Abstract
Midlife metabolic and vascular risk factors (MVRFs) predict cognitive decline and dementia; however, these risk factors tend to overlap, and the mechanisms underlying their effects on cognitive performance are not well understood. This cross‐sectional study investigates the contributions of MVRFs to regional cerebral blood flow (CBF) and verbal learning & memory among middle‐aged adults. We used partial least squares (PLS) analysis to create latent risk factor profiles and examine their associations to CBF in 93 regions of interest among 451 participants (age 50.3 ± 3.5 years) of the Coronary Artery Risk Development in Young Adults. This multivariate analysis revealed regional CBF was lower in relation to obesity (higher body mass index and waist circumference), dysregulated glucose homeostasis (higher fasting glucose, oral glucose tolerance, and higher fasting insulin), and adverse fasting lipid profile (lower high‐density lipoprotein cholesterol and higher triglycerides). In a sensitivity analysis, we found that significant associations between MVRFs and CBF were prominent in the hypertension‐medicated subgroup. In a mediation model, the PLS‐based MVRFs profile was associated with memory performance (rey auditory verbal learning test); however, CBF was not a significant mediator of this association. The results describe an adverse midlife metabolic profile that might set the stage for incipient dementia and contribute to widespread changes in CBF.
Keywords: blood pressure, body mass index, cerebral blood flow, fasting glucose, memory, partial least squares, vascular risk factors
1. INTRODUCTION
Obesity, diabetes, high blood pressure, and altered lipids are commonly comorbid metabolic and vascular risk factors (MVRFs), often conceptualized collectively as the “metabolic syndrome.” Each of these variables is associated with an increased risk of dementia. The impacts of midlife obesity and impaired glucose tolerance stretch over 25 years of follow‐up (Curb et al., 1999; Qiu & Fratiglioni, 2015; Whitmer, Gunderson, Barrett‐Connor, Quesenberry, & Yaffe, 2005). Data‐driven approaches suggest that vascular changes are among the earliest to occur in incipient Alzheimer's disease (AD) (Iturria‐Medina et al., 2016). Accordingly, trials designed to prevent or delay cognitive decline focus increasingly on managing MVRFs (Chuang et al., 2016), but the impacts of these MVRFs on the brain and cognitive decline remain incompletely understood.
MVRFs at midlife, a critical turning point in brain aging, likely contribute to endothelial dysfunction, inflammatory processes, and to morphological and functional alterations to the arteriolar walls (Joutel et al., 2010). MVRFs are thought to induce changes in neural circuits, such as the prefrontal and ventral striatal regions involved in food reward processing, self‐regulation and stress (Dunn et al., 2012; Ottino‐González et al., 2017), which could influence regional neurovascular metabolism and hence, CBF. Evidence in support of brain changes in relation to MVRFs is however mixed (Cavalieri et al., 2010). Among the MVRFs, the most is known about the effects of hypertension and diabetes on the brain. A meta‐analysis on the role of blood pressure implicates loss of brain tissue in the frontal and temporal lobes (Beauchet et al., 2013; Schneider et al., 2017). A case–control comparison of people with and without metabolic syndrome reported reduced white matter, in addition to gray matter (Sala et al., 2014). Another study reported that it is visceral adipose tissue that appears to be tied to microstructural changes in the brain (Widya et al., 2015). Typically, these MVRFs co‐occur in people, requiring precise physiological endpoints and large samples to disentangle their effects.
Longitudinal studies show that arterial stiffness and CBF are important markers in the context of vascular aging (De Vis et al., 2018)(Seals, Moreau, Gates, & Eskurza, 2006). Meanwhile, studying midlife adults is also key as it is during this period that cerebrovascular reactivity appears to change significantly (Peng et al., 2018). The current study focuses on CBF because cerebrovascular imaging remains under‐represented in studies of MVRFs (Jansen et al., 2016; Last et al., 2007; Tchistiakova, Anderson, Greenwood, & Macintosh, 2014). Among 60‐year‐old adults with and without metabolic syndrome, high waist circumference and triglycerides were associated with reduced CBF (Birdsill et al., 2013). Yet, the influences of particular components of the metabolic syndrome—obesity, dyslipidemia, dysregulated glucose homeostasis and hypertension—have yet to be adequately characterized. To examine these inter‐related MVRFs, we employ multivariate partial least squares, an analytical approach that is designed to handle collinear independent variables (McIntosh, Bookstein, Haxby, & Grady, 1996). The outcome measures in this multivariate PLS model are the CBF values from within regions of interest (ROI) in a sample of adults that are 50 years old. We hypothesize there will be at least one latent variable that relates MVRFs and CBF, and specifically, that a higher MVRF burden will be associated with lower regional CBF levels. Since this cohort has a sizable subgroup of participants with hypertension (Launer et al., 2015), we conducted a sensitivity analysis stratified by hypertension status. To provide clinical context, we tested if CBF mediated an indirect relationship between MVRF burden and memory performance, simultaneously testing a direct relationship between MVRF burden and memory (i.e., one that is not mediated by CBF).
2. METHODS
2.1. Participants
Data in this study are from a sub‐sample of black and white men and women who participated in the community‐based coronary artery risk development in young adults (CARDIA) study, examined at year‐25. CARDIA is a longitudinal study of the development and determinants of cardiovascular disease in 5115 young adults who were aged 18–30 years at baseline in 1985–1986. The community‐based sample for the current study was recruited from three US cities (Birmingham, Minneapolis, and Oakland) to be approximately balanced by sex, age (18–24 years and 25–30 years), race (white, black), and education (high school, >high school) (Friedman et al., 1988).
Between 2010 and 2011, 72% of the surviving CARDIA cohort attended the year‐25 exam assessments. Participants provided written informed consent at each exam, and institutional review boards from each field center and the coordinating center (University of Minnesota Institutional Review Board, Kaiser Permanente Northern California Institutional Review Board) approved this study annually. A sub‐sample of CARDIA participants underwent MRI scanning at year 25 to characterize brain morphology, pathology, physiology, and function. Other standard exclusions for the MRI sub‐study were applied, which included implants not safe at 3.0 T and physical body size of participants precluding comfortable positioning in the magnet bore.
2.2. Clinical assessments
Risk factor measures were obtained at the 25‐year follow‐up, and were characterized by: (a) body mass index (BMI; from height and weight) and waist circumference to index obesity; (b) diastolic and systolic blood pressure were assessed using a digital blood pressure monitor (Omron HEM‐907XL; Online Fitness, CA); (c) participants were instructed to fast and abstain from smoking or heavy physical exertion for 12 hr prior to the blood draw to measure fasting glucose and insulin levels. An oral glucose tolerance test (OGTT) was performed to measure the acute (2‐hr) blood glucose response to a high glucose drink; (d) blood samples were also analyzed for high‐density lipoprotein (HDL) and low‐density lipoprotein (LDL) cholesterol, and triglyceride concentrations. Diabetes was defined following the American Diabetes Association criteria for levels of fasting, non‐fasting, or postprandial OGTT results, HbA1c percent, or use of anti‐diabetes medication (American Diabetes Association, 2011).
2.3. Cognitive assessments
To index memory performance, we used the Rey auditory verbal learning test (RAVLT) as the primary cognitive measure. The RAVLT was tabulated as five scores from verbal learning trials, and two free recall trials, the first after a distractor list, and the second after a 30‐min delay. This test has demonstrated utility in type 2 diabetes, obesity and/or cardiovascular disease (Cournot et al., 2006; MacIntosh et al., 2015; Punthakee et al., 2012).
2.4. Brain MRI
MR imaging consisted of T1‐weighted anatomical imaging and two CBF techniques: pseudo‐continuous arterial spin labeling (ASL) was used to measure whole brain and regional CBF; after the ASL sequence, phase‐contrast angiography was performed and these data were used to provide a secondary estimate for whole brain CBF in the current study. MRI details are described previously (Launer et al., 2015). T1‐weighted images were acquired in three‐dimensions using an MPRAGE sequence, along the sagittal plane, and with the following parameters: voxel = 1 × 1 × 1 mm3, TR/TE/TI = 1900/2.9/900 ms, matrix = 256 × 256, slices = 176, FOV = 250 mm, flip angle = 90, GRAPPA = 2, and bandwidth = 170 Hz/pixel. ASL used gradient‐echo echo planar imaging with repetition time (TR) and echo time (TE) of 4 s, 11 ms, respectively. ASL was performed with a label duration of 1.48 s, offset by a fixed distance of 90 mm from the central ASL imaging volume, and post labeling delay (PLD) to the most inferior slice by 1.5 s, with a radio‐frequency pulse gap of 0.36 ms, pulse duration of 0.5 ms, and mean z‐direction gradient of 0.6 mT/m, and no background suppression. Forty label and control pairs were acquired for a 5 min and 20 s acquisition. Other imaging parameters include: voxels = 3.4 × 3.4 × 5 mm3, matrix = 64 × 64, flip angle = 90°, FOV = 220 mm, bandwidth = 3,004 Hz/pixel, echo spacing = 0.44 ms and an EPI factor = 64. Twenty slices with a distance factor of 20% were acquired from inferior to superior in a sequential order. Phase‐contrast angiography was prescribed at the level of ASL labeling plane (Dolui et al., 2016). Angiographic data were acquired at eight phases within a cardiac cycle with a maximum velocity encoding of 100 cm/s, voxels = 0.8 × 0.8 × 5.0 mm3, FOV = 20 cm, TR = 140 ms, TE = 10 ms, flip angle = 150 and bandwidth = 260 Hz/pixel. As in (Dolui et al., 2016) and as a quality control procedure, we used the phase contrast data to estimate whole brain CBF, normalized by brain volume. We compared this estimate against total CBF from ASL.
2.5. Image processing
MR images were processed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) and in‐house programs developed in MATLAB (Mathworks Inc., Natick, MA). Raw ASL label‐control time series data were first motion corrected followed by removal of residual motion (Wang, 2012). Images were smoothed using an isotropic Gaussian kernel with full‐width‐half‐max of 5 mm. Thereafter, a CBF time series was obtained by pairwise control‐label subtraction. We converted the CBF image intensity to absolute units after averaging the difference images and used the ASL control image as the estimate of the equilibrium magnetization (Alsop et al., 2014).
CBF images were effectively down sampled to participant‐specific and anatomy defined gray matter ROI, based probabilistic segmentation of T1 images using SPM8. This step was chosen to reduce the number of output variables that were used in the PLS. A total of 93 ROIs were extracted using a Talairach‐based brain anatomy template and a previously developed segmentation algorithm (Goldszal et al., 1998). These data were represented in a matrix grid (i.e., an 8 × 12 matrix with three empty cells). Total brain volume was based on tissue segmentation maps for gray and white matter maps. Whole brain ASL was computed as mean CBF across all ROIs. Phase contrast angiography data were analyzed by a semiautomated procedure of isolating the internal carotid and vertebral arteries and growing the lumen mask using a flood‐filling algorithm of neighboring voxels (Dolui et al., 2016). Blood flow velocity was obtained for each of the eight phases at each voxel in the mask, which was then multiplied by cross‐sectional area to produce a volume flow rate estimate. Global estimates were adjusted by brain density (assumed to be 1.06 g/ml) (Aslan et al., 2010) to produce CBF with units of ml/100 g/min. The ratio of phase contrast to ASL whole brain CBF estimates was used to evaluate potential bias. The whole brain CBF ratio was not influenced by BMI (t = −0.67, df = 380, r = .034, p = .50; Figure S1), thus BMI did not confound the ASL measurement.
2.6. Statistical analyses
Clinical variables and outcomes were summarized using counts and percentages or means and SDs. Regional CBF estimates were adjusted for age, sex, years of education, and site. Also a priori, CBF was adjusted for total brain volume to account for global tissue volume and further account for partial volume effects. The PLS model included CBF ROI as dependent and MVRFs as independent variables.
PLS was implemented in Matlab and software is available from Rotman Research Institute (A. R. McIntosh et al., 1996; A. R. McIntosh & Lobaugh, 2004). In PLS models, dependent and independent variables are combined into one cross‐correlation matrix. The objective is to reduce the dimensionality of this matrix, which in this case used singular value decomposition, a technique similar to principal component analysis. This procedure generates a set of orthogonal latent variables (consisting of pairs of singular matrices and singular values per latent variable). A summary metric, that is, a brain score, was computed for each latent variable (the matrix manipulation was a dot product between the singular images and the original data matrix). Latent variables were evaluated for statistical significance by nonparametric permutation testing. A latent variable was deemed significant if the permutations revealed a p‐value threshold, that is, p < .05. The bootstrap procedure is designed to determine which ROIs show the latent variable effects. As described previously (A. R. McIntosh & Lobaugh, 2004), no correction for multiple comparisons is necessary since no statistical test is performed for this step. An absolute value of the bootstrapping ratio (BSR > 2.0) was used as the threshold for individual ROIs.
To support the primary hypothesis, sensitivity and exploratory analyses were performed. In a sensitivity analysis, the PLS model was run in subgroups based on treatment for a diagnosis of hypertension as a potential source of heterogeneity. A second sensitivity analysis was also run after additional adjusting CBF data by race (i.e., black and white). In an exploratory analysis, we used three summary measures to conduct a mediation analysis (Sobel function in the Multilevel package in R, version 3.3.3). This mediation model tested for direct and indirect associations between MVRFs and memory performance; the indirect association included CBF as a potential mediator. The MVRF composite consisted of the primary factor among the MVRF variables identified by the PLS. The CBF composite was the average across the significant ROIs identified by the PLS—covariate adjustments as previously. The third variable was the aggregate score from the RAVLT memory performance test, which was extracted as a single factor score in a factor analysis, after adjusting for age, sex, years of education, and site.
3. RESULTS
There were 451 CARDIA participants (220 male/231 female) included in this study. Table 1 shows sample details. Many of the metabolic and vascular risk factors showed a high degree of bivariate correlation, except for LDL cholesterol, which was significantly related only to HDL cholesterol (Figure 1). A representative CBF image is provided in Figure 2.
Table 1.
Demographic, metabolic syndrome, and study variables (mean ± SD, or count)
| Age (years) | 50.3 ± 3.5 |
| Sex (male/female) | 220/231 |
| Race (black/white) | 160/291 |
| History of hypertension (yes/no/not known) | 99/347/5 |
| History of hypercholesterolemia (yes/no/not known) | 108/328/15 |
| History of diabetes (yes/no) | 11/440 |
| Body mass index (kg/m2) | 28.2 ± 5.1 |
| Waist circumference (cm) | 90.9 ± 13.2 |
| Diastolic blood pressure (mmHg) | 73.7 ± 11.0 |
| Systolic blood pressure (mmHg) | 118.1 ± 14.5 |
| Diabetes diagnosis (no/yes) | 441/10 |
| Fasting glucose (mg/dl) | 94.3 ± 20.4 |
| Glucose tolerance test at 2‐hr (mg/dl) | 104.6 ± 41.8 |
| Fasting insulin (pmol/L) | 28.0 ± 6.9 |
| High‐density lipoprotein (mg/dl) | 58.9 ± 16.8 |
| Low‐density lipoprotein level (mg/dl) | 115.7 ± 31.5 |
| Triglycerides (mg/dl) | 108.7 ± 62.8 |
| MRI scanning site (1/2/3) | 5/230/216 |
Figure 1.
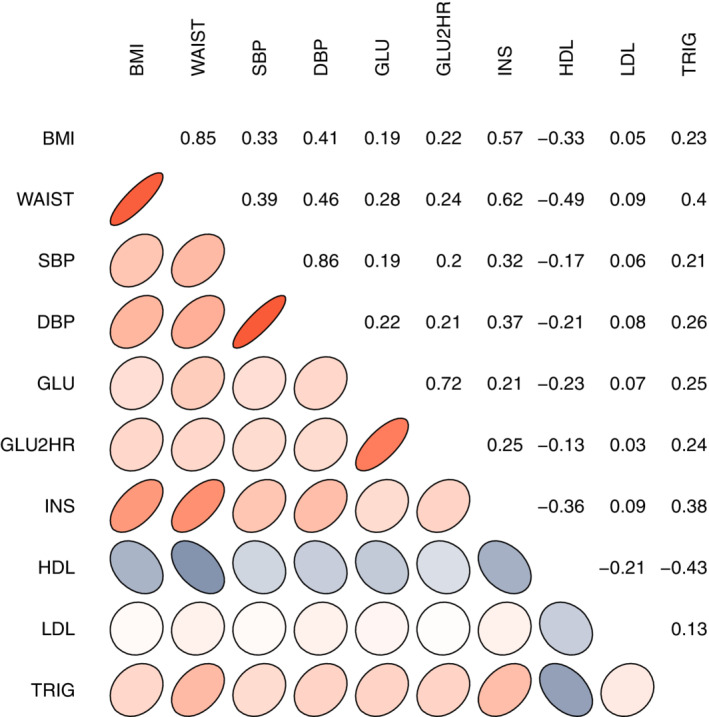
A summary of the bivariate correlation coefficients between each of the metabolic and vascular risk factors. Ellipse shapes denote the direction of the correlation and numerical values are also provided in each of the corresponding correlation panels. Red color denotes positive correlation, while blue denotes negative correlation. BMI, body mass index; waist, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; GLU, fasting glucose; GLU2HR, oral glucose tolerance test; INS, insulin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; TRIG: triglycerides
Figure 2.
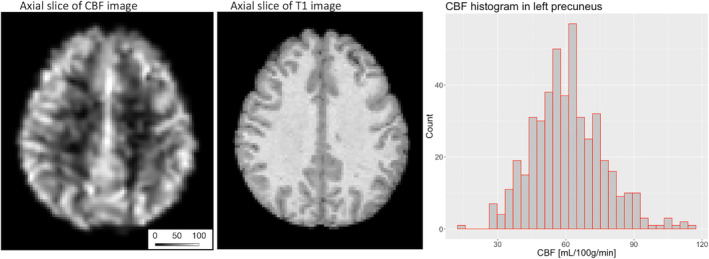
The left panel image shows an axial view of the absolute CBF image for a single participant. The scale bar denotes the CBF level as the image intensity (units: ml/100 g/min). The middle panel shows the corresponding T1‐weighted image. The right panel is the histogram of CBF values across all participants for one ROI (i.e., the left precuneus region). CBF, cerebral blood flow; ROI, regions of interest
3.1. PLS analysis
PLS analysis produced one significant latent variable (p = .005) that explained 79% of the variance between the CBF ROIs (outputs) and the MVRFs (Figure 3). BMI, waist circumference, fasting glucose, OGTT, insulin, HDL, and triglycerides each contributed significantly to this latent variable. Each variable showed a partial correlation with CBF that was an inverse relationship, except for HDL cholesterol that showed a positive partial correlation. The latent variable, indexing a composite metabolic risk factor profile of obesity, glucose dysregulation, and dyslipidemia, was related to CBF in the ROIs provided in the Supplementary Table. The identified ROIs included regions in the temporal lobe (e.g., hippocampus, temporal pole, and superior temporal gyrus), the frontal lobe (e.g., amygdala, insula, and medial and middle frontal regions), the parietal lobe (e.g., angular gyri, cuneus, and cingulate), the occipital lobe (e.g., lingual gyrus, lateral, inferior, and superior occipital cortex, and occipital pole), and basal ganglia (e.g., caudate, globus pallidus, and putamen).
Figure 3.
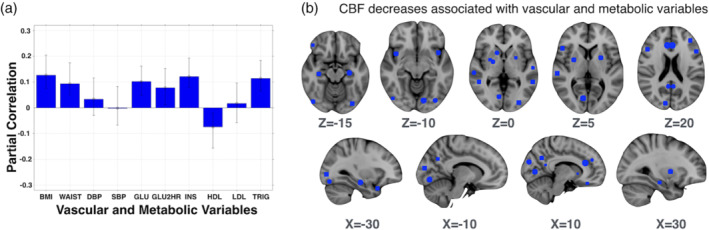
(a) The PLS model demonstrates there is an association between regional CBF and MVRFs, producing one significant latent variable (permutation‐based p = .005). MVRFs with a partial correlation above zero, based on the error bounds, contribute to the latent variable effect. (b) The color overlay (blue) on the standard brain demonstrates regions that contributed to the latent variable based on the calculated bootstrap ratio (BSR > 2; the size of the color denotes the bootstrap ratio). Given that the PLS was performed on an ROI basis, the corresponding brain locations reflect the approximate neuroanatomical coordinates. CBF, cerebral blood flow; BSR, bootstrapping ratio; PLS, partial least squares; ROI, regions of interest
3.2. Mediation analysis
The mediation model is shown in Figure 4. There was a significant association between the MVRF composite score and the CBF composite (t = −2.10, p = .03). The MVRF composite was also associated with the memory composite (i.e., a direct effect with the single factor from the seven RAVLT test scores; t = −2.06, p = .03). The composite CBF was not associated with the RAVLT composite score (t = 0.46, p = .60), and there was no evidence of an indirect effect (i.e., CBF did not mediate an association between MVRFs and memory, Z‐statistic = −0.45, p = .65).
Figure 4.
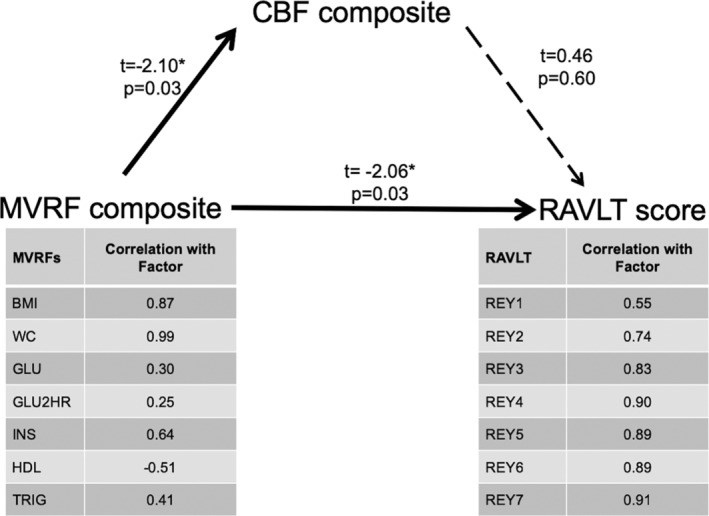
The mediation model tested for direct and indirect associations between the MVRF composite and the RAVLT score. There were significant direct associations between MVRFs and CBF (t = −2.10, p = .03; as expected from PLS), and MVRFs and RAVLT (t = −2.06, p = .03); however, the indirect CBF mediation pathway was not significant. For context, the table shows the correlation between the summary metrics for MVRFs and RAVLT in relation to their factor analysis inputs. CBF, cerebral blood flow; PLS, partial least squares; RAVLT, Rey auditory verbal learning test; MVRFs, metabolic and vascular risk factors
3.3. Sensitivity analyses
In a subgroup without hypertension (N = 347), a latent variable consistent with previous PLS models was seen; although this latent variable explained 57% of the variance, it was not significant (p = .18) based on the permutation‐based criteria. In contrast, among those with hypertension (N = 99), the latent variable was significant with p < .001, which explained 81% of the variance (see Figure S2). Additionally, accounting for the influence of race on CBF (i.e., covarying for race a priori to the PLS model) did not affect the significant latent variable and had the effect of increasing the explained variance in the PLS model (i.e., 86%). We did observe, however, that diastolic blood pressure contributed significantly to this race‐adjusted PLS model.
4. DISCUSSION
A cluster of metabolic and vascular risk factors explained widespread regional decrements in CBF at midlife. The PLS analysis pooled the shared variance in a principled manner to identify the set of MVRFs that were associated with CBF. The MVRF profile comprised obesity, impaired glucose homeostasis, and dyslipidemia variables. Although blood pressure did not contribute to the latent pattern associated with altered CBF in the PLS model, sensitivity analysis showed that the hypertension‐medicated subgroup contributed significantly to the PLS latent variable. Exploration of these MVRFs and CBF data in relation to memory suggested a direct association between MVRFs and memory, but an indirect effect mediated by a composite of the CBF regions was not significant.
This study adds to the literature linking MVRFs and brain health. An autopsy study showed that individuals with more severe diabetes were more likely to have reduced brain tissue volumes, including the hippocampus, compared to people without diabetes, whereas adults with prediabetes did not exhibit brain changes relative to controls (Schneider et al., 2017). Hippocampal volume was almost exclusively found to differ between people with and without diabetes, as determined by voxel‐based morphometry MRI analyses (Gold et al., 2007); however, that diabetes group also had higher BMI, higher serum triglycerides, and lower serum HDL concentrations compared to controls (Gold et al., 2007). The vast majority of participants in the current study did not have diabetes (i.e., 97%). Thus, the present results of reduced CBF among those with increased MVRFs suggest that previous anatomical findings may underestimate the effect of MVRFs on the brain. The PLS latent variable implicated numerous ROIs that spanned all four lobes of the brain and included subcortical gray matter. The regions identified serve numerous brain functions, but collectively could be viewed as regions involved in neurodegeneration. Discussion on the specific MVRFs in relation to CBF are to follow; however, we speculate that MVRFs may induce changes in CBF more broadly than, and earlier than, neuroanatomical decline.
The current study focused primarily on gray matter CBF. White matter CBF accounted for a select number of ROIs, however, and is of interest as evidence points to neuropathological white matter changes and a pattern of reduced CBF from cortical gray to periventricular white matter (Tomimoto et al., 2003)(Makedonov, Black, & MacIntosh, 2013). Thus, further investigation on the physiological imaging of white matter is warranted, as newer approaches with background suppression and segmented 3D acquisition can provide the required sensitivity and spatial resolution (van Gelderen, de Zwart, & Duyn, 2008; Vidorreta et al., 2012).
The PLS identified regions where CBF contributed significantly to the latent variable. One previous report suggested that lower CBF among adults with type 2 diabetes, compared to controls, was mostly confined to temporal and parietal‐occipital regions (Last et al., 2007). In contrast, CBF decrements were observed, in all lobes in the current study. Some of the pertinent brain regions, often implicated in functional neuroimaging studies of neurodegeneration, that were identified in this study include the angular gyrus, cuneus, basal ganglia structures, hippocampus, insula, cingulate regions, among others. The large number of CBF ROI detected was not the result of an ASL signal quality bias, that is, radio frequency coil‐loading bias, as the ratio of whole brain CBF between ASL and phase contrast angiography was not influenced by between‐subject differences in BMI. Instead, the multivariate PLS proved to be a robust method of integrating the between‐subject variance and ascertain a feature in the data that were consistent across the many CBF regions. In addition, to address the potential partial volume effects, CBF estimates were first down sampled to participant specific regions of interest to account for regional tissue difference. Second, CBF estimates were adjusted prior to the PLS analyses by total brain volume.
Neither systolic nor diastolic blood pressure contributed to the latent variables in the PLS models. There are two important points to make regarding these findings. First, a second latent variable from the PLS model clearly reflected blood pressure, but it explained only a small amount of variance (i.e., 9.3%) and it was not significantly related to CBF. The lack of blood pressure influence may appear paradoxical; however, other cohorts, that is, men with coronary artery disease and adults in their 80s, also report no associations between blood pressure and CBF features (Foster‐Dingley et al., 2015; MacIntosh et al., 2015). Second, treatment for hypertension may have a confounding effect. To address this, we considered sensitivity analyses in subgroups based on treatment for a history of hypertension. Although the subgroup without hypertension showed a qualitatively similar pattern, the strength of the partial correlations was more modest, and the latent variable did not reach significance. Among those with a history of hypertension, the effects of the other MVRFs on CBF were larger and significant. This result on midlife CBF extends previous neuroanatomical findings that document additive effects of these MVRFs on atrophy in older people (Tchistiakova & MacIntosh, 2016). It might be considered that although treatment for hypertension can bring many people with a history of hypertension into normal blood pressure ranges, it may not correct other abnormalities in vessel function that confer vulnerability to other MVRFs. Further investigation is warranting, however, as we did not examine the influence that specific hypertension medications or adherence may have on the current results.
Hyperinsulinemia featured prominently in the PLS latent variable. Although particular underlying mechanisms remain hypothetical, one theory suggests that insulin resistance might reduce transport of insulin into the brain, leading to a paradoxical cerebral insulin deficiency that tends to target temporal and frontal brain regions where insulin receptors are expressed (Craft, 2005). Insulin may be involved in clearance of the beta‐amyloid protein, and modulate inflammatory cytokine signaling (Craft, 2005; Watson et al., 2003), which could alter brain structure, function, and CBF. Hyperinsulinemia was closely related to hyperglycemia, which in animal models can be harmful during hypoxia–ischemia conditions (Chang et al., 1998) and which can affect endothelial function through increased oxidative stress, reduced nitric oxide availability and increased endothelin‐1 mediated vasoconstriction, among other potential mechanisms (Kalani, 2008; Monnier et al., 2006).
Two obesity metrics, BMI and waist circumference, contributed to the significant latent variable, adding to previous brain imaging obesity findings (Debette et al., 2010; Ho et al., 2010; Raji et al., 2010). While these measures were chosen based on established clinical relevance, they cannot speak directly to adiposity or fat distribution pattern (e.g., visceral vs. subcutaneous), and more precise obesity metrics might be useful in future studies to better inform underlying mechanisms.
The PLS findings implicated triglycerides and HDL variables, which are influenced by eating habits, smoking, sedentary lifestyle, and obesity. Our findings appear at odds with one study that reported that these variables did not influence cognition over a 7‐year period among non‐demented adults living in their 70s (Reitz, Luchsinger, Tang, Manly, & Mayeux, 2005); however, a more recent study found that high triglycerides in midlife were associated with increased risk of AD markers, that is, protein concentrations in cerebrospinal fluid, and evidence of amyloid in tissue during 20 year follow‐up imaging (Nägga et al., 2018). The findings in to, lend further support to the notion that midlife may be a crucial juncture at which MVRFs set the stage for later cognitive decline.
In our sample of largely nondiabetic participants, we observed a direct association between MVRFs and memory performance, which concurs with work by Gold et al., who found that short and long delayed recall were affected in diabetes (Gold et al., 2007). We, however, did not find evidence that CBF mediated the relationship between MVRFs and memory performance in midlife that was described previously (Yaffe et al., 2014). Notably, the mean of the PLS‐defined regional CBF values was not associated with memory performance. It is possible that averaging CBF across the ROIs failed to account for regional differences that may be important sources of heterogeneity between participants. Another possibility is the potential of ceiling effects in the CBF or RAVLT measures, as this group included cognitively intact 50‐year‐old adults. The results do not preclude longitudinal relationships, or cerebrovascular reactivity, which was not assessed here, as a potential mediator (Albanese et al., 2017; Tchistiakova & MacIntosh, 2016).
The current study is limited to cross‐sectional observations; therefore, additional work is required to understand whether CBF predicts future clinical cognitive outcomes. The narrow age range in this study is strength; however, this along with the cross‐sectional data preclude our ability to test for an age‐effect in relationships between MVRFs, CBF, and cognitive changes (Barrett‐Connor, Edelstein, Corey‐Bloom, & Wiederholt, 1996). We are also not able to address the potential influence of large artery vascular disease, such as steno‐occlusive disease, as angiography data were not collected; thus, this could be a source of between‐subject differences in CBF. As for the arteries in the neck, the phase contrast angiography was prescribed the same physical distance below the ASL imaging volume. Our choice of the upper velocity cut‐off that was comparable to previous work (Khan et al., 2017), but could have introduced some bias in the phase contrast CBF quantification. The phase contrast images were therefore visually inspected to ensure that all four neck arteries were visible in each participant in this analysis. Lastly, amyloid and tau biomarkers were not available to exclude potential early stage AD.
Adults in midlife with increased MVRFs, decreased CBF, and poor memory performance might be viewed as an important target population for preventative interventions. Therefore, understanding cognitive performance in this cohort may be of paramount clinical importance. Additional work will be needed to better characterize neuroendocrine changes that affect trajectories of CBF and cognitive performance in middle‐aged adults with and without early neurodegenerative changes (Ishii & Iadecola, 2015).
Supporting information
Supplementary Figure 1 To account for potential bias in the ASL‐based CBF estimates, we measured whole brain CBF estimates using two approaches: ASL and phase contrast angiography. The whole brain CBF ratio, between ASL and phase contrast angiography, was not related to body mass index (r = .034, p = .50)
Supplementary Figure 2 To account for hypertension as a potential driver of subgroup differences, we split the cohort based on hypertension diagnosis status and re‐ran the PLS model. (a) We found that the hypertensive adults showed significant PLS latent variable effect (n = 99, p < .001, that is, consistent with Figure 2); (b) whereas the larger sample non‐hypertensive subgroup did not produce a significant PLS latent variable (n = 347, p = .18)
ACKNOWLEDGMENTS
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). This manuscript has been reviewed by CARDIA for scientific content. BJM acknowledges support from the Brain and Behavior Foundation Independent Investigator Award. BM and WS report support from the Canadian Institutes for Health Research, and the Natural Sciences and Engineering Council.
MacIntosh BJ, Shirzadi Z, Atwi S, et al. Metabolic and vascular risk factors are associated with reduced cerebral blood flow and poorer midlife memory performance. Hum Brain Mapp. 2020;41:855–864. 10.1002/hbm.24844
Funding information Brain and Behaviour Foundation Independent Investigator Award; Canadian Institutes for Health Research; National Heart, Lung, and Blood Institute, Grant/Award Numbers: HHSN268201800003I, HHSN268201800004I, HHSN268201800005I, HHSN268201800006I, HHSN268201800007I; Natural Sciences and Engineering Council
DATA AVAILABILITY STATEMENT
Data in this study are from a sub‐sample of black and white men and women who participated in the community‐based Coronary Artery Risk Development in Young Adults (CARDIA) study, examined at year‐25. CARDIA is a longitudinal study of the development and determinants of cardiovascular disease in 5115 young adults who were aged 18–30 years at baseline in 1985–1986.
REFERENCES
- Albanese, E. , Launer, L. J. , Egger, M. , Prince, M. J. , Giannakopoulos, P. , Wolters, F. J. , & Egan, K. (2017). Body mass index in midlife and dementia: Systematic review and meta‐regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimer's & Dementia (Amsterdam, Netherlands), 8, 165–178. 10.1016/j.dadm.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop, D. C. , Detre, J. A. , Golay, X. , Günther, M. , Hendrikse, J. , Hernandez‐Garcia, L. , … Zaharchuk, G. (2014). Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine : Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine, 73(1), 102–116. 10.1002/mrm.25197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association . (2011). Diagnosis and classification of diabetes mellitus. Diabetes Care, 34(1), S62–S69. 10.2337/dc11-S062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan, S. , Xu, F. , Wang, P. L. , Uh, J. , Yezhuvath, U. S. , van Osch, M. , & Lu, H. (2010). Estimation of labeling efficiency in pseudocontinuous arterial spin labeling. Magnetic Resonance in Medicine : Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine, 63(3), 765–771. 10.1002/mrm.22245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett‐Connor, E. , Edelstein, S. L. , Corey‐Bloom, J. , & Wiederholt, W. C. (1996). Weight loss precedes dementia in community‐dwelling older adults. Journal of the American Geriatrics Society, 44(10), 1147–1152. http://www.ncbi.nlm.nih.gov/pubmed/8855991 [DOI] [PubMed] [Google Scholar]
- Beauchet, O. , Celle, S. , Roche, F. , Bartha, R. , Montero‐Odasso, M. , Allali, G. , & Annweiler, C. (2013). Blood pressure levels and brain volume reduction: A systematic review and meta‐analysis. Journal of Hypertension, 31(8), 1502–1516. 10.1097/HJH.0b013e32836184b5 [DOI] [PubMed] [Google Scholar]
- Birdsill, A. C. , Carlsson, C. M. , Willette, A. A. , Okonkwo, O. C. , Johnson, S. C. , Xu, G. , … Bendlin, B. B. (2013). Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity (Silver Spring, Md.), 21(7), 1313–1320. 10.1002/oby.20170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri, M. , Ropele, S. , Petrovic, K. , Pluta‐Fuerst, A. , Homayoon, N. , Enzinger, C. , … Schmidt, R. (2010). Metabolic syndrome, brain magnetic resonance imaging, and cognition. Diabetes Care, 33(12), 2489–2495. 10.2337/dc10-0851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, Y. S. , Park, W. S. , Lee, M. , Kim, K. S. , Shin, S. M. , & Choi, J. H. (1998). Effect of hyperglycemia on brain cell membrane function and energy metabolism during hypoxia‐ischemia in newborn piglets. Brain Research, 798(1–2), 271–280. http://www.ncbi.nlm.nih.gov/pubmed/9666146 [DOI] [PubMed] [Google Scholar]
- Chuang, Y.‐F. , An, Y. , Bilgel, M. , Wong, D. F. , Troncoso, J. C. , O'Brien, R. J. , … Thambisetty, M. (2016). Midlife adiposity predicts earlier onset of Alzheimer's dementia, neuropathology and presymptomatic cerebral amyloid accumulation. Molecular Psychiatry, 21(7), 910–915. 10.1038/mp.2015.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournot, M. , Marquié, J. C. , Ansiau, D. , Martinaud, C. , Fonds, H. , Ferrières, J. , & Ruidavets, J. B. (2006). Relation between body mass index and cognitive function in healthy middle‐aged men and women. Neurology, 67(7), 1208–1214. 10.1212/01.wnl.0000238082.13860.50 [DOI] [PubMed] [Google Scholar]
- Craft, S. (2005). Insulin resistance syndrome and Alzheimer's disease: Age‐ and obesity‐related effects on memory, amyloid, and inflammation. Neurobiology of Aging, 26(1), 65–69. 10.1016/j.neurobiolaging.2005.08.021 [DOI] [PubMed] [Google Scholar]
- Curb, J. D. , Rodriguez, B. L. , Abbott, R. D. , Petrovitch, H. , Ross, G. W. , Masaki, K. H. , … White, L. R. (1999). Longitudinal association of vascular and Alzheimer's dementias, diabetes, and glucose tolerance. Neurology, 52(5), 971–975. http://www.ncbi.nlm.nih.gov/pubmed/10102414 [DOI] [PubMed] [Google Scholar]
- De Vis, J. B. , Peng, S.‐L. , Chen, X. , Li, Y. , Liu, P. , Sur, S. , … Lu, H. (2018). Arterial‐spin‐labeling (ASL) perfusion MRI predicts cognitive function in elderly individuals: A 4‐year longitudinal study. Journal of Magnetic Resonance Imaging, 48(2), 449–458. 10.1002/jmri.25938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette, S. , Beiser, A. , Hoffmann, U. , Decarli, C. , O'Donnell, C. J. , Massaro, J. M. , … Seshadri, S. (2010). Visceral fat is associated with lower brain volume in healthy middle‐aged adults. Annals of Neurology, 68(2), 136–144. 10.1002/ana.22062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolui, S. , Wang, Z. , Wang, D. J. J. , Mattay, R. , Finkel, M. , Elliott, M. , … Detre, J. A. (2016). Comparison of non‐invasive MRI measurements of cerebral blood flow in a large multisite cohort. Journal of Cerebral Blood Flow and Metabolism, 36(7), 1244–1256. 10.1177/0271678X16646124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, J. P. , Kessler, R. M. , Feurer, I. D. , Volkow, N. D. , Patterson, B. W. , Ansari, M. S. , … Abumrad, N. N. (2012). Relationship of dopamine type 2 receptor binding potential with fasting neuroendocrine hormones and insulin sensitivity in human obesity. Diabetes Care, 35(5), 1105–1111. 10.2337/dc11-2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster‐Dingley, J. C. , Moonen, J. E. F. , de Craen, A. J. M. , de Ruijter, W. , van der Mast, R. C. , & van der Grond, J. (2015). Blood pressure is not associated with cerebral blood flow in older persons. Hypertension (Dallas, Tex.: 1979), 66(5), 954–960. 10.1161/HYPERTENSIONAHA.115.05799 [DOI] [PubMed] [Google Scholar]
- Friedman, G. D. , Cutter, G. R. , Donahue, R. P. , Hughes, G. H. , Hulley, S. B. , Jacobs, D. R. , … Savage, P. J. (1988). CARDIA: Study design, recruitment, and some characteristics of the examined subjects. Journal of Clinical Epidemiology, 41(11), 1105–1116. http://www.ncbi.nlm.nih.gov/pubmed/3204420 [DOI] [PubMed] [Google Scholar]
- Gold, S. M. , Dziobek, I. , Sweat, V. , Tirsi, A. , Rogers, K. , Bruehl, H. , … Convit, A. (2007). Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia, 50(4), 711–719. 10.1007/s00125-007-0602-7 [DOI] [PubMed] [Google Scholar]
- Goldszal, A. F. , Davatzikos, C. , Pham, D. L. , Yan, M. X. , Bryan, R. N. , & Resnick, S. M. (1998). An image‐processing system for qualitative and quantitative volumetric analysis of brain images. Journal of Computer Assisted Tomography, 22(5), 827–837. [DOI] [PubMed] [Google Scholar]
- Ho, A. J. , Raji, C. A. , Becker, J. T. , Lopez, O. L. , Kuller, L. H. , Hua, X. , … Thompson, P. M. (2010). Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiology of Aging, 31(8), 1326–1339. 10.1016/j.neurobiolaging.2010.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii, M. , & Iadecola, C. (2015). Metabolic and non‐cognitive manifestations of Alzheimer's disease: The hypothalamus as both culprit and target of pathology. Cell Metabolism, 22(5), 761–776. 10.1016/j.cmet.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturria‐Medina, Y. , Sotero, R. C. , Toussaint, P. J. , Mateos‐Pérez, J. M. , Evans, A. C. , Weiner, M. W. , … Furst, A. J. (2016). Early role of vascular dysregulation on late‐onset Alzheimer's disease based on multifactorial data‐driven analysis. Nature Communications, 7, 11934 10.1038/ncomms11934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen, J. F. A. , van Bussel, F. C. G. , van de Haar, H. J. , van Osch, M. J. P. , Hofman, P. A. M. , van Boxtel, M. P. J. , … Backes, W. H. (2016). Cerebral blood flow, blood supply, and cognition in type 2 diabetes mellitus. Scientific Reports, 6(1), 10 10.1038/s41598-016-0003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutel, A. , Monet‐Leprêtre, M. , Gosele, C. , Baron‐Menguy, C. , Hammes, A. , Schmidt, S. , … Hubner, N. (2010). Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. The Journal of Clinical Investigation, 120(2), 433–445. 10.1172/JCI39733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani, M. (2008). The importance of endothelin‐1 for microvascular dysfunction in diabetes. Vascular Health and Risk Management, 4(5), 1061–1068. http://www.ncbi.nlm.nih.gov/pubmed/19183753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, M. A. , Liu, J. , Tarumi, T. , Lawley, J. S. , Liu, P. , Zhu, D. C. , … Zhang, R. (2017). Measurement of cerebral blood flow using phase contrast magnetic resonance imaging and duplex ultrasonography. Journal of Cerebral Blood Flow and Metabolism, 37(2), 541–549. 10.1177/0271678X16631149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last, D. , Alsop, D. C. , Abduljalil, A. M. , Marquis, R. P. , de Bazelaire, C. , Hu, K. , … Novak, V. (2007). Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care, 30(5), 1193–1199. 10.2337/dc06-2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer, L. J. , Lewis, C. E. , Schreiner, P. J. , Sidney, S. , Battapady, H. , Jacobs, D. R. , … Bryan, R. N. (2015). Vascular factors and multiple measures of early brain health: CARDIA brain MRI study. PLoS ONE, 10(3), e0122138 10.1371/journal.pone.0122138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntosh, B. J. , Swardfager, W. , Robertson, A. D. , Tchistiakova, E. , Saleem, M. , Oh, P. I. , … Lanctôt, K. L. (2015). Regional cerebral arterial transit time hemodynamics correlate with vascular risk factors and cognitive function in men with coronary artery disease. American Journal of Neuroradiology, 36(2), 295–301. 10.3174/ajnr.A4094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makedonov, I. , Black, S. E. , & MacIntosh, B. J. (2013). Cerebral small vessel disease in aging and Alzheimer's disease: A comparative study using MRI and SPECT. European Journal of Neurology : The Official Journal of the European Federation of Neurological Societies, 20(2), 243–250. 10.1111/j.1468-1331.2012.03785.x [DOI] [PubMed] [Google Scholar]
- McIntosh, A. R. , Bookstein, F. L. , Haxby, J. V. , & Grady, C. L. (1996). Spatial pattern analysis of functional brain images using partial least squares. NeuroImage, 3(3 Pt 1), 143–157. 10.1006/nimg.1996.0016 [DOI] [PubMed] [Google Scholar]
- McIntosh, A. R. , & Lobaugh, N. J. (2004). Partial least squares analysis of neuroimaging data: Applications and advances. NeuroImage, 23(Suppl 1), S250–S263. 10.1016/j.neuroimage.2004.07.020 [DOI] [PubMed] [Google Scholar]
- Monnier, L. , Mas, E. , Ginet, C. , Michel, F. , Villon, L. , Cristol, J.‐P. , & Colette, C. (2006). Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic Hyperglycemia in patients with type 2 diabetes. JAMA, 295(14), 1681–1687. 10.1001/jama.295.14.1681 [DOI] [PubMed] [Google Scholar]
- Nägga, K. , Gustavsson, A.‐M. , Stomrud, E. , Lindqvist, D. , van Westen, D. , Blennow, K. , … Hansson, O. (2018). Increased midlife triglycerides predict brain β‐amyloid and tau pathology 20 years later. Neurology, 90(1), e73–e81. 10.1212/WNL.0000000000004749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottino‐González, J. , Jurado, M. A. , García‐García, I. , Segura, B. , Marqués‐Iturria, I. , Sender‐Palacios, M. J. , … Garolera, M. (2017). Allostatic load is linked to cortical thickness changes depending on body‐weight status. Frontiers in Human Neuroscience, 11, 639 10.3389/fnhum.2017.00639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, S.‐L. , Chen, X. , Li, Y. , Rodrigue, K. M. , Park, D. C. , & Lu, H. (2018). Age‐related changes in cerebrovascular reactivity and their relationship to cognition: A four‐year longitudinal study. NeuroImage, 174, 257–262. 10.1016/j.neuroimage.2018.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punthakee, Z. , Miller, M. E. , Launer, L. J. , Williamson, J. D. , Lazar, R. M. , Cukierman‐Yaffee, T. , … ACCORD‐MIND Investigators . (2012). Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: Post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care, 35(4), 787–793. 10.2337/dc11-1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, C. , & Fratiglioni, L. (2015). A major role for cardiovascular burden in age‐related cognitive decline. Nature Reviews. Cardiology, 12(5), 267–277. 10.1038/nrcardio.2014.223 [DOI] [PubMed] [Google Scholar]
- Raji, C. A. , Ho, A. J. , Parikshak, N. N. , Becker, J. T. , Lopez, O. L. , Kuller, L. H. , … Thompson, P. M. (2010). Brain structure and obesity. Human Brain Mapping, 31(3), 353–364. 10.1002/hbm.20870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz, C. , Luchsinger, J. , Tang, M.‐X. , Manly, J. , & Mayeux, R. (2005). Impact of plasma lipids and time on memory performance in healthy elderly without dementia. Neurology, 64(8), 1378–1383. 10.1212/01.WNL.0000158274.31318.3C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala, M. , de Roos, A. , van den Berg, A. , Altmann‐Schneider, I. , Slagboom, P. E. , Westendorp, R. G. , … van der Grond, J. (2014). Microstructural brain tissue damage in metabolic syndrome. Diabetes Care, 37(2), 493–500. 10.2337/dc13-1160 [DOI] [PubMed] [Google Scholar]
- Schneider, A. L. C. , Selvin, E. , Sharrett, A. R. , Griswold, M. , Coresh, J. , Jack, C. R. , … Gottesman, R. F. (2017). Diabetes, prediabetes, and brain volumes and subclinical cerebrovascular disease on MRI: The atherosclerosis risk in communities neurocognitive study (ARIC‐NCS). Diabetes Care, 40(11), 1514–1521. 10.2337/dc17-1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals, D. R. , Moreau, K. L. , Gates, P. E. , & Eskurza, I. (2006). Modulatory influences on ageing of the vasculature in healthy humans. Experimental Gerontology, 41(5), 501–507. 10.1016/j.exger.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Tchistiakova, E. , Anderson, N. D. , Greenwood, C. E. , & Macintosh, B. J. (2014). Combined effects of type 2 diabetes and hypertension associated with cortical thinning and impaired cerebrovascular reactivity relative to hypertension alone in older adults. NeuroImage: Clinical, 5, 36–41. 10.1016/j.nicl.2014.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchistiakova, E. , & MacIntosh, B. J. (2016). Summative effects of vascular risk factors on cortical thickness in mild cognitive impairment. Neurobiology of Aging, 45, 98–106. 10.1016/j.neurobiolaging.2016.05.011 [DOI] [PubMed] [Google Scholar]
- Tomimoto, H. , Ihara, M. , Wakita, H. , Ohtani, R. , Lin, J.‐X. , Akiguchi, I. , … Shibasaki, H. (2003). Chronic cerebral hypoperfusion induces white matter lesions and loss of oligodendroglia with DNA fragmentation in the rat. Acta Neuropathologica, 106(6), 527–534. 10.1007/s00401-003-0749-3 [DOI] [PubMed] [Google Scholar]
- van Gelderen, P. , de Zwart, J. A. , & Duyn, J. H. (2008). Pittfalls of MRI measurement of white matter perfusion based on arterial spin labeling. Magnetic Resonance in Medicine : Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine, 59(4), 788–795. 10.1002/mrm.21515 [DOI] [PubMed] [Google Scholar]
- Vidorreta, M. , Wang, Z. , Rodríguez, I. , Pastor, M. a. , Detre, J. a. , & Fernández‐Seara, M. a. (2012). Comparison of 2D and 3D single‐shot ASL perfusion fMRI sequences. NeuroImage, 66C, 662–671. 10.1016/j.neuroimage.2012.10.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. (2012). Improving cerebral blood flow quantification for arterial spin labeled perfusion MRI by removing residual motion artifacts and global signal fluctuations. Magnetic Resonance Imaging, 30(10), 1409–1415. 10.1016/j.mri.2012.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, G. S. , Peskind, E. R. , Asthana, S. , Purganan, K. , Wait, C. , Chapman, D. , … Craft, S. (2003). Insulin increases CSF Abeta42 levels in normal older adults. Neurology, 60(12), 1899–1903. http://www.ncbi.nlm.nih.gov/pubmed/12821730 [DOI] [PubMed] [Google Scholar]
- Whitmer, R. A. , Gunderson, E. P. , Barrett‐Connor, E. , Quesenberry, C. P. , & Yaffe, K. (2005). Obesity in middle age and future risk of dementia: A 27 year longitudinal population based study. BMJ (Clinical Research Ed.), 330(7504), 1360 10.1136/bmj.38446.466238.E0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widya, R. L. , Kroft, L. J. M. , Altmann‐Schneider, I. , van den Berg‐Huysmans, A. A. , van der Bijl, N. , de Roos, A. , … Leiden Longevity Study Group . (2015). Visceral adipose tissue is associated with microstructural brain tissue damage. Obesity (Silver Spring, Md.), 23(5), 1092–1096. 10.1002/oby.21048 [DOI] [PubMed] [Google Scholar]
- Yaffe, K. , Vittinghoff, E. , Pletcher, M. J. , Hoang, T. D. , Launer, L. J. , Whitmer, R. A. , … Sidney, S. (2014). Early adult to midlife cardiovascular risk factors and cognitive function. Circulation, 129(15), 1560–1567. 10.1161/CIRCULATIONAHA.113.004798 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 To account for potential bias in the ASL‐based CBF estimates, we measured whole brain CBF estimates using two approaches: ASL and phase contrast angiography. The whole brain CBF ratio, between ASL and phase contrast angiography, was not related to body mass index (r = .034, p = .50)
Supplementary Figure 2 To account for hypertension as a potential driver of subgroup differences, we split the cohort based on hypertension diagnosis status and re‐ran the PLS model. (a) We found that the hypertensive adults showed significant PLS latent variable effect (n = 99, p < .001, that is, consistent with Figure 2); (b) whereas the larger sample non‐hypertensive subgroup did not produce a significant PLS latent variable (n = 347, p = .18)
Data Availability Statement
Data in this study are from a sub‐sample of black and white men and women who participated in the community‐based Coronary Artery Risk Development in Young Adults (CARDIA) study, examined at year‐25. CARDIA is a longitudinal study of the development and determinants of cardiovascular disease in 5115 young adults who were aged 18–30 years at baseline in 1985–1986.


