Abstract
Evidence indicates better cognitive and behavioral outcomes for females born very preterm (≤32 weeks gestation) compared to males, but the neurophysiology underlying this apparent resiliency of the female brain remains poorly understood. Here we test the hypothesis that very preterm males express more pronounced connectivity alterations as a reflection of higher male vulnerability. Resting state MEG recordings, neonatal and psychometric data were collected from 100 children at age 8 years: very preterm boys (n = 27), very preterm girls (n = 34), full‐term boys (n = 15) and full‐term girls (n = 24). Neuromagnetic source dynamics were reconstructed from 76 cortical brain regions. Functional connectivity was estimated using inter‐regional phase‐synchronization. We performed a series of multivariate analyses to test for differences across groups as well as to explore relationships between deviations in functional connectivity and psychometric scores and neonatal factors for very preterm children. Very preterm boys displayed significantly higher (p < .001) absolute deviation from average connectivity of same‐sex full‐term group, compared to very preterm girls versus full‐term girls. In the connectivity comparison between very preterm and full‐term groups separately for boys and girls, significant group differences (p < .05) were observed for boys, but not girls. Sex differences in connectivity (p < .01) were observed in very preterm children but not in full‐term groups. Our findings indicate that very preterm boys have greater alterations in resting neurophysiological network communication than girls. Such uneven brain communication disruption in very preterm boys and girls suggests that stronger connectivity alterations might contribute to male vulnerability in long‐term behavioral and cognitive outcome.
Keywords: neuromagnetic brain connectivity, sex differences, very preterm children
1. INTRODUCTION
Preterm birth (≤37 weeks gestation) is the greatest cause of neonatal mortality and morbidity and, in case of survival, leads to long‐term negative health consequences (Lawn et al., 2005; Moster, Lie, & Markestad, 2008). Mounting evidence suggests strong male disadvantage in the preterm population including increased preterm birth incidence, lower survival rates and worse long‐term outcomes (Hindmarsh, O'Callaghan, Mohay, & Rogers, 2000; Hintz et al., 2006; Ingemarsson, 2003; Månsson et al., 2015; Peacock, Marston, Marlow, Calvert, & Greenough, 2012; Rose et al., 2009; Schindler et al., 2017; Whitfield, Grunau, & Holsti, 1997). Even in the absence of major pathologies and developmental impairments, preterm boys tend to experience more behavioral and cognitive difficulties, in particular: executive control (Urben et al., 2017); language and speech problems (Wolke, Samara, Bracewell, & Marlow, 2008); language and cognitive ability (Skiöld et al., 2014; Wood et al., 2005; Young et al., 2016).
A number of neuroimaging studies aimed to clarify the underlying mechanism of male disadvantage, investigating sex differences in brain structure in the preterm population. The most consistent finding was a larger intracranial volume in preterm boys compared to girls (Ball et al., 2017; Benavides et al., 2018; Kersbergen et al., 2016; Liu et al., 2011; Skiöld et al., 2014; Thompson et al., 2018; Urben et al., 2017; Vasileiadis, Thompson, Han, & Gelman, 2009). However, bigger brain volume in boys is also observed in the full‐term population and was not shown to be related to preterm birth or long‐term outcome. It has also been shown that preterm girls have more cortical folding despite smaller brain volume, suggesting more “compact” brain development compared to preterm boys (Vasileiadis et al., 2009). The proportional cerebral white matter volume has been shown to be larger in preterm boys, whereas proportional gray matter volume was larger in preterm girls (Benavides et al., 2018). Diffusion tensor imaging (DTI) studies also reported sex differences in the preterm population, which includes lower regional and mean fractional anisotropy (FA) and higher medium diffusivity in boys indicating less organized white matter microstructure (Constable et al., 2008; Liu et al., 2011; Thompson et al., 2018). Similarly, other studies have reported male sex to be associated with reduced corpus callosum microstructural growth trajectory during first 6 months of life as well as being a risk factor for diffuse white matter injury (Barnett et al., 2017; Teli et al., 2018). White matter features have also been shown to have different associations with long‐term outcome in boys and girls (van Kooij et al., 2011). Importantly, mean FA was shown to increase in preterm girls but not in boys in response to erythropoietin treatment indicating sex‐specific medical therapy effectiveness in preterm population (Phillips et al., 2017). Such evidence of white matter alterations suggests that exploration of brain connectivity is a promising direction toward understanding of unequal consequences of preterm birth in males and females.
In support of the view that neurophysiological connectivity may be associated with developmental outcomes in very preterm children, we recently found that extremely preterm (≤28 weeks gestation) and very preterm children (28–32 weeks gestation) had pronounced alterations in connectivity which were associated with both adverse neonatal experience and poorer behavioral and cognitive performance at school age (Kozhemiako et al., 2019). These findings, combined with the long‐established evidence of males being more affected by preterm birth that leads to poorer long‐term outcome, led us to hypothesize that preterm males are characterized by more pronounced inter‐regional connectivity alterations than preterm females. Additionally, we predicted that such larger deviations from the full‐term typical connectivity would be associated with adverse neonatal experience and poorer long‐term outcome in both preterm males and females. We also investigated sex differences in connectivity in full‐term individuals and how typical sex differences are affected by very preterm birth.
2. MATERIALS AND METHODS
2.1. Participants
Resting state MEG data were recorded from 100 children at age of eight. Participants were divided into four groups: preterm boys (24–32 weeks GA, n = 27), preterm girls (24–32 weeks GA, n = 34), full‐term boys (40 weeks GA, n = 15) and full‐term girls (40 weeks GA, n = 24). Children with major brain injury (periventricular leukomalacia or grade III–IV intraventricular hemorrhage on neonatal cranial ultrasound) or sensory or cognitive impairments were excluded. Clinical, demographic and psychometric data are presented in Table 1. The recruitment of the participants was conducted within a prospective longitudinal study investigating effects of neonatal pain‐related stress on neurodevelopment of very preterm children, for example, (Grunau et al., 2007; Grunau et al., 2009). This study was approved by the Clinical Research Ethics board of the University of British Columbia and the Research Ethics Board of the Children's & Women's Health Centre of BC. Written informed consent was obtained for every participant and their parent(s).
Table 1.
Characteristics of the participants
| Characteristics | Preterm | Full‐term | ||
|---|---|---|---|---|
| Boys | Girls | Boys | Girls | |
| Number of subjects | 27 | 34 | 15 | 24 |
| Age, years | 7.8 (0.39) | 7.7 (0.39) | 8.0 (0.91) | 8.0 (1.11) |
| MRI scans | 16 | 25 | 5 | 8 |
| Head circumference at age 8 years | 51.5 (2.38) | 51.7 (1.79) | 52.8 (1.64) | 52.3 (1.96) |
| GA at birth, weeks | 29.7 (2.29)*** | 29.5 (2.45)*** | 39.6 (1.21) | 40.1 (0.68) |
| Birth weight, g | 1,382.9 (501.46)*** | 1,286.9 (370.42)*** | 3,496.4 (732.20) | 3,415.8 (300.91) |
| Number of subjects small for GA | 2 | 2 | 0 | 0 |
| Number of skin‐breaking procedures | 103.6 (82.94) | 94.6 (73.40) | n/a | n/a |
| Morphine dosage | 0.9 (1.67) | 0.9 (3.70) | n/a | n/a |
| Days on mechanical ventilation | 8.7 (13.29) | 10.18 (18.02) | n/a | n/a |
| Early illness severity (SNAP‐II) | 13.8 (10.08) | 10.3 (10.71) | n/a | n/a |
| Verbal comprehension index (WISC‐IV) | 100.9 (18.33) | 98.2 (11.26)** | 110.1 (14.87) | 107.9 (12.15) |
| Perceptual reasoning index (WISC‐IV) | 101.0 (18.79)* | 100.7 (12.86)** | 113.6 (14.71) | 112.8 (12.69) |
| Working memory index (WISC‐IV) | 95.2 (11.92) | 99.7 (12.18) | 102.1 (12.16) | 102.3 (10.92) |
| Processing speed index (WISC‐IV) | 92.0 (10.47) | 96.9 (14.64)* | 98.4 (14.46) | 108.5 (16.01) |
| Full‐scale IQ (WISC‐IV) | 97.0 (16.40)* | 98.7 (12.38)** | 108.7 (13.67) | 110.6 (12.40) |
| Internalizing behavior (CBCL) | 51.4 (12.49) | 50.6 (9.23) | 51.9 (10.96) | 48.4 (12.49) |
| Externalizing behavior (CBCL) | 49.0 (11.60) | 46.1 (9.23) | 48.6 (10.83) | 46.3 (10.52) |
| Behavioral regulation index (BRIEF) | 54.6 (13.07) | 49.2 (10.58) | 50.6 (8.84) | 50.0 (12.27) |
| Metacognition index (BRIEF) | 56.5 (11.99) | 52.4 (14.52) | 51.6 (6.01) | 48.0 (12.41) |
| Visual‐motor integration (BEERY) | 93.6 (7.66)* | 94.5 (10.04)** | 100.7 (15.47) | 103.4 (10.36) |
| Visual perception (BEERY) | 101.8 (15.45)* | 102.2 (15.40)** | 114.4 (16.33) | 113.5 (14.99) |
| Motor coordination (BEERY) | 90.9 (9.61) | 92.5 (10.18)* | 95.2 (11.60) | 99.2 (10.90) |
Note: The statistics are reported in the terms of group mean (SD), unless the number of subjects or scans is reported.
Abbreviations: BEERY, Beery‐Buktenica Developmental Test of Visual‐Motor Integration (5th ed.); BRIEF, Behavior Rating Inventory of Executive Function; CBCL, Child Behavior Checklist; GA, gestational age; n/a, not applicable; WISC‐IV, Wechsler Intelligence Scale for Children.
p < .05
p < .01
p < .001 group difference (preterm—full‐term the same sex).
2.2. Data collection
Resting state MEG data were recorded for 2 min in a magnetically shielded room using a 151 channel MEG system (CTF Systems, Coquitlam, Canada). During the recording participants were supine and were instructed to fixate on a smiling face at the center of a screen. A research assistant accompanied participants in the magnetically shielded room to ensure that the children were following the instructions. Three fiducial coils were placed at the nasion and the left and right preauricular locations to enable continuous head position tracking. Data were collected at the sampling rate of 1,200 Hz and stored for future analysis. The shape of each participant's head surface was also digitized using a Polhemus FASTRACK digitizer.
T1‐weighted volumetric MRI images (1.5 T) were also collected, but only for 54 children (the information on MRI availability for each group is provided in the Table 1). Additionally, 19 children who participated in this study had MRI, but no MEG data. In total, we had a pool of 73 T1‐weighted MRI images. Each of those participants who did not have the T1 MRI, was best‐matched with one of the T1 MRI images from this pool with the goal to create a head model (a detailed description is provided below in the Data Analysis Section 2.5).
2.3. Psychometric assessment
Cognitive assessment was performed following MEG data collection by a psychometrician who was blind to the participant's group status, and a parent completed questionnaires in a separate room. The assessment targeted the specific domains, which frequently are reported to be altered in very preterm children. Those comprised verbal and nonverbal intelligence, visual‐motor abilities, internalizing and externalizing behavior, and executive functions (Aarnoudse‐Moens, Weisglas‐Kuperus, van Goudoever, & Oosterlaan, 2009; Bhutta, Cleves, Casey, Cradock, & Anand, 2002; Grunau, Whitfield, & Fay, 2004; Loe, Lee, Luna, & Feldman, 2011; Ranger, Synnes, Vinall, & Grunau, 2014). Psychometric tests included the Wechsler Intelligence Scale for Children (WISC‐IV; Wechsler, 2003) and Beery‐Buktenica Developmental Test of Visual‐Motor Integration (5th ed.; Beery, Buktenika, & Beery, 2004), and questionnaires included the Child Behavior Checklist (Achenbach & Rescorla, 2001), the Behavior Rating Inventory of Executive Function (BRIEF; Gioia, Isquith, & Guy, 2000).
2.4. Neonatal data collection
During neonatal intensive care of the very preterm participants, daily chart reviews were conducted by an experienced research nurse to collect clinical information (for details see Grunau et al., 2009). In the present study we used GA, number of skin‐breaking procedures, cumulative morphine dose adjusted for daily weight, days on mechanical ventilation and early illness severity (SNAP‐II).
2.5. MEG analysis
Dipolar source solutions were calculated for three fiducial coils 30 times/s resulting in a continuous record of head position in the dewar for all participants individually. MEG data were down‐sampled to 600 Hz. The 60 Hz line noise was eliminated by applying a 2 Hz‐wide notch filter. For each participant, 15 segments of data were selected such that the deviations of the position of any fiducial coil from its median position were within 5 mm for any direction. The segments were 4 s long and did not overlap. Due to excessive movement in four subjects only 11–13 segments were available (one preterm girl, two preterm boys, one full‐term girl), which were still included in the analysis.
The following preprocessing steps were used to estimate comprised a reconstruction of neuromagnetic source activity using a beamformer. A head model was created for each subject using a single shell method as implemented in FieldTrip toolbox (Oostenveld, Fries, Maris, & Schoffelen, 2011). For those participants who did not have a T1 MRI image recorded, the pool of 73 child MRIs was searched for the best‐matched MRI for participant, as implemented in our previous study (Kozhemiako et al., 2019). Specifically, to choose the best match we calculated the mean distance between each Polhemus point and the closest point on the skull surface, which was derived from segmented MRI image using Fieldtrip toolbox. Subsequently, the MRI scans with the smallest aggregated distance between skull surface points and Polhemus points were selected for each participant. To ensure that the average distance was not different between our groups, we performed six two‐sampled t tests performed on a pair‐wise basis. The results are reported in Section 3. MEG and MRI were then co‐registered using fiducial and head shape information.
To estimate neuromagnetic activity originating from the cerebral cortex all 76 cortical regions of interests (ROIs) were selected from the automated anatomical labeling (AAL) atlas (Tzourio‐Mazoyer et al., 2002). Each brain region was represented by one seed computed as the center of mass, under an extra condition that the center is within the given region. MEG data were band‐pass filtered from 1 to 150 Hz to remove low‐frequency drifts and ultra‐high frequencies. Then, for each pair of brain regions, we applied a multiple constrained minimum variance (MCMV) beamformer to estimate neuromagnetic activity at two selected locations (Moiseev, Gaspar, Schneider, & Herdman, 2011). The main advantage of such approach is that it computes a unique inverse solution for each source pair nulling the contribution of one source onto another and alleviating a signal leakage (Moiseev et al., 2011).
2.6. Connectivity and statistical analysis
Functional connectivity is often quantified in terms of coordinated phases of neural dynamics reconstructed at spatially distinctive locations (Vakorin & Doesburg, 2016). In our case, for each seed location, a complex wavelet decomposition was applied for each segment to estimate phase dynamics for each epoch at 50 frequency points equally spaced on a logarithmic scale from 4 to 50 Hz (Flandrin, Auger, Gonçalvès, & Lemoine, 1996). More specifically, to estimate MEG functional connectivity, we calculated the phase‐locking value (PLV; Lachaux, Rodriguez, Martinerie, & Varela, 1999), a measure which quantifies stability of phase differences across time, for each pair of reconstructed neuromagnetic dynamics at each frequency point. PLV values were subsequently averaged across epochs for each source pair.
Partial least square (PLS) analysis was applied to test the significance of group differences in inter‐regional connectivity and its association with neonatal and long‐term behavioral and cognitive outcome in our participants. PLS is a multivariate technique, based on the singular value decomposition to decompose the entire data matrix (all the subjects within the groups by all the features) into latent variables (LVs) which explain the variance in the data, similar to principal component analysis (Lobaugh, West, & McIntosh, 2001; McIntosh & Lobaugh, 2004). Each LV has three components: (a) a vector, which can be interpreted as a group contrast; (b) a scalar value, which is ultimately related to explained variance; and (c) a vector of saliences, representing a contribution of each feature (a combination of frequency and source pairing) to the group contrast. Two types of PLS analysis were employed in the present study: mean‐centered PLS and behavioral PLS. Both versions of PLS analysis comprise a global test for significance of overall group contrast in connectivity (in mean‐centered PLS) or correlations between connectivity and neonatal or psychometric data (in behavioral PLS). The global test is based on random permutations of subjects across groups, resulting in one p‐value for a group contrast. Additionally, there are series of feature‐specific (local) tests based on bootstrapping subjects within the groups, performed with the goal to estimate the contribution of individual MEG features (PLV for unique combinations of frequencies and source pairs, in our case) to the overall group contrast or correlations. The resulting measures from the bootstrap resampling can be interpreted as z‐scores. The positive or negative z‐scores of 2.5 or −2.5 approximately corresponds to the 95% confidence interval (McIntosh & Lobaugh, 2004). In this study, the number of permutations and the number of bootstrap resamplings were equal to 5,000.
In Section 3, each significant LV obtained from the mean‐centered PLS indicates a contrast representing overall group differences, a corresponding p‐value from the global test, and a set of z‐scores, each expressing the robustness of contribution of a given source pair at a specific frequency to the overall group contrast. To assess the overall directionality of the group differences we compute the sample skewness (Sk) the sample median (Md) for each z‐score distribution. For the behavioral PLS p‐values and overall correlations between connectivity alterations and neonatal or psychometric scores are presented. Statistical differences in neonatal and psychometric scores were investigated by series of pair‐wise two‐tailed t tests with subsequent correction for multiple comparisons using Bonferroni method.
2.7. Testing the hypothesis of male disadvantage
To test our main hypothesis that very preterm boys are characterized by more pronounced connectivity alteration from same‐sex full‐term group than girls, the magnitude of connectivity alterations in preterm boys and girls were compared. For each subject from the preterm groups, the magnitude of connectivity alterations was defined for each unique combination of source pairing and frequency point as the absolute connectivity deviation from the same‐sex full‐term connectivity averaged across the full‐term boys or girls. Accordingly, the average connectivity for each connection and frequency bin was computed for full‐term boys and girls separately. Then for each individual male from the preterm group and for each MEG feature, the mean connectivity values of the full‐term male group were subtracted which resulted in connectivity deviation matrix for preterm boys. Similar procedures were conducted for preterm girls by subtracting the average connectivity values of the full‐term female group. Subsequently, a mean‐centered PLS analysis was performed to compare the absolute connectivity deviations from full‐term group between preterm boys and girls. Additionally, two separate mean‐centered PLS analyses were used to compare connectivity between preterm and full‐term participants for boys and girls separately.
To test if the deviations from the full‐term averaged connectivity in preterm groups are related to adverse neonatal experience and behavioral and cognitive abilities at school age, four behavioral PLS analyses were run. Thus, we investigated correlations, separately for preterm girls and boys, between the absolute deviations in functional connectivity, and two clusters of “behavioral” data: (a) neonatal factors and (b) behavioral and cognitive scores collected on the same day as MEG acquisition.
2.8. Analysis of sex differences in connectivity
To investigate sex differences in connectivity between the control groups, as well as how these differences are affected by very preterm birth, two mean‐centered PLS analyses were performed comparing the phase‐locking values between boys and girls in the full‐term and preterm groups, separately. Additionally, similarities in general sex differences pattern in full‐term and very preterm children were assessed by comparing the female/male connectivity ratio between the preterm and the full‐term group. The mean of connectivity across subjects was computed for each group resulting in one estimate of averaged connectivity for each MEG feature, specifically, for every connection at each frequency, per group. These values were used to calculate the female to male ratio by dividing the females' average connectivity estimate by averaged connectivity of males from the same group for each MEG feature. Two distributions of the ratio values (for full‐term and preterm groups) were thus obtained for each unique combination of source paring and frequency: ratio value >1 reflects higher connectivity in females, whereas ratio value <1 implies higher connectivity in males. To quantify the size effects of differences between two distributions Cohen's d was calculated. To assess spatial and temporal variations in the effect size of differences in female/male connectivity ratio Cohen's d was computed across source pairings within lobes (frontal, parietal, temporal, occipital and limbic) within the canonical bands (theta [4–8 Hz], alpha [8–13 Hz], beta [13–35 Hz], and gamma [35–50 Hz]), resulting in 16 distributions, in total, separately for preterm and full‐term cohorts. To check the significance of female/male ratio 20 random subsamples of 10 participants for all four groups were generated. Then, for each subsample female/male connectivity ratio for preterm and full‐term groups were computed separately, resulting in two matrices (subsamples × connections × frequency bins). Mean‐centered PLS analysis was performed comparing female/male connectivity ratio between the full‐term and preterm groups. It was performed similar to how the group differences in connectivity (regional synchrony) were explored, with two distinctions: (a) instead of phase‐locking value (PLV), our variable of interest was the female/male ratio in PLV; and (b) instead of subjects, our observations were subsamples.
3. RESULTS
3.1. Demographic, neonatal, and behavioral characteristics of the cohort
The results of statistical comparison between groups in clinical scores collected during neonatal period and psychometric scores assessed at school age are provided in the Table 1. The only significant differences in neonatal characteristics which survived after Bonferroni correction were lower GA and weight at birth for preterm boys versus full‐term boys, and preterm girls versus full‐term girls.
3.2. Best‐matched MRI selection
On average, the distance between the Polhemus digitization and reconstructed MRI‐based head surface was slightly smaller in the MRI‐matched participants compared to those with native MRI (2.1 ± 0.28 mm and 1.9 ± 0.34 mm, respectively). These differences, however, were not statistically significant across the four groups, according to the six two‐sampled t tests performed on a pair‐wise basis.
3.3. Preterm boys demonstrate more pronounced connectivity alterations
Using mean‐centered PLS analysis deviations in PLV from same‐sex full‐term groups in preterm boys and girls were compared. This analysis revealed significantly (p < .001) larger deviations in functional connectivity for the preterm boys with respect to the preterm girls, as illustrated on Figure 1a,b. The overall distribution of all the z‐scores for all connections and frequencies is shown in Figure 1c wherein the largest negative (z‐score <−2.5) and positive (z‐scores >2.5) tails are marked in blue and red color, respectively. This distribution was skewed toward positive z‐scores: the sample skewness Sk = −0.23, and the sample median Md = 0.39. In Figure 1d, the distribution of the tails, and c across frequencies. Specifically, for each frequency point, we calculated the number of connections associated with z‐scores, which were either higher than 2.5 or lower than −2.5 (the most robust effects). Such measures represent the strength of positive (deviations are higher for preterm boys) and negative (deviations are higher for preterm girls) effects. On average, preterm boys had larger deviations in connectivity from full‐term boys across all the frequencies with peaks around theta (4–8 Hz) and beta (13–25 Hz) bands, as compared to preterm girls' deviations from full‐term girls' connectivity.
Figure 1.
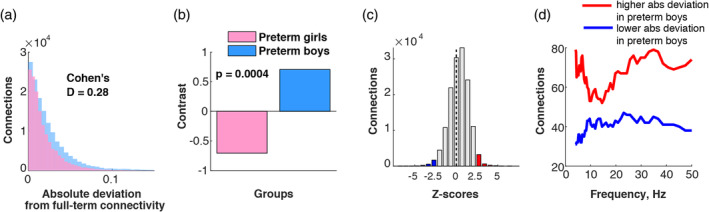
More pronounced connectivity alterations in preterm boys than girls: (a) distribution of absolute connectivity deviation from full‐term same‐sex groups in preterm males and females; (b) PLS contrast reflecting significant group difference in absolute connectivity deviation from full‐term groups; (c) distribution of z‐scores; (d) distribution of z‐scores across different frequencies [Color figure can be viewed at http://wileyonlinelibrary.com]
Another PLS analysis performed separately for boys and girls to investigate differences in connectivity between preterm and full‐term groups, revealed significant differences only for the boys (p = .03; Figure 2a). Comparisons of the preterm and full‐term girls in terms of their inter‐regional connectivity did not reach statistical significance (p = .13; Figure 2f). On average, preterm boys had significantly higher connectivity than full‐term boys of the same sex as illustrated in the group contrast (Figure 2a). Figure 2b displays the corresponding overall distribution of z‐scores, marking the two tails in red and blue in the same way as described above. The sample skewness of this distribution was close to zero (Sk = −0.003) but the median of 0.50 suggested that the distribution was skewed toward positive z‐scores. Note that given the contrast in Figure 2a, positive z‐scores reflect increases in connectivity in the preterm boys compared to the full‐term boys. Negative z‐scores in this case are associated with lower connectivity in the preterm boys. To compare the strength of these two effects and to identify the temporal patterns for increases and decreases in connectivity, we calculated the number of connections with z‐score > 2.5 and negative connections with z‐score <−2.5 for each frequency point (Figure 2c). On average, increased connectivity in the preterm boys dominated over decreased connectivity. The effects associated with increased connectivity in preterm boys were mostly located in lower frequencies including higher theta (6–8 Hz) and alpha (8–13 Hz). The spatial localizations of increased connectivity were scattered across the brain, mostly involving long‐range connections between frontal areas and the rest of the brain (Figure 2d,e).
Figure 2.
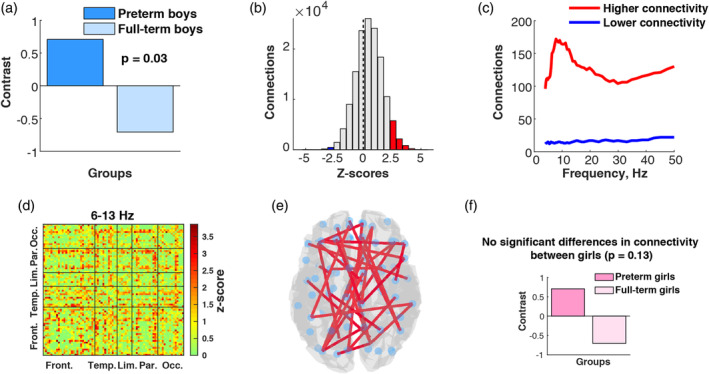
Connectivity differences between preterm and full‐term groups: (a) PLS contrast illustrating connectivity difference between preterm boys and full‐term boys; (b) distribution of z‐scores associated with PLS contrast between preterm boys and full‐term boys; (c) distribution of z‐scores across different frequencies that indicates on which frequencies the connectivity differences between preterm and full‐term boys were the most pronounced; (d) connectivity matrix reflecting spatial distribution of connections with positive z‐scores for 5.5–13 Hz; (e) 1% connections with highest z‐scores for the same frequency range in the brain space demonstrating higher connectivity in preterm boys compared to full‐term boys; (f) PLS contrast between preterm girls and full‐term girls [Color figure can be viewed at http://wileyonlinelibrary.com]
3.4. Association with neonatal factors and long‐term behavioral and cognitive outcome
Behavioral PLS analysis of associations between deviations in connectivity from the full‐term same‐sex group and adverse neonatal experience revealed significant overall associations (p = .024) for preterm girls (Figure 3I). The z‐score distribution was almost symmetrical, with skewness of −0.03, and positive median of 0.10 (Figure 3b). The distribution across frequencies illustrates that for most of the frequency bins there were more positive connections with z‐score >2.5 than negative connections with z‐score <−2.5 indicating that in general higher deviation from average full‐term connectivity in preterm girls was negatively correlated with gestational age and positively correlated with number of skin‐breaking procedures, total dose of morphine, duration of mechanical ventilation and early illness severity (Figure 3I,a). In preterm boys we found similar pattern of association with neonatal factors; however, it was significant only at 90% confidence interval (p = .098; Figure 3II). The z‐score distribution was negatively skewed (Sk = −0.32) with positive median (Md = 0.20) indicating more connections with positive z‐score and suggesting that, similarly to girls, the higher absolute deviation from averaged full‐term connectivity was positively associated with adverse neonatal experience and lower GA.
Figure 3.
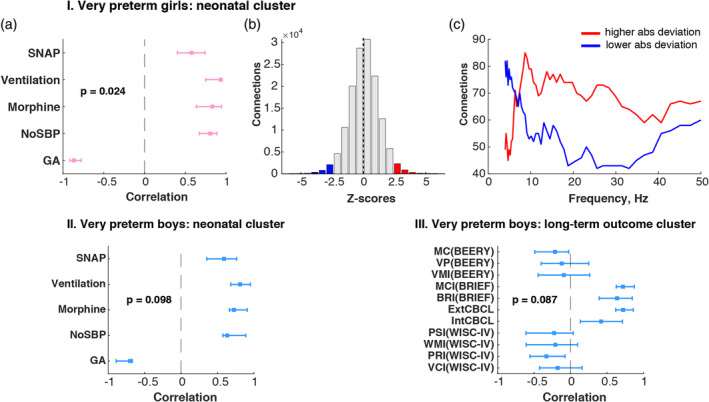
Associations between absolute connectivity deviation and adverse neonatal experience and long‐term behavioral and cognitive outcome: the graphs I, II and III illustrate the results from three separate behavioral PLS analyses investigating correlations between the absolute deviation in connectivity from the same‐sex full‐term group I. in preterm girls and neonatal factors: (I, a) PLS correlation coefficients are shown as a tick mark with whiskers representing bootstrap upper and lower boundary for correlation coefficient for each neonatal factor; (I, b) overall z‐score distribution; (I, c) z‐score distribution across frequencies. (II) correlations between connectivity deviations in preterm boys and neonatal factors; (III) correlations between connectivity deviations in preterm boys and behavioral and cognitive outcome at school age. Neonatal cluster: GA—gestational age, NoSBP—number of skin‐breaking procedures, Morphine—cumulative morphine dose with dosing adjusted for weight, Ventilation—days on mechanical ventilation and SNAP—early illness severity. Long‐term outcome cluster: VC(WISC‐IV)—verbal comprehension, PRI(WISC‐IV)—perceptual reasoning index, WMI(WISC‐IV)—working memory index, PSI(WISC‐IV)—processing speed index from the Wechsler Intelligence Scale for Children; IntCBCL—internalizing index, ExtCBCL—internalizing index from the Child Behavior Checklist; BRI(BRIEF)—behavioral regulation index, MC(BRIEF)—metacognition index from the Behavior Rating Inventory of Executive Function; VMI(BEERY)—visual‐motor integration, VP(BEERY)—visual perception from the Beery‐Buktenica Developmental Test of Visual‐Motor Integration (5th ed.) [Color figure can be viewed at http://wileyonlinelibrary.com]
When investigating the relationships between the deviations in connectivity and the long‐term behavioral and cognitive outcomes we did not find significant relationships for either of the preterm groups. However, in preterm boys we again registered a “trend” toward an association (p = .087). Slightly negative skewness (Sk = −0.13) of z‐score distribution and positive median (Md = 0.4) indicated that the majority of connections had positive z‐scores. Thus, on average higher absolute deviation from full‐term group in preterm boys was positively associated with Metacognition and Behavior Regulation Indices (BRIEF), Externalizing and Internalizing Behavior (CBCL) and had a negative relationship with visual‐motor integration (BEERY) and the Perceptual Reasoning Index (WISC‐IV). Note that for BRIEF and CBCL scores, higher score indicates more difficulties in corresponding behavioral or cognitive domains.
3.5. Sex differences in connectivity within full‐term and preterm groups
To investigate whether sex differences in connectivity are altered in preterm children, we ran two mean‐centered PLS analyses: preterm boys versus preterm girls and full‐term boys versus full‐term girls. While we did not find significant sex differences in the full‐term group (Figure 4f; p = .28), the comparison within preterm participants showed significant differences in inter‐regional connectivity between boys and girls (p = .002, Figure 4a). The distribution of z‐scores with sample skewness of −0.21 and a median of 0.46 indicated that the majority of connections had positive z‐scores (Figure 4b). That indicates overall increased connectivity in boys compared to girls. In particular, such overconnectivity was present in the low theta (4–6 Hz), alpha (8–13 Hz) and low beta (13–20 Hz) bands (Figure 4c). The spatial localization of the connectivity differences around 8–20 Hz was evident across the brain and involved mostly frontal and parietal connections (Figure 4d,e).
Figure 4.
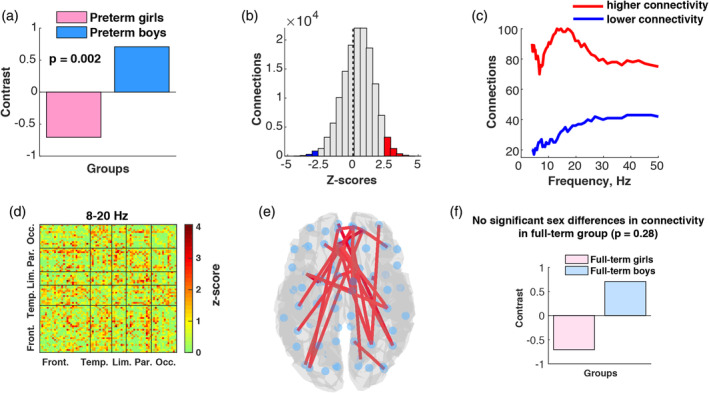
Sex differences in connectivity within full‐term and preterm groups: (a) PLS contrast demonstrating significant sex differences in connectivity in preterm groups; (b) z‐scores distribution associated with significant contrast between preterm boys and girls; (c) z‐scores distribution across frequency bins that illustrates which frequencies contributed the most to the revealed differences between preterm boys and girls; (d) spatial distribution of positive z‐scores in 8–20 Hz frequency range; (e) connections with the highest z‐score (top 1%) in the brain space reflecting higher connectivity in preterm boys compared to preterm girls; (f) PLS contrast between full‐term girls and full‐term boys [Color figure can be viewed at http://wileyonlinelibrary.com]
To further investigate the revealed sex differences in preterm children, we compared the average female/male connectivity ratio in preterm and full‐term children. Specifically, we were interested whether preterm children display exaggerated sex differences, which are present in full‐term group to a much smaller extent. Thus, we compared the distributions of female/male connectivity ratios between preterm and full‐term groups (Figure 5). The ratio values >1 mean higher connectivity in girls and ratio values <1 mean higher connectivity in boys. As illustrated in Figure 5, on average full‐term girls tended to have higher connectivity than boys, whereas the preterm children showed the opposite trend of higher connectivity in boys compared to girls. The largest differences between female/male ratio distribution in preterm and full‐term children as measured by Cohen's d, were observed in alpha frequency in parietal, limbic and temporal areas. Additionally, we run mean‐centered PLS analysis using randomly generated subsamples and confirmed that there was a significant difference (p < .001) in female/male connectivity ratio between full‐term and preterm groups.
Figure 5.
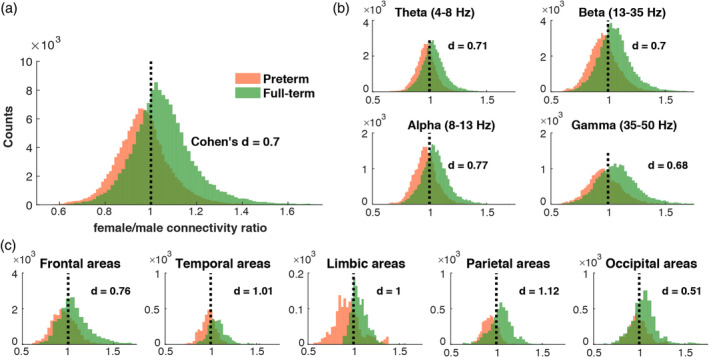
Female/male connectivity ratio distributions in preterm and full‐term groups: (a) averaged female/male connectivity ratio across all connections and frequencies; (b) averaged female/male connectivity ratio across canonical frequency bands; (c) averaged female/male connectivity ratio across brain areas [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
The present study investigated the neurophysiological bases of male vulnerability to adverse effects of very preterm birth resulting in poorer behavioral and cognitive outcomes later in life. We tested the hypothesis that very preterm boys would express more pronounced alterations in inter‐regional brain connectivity compared to very preterm girls. We found greater absolute deviation from the full‐term connectivity pattern in preterm boys compared to preterm girls and significant group differences in connectivity between preterm and full‐term boys, but not girls. These findings indicate that connectivity alterations, as apparent in preterm boys, were not present or at least not to the same extent in preterm girls further supporting our hypothesis. Similarly, in volumetric MRI studies, differences between preterm and full‐term boys have been previously reported at school age, with no differences among girls. For example, lower gray matter volumes in prefrontal cortex, basal ganglia, temporal lobe and lower white matter volumes in corpus callosum, corticospinal tract, prefrontal cortex have been reported in preterm boys compared to full‐term (Kesler et al., 2008). However, those results were shown not to be correlated with neonatal variables or cognitive outcome. Another study reported reduced white matter volume in preterm boys but not in girls; however, lower white matter volume was significantly associated with birth weight in both boys and girls (Reiss et al., 2004). These findings are in line with white matter dysmaturation frequently reported in the preterm population (Batalle et al., 2017; Fischi‐Gomez et al., 2016; Karolis et al., 2016). It was hypothesized that in the condition of white matter scarcity there is a tendency to prioritize rich‐club connections (maintain communication between “rich‐club” nodes in distributed brain networks) over local connections that sometimes even leads to stronger rich‐club architecture in preterms (Karolis et al., 2016). Thus, increased long‐range connectivity involving frontal connection in low frequencies reported in this study between very preterm and full‐term boys might reflect such prioritization strategy in very preterm brain. In contrast Kontis et al. (2009), in their study on adults born very preterm found that preterm females had increased mean diffusivity in corpus callosum compared to full‐term females which also correlated with lower Performance IQ, but neither differences in white matter microstructure nor its association with cognitive outcome were found for males (Kontis et al., 2009). However, due to the lack of demographic details separately for very preterm males and females, it is unknown if there were differences in sample size, age, GA, or cognitive outcome for males and females, which could possibly explain such unusual results.
Alternatively, the resting state overconnectivity might be an indicator of abnormal cortical maturation in very preterm boys. At school age, the brain is still in the process of tuning the connections to increase overall efficiency of information transduction and processing (Benasich & Ribary, 2018). Such age‐related brain reorganization is a complex dynamic process that involves axons growing and retracting with new synapses being formed while others being eliminated by pruning (Spear, 2013). It has been shown in animal models that during preadolescence dendritic ramification in prefrontal cortex follows a sex‐specific pattern which gets disrupted in males exposed to prenatal stress, whereas females remain unaffected (Markham, Mullins, & Koenig, 2013). Nevertheless, current understanding of the premature birth long‐term implications is far from complete and future investigations of the neurophysiological mechanisms of altered connectivity in children born very preterm will be of great importance.
Our analysis of associations between absolute deviation from full‐term connectivity and adverse neonatal experience and long‐term outcomes revealed a strong link between connectivity alterations and neonatal events in preterm girls, whereas no such correlation was observed with cognitive and behavioral performance at school age. Preterm boys, in turn, demonstrated a “trend” toward significant association between connectivity deviation and both neonatal factors and long‐term outcomes. These results suggest that connectivity alterations in girls might be only partially impacted by adverse neonatal events but do not relate to later behavioral and cognitive abilities whereas connectivity alteration in boys seem to be indicative of both, although only at the 90% confidence interval.
In the present study, we showed that significant sex differences in connectivity are present between preterm boys and girls, but not in full‐term children. Interestingly, the spatial and temporal pattern of connectivity differences between preterm girls and preterm boys were similar to the differences between preterm boys and full‐term boys. Potentially, this might indicate that pathological connectivity alterations in preterm boys were differentiating them from all other groups. In a study on ERP oddball task responses in children at age 6 years, there were opposite results of significant sex differences in the full‐term group showing larger amplitude of P3 component in full‐term boys, and no sex difference in the extremely preterm group (Lavoie, Robaey, Stauder, Glorieux, & Lefebvre, 1998). However, similar to our results, the discrepancy between sex differences in full‐term but not in the extremely preterm group was due to atypical preterm male brain response. Extremely preterm boys had diminished amplitude of P3, while extremely preterm girls displayed amplitudes typical to full‐term girls. This motivated us to compare the overall pattern of full‐term and preterm female/male connectivity ratio that distinctly showed that sex differences pattern in preterm children does not resemble the full‐term. We confirmed that sex differences observed between preterm boys and preterm girls do not represent magnified full‐term sex differences, but rather appear to reflect unequal alteration in brain connectivity in this vulnerable population.
This study provides the first evidence of more pronounced alterations of inter‐regional neurophysiological connectivity in very preterm boys than in girls that potentially might reflect neurophysiological basis of male disadvantage in very preterm children. The investigation of sex differences in expression of preterm birth consequences on brain function is of great importance, to delineate underlying neurophysiological mechanisms which are responsible for the long‐term behavioral and cognitive alterations related to prematurity. Moreover, understanding sex‐specific mechanisms is important for development of targeted therapeutic strategies.
4.1. Limitations
The data collection did not include EOG or ECG electrodes, limiting capacity for accurate monitoring of eye movements, given that children born very preterm tend to display high anxiety in novel situations and poor attention span, therefore might ask to end the session. The length of resting state recording was only 3 min, which was further reduced to 60 s after discarding the segments of data with head motion exceeding 5 mm in any direction.
For some participants, the structural MRI was not acquired so the best match of other T1 scans was used for the headmodel creation. Although for the MEG data analysis the best match approach was applied, the absence of native MRI scans for some children limited our investigation in terms of volumetric properties of gray matter and white matter and their association with higher connectivity in very preterm boys. Despite these limitations inherent to studying children born very preterm at age 7–8 years, our findings provide novel information on neurophysiological function in very preterm boys that will inform future studies in this field.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
ACKNOWLEDGMENTS
We are grateful to Dr. Ken Poskitt, pediatric neuroradiologist, for his invaluable assistance in obtaining the MRI scans. In addition, we thank Ivan Cepeda and Gisela Gosse for coordinating the study, Katia Jitlina, Amanda Degenhardt, Teresa Cheung and Julie Unterman for their help in data collection. This study was supported by grant R01 HD039783 from the Eunice Kennedy Shriver Institute of Child Health and Human Development (R.E.G.), the Canadian Institutes of Health Research grants MOP42469 (R.E.G.) and MOP‐136935 (S.M.D.). This research was enabled in part by support provided by WestGrid (http://www.westgrid.ca) and Compute Canada (http://www.computecanada.ca).
Kozhemiako N, Nunes AS, Vakorin VA, et al. Sex differences in brain connectivity and male vulnerability in very preterm children. Hum Brain Mapp. 2020;41:388–400. 10.1002/hbm.24809
Ruth E. Grunau and Sam M. Doesburg are co‐senior authors.
Funding information Canadian Institutes of Health Research, Grant/Award Numbers: MOP‐136935, MOP42469; Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: R01 HD039783
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request, pending permission from the Clinical Research Ethics Board at the University of British Columbia and BC Children's & Women's Hospitals.
REFERENCES
- Aarnoudse‐Moens, C. S. H. , Weisglas‐Kuperus, N. , van Goudoever, J. B. , & Oosterlaan, J. (2009). Meta‐analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics, 124, 717–728 Retrieved from http://pediatrics.aappublications.org/cgi/doi/10.1542/peds.2008-2816 [DOI] [PubMed] [Google Scholar]
- Achenbach, T. , & Rescorla, L. (2001). Manual for the ASEBA school‐age forms & profiles: An integrated system of multi‐informant assessment. Burlington: University of Vermont, Research Center for Children, Youth & Families. [Google Scholar]
- Ball, G. , Aljabar, P. , Nongena, P. , Kennea, N. , Gonzalez‐Cinca, N. , Falconer, S. , … Edwards, A. D. (2017). Multimodal image analysis of clinical influences on preterm brain development. Annals of Neurology, 82, 233–246 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/28719076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett, M. L. , Tusor, N. , Ball, G. , Chew, A. , Falconer, S. , Aljabar, P. , … Counsell, S. J. (2017). Exploring the multiple‐hit hypothesis of preterm white matter damage using diffusion MRI. NeuroImage: Clinical, 17, 596–606 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/29234596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalle, D. , Hughes, E. J. , Zhang, H. , Tournier, J.‐D. , Tusor, N. , Aljabar, P. , … Counsell, S. J. (2017). Early development of structural networks and the impact of prematurity on brain connectivity. NeuroImage, 149, 379–392 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/28153637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery, R. , Buktenika, N. , & Beery, N. (2004). Beery‐Buktenica developmental test of visual‐motor integration (5th ed.). Minneapolis, MN: Psychological Corporation; Retrieved from http://www.pearsonclinical.co.uk/Psychology/ChildCognitionNeuropsychologyandLanguage/ChildPerceptionandVisuomotorAbilities/Beery-BuktenicaDevelopmental(BeeryVMI)/Beery-BuktenicaDevelopmentalTestofVisual-MotorIntegrationSixthEdition(BeeryVMI).aspx [Google Scholar]
- Benasich, A. , & Ribary, U. (2018). In Benasich A. & Ribary U. (Eds.), Emergent brain dynamics: Prebirth to adolescence. Cambridge, MA: The MIT Press. [Google Scholar]
- Benavides, A. , Metzger, A. , Tereschenko, A. , Conrad, A. , Bell, E. F. , Spencer, J. , … Nopoulos, P. (2018). Sex‐specific alterations in preterm brain. Pediatric Research, 85, 55–62 Retrieved from http://www.nature.com/articles/s41390-018-0187-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutta, A. T. , Cleves, M. A. , Casey, P. H. , Cradock, M. M. , & Anand, K. J. S. (2002). Cognitive and behavioral outcomes of school‐aged children who were born preterm. JAMA, 288, 728 Retrieved from http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.288.6.728 [DOI] [PubMed] [Google Scholar]
- Constable, R. T. , Ment, L. R. , Vohr, B. R. , Kesler, S. R. , Fulbright, R. K. , Lacadie, C. , … Reiss, A. R. (2008). Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: An investigation of group and gender effects. Pediatrics, 121, 306–316 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18245422 [DOI] [PubMed] [Google Scholar]
- Fischi‐Gomez, E. , Muñoz‐Moreno, E. , Vasung, L. , Griffa, A. , Borradori‐Tolsa, C. , Monnier, M. , … Hüppi, P. S. (2016). Brain network characterization of high‐risk preterm‐born school‐age children. NeuroImage: Clinical, 11, 195–209 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/26955515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandrin, P. , Auger, F. , Gonçalvès, P. , & Lemoine, O. (1996). Time‐frequency toolbox for use with MATLAB reference guide. Retrieved from http://tftb.nongnu.org/refguide.pdf
- Gioia, G. , Isquith, P. , Guy, S. , & Kenworthy, L. (2000). BRIEF—Behavior rating inventory of executive function, professional manual. In Child neuropsychology, 6, 235–238 Retrieved from https://www.researchgate.net/profile/Gerard_Gioia/publication/233366509_TEST_REVIEW_behavior_rating_inventory_of_executive_function/links/5bfac72792851ced67d6f109/TEST-REVIEW-behavior-rating-inventory-of-executive-function.pdf [Google Scholar]
- Grunau, R. E. , Haley, D. W. , Whitfield, M. F. , Weinberg, J. , Yu, W. , & Thiessen, P. (2007). Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. The Journal of Pediatrics, 150, 151–156 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17236892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau, R. E. , Whitfield, M. F. , & Fay, T. B. (2004). Psychosocial and academic characteristics of extremely low birth weight (< or = 800 g) adolescents who are free of major impairment compared with term‐born control subjects. Pediatrics, 114, e725–e732 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7936899 [DOI] [PubMed] [Google Scholar]
- Grunau, R. E. , Whitfield, M. F. , Petrie‐Thomas, J. , Synnes, A. R. , Cepeda, I. L. , Keidar, A. , … Johannesen, D. (2009). Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain, 143, 138–146 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19307058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindmarsh, G. J. , O'Callaghan, M. J. , Mohay, H. A. , & Rogers, Y. M. (2000). Gender differences in cognitive abilities at 2 years in ELBW infants: Extremely low birth weight. Early Human Development, 60, 115–122 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11121674 [DOI] [PubMed] [Google Scholar]
- Hintz, S. , Kendrick, D. , Vohr, B. , Kenneth Poole, W. , Higgins, R. , & The NICHD Neonatal Research Network . (2006). Gender differences in neurodevelopmental outcomes among extremely preterm, extremely‐low‐birthweight infants. Acta Paediatrica, 95, 1239–1248 Retrieved from http://doi.wiley.com/10.1080/08035250600599727 [DOI] [PubMed] [Google Scholar]
- Ingemarsson, I. (2003). Gender aspects of preterm birth. BJOG, 110, 34–38 Retrieved from http://doi.wiley.com/10.1046/j.1471-0528.2003.00022.x [DOI] [PubMed] [Google Scholar]
- Karolis, V. R. , Froudist‐Walsh, S. , Brittain, P. J. , Kroll, J. , Ball, G. , Edwards, A. D. , … Nosarti, C. (2016). Reinforcement of the Brain's Rich‐Club architecture following early neurodevelopmental disruption caused by very preterm birth. Cerebral Cortex, 26, 1322–1335 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/26742566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersbergen, K. J. , Makropoulos, A. , Aljabar, P. , Groenendaal, F. , de Vries, L. S. , Counsell, S. J. , & Benders, M. J. N. L. (2016). Longitudinal regional brain development and clinical risk factors in extremely preterm infants. The Journal of Pediatrics, 178, 93–100.e6 Retrieved from https://www.sciencedirect.com/science/article/pii/S0022347616306916?via%3Dihub [DOI] [PubMed] [Google Scholar]
- Kesler, S. R. , Reiss, A. L. , Vohr, B. , Watson, C. , Schneider, K. C. , Katz, K. H. , … Ment, L. R. (2008). Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. The Journal of Pediatrics, 152, 513–520.e1 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18346506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontis, D. , Catani, M. , Cuddy, M. , Walshe, M. , Nosarti, C. , Jones, D. , … Allin, M. (2009). Diffusion tensor MRI of the corpus callosum and cognitive function in adults born preterm. Neuroreport, 20, 424–428 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19218872 [DOI] [PubMed] [Google Scholar]
- Kozhemiako, N. , Nunes, A. , Vakorin, V. A. , Chau, C. M. Y. , Moiseev, A. , Ribary, U. , …, Doesburg, S. M. . (2019). Atypical resting state neuromagnetic connectivity and spectral power in very preterm children. Retrieved from https://authorservices.wiley.com/api/pdf/fullArticle/16319025 [DOI] [PubMed]
- Lachaux, J.‐P. , Rodriguez, E. , Martinerie, J. , & Varela, F. J. (1999). Measuring phase synchrony in brain signals. Human Brain Mapping, 8, 194–208 Retrieved from http://doi.wiley.com/10.1002/%28SICI%291097-0193%281999%298%3A4%3C194%3A%3AAID-HBM4%3E3.0.CO%3B2-C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie, M. E. , Robaey, P. , Stauder, J. E. A. , Glorieux, J. , & Lefebvre, F. (1998). Extreme prematurity in healthy 5‐year‐old children: A re‐analysis of sex effects on event‐related brain activity. Psychophysiology, 35, 679–689 Retrieved from http://doi.wiley.com/10.1111/1469-8986.3560679 [PubMed] [Google Scholar]
- Lawn, J. E. , Cousens, S. , Zupan, J. , & Lancet Neonatal Survival Steering Team . (2005). 4 million neonatal deaths: When? Where? Why? Lancet, 365, 891–900 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15752534 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Metens, T. , Absil, J. , De Maertelaer, V. , Balériaux, D. , David, P. , … Aeby, A. (2011). Gender differences in language and motor‐related fibers in a population of healthy preterm neonates at term‐equivalent age: A diffusion tensor and probabilistic Tractography study. American Journal of Neuroradiology, 32, 2011–2016 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21940804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobaugh, N. J. , West, R. , & McIntosh, A. R. (2001). Spatiotemporal analysis of experimental differences in event‐related potential data with partial least squares. Psychophysiology, 38, 517–530 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11352141 [DOI] [PubMed] [Google Scholar]
- Loe, I. M. , Lee, E. S. , Luna, B. , & Feldman, H. M. (2011). Behavior problems of 9–16 year old preterm children: Biological, sociodemographic, and intellectual contributions. Early Human Development, 87, 247–252 Retrieved from https://www.sciencedirect.com/science/article/pii/S0378378211000582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Månsson, J. , Fellman, V. , Stjernqvist, K. , & EXPRESS Study Group (authors) . (2015). Extremely preterm birth affects boys more and socio‐economic and neonatal variables pose sex‐specific risks. Acta Paediatrica, 104, 514–521 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25620552 [DOI] [PubMed] [Google Scholar]
- Markham, J. A. , Mullins, S. E. , & Koenig, J. I. (2013). Periadolescent maturation of the prefrontal cortex is sex‐specific and is disrupted by prenatal stress. The Journal of Comparative Neurology, 521, 1828–1843 Retrieved from http://doi.wiley.com/10.1002/cne.23262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh, A. R. , & Lobaugh, N. J. (2004). Partial least squares analysis of neuroimaging data: Applications and advances. NeuroImage, 23, 250–263. [DOI] [PubMed] [Google Scholar]
- Moiseev, A. , Gaspar, J. M. , Schneider, J. A. , & Herdman, A. T. (2011). Application of multi‐source minimum variance beamformers for reconstruction of correlated neural activity. NeuroImage, 58, 481–496 Retrieved from https://www.sciencedirect.com/science/article/pii/S1053811911006276 [DOI] [PubMed] [Google Scholar]
- Moster, D. , Lie, R. T. , & Markestad, T. (2008). Long‐term medical and social consequences of preterm birth. The New England Journal of Medicine, 359, 262–273 Retrieved from http://www.nejm.org/doi/abs/10.1056/NEJMoa0706475 [DOI] [PubMed] [Google Scholar]
- Oostenveld, R. , Fries, P. , Maris, E. , & Schoffelen, J.‐M. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011, 1–9 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21253357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock, J. L. , Marston, L. , Marlow, N. , Calvert, S. A. , & Greenough, A. (2012). Neonatal and infant outcome in boys and girls born very prematurely. Pediatric Research, 71, 305–310. http://www.nature.com/articles/pr201150 [DOI] [PubMed] [Google Scholar]
- Phillips, J. , Yeo, R. A. , Caprihan, A. , Cannon, D. C. , Patel, S. , Winter, S. , … Ohls, R. K. (2017). Neuroimaging in former preterm children who received erythropoiesis stimulating agents. Pediatric Research, 82, 685–690 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/28553989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranger, M. , Synnes, A. R. , Vinall, J. , & Grunau, R. E. (2014). Internalizing behaviours in school‐age children born very preterm are predicted by neonatal pain and morphine exposure. European Journal of Pain, 18, 844–852 Retrieved from http://doi.wiley.com/10.1002/j.1532-2149.2013.00431.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss, A. L. , Kesler, S. R. , Vohr, B. , Duncan, C. C. , Katz, K. H. , Pajot, S. , … Ment, L. R. (2004). Sex differences in cerebral volumes of 8‐year‐olds born preterm. The Journal of Pediatrics, 145, 242–249 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15289777 [DOI] [PubMed] [Google Scholar]
- Rose, J. , Butler, E. E. , Lamont, L. E. , Barnes, P. D. , Atlas, S. W. , & Stevenson, D. K. (2009). Neonatal brain structure on MRI and diffusion tensor imaging, sex, and neurodevelopment in very‐low‐birthweight preterm children. Developmental Medicine and Child Neurology, 51, 526–535 Retrieved from http://doi.wiley.com/10.1111/j.1469-8749.2008.03231.x [DOI] [PubMed] [Google Scholar]
- Schindler, T. , Koller‐Smith, L. , Lui, K. , Bajuk, B. , Bolisetty, S. , & New South Wales and Australian Capital Territory Neonatal Intensive Care Units' Data Collection NSW and ACTNICUD . (2017). Causes of death in very preterm infants cared for in neonatal intensive care units: A population‐based retrospective cohort study. BMC Pediatrics, 17, 59 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/28222717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiöld, B. , Alexandrou, G. , Padilla, N. , Blennow, M. , Vollmer, B. , & Adén, U. (2014). Sex differences in outcome and associations with neonatal brain morphology in extremely preterm children. The Journal of Pediatrics, 164, 1012–1018 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24530122 [DOI] [PubMed] [Google Scholar]
- Spear, L. P. (2013). Adolescent Neurodevelopment. Journal of Adolescent Health, 52, S7–S13 Retrieved from https://www.sciencedirect.com/science/article/pii/S1054139X12002078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teli, R. , Hay, M. , Hershey, A. , Kumar, M. , Yin, H. , & Parikh, N. A. (2018). Postnatal microstructural developmental trajectory of corpus callosum subregions and relationship to clinical factors in very preterm infants. Scientific Reports, 8, 7550 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/29765059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, D. K. , Kelly, C. E. , Chen, J. , Beare, R. , Alexander, B. , Seal, M. L. , … Cheong, J. L. Y. (2018). Early life predictors of brain development at term‐equivalent age in infants born across the gestational age spectrum. NeuroImage, 185, 813–824 Retrieved from https://www.sciencedirect.com/science/article/pii/S1053811918303409?via%3Dihub [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15, 273–289 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11771995 [DOI] [PubMed] [Google Scholar]
- Urben, S. , Van Hanswijck De Jonge, L. , Barisnikov, K. , Pizzo, R. , Monnier, M. , Lazeyras, F. , … Hüppi, P. S. (2017). Gestational age and gender influence on executive control and its related neural structures in preterm‐born children at 6 years of age. Child Neuropsychol, 23, 188–207 Retrieved from https://www.tandfonline.com/doi/full/10.1080/09297049.2015.1099619 [DOI] [PubMed] [Google Scholar]
- Vakorin, V. A. , & Doesburg, S. M. (2016). Development of human neurophysiological activity and network dynamics In Palva S. (Ed.), Multimodal oscilation‐based connectivity theory (pp. 107–122). AG Switzerland: Springer. [Google Scholar]
- van Kooij, B. J. M. , van Pul, C. , Benders, M. J. N. L. , van Haastert, I. C. , de Vries, L. S. , & Groenendaal, F. (2011). Fiber tracking at term displays gender differences regarding cognitive and motor outcome at 2 years of age in preterm infants. Pediatric Research, 70, 626–632 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21857376 [DOI] [PubMed] [Google Scholar]
- Vasileiadis, G. T. , Thompson, R. T. , Han, V. K. M. , & Gelman, N. (2009). Females follow a more “compact” early human brain development model than males. A case–control study of preterm neonates. Pediatric Research, 66, 551–554 Retrieved from http://www.nature.com/doifinder/10.1203/PDR.0b013e3181ba1ae7 [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (2003). Wechsler intelligence scale for children. 4th ed. (WISC‐IV). San‐Antonio, TX: Psychological Corporation; Retrieved from http://ux1.eiu.edu/~glcanivez/Adobepdf/Publications-Papers/Canivez(2014)BurosMMYWPPSI-IVReview.pdf [Google Scholar]
- Whitfield, M. F. , Grunau, R. E. , & Holsti, L. (1997). Extremely premature (< or = 800 g) schoolchildren: Multiple areas of hidden disability. Archives of Disease in Childhood. Fetal and Neonatal Edition, 77, F85–F90 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9377151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolke, D. , Samara, M. , Bracewell, M. , & Marlow, N. (2008). Specific language difficulties and school achievement in children born at 25 weeks of gestation or less. The Journal of Pediatrics, 152, 256–262.e1 Retrieved from https://www.sciencedirect.com/science/article/pii/S0022347607006506?via%3Dihub [DOI] [PubMed] [Google Scholar]
- Wood, N. S. , Costeloe, K. , Gibson, A. T. , Hennessy, E. M. , Marlow, N. , Wilkinson, A. R. , & EPICure Study Group . (2005). The EPICure study: Associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Archives of Disease in Childhood. Fetal and Neonatal Edition, 90, F134–F140 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15724037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, J. M. , Morgan, B. R. , Powell, T. L. , Moore, A. M. , Whyte, H. E. A. , Lou, S. M. , & Taylor, M. J. (2016). Associations of perinatal clinical and magnetic resonance imaging measures with developmental outcomes in children born very preterm. The Journal of Pediatrics, 170, 90–96 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/26707586 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request, pending permission from the Clinical Research Ethics Board at the University of British Columbia and BC Children's & Women's Hospitals.


