessed March 20, 2020). Applications for ADLT status are a separate process from PLA code obtainment through AMA and are processed through CMS on a quarterly basis (Centers for Medicaid & Medicare Services, https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Advanced-Diagnostic-Laboratory-Tests.html, last accessed January 3, 2019).
G Codes
In addition to the level 1 HCPCS CPT codes, during generation of the HCPCS codes, CMS also developed level 2 HCPCS codes to identify and submit claims for devices, drugs, durable medical equipment, and supplies. These items are not identified by CPT and are alphanumeric codes. In addition to these typical level 2 codes, G codes (ie, G plus four numerical digits) are also HCPCS level 2 codes, generated and used by CMS, and they represent medical procedures. Typically, CMS generates G codes for procedures when it has requirements that are not currently served by AMA CPT category I or III codes. When CMS defines a G code for a laboratory service, then the G code has to be used for Medicare claims for that service under the applicable circumstances. Private payers, however, may not recognize the G code, and other CPT codes must be used for the claim (Centers for Medicaid & Medicare Services, https://www.cms.gov/Medicare/Coding/MedHCPCSGenInfo/HCPCS_Coding_Questions.html, last accessed December 3, 2016).
In developing the new molecular pathology CPT codes, the intent had been to include the professional component with each code and place the codes under the PFS. However, when CMS decided that the individual codes only consisted of the technical component and placed all of the molecular pathology CPT codes under the CLFS, they generated the G code G0452 to identify any physician work involved for interpretation of molecular pathology procedures. The requirements of the G0452 code are interpretation is requested by submitted physician (standing orders are included); the results are a narrative report in the patient's record; and the interpretation requires medical judgment (Centers for Medicaid & Medicare Services, https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/Downloads/2013-Medicare-Physician-Fee-Schedule-Final-Rule.pdf, last accessed January 3, 2019).
The 2020 Medicare payment for the G0452 code is $19.13 (may be higher or lower in some regions of the country) for any molecular test interpretation, regardless of complexity.
Coding Modifiers
Sometimes submission of a CPT or other HCPCS code to describe a rendered service does not fully explain the nature of the billed service, and additional information, such as a code modifier, is required to indicate that the procedure has been altered by a specific circumstance. This may allow preemptive explanation of a procedure code, which would otherwise be denied, or further classification of the nature of a billed service, providing additional information to improve accuracy. Modifiers are always two digits.
Level I modifiers are normally referred to as CPT modifiers, consist of two numeric digits, and are updated annually by the AMA. Level II modifiers are typically referred to as HCPCS modifiers, consist of two alphanumeric characters, and are updated annually by CMS. A comprehensive list of modifiers is available online (Noridian Healthcare Solutions, LLC, https://med.noridianmedicare.com/web/jeb/topics/modifiers, last accessed February 7, 2019). The most commonly utilized modifiers relevant to molecular pathology are discussed below.
Rates for laboratory services reimbursed on the PFS are designed to include both professional and technical components, although the value attributed to each component is not explicitly stated. In the practice of split billing, a -TC and -26 modifier can be used, which serves to dissociate the technical component of a service from the professional component. To represent an isolated technical component bill, the CPT code is appended by the modifier ∗TC (a level II modifier). Conversely, if the intent is to bill only a professional component of a service, the modifier -26 (a level I modifier) is appended.
Another commonly used modifier, -59, is used to define distinct procedural services that are not normally reported together but are appropriate under certain circumstances. One example in molecular pathology would be the inclusion of CPT codes for fluorescence in situ hybridization and microdissection (done in conjunction with tumor testing) for the same patient on the same date of service. Normally, fluorescence in situ hybridization and microdissection codes billed in combination would be flagged as incompatible (and therefore denied); however, because the fluorescence in situ hybridization analysis and microdissection are for separate assays, the −59 modifier would be appropriate. The definition of CPT modifiers is identified in Appendix A of the CPT manual.2
MolDx Program Z Codes
The Molecular Diagnostic Services (MolDx) Program, generated in 2011, is a process established and administered by the MAC Palmetto GBA to evaluate the analytical and clinical validity as well as clinical utility of individual assays, including laboratory-developed molecular tests, to determine coverage and reimbursement. The program professes to hinge on three components: i) test registration and identification, ii) review of an application for coverage, and iii) determination of coverage (if any) and price (if the service is not already nationally priced by CMS) (Palmetto GBA, https://www.palmettogba.com/palmetto/moldx.nsf/docsCat/MolDx%20Website∼MolDx∼Browse%20By%20Topic∼General, last accessed August 28, 2018). The MolDx Program currently covers multiple jurisdictions, 28 states total, which are otherwise overseen by other MACs, as numerous MACs have adopted it (Figure 1).
In the MolDx model, each individual molecular assay is tracked by a Z code. The Z codes are unique five-character alphanumeric tracking codes used to identify an individual molecular diagnostic laboratory test and allow transparent tracking of relevant utilization and technical information about a test on the shareable Change Healthcare Diagnostics Exchange.3 Laboratories submit a request for a Z code and, in some cases, submit validation documentation and references for a technology assessment, which is reviewed by Palmetto in its coverage determination process. Z codes are submitted on claim forms along with the relevant CPT code for that service. According to the MolDx program, the combination of Z codes and CPT codes is meant to facilitate test identification, facilitate coverage determination, and establish reimbursement (Palmetto GBA, https://www.palmettogba.com/palmetto/MolDX.nsf/vMasterDID/8N3ELL4072?open, last accessed February 16, 2018).
There is concern in the laboratory community that in its technical reviews, the MolDx program is redundant to regulatory oversight established by the Clinical Laboratory Improvement Act and the New York State Clinical Laboratory Evaluation Program. In addition, MolDx may use Z codes to assist in its claims processing of codes that would otherwise be represented by the code for unlisted molecular procedure, 81479. However, there is no agreement that 81479 appropriately captures medical service utilization for further analysis.
ICD System
The ICD, of which ICD-10-Clinical Modification is the current edition, is a system of classification that describes the clinical diagnosis, condition, or scenario associated with a specific health care encounter. Appropriate ICD codes may accompany the diagnostic or clinical procedure code (CDC, https://www.cdc.gov/nchs/icd/icd10cm.htm, last accessed May 15, 2017). The ICD is copyrighted and published by the World Health Organization. In the setting of molecular pathology testing reimbursement, ICD codes help providers support the billing of a CPT code by providing information on the clinical context for which the procedure was performed. Payers use ICD codes to determine whether the patient circumstances for the procedure meet coverage criteria.
AMA Committees, Subcommittees, and Working Groups Relevant to Coding
A variety of committees and subcommittees through the AMA and other professional societies influence the coding system, and those relevant to molecular pathology are described below and summarized in Figure 2 .
Figure 2.
Various organizations impact the landscape of molecular pathology economics and interact in complex ways. Figure lists and describes the relationship between select key entities, including groups affecting Current Procedural Terminology (top left), Centers for Medicare & Medicaid Services (CMS) committees and working groups (top right), and private payers and associated groups (bottom right).
The CPT Editorial Panel is authorized and charged by the AMA board of trustees to develop, maintain, and revise the CPT (American Medical Association, https://www.ama-assn.org/about/cpt-editorial-panel/cpt-purpose-mission, last accessed March 20, 2020). The CPT Editorial Panel comprises 17 members, 11 of whom are physicians nominated by the national medical specialty societies. In addition, physician representatives from Blue Cross/Blue Shield, America's Health Insurance Plans, the American Hospital Association, and CMS are included as part of the panel. Finally, two representatives from the Healthcare Professional Advisory Committee round out the group. These professionals include pharmacists, psychologists, physical therapists, and other groups. The CPT Editorial Panel also has an Executive Committee. These five individuals comprise the chair, the co-chair, and three members at-large. At least one Executive Committee member at-large must be from a payer group.
Additional groups advise the CPT Editorial Panel on several matters related to molecular pathology (Figure 2). The CPT Advisory Committee, a group of mainly physicians nominated from the national medical specialty societies who are seated in the AMA House of Delegates, exists to support the work of the CPT Editorial Panel. Nonphysician members drawn from the AMA Health Care Professionals Advisory Committee make up part of the CPT Advisory Committee as well. The primary aim of the committee is to advise the CPT Editorial Panel in developing, approving, and editing the CPT code set by providing expert advice on current medical practice. New codes proposals, as well as requests to modify codes, are circulated to this group for comment. The advisory committee will recommend nomenclature for procedures, provide references from the medical literature in support or denial of a specific procedure, and advise on necessary revisions in the code set relevant to each member's area of expertise. The CPT Advisory Committee is also involved in the generation and editing of AMA educational materials addressing CPT issues.
Ad hoc workgroups with subspecialty expertise advise the editorial board. There are two advisory groups that are specific to pathology and laboratory medicine, which advise on matters specific to molecular codes as the need arises. The MPAG, which is a standing subcommittee that advises the CPT Editorial Panel on nomenclature and applicability of new and revised codes in the molecular diagnostics category, is tasked with review of all coding change applications (CCAs) that are submitted to the AMA regarding molecular pathology. The MPAG then makes its recommendations to the panel and makes them available to the PCC to assist with its deliberations.
The PCC reviews all laboratory and pathology codes, including molecular codes that have also been reviewed by the MPAG. The PCC, generated by the AMA but supported by the College of American Pathologists' staff, provides formal recommendations to the CPT Editorial Panel. The charge of this committee is to develop a consensus on new and revised codes in pathology and laboratory medicine and bring this recommendation to the CPT Editorial Panel. The PCC was generated because many CPT Editorial Panel members are unfamiliar with the unique aspects of laboratory medicine and the PCC also allows the broader laboratory community (including ACLA, the American Society for Microbiology, Advamed, and other groups) to have a direct voice in the generation of CPT codes relevant to the practice of anatomic and clinical pathology. The chair of the PCC is responsible for presentation and defense of all codes presented to the editorial panel that fall in the purview of pathology and laboratory medicine. The AMP currently occupies a rotating seat on the PCC.
The newest relevant committee is the Proprietary Laboratory Analysis Technical Advisory Group and was formed in response to requirements set out by PAMA and the resultant generation of PLA codes by the AMA. Requests for new PLA codes are submitted quarterly by the laboratory or manufacturer offering the test. The requests are reviewed by the PLA Technical Advisory Group, whose primary responsibility is to ensure that the assay meets claims made by the manufacturer and to edit the descriptor for consistency with CPT format. The PLA Technical Advisory Group then makes recommendations on the new PLA codes to the CPT Editorial Panel, who will ultimately vote to approve the codes. A summary of the various groups in the AMA (as well as CMS and other professional societies) with impact on the economics of molecular pathology is provided in Figure 2.
Proposing a New CPT Code
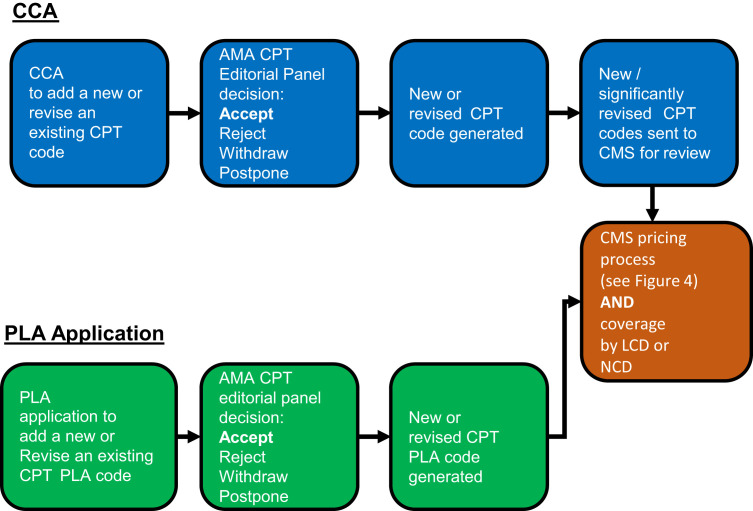
The AMA CPT Editorial Panel oversees the code change application process for new codes or revisions to existing codes. The initial step in the process is completion of a CCA and submission to the AMA by one of the three annual deadlines. Some of the components required by the AMA, along with the proposed code descriptor for the procedure, include the following: a clinical vignette describing the typical patient; a clear description of the service; the rationale for the proposed CPT code relative to existing CPT codes; reference to the supporting literature; the number of laboratories performing the procedure and the annual test volume; and copies of laboratory protocols and example reports (American Medical Association, https://www.ama-assn.org/practice-management/CPT-coding-change-request-instructions, last accessed August 28, 2018).
The CCA can be completed by any interested party, including an individual physician, physician practice, hospital, payer, or company. The CCA process is administered by AMA CPT Editorial Panel staff. The panel requires all laboratory-related code applications be reviewed by the PCC, which is managed by the College of American Pathologists. The PCC is composed of representatives of pathology and laboratory organizations. In addition, the panel generated the MPAG to review all molecular pathology–related applications and provide recommendations to the PCC. The PCC will then make recommendations for each pathology CCA to the CPT Editorial Panel. The CPT Editorial Panel then meets to discuss and votes whether to accept or reject each application during each CPT Editorial Panel meeting. Final votes are posted to the AMA CPT website about a month after each CPT Editorial Panel meeting. Applicants have the option to participate and answer questions by the MPAG, PCC, and/or CPT Editorial Panel. A summary of a code's lifecycle is provided in Figure 3 .
Figure 3.
Coding life cycle. Codes are generated or revised through either the coding change application (CCA) process for submission of a new or revised Current Procedural Terminology (CPT) code (blue) or the proprietary laboratory analysis (PLA) application process for submissions of a new or revised CPT PLA code (green). The CPT Editorial Panel takes into account comments and recommendations from the Molecular Pathology Advisory Group and the Pathology Coding Caucus. Once a code is generated, subsequent and separate processes exist for both pricing and coverage of that code. AMA, American Medical Association; CMS, Centers for Medicare & Medicaid Services; LCD, local coverage determination; NCD, national coverage determination.
CPT Codes for Molecular Testing
This section will lay out the history of how CPT codes for molecular pathology procedures have evolved and then describe each group of category I CPT codes for molecular testing, focusing on germline and somatic testing; those groups are molecular pathology procedures (tier 1 and tier 2), multianalyte assays with algorithmic analyses, genomic sequencing procedures, and the molecular pathology unlisted procedure code.
The first appearance of molecular-focused CPT codes was in 1993, with the addition of a molecular diagnostics section to the chemistry laboratory section, which included several separate codes for individual steps of molecular testing. From 1993 to 2002, additional CPT codes were added for other molecular services. These codes were structured differently than today's molecular codes as codes existed for each step in a molecular procedure (eg, nucleic acid extraction, amplification, and molecular probes). A laboratory performing an individual molecular assay identified each technical step within the assay and billed per the assembled codes. This practice became known as stacking codes because they were used in sets and multiples (eg, DNA extraction × 1 plus probe amplification × 10). By the early 2000s, some flaws in this coding system became evident; chief among them was that payers were only able to discern that a molecular assay had been performed, but the specific analyte could not be identified.
Between 2009 and 2013, intensive efforts were undertaken to overhaul molecular pathology coding. AMP sponsored an effort to develop a new coding scheme for molecular tests and produced a white paper describing one possible approach (Association for Molecular Pathology, https://www.amp.org/AMP/assets/File/position-statements/2009/AMPCPTReformProposal_Final.pdf, last accessed September 4, 2018). In 2009, the AMA CPT Editorial Panel generated the Molecular Pathology Working Group and charged it with the development of a new coding scheme. The development of the current CPT codes for molecular pathology services was the result of several years of work from a diverse group of stakeholders, but was based heavily on AMP's recommended structure. Of key importance to this newly devised coding strategy was a method-agnostic approach, focused on specific genes and/or conditions, which by design was different from the previous method-based approach.
Category I CPT molecular pathology procedure CPT codes are divided into two sections, or tiers: tier 1 and tier 2. Tier 1 codes (81170 to 81355) include commonly performed analyte-specific or other well-described analytic targets (eg, CFTR screening, EGFR mutation testing, and chimerism analysis). Tier 2 includes less commonly evaluated analytes, and they are grouped into nine tier 2 codes. The nine codes in tier 2 (81400 to 81408) correspond to nine levels of increasing technical complexity.
The initial distribution of analytes (typically genes) into tier 1 and tier 2 designations was determined based on surveys of some laboratories to determine tests that had the highest volume. Each year, some analytes are moved from tier 2 to tier 1 on the basis of volume increases and the need for more specific and granular coding. For example, in 2017, gene IDH1 went from classified under tier 2 code 81403 to its own tier 1 code, 81120. These changes are proposed and reviewed through the code change process.
Although placement of an analyte into a tier 2 code is not intended to reflect reduced clinical usefulness or that it is research, some payers viewed the tier 2 category codes in this way. However, the challenge remains that generation of a separate code for all clinical genes and targets used in human clinical testing may not be feasible because of the number of targets.
The multianalyte assays with algorithmic analyses code group was also proposed and developed by the AMA ad hoc Molecular Pathology Work Group for addressing assays using multiple analytes and reporting a result based on a proprietary algorithm. The analytes may be nucleic acid, protein, or chemistry based and performed in various combinations to arrive at a computationally derived result, typically reported as a numeric score or probability assessment. Because of the algorithmic nature of these assays, they are generally (but not always) proprietary assays offered by a single laboratory provider.
Analyte-specific CPT molecular codes were first published in January 2012; however, CMS deferred adoption of the new codes until 2013. Around this same time frame, stakeholders recognized the growing utilization of then-new technology, next-generation sequencing (NGS; alias massively parallel sequencing). This technology coincided with and enabled the increasing clinical deployment of testing that targeted multiple genomic regions simultaneously, thus reducing the need for sequential or iterative testing of one gene or target at a time.
To address this new coding challenge, AMP developed a draft coding structure to describe genomic sequencing procedures that was presented at a subsequent AMA stakeholder meeting in 2013 (Association for Molecular Pathology, https://www.amp.org/AMP/assets/File/position-statements/2013/AMPProposaltoAddressCodingforGenomicSequencingProcedures_FINAL.pdf, last accessed December 20, 2018). AMA formed disease-related expert workgroups to develop the code descriptors, which led to publication of the first set of genomic sequencing procedure codes on January 1, 2015. These codes are designed to capture panel-based testing across a spectrum of clinical scenarios, generally grouped by clinical indication, as well as larger assays that query a much larger fraction of the genome (eg, exome and genome). These codes are also designed to be method agnostic, and it is not imperative that NGS is utilized if the panel-based descriptor of testing has been satisfied. As in other areas of coding, since the original publication of these genomic sequencing procedure codes, new codes have been proposed and adopted, and some codes have undergone changes [eg, the minimum gene list for hereditary colon cancer disorders (CPT 81436) increased from 7 in 2015 to 10 in 2017].
In molecular testing, the unlisted procedure code 81479 may be used until a new specific code is established (American Medical Association, 2017). This code should only be used when no existing code appropriately describes the service provided, and it cannot be multiplexed. Submitting claims using an unlisted procedure code generally involves inclusion of additional documentation describing the procedure and its medical necessity. Claims without supporting documentation are generally denied. In addition, if an appropriate existing code applies, the claim will be denied.
Coding Edits
Procedure-to-procedure edits and medically unlikely edits (MUEs) were developed by CMS to reduce claims error rates and fraud in Part B billing for Medicare claims, and the program was implemented on January 1, 2007 (Centers for Medicare & Medicaid Services, https://www.cms.gov/medicare/coding/nationalcorrectcodinited/mue.html, last accessed August 28, 2018). CMS's national correct coding program provides computer-driven edits on tens of thousands of services where the edit is considered so evident that it does not require a local coverage determination (LCD) or a national coverage determination (NCD; Centers for Medicare & Medicaid Services, https://www.cms.gov/Medicare/Coding/NationalCorrectCodInitEd/index.html, last accessed August 28, 2018).
There are two classes of edits. Procedure-to-procedure edits block payment for two CPT codes on the same day of service. For example, a comprehensive code for sequencing and duplication deletion analysis of gene X would not allow additional separate billing on the same day for sequencing of gene X. Some of these P2P edits are hard coded (type 1), and some are soft coded (type 2), which can be overcome by a modifier (Centers for Medicare & Medicaid Services, https://www.cms.gov/Medicare/Coding/NationalCorrectCodInitEd/NCCI-Coding-Edits.html, last accessed August 28, 2018).
A second type of edit, MUEs, is designed to limit the number of units of a CPT code on a given date of service for a given beneficiary. Often, this is 1, but it may be a higher multiple. Some are driven by medical logic (eg, a patient can have only 1 appendectomy). Others are driven by volume (eg, >3 of a certain service is considered unlikely and unreasonable, so an MUE edit stops payment at n = 3). Although many MUEs are considered absolute, some are soft edits in that they can be appealed through the regular claims appeal process, whereas others can be overridden with the use of the −59 coding modifier.
Both procedure-to-procedure edits and MUEs are updated quarterly by the National Correct Coding Initiative (an independent contractor for CMS) and published annually. Before edit implementation, the proposed edits are released for review and comment to the AMA, national medical/surgical societies, and other national health care organizations, including nonphysician professional societies, hospital organizations, laboratory organizations, and durable medical equipment organizations. Most MUE values are published on the CMS website, but a few are confidential and maintained in CMS and between contractors. The National Correct Coding Initiative Policy Manual for Medicare Services is updated annually and includes both a rationale for some of the code edits as well as additional coding guidance that is written by National Correct Coding Initiative with input and approval of CMS. The policy manual can be accessed on the CMS website, which also contains a listing of the National Correct Coding Initiative procedure-to-procedure edits and published MUEs (Centers for Medicare & Medicaid Services, National Correct Coding Initiative Edits).
If a provider/supplier, health care organization, or other interested party believes that an MUE value should be modified, the party may contact the private company to which CMS contracts these services. The current contractor is Capitol Bridge, LLC. The party should include an alternative MUE value, the rationale for the recommended value, and any supporting documentation (Centers for Medicare & Medicaid Services, https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/How-To-Use-NCCI-Tools.pdf, last accessed October 16, 2018). The pace of advances in molecular diagnostics is challenging to those seeking to capture its complexity while maintaining granularity of information. As the field of molecular pathology is undergoing rapid evolution, it is clear that the CPT codes in existence today are likely to require continued modifications. The pace and breadth of change in this discipline present an ongoing challenge for molecular professionals, payers, and their representative organizations.
Coverage
There are two formal processes Medicare uses to develop and disseminate coverage decisions and criteria for utilization of medical services: NCDs and LCDs.4 The main outcome of either process is to define the clinical scenario and parameters for a medical service, which are paired with appropriate ICD-10 codes and CPT codes, to establish the coverage criteria. NCDs and LCDs define the clinical scenario necessary for coverage, as well as limited or noncoverage, of specific clinical procedures. They involve services in benefit categories that fall under Medicare Part A or B (Centers for Medicare & Medicaid Services, https://www.cms.gov/Medicare/Coverage/DeterminationProcess/Downloads/FR09262003.pdf, last accessed August 28, 2018). NCDs are binding across every Medicare geography, whereas LCDs only apply in a specific jurisdiction in which they have been promulgated. If there is contradicting information between an NCD and an LCD, the NCD supersedes the LCD (Noridian, https://med.noridianmedicare.com/web/jea/policies/ncd, last accessed August 28, 2018).
Local Coverage Determinations
As nearly all CMS coverage decisions for molecular tests are LCDs, understanding the LCD process is crucial for understanding molecular pathology reimbursement and coverage by CMS. In addition, because many private insurers and states promulgate Medicaid base coverage and reimbursement decisions in part on Medicare LCDs, understanding the role of LCDs helps to broadly frame the context for coverage decisions across the board. Chapter 13 of the CMS Program Integrity Manual governs the process for generating and revising LCDs (Centers for Medicare & Medicaid Services, https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/pim83c13.pdf, last accessed August 28, 2018).
LCDs may be generated at the initiative of a MAC or based on a request submitted to the MAC from stakeholders within its jurisdiction. There are five main reasons that a MAC will develop an LCD: the MAC identified an item or service that is not covered under certain circumstances and wishes to establish automated review; frequent denials are issued or anticipated; a contractor has assumed the LCD development workload of another contractor and is undertaking to make LCDs more uniform across jurisdictions; a multistate contractor is undertaking an initiative to generate uniform LCDs across its jurisdiction; and an LCD is needed to ensure beneficiary access to care.
LCD coverage decisions should be based primarily on published authoritative evidence derived from randomized clinical trials or other definitive studies as well as general acceptance by the medical community (standard of practice; Centers for Medicare & Medicaid Services, https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/pim83c13.pdf, last accessed August 28, 2018). In addition, the medical community practice should be based on scientific data or research studies published in peer-reviewed medical journals, consensus of expert medical opinion (ie, recognized authorities in the field), technology assessments, or medical opinion derived from consultations with medical associations or other health care experts.
Ultimately, the decision to publish and implement an LCD rests with the MAC medical director. However, there are two formal processes for input on a proposed LCD. First, every proposed LCD must first be reviewed by a Contractor Advisory Committee (CAC) before finalization. Each state hosts a CAC, and CAC membership draws from physicians, beneficiaries, and other health care providers; relevant medical specialties are typically represented by one member from that specialty. The CAC serves as a formal mechanism for physicians in the state to be informed of and participate in the development of an LCD in an advisory capacity. CAC members often relay input from professional organizations; within pathology, the CAP and AMP are both involved in drafting responses to proposed LCDs relevant to pathology practice. Second, LCDs are posted for public comment for 45 days, allowing any stakeholder (including professional organizations) to submit a public comment. There is a legal process for appealing an LCD to a CMS administrative law judge. Also, individual claims denied under an LCD can be appealed on a one-by-one basis based on unique circumstances of the individual case.
Relevant stakeholders, especially physicians, have voiced several criticisms of the LCD process. The accrual of background information leading to an LCD is often not as systematic and comprehensive as desired. This has led to the perception by some that the decision-making process behind LCDs often lacks a degree of transparency. Also, some CAC members believe that their input into proposed LCDs may not always be deeply considered by their MAC before LCD finalization. In response to these and other criticisms, legislation broadly supported by the laboratory community was introduced in the House and Senate in 2017 (Congress.gov, https://www.congress.gov/bill/115th-congress/senate-bill/794 and https://www.congress.gov/bill/115th-congress/house-bill/3635, last accessed July 19, 2019). The Local Coverage Determination Act of 2017 included several provisions, including open meetings, upfront disclosure, meaningful reconsideration and options for appeal, and discouraging the use of LCDs as a backdoor for national coverage; as many MACs have adopted the MolDx program, LCDs issued by Palmetto and then promulgated to all other MolDx jurisdictions result in a de facto NCD (Association for Molecular Pathology, https://www.amp.org/AMP/assets/File/position-statements/2013/MolDx%20Coverage%20Letter%20and%20Attachments%2010302013%20FINAL.pdf, last accessed September 4, 2018). Ultimately, the bill was passed in the House but not the Senate. The future of any LCD legislation is unclear. However, and likely in response to this legislation, CMS announced in October 2018, revisions to Chapter 13 of the Program Integrity Manual, where CMS adopted some of the recommendations contained within The Local Coverage Determination Act of 2017 (Centers for Medicare & Medicaid Services, https://www.cms.gov/newsroom/fact-sheets/summary-significant-changes-medicare-program-integrity-manual-chapter-13-local-coverage, last accessed July 19, 2019).
National Coverage Determinations
In contrast to LCDs, NCDs are written, reviewed, and issued by CMS directly rather than through a MAC. Requests are made through a formal request letter with accompanying documentation that includes supporting medical and scientific literature along with other relevant information, such as any clinical trials or studies currently underway with bearing on the request (Centers for Medicare & Medicaid Services, https://www.cms.gov/Medicare/Coverage/DeterminationProcess/Downloads/FR08072013.pdf, last accessed August 28, 2018). The NCD process is reliant on a systematic written review of the relevant literature, technology assessments, and input from various stakeholders. For each NCD, CMS may also commission a formal health technology assessment by the Agency for Healthcare Research and Quality (usually via its academic medical center subcontractors) or review by the Medicare Evidence Development and Coverage Advisory Committee that reviews the evidence for an NCD and makes recommendations to CMS on coverage. NCDs are considered binding positions of the CMS agency. Although its use is rare, there is also a legal appeal process to challenge NCDs that are not based on reasonable conclusions from the evidence (Centers for Medicare & Medicaid Services, https://www.cms.gov/Medicare/Coverage/DeterminationProcess/Downloads/FR09262003.pdf, last accessed August 28, 2018).
At the time of writing, CMS lists NCDs for 25 clinical laboratory tests (Centers for Medicare & Medicaid Services, https://www.cms.gov/medicare-coverage-database/indexes/lab-ncd-index.aspx?bc=AgAAAAAAAAAAAA%3d%3d&, last accessed August 28, 2018). Tests under those NCDs are primarily routine and highly automated assays; most have been in use for decades. Notably, few molecular pathology tests are currently covered by NCDs. Examples of molecular pathology NCDs include HIV Testing–Diagnosis and HIV Testing–Prognosis Including Monitoring; both HIV-1 RNA detection and monitoring share the CPT code 87536 and an NCD that covers genetic testing for warfarin dosing only in the context of a clinical trial. In March 2018, CMS finalized a molecular pathology NCD for next-generation sequencing of tumor samples (Centers for Medicare & Medicaid Services, https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId = 290&bc = AAAAAAAAACAA, last accessed October 20, 2018). This NCD covered FDA approved or cleared NGS assays with companion diagnostic indications in advanced stage tumors in conjunction with the FDA approval of a commercial NGS assay. Coverage of NGS assays not approved by the FDA was left to the discretion of LCDs from local MACs. On implementation of this policy, stakeholder concerns were raised when it was discovered that NGS-based germline testing was included in the scope of this policy. The implication of this interpretation is both germline and somatic tumor NGS-based testing are now noncovered for Medicare beneficiaries with early-stage cancer. In response to this, AMP spearheaded a stakeholder sign-on letter with 62 other organizations to urge CMS to revise its current interpretation of the NCD by limiting it to somatic tumor testing and to communicate this change to the MACs (Association for Molecular Pathology, https://www.amp.org/AMP/assets/File/advocacy/Group_Stakeholder_Letter_NGSNCD-FINAL-1-31-2019.pdf, last accessed July 19, 2019).
In response to stakeholder concern, on March 26, 2019, the CMS Coverage and Analysis Group announced its plans to reopen the NCD. At the end of April 2019, CMS opened a formal reconsideration of the NCD (Centers for Medicare & Medicaid Services, https://www.cms.gov/medicare-coverage-database/details/nca-tracking-sheet.aspx?NCAId=296, last accessed July 19, 2019). A proposed decision memo was released in late fall, and a final decision was released in January 2020. The final NCD released in 2020 did make changes in section 90.2 of the National Coverage Determinations Manual to clarify that the NCD is only applicable to NGS-based tests for somatic and germline cancer. Under the NCD, CMS nationally covers tests using NGS technology for breast and ovarian cancer when the NGS test has FDA approval or clearance for use in the beneficiary's cancer and when the beneficiary meets some additional criteria, such as having clinical indications for germline testing. In addition, the local coverage section of the NCD expands MAC discretion to cover germline cancer tests using NGS technology for any cancer diagnosis, including breast and ovarian cancer (regardless of stage). MAC discretion for these tests is also dependent on additional and similar beneficiary criteria, as described for national coverage. Thus, although the final NCD did not remove germline testing from the scope, as requested by many stakeholders, the final policy does allow MAC discretion for NGS-based germline testing going forward (Centers for Medicare & Medicaid Services, https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=296&bc=AAAAAAAAABAA&, last accessed April 8, 2020).
Private Payer Coverage Policy
Plans offered by commercial carriers vary considerably among each other and compared with Medicare coverage policy. Individual laboratories may strategically develop preferred vendor status with a commercial carrier on a competitive basis to capture market share. In large, complex health care systems with complicated contracts and the needs of the contracting hospital (and insurer) are primary: individual laboratories, including the molecular pathology laboratories, are typically not a top priority for contracting considerations.
Some insurer websites list the genetic tests they will potentially cover. The mechanism for policy decisions on coverage is not usually given beyond general statements about using the best evidence available. The Blue Cross Blue Shield Association had its own rigorous and publicly available Technology Evaluation Center but has replaced it with Evidence Street, a web-based platform for subscribers only, which curates evidence and reviews on clinical utility submitted from a variety of sources and includes an extensive disclaimer (Blue Cross Blue Shield Association, https://www.bcbs.com/news/press-releases/blue-cross-blue-shield-association-launches-evidence-street-website-streamline, last accessed July 26, 2019).
Insurers will not have in-house expertise on every molecular diagnostic procedure and may utilize the services of various providers of technology assessments. Alternatively, payers often use Medicare coverage policies as benchmarks or engage consultants who advise on coverage policies of other payers. Evidence-based guidelines from professional groups, including AMP, CAP, American College of Obstetricians and Gynecologists, American College of Medical Genetics and Genomics, American Society for Clinical Pathology, American Society of Clinical Oncology, and the National Comprehensive Cancer Network (to name a few), provide thought leadership, which can significantly impact reimbursement policies. Two notable examples are the AMP-CAP–International Association for the Study of Lung Cancer report on molecular testing in lung cancer5 and the AMP-CAP report on molecular testing for colorectal cancer.6 This still leaves a large number of tests to evaluate. To fill this policy gap, and at the same time to streamline the process of authorizing/denying actual patient coverage requests, new organizations have arisen, called Laboratory Benefit Managers (LBMs; GenomeWeb, https://www.genomeweb.com/molecular-diagnostics/insurers-turn-automated-prior-authorization-programs-rein-genetic-testing-use#.W4V-VbonZhE, last accessed August 28, 2018).
In recent years, health insurers have begun to utilize or build management systems that assist with various aspects of insurance policy. Major players in this space include LBMs and other evidentiary review organizations that are increasingly significant players for laboratories and their relationship with health insurers. Health plans have generated, or contracted with, companies to deploy new systems to manage laboratory services; these services include medical and coverage policy development, technical assessments, claims editing, and network services. Most notably, a major focus of LBMs is administering prior authorization (or preauthorization) programs, which require that laboratories obtain approval from the health plan before it will cover the cost of a laboratory procedure. Preauthorization is increasingly becoming required for genetic and molecular oncology testing, with LBMs facilitating decision making. LBMs vary in operating style and focus and include companies such as AIM Specialty Health, Beacon Laboratory Benefit Solutions, Avalon Healthcare Solutions, Kentmere Lab Benefit Management System, and eviCore (Health Affairs, https://www.healthaffairs.org/do/10.1377/hblog20191021.563154/full, last accessed March 31, 2020). LBMs and their role within the health care system continue to grow as they expand their services. The variable degree of transparency with respect to decision making in these institutions presents a potential challenge for molecular professionals. The level of success of LBMs in achieving purported goals, such as cost savings, while maintaining patient access to appropriate testing remains to be determined.
Pricing
CMS pays for laboratory tests using two different fee schedules with different policy mechanisms. As noted previously, the PFS is used to pay for physician services. Making a diagnosis by taking a history and performing a physical examination is an archetypal example. For surgical pathologists, the counterpart would be making a diagnosis by microscopic assessment. In contrast, most clinical laboratory results, especially those on automated instruments, do not receive pathologist interpretation payment and are reimbursed on the CLFS, where the payment is intended to include both the technical and interpretative work (Centers for Medicare & Medicaid Services, https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/index.html, last accessed September 4, 2018).
In 2013, CMS determined that the newly developed molecular pathology codes should reside on the CLFS, not the PFS. CMS based this decision on its finding that most molecular pathology codes did not require the services specifically of a physician. The advantage of this arrangement across molecular pathology is the global inclusion of molecular pathology professionals across the spectrum of training, and does not exclude non-M.D. laboratorians from billing for these services (as would be expected if the codes were placed on the PFS). In contrast, a significant disadvantage to this approach is the general inability to recover the costs associated with complex and time-consuming interpretive services (including labor time, software, and databases) associated with molecular testing, which can be highly variable, depending on the technical details of an assay and the patient circumstance. As noted above in the section on coding, there currently exists only one option for coding of professional (physician) interpretation of molecular testing (G0452), which provides one fixed payment for any molecular pathology interpretation (currently approximately $19).
PFS and the RUC Process
Before 1992, physician payments by Medicare were made on the basis of usual, customary, and reasonable charges, as set by local claims processing contractors (alias customary, prevailing, and reasonable). As a result of escalating expenditures in the 1980s, the Omnibus Budget Reconciliation Act of 1989 was adopted, mandating that professional service payment would be determined by the Physician Fee Schedule. The schedule is organized by codes, and the relative value of each activity is determined by the Resource-Based Relative Value Scale Update Committee (RUC) of the AMA.
The RUC is composed of 31 physician-voting members and 300 medical advisors who aid in the valuation process. The committee meets three times annually to value codes added or revised at the prior CPT Editorial Panel meeting. Members of the RUC make recommendations to CMS on the financial resources required to perform a medical service, including professional time, supplies, and equipment. Codes go to the RUC for consideration under one of three circumstances: AMA has adopted a new CPT code and recommends it for the PFS; CMS has identified a misvalued code (this might be as a result of error in initial valuation, flagged by a spike in testing activity signaling overly generous reimbursement, or because, as technology matures, procedures become easier); or every code is reviewed on a rolling 5-year cycle (American Medical Association, https://www.ama-assn.org/about/rvs-update-committee-ruc, last accessed July 26, 2019).
To set a resource-based relative value, the resources required for the procedure are tallied. The RUC began with the results of a Harvard-AMA study in 1988, which formed the foundation for the Resource-Based Relative Value Scale. Now, for a new code, the appropriate professional society and its RUC advisors survey members via a variety of mechanisms of their professional society and other experts on three aspects: physician time required; practice expense for performing the procedure (ie, supplies, equipment, and labor); and liability insurance.
Each of these three components is evaluated via survey of professionals performing the service in question, and a reimbursement for each component is separately derived. Variation in cost across regions is accounted for by adjustment using the Geographic Practice Cost Index. The advisors present their determination of the relative value units for a new CPT code to the RUC for a vote. RUC meeting dates, minutes, and vote totals are posted on the AMA website, adding transparency to the process [American Medical Association, About–Relative Value Scale Update Committee (RUC)]. The RUC then sends the recommendations to CMS.
CMS is not obligated to accept the RUC recommendation and can generate its own relative value unit evaluation. CMS publishes its final fee schedules annually. More important, CMS also determines the conversion factor: the dollar amount assigned per single relative value unit. In 1992, it was $31; and in 2019, it was $36. The conversion factor has historically been updated annually, in part based on the Medicare Economic Index, and capped by the sustainable growth rate but has recently been changed to be determined by federal legislation.
Clinical Laboratory Fee Schedule Pricing
The CLFS was authorized by the Deficit Reduction Act of 1984. It froze prices on the basis of regional contractor fee schedules (which varied considerably) while updating them gradually on the basis of inflation or congressional actions. It also set a national limitation amount, which capped the maximum national payment, resulting in lowered payments in some jurisdictions. National limitation amount is now essentially a historical term, because test prices will be reset every 3 years by national private payer rate surveys (see PAMAof 2014 below).
In 2014, payment for laboratory tests in hospital outpatient settings was folded into a single charge for the visit (the Hospital Outpatient Prospective Payment System), with the exception of molecular diagnostic tests, which remain separately reimbursed using the CLFS. Hospital outpatient settings may include hospital outpatient clinics, emergency departments, and affiliated medical clinics that are owned by a hospital and classified as outpatient centers.
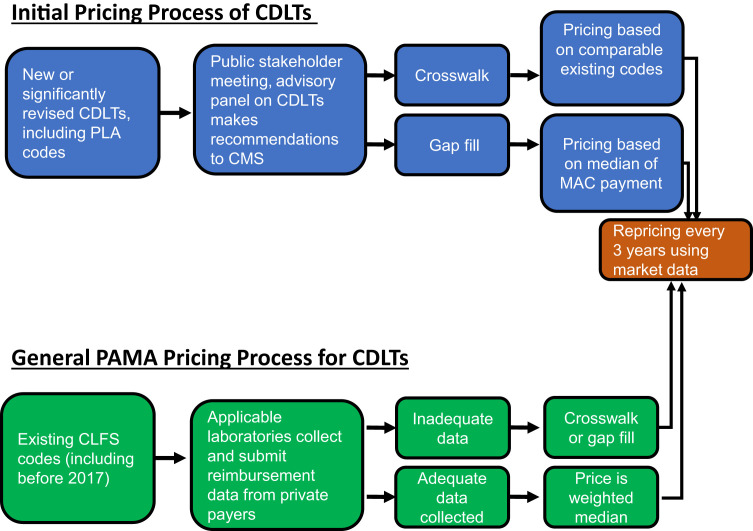
Before 2017, new codes would receive a price from CMS using one of two processes, crosswalking or gap filling, and except for minor inflation or other administrative adjustments, laboratory prices were not reset. Since 2017, there are complex processes that reset the whole CLFS fee schedule every 3 years, established by PAMA (see below) (Figure 4 ). However, as set forth in 42 CFR § 414.506, crosswalking and gap filling are still used to determine initial pricing of most new or reconsidered codes (Centers for Medicare & Medicaid Services, https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Laboratory_Public_Meetings.html, last accessed July 19, 2019).
Figure 4.
The process for pricing new Current Procedural Terminology (CPT) codes and repricing existing CPT codes under Protecting Access to Medicare Act (PAMA). New codes are discussed at the annual stakeholder meeting, the result of which is a recommendation by the Medicare Advisory Panel on Clinical Diagnostic Laboratory Tests (CDLTs) to Centers for Medicare & Medicaid Services (CMS) for utilizing the crosswalk or gap fill method to price a new code. Ultimately, CMS decides the pricing mechanism. Existing CPT codes were repriced in 2017 on the basis of the weighted median of private payer rates submitted by applicable laboratories. If adequate data from applicable laboratories are not provided, CMS reverts pricing back to gap fill or crosswalk. Every 3 years, CPT codes on the Clinical Laboratory Fee Schedule (CLFS) will be revalued to align with market data from applicable laboratories, as described above. MAC, Medicare administrative contractor; PLA, proprietary laboratory analysis.
Crosswalking occurs when a new test or substantially revised test is determined to be similar to an existing test, multiple existing test codes, or a portion of an existing test code, which is then used to set the payment. The price is set by CMS, although CMS accepts public comment on its proposals as well as input from the Advisory Panel on Clinical Diagnostic Laboratory Tests (CDLTs) during the summer before when the rates will go into effect. Gap filling is used when no comparable existing CDLT is available. In the first year of the gap-filling process, MAC specific amounts are established for the new CDLT using the charges for the test, including any routine discounts to these charges; resources required to perform the test; payment amounts determined by other payors; charges, payment amounts, and resources required for other tests that may be comparable or relevant; and any other criteria that CMS deems relevant. To set the national rate for the first year during gap fill, CMS takes the median of the rates determined by individual MACs during each separate gap fill process.
PAMA of 2014
On April 1, 2014, the PAMA of 2014 was enacted (Protecting Access to Medicare Act, Pub L. No. 113-93, 128 Stat. 1040), and became effective January 1, 2018. Section 216 of PAMA aimed to bring the CLFS rates into closer alignment with market rates. To do this, PAMA requires collection and reporting of data from applicable laboratories on payments that the laboratory receives from commercial payers for every unbundled test (ie, not part of a bundled payment, as seen in diagnosis-related groups or the Hospital Outpatient Prospective Payment System). CMS then sets the CLFS rate for most clinical tests for the next 3-year period on the basis of the weighted median of the reported prices. The legislation was prompted by a 2013 Office of the Inspector General Report, which found that in 2011 Medicare paid between 18% and 30% more than other insurer for 20 high-volume and/or high-expenditure laboratory tests and recommended that CMS seek legislation that would establish lower payment rates for tests (US Department of Health and Human Services Office of Inspector General, https://oig.hhs.gov/oei/reports/oei-07-11-00010.pdf, last accessed April 10, 2020).
In addition to the new data reporting and collection of private payer rates, PAMA also included several other provisions, including generation of a new subcategory of clinical diagnostic tests, ADLTs [Centers for Medicare & Medicaid Services, Application for Advanced Diagnostic Laboratory Test (ADLT) Status under the Medicare Clinical Laboratory Fee Schedule (CLFS)]. To be acknowledged as an ADLT, tests must be covered under Medicare Part B and offered and furnished by a single laboratory. In addition, the test must meet additional special criteria established by CMS and must go through an application process to receive ADLT status. ADLTs also have separate report and payment requirements to those of CDLTs under PAMA.
PAMA also included provisions related to MAC consolidation and the LCD process, gap fill transparency, and generation of an Advisory Panel on Clinical Diagnostic Laboratory Tests to advise the secretary of the Department of Health and Human Services and the administrator of the CMS on payment and policy issues. The panel charter calls for up to 15 members with expertise, which may include molecular pathologists, clinical laboratory researchers, and individuals with expertise in clinical laboratory science or health economics (Centers for Medicare & Medicaid Services, https://www.cms.gov/Regulations-and-Guidance/Guidance/FACA/AdvisoryPanelonClinicalDiagnosticLaboratoryTests.html, last accessed August 28, 2018). The meetings are open, with public comment invited before the meeting, and the proceedings are posted on a CMS website. Much of the charge of the panel is devoted to making recommendations to CMS for determinations of pricing for new tests or for substantially altered tests, using crosswalking or gap filling.
PAMA passage and implementation has been a lengthy process and paved with road bumps, with stakeholders expressing concerns about several issues, including which laboratories are required to report; data collection and reporting difficulties; civil monetary penalties; and the integrity of the data used to calculate the rates. One major issue for stakeholders has been how CMS defines applicable laboratories (ie, those required to report reimbursement data). There was concern by some that the way that applicable laboratories were defined biased the outcome toward significantly lower payments than are actually seen in the overall marketplace. Smaller laboratories and hospital referral laboratories, which often have higher reimbursement rates, were mostly left out of the original definition. AMP and others expressed concern that it would be difficult for hospital and other laboratories to accurately extract payer information from their records as many laboratories do not have the systems in place to determine the private payer payment rates for each test in addition to the associated volume of those tests and do not have the resources to significantly change their systems as reimbursement levels decrease under PAMA. In December 2017, ACLA filed a lawsuit against CMS, asserting that CMS ignored congressional intent and instituted a highly flawed data reporting process in advance of setting market rates under PAMA (American Chemical Laboratory Association, http://www.acla.com/cms-ignored-congressional-intent-in-implementing-new-clinical-lab-payment-system-under-pama-acla-charges-in-suit, last accessed August 28, 2018). In February 2018, CAP submitted a friend of the court or amicus curiae brief in support of ACLA's legal case (Cision, https://www.prnewswire.com/news-releases/cap-files-amicus-brief-in-support-of-acla-motion-for-summary-judgement-in-pama-lawsuit-300602935.html, last accessed August 28, 2018). The case was dismissed by the US District Court for the District of Columbia on the grounds they lacked jurisdiction; ACLA appealed the decision, and in July 2019, the US Court of Appeal for the District of Columbia reversed the lower court decision and remanded the case [American Clinical Laboratory Association v. Alex Michael Azar, II, 334 F Supp 3d 301 (D.D.C. 2018); and American Clinical Laboratory Association v. Alex Michael Azar, II, 931 F 3d 1195 (D.C. Cir 2019)]. In addition, CMS did expand the definition of applicable laboratories, effective January 1, 2019, to include laboratories that use the 14X bill type. This change will result in many more hospital outreach laboratories having to report.
The summary of PAMA data reporting in 2017 shows that 75% of tests on the CLFS had rates decreased, 10% of rates increased, and 15% did not change (Centers for Medicaid & Medicare Services, https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Downloads/CY2018-CLFS-Payment-System-Summary-Data.pdf, last accessed April 10, 2020). Although it was projected that PAMA would save >$300 million in a calendar year, CMS analysis of the first reporting period found that the impact for calendar year 2018 would be closer to $670 million. According to the Office of Inspector General's July 2018 report, during the first round of PAMA, laboratories reported difficulty determining if they met CMS's criteria to report applicable information (US Department of Health and Human Services Office of the Inspector General, https://oig.hhs.gov/oei/reports/oei-09-17-00050.pdf, last accessed April 10, 2020). The Office of Inspector General reported that at least 20 high-volume independent laboratories did not report in 2017 that likely met the majority criterion. Furthermore, CMS reported that 37% of reporting laboratories were exempt from reporting because they did not meet the requirements of the low-expenditure threshold. The report concluded that these problems may not have had a meaningful impact on the 2018 rates, but pose a risk in future reporting periods.
Significant concern about many aspects of PAMA continue. The second data collection period occurred from January 1, 2019, to June 30, 2019, and the data collected during that time would have needed to be reported to CMS by applicable laboratories starting in January 2020. However, during 2019, there were legislative efforts to stall this reporting period to give laboratories more time to report and collect data as well as commission a study to assess how to improve PAMA implementation, the Laboratory Access for Beneficiaries Act of 2019. In December, Congress passed the Laboratory Access for Beneficiaries Act [included in the Further Consolidated Appropriations Act, Pub L 116-94 (2020)], and PAMA reporting for CDLTs that are not ADLTs was delayed by 1 year. The Laboratory Access for Beneficiaries Act did not eliminate the statutory phase in of rate reductions, but it reduced the maximum cut that could be applied. For 2020, the rates for CDLTs that are not ADLTs or new CLDTs may not be reduced by >10% of the rates for 2019. There will be a 15% reduction cap for each of 2021, 2022, and 2023 (Centers for Medicare & Medicaid Services, https://www.cms.gov/Outreach-and-Education/Medicare-Learning-NetworkMLN/MLNMattersArticles/Downloads/SE19006.pdf, last accessed March 30, 2020).
In addition, in response to the 2019 coronavirus disease pandemic, Congress passed and the President signed into law a piece of legislation in March of 2020 to respond to the economic toll 2019 coronavirus disease had taken on the United States. The Coronavirus Aid, Relief, and Economic Security Act provided $2 trillion to stimulate the economy and respond to the 2019 coronavirus disease pandemic [Coronavirus Aid, Relief, and Economic Security Act, Pub L No. 116-136 (2020)]. The legislation included a PAMA provision in Section 3718, which prevented scheduled reductions in Medicare payments for clinical diagnostic laboratory tests furnished to beneficiaries in 2021 and delays the upcoming laboratory data reporting period by an additional year beyond the Laboratory Access for Beneficiaries Act. Following these two pieces of legislation, the next round of PAMA reporting will begin on January 1, 2022, and will end on March 31, 2022.
Concluding Statement
The economic landscape of molecular pathology diagnostics is a complicated interplay between many stakeholders and distinct from that of other laboratory tests. Adding to the complexity is the rapid pace of adoption of novel technologies in molecular pathology requiring constant changes to the coding, coverage, and reimbursement landscape. Please refer to Table 1 for a list of helpful websites to stay up to date on these programs and policies. It is the right and the responsibility of molecular professionals to be part of the conversation surrounding these changes to the system, as decisions made will ultimately impact the ability to offer molecular testing and patients' access to novel and potentially life-saving diagnostics.
Table 1.
Molecular Pathology Economics Website Resources
All online resources last accessed May 18, 2020.
AMA, American Medical Association; CLFS, Clinical Laboratory Fee Schedule; CMS, Centers for Medicare & Medicaid Services; CPT, Current Procedural Terminology; MolDx, Molecular Diagnostic Services; PAMA, Protecting Access to Medicare Act.
Disclaimer
The Association for Molecular Pathology (AMP) Special Reports are developed to be of assistance to laboratory and other health care professionals by providing guidance and recommendations for particular areas of practice. The guidelines or reports should not be considered inclusive of all proper approaches or methods, or exclusive to others. The guidelines or reports cannot guarantee any specific outcome, nor do they establish a standard of care. The guidelines or reports are not intended to dictate the treatment of a particular patient. Treatment decisions must be made on the basis of the independent judgement of health care providers and each patient's individual circumstances. AMP makes no warranty, express or implied, regarding the guidelines or reports and specifically excludes any warranties of merchantability and fitness for a particular use or purpose. AMP shall not be liable for direct, indirect, special, incidental, or consequential damages related to the use of the information contained herein.
Acknowledgments
We thank Bruce Quinn, Marc Synovec, and Katherine Tynan for review and comments on this work and former members and current advisors to the Economic Affairs Committee, Jan Nowak and Aaron Bossler, for contributions.
Footnotes
See related Editorial on page 967
Supported exclusively by the Association for Molecular Pathology.
A.N.S. and J.L.P. contributed equally to this work.
Disclosures: A.N.S. is an employee of Loxo Oncology, a wholly owned subsidiary of Eli Lilly, and was the past Vice-Chair for New Codes for the Association for Molecular Pathology (AMP) Economic Affairs Committee. J.L.P. is an employee of the University of Utah and ARUP Laboratories and is the Vice-Chair for New Codes of the AMP Economic Affairs Committee. M.C.H. served as a consultant at Guardant Health. S.K.C. is a consultant and speaker for Astra-Zeneca, a consultant and speaker for Bristol-Myers Squibb, a consultant and speaker for Genentech, a speaker for Merck, an independent consultant for Viracor-IBT, an employee of MAWD Pathology Group, a Board Member for the American Pathology Foundation, the Vice-President and Board Member for the Missouri Society of Pathologists, and the Chair of the AMP Economic Affairs Committee. D.L.A. is on the National Comprehensive Cancer Network Non-Small Cell Lung Cancer Committee, is on the College of American Pathologists Genomic Medicine Resource Committee, and was a member of the AMP Economic Affairs Committee.
Standard of practice is not defined by this article, and there may be alternatives. See Disclaimer for further details.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the Association for Molecular Pathology.
The EAC101 Working Group is a Working Group of the Association for Molecular Pathology Economic Affairs Committee. The EAC101 Working Group members consisted of Anthony N. Sireci, Jay L. Patel, Loren Joseph, Matthew C. Hiemenz, Oana C. Rosca, Samuel K. Caughron, and Dara L. Aisner. The 2020 Economic Affairs Committee consisted of Samuel K. Caughron (2020 Chair), Pranil Chandra (Vice-Chair: Coverage Decisions), Jay L. Patel (Vice-Chair: New Codes and Pricing), Jennifer Dien Bard, Aaron D. Bossler (Advisor), Rajyasree Emmadi, Jeffrey Gagan, Tanner Hagelstrom, Amanda Harrington, Matthew Hiemenz, Susan Hsaio, Lloyd Hutchinson, Loren Joseph, Federico Monzon, Jan Novak (Advisor), Victoria M. Pratt, Salvatore Priore, Aparna Rajadhyaksha, Oana C. Rosca, Antonia R. Sepulveda, Anthony N. Sireci, Ester Stein, Katherine Tynan, and Eric Vail.
References
- 1.Hirsch J.A., Leslie-Mazwi T.M., Nicola G.N., Barr R.M., Bello J.A., Donovan W.D., Tu R., Alson M.D., Manchikanti L. Current procedural terminology: a primer. J Neurointerv Surg. 2015;7:309–312. doi: 10.1136/neurintsurg-2014-011156. [DOI] [PubMed] [Google Scholar]
- 2.American Medical Association . American Medical Association; Chicago, IL: 2017. CPT 2018: Standard Edition. [Google Scholar]
- 3.Peabody J.W., Shimkhada R., Tong K.B., Zubiller M.B. New thinking on clinical utility: hard lessons for molecular diagnostics. Am J Manag Care. 2014;20:750–756. [PubMed] [Google Scholar]
- 4.Burken M.I., Wilson K.S., Heller K., Pratt V.M., Schoonmaker M.M., Seifter E. The interface of Medicare coverage decision-making and emerging molecular-based laboratory testing. Genet Med. 2009;11:225–231. doi: 10.1097/GIM.0b013e3181976829. [DOI] [PubMed] [Google Scholar]
- 5.Lindeman N.I., Cagle P.T., Aisner D.L., Arcila M.E., Beasley M.B., Bernicker E.H., Colasacco C., Dacic S., Hirsch F.R., Kerr K., Kwiatkowski D.J., Ladanyi M., Nowak J.A., Sholl L., Temple-Smolkin R., Solomon B., Souter L.H., Thunnissen E., Tsao M.S., Ventura C.B., Wynes M.W., Yatabe Y. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors. J Mol Diagn. 2018;20:129–159. doi: 10.1016/j.jmoldx.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Sepulveda A.R., Hamilton S.R., Allegra C.J., Grody W., Cushman-Vokoun A.M., Funkhouser W.K., Kopetz S.E., Lieu C., Lindor N.M., Minsky B.D., Monzon F.A., Sargent D.J., Singh V.M., Willis J., Clark J., Colasacco C., Rumble R.B., Temple-Smolkin R., Ventura C.B., Nowak J.A. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J Mol Diagn. 2017;19:187–225. doi: 10.1016/j.jmoldx.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]