Abstract
Hippocampal atrophy and abnormal β‐Amyloid (Aβ) deposition are established markers of Alzheimer's disease (AD). Nonetheless, longitudinal trajectory of Aβ‐associated hippocampal subfield atrophy prior to dementia remains unclear. We hypothesized that elevated Aβ correlated with longitudinal subfield atrophy selectively in no cognitive impairment (NCI), spreading to other subfields in mild cognitive impairment (MCI). We analyzed data from two independent longitudinal cohorts of nondemented elderly, including global PET‐Aβ in AD‐vulnerable cortical regions and longitudinal subfield volumes quantified with a novel auto‐segmentation method (FreeSurfer v.6.0). Moreover, we investigated associations of Aβ‐related progressive subfield atrophy with memory decline. Across both datasets, we found a converging pattern that higher Aβ correlated with faster CA1 volume decline in NCI. This pattern spread to other hippocampal subfields in MCI group, correlating with memory decline. Our results for the first time suggest a longitudinal focal‐to‐widespread trajectory of Aβ‐associated hippocampal subfield atrophy over disease progression in nondemented elderly.
Keywords: hippocampal subfield atrophy, longitudinal atrophy trajectory, nondemented elderly, β‐Amyloid
1. INTRODUCTION
Hippocampal atrophy and abnormal β‐Amyloid (Aβ) are characteristic of Alzheimer's disease (AD; Barnes et al., 2009; Jack Jr. et al., 2013). Evidence from animal models suggest that Aβ burden may affect hippocampal neurodegeneration via synaptic or neuronal dysfunctions (Busche et al., 2012; Busche & Konnerth, 2015; Masters, Cappai, Barnham, & Villemagne, 2006; Perez‐Cruz et al., 2011). Indeed, greater PET‐Aβ burden was associated with smaller volume cross‐sectionally (Chetelat et al., 2013; Hanseeuw, Dricot, Lhommel, Quenon, & Ivanoiu, 2016; Mormino et al., 2009) and faster volume decline longitudinally in the whole hippocampus (Villemagne et al., 2013) in nondemented elderly. Nevertheless, lack of association between PET‐Aβ and whole hippocampal atrophy was also found in cognitively normal elderly (Becker et al., 2011). Given Aβ‐associated hippocampal atrophy in nondemented elderly but not in patients with AD‐dementia (Chetelat et al., 2010), it may be implied that Aβ affects hippocampal degeneration more robustly in early disease stage while other pathologies contribute more in dementia stage. This is in line with the AD Pathological Cascade model (Jack Jr. et al., 2013) proposing that Aβ accumulation precedes hippocampal atrophy and both reach a plateau at dementia or earlier. Nonetheless, the longitudinal trajectory of Aβ‐associated hippocampal atrophy in predementia remains unclear.
Notably, the hippocampus consists of several subfields (Bakker, Kirwan, Miller, & Stark, 2008; Small, Schobel, Buxton, Witter, & Barnes, 2011). A review concluded selective subfield atrophy before dementia, more consistently in the CA1/subiculum (de Flores, La Joie, & Chetelat, 2015). Few studies tested Aβ‐associated subfield atrophy in nondemented elderly, showing smaller subiculum/hippocampal‐tail/presubiculum in Aβ‐positive no cognitive impairment (NCI; vs. Aβ‐negative NCI) (Hsu et al., 2015), and smaller CA1/subiculum in Aβ‐positive mild cognitive impairment (MCI) compared with NCI (but no difference compared with Aβ‐negative MCI) (La Joie et al., 2013). These findings suggest selective hippocampal subfield atrophy related to Aβ in predementia.
However, previous studies relied on manual segmentation (La Joie et al., 2013) or earlier in‐vivo atlas‐based FreeSurfer approach (Hsu et al., 2015), leading to hippocampal segmentation inaccuracy (Iglesias et al., 2015; Wisse, Biessels, & Geerlings, 2014). Moreover, cross‐sectional design and dichotomized Aβ‐status prevented them from testing longitudinal trajectories of Aβ‐associated subfield atrophy accurately.
Therefore, we aimed to investigate how Aβ influence hippocampal‐subfield atrophy in NCI/MCI longitudinally in two independent datasets, applying a novel ex‐vivo ultra‐high‐resolution atlas‐based automatic hippocampal segmentation (FreeSurfer v.6.0). We hypothesized Aβ‐associated longitudinal atrophy in selective subfields (e.g., CA1/subiculum), spreading to other subfields over disease deterioration in nondemented elderly. Furthermore, we tested whether Aβ‐associated progressive subfield atrophy correlated with memory decline.
2. METHODS
2.1. Participants
This study recruited participants from two independent datasets: (a) 75 NCI and 104 MCI who were new patients from the Alzheimer's Disease Neuroimaging Initiative (ADNI2) study as defined on July 10, 2018 and (b) 20 NCI and 93 MCI from Memory Aging and Cognition Centre (MACC) dataset recruited from memory clinics/community in Singapore (Chong et al., 2017). ADNI data were obtained from the ADNI database (http://adni.loni.usc.edu/). ADNI is a public‐private partnership (PI: Dr. Michael W. Weiner), testing whether MRI, PET, clinical/neuropsychological assessment, and other biological markers can be combined to measure the progression of MCI and early AD.
For the MACC dataset, weekly consensus meetings were organized between neurologists, psychologists, and research personnel to make diagnosis based on clinical observations, CT and/or MRI scans, and laboratory tests. Diagnosis of MCI was made if patients had subjective cognitive complaint and objective impairment in at least one domain on the locally validated neuropsychological assessment battery (see Section 2.2), but remained functionally independent and not demented. For MACC participants, exclusion criteria included: (a) comorbidity with neuropsychiatric disorders (e.g., bipolar disorder) or epilepsy associated with cognitive impairment; (b) disorders that may influence the central nervous system (e.g., current or past substance abuse disorder, nutritional, toxic, or traumatic disorder) in accordance with the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV); (c) central nervous system infections, such as bacterium, Creutzfield–Jacob disease, viral encephalitis, fungi, syphilis, tuberculosis, or rickettsiae); (d) hypo‐/hyper‐tensive, hypoxic/anoxic, uremic, or hepatic encephalopathy; (e) intracerebral hemorrhage possibly leading to impaired cognition; (f) space occupying intracranial lesion (e.g., tumor); (g) inflammatory vasculitides, cranial arteritis, or moyamoya disease in the central nervous system; (h) normal or obstructive pressure hydrocephalus; (i) cortical infarct leading to surface model reconstruction errors. Moreover, the following exclusion criteria were applied for both datasets, including (a) MRI‐incompatibilities (e.g., mental implants, pregnancy); (b) surface model reconstruction errors for structural images (see MRI preprocessing); (c) NCI participants with a score of <26 on the Mini‐Mental State Examination (MMSE).
After image quality control (see Section 2.4), 52 NCI and 77 MCI from the ADNI were included in the final cross‐sectional and longitudinal analyses with annual follow‐ups up to 4‐years (Table 1). For the MACC dataset, 15 NCI and 76 MCI were included in the cross‐sectional analyses. A subset of 12 NCI and 45 MCI who had two MRI scans were selected for the longitudinal analyses (Table 1; see Section 2.3 for MACC selection note). There was no difference in follow‐up time between NCI and MCI for both datasets, although the follow‐up time was in general shorter in the MACC dataset compared with the ADNI dataset (Table 1).
Table 1.
Demographic and neuropsychological features of participants included in the cross‐sectional analysis of the ADNI dataset and MACC dataset
| ADNI dataset | MACC dataset | |||||||
|---|---|---|---|---|---|---|---|---|
| NCI | MCI | NCI | MCI | |||||
| (n = 52) | (n = 77) | t/χ 2 | p | (n = 15) | (n = 76) | t/χ 2 | p | |
| Age, M(SD)f | 73.7 (6.7) | 68.6 (6.5) | 4.40 | <.001* | 73.2 (7.4) | 76.0 (5.9) | 1.59 | .12 |
| Male/female | 24/28 | 35/42 | 0.006 | .94 | 8/7 | 37/39 | 0.11 | .74 |
| Handedness, R/Lf | 46/6 | 68/9 | 0.001 | .98 | 15/0 | 74/2 | 0.40 | .53 |
| Ethnicity, H,L/non‐H,L or C/non‐C | 2/50 | 0/77 | 3.01 | .08 | 15/0 | 65/11 | 2.47 | .12 |
| Education, M (SD)e , f | 16.9 (2.4) | 16.5 (2.6) | 0.85 | .40 | 8.0 (4.8) | 8.6 (4.7) | 0.41 | .68 |
| CDR‐SOB, M (SD)a , f | 0.02 (0.1) | 1.3 (0.9) | 12.16 | <.001* | 0.0 (0.0) | 0.8 (0.9) | 7.39 | <.001* |
| MMSE, M (SD)a , f | 29.3 (1.1) | 28.3 (1.6) | 4.37 | <.001* | 28.6 (1.2) | 24.4 (3.6) | 7.17 | .001* |
| Aβ burden, M (SD)b | 0.11 (0.4) | 0.24 (0.4) | 1.61 | .11 | 0.12 (0.3) | 0.40 (0.5) | 2.88 | .007* |
| Aβ burden, rangeb | −0.4~1.1 | −0.4~1.1 | ‐ | ‐ | −0.2~0.9 | −0.2~1.6 | ‐ | ‐ |
| WMH burden, M (SD)b | 0.94 (1.3) | 0.81 (1.1) | 0.61 | .55 | 1.6 (2.3) | 1.8 (1.8) | 0.34 | .73 |
| Dementia convertor, no. (%) | 0 (0) | 17 (22.1) | ‐ | ‐ | 0 (0) | 3 (4.0) | ‐ | ‐ |
| Remitter, no.c (%) | 0 (0) | 0 (0) | ‐ | ‐ | 0 (0) | 1 (1.3) | ‐ | ‐ |
| Follow‐up months, M (SD)d | 48.4 (6.9) | 46.3 (8.2) | 1.58 | .118 | 23.3 (1.8) | 23.7 (2.0) | 0.59 | .556 |
Note: All NCI included in the present study had a MMSE score of ≥26. Groups were compared within each dataset or between datasets on the listed variables, using independent‐samples T test or chi‐square tests where appropriate, with a threshold of p < .05 (*, two‐tailed). We did not compare Aβ, WMH, and ethnicity between datasets due to difference in radiotracer, WMH quantification method and recruited population, respectively.
Abbreviations: C/non‐C, Chinese/non‐Chinese; CDR‐SOB, Clinical Dementia Rating Sum of Boxes score; H,L/non‐H,L, Hispanic or Latino/non‐Hispanic or Latino; L, left; M, mean; MCI, mild cognitive impairment; MMSE, Mini‐Mental State Examination; N, number; NCI, no cognitive impairment; R, right; SD, standard deviation; WMH, white matter hyperintensity.
Six NCI did not have MMSE and CDR‐SOB scores, and CDR‐SOB score was not available for two MCI from the MACC dataset.
Aβ and WMH represented log‐transformed SUVR score with PVC and log‐transformed WMH volume respectively.
This participant remitted from dementia (baseline) to NCI (Year 2), and then deteriorated to MCI during subsequent PET scanning, based on which we included this participant in cross‐sectional analyses but excluded from the longitudinal investigation.
For MACC dataset, a subset of 12 NCI and 45 MCI were included in the longitudinal analyses.
We compared the same group between datasets, with significance being indicated for NCI.
We compared the same group between datasets, with significance being indicated for MCI.
This study was conducted in line with the Helsinki Declaration, and the MACC study was approved by the National Healthcare Group Domain‐Specific Review Board and SingHealth Institutional Review Board. All participants have provided written consent.
2.2. Neuropsychological assessments
In both datasets, the MMSE and Clinical Dementia Rating (CDR) were performed. Regarding the MACC dataset, a locally validated neuropsychological assessment battery was administered to all participants, including two memory domains (verbal and visual memory), and five nonmemory domains (language, attention, executive function, visuoconstruction, and visuomotor speed) following our previous work (Ji et al., 2017; Vipin et al., 2018). Standardized z‐score for each domain was calculated following previous publication (Xu et al., 2015). A memory z‐score was calculated by averaging the z‐scores between the two memory domains for MACC. For ADNI, we used the ADNI memory z‐score as validated by previous work (Crane et al., 2012).
2.3. Image acquisition
For both datasets, whole‐brain T1‐weighted structural MRI images were collected using the Magnetization prepared rapid gradient recalled echo (MPRAGE) sequence. ADNI participants were scanned in a 3T scanner (SIEMENS Magnetom Tim Trio, Skyra and Verio scanner, or PHILIPS Magnetom Achieva, Intera, and Ingenia scanner depending on the scanning site) at baseline and annual follow‐up (176 sagittal slices, voxel size = 1 × 1 × 1.2 mm3, TR/TE/TI = 2300/2.98/900 ms, FOV = 240 × 256 mm2, flip angle = 9°). MACC participants were scanned in a 3T Siemens Magnetom Tim Trio scanner (32‐channel head coil) at baseline and Year 2 (192 sagittal slices, voxel size = 1 × 1 × 1 mm3, TR/TE/TI = 2300/1.9/900 ms, FOV = 256 × 256 mm2, flip angle = 9°). The MACC MRI scanning protocol also included a Fluid attenuation inversion recover (FLAIR) image (48 axial slices, voxel size = 1 × 1 × 3 mm3, TR/TE/TI = 9000/82/2500 ms, flip angle = 180°, FOV = 232 × 256 mm2).
The PET emission protocols were as follows: (a) ADNI dataset: 370 MBq 18F‐AV‐45 injection was followed by dynamic imaging (four 5‐min frames) from 50 to 70 min postinjection, and PET scan was performed within 28 days from the baseline MRI scan. Other scanning parameters depended on the scanner used at different sites, as described on the ADNI website (ADNI, 2011); (b) MACC dataset: PET scanning was performed on a Siemens Biograph mMR scanner (Delso et al., 2011) at the Clinical Imaging Research Centre (National University of Singapore, Singapore), 370 MBq 11C‐PIB injection was followed by static data acquisition from 40 to 70 min post injection (176 axial slices, voxel size = 1 × 1 × 3 mm3, FOV = 256 × 256 mm2). For MACC dataset, PET data were acquired per participant once during the whole longitudinal project. Therefore, we selected the MRI scan closest in time to the PET scan for the cross‐sectional analysis (not necessarily baseline MRI scan, mean ± SD: 3.1 ± 7.3 months). Given the slow Aβ accumulation (Villemagne et al., 2013) and for the purpose of increasing sample size for longitudinal analyses, baseline and Year 2 MRI scans were included in the MACC longitudinal analyses irrespective of PET scan time (baseline MRI‐PET interval [mean ± SD]: 33.1 ± 14.7 months).
2.4. Data preprocessing
2.4.1. MRI preprocessing
Structural images were first checked for motion via visual inspection, and then preprocessed using the FreeSurfer pipeline (v.6.0) (Fischl et al., 2002; Fischl et al., 2004; Reuter, Schmansky, Rosas, & Fischl, 2012). The longitudinal data were further preprocessed with the standard longitudinal FreeSurfer pipeline, creating an unbiased subject‐specific template for re‐processing structural image at each time point. This longitudinal pipeline has shown to reduce within‐subject variability and improve sensitivity to detect subtle changes (Reuter, Rosas, & Fischl, 2010). Manual editing on structural images was applied where appropriate blinded to diagnoses. Participants with surface reconstruction errors that cannot be addressed by manual editing were excluded.
2.4.2. PET preprocessing
We downloaded from the ADNI website the mean aligned PET images (native space) for further preprocessing (http://adni.loni.usc.edu/methods/pet-analysis-method/pet-analysis/ n.d.). Briefly, the four 5‐min frame images were aligned to the first one and then averaged across the emission frames using the Statistical Parametric Mapping (SPM5). The MRI FreeSurfer parcellations (MRI preprocessing) were used to define the regions of interest (ROI) for subsequent calculation of standardized uptake value ratio (SUVR) from PetSurfer (Greve et al., 2016). Specifically, a high‐resolution segmentation was created based on the preprocessed structural image per participant, followed by coregistering the mean aligned PET image to the high‐resolution T1‐MPRAGE segmentation. Afterward, partial volume correction (PVC) was applied using the symmetric geometric transfer matrix, and SUVR scores were derived for each ROI from the FreeSurfer‐defined parcellation (cerebellar gray matter as the reference region). Finally, a global SUVR score was calculated by extracting means from four main cortical regions (i.e., frontal, cingulate cortex, lateral temporal, and lateral parietal) as used in previous literature (Landau & Jagust, 2015). A global SUVR score without applying PVC was also obtained.
For MACC PET data, motion correction was performed prior to tomographic reconstruction into a single static frame, using an in‐house developed rebinner with rigid motion correction, binning into 20s frames (Reilhac et al., 2018). Motion corrected images were reconstructed into a volume of 344 × 344 × 344 voxels (voxel size = 2.09 × 2.09 × 2.03 mm3) using 3D Ordinary Poisson Ordered‐subsets Expectation Maximization algorithm (Panin, Kehren, Michel, & Casey, 2006) with all corrections performed (including resolution modeling) and using three iterations and 21 subsets. Subsequently, same PetSurfer based preprocessing steps were applied as used for the ADNI dataset to obtain the global SUVR score, both with and without PVC. We used the global SUVR score for subsequent statistical analyses.
2.5. Hippocampal subfield segmentation
Based on the preprocessed structural‐images, a subject‐specific atlas based longitudinal pipeline of automated hippocampal subfield segmentation (FreeSurfer v.6.0) was used to generate more sensitive subfield volume (Iglesias et al., 2016). Since not all MACC baseline structural‐images were used for longitudinal investigation (see Section 2.3), cross‐sectional pipeline of hippocampal subfield segmentation (FreeSurfer v.6.0) (Iglesias et al., 2015) was applied for the MACC cross‐sectional analysis, which was similar to the longitudinal pipeline without creating a subject‐specific template. Importantly, both algorithms were based on a probabilistic atlas acquired from ultra‐high resolution (~0.1 mm) ex vivo MRI data, showing significantly improved segmentation accuracy compared with previous method based on in vivo atlas from the earlier FreeSurfer v.5.3, and better alignment with previous histological studies (Iglesias et al., 2015; Iglesias et al., 2016). Twelve subfields were obtained (Figure 1), and we focused on the seven gray‐matter subfields following previous studies (Ho et al., 2017), including the granule cell layer of dentate gyrus (GCL), CA1, CA2/3, CA4, molecular layer (ML), hippocampal tail, and subiculum. Bilateral volume was averaged per subfield for subsequent analyses.
Figure 1.
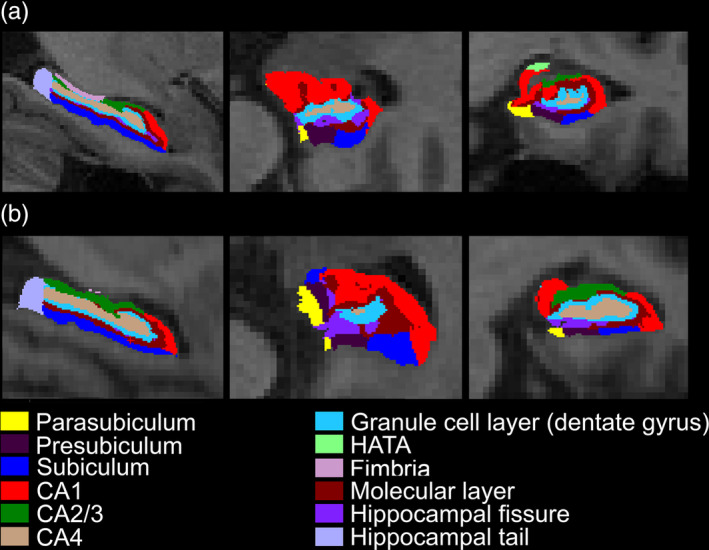
Exemplar illustration of hippocampal subfield segmentation based on FreeSurfer (v.6.0) in a representative NCI. One representative NCI was selected from (a) ADNI dataset and (b) MACC dataset each. The planes of sagittal (left), axial (middle), and coronal (right) are shown. Abbreviations: NCI, no cognitive impairment
2.6. Statistical analyses
2.6.1. Longitudinal analyses
Linear mixed model (LMM) analyses (R software, v.3.5.1) were conducted to test global Aβ‐associated progressive hippocampal atrophy in NCI and MCI separately (Model 1). To provide a reference for any observed Aβ‐associated effects, we also explored the time effects only on hippocampal subfield atrophy in groups separately (Model 2).
where Volumeij represented volume of individual subfield or whole hippocampus at time j for participant i, Aβ was indexed with the log‐transformed SUVR score, and time was the interval from baseline MRI for each time point (in month, baseline = 0). Baseline age and total intracranial volume (TIV) were added as covariates. β represented estimates for the fixed effects (i.e., Aβ, Time, Age, TIV, and the interaction), and individual intercept and slope of time were modeled as subject specific random effects such that participants could have different baseline measures and longitudinal changes (estimates denoted by b).
The MACC dataset included participants with varying cerebrovascular disease markers (e.g., cortical infarct, lacune, white matter hyperintensity [WMH], and microbleed) while ADNI dataset excluded those with significant neurologic conditions (e.g., multiple infarcts, lacunes) and had low level of WMH (Weiner et al., 2017). As such, ADNI MCI possibly had a more pure AD pathology than MACC MCI who more suffered from mixed pathology, potentially leading to different extents of atrophy progression. To test this possibility, we derived a subsample of MCI patients that were close in the WMH volume between the two datasets and repeated the analyses (Supporting Information). We did not match WMH on NCI, due to limited MACC NCI sample.
Moreover, we performed validation analyses by further controlling for age and sex, PET‐MRI interval, excluding dementia convertors (ADNI: 17 MCI converted to AD; MACC: three MCI converted to AD) and repeating analyses using global SUVR without PVC. To facilitate results comparison between the two datasets, we also repeated the analysis for the ADNI dataset with the first 2‐year follow‐up data.
For all analyses, age and TIV were included as covariates, and statistical threshold was set at p ≤ .05. We applied Holm–Bonferroni multiple comparison correction for the number of subfields (n = 7).
2.6.2. Cross‐sectional analyses
To provide more complete information, we also built separate cross‐sectional general linear regression models for each subfield and whole hippocampus in NCI and MCI groups, with global Aβ (log‐transformed SUVR) as the predictor and hippocampal volume of interest as the dependent variable.
Due to the MACC MRI scan selection (see Section 2.3), we repeated the cross‐sectional analyses in NCI and MCI separately using the baseline MRI scan. Additionally, as a sanity check, we compared hippocampal subfield volume between NCI and MCI groups cross‐sectionally.
2.6.3. Associations between progressive hippocampal subfield atrophy and memory decline
We first built LMMs to examine whether there was memory decline over time (Model 3). For subfields showing global Aβ‐associated progressive atrophy, we investigated whether their longitudinal atrophy related to memory decline, using separate LMMs (Model 4). Analyses were performed in NCI and MCI separately. We controlled for age and TIV. Threshold was set at p ≤ .05, and we applied Holm–Bonferroni multiple comparison correction for the number of subfields showing longitudinal associations with Aβ.
where Memoryij was the memory z‐score at time j for participant i, and volumeij represented individual subfield volume of interest. Denotations were the same for other parameters as stated for model 1,2.
3. RESULTS
3.1. Longitudinal trajectories of Aβ‐associated hippocampal subfield atrophy in NCI and MCI
There were significant Aβ and time interaction in ADNI‐participants (NCI: β = −1.38, p = .02; MCI: β = −4.09, p < .001) and MACC MCI group (β = −2.88, p = .05), but not in MACC NCI group (p = .13).
Regarding subfields, compared with the focal Aβ‐related longitudinal volume decline in NCI, MCI exhibited more general involvement of subfields over time. Specifically, in ADNI MCI, Aβ‐related progressive atrophy widely spread to all the subfields (ps ≤.05 after multiple comparison correction) (Figure 2, Table Se‐2). For MACC MCI, we found longitudinal atrophy in the CA1 (β = −.50, p = .014), ML (β = −.53, p = .027), and the subiculum (trend‐wise, β = −.38, p = .051; Figure 2, Table Se‐2). Additionally, WMH matched MCI subsamples showed similar patterns for both datasets, with the MACC MCI subsample showing more robust Aβ‐associated progressive atrophy in the same subfields compared with the whole MACC MCI sample, surviving multiple comparison correction (Table Se‐3).
Figure 2.
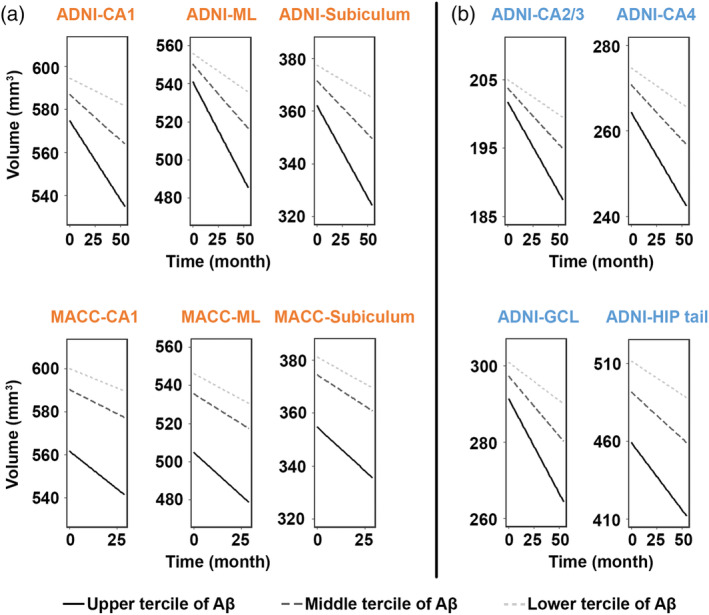
Widespread progressive hippocampal subfield atrophy over time with greater Aβ burden in MCI across datasets. In ADNI dataset, higher level of Aβ correlated to faster decline in volume in all the seven hippocampal subfields, surviving Holm–Bonferroni multiple comparison correction. Similar patterns were observed in the CA1, ML, and subiculum (trend‐wise, p = .051) for the MACC dataset. Data were divided into three approximately equal‐sized groups in terms of the log‐transformed SUVR scores, represented by the solid line (upper tercile), dark gray dotted line (middle tercile), and the light gray dotted line (lower tercile). Hippocampal subfields in orange represented overlapping patterns (a), while those in blue represented distinct patterns between the two datasets (b). Abbreviations: GCL, Granule cell layer of the dentate gyrus; HIP tail, hippocampal tail; MCI, mild cognitive impairment; ML, molecular layer
Regarding cognitively normal individuals, we found progressive volume decline in the CA1 (β = −.21, p = .028), CA4 (β = −.14, p = .024), HIP tail (β = −.34, p = .027), ML (β = −.25, p = .018), and GCL (β = −.16, p = .023) that were modulated by Aβ in ADNI NCI group (Figure 3, Table Se‐4). In MACC NCI, we replicated the findings of Aβ‐associated CA1 volume decline (β = −.55, p = .046; Figure 3, Table Se‐4). None survived multiple comparison correction.
Figure 3.
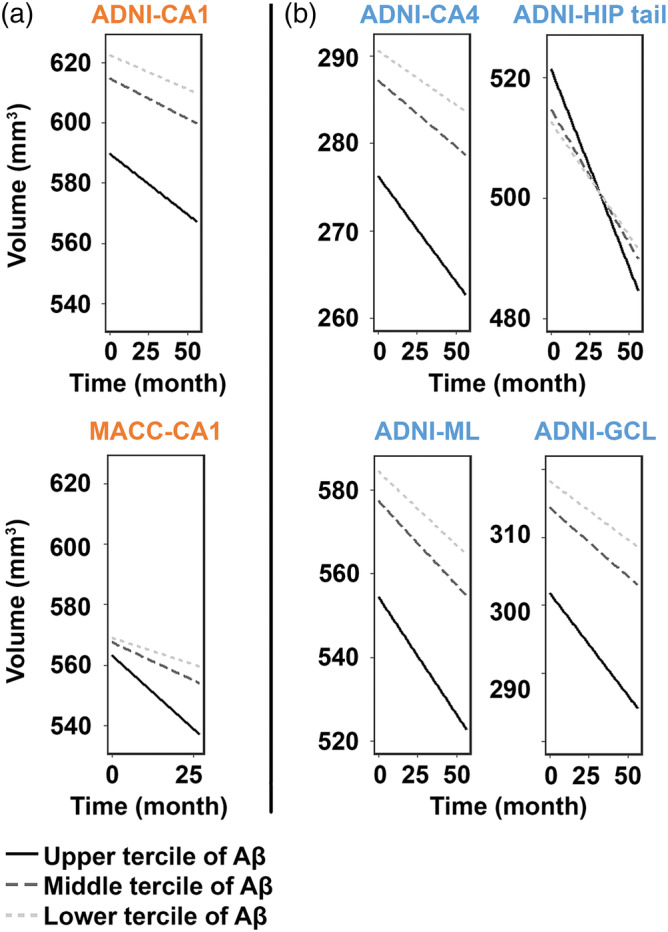
Faster volume decline in the CA1 with greater Aβ burden in NCI across datasets. Similar between the ADNI dataset and MACC dataset (denoted by orange color), higher level of Aβ was associated with faster atrophy in the CA1 (a). Differently (denoted by blue color), NCI participants in the ADNI dataset also presented faster atrophy in the CA4, HIP tail, ML, and GCL (b). Data were divided into three approximately equal‐sized groups in terms of the log‐transformed SUVR scores, represented by the solid line (upper tercile), dark gray dotted line (middle tercile), and the light gray dotted line (lower tercile). Hippocampal subfields in orange represented overlapping patterns, while those in blue represented distinct patterns between the two datasets. None survived Holm–Bonferroni multiple comparison correction. Abbreviations: GCL, granule cell layer of the dentate gyrus; HIP tail, hippocampal tail; ML, molecular layer; NCI, no cognitive impairment
As a sanity check, we tested the time effects on hippocampal subfield atrophy. Unsurprisingly, all subfields showed longitudinal atrophy irrespective of Aβ burden in NCI and MCI for both ADNI and MACC datasets (ps ≤.05 after multiple comparison correction, Table Se‐5,6).
Controlling for age and gender, PET‐MRI interval, and repeating analyses after excluding dementia convertors and using global SUVR without PVC demonstrated largely similar results (Table Se‐7,8). Due to the follow‐up time difference between ADNI and MACC, exploratory analyses with the first 2‐year follow‐up data in the ADNI dataset revealed comparable results (Table Se‐9).
Furthermore, the associations of Aβ with hippocampal subfield atrophy cross‐sectionally were rather weak, which did not survive multiple comparison correction (Figure Se‐1, 2). Also, as expected, MCI showed smaller subfield volume in all subfields than NCI cross‐sectionally for both datasets (Table Se‐10).
3.2. Progressive hippocampal subfield atrophy correlated with memory decline in MCI
We found significant memory decline in MACC‐participants (NCI: β = −.05, p = .002; MCI: β = −.03, p = .003), but not in ADNI‐participants (ps > .05). However, Aβ‐associated longitudinal subfield atrophy was related to memory deterioration in MCI for both MACC (Figure 4; ps ≤.05 for the CA1 and ML after multiple comparison correction) and ADNI (ps ≤.05 after multiple comparison correction; Table Se‐11), which was absent in NCI (ps > .05).
Figure 4.
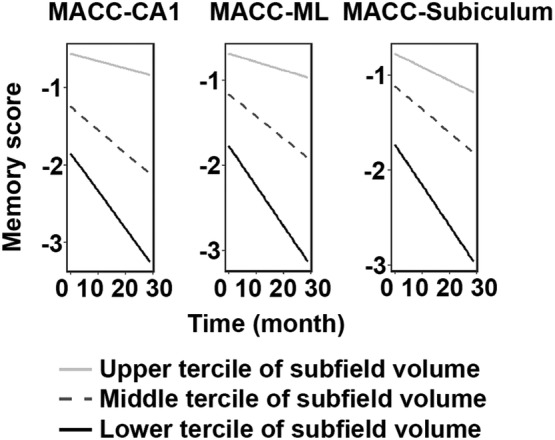
Progressive hippocampal subfield atrophy was associated with faster memory decline over time in MACC MCI. For hippocampal subfields that showed progressive atrophy in association with Aβ, faster decline in volume in these subfields correlated to faster memory decline in MCI for the MACC dataset, as well as ADNI dataset (described in text, and not shown in figure). All survived Holm–Bonferroni multiple comparison correction, except a trend‐wise effect in the subiculum (p = .07). Data were divided into three approximately equal‐sized groups in terms of the hippocampal subfields volume, represented by the light gray solid line (upper tercile), dotted line (middle tercile), and the black solid line (lower tercile). Abbreviations: MCI, mild cognitive impairment; ML, molecular layer
Correlation between global Aβ and memory decline in MCI was nonsignificant for both datasets (ps > .1). Exploratory analyses showed region‐specific associations between Aβ in regions with early deposition (i.e., posterior cingulate cortex [PCC], precuneus, and medial orbital frontal gyrus; Palmqvist et al., 2017) and memory decline over time in MCI group of both datasets (Table Se‐12). Such effects were also found between Aβ in the precuneus and medial orbital frontal gyrus and memory decline in ADNI NCI group, but not in MACC NCI group due to the small sample size (Table Se‐13).
4. DISCUSSION
This study is the first demonstration of a longitudinal focal‐to‐widespread trajectory of hippocampal subfield atrophy in association with Aβ over disease progression in nondemented elderly, replicated in two independent datasets. Compared with the subtle CA1 volume decline in NCI, MCI patients demonstrated more widespread subfield atrophy over time. Moreover, Aβ‐associated subfield atrophy rate was related to the rate of memory decline over time in MCI group. Our findings added novel knowledge on the association between Aβ and neurodegeneration, and possible mechanisms of how Aβ contributes to memory deterioration along disease progression prior to dementia.
4.1. Focal‐to‐widespread progressive hippocampal subfield atrophy over time with greater Aβ burden in nondemented elderly
Importantly, both datasets consistently showed Aβ‐associated longitudinal atrophy progression in the CA1, ML, and subiculum. The CA1 and subiculum are key regions of the hippocampal circuit, being associated with input integration and memory retrieval, respectively (Small et al., 2011). Also, smaller ML has been correlated with poorer performance in memory retrieval (Zheng et al., 2018). Altogether, it may be implied that progressive atrophy in the CA1, ML, and subiculum relates to impaired memory functioning. That volume decline in these subfields correlated with memory decline in MCI lent further support.
Moreover, more widespread Aβ‐associated atrophy progression in ADNI MCI (versus MACC MCI) was not due to its longer follow‐up (Table Se‐9, ADNI 2‐year longitudinal results), while it might be partly explained by cerebrovascular burden. Evidence has shown longitudinal hippocampal atrophy in association with baseline WMH before dementia (den Heijer et al., 2012), which has been suggested to be Aβ‐independent (Vemuri et al., 2015). Indeed, MACC MCI subsample after excluding those with more severe WMH showed Aβ‐associated volume decline in the same subfields, but to a stronger extent. Future studies need to investigate the relationship between Aβ, individual cerebrovascular markers, and hippocampal subfield atrophy. In contrast to the observed widespread volume decline in MCI, NCI showed subtle volume decline in the CA1 selectively across both datasets. Interestingly, CA1 atrophy has also been observed in other disorders (e.g., bipolar disorder (Bearden et al., 2008), and schizophrenia (Ho et al., 2017)), suggesting a general CA1 vulnerability.
Taken together, we propose that Aβ‐associated subfield atrophy progression takes the form of a focal‐to‐widespread pattern as disease progresses from NCI to MCI. The AD Pathological Cascade model (Jack Jr. et al., 2013) and Amyloid/Tau/Neurodegeneration AD biomarkers system (Jack Jr. et al., 2016) have highlighted the role of individual AD biomarkers. Our results underscore the importance of associations between individual biomarkers, potentially leading to a better understanding of the underlying disease mechanism.
4.2. Possible underlying mechanisms of Aβ modulation on the hippocampal subfield atrophy
Although the underlying neurotoxic mechanism of Aβ remains controversial, it has been suggested that Aβ induces synaptic loss and impairs neuronal function, resulting in neurodegeneration (Masters et al., 2006). Our study replicated previous findings of neocortex Aβ‐hippocampal atrophy association in nondemented elderly (Chetelat et al., 2010; Mormino et al., 2009). This could not be contributed to hippocampal Aβ deposition (Chetelat et al., 2010), which is none or low in early stage (Thal, Rub, Orantes, & Braak, 2002). Several possibilities may be proposed. Specifically, key cortical regions for earliest Aβ deposition (e.g., PCC/precuneus, orbital frontal; Palmqvist et al., 2017) could connect to hippocampus via white matter tracts (e.g., from the orbital frontal via uncinate fasciculus, and from the PCC/retrosplenial via cingulum; Greicius, Supekar, Menon, & Dougherty, 2009; Vipin et al., 2019). This implies a structural pathway where abnormal Aβ may modulate. Indeed, Aβ has been associated with longitudinal impairment of white matter integrity before dementia (Vipin et al., 2019). Also, reduced precuneus–hippocampus functional connectivity has been found in nondemented participants with positive Aβ compared with those with negative Aβ (Sheline et al., 2010), providing a potential functional pathway. Notably, we showed that regional Aβ burden in these regions was associated with memory decline especially in MCI patients (Table Se‐12). We did not find global Aβ‐memory decline association, which was in line with previous animal/human literature (i.e., association between global Aβ and cognition was generally absent or weak if any; Chetelat et al., 2012; Foley, Ammar, Lee, & Mitchell, 2015). Our findings may indicate a possible region‐specific influence of Aβ on longitudinal memory decline. Nonetheless, future work is needed to study the spatiotemporal relationship between regional Aβ, hippocampal subfield degeneration, and memory decline in longer follow‐up period. Another possibility links to soluble Aβ that precedes Aβ plaques formation, and has been suggested to affect hippocampal neurodegeneration via disturbing synaptic processing based on transgenic mice model of AD (Perez‐Cruz et al., 2011). Soluble Aβ has also been shown to induce hippocampal neuronal hyperactivity at early disease stage in transgenic AD mice, which has been hypothesized to be associated with neuronal dysfunction and cell death at a later stage (Busche et al., 2012; Busche & Konnerth, 2015).
Furthermore, given the association between tau deposition and atrophy in the whole hippocampus or hippocampal subfields (Apostolova et al., 2015), hippocampal subfield atrophy might be contributed by hippocampal tau deposition, which coincides with neocortex Aβ accumulation in early stage (Braak, Alafuzoff, Arzberger, Kretzschmar, & Del Tredici, 2006). Supporting preliminary evidence from ADNI MCI group suggested that Aβ‐associated progressive hippocampal subfield atrophy was more pronounced in MCI with p‐tau positivity, but not in MCI with p‐tau negativity (Table Se‐14). Further systematic investigation into the interactions between region‐specific tau and Aβ deposition and their impact on neurodegeration over time is needed using tau‐PET method (Hall et al., 2017).
Regarding the selective CA1 volume decline in NCI, the CA1 has more than 21 types of inhibitory interneurons (Klausberger & Somogyi, 2008), and CA1 interneurons reduction has been found in transgenic AD mice (Takahashi et al., 2010). This might lead to an excitation‐inhibition imbalance in the CA1, being associated with synaptic dysfunction and neuronal loss. The largest number of neurons (West & Gundersen, 1990) and capillaries (Lokkegaard, Nyengaard, & West, 2001) as estimated in the CA1 (vs. other subfields) might also contribute. Altogether, we propose that the CA1 atrophy represents a general and early vulnerability to AD and the atrophy spreads to other subfields over time via possible mechanisms as mentioned above. Although we could not pinpoint the exact neocortex Aβ‐hippocampal atrophy pathway(s), our result replications in two datasets provide convincing evidence for the focal‐to‐widespread pattern of Aβ‐associated subfield atrophy over time.
4.3. Limitations and future directions
There were some limitations. Firstly, MACC‐dataset had fewer NCI (n = 15) than ADNI‐dataset (n = 52). However, similar results were observed between datasets. Secondly, other variables may also contribute to hippocampal subfield atrophy and disease progression (e.g., tau pathology and cerebrovascular factors) in addition to Aβ (Apostolova et al., 2015; den Heijer et al., 2012). Future studies need to take into consideration the spatial quantification of Aβ instead of an average Aβ burden, and determine the contributing variables and the temporal relationships underlying disease progression. Finally, it would be interesting to test how Aβ affects the hippocampus along the anterior–posterior axis in predementia stages.
5. CONCLUSION
To conclude, converging results from two independent datasets showed that greater Aβ burden correlated with selective CA1 volume decline in NCI and longitudinal atrophy extension into other subfields in MCI, resulting in a focal‐to‐widespread longitudinal trajectory. Moreover, the Aβ‐associated progressive subfield atrophy correlated with memory decline in MCI, hence, potentially serving as imaging markers for disease progression monitoring.
CONFLICT OF INTEREST
Dr. John T. O'Brien reports grant from Avid/Lilly, personal fees from TauRx, Axon, Eisai, and GE Healthcare outside the submitted work. Other coauthors report no potential conflicts of interest.
Supporting information
Appendix S1: SUPPLEMENTARY MATERIALS
ACKNOWLEDGMENTS
We thank all the participants for their contributions to the study. This research was funded by the National Medical Research Council (NMRC) Centre Grant (NMRC/CG/013/2013 and NMRC/CG/NUHS/2010 to Dr. Christopher Chen), the Biomedical Research Council, Singapore (BMRC 04/1/36/372 to Dr. Juan Zhou), the National Medical Research Council, Singapore (NMRC/CBRG/0088/2015, NMRC/CIRG/1390/2014 to Dr. Juan Zhou, and NMRC/CIRG/1446/2016 to Dr. Christopher Chen), and Duke‐NUS Medical School Signature Research Program funded by Ministry of Health, Singapore. Dr. John T. O'Brien is supported by the NIHR Cambridge Biomedical Research Centre. The present study also received support from the Cambridge‐NUHS Seed Funding (NUHSRO/2017/014/Cambridge/01) awarded to Dr. Christopher Chen and Dr. John T. O'Brien jointly.
Moreover, genuine acknowledgements should go to all researchers who involves in the data collection and sharing from the Alzheimer's Disease Neuroimaging Initiative (ADNI; http://adni.loni.usc.edu/). Data collection and sharing of ADNI for this project was funded by the ADNI (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH‐12‐2‐0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol‐Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann‐La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. ADNI clinical sites in Canada is receiving funds from the Canadian Institutes of Health Research. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Zhang L, Mak E, Reilhac A, et al. Longitudinal trajectory of Amyloid‐related hippocampal subfield atrophy in nondemented elderly. Hum Brain Mapp. 2020;41:2037–2047. 10.1002/hbm.24928
Funding information Biomedical Research Council Singapore, Grant/Award Number: BMRC 04/1/36/372; Cambridge‐NUHS Seed Funding, Grant/Award Number: NUHSRO/2017/014/Cambridge/01; Duke‐NUS Medical School Signature Research Program; National Institute for Health Research Cambridge Biomedical Research Centre; Center grant, collaborative basic‐scientist individual research grant and clinical individual research grant, Grant/Award Numbers: NMRC/CBRG/0088/2015, NMRC/CG/013/2013, NMRC/CG/NUHS/2010, NMRC/CIRG/1390/2014, NMRC/CIRG/1446/2016
Contributor Information
Juan H. Zhou, Email: helen.zhou@nus.edu.sg.
Christopher L.H. Chen, Email: phccclh@nus.edu.sg.
DATA AVAILABILITY STATEMENT
De‐identified data are available upon requests from the corresponding authors.
REFERENCES
- ADNI . (2011) ADNI 2 PET Technical Procedures Manual.
- ADNI . PET Pre‐processing.
- Apostolova, L. G. , Zarow, C. , Biado, K. , Hurtz, S. , Boccardi, M. , Somme, J. , … EADC‐ADNI Working Group on the Harmonized Protocol for Manual Hippocampal Segmentation . (2015). Relationship between hippocampal atrophy and neuropathology markers: A 7T MRI validation study of the EADC‐ADNI harmonized hippocampal Segmentation protocol. Alzheimers Dement, 11, 139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker, A. , Kirwan, C. B. , Miller, M. , & Stark, C. E. (2008). Pattern separation in the human hippocampal CA3 and dentate gyrus. Science, 319, 1640–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, J. , Bartlett, J. W. , van de Pol, L. A. , Loy, C. T. , Scahill, R. I. , Frost, C. , … Fox, N. C. (2009). A meta‐analysis of hippocampal atrophy rates in Alzheimer's disease. Neurobiology of Aging, 30, 1711–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden, C. E. , Thompson, P. M. , Dutton, R. A. , Frey, B. N. , Peluso, M. A. , Nicoletti, M. , … Soares, J. C. (2008). Three‐dimensional mapping of hippocampal anatomy in unmedicated and lithium‐treated patients with bipolar disorder. Neuropsychopharmacology, 33, 1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, J. A. , Hedden, T. , Carmasin, J. , Maye, J. , Rentz, D. M. , Putcha, D. , … Johnson, K. A. (2011). Amyloid‐beta associated cortical thinning in clinically normal elderly. Annals of Neurology, 69, 1032–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak, H. , Alafuzoff, I. , Arzberger, T. , Kretzschmar, H. , & Del Tredici, K. (2006). Staging of Alzheimer disease‐associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathologica, 112, 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche, M. A. , Chen, X. , Henning, H. A. , Reichwald, J. , Staufenbiel, M. , Sakmann, B. , & Konnerth, A. (2012). Critical role of soluble amyloid‐β for early hippocampal hyperactivity in a mouse model of Alzheimer's disease. Proceedings of the National Academy of Sciences, 109, 8740–8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche, M. A. , & Konnerth, A. (2015). Neuronal hyperactivity–A key defect in Alzheimer's disease? BioEssays, 37, 624–632. [DOI] [PubMed] [Google Scholar]
- Chetelat, G. , La Joie, R. , Villain, N. , Perrotin, A. , de La Sayette, V. , Eustache, F. , & Vandenberghe, R. (2013). Amyloid imaging in cognitively normal individuals, at‐risk populations and preclinical Alzheimer's disease. NeuroImage: Clinical, 2, 356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetelat, G. , Villemagne, V. L. , Bourgeat, P. , Pike, K. E. , Jones, G. , Ames, D. , … Lifestyle Research, G. (2010). Relationship between atrophy and beta‐amyloid deposition in Alzheimer disease. Annals of Neurology, 67, 317–324. [DOI] [PubMed] [Google Scholar]
- Chetelat, G. , Villemagne, V. L. , Pike, K. E. , Ellis, K. A. , Ames, D. , Masters, C. L. , … Australian Imaging, B., Lifestyle Study of Ageing Research, G . (2012). Relationship between memory performance and beta‐amyloid deposition at different stages of Alzheimer's disease. Neurodegenerative Diseases, 10, 141–144. [DOI] [PubMed] [Google Scholar]
- Chong, J. S. X. , Liu, S. , Loke, Y. M. , Hilal, S. , Ikram, M. K. , Xu, X. , … Zhou, J. (2017). Influence of cerebrovascular disease on brain networks in prodromal and clinical Alzheimer's disease. Brain, 140, 3012–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane, P. K. , Carle, A. , Gibbons, L. E. , Insel, P. , Mackin, R. S. , Gross, A. , … Alzheimer's Disease Neuroimaging Initiative . (2012). Development and assessment of a composite score for memory in the Alzheimer's Disease Neuroimaging Initiative (ADNI). Brain Imaging and Behavior, 6, 502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Flores, R. , La Joie, R. , & Chetelat, G. (2015). Structural imaging of hippocampal subfields in healthy aging and Alzheimer's disease. Neuroscience, 309, 29–50. [DOI] [PubMed] [Google Scholar]
- Delso, G. , Furst, S. , Jakoby, B. , Ladebeck, R. , Ganter, C. , Nekolla, S. G. , … Ziegler, S. I. (2011). Performance measurements of the Siemens mMR integrated whole‐body PET/MR scanner. Journal of Nuclear Medicine, 52, 1914–1922. [DOI] [PubMed] [Google Scholar]
- den Heijer, T. , van der Lijn, F. , Ikram, A. , Koudstaal, P. J. , van der Lugt, A. , Krestin, G. P. , … Breteler, M. M. (2012). Vascular risk factors, apolipoprotein E, and hippocampal decline on magnetic resonance imaging over a 10‐year follow‐up. Alzheimers Dement, 8, 417–425. [DOI] [PubMed] [Google Scholar]
- Fischl, B. , Salat, D. H. , Busa, E. , Albert, M. , Dieterich, M. , Haselgrove, C. , … Dale, A. M. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33, 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl, B. , van der Kouwe, A. , Destrieux, C. , Halgren, E. , Segonne, F. , Salat, D. H. , … Dale, A. M. (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14, 11–22. [DOI] [PubMed] [Google Scholar]
- Foley, A. M. , Ammar, Z. M. , Lee, R. H. , & Mitchell, C. S. (2015). Systematic review of the relationship between amyloid‐beta levels and measures of transgenic mouse cognitive deficit in Alzheimer's disease. Journal of Alzheimer's Disease, 44, 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius, M. D. , Supekar, K. , Menon, V. , & Dougherty, R. F. (2009). Resting‐state functional connectivity reflects structural connectivity in the default mode network. Cerebral Cortex, 19, 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve, D. N. , Salat, D. H. , Bowen, S. L. , Izquierdo‐Garcia, D. , Schultz, A. P. , Catana, C. , … Johnson, K. A. (2016). Different partial volume correction methods lead to different conclusions: An (18)F‐FDG‐PET study of aging. NeuroImage, 132, 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, B. , Mak, E. , Cervenka, S. , Aigbirhio, F. I. , Rowe, J. B. , & O'Brien, J. T. (2017). In vivo tau PET imaging in dementia: Pathophysiology, radiotracer quantification, and a systematic review of clinical findings. Ageing Research Reviews, 36, 50–63. [DOI] [PubMed] [Google Scholar]
- Hanseeuw, B. , Dricot, L. , Lhommel, R. , Quenon, L. , & Ivanoiu, A. (2016). Patients with amyloid‐negative mild cognitive impairment have cortical Hypometabolism but the hippocampus is preserved. Journal of Alzheimer's Disease, 53, 651–660. [DOI] [PubMed] [Google Scholar]
- Ho, N. F. , Iglesias, J. E. , Sum, M. Y. , Kuswanto, C. N. , Sitoh, Y. Y. , De Souza, J. , … Holt, D. J. (2017). Progression from selective to general involvement of hippocampal subfields in schizophrenia. Molecular Psychiatry, 22, 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, P. J. , Shou, H. , Benzinger, T. , Marcus, D. , Durbin, T. , Morris, J. C. , & Sheline, Y. I. (2015). Amyloid burden in cognitively normal elderly is associated with preferential hippocampal subfield volume loss. Journal of Alzheimer's Disease, 45, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias, J. E. , Augustinack, J. C. , Nguyen, K. , Player, C. M. , Player, A. , Wright, M. , … Alzheimer's Disease Neuroimaging Initiative . (2015). A computational atlas of the hippocampal formation using ex vivo, ultra‐high resolution MRI: Application to adaptive segmentation of in vivo MRI. NeuroImage, 115, 117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias, J. E. , Van Leemput, K. , Augustinack, J. , Insausti, R. , Fischl, B. , Reuter, M. , & Alzheimer's Disease Neuroimaging Initiative . (2016). Bayesian longitudinal segmentation of hippocampal substructures in brain MRI using subject‐specific atlases. NeuroImage, 141, 542–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack, C. R., Jr. , Bennett, D. A. , Blennow, K. , Carrillo, M. C. , Feldman, H. H. , Frisoni, G. B. , … Dubois, B. (2016). A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology, 87, 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack, C. R., Jr. , Knopman, D. S. , Jagust, W. J. , Petersen, R. C. , Weiner, M. W. , Aisen, P. S. , … Trojanowski, J. Q. (2013). Tracking pathophysiological processes in Alzheimer's disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurology, 12, 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, F. , Pasternak, O. , Liu, S. , Loke, Y. M. , Choo, B. L. , Hilal, S. , … Zhou, J. (2017). Distinct white matter microstructural abnormalities and extracellular water increases relate to cognitive impairment in Alzheimer's disease with and without cerebrovascular disease. Alzheimer's Research & Therapy, 9, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger, T. , & Somogyi, P. (2008). Neuronal diversity and temporal dynamics: The unity of hippocampal circuit operations. Science, 321, 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Joie, R. , Perrotin, A. , de La Sayette, V. , Egret, S. , Doeuvre, L. , Belliard, S. , … Chetelat, G. (2013). Hippocampal subfield volumetry in mild cognitive impairment, Alzheimer's disease and semantic dementia. NeuroImage: Clinical, 3, 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau, S. , Jagust, W. (2015) Florbetapir processing methods 2014.
- Lokkegaard, A. , Nyengaard, J. R. , & West, M. J. (2001). Stereological estimates of number and length of capillaries in subdivisions of the human hippocampal region. Hippocampus, 11, 726–740. [DOI] [PubMed] [Google Scholar]
- Masters, C. L. , Cappai, R. , Barnham, K. J. , & Villemagne, V. L. (2006). Molecular mechanisms for Alzheimer's disease: Implications for neuroimaging and therapeutics. Journal of Neurochemistry, 97, 1700–1725. [DOI] [PubMed] [Google Scholar]
- Mormino, E. C. , Kluth, J. T. , Madison, C. M. , Rabinovici, G. D. , Baker, S. L. , Miller, B. L. , … Alzheimer's Disease Neuroimaging Initiative . (2009). Episodic memory loss is related to hippocampal‐mediated beta‐amyloid deposition in elderly subjects. Brain, 132, 1310–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist, S. , Scholl, M. , Strandberg, O. , Mattsson, N. , Stomrud, E. , Zetterberg, H. , … Hansson, O. (2017). Earliest accumulation of beta‐amyloid occurs within the default‐mode network and concurrently affects brain connectivity. Nature Communications, 8, 1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panin, V. Y. , Kehren, F. , Michel, C. , & Casey, M. (2006). Fully 3‐D PET reconstruction with system matrix derived from point source measurements. IEEE Transactions on Medical Imaging, 25, 907–921. [DOI] [PubMed] [Google Scholar]
- Perez‐Cruz, C. , Nolte, M. W. , van Gaalen, M. M. , Rustay, N. R. , Termont, A. , Tanghe, A. , … Ebert, U. (2011). Reduced spine density in specific regions of CA1 pyramidal neurons in two transgenic mouse models of Alzheimer's disease. The Journal of Neuroscience, 31, 3926–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilhac, A. , Merida, I. , Irace, Z. , Stephenson, M. C. , Weekes, A. A. , Chen, C. , … Costes, N. (2018). Development of a dedicated Rebinner with rigid motion correction for the mMR PET/MR scanner, and validation in a large cohort of (11)C‐PIB scans. Journal of Nuclear Medicine, 59, 1761–1767. [DOI] [PubMed] [Google Scholar]
- Reuter, M. , Rosas, H. D. , & Fischl, B. (2010). Highly accurate inverse consistent registration: A robust approach. NeuroImage, 53, 1181–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter, M. , Schmansky, N. J. , Rosas, H. D. , & Fischl, B. (2012). Within‐subject template estimation for unbiased longitudinal image analysis. NeuroImage, 61, 1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline, Y. I. , Raichle, M. E. , Snyder, A. Z. , Morris, J. C. , Head, D. , Wang, S. , & Mintun, M. A. (2010). Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biological Psychiatry, 67, 584–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small, S. A. , Schobel, S. A. , Buxton, R. B. , Witter, M. P. , & Barnes, C. A. (2011). A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nature Reviews. Neuroscience, 12, 585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, H. , Brasnjevic, I. , Rutten, B. P. , Van Der Kolk, N. , Perl, D. P. , Bouras, C. , … Dickstein, D. L. (2010). Hippocampal interneuron loss in an APP/PS1 double mutant mouse and in Alzheimer's disease. Brain Structure & Function, 214, 145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal, D. R. , Rub, U. , Orantes, M. , & Braak, H. (2002). Phases of a beta‐deposition in the human brain and its relevance for the development of AD. Neurology, 58, 1791–1800. [DOI] [PubMed] [Google Scholar]
- Vemuri, P. , Lesnick, T. G. , Przybelski, S. A. , Knopman, D. S. , Preboske, G. M. , Kantarci, K. , … Jack, C. R., Jr. (2015). Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain, 138, 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne, V. L. , Burnham, S. , Bourgeat, P. , Brown, B. , Ellis, K. A. , Salvado, O. , … Lifestyle Research, G. (2013). Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: A prospective cohort study. Lancet Neurology, 12, 357–367. [DOI] [PubMed] [Google Scholar]
- Vipin, A. , Loke, Y. M. , Liu, S. , Hilal, S. , Shim, H. Y. , Xu, X. , … Zhou, J. (2018). Cerebrovascular disease influences functional and structural network connectivity in patients with amnestic mild cognitive impairment and Alzheimer's disease. Alzheimer's Research & Therapy, 10, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vipin, A. , Ng, K. K. , Ji, F. , Shim, H. Y. , Lim, J. K. W. , Pasternak, O. , … Alzheimer's Disease Neuroimaging Initiative . (2019). Amyloid burden accelerates white matter degradation in cognitively normal elderly individuals. Human Brain Mapping, 40, 2065–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner, M. W. , Veitch, D. P. , Aisen, P. S. , Beckett, L. A. , Cairns, N. J. , Green, R. C. , … Alzheimer's Disease Neuroimaging Initiative . (2017). Recent publications from the Alzheimer's Disease Neuroimaging Initiative: Reviewing progress toward improved AD clinical trials. Alzheimers Dement, 13, e1–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, M. J. , & Gundersen, H. J. (1990). Unbiased stereological estimation of the number of neurons in the human hippocampus. The Journal of Comparative Neurology, 296, 1–22. [DOI] [PubMed] [Google Scholar]
- Wisse, L. E. , Biessels, G. J. , & Geerlings, M. I. (2014). A critical appraisal of the hippocampal subfield segmentation package in FreeSurfer. Frontiers in Aging Neuroscience, 6, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X. , Hilal, S. , Collinson, S. L. , Chong, E. J. , Ikram, M. K. , Venketasubramanian, N. , & Chen, C. L. (2015). Association of magnetic resonance imaging markers of cerebrovascular disease burden and cognition. Stroke, 46, 2808–2814. [DOI] [PubMed] [Google Scholar]
- Zheng, F. , Cui, D. , Zhang, L. , Zhang, S. , Zhao, Y. , Liu, X. , … Qiu, J. (2018). The volume of hippocampal subfields in relation to decline of memory recall across the adult lifespan. Frontiers in Aging Neuroscience, 10, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: SUPPLEMENTARY MATERIALS
Data Availability Statement
De‐identified data are available upon requests from the corresponding authors.


