Abstract
Purpose
To evaluate foveal outer nuclear layer (ONL) thickness and the difference thereof between bilateral eyes and their possible associations with clinical characteristics in a healthy Chinese population.
Materials and Methods
Normal subjects were enrolled. Generalized linear models were used to assess the associations of foveal ONL thickness with sex, age, and spherical equivalents (SEs) and the associations of the difference in foveal ONL thickness between bilateral eyes with sex, age, and difference in SEs between bilateral eyes.
Results
Totally, 304 subjects were included. The average foveal ONL thickness was 103.19 ± 14.25 (range 70–151) μm in the right eye and 103.90 ± 14.63 (range 69–155) μm in the left eye. The mean difference in foveal ONL thickness between right and left eyes was −0.71 ± 4.36 (range −13 to +12) μm. Men had slightly greater foveal ONL thickness values in both right and left eyes compared with women (both P < 0.05); however, some women had a thicker foveal ONL than that of men (85/198 vs. 46/106 in the right eye; 79/198 vs. 52/106in the left eye). Age and SEs were not associated with foveal ONL thickness in either eye (all P > 0.05). Sex, age, and difference in SEs between bilateral eyes were not associated with the difference in foveal ONL thickness between bilateral eyes (all P > 0.05).
Conclusions
Foveal ONL thickness showed wide variation in a normal Chinese population but little difference between bilateral eyes. Both these parameters could not be adjusted by sex, age, SEs, or the SEs difference between bilateral eyes. Thus, in those diseases involving only one eye, the difference or ratio of foveal ONL thickness between the affected eye and normal fellow eye may reflect the actual degree of the disease, rather than the foveal ONL thickness in the affected eye alone.
1. Introduction
The outer nuclear layer (ONL) of the retina contains the nuclei of cone and rod photoreceptors [1], and a reduction in ONL thickness is considered to be caused by photoreceptor cell death [2–6]. Thinning of the foveal ONL is associated with decreased visual acuity in various diseases; thus, foveal ONL thickness on optical coherence tomography (OCT) images is considered an important biomarker of retinal degeneration in clinical studies [7–14].
The great inter individual variability of the foveal ONL thickness among ethnicities has been reported [15]. However, the degree of variability in foveal ONL thickness in the normal Asian population and the associations of foveal ONL thickness with clinical characteristics remain unclear. Therefore, in this study, foveal ONL thickness and the difference thereof between bilateral eyes of the same subject and their possible associations with clinical characteristics were evaluated in a normal Chinese population.
2. Materials and Methods
The approval of the Ethics Committee of Eye and Ear Nose Throat (ENT) Hospital Fudan University was obtained, and written informed consent was obtained from all subjects before their enrolment in the study. The study adhered to the tenets of the Declaration of Helsinki.
2.1. Subjects
This was a prospective cross-sectional study that enrolled normal subjects of East Asian descent originating from mainland China, based on self-declaration, during routine ophthalmological examinations in the Eye, Ear, Nose, and Throat Hospital of Fudan University, Shanghai, China, from September 2014 to November 2019.
Data from both eyes were collected, including best corrected visual acuity (BCVA), measured using a standard Snellen chart and converted to logarithm of the minimum angle of resolution (logMAR) for statistical analysis; refraction data, converted to spherical equivalents (SEs), which were calculated as the spherical dioptric power plus one-half the cylindrical dioptric power; intraocular pressure (IOP), measured using a noncontact tonometer (TX-20, Canon, Tokyo, Japan); as well as the physical examination information of slit-lamp biomicroscopy and OCT examination.
The inclusion criteria of this study were SEs difference, defined as a difference in SEs between the right and left eyes of the same subject within −2.5 to +2.5 dioptres [D]; SEs in both eyes within −4 to +3 D; BCVA ≥ 20/25 in both eyes; no history of refractive surgery; IOP of 12–21 mmHg; no clinical signs or history of intraocular disease; and no staphyloma or choroidal excavation on any OCT image.
2.2. OCT Protocol
All OCT images were obtained through a dilated pupil using a high-definition 5-line raster scan protocol (length 6 mm, spacing 0.075 mm; Cirrus HD-OCT, Carl Zeiss Meditec, Dublin, CA, USA). In each subject, this protocol was applied horizontally and centred on the fovea. The OCT images that passed through the central fovea showing the steepest foveal excavation and the thinnest highly reflective Henle fibre layer were selected for measurement of ONL thickness [12]. Considering the potential diurnal variations in retinal layer thickness [16], we acquired all OCT images between 1 : 00 pm and 5 : 00 pm and within an interval of 3 min between bilateral eyes.
2.3. OCT Image Analysis
Foveal ONL thickness was defined as the distances between the internal limiting membrane (ILM) and the external limiting membrane (ELM) at the centre of the fovea (Figure 1). The difference in the foveal ONL thickness was defined as the difference between the foveal ONL thickness measured from the right eye and that from the left eye in the same subject. The measurements were made manually using the supplied software (SW version 7.0.1.290, Carl Zeiss Meditec).
Figure 1.
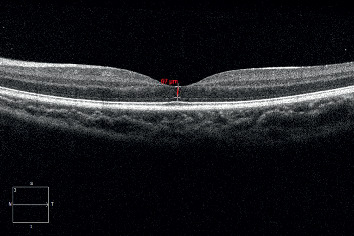
Illustration of foveal outer nuclear layer (ONL) thickness measurement in optical coherence tomography images. Foveal ONL thickness was defined as the distance between the internal limiting membrane and the external limiting membrane at the centre of the fovea.
All scans and measurements were made by Y.J. The repeatability of the measurements was calculated from two horizontal scans taken in each eye during a single visit; 22 normal eyes were included to assess the repeatability of the measurements. Both the paired t-test and intraclass correlation coefficients (ICCs) were used to assess the repeatability of the measurements (an ICC of 0.81–1.00 indicated almost perfect agreement between repeated measurements, and an ICC < 0.40 indicated poor to fair agreement) [17].
2.4. Statistical Analysis
The data were analysed using SPSS for Windows version 21.0 (SPSS, Chicago, IL, USA). The calculated values are presented as either medians (P25 and P75), means ± standard deviations (SDs), or frequencies (proportions). The Kolmogorov–Smirnov test was used to confirm the normality of the data. The generalized linear models were used to assess the associations of foveal ONL thickness (dependent variable of interest) with sex, age, and SEs (independent variables), and the associations of the difference in foveal ONL thickness between bilateral eyes (dependent variable of interest) with sex, age, and the difference in SEs between bilateral eyes (independent variables). The paired t-test was used to assess the difference in foveal ONL thickness between bilateral eyes. Either Pearson correlation coefficient or Spearman correlation coefficient was used to examine the correlation between foveal ONL thickness of the bilateral eyes. A P value < 0.05 was considered statistically significant.
3. Results
A total of 304 subjects (608 eyes) were enrolled in the study. The demographic data for the subjects and the values for their clinical characteristics are listed in Table 1. The foveal ONL thickness varied greatly among individuals, while the difference in foveal ONL thickness between bilateral eyes was small (Table 1, Figure 2). The foveal ONL thickness measurements in normal eyes showed good repeatability (t = 1.183, P=0.250, paired t-test), with an ICC of 0.990.
Table 1.
The demographic data for the subjects and the values for their clinical characteristics.
| Mean | SD | Median | Min | Max | P ∗ | |
|---|---|---|---|---|---|---|
| Age (years) | 50.43 | 16.06 | 53.00 | 20 | 80 | 0.0001 |
| Male, n (%) | 106 (34.9%) | NA | NA | NA | NA | NA |
| BCVA (logMAR) of the right eye | 0.02 | 0.04 | 0.00 | 0.00 | 0.10 | 0.0001 |
| BCVA (logMAR) of the left eye | 0.02 | 0.04 | 0.00 | 0.00 | 0.10 | 0.0001 |
| SEs of the right eye | −0.25 | 1.35 | 0.00 | −3.75 | 2.75 | 0.0001 |
| SEs of the left eye | −0.14 | 1.29 | 0.13 | −3.88 | 2.88 | 0.0001 |
| SEs difference | −0.12 | 0.63 | −0.13 | −2.38 | 1.88 | 0.0001 |
| Foveal ONL thickness in the right eyes (μm) | 103.19 | 14.25 | 101.50 | 70 | 151 | 0.177 |
| Foveal ONL thickness in the left eyes (μm) | 103.90 | 14.63 | 102.00 | 69 | 155 | 0.050 |
| Difference in foveal ONL thickness | −0.71 | 4.36 | 0.00 | −13 | 12 | 0.093 |
BCVA = best corrected visual acuity; NA = not applicable; logMAR = logarithm of the minimum angle of resolution; ONL = outer nuclear layer; SEs = spherical equivalents; SD = standard deviation; SEs difference was defined as a difference in SEs between the right and left eyes of the same subject. Difference in foveal ONL thickness was defined as the difference between the foveal ONL thickness measured from the right eye and that from the left eye in the same subject. ∗The Kolmogorov–Smirnov test was used to confirm the normality of the data. P ≥ 0.05 indicates that the data are normally distributed, and the mean value is shown in bold. P < 0.05 indicates that the data are not normally distributed, and the median value is shown in bold.
Figure 2.
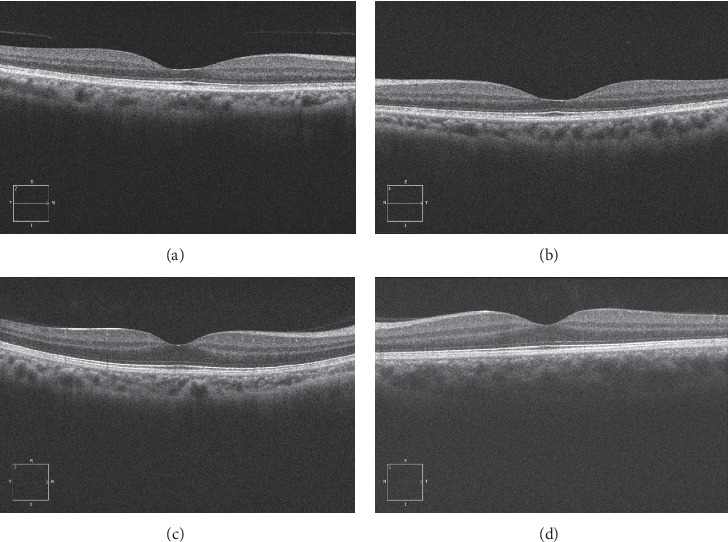
Representative images showing variation in the thickness of the foveal outer nuclear layer (ONL) among individuals in a normal Chinese population. (a, b) Horizontal line scans of the right and left eyes of a 61-year-old female subject with best corrected visual acuity (BCVA) of 20/20 in both eyes. The spherical equivalents (SEs) in the right and left eyes were +1 dioptres D and +1.25 D, respectively. The foveal ONL thicknesses in the right and left eyes were 75 and 70 μm, respectively. (c, d) Horizontal line scans of the right and left eyes of a 29-year-old male subject with BCVA of 20/20 in both eyes. The SEs in the right and left eyes were −2 D and −2.5 D, respectively. The foveal ONL thicknesses in the right and left eyes were both 134 μm.
Table 2 shows the regression coefficients (β) obtained from linear regression analyses of the associations of sex, age, and SEs (exposure variables of interest) with foveal ONL thickness (dependent variable).Men had slightly greater foveal ONL thickness values in both the right and left eyes compared with women (Table 2, both P < 0.05). However, some women had a thicker foveal ONL than that of men in either the right or left eye. In the right eye, 85 (42.9%) women had a foveal ONL thickness greater than the mean value (103.19), whereas 46 (43.4%) men had a foveal ONL thickness less than 103.19; in the left eye, 79 (39.9%) women had a foveal ONL thickness greater than the mean value (103.90), whereas 52 (49.1%) men had a foveal ONL thickness less than 103.90. Age and SEs were not associated with foveal ONL thickness in either the right or left eye (Table 2, both P > 0.05).
Table 2.
The associations of foveal outer nuclear layer thickness with clinical characteristics using generalized linear models.
| The foveal ONL thickness in the right eyes | The foveal ONL thickness in the left eyes | The difference in foveal ONL thickness between the right eye and the left eye of the same subject | |||||
|---|---|---|---|---|---|---|---|
| Beta (95% CI) | P value | Beta (95% CI) | P value | Beta (95% CI) | P value | ||
| Sex (M : F) | 4.343 (0.967, 7.719) | 0.012a | 4.520 (1.069, 7.970) | 0.010a | −0.166(−1.208, 0.877) | 0.755a | |
| Age | 0.064 (−0.039, 0.167) | 0.225a | 0.075 (−0.030, 0.181) | 0.162a | −0.010 (−0.041, 0.021) | 0.513a | |
| SEs | −0.354 (−1.581, 0.873) | 0.571a | −0.430 (−1.734, 0.875) | 0.518a | |||
| The difference in SEs between the right eye and the left eye of the same subject | −0.178 (−0.954, 0.597) | 0.653a | |||||
F = female; M = male; ONL = outer nuclear layer; SEs = spherical equivalents. Note that men had slightly greater foveal ONL thickness values in both the right and left eyes compared with women (both P < 0.05), whereas age and SEs were not associated with foveal ONL thickness in either the right or left eye (all P > 0.05). No association of sex, age, or SEs difference between bilateral eyes with the difference in foveal ONL thickness between bilateral eyes was found (P=0.755, 0.513, or 0.653, respectively) a generalized linear models.
Table 2 also shows the regression coefficients determined by linear regression analyses of the associations of sex, age, and difference in SEs between bilateral eyes (exposure variables of interest) with the difference in foveal ONL thickness between bilateral eyes (dependent variable). No association of sex, age, or SEs difference between bilateral eyes with the difference in foveal ONL thickness between bilateral eyes was found (P=0.755, 0.513, or 0.653, respectively). The foveal ONL thickness was statistically different between bilateral eyes of the same subject (t = −2.831, P=0.005, paired t-test). However, the mean difference in the foveal ONL thickness of the right eye and the left eye was only −0.71 ± 4.36 μm, and the foveal ONL thickness values of the bilateral eyes of the same subject were closely correlated (r = 0.955, P=0.0001).
4. Discussion
The study showed that in a healthy Chinese population, the foveal ONL thickness varied greatly, from 69 to 155 μm with a SD of 15 μm, while the difference in foveal ONL thickness between bilateral eyes was small (0.71 μm), ranging from −13 to +12 μm with a SD of 4 μm. The foveal ONL thickness and the difference thereof between bilateral eyes were not associated or only weakly associated with sex, age, SEs, or the SEs difference between bilateral eyes.
Pilat et al. reported a notable difference in foveal ONL thickness among ethnicities [15]. In the present study, although strictly controlled healthy Chinese subjects were enrolled, the foveal ONL thickness varied greatly with the maximum value more than double the minimum value (155 vs. 69 μm, respectively).This was probably related to the significant variation in foveal pit morphology among individuals [18–20]. Scheibe et al. evaluated the shape of the fovea in normal Caucasian subjects of European descent using OCT parameters including the foveal radius, foveal bowl area, and foveal rim height, all of which showed large differences among individuals [20]. This may partially explain the significant variation in foveal ONL thickness among the normal Chinese subjects in the present study. In contrast to the high variation among subjects, the foveal ONL thickness showed minimal differences between bilateral eyes of the same subject. This may be because the foveal region in bilateral eyes has good symmetry: foveal radius, foveal bowl area, foveal pit depth, and maximum slope, all of which exhibited high correlations between the right and left eyes of the same subject [18, 20].
It has been reported that the foveal cone density is highly variable among individuals, whereas the foveal cone densities of bilateral eyes are very similar [21–25].These findings may also partially explain the inter- and intra-individual variability in foveal ONL thickness in the present study.
Our study showed that foveal ONL thickness was associated with sex but not with age or SEs (Table 2). No previous study has examined these correlations using manual measurements of foveal ONL thickness. However, it has been reported that the minimum central retinal thickness (the distance between the ILM and retinal pigment epithelium at the foveal dip) is greater in men than women [26]. This finding was comparable to that of the current study. Although sex was associated with foveal ONL thickness, some women had a thicker foveal ONL than that of men (85/198 vs. 46/106 in the right eye; and 79/198 vs. 52/106 in the left eye). This finding suggested that only adjusting for sex in the comparison of foveal ONL thickness among individuals may be insufficient.
In addition, the ONL thickness within 1 mm region in early treatment diabetic retinopathy study circles by automated segmentations was not related to either age or SEs [27–30].These findings are similar to those of the current study. The present findings revealed a large variation in foveal ONL thickness in a normal Chinese population, and this variation was not associated with age or SEs. It suggested that the foveal ONL thickness cannot be adjusted by age or SEs when compared among individuals. Until the predictors of foveal ONL thickness are identified, directly comparing foveal ONL thickness values in the affected eyes among individuals could introduce bias or errors, especially in those clinical studies designed to detect only a very small degree of change in foveal ONL thickness.
In contrast, the difference in foveal ONL thickness between bilateral eyes was minimal (−0.71 ± 4.36 μm) and was not associated with sex, age, or SEs difference. Although the foveal ONL thickness values of the bilateral eyes were statistically different (t = −2.831, P=0.005, paired t-test), they were closely correlated (r = 0.955, P=0.0001). This indicated that the foveal ONL thickness values in bilateral eyes of the same subject were almost the same, regardless of sex, age, or small SEs differences between bilateral eyes. Thus, in diseases involving only one eye, such as retinal detachment, and central serous chorioretinopathy (CSC), the normal fellow eye could serve as a reliable control; the difference or ratio of foveal ONL thickness between the affected eye and normal fellow eye may reflect the actual degree of the disease, rather than the foveal ONL thickness in the affected eye alone.
The normal ONL contains rod and cone photoreceptor nuclei throughout the retina with the exception of a rod-free zone approximately 350 μm in diameter, extending 100 to 200 μm from the foveal centre, in which only cones are present [21, 22]. A reduction in ONL thickness is considered to be caused by photoreceptor cell death [2–6]. Therefore, the ONL thickness can provide an indirect measure for the photoreceptor survival both to study the natural cause of degenerative retinal diseases and therapeutic effects of potential treatments. Henle fibre layer (HFL) consists of the photoreceptor axons, and Müller cell processes that are substantial in the human macula [31]. Several studies have reported that the HFL is routinely included in manual and automated segmentations of the apparent ONL, thus resulting in an artificially thick assessment of the true ONL thickness in spectral domain OCT images [32–38]. However, at the centre of the fovea, the Henle fibre layer is the thinnest, comprising less than 10.7% of the measured thickness [32]. Compared with using directional OCT followed by analyses in custom software [32], which can provide accurate ONL measurements, manual measurement of the ONL thickness at the centre of the fovea seems to be a simple and relatively accurate method in studies using spectral domain OCT, especially under pathological conditions such as retinal detachment and CSC.
Our study has several limitations: (1) axial length was not measured. As the range in SEs was small and the BCVA values were normal, the axial length may not have significantly affected the results; (2) only eyes with low refractive errors were included; (3) visual function tests were incomplete, including visual field test and colour vision test; and (4) the sample size was relatively small. Further studies that include axial length, complete visual function tests, and a larger sample size with greater refractive errors could provide more information.
5. Conclusion
In a normal Chinese population, the foveal ONL thickness varied greatly, while the difference in foveal ONL thickness between bilateral eyes was small. These two values could not be adjusted by sex, age, SEs, or the SEs difference between bilateral eyes. Thus, in those diseases involving only one eye, the difference or ratio of foveal ONL thickness between the affected eye and normal fellow eye may reflect the actual degree of the disease, rather than the foveal ONL thickness value of the affected eye alone.
Acknowledgments
This work was supported by the Science and Technology Commission of Shanghai Municipality (grant no. 18411965100), the National Key Research & Development Plan (grant no. 2017YFC0108200), and the National Natural Science Foundation of China (grant no. 81900867).
Abbreviations
- BCVA:
Best corrected visual acuity
- CSC:
Central serous chorioretinopathy
- ELM:
External limiting membrane
- ICCs:
Intraclass correlation coefficients
- ILM:
Internal limiting membrane
- IOP:
Intraocular pressure
- logMAR:
Logarithm of the minimum angle of resolution
- OCT:
Optical coherence tomography
- ONL:
Outer nuclear layer
- SDs:
Standard deviations
- SEs:
Spherical equivalents.
Data Availability
The data used to support the findings of this study are restricted by the Ethics Committee of Eye and Ear Nose Throat Hospital Fudan University in order to protect patient privacy. Data are available from Qing Chang (qngchang@aliyun.com) for researchers who meet the criteria for access to confidential data.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Hogan MJ., Alvarado J., Weddell J. Histology of the Human Eye. Philadelphia, PA, USA: W.B. Saunders; 1971. pp. 444–457. [Google Scholar]
- 2.Sipperley J. O., Quigley H. A., Gass D. M. Traumatic retinopathy in primates. Archives of Ophthalmology. 1978;96(12):2267–2273. doi: 10.1001/archopht.1978.03910060563021. [DOI] [PubMed] [Google Scholar]
- 3.Lewis G. P., Charteris D. G., Sethi C. S., Fisher S. K. Animal models of retinal detachment and reattachment: identifying cellular events that may affect visual recovery. Eye. 2002;16(4):375–387. doi: 10.1038/sj.eye.6700202. [DOI] [PubMed] [Google Scholar]
- 4.Cook B., Lewis G. P., Fisher S. K., Adler R. Apoptotic photoreceptor degeneration in experimental retinal detachment. Investigative Ophthalmology & Visual Science. 1995;36(36):990–996. [PubMed] [Google Scholar]
- 5.Hisatomi T., Sakamoto T., Goto Y., et al. Critical role of photoreceptor apoptosis in functional damage after retinal detachment. Current Eye Research. 2002;24(3):161–172. doi: 10.1076/ceyr.24.3.161.8305. [DOI] [PubMed] [Google Scholar]
- 6.Arroyo J. G., Yang L., Bula D., Chen D. F. Photoreceptor apoptosis in human retinal detachment. American Journal of Ophthalmology. 2005;139(4):605–610. doi: 10.1016/j.ajo.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 7.Gharbiya M., Grandinetti F., Scavella V., et al. Correlation between spectral-domain optical coherence tomography findings and visual outcome after primary rhegmatogenous retinal detachment repair. Retina. 2012;32(1):43–53. doi: 10.1097/iae.0b013e3182180114. [DOI] [PubMed] [Google Scholar]
- 8.Kim J. H., Park D. Y., Ha H. S., Kang S. W. Topographic changes of retinal layers after resolution of acute retinal detachment. Investigative Opthalmology & Visual Science. 2012;53(11):7316–7321. doi: 10.1167/iovs.12-10155. [DOI] [PubMed] [Google Scholar]
- 9.Chen H., Lu Y., Huang H., Zheng J., Hou P., Chen W. Prediction of visual prognosis with spectral-domain optical coherence tomography in outer retinal atrophy secondary to closed globe trauma. Retina. 2013;33(6):1258–1262. doi: 10.1097/iae.0b013e31827b63ba. [DOI] [PubMed] [Google Scholar]
- 10.Pappuru R. R., Ouyang Y., Nittala M. G., et al. Relationship between outer retinal thickness substructures and visual acuity in eyes with dry age-related macular degeneration. Investigative Opthalmology & Visual Science. 2011;52(9):6743–6748. doi: 10.1167/iovs.10-6723. [DOI] [PubMed] [Google Scholar]
- 11.Maruko I., Iida T., Sekiryu T., Saito M. Morphologic changes in the outer layer of the detached retina in rhegmatogenous retinal detachment and central serous chorioretinopathy. American Journal of Ophthalmology. 2009;147(3):489–494. doi: 10.1016/j.ajo.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto H., Sato T., Kishi S. Outer nuclear layer thickness at the fovea determines visual outcomes in resolved central serous chorioretinopathy. American Journal of Ophthalmology. 2009;148(1):105–110. doi: 10.1016/j.ajo.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Hata M., Oishi A., Shimozono M., Mandai M., Nishida A., Kurimoto Y. Early changes in foveal thickness in eyes with central serous chorioretinopathy. Retina. 2013;33(2):296–301. doi: 10.1097/iae.0b013e31826710a0. [DOI] [PubMed] [Google Scholar]
- 14.Ohkuma Y., Hayashi T., Sakai T., Watanabe A., Tsuneoka H. One-year results of reduced fluence photodynamic therapy for central serous chorioretinopathy: the outer nuclear layer thickness is associated with visual prognosis. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2013;251(8):1909–1917. doi: 10.1007/s00417-013-2289-4. [DOI] [PubMed] [Google Scholar]
- 15.Pilat A. V., Proudlock F. A., Mohammad S., Gottlob I. Normal macular structure measured with optical coherence tomography across ethnicity. British Journal of Ophthalmology. 2014;98(7):941–945. doi: 10.1136/bjophthalmol-2013-303119. [DOI] [PubMed] [Google Scholar]
- 16.Jo Y.-J., Heo D.-W., Shin Y.-I., Kim J.-Y. Diurnal variation of retina thickness measured with time domain and spectral domain optical coherence tomography in healthy subjects. Investigative Opthalmology & Visual Science. 2011;52(9):6497–6500. doi: 10.1167/iovs.11-7403. [DOI] [PubMed] [Google Scholar]
- 17.Yu J., Jiang C., Wang X., et al. Macular perfusion in healthy Chinese: an optical coherence tomography angiogram study. Investigative Opthalmology & Visual Science. 2015;56(5):3212–3217. doi: 10.1167/iovs.14-16270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner-Schuman M., Dubis A. M., Nordgren R. N., et al. Race- and sex-related differences in retinal thickness and foveal pit morphology. Investigative Opthalmology & Visual Science. 2011;52(1):625–634. doi: 10.1167/iovs.10-5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubis A. M., Hansen B. R., Cooper R. F., Beringer J., Dubra A., Carroll J. Relationship between the foveal avascular zone and foveal pit morphology. Investigative Opthalmology & Visual Science. 2012;53(3):1628–1636. doi: 10.1167/iovs.11-8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheibe P., Zocher M. T., Francke M., Rauscher F. G. Analysis of foveal characteristics and their asymmetries in the normal population. Experimental Eye Research. 2016;148:1–11. doi: 10.1016/j.exer.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Curcio C. A., Sloan K. R., Kalina R. E., Hendrickson A. E. Human photoreceptor topography. The Journal of Comparative Neurology. 1990;292(4):497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- 22.Li K. Y., Tiruveedhula P., Roorda A. Intersubject variability of foveal cone photoreceptor density in relation to eye length. Investigative Opthalmology & Visual Science. 2010;51(12):6858–6867. doi: 10.1167/iovs.10-5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menghini M., Lujan B. J., Zayit-Soudry S., et al. Correlation of outer nuclear layer thickness with cone density values in patients with retinitis pigmentosa and healthy subjects. Investigative Ophthalmology & Visual Science. 2014;56(1):372–381. doi: 10.1167/iovs.14-15521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang T., Godara P., Blanco E. R., et al. Variability in human cone topography assessed by adaptive optics scanning laser ophthalmoscopy. American Journal of Ophthalmology. 2015;160(2):290–300. doi: 10.1016/j.ajo.2015.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilk M. A., Wilk B. M., Langlo C. S., Cooper R. F., Carroll J. Evaluating outer segment length as a surrogate measure of peak foveal cone density. Vision Research. 2017;130:57–66. doi: 10.1016/j.visres.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bafiq R., Mathew R., Pearce E., et al. Age, sex, and ethnic variations in inner and outer retinal and choroidal thickness on spectral-domain optical coherence tomography. American Journal of Ophthalmology. 2015;160(5):1034–1043. doi: 10.1016/j.ajo.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 27.Won J. Y., Kim S. E., Park Y. H. Effect of age and sex on retinal layer thickness and volume in normal eyes. Medicine (Baltimore) 2016;95 doi: 10.1097/md.0000000000005441.e5441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdolrahimzadeh S., Parisi F., Scavella V., Recupero S. M. Optical coherence tomography evidence on the correlation of choroidal thickness and age with vascularized retinal layers in normal eyes. Retina. 2016;36(12):2329–2338. doi: 10.1097/iae.0000000000001097. [DOI] [PubMed] [Google Scholar]
- 29.Liu X., Shen M., Yuan Y., et al. Macular thickness profiles of intraretinal layers in myopia evaluated by ultrahigh-resolution optical coherence tomography. American Journal of Ophthalmology. 2015;160(1):53–61. doi: 10.1016/j.ajo.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Kamal Abdellatif M., Abdelmaguid Mohamed Elzankalony Y., Abdelmonsef Abdelhamid Ebeid A., Mohamed Ebeid W. Outer retinal layers’ thickness changes in relation to age and choroidal thickness in normal eyes. J Ophthalmol. 2019;2019:p. 1698967. doi: 10.1155/2019/1698967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curcio C. A., Messinger J. D., Sloan K. R., Mitra A., McGwin G., Spaide R. F. Human chorioretinal layer thicknesses measured in macula-wide, high-resolution histologic sections. Investigative Opthalmology & Visual Science. 2011;52(7):3943–3954. doi: 10.1167/iovs.10-6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lujan B. J., Roorda A., Croskrey J. A., et al. Directional optical coherence tomography provides accurate outer nuclear layer and Henle fiber layer measurements. Retina. 2015;35(8):1511–1520. doi: 10.1097/iae.0000000000000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ooto S., Hangai M., Tomidokoro A., et al. Effects of age, sex, and axial length on the three-dimensional profile of normal macular layer structures. Investigative Opthalmology & Visual Science. 2011;52(12):8769–8779. doi: 10.1167/iovs.11-8388. [DOI] [PubMed] [Google Scholar]
- 34.Srinivasan V. J., Monson B. K., Wojtkowski M., et al. Characterization of outer retinal morphology with high-speed, ultrahigh-resolution optical coherence tomography. Investigative Opthalmology & Visual Science. 2008;49(4):1571–1579. doi: 10.1167/iovs.07-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demirkaya N., van Dijk H. W., van Schuppen S. M., et al. Effect of age on individual retinal layer thickness in normal eyes as measured with spectral-domain optical coherence tomography. Investigative Opthalmology & Visual Science. 2013;54(7):4934–4940. doi: 10.1167/iovs.13-11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yanni S. E., Wang J., Cheng C. S., et al. Normative reference ranges for the retinal nerve fiber layer, macula, and retinal layer thicknesses in children. American Journal of Ophthalmology. 2013;155(2):354–360. doi: 10.1016/j.ajo.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bagci A. M., Shahidi M., Ansari R., Blair M., Blair N. P., Zelkha R. Thickness profiles of retinal layers by optical coherence tomography image segmentation. American Journal of Ophthalmology. 2008;146(5):679–687. doi: 10.1016/j.ajo.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee D. J., Woertz E. N., Visotcky A., et al. The Henle fiber layer in albinism: comparison to normal and relationship to outer nuclear layer thickness and foveal cone density. Investigative Opthalmology & Visual Science. 2018;59(13):5336–5348. doi: 10.1167/iovs.18-24145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are restricted by the Ethics Committee of Eye and Ear Nose Throat Hospital Fudan University in order to protect patient privacy. Data are available from Qing Chang (qngchang@aliyun.com) for researchers who meet the criteria for access to confidential data.


