Key Points
Question
Does antibiotic treatment with C-reactive protein (CRP)–guided duration or fixed 7-day duration, compared with fixed 14-day duration, provide noninferior clinical failure rates in patients with uncomplicated gram-negative bacteremia?
Findings
In this randomized clinical trial that included 504 adults with uncomplicated gram-negative bacteremia, 30-day clinical failure occurred in 2.4% of patients assigned to receive CRP-guided antibiotic treatment duration, 6.6% assigned to 7-day treatment, and 5.5% assigned to 14-day treatment. The differences between CRP-guided treatment and 7-day treatment compared with 14-day treatment met the noninferiority criterion of 10%, but adherence to the CRP strategy or planned follow-up did not occur in 23% of patients.
Meaning
CRP-guided treatment and 7-day treatment were noninferior to 14-day treatment in patients with uncomplicated gram-negative bacteremia, but interpretation is limited by the large noninferiority margin compared with the low observed event rate and lower adherence in the CRP-guided group.
Abstract
Importance
Antibiotic overuse drives antibiotic resistance. Gram-negative bacteremia is a common infection that results in substantial antibiotic use.
Objective
To compare the clinical effectiveness of C-reactive protein (CRP)–guided, 7-day, and 14-day antibiotic durations 30, 60, and 90 days after treatment initiation.
Design, Setting, and Participants
Multicenter, noninferiority, point-of-care randomized clinical trial including adults hospitalized with gram-negative bacteremia conducted in 3 Swiss tertiary care hospitals between April 2017 and May 2019, with follow-up until August 2019. Patients and physicians were blinded between randomization and antibiotic discontinuation. Adults (aged ≥18 years) were eligible for randomization on day 5 (±1 d) of microbiologically efficacious therapy for fermenting, gram-negative bacteria in blood culture(s) if they were afebrile for 24 hours without evidence for complicated infection (eg, abscess) or severe immunosuppression.
Intervention
Randomization in a 1:1:1 ratio to an individualized CRP-guided antibiotic treatment duration (discontinuation once CRP declined by 75% from peak; n = 170), fixed 7-day treatment duration (n = 169), or fixed 14-day treatment duration (n = 165).
Main Outcomes and Measures
The primary outcome was the clinical failure rate at day 30, defined as the presence of at least 1 of the following, with a non-inferiority margin of 10%: recurrent bacteremia, local suppurative complication, distant complication (growth of the same organism causing the initial bacteremia), restarting gram-negative–directed antibiotic therapy due to clinical worsening suspected to be due to the initial organism, or death due to any cause. Secondary outcomes included the clinical failure rate on day 90 of follow-up.
Results
Among 504 patients randomized (median [interquartile range] age, 79 [68-86] years; 306 of 503 [61%] were women), 493 (98%) completed 30-day follow-up and 448 (89%) completed 90-day follow-up. Median antibiotic duration in the CRP group was 7 (interquartile range, 6-10; range, 5-28) days; 34 of the 164 patients (21%) who completed the 30-day follow-up had protocol violations related to treatment assignment. The primary outcome occurred in 4 of 164 (2.4%) patients in the CRP group, 11 of 166 (6.6%) in the 7-day group, and 9 of 163 (5.5%) in the 14-day group (difference in CRP vs 14-day group, −3.1% [1-sided 97.5% CI, −∞ to 1.1]; P < .001; difference in 7-day vs 14-day group, 1.1% [1-sided 97.5% CI, −∞ to 6.3]; P < .001). By day 90, clinical failure occurred in 10 of 143 patients (7.0%) in the CRP group, 16 of 151 (10.6%) in the 7-day group, and 16 of 153 (10.5%) in the 14-day group.
Conclusions and Relevance
Among adults with uncomplicated gram-negative bacteremia, 30-day rates of clinical failure for CRP-guided antibiotic treatment duration and fixed 7-day treatment were noninferior to fixed 14-day treatment. However, interpretation is limited by the large noninferiority margin compared with the low observed event rate, as well as low adherence and wide range of treatment durations in the CRP-guided group.
Trial Registration
ClinicalTrials.gov Identifier: NCT03101072
This randomized trial compares the effects of C-reactive protein (CRP)–guided antibiotic duration (discontinuation once CRP declined by 75% of peak value) vs fixed 7- vs 14-day durations on 30-day clinical failure rate among patients with gram-negative bacteremia.
Introduction
Prolonged antibiotic exposure drives emergence of resistance and increases the occurrence of adverse effects, such as Clostridioides difficile infection and other microbiotic disruptions; longer courses of antibiotics are also associated with lengthier hospitalizations and higher costs.1,2 Gram-negative bacteremia, particularly due to Escherichia coli, is a frequent community-acquired and health care–associated infection.3 There is marked heterogeneity in clinicians’ choice of antibiotic duration for this infection. Most patients with bacterial bloodstream infections still receive between 10 and 14 days of antibiotics, although these durations are based largely on expert opinion, and recent evidence from retrospective analyses and a randomized trial indicate clinical noninferiority between 7-day and 14-day courses.4,5
Fixed antibiotic durations provide straightforward guidance but do not take into account host characteristics or treatment response. Given high diversity among pathogens and their hosts, another approach would be to individualize durations via biomarker-assisted guidance. Although procalcitonin provides guidance, this biomarker is not always available, affordable, or antibiotic-sparing.6,7,8 C-reactive protein (CRP), an acute-phase protein released by hepatocytes to coactivate the complement system in response to antigenic and other stimuli, is a widely available, inexpensive, and highly sensitive marker of the inflammatory state.9,10 The objective of this clinical trial was to compare the effects of individualized CRP-guided antibiotic durations and fixed 7-day durations with 14-day durations on clinical failure by day 30 among patients with gram-negative bacteremia.
Methods
Study Design and Population
This multicenter, point-of-care randomized clinical trial was approved by each site’s ethics committee (Swissethics 2017-00108). Patients with capacity provided consent either in writing or orally before a witness not involved in the study; patients without capacity could be included with written proxy consent. The statistical analysis plan and full trial protocol are available in Supplement 1 and Supplement 2.
The trial was conducted from April 2017 to August 2019 and included adults aged at least 18 years who were hospitalized at 3 tertiary care hospitals in Geneva, Lausanne, and St Gallen, Switzerland. Inclusion criteria were growth of gram-negative bacteria in at least 1 blood culture and treatment with a microbiologically efficacious antibiotic. Patients with fever or hemodynamic instability in the 24 hours prior to recruitment, severe immunosuppression (see eAppendix 1 in Supplement 3), bacteremia with nonfermenting bacilli or polymicrobial, gram-positive growth, recurrent bacteremia (initial bacteremia in the preceding 60 days), or complicated infections (eg, abscess, endocarditis) were not eligible for inclusion.11 Race or ethnicity was recorded to allow clinician readers to assess the demographic similarity of this trial population with that of their own clinical populations. Race/ethnicity was determined by self-report when feasible and otherwise requested from the patient’s representative, and was reported according to fixed categories per US Food and Drug Administration guidance.12
Patients were randomized on day 5 (±1 d) of microbiologically efficacious antibiotic therapy and followed up through day 90. Patients, investigators, and inpatient physicians were blinded to randomization groups between randomization and antibiotic discontinuation. Outcome assessors and data analysts were blinded throughout; inpatient physicians were additionally blinded to the algorithm used for antibiotic discontinuation in the individualized group. The algorithm was not disclosed so physicians would not be able to determine for themselves when the discontinuation date would be (eAppendix 2 in Supplement 3). An independent safety monitoring board evaluated results of 2 blinded interim safety analyses and recommended trial continuation at both instances.
Randomization
The randomization sequence was stratified by site; computer-generated; and used randomly permuted blocks of 3, 6, 9, or 12 allocations. Assignments were concealed from study investigators via opaque, sealed envelopes until patient enrollment and randomized treatment durations in a 1:1:1 ratio.
Intervention and Procedures
Day 1 was the first day of microbiologically efficacious antibiotic therapy, based on reported susceptibilities via routine resistance testing. On day 5 (±1 d), participants were randomly assigned to an individualized CRP-guided treatment duration, a fixed 7-day duration, or a fixed 14-day duration (control group). Inpatient physicians followed local guidelines for antibiotic choice and administration route; a switch from intravenous to oral administration was allowed per routine practice.
For patients in the CRP-guided group, inpatient physicians were notified by the study team, beginning on day 5 of microbiologically efficacious antibiotic therapy, to discontinue antibiotic(s) once serum CRP had decreased 75% from its peak10 and the patient had been afebrile for 48 hours. Per local practice, CRP levels were drawn every 1 to 2 days from onset of illness until a descent from peak was documented. If not already performed, CRP testing was recommended on days 8 (±2 d) and 12 (±2 d) in all patients whose antibiotic therapy was not already discontinued. If by day 14 the CRP level had not decreased by 75%, it was no longer used to guide therapy (see eAppendix 3 in Supplement 3 for details on the algorithm’s construction and rationale). Follow-up visits were conducted by telephone on days 30 (±7 d), 60 (±14 d), and 90 (±21 d).
Outcomes and Definitions
The primary outcome was the occurrence of clinical failure by day 30, with failure defined by the presence of at least 1 of the following: relapse (recurrent bacteremia through day 30); local suppurative complication (eg, kidney abscess in pyelonephritis); distant complication, defined by growth of the same organism causing the initial bacteremia (as determined by antibiotic susceptibility profiling); the restarting of gram-negative–directed antibiotic therapy because of clinical worsening suspected to be due to the initial organism; or death due to any cause through day 30. The cases of patients deemed by site investigators to fulfill criterion of restarting gram-negative–directed antibiotic therapy because of clinical worsening suspected to be due to the initial organism were presented to a blinded panel of non–site investigators for final adjudication.
Secondary outcomes included the incidence of clinical failure at days 60 and 90; all-cause mortality at days 30, 60, and 90; the incidence of antibiotic-related adverse events through day 90, including C difficile infection (by study group and length of antibiotic therapy); the incidence of emergence of resistance to study antibiotic(s) in any clinical isolate positive for the initially infecting organism (by study group and length of antibiotic therapy); overall number of days of antibiotic therapy in the 90-day study period (eAppendix 4 in Supplement 3); length of hospital stay; the number of patients in each group whose deviation from the assigned treatment duration was due to physician decision in the absence of suspected complication, failure, or adverse effect; and cost-effectiveness. The latter outcome has not yet been analyzed and is not reported here. Because CRP is excreted through the kidneys, antibiotic durations in the CRP-guided group were further assessed by kidney function according to chronic kidney disease stages.
Bacteremia was characterized as nosocomial if the first positive blood culture was drawn at least 48 hours after admission; health care–associated if drawn in the first 48 hours of hospitalization in a patient who was transferred from another health care facility, receiving chronic dialysis, or diagnosed with metastatic cancer13; or community-acquired if none of these conditions were met. Severity of illness was defined by the quick Sequential Organ Failure Assessment score.14
Laboratory Methods
Blood culturing and CRP quantification methods are described in eAppendix 5 in Supplement 3.
Statistical Methods
The sample size calculation was driven by a noninferiority hypothesis based on reported failure rates between 10% and 30% in settings with access to broad-spectrum antibiotics and resistance prevalence similar to those in Switzerland.10,13,15,16 The noninferiority margin of 10% was chosen in line with guidance provided by the Infectious Disease Society of America17 given the expected gain from reduced antibiotic use and therewith a reduced risk for adverse effects and emergence and spread of resistance.18 Assuming a 1-sided type I error (α) of .025, attrition of up to 5%, and potential treatment switching of up to 12%, 167 patients were needed in each group at a power of 0.80, making the target sample size 500 participants.
The primary analysis set consisted of all randomized patients with any baseline data available; the per-protocol population included patients who were randomized, received the antibiotic duration assigned (±48 h), had 30-day (primary outcome) follow-up data available, and for whom no major protocol deviations were documented throughout the study. The primary analysis used a 1-sided 97.5% CI for noninferiority, which would be demonstrated if the lower bound of the CI of the treatment duration effect difference did not exceed 10%.
Analyses were performed with blinding to treatment duration randomization. Baseline characteristics are reported as percentages, medians, and interquartile ranges (IQRs). Clinical failure was compared among groups using Fisher exact tests and adverse events were compared using χ2 tests with events numbering greater than 10. Patients who had documented failure and were thereafter lost to follow-up were included in the primary outcome analysis of any failure by day 30, while those whose response status was unknown before dropout were considered missing. Missing values were listed and excluded from the primary analysis; sensitivity analyses were conducted with best-worst– and worst-best–case scenarios.
Bivariable and multivariable regression models were constructed to assess associations between failure and baseline demographic and clinical characteristics. The multivariable model assumed independence of observations and minimal multicollinearity of independent variables and was constructed to include age, sex, comorbidity status, severity of illness, and variables emerging from bivariable analyses with an arbitrary P value of ≤.20; a more parsimonious model including age and variables with P ≤.20 in bivariable models was created post hoc given the relatively low number of outcome events. Goodness of fit was assessed via the Hosmer-Lemeshow test. Post hoc mixed-effects logistic regression with site as a random intercept was conducted to assess for potential site effects in this multicenter trial. For all non–primary outcome comparisons, statistical tests were 2-sided with a significance level of .05; because there was no adjustment for multiple comparisons, secondary end point analyses should be interpreted as exploratory. All analyses were performed using Stata, version 15.1 (StataCorp).
Results
Study Population
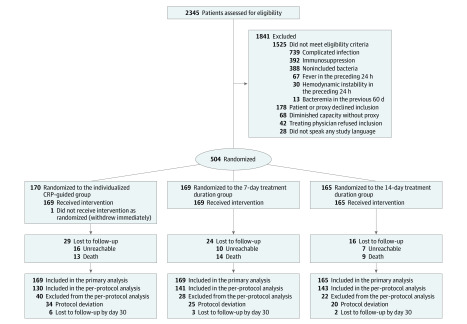
Of 2345 patients screened, 504 were enrolled and randomized (170 to the CRP-guided antibiotic therapy duration group, 169 to the 7-day antibiotic therapy group, and 165 to the 14-day antibiotic therapy group). A total of 493 patients (98%) completed the 30-day follow-up and 448 (89%) completed the 90-day follow-up (Figure 1). There were no significant differences between primary analysis and per-protocol populations in baseline characteristics and outcomes (eTables 1 and 2 in Supplement 3).
Figure 1. Flow of Participants in a Study of the Effect of C-Reactive Protein (CRP)–Guided Antibiotic Treatment Duration, 7-Day Treatment, or 14-Day Treatment on Clinical Failure in Patients With Uncomplicated Gram-Negative Bacteremia.
Some patients had more than 1 exclusion criterion. Informed consent documents were available in French, German, and English. No antibiotic duration data were available for 1 patient in the CRP-guided group and 1 in the 14-day group.
The median (IQR) baseline age of patients was 79 (68-86) years; 306 of 503 patients (61%) were women and the median (IQR) Charlson comorbidity index of patients was 1 (0-2). Among the 503 included patients, 107 (21%) were diagnosed with diabetes; 153 (30%) had mild kidney insufficiency; 209 (42%) had moderate kidney insufficiency; 78 (16%) had severe kidney insufficiency; and 108 had foreign body material, including prosthetic material and devices (Table 1). Presumed bacteremia source was urinary in 348 patients (69%), abdominal in 87 (17%), and pulmonary in 36 (7%). The median (IQR) overall quick Sequential Organ Failure Assessment score was 1 (0-2). Bacteremia was caused by E coli in 373 patients (74%), Klebsiella spp in 88 patients (17%), and Proteus spp in 20 patients (4%) (Table 2).
Table 1. Baseline Characteristics of Patients in a Study of the Effect of C-Reactive Protein (CRP)–Guided, 7-Day, or 14-Day Antibiotic Treatment Duration on Clinical Failure in Patients With Gram-Negative Bacteremia.
| Characteristic | Antibiotic therapy duration group, No. (%) | ||
|---|---|---|---|
| CRP-guided (n = 169)a | 7 d (n = 169) | 14 d (n = 165) | |
| Sex | |||
| Women | 105 (62) | 107 (63) | 94 (57) |
| Men | 64 (38) | 62 (37) | 71 (43) |
| Age, median (IQR), y | 78 (69-86) | 78 (69-86) | 80 (67-85) |
| Race/ethnicity | |||
| White | 163 (96) | 160 (95) | 155 (94) |
| Asian | 3 (2) | 3 (2) | 2 (1) |
| Hispanic | 1 (1) | 2 (1) | 4 (2) |
| Black | 1 (1) | 2 (1) | 2 (1) |
| BMI, median (IQR) | 26 (23-30) | 26 (23-30) | 26 (23-29) |
| eGFR at inclusion, median (IQR), mL/min/1.73 m2 | 51 (37-72) | 51 (32-73) | 55 (38-74) |
| Charlson Comorbidity Index score, median (IQR)b | 1 (0-2) | 1 (0-2) | 1 (0-2) |
| Diabetes mellitus | 38 (22) | 33 (20) | 36 (22) |
| Presence of removable urethral catheter | 17 (10) | 7 (4) | 24 (14) |
| Presence of implanted material | 41 (24) | 36 (21) | 31 (19) |
| Artificial joint | 4 (2) | 5 (3) | 10 (6) |
| Implanted urinary tract devicec | 6 (4) | 3 (2) | 5 (3) |
| Endovascular device | 6 (4) | 2 (1) | 1 (1) |
| Artificial valve | 4 (2) | 2 (1) | 0 (0) |
| Otherd | 22 (13) | 25 (15) | 16 (10) |
| Source of bacteremia | |||
| Urinary | 124 (73) | 107 (63) | 117 (71) |
| Abdominal | 30 (18) | 37 (22) | 20 (12) |
| Pulmonary | 6 (4) | 14 (8) | 16 (10) |
| Endovascular device | 1 (1) | 5 (3) | 5 (3) |
| Wound | 0 | 2 (1) | 2 (1) |
| Unknown | 8 (5) | 4 (2) | 5 (3) |
| Bacteremia acquisitione | |||
| Community-acquired | 108 (64) | 108 (64) | 99 (60) |
| Nosocomial | 45 (27) | 45 (27) | 45 (27) |
| Health care–associated | 16 (9) | 16 (9 | 21 (13) |
| qSOFA score, median (IQR)f | 1 (0-2) | 1 (0-2) | 1 (0-1) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); eGFR, estimated glomerular filtration rate; IQR, interquartile range.
One patient withdrew from the study immediately after randomization and thus was not included in the primary analysis set because there was no baseline data available.
Charlson Comorbidity Index scores range from 0 to 37, with higher values indicating greater number or degree of underlying comorbidities.
Includes suprapubic catheter (n = 6), jejunostomy tube (“pigtail”) catheter (n = 3), nephrostomy tube (n = 3), and ileal conduit (n = 2).
Other foreign body material included metal screws, pins, rods, and plates; artificial spinal discs; and breast implants.
Bacteremia was characterized as nosocomial if the first positive blood culture was drawn at least 48 hours after admission; health care–associated if drawn in the first 48 hours of hospitalization in a patient who was transferred from another health care facility, receiving chronic dialysis, or diagnosed with metastatic cancer13; and community-acquired if none of these conditions were met.
The quick Sequential Organ Failure Assessment (qSOFA) score was developed for early identification of patients at high risk for poor outcome with an infection. The patient receives 1 point for each of the following: low blood pressure (systolic blood pressure ≤100 mm Hg), high respiratory rate (≥22 breaths/min), and altered mentation (Glasgow Coma Scale score ≤14). A score of at least 2 is associated with a higher risk of death or prolonged intensive care unit stay.14
Table 2. Baseline Blood Culture Isolates in a Study of the Effect of C-Reactive Protein (CRP)–Guided, 7-Day, or 14-Day Antibiotic Treatment Duration on Clinical Failure in Patients With Gram-Negative Bacteremia.
| Blood culture isolate | Antibiotic therapy duration group, No. (%) | ||
|---|---|---|---|
| CRP-guided (n = 169) | 7 d (n = 169) | 14 d (n = 165) | |
| Escherichia coli | 126 (75) | 123 (73) | 124 (75) |
| Extended-spectrum β-lactamase | 14 (8) | 9 (5) | 12 (7) |
| Klebsiella spp | 27 (16) | 35 (21) | 26 (16) |
| Klebsiella pneumoniae | 25 (15) | 23 (14) | 21 (13) |
| Extended-spectrum β-lactamase | 1 (1) | 2 (1) | 1 (1) |
| Klebsiella oxytoca | 1 (1) | 11 (7) | 1 (1) |
| Extended-spectrum β-lactamase | 0 | 0 | 0 |
| Klebsiella aerogenes | 1 (1) | 1 (1) | 4 (2) |
| Extended-spectrum β-lactamase | 1 (1) | 0 | 0 |
| Proteus spp | 7 (4) | 7 (4) | 6 (4) |
| Proteus mirabilis | 5 (3) | 4 (2) | 6 (4) |
| Extended-spectrum β-lactamase | 0 | 0 (0) | 0 |
| Proteus vulgaris | 2 (1) | 3 (2) | 0 |
| Extended-spectrum β-lactamase | 0 | 0 | 0 |
| Enterobacter cloacae | 6 (4) | 3 (2) | 1 (1) |
| Extended-spectrum β-lactamase | 0 | 0 | 0 |
| Other (%)a | 9 (5) | 10 (6) | 12 (7) |
Serratia spp (n = 10), Bacteroides fragilis (n = 8), Citrobacter spp (n = 5), Hafnia alvei (n = 3), Haemophilus influenzae (n = 2), Tatumella ptyseos (n = 1), Salmonella typhimurium (n = 1), Providencia rettgeri (n = 1).
Treatment Durations in the CRP-Guided Group
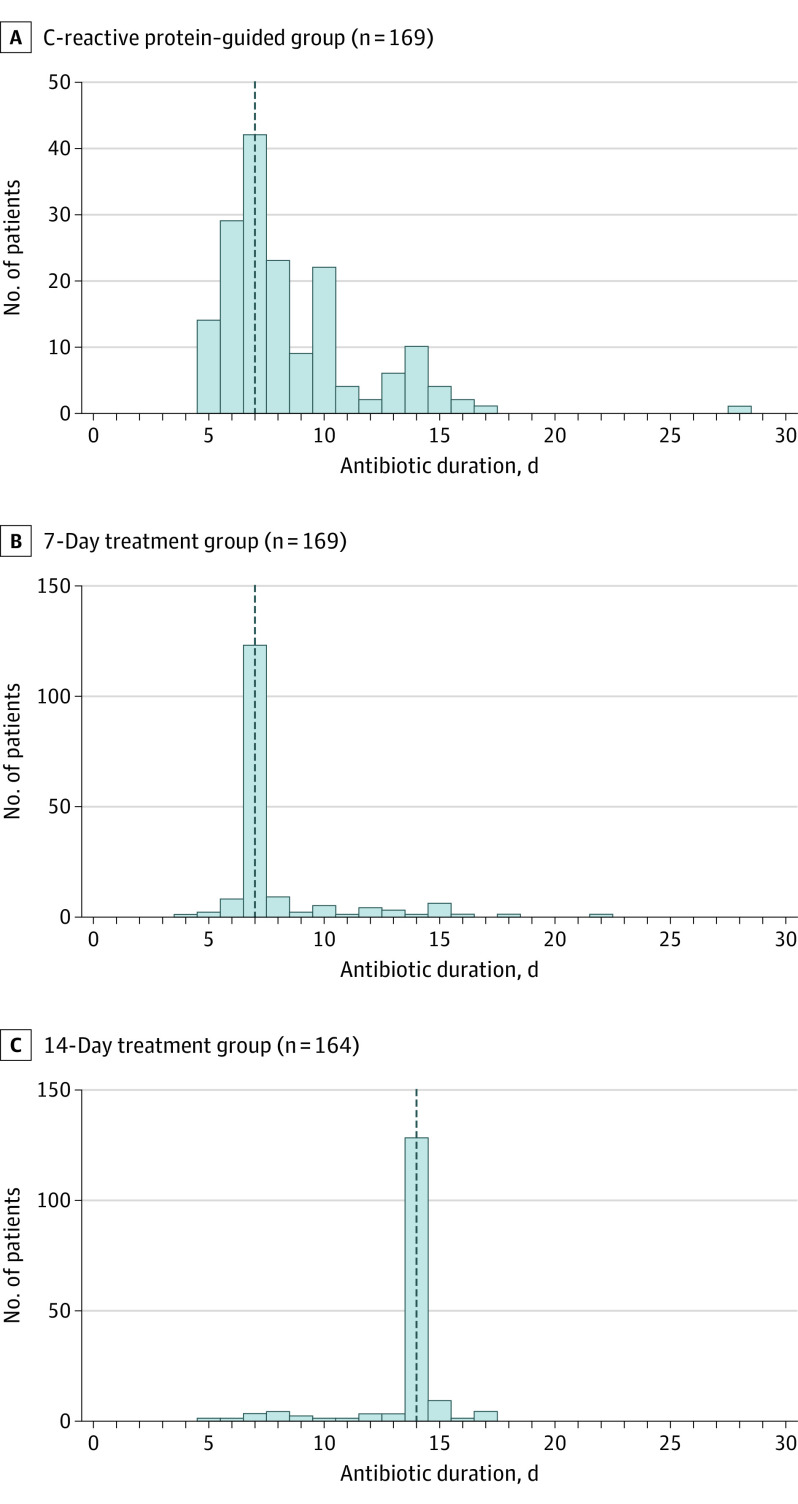
Median therapy duration in the intention-to-treat CRP-guided group was 7 (IQR, 6-10; range, 5-28) days (Figure 2). The patient who underwent 28 days of treatment was discovered to have multiple kidney abscesses after randomization, and CRP was not used to guide therapy. In patients with normal kidney function or mild insufficiency (n = 69), the median (IQR) therapy duration was 7 (6-8) days; in patients with moderate or severe insufficiency (n = 100), the median (IQR) therapy duration was 8 (6.5-10.5) days. Logistic difficulties, such as rapid discharge from hospital before a 75% reduction from peak could be documented (the most common logistic difficulty), blood draws declined by patients, or samples that were lost or had insufficient volume, impeded the per-protocol collection of serial CRP levels in 12 of 170 patients (7%) and led to an overall higher number of treatment-related per-protocol violations among patients reaching 30-day follow-up in the CRP-guided group (34 of 164 [21%] patients) compared with the 7-day (25 of 166 [15%]) and 14-day (20 of 163 [12%]) treatment duration groups. Reasons for protocol deviations in all study groups are described in eTable 3 in Supplement 3. In all study groups, patients who had longer-than-assigned treatment durations did not differ significantly from their per-protocol counterparts in baseline characteristics or clinical outcomes (eTable 4 in Supplement 3). Patients in the per-protocol population had shorter durations, although differences were not significant (eTable 5A in Supplement 3). Patients in all study groups had similar CRP kinetics (eTable 5B in Supplement 3).
Figure 2. Durations of Antibiotic Therapy in the Primary Analysis Set in a Study of the Effect of C-Reactive Protein (CRP)–Guided Antibiotic Treatment Duration, 7-Day Treatment, or 14-Day Treatment on Clinical Failure in Patients With Uncomplicated Gram-Negative Bacteremia.
The dotted vertical line indicates the median antibiotic duration for that group. The patient in the CRP group who received 28 days of antibiotics was diagnosed with multiple kidney abscesses the day after randomization, so CRP was not used to guide therapy.
Clinical Outcomes
By day 30, data were missing for 11 randomized patients (2%). Noninferiority of both CRP-guided and fixed 7-day antibiotic courses to 14-day courses was observed; clinical failure occurred by day 30 in 4 of 164 patients (2.4%) in the CRP-guided group, 11 of 166 (6.6%) in the 7-day treatment duration group, and 9 of 163 (5.5%) in the 14-day treatment duration group (CRP-guided vs 14-day group: difference, −3.1% [1-sided 97.5% CI, −∞ to 1.1]; 7- vs 14-day group: difference, 1.1% [1-sided 97.5% CI, −∞ to 6.3]; Table 3). These patterns held through days 60 and 90; at final follow-up, clinical failure occurred in 10 of 143 patients (7.0%) in the CRP-guided group, 16 of 151 (10.6%) in the 7-day group, and 16 of 153 (10.5%) in the 14-day group. Reasons for failure were primarily all-cause mortality in the first 30 days or the restarting of antibiotic therapy because of suspected recurrence (Table 3). There were 3 instances of recurrent bacteremia, 2 local suppurative complications, and no known hematogenous seeding of distant foci. All-cause mortality through day 90 occurred in 13 of 154 patients (8%) in the CRP-guided group, 14 of 159 (9%) in the 7-day group, and 9 of 158 (6%) in the 14-day group (eTable 6 in Supplement 3).
Table 3. Clinical Outcomes in a Study of the Effect of C-Reactive Protein (CRP)–Guided, 7-Day, or 14-Day Antibiotic Treatment Duration on Clinical Failure in Patients With Gram-Negative Bacteremia.
| Outcome | Antibiotic therapy duration group, No. (%) | CRP-guided vs 14 d | 7 d vs 14 d | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CRP-guided (n = 169) | 7 d (n = 169) | 14 d (n = 165) | Difference, % (1-sided 97.5% CI) | P valuea | Difference, % (1-sided 97.5% CI) | P valuea | ||||
| Primary outcome | ||||||||||
| Clinical response through day 30 | −3.1 (−∞ to 1.1) | <.001 | 1.1 (−∞ to 6.3) | <.001 | ||||||
| Clinical success | 160 (97.6) | 155 (93.4) | 154 (94.5) | |||||||
| Clinical failure | 4 (2.4) | 11 (6.6) | 9 (5.5) | |||||||
| Recurrent bacteremia | 0 | 1 (9)a | 2 (22) | |||||||
| Suppurative local complication | 0 | 2 (18)b | 1 (11) | |||||||
| Distal complication | 0 | 0 | 0 | |||||||
| Targeted therapy restart | 2 (50) | 3 (27) | 2 (22) | |||||||
| 30-d all-cause mortalityc | 2 (50) | 6 (55) | 4 (44) | |||||||
| Missingd | 5 (2.9) | 3 (1.8) | 2 (1.2) | |||||||
| Secondary outcomes | ||||||||||
| Clinical response through day 60 | −1.8 (−∞ to 3.7) | <.001 | 2.6 (−∞ to 8.9) | .010 | ||||||
| Clinical success | 146 (94.2) | 141 (89.8) | 146 (92.4) | |||||||
| Clinical failure | 9 (5.8) | 16 (10.2) | 12 (7.6) | |||||||
| Recurrent bacteremia | 0 | 1 (6)b | 2 (17) | |||||||
| Suppurative local complication | 0 | 1 (6)b | 1 (8) | |||||||
| Distal complication | 0 | 0 | 0 | |||||||
| Targeted therapy restart | 7 (78) | 9 (56) | 5 (42) | |||||||
| 30-d all-cause mortalityc | 2 (22) | 6 (38) | 4 (33) | |||||||
| Missingd | 9 (5.3) | 7 (4.1) | 3 (1.8) | |||||||
| Death after day 30 | 5 (3.0) | 5 (3.0) | 4 (2.4) | |||||||
| Clinical response through day 90 | −3.5 (−∞ to 2.9) | <.001 | 0.1 (−∞ to 7.0) | .002 | ||||||
| Clinical success | 133 (93.0) | 135 (89.4) | 137 (89.5) | |||||||
| Clinical failure | 10 (7.0) | 16 (10.6) | 16 (10.5) | |||||||
| Recurrent bacteremia | 0 | 1 (6)b | 2 (13) | |||||||
| Suppurative local complication | 0 | 1 (6)b | 1 (6) | |||||||
| Distal complication | 0 | 0 | 0 | |||||||
| Targeted therapy restart | 8 (80) | 9 (56) | 9 (56) | |||||||
| 30-d all-cause mortalityc | 2 (20) | 6 (38) | 4 (25) | |||||||
| Missingd | 15 (8.9) | 10 (5.9) | 7 (4.2) | |||||||
| Death after day 30 | 11 (6.5) | 8 (4.7) | 5 (3.0) | |||||||
P value for noninferiority (see eAppendix 6 in Supplement 3 for calculation).
One patient had both recurrent bacteremia and a suppurative local complication by day 30.
Through day 30.
The CRP-guided group had more missing data throughout because of early withdrawals (n = 5). (Missing data are cumulative over the 3 measured time points.)
Sensitivity analyses for missing data with best-worst– and worst-best–case scenarios confirmed noninferiority in all scenarios (eTable 7 in Supplement 3). Mixed-effects logistic regression modeling with site as a random intercept revealed no significant site effect (eTable 8 in Supplement 3).
In bivariable analyses, sex, advanced age (≥70 years), comorbidity status, severity of illness, switch from an intravenous to oral antibiotic, and delays in appropriate therapy were not associated with clinical failure, while the presence of foreign body material, bacteremia of pulmonary origin or due to Klebsiella spp or Proteus spp, and empirical therapy with piperacillin-tazobactam were (eTable 9 in Supplement 3). However, in multivariable analyses only foreign body material (odds ratio [OR], 2.82 [95% CI, 1.15-6.92]) and bacteremia of pulmonary origin (OR, 3.88 [95% CI, 1.27-11.83]) remained significant risk factors, while younger age (<70 years) was protective (OR, 0.10 [95% CI, 0.01-0.79]) (eTable 10 in Supplement 3).
None of the 44 patients in the CRP-guided group with bacteremia of pulmonary origin and/or foreign body material experienced clinical failure by day 30, while 8 of 45 patients (18%; P = .006) in the 7-day group and 7 of 44 (16%; P = .01) in the 14-day group did have clinical failure. At 90 days, 2 of 39 evaluable patients (5%) in the CRP-guided group, 10 of 42 (24%; P = .03) in the 7-day group, and 9 of 41 (22%; P = .05) in the 14-day group had clinical failure.
Adverse events were relatively infrequent and occurred among similar percentages of patients in all treatment groups. C difficile infection was the most frequent adverse event (13 of 493 patients [3%]), followed by diarrhea not related to C difficile (5 of 493 patients [1%]) (eTable 11 in Supplement 3). Six serious adverse events considered possibly or probably related to antibiotic therapy occurred: 3 hospitalizations for C difficile infection, 1 hospitalization for abdominal pain, 1 hospitalization for headache, and 1 death of unknown cause shortly after antibiotic discontinuation (eTable 12 in Supplement 3).
C difficile infection occurred in 7 of 164 patients (4%) in the CRP-guided group, 2 of 166 (1%) in the 7-day group, and 4 of 163 (2%) in the 14-day group. By actual length of therapy, C difficile infection occurred in 0 of 49 patients who received less than 7 days of antibiotic therapy, 5 of 245 (1%) who received 7 to 10 days, and 8 of 201 (4%) who received more than 10 days.
Emergence of resistance to study antibiotic(s) was rarely detected in subsequent isolates because follow-up clinical specimens were only rarely collected, and not for study purposes. Seven patients in the CRP-guided group, 4 in the 7-day group, and 2 in the 14-day group had additional clinical specimens collected. Of these patients, 3 (43%) in the CRP-guided group and 3 (75%) in the 7-day group had specimen positive for organisms resistant to the antibiotic(s) used to manage the bacteremia. By length of therapy, antibiotic resistance was detected in 1 of 3 patients (33%) who received less than 7 days of antibiotic therapy, 5 of 7 (71%) who received 7 to 10 days of antibiotic therapy, and 0 of 3 who received more than 10 days of antibiotic therapy.
The median (IQR) number of antibiotic days of therapy throughout the 90-day study period was 9 (8-14) in the CRP-guided group, 9 (7-14) in the 7-day group, and 16 (14-18) in the 14-day group (P < .001 for CRP-guided vs 14-day and 7-day vs 14-day groups). Median (IQR) hospital length of stay was similar across groups, with 10 (6-19) days in the CRP-guided group, 10 (6-17) in the 7-day group, and 9 (5-19) in the 14-day group. Deviations from the assigned treatment duration were due to the physician’s decision in the absence of suspected complication, failure, or adverse effect in 4 of 34 patients (12%) in the CRP-guided group, 10 of 25 (40%) in the 7-day group, and 2 of 20 (10%) in the 14-day group (eTable 3 in Supplement 3).
Discussion
In this randomized clinical trial, CRP-guided antibiotic durations and fixed 7-day durations of antibiotic therapy were noninferior to 14-day durations of antibiotic therapy in 30-day rates of clinical failure among patients with uncomplicated gram-negative bacteremia. These patients, all with source control and some evidence of response to antibiotics by day 5, experienced little clinical failure in general, whether treated with 14 or 5 days of antibiotics (the latter as long as a 75% CRP reduction was observed). This observation reinforces the principle that antibiotic duration need not be predefined in the first days of acute illness; rather, it should be determined in the days thereafter, in line with the patient’s ongoing response.
Reasons for clinical failure were primarily all-cause mortality in the first 30 days or the restarting of antibiotic therapy because of suspected recurrence; among 500 patients, there were only 3 patients with recurrent bacteremia and 2 with local suppurative complications, and there was no hematogenous seeding of distant foci identified. Foreign body material and pulmonary source were found to be risk factors for failure in multivariable analyses, and younger age (<70 years) was found to be protective. Escherichia coli was associated with fewer failures compared with Klebsiella spp and Proteus spp in crude comparisons, but none were significantly associated with success or failure in adjusted analyses.
With regard to fixed 7-day antibiotic courses, these findings support those of Yahav et al,4 who demonstrated noninferiority to 14-day courses for gram-negative bacteremia in a similar population. However, the overall rate of clinical failure in the present study was lower because all-cause hospitalizations were not included as a criterion for failure and this primary outcome was assessed at day 30 rather than day 90.
The viability of individualized antibiotic durations was also assessed in this study, because fixed courses cannot address the wide diversity among patients, pathogens, and sources of infection or allow for early identification of patients whose recovery is slower and thus require prolonged antibiotic therapy or, conversely, those whose infections resolve swiftly and thus need less antibiotic support.
CRP is a highly sensitive, real-time marker of the inflammatory state. Its guidance for antibiotic discontinuation proved effective and antibiotic sparing compared with the 14-day control: the CRP-guided group had the fewest failures at every time point, although this finding requires confirmation in larger trials, and the median antibiotic duration was 7 days.
The ability of the biomarker to reduce antibiotic consumption compared with the control group receiving 14 days could not have been assumed; CRP elevation is not specific to infection-related inflammation. Postoperative patients or patients who have an underlying neoplasm may not rapidly normalize CRP concentrations even though the infection is resolving. In these cases, clinical judgment should play a greater role than an algorithm in treatment decisions. In addition, obtaining CRP levels requires sequential blood draws that occasionally proved difficult to realize, particularly in patients who were rapidly improving and planning for discharge. Although the requirement for a 75% reduction from peak was a successful approach in terms of clinical response and reduced antibiotic consumption, other algorithms could be explored to ensure both these and more minimized logistic difficulties, as could the use of rapid, point-of-care CRP testing.19
Cost-effectiveness analyses are pending; in the absence of this information, the value and efficiency of either CRP-guided or 7-day antibiotic durations against 14-day durations cannot be determined.
Limitations
This study has several limitations. First, blinding was only between randomization and antibiotic discontinuation because dummy formulations for several different intravenous and oral antibiotics were not feasible. Second, daily CRP measurements were not strictly enforced in this point-of-care randomized trial, which, by design, was integrated into routine care and thus expressly allowed patients to remain in their usual clinical milieu without the addition of extra testing or visits. Rather, inpatient physicians requested CRP measurements per routine practice, such that levels were drawn in general at least every 2 days. Third, interpretation of the results is limited by an event rate that was lower than the prespecified noninferiority margin and the relatively lower adherence to assigned treatment durations in the CRP-guided group.
Conclusions
Among adults with uncomplicated gram-negative bacteremia, 30-day rates of clinical failure for CRP-guided treatment duration and fixed 7-day treatment were noninferior to fixed 14-day treatment duration. However, interpretation is limited by the large noninferiority margin compared with the low observed event rate, as well as low adherence and wide range of treatment durations in the CRP-guided group.
Trial protocol
Statistical analysis plan
eAppendix
eResults
eReferences
Data sharing statement
References
- 1.Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis. 2014;14:13. doi: 10.1186/1471-2334-14-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuchs BB, Tharmalingam N, Mylonakis E. Vulnerability of long-term care facility residents to Clostridium difficile infection due to microbiome disruptions. Future Microbiol. 2018;13:1537-1547. doi: 10.2217/fmb-2018-0157 [DOI] [PubMed] [Google Scholar]
- 3.Laupland KB. Incidence of bloodstream infection: a review of population-based studies. Clin Microbiol Infect. 2013;19(6):492-500. doi: 10.1111/1469-0691.12144 [DOI] [PubMed] [Google Scholar]
- 4.Yahav D, Franceschini E, Koppel F, et al. ; Bacteremia Duration Study Group . Seven versus 14 days of antibiotic therapy for uncomplicated gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis. 2019;69(7):1091-1098. doi: 10.1093/cid/ciy1054 [DOI] [PubMed] [Google Scholar]
- 5.Chotiprasitsakul D, Han JH, Cosgrove SE, et al. ; Antibacterial Resistance Leadership Group . Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis. 2018;66(2):172-177. doi: 10.1093/cid/cix767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819-827. doi: 10.1016/S1473-3099(16)00053-0 [DOI] [PubMed] [Google Scholar]
- 7.Bouadma L, Luyt CE, Tubach F, et al. ; PRORATA trial group . Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375(9713):463-474. doi: 10.1016/S0140-6736(09)61879-1 [DOI] [PubMed] [Google Scholar]
- 8.Albrich WC, Harbarth S. Pros and cons of using biomarkers versus clinical decisions in start and stop decisions for antibiotics in the critical care setting. Intensive Care Med. 2015;41(10):1739-1751. doi: 10.1007/s00134-015-3978-8 [DOI] [PubMed] [Google Scholar]
- 9.Lin KH, Wang FL, Wu MS, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection in patients with liver cirrhosis: a systematic review and meta-analysis. Diagn Microbiol Infect Dis. 2014;80(1):72-78. doi: 10.1016/j.diagmicrobio.2014.03.029 [DOI] [PubMed] [Google Scholar]
- 10.Oliveira CF, Botoni FA, Oliveira CR, et al. Procalcitonin versus C-reactive protein for guiding antibiotic therapy in sepsis: a randomized trial. Crit Care Med. 2013;41(10):2336-2343. doi: 10.1097/CCM.0b013e31828e969f [DOI] [PubMed] [Google Scholar]
- 11.Huttner A, Albrich WC, Bochud PY, et al. PIRATE project: point-of-care, informatics-based randomised controlled trial for decreasing overuse of antibiotic therapy in gram-negative bacteraemia. BMJ Open. 2017;7(7):e017996. doi: 10.1136/bmjopen-2017-017996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration Guidance for Industry: Collection of Race and Ethnicity Data in Clinical Trials US Food and Drug Administration; 2016.
- 13.Fitzpatrick JM, Biswas JS, Edgeworth JD, et al. ; United Kingdom Clinical Infection Research Group . Gram-negative bacteraemia; a multi-centre prospective evaluation of empiric antibiotic therapy and outcome in English acute hospitals. Clin Microbiol Infect. 2016;22(3):244-251. doi: 10.1016/j.cmi.2015.10.034 [DOI] [PubMed] [Google Scholar]
- 14.Angus DC, Seymour CW, Coopersmith CM, et al. A framework for the development and interpretation of different sepsis definitions and clinical criteria. Crit Care Med. 2016;44(3):e113-e121. doi: 10.1097/CCM.0000000000001730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pittet D, Li N, Wenzel RP. Association of secondary and polymicrobial nosocomial bloodstream infections with higher mortality. Eur J Clin Microbiol Infect Dis. 1993;12(11):813-819. doi: 10.1007/BF02000400 [DOI] [PubMed] [Google Scholar]
- 16.Tellier G, Niederman MS, Nusrat R, Patel M, Lavin B. Clinical and bacteriological efficacy and safety of 5 and 7 day regimens of telithromycin once daily compared with a 10 day regimen of clarithromycin twice daily in patients with mild to moderate community-acquired pneumonia. J Antimicrob Chemother. 2004;54(2):515-523. doi: 10.1093/jac/dkh356 [DOI] [PubMed] [Google Scholar]
- 17.Infectious Diseases Society of America White paper: recommendations on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens. Clin Infect Dis. 2012;55(8):1031-1046. doi: 10.1093/cid/cis688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandberg T, Skoog G, Hermansson AB, et al. Ciprofloxacin for 7 days versus 14 days in women with acute pyelonephritis: a randomised, open-label and double-blind, placebo-controlled, non-inferiority trial. Lancet. 2012;380(9840):484-490. doi: 10.1016/S0140-6736(12)60608-4 [DOI] [PubMed] [Google Scholar]
- 19.Ward C. Point-of-care C-reactive protein testing to optimise antibiotic use in a primary care urgent care centre setting. BMJ Open Qual. 2018;7(4):e000391. doi: 10.1136/bmjoq-2018-000391 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eAppendix
eResults
eReferences
Data sharing statement