Abstract
The cellular neurobiology of schizophrenia remains poorly understood. We discuss neuroimaging studies, pathological findings, and experimental work supporting the idea that glial cells might contribute to the development of schizophrenia. Experimental studies suggest that abnormalities in the differentiation competence of glial progenitor cells lead to failure in the morphological and functional maturation of oligodendrocytes and astrocytes. We propose that immune activation of microglial cells during development, superimposed upon genetic risk factors, could contribute to defective differentiation competence of glial progenitor cells. The resulting hypomyelination and disrupted white matter integrity might contribute to transmission desynchronisation and dysconnectivity, whereas the failure of astrocytic differentiation results in abnormal glial coverage and support of synapses. The delayed and deficient maturation of astrocytes might, in parallel, lead to disruption of glutamatergic, potassium, and neuromodulatory homoeostasis, resulting in dysregulated synaptic transmission. By highlighting a role for glial cells in schizophrenia, these studies potentially point to new mechanisms for disease modification.
Introduction
Schizophrenia is a chronic and debilitating psychiatric disorder, with accelerated mortality and profound morbidity1. As a syndrome, schizophrenia progresses through at least three phases: the prodromal (prepsychotic) phase, the initial onset of psychosis, and chronic illness2. The core symptomology of schizophrenia includes social, emotional, perceptive, and cognitive domains, and its clinical phenotype can be subdivided into positive and negative symptoms, and those of cognitive impairment2. In addition, most patients have abnormal sleep patterns, and sleep disturbances tend to precede clinical onset3. Cognitive impairments are apparent early in the prodromal phase, and can be used to follow disease progression and therapeutic outcomes in patients4. An established core feature of cognitive impairment in schizophrenia is diminished working memory, which can occur in patients who are drug naive or medicated, and in both early and chronic phases of the disease5. The dorsolateral prefrontal cortex is a primary site involved in working memory, and its functional impairment parallels the cognitive decline in schizophrenia6. Patients with schizophrenia have altered neural oscillatory patterns in the dorsolateral prefrontal cortex when performing working memory tasks7, and abnormal neural oscillations occur in other cortical areas throughout all clinical phases of schizophrenia, independent of disease phenotype7. This observation has led to the hypothesis that oscillatory abnormalities are a core endophenotype of schizophrenia, which suggests that broad cortical dysconnectivity underpins the symptomology of this disorder7.
Schizophrenia has a substantial genetic component, but its penetrance, phenotype, and severity are environmentally modulated8; its clinical manifestations comprise a final common pathway of multiple genetic and epigenetic influences8. Twin studies have established a strong genetic basis for schizophrenia, with concordance rates estimated at 33% for monozygotic twin pairs, compared with 7% in dizygotic twin pairs9. In adoption studies, children with a high genetic risk of developing schizophrenia are more sensitive to adverse rearing than children at lower genetic risk10. The genetic susceptibility for schizophrenia is therefore sensitive to environmental and social risk factors, such that a high prevalence of adverse developmental events increases the risk of schizophrenia later in life8. Developmental risk factors include physical or emotional maternal stress during the first trimester of pregnancy, maternal infections during the second and third trimesters, obstetric complications, and exposure to various infectious agents and their attendant inflammatory responses11. To what extent any single factor increases susceptibility to schizophrenia remains unclear8,11.
Disease phenotype and the associated genetic, histopathological, and imaging biomarkers vary greatly across patients, as well as by brain region and even in between cortical layers12-17. Complicating matters further, schizophrenia research is highly diverse, and the heterogeneity in study designs has led to a difficulty in controlling and standardising variables, such as the brain regions studied, the rate of disease progression, drug treatment history, lifestyle factors, and cause of death12-15,18,19.
White matter pathology in schizophrenia
At the onset of psychosis, the anatomic pathology of schizophrenia includes structural changes in both grey and white matter across multiple brain regions, with the most severe changes being in forebrain tract connectivity18,20. White matter loss can precede obvious clinical symptoms in patients who are drug naive20, and progresses throughout the course of clinical manifestation20. The structural environment of axons can be assessed using diffusion tensor imaging, which is a technique based on MRI that assesses changes in white matter fractional anisotropy—a measure of molecular water diffusibility21. In patients with schizophrenia, abnormal fractional anisotropy appears in multiple subcortical tracts, suggesting reduced white matter tract integrity. Much of the forebrain subcortical white matter is affected, including the cingulum, as is the white matter underlying the parietal regions, occipital regions, and prefrontal cortex21. Fractional anisotropy reductions are especially evident in the white matter below the cingulum and prefrontal cortex, and across the entire superior longitudinal fasciculus, which is the principal frontoparietal white matter connection21, as well as in the arcuate and uncinate fasciculi, which connect limbic regions21.
The frontoparietal circuitry is particularly associated with working memory6. Using a combination of diffusion tensor imaging and functional MRI during a memory task, a direct correlation was noted between reduced fractional anisotropy in the right medial temporal and right frontal lobes and relative hypoactivation in the prefrontal, superior parietal, and occipital regions of patients with schizophrenia (with hypoactivation detected as a diminished blood-oxygen-level dependent signal) 22. Thus, deficits in frontoparietal network coordination might underlie the working memory deficits often observed in patients with schizophrenia23.
Abnormalities in fractional anisotropy are associated with other typical symptoms of schizophrenia, including auditory hallucinations23, the passivity of abulia, and impaired executive functions24. Furthermore, the extent of white matter abnormalities is associated with the severity of symptoms in schizophrenia; in patients with first-episode schizophrenia who are drug naive, those patients with more widespread white matter abnormalities show more severe negative symptoms than those with less widely distributed abnormalities25. This finding is consistent with observations that the internal capsule is smaller in patients with schizophrenia who have a poor outcome than in those who have a good outcome26. Chronic administration of the dopamine, serotonin, norepinephrine antagonist clozapine leads to increased fractional anisotropy values in several brain regions, suggesting that clozapine treatment can restore white matter microstructural integrity27, with attendant symptomatic improvement.
Symptoms of psychosis are observed in other diseases affecting white matter integrity, including the leukodystrophies, demyelinating disorders, infiltrating glial neoplasms, and callosal anomalies, among others28. These diverse diseases share a disruption of normal myelin development or maintenance, and psychosis is more severe in diseases with nonfunctional myelin formation than in those with disrupted myelinated structures28. In addition, the concentration of N-acetylaspartate is raised in the white matter of patients with schizophrenia, suggesting both myelin loss and axonal abnormalities29. Together, these data demonstrate an association between white matter changes, myelin loss, and altered circuit activation in distal regions22, thereby linking intracortical dysconnectivity with the core features of schizophrenia. An open question is whether defective oligodendrocytic function is primary or secondary to dysfunctional neural circuits in schizophrenia. We will argue that white matter pathology is a common feature of schizophrenia, and that it results from a primary, cell-intrinsic failure in the differentiation of glial progenitor cells.
Glial dysfunction in schizophrenia
The hypothesis that higher brain function solely comprises the integrated product of neuronal activity has been challenged by evidence showing the importance of glial cells to both the development and structure of local neural networks. Both macroglia—including oligodendrocytes30, glial progenitor cells31, and astrocytes32—and microglial cells33 contribute to the modulation and synchronization of neuronal activity. As such, macroglia and microglial cells influence information processing and the structural and functional plasticity of neural networks. Together, these functional roles, the evolution of astrocyte complexity with phylogeny, and the coincident appearance of neuropsychiatric disorders with hominid evolution all combine to suggest the strong contribution of glial pathology to psychiatric diseases34,35.
Although glial involvement in schizophrenia has long been considered12,14,15,18,19,34-36, the nature of its contribution to schizophrenia pathology has remained unclear. All four glial cell types have been reported as showing dysregulated patterns of gene expression in postmortem tissue from patients with schizophrenia, in both cortical and subcortical regions35. The involvement of glial cells in schizophrenia is supported by RNA sequencing data37,38 and genome-wide association studies39,40. These data point to the enrichment of genes related to development in schizophrenia risk loci37,40, and in particular suggest the dysregulation of neuroinflammatory pathways and upregulation of genes related to astrocytes in schizophrenia38,39. RNA expression data suggested a predominant neuronal pathogenesis of schizophrenia41, but that particular study was limited to postmortem analysis of adult brain tissue. Limiting data to postmortem analysis of adult brain tissue is insensitive to the transcriptional dysregulation of glial development, which might have profound consequences for adult neural network structure42. Indeed, substantial glial developmental pathology and consequent neural network dysfunction might leave only a weak RNA expression signature in adult glial cells. As such, defining the extent to which glial pathology in schizophrenia is causal or merely secondary to neuronal pathology has been difficult to establish.
Fundamentally, an absence of adequate experimental tools32 and the evolutionary gap between rodent and human astrocytes43 have underlaid the difficulties in assessing the relative causal contribution of glial pathology to schizophrenia. To address this question, Windrem and colleagues42 developed a humanised chimeric mouse model by engrafting glial progenitor cells—prepared from induced pluripotent stem cells derived from patients with juvenile-onset schizophrenia or their age-matched controls—into neonatal, congenitally hypomyelinated mice. The human glial progenitor cells outcompeted their host counterparts to colonise the recipient mouse brains, where they differentiated into myelinating oligodendrocytes and astrocytes; the resultant mice developed patient-specific, largely humanised forebrain white matter42. The schizophrenia-derived human glial chimeras manifested delayed and deficient glial differentiation, which was associated with diminished oligodendrocyte maturation and impaired central myelination42. The structural maturation of astrocytes was likewise impaired, and showed fewer primary processes, less proximal branching, and less coherent domain structure than did littermate controls42. These schizophrenia-derived glial chimeric mice displayed higher anxiety levels, diminished auditory prepulse inhibition, anhedonia, social deficits, impairment in executive memory, hyperactivity, and disrupted sleep compared with littermate controls42. These signs are all consistent with the hypothesis that a deficit in glial functions contributes to the behavioural phenotype of these mice. Furthermore, RNA sequencing of glial progenitor cells derived from patients with schizophrenia—the parental source of both oligodendrocytes and astrocytes—revealed substantially downregulated expression of a host of genes associated with glial differentiation relative to normal glia42. The defective differentiation of glial progenitor cells was linked to the impairment of potassium uptake, which was rescued by the shRNAi-mediated knock-down of the REST repressor, binding sites that were noted in the regulatory regions of multiple glial potassium transporters. These data indicated that targeting REST-dependent transcriptional repression might rescue astroglial differentiation and potassium transport in schizophrenia44. This group of experimental observations suggests that glial progenitor cells contribute to schizophrenia, or at least to juvenileonset disease in an in vivo, humanised glial chimeric mouse devoid of pre-existing neuronal pathology. In the following sections, we discuss each of the four types of glial cells in terms of their function and their connection to schizophrenia.
Oligodendrocytes
Myelination is a dynamic process that adapts to external stimuli, conforming to the activity-dependent requirements of the maturing neural network long after early ontogeny. Acquiring new skills is associated with an increase in myelination45, whereas sensory deprivation46 and maternal rearing of mice results in hypomyelination47, including reduced myelin thickness and gene expression in the prefrontal cortex46, and irreversible myelination alterations in the medial prefrontal cortex47. The medial prefrontal cortex alterations are accompanied by clinically signifiant social and cognitive deficits, indicating that proper myelination of the prefrontal cortex is central to cognition, and that myelation dependent on social experience can regulate cognitive functions47. Furthermore, myelinated and unmyelinated segments occur on the same axon in the neocortex in mice30, and linear myelin ensheathment varies between cortical layers of the same region48. The internodal distances of single myelin segments can also vary substantially, with major implications for conduction properties and neuronal synchrony within distributed neural networks49. Such complexity in myelination predisposes it to the types of desynchronising events that are characteristics of schizophrenia.
Oligodendrocytes are the principal myelinating cell type of the CNS50. Myelination might depend in part upon neuronal activity, through activation of oligodendrocytic NMDA receptors50. The presence of activity-dependent growth factors (eg, neuregulin) accelerates myelination, and neuronal activity is therefore a key determinant of myelinogenesis. Accordingly, myelination is reduced by neuronal NMDA receptor inhibition and by suppression of action potentials50. In mice reared alone, mRNA levels of neuregulin 1 are lower than in mice reared together in groups, suggesting a link between social isolation and central hypomyelination47. However, knocking out oligodendrocyte-specific ErbB3, a growth factor receptor, produces a phenotype only when done during early postnatal development47; similarly, developmental overexpression of neuregulin 1 leads to hypermyelination51. These observations suggest that a crucial time window exists during ontogeny, during which oligodendrocytes are particularly sensitive to changes in the neural environment and developmental myelination is most at risk of disruption.
In patients with schizophrenia, postmortem stereological analyses have shown a substantial decrease in the numbers and density of oligodendrocytes in cortical layers III52 and V16 of the dorsolateral and medial prefrontal cortices, and in the superior frontal gyrus16. Other postmortem findings in the brains of patients with schizophrenia include a substantial decrease in both volume and mitochondrial number of oligodendrocytes within the caudate nucleus and prefrontal areas53. Similarly, in patients with chronic schizophrenia treated with antipsychotics, both oligodendrocyte numbers and myelin volume were significantly diminished in the anterior thalamic nucleus54. Other postmortem studies have documented differentially diminished mRNA expression of several proteins associated with oligodendrocytes and myelin (eg, QKI55 and CNP56) in patients with schizophrenia as compared with healthy controls. This decline in mRNA expression of oligodendrocyte markers might reflect a developmental failure in oligodendrocyte maturation, as has been shown in oligodendrocyte progenitor cells derived from patients42,44. Taken together, these studies indicate that schizophrenia is associated with impaired oligodendrocyte differentiation and consequent dysmyelination.
Glial cell progenitors
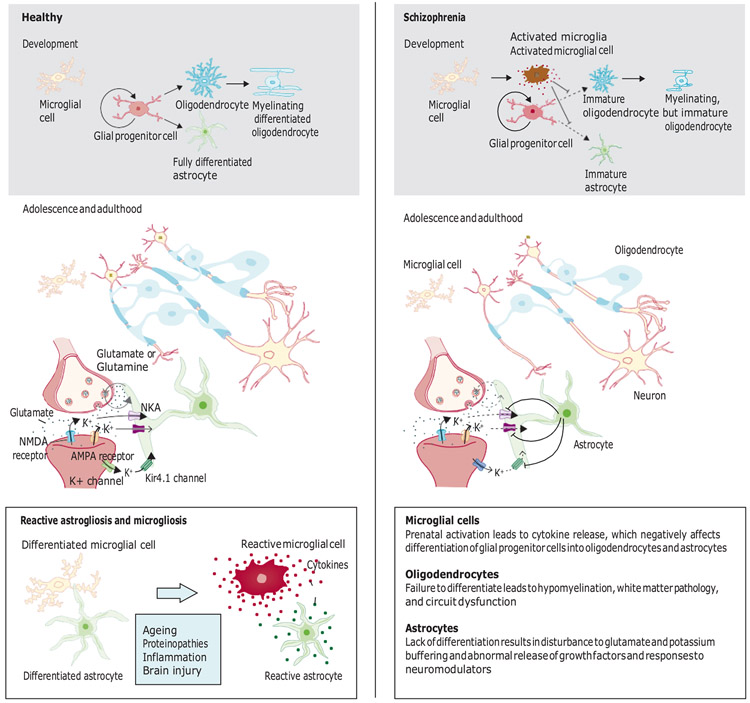
In the adult CNS, myelinating oligodendrocytes can be replenished from a pool of glial progenitor cells57. Glial progenitor cells represent the majority of proliferating cells in the adult CNS; most persist at the G1 phase of the cell cycle58. The transition of glial progenitor cells into the S phase is positively regulated by cyclins D and E, and negatively regulated by members of the Kip family of cell cycle inhibitors59. Both adult and fetal glial progenitor cells retain the capacity to differentiate into oligodendrocytes and astrocytes (figure),31 following a tightly regulated differentiation pathway31. Oligodendrocyte lineage transcription factor 2 (Olig2) is required for glial progenitor cells to initiate the oligodendrocytic differentiation programme60, while simultaneously inhibiting astrocytic differentiation, as signalled by the transcription of astrocytic glial fibrillary acidic protein31. Overexpression of Olig2 in adult glial progenitor cells in mice enhances differentiation and remyelination, while overexpression in embryonic glial progenitor cells boosts the generation of oligodendrocytes, which is accompanied by an increase in glial progenitor cell migration velocity60. In contrast, deletion of Olig2 in glial progenitor cells of adult mice potentiates their astrocytic differentiation, significantly reducing the number of oligodendrocytes and thereby suppressing myelination31. Other elements crucial in determining the fate of glial progenitor cells include growth factors, neurotransmitters, redox state31, and neural activity31,61. The developmental fate of glial progenitor cells is therefore highly sensitive to the surrounding neural environment31,61.
Figure: A unified gliocentric model of schizophrenia.
In development, glial progenitor cells give rise to oligodendrocytes and astrocytes, following a tightly regulated differentiation pathway. We hypothesise that untimely immune activation of microglia during fetal development suppresses the differentiation of glial progenitor cells, resulting in immature morphology and abnormal functionality and numbers of oligodendrocytes and astrocytes. Deficits in oligodendrocyte maturation competence lead to structural hypomyelination and loss of white matter integrity. Failure of astrocytic differentiation results in reduced synaptic coverage and abnormal buffering of glutamate and potassium. The proposed model links disrupted glial cell differentiation to the initial event in schizophrenia, which differs from other conditions involving glial cells, in which fully differentiated glial cells undergo reactive changes (lower left panel). Reactive changes in glial cells might, however, occur in schizophrenia as secondary responses to, for example, synaptic dysregulation, lack of sleep, or drug exposure. NKA=sodium−potassium pump. K+=potassium ions.
In postmortem brain samples from patients with chronic and severe forms of schizophrenia, the expression of the genes encoding cyclins D1 and D2 is increased, whereas expression of those genes encoding the Kip cycle cell inhibitors is decreased36. In patients with less severe symptoms, expression of genes associated with maintenance of the cell cycle in glial progenitor cells is increased, whereas expression of genes associated with cell cycle arrest is decreased14. Thus, glial progenitor cells tend to remain proliferative, and are less likely to exit the cell cycle in patients with schizophrenia. In genetically unrelated patients with schizophrenia, genotyping and polymorphism detection have revealed that an interaction between OLIG2 and ERBB4, and an interaction between OLIG2 and CNP both affect disease risk62. A linkage analysis for trans-effects on the expression of these two pairs of genes suggests that each locus regulates the expression of the other62. Furthermore, diffusion tensor imaging studies have shown an association of a single nucleotide polymorphism in OLIG2 with decreased white matter integrity63, implicating impaired glial progenitor cell differentiation in white matter pathology. These findings collectively suggest that the cell cycle of glial progenitor cells is dysregulated in patients with schizophrenia, resulting in arrested differentiation and delayed maturation of oligodendrocytes and astrocytes.
Astrocytes
Astrocytes play several key roles in the CNS during development and in adulthood. During development, they are essential for synapse formation64. Astrocytes also produce and deliver glutamate to neurons65, buffer extra- cellular potassium66, and respond to neuromodulator activity32. Inhibition of the α1 noradrenaline receptor eliminates more than 90% of spontaneous astrocytic calcium signalling in awake behaving mice32. In turn, astrocytic calcium signalling modulates potassium buffering, glycogen mobilisation, and functional hyperaemia32. Astrocytes are therefore crucial to the regulation of multiple homoeostatic functions that determine the precision of synaptic activity. They not only provide metabolic support for synapses, but are also necessary for synapse formation and maintenance during development and adulthood64.
In rodent models, changes in astrocytic numbers and morphological phenotype have been shown to trigger cognitive dysfunction. Selective elimination of astrocytes using a toxin specific to astrocytes67, or in a transgenic mouse line,68 reduces the density of astrocytes in the prefrontal cortex, resulting in mice with deficits in attentional set-shifting, working memory, reversal learning67, and recognition memory, and abnormal cortical gamma oscillations68. Furthermore, mice overexpressing S100-b (a calcium-binding protein, which is important for the migration, maintenance, and morphology of mature astrocytes) show altered patterns of spatial and temporal exploration, suggesting an impairment in short term memory69. In contrast, S100-b deletion enhances longterm potentiation in the CA1 hippocampal region, accompanied by improved spatial memory and fearassociated memory70. Similarly, diminished expression of the astrocytic glutamate transporter reduces prepulse inhibition of the acoustic startle response71, a well established feature of schizophrenia.
Postmortem data regarding disease-dependent changes in protoplasmic and fibrous astrocytes vary greatly. In one study of the anterior cingulate gyrus of patients with schizophrenia, significant abnormalities in various astrocytic markers (DIO2, AQP-4, S100-B, glutaminase, thrombospondin, and excitatory amino acid transporter 2) were observed in the deep cortical layers, as compared with those of healthy individuals13. No corresponding differences were found in the superficial layers or in the underlying white matter of the anterior cingulate gyrus13, suggesting that astrocytic pathology in those cases was region specific, with a regional selectivity that predicted symptomatic heterogeneity. In another study, fewer fibrous astrocytes were observed in the subgenual cingulate cortex of patients with schizophrenia than in healthy controls72. This finding was of particular interest since other white matter diseases associated with psychosis, such as some leukodystrophies, have been associated with abnormalities in fibrous astrocytes73, suggesting a possible connection between thought disorders, white matter alterations, and astrocyte pathology.
We propose that failure of glial differentiation is an initial event in schizophrenia, whereas astrogliosis and microgliosis might occur as secondary events later in disease development, possibly triggered by disturbances in network connectivity, homoeostatic failure of glutamate and potassium regulation, changes in neuromodulator release, neuroinflammation, sleep deficits, and drugrelated effects (figure). The effects associated with drugs might explain the large variation in postmortem data from patients with schizophrenia, as reactive gliosis is not a prerequisite for developing schizophrenia. Thus, the focus on reactive glia, rather than on abnormal glial developmental trajectories, might constitute a major obstacle in determining the role of glial cells in schizophrenia.
The contribution of glutamate to the pathology of schizophrenia has been studied extensively. Postmortem analyses suggest layer-specific alterations in both glutamatergic neurons and receptors, as well as in the glutamate metabolic pathway65. These include reductions that are associated with schizophrenia in the expression of the astrocytic glutamate transporter65, glutamine synthetase65, glutamate dehydrogenase74, glutaminase65, and D-serine75. However, the extent to which these changes in glutamate homoeostasis occur autonomously in neurons, rather than being downstream of astrocytic failure in glutamate reuptake and processing, remains unclear. S100-B expression is increased in astrogliosis, in neurodegenerative and neuroinflammatory diseases76, and in the glial cells of patients with schizophrenia77. When compared with healthy individuals, patients with schizophrenia who have frequent hallucinations have more abundant glial cells containing S100-B in the grey matter of the dorsolateral prefrontal cortex, whereas other studies found significantly fewer astrocytes in patients with predominantly negative symptoms77. In sum, these data suggest an association between astrocyte pathology, glutamatergic signalling, and schizophrenia.
Astrocytes might regulate oligodendrocyte function, whether by directly regulating oligodendrocyte differentiation, or by indirectly affecting myelination competence, or both78. During development, the ATP-sensitive inward rectifier potassium channel (Kir4.1) is regulated in both astrocytes and oligodendrocytes, resulting in distinct potassium influxes at embryonic and postnatal stages in the oligodendrocyte cell lineage79. Kir4.1 is an important regulator of oligodendrocyte differentiation80 and myelinating function79. Kir4.1 regulation of potassium influxes influences the thickness and extent of the myelin sheath according to the maturation stage of oligodendrocytes81. Oligodendrocytes cultured from Kir4.1 knockout mice display an immature morphology and a depolarised membrane potential79, and these mice show pronounced pathology in the entire CNS, and die before reaching adulthood79. Immunohistochemical studies of rat white matter have shown Kir4.1 immunoreactivity on the fine processes of astrocytes, beginning at postnatal day 10 (P10) and peaking at P1580. These changes in potassium channel expression correspond to developmental shifts in resting membrane potential, whereby the resting membrane potential declines as glial progenitor cells differentiate into mature oligodendrocytes81. Kir4.1 can also modulate the shape of action potentials82, and two papers have reported the transcriptional downregulation of KCNJ10 (the gene encoding Kir4.1) in glial cells derived from patients with childhood-onset schizophrenia42,44.
Astrocytes can affect oligodendrocyte differentiation by secreting growth factors, including platelet-derived growth factor83. Decreased platelet-derived growth factor secretion results in sustained glial progenitor cell maintenance at the expense of oligodendrocyte maturation83. Astrocytes also secrete the signal effectors neuregulin and brain-derived neurotrophic factor, both of which mediate neural activity-dependent myelination50. Polymorphisms of both NRG1 and ERBB4 have been observed in patients with schizophrenia84, and mice with impaired ErbB signalling show alterations in oligodendrocyte number, morphology, and myelin thickness, with correspondingly reduced conduction velocity85, as well as schizophreniform behavioural abnormalities and cognitive impairments86. These mice also show perturbation of mesolimbic dopaminergic function, a common feature of clinical schizophrenia85. Together, this literature suggests that downregulation of Kir4.1 in oligodendrocytes and astrocytes leads to hypomyelination. Furthermore, astrocytes secrete growth factors that promote oligodendrocytic differentiation, a shortage of which might exacerbate oligodendrocyte pathology in schizophrenia, at least in part by altering myelination-associated plasticity.
Neuromodulator-related abnormalities are an established feature of schizophrenia pathology87, and are likely to contribute to the sleep deficits3 and cognitive impairments2,4,5 of patients with schizophrenia. Evidence from two-photon imaging experiments on awake behaving mice have shown that neuromodulators—in particular noradrenaline—regulate astrocytic calcium signalling, which in turn regulates extracellular potassium concentrations during the sleep–wake cycle66. As such, it is possible that the defects in neuromodulator signalling in schizophrenia lead at least partially to downstream abnormalities in extracellular potassium concentrations, with unpredictable consequences for synaptic transmission.
Finally, astrocytic complexity has expanded markedly with phylogenetic evolution, with both increased cell autonomous complexity and pleomorphism43. Human astrocytes are distinguishable both structurally and functionally from those of other primates43,88, suggesting that astrocytes are crucial to the cognitive capabilities of the human species43. In humanised chimeric mice, engrafted human glial progenitor cells differentiated into astrocytes that maintained the large size and complexity of human astrocytes, and humanised chimeric mice outperform mice transplanted with mouse glial progenitor cells on all cognitive tests89. This observation suggests that evolutionary changes in astrocytes are central to higher brain function, possibly implicating astrocytes in the pathology of schizophrenia—a disease unique to humans43. The increased structural and functional complexity of human astrocytes is expected to render these highly differentiated cells more vulnerable to environmental and social risk factors, such as maternal stress and inflammation during pregnancy, which are known to increase the risk of schizophrenia8,11.
Microglia
Microglia are the resident immune cells of the CNS90, and are responsible for synaptic pruning during development33. The role of microglial activation and neuroinflammation in schizophrenia has been a recent focus of investigation. Although some meta-analyses suggest an increase of both cellular and molecular concentrations of inflammatory markers in the brains of patients with schizophrenia15, other analyses have found no consistent association with microglial activation12,17. Genes associated with positive loci for schizophrenia in genome-wide association studies have been shown to be enriched among genes dynamically regulated in the prefrontal cortex during early brain development; this enrichment was notable given the many genes in the dorsolateral prefrontal cortex that undergo fetal-to-postnatal isoform switching during second trimester development37. Thus, genetic alterations during early brain development could result in altered developmental trajectories19.
Activated microglia have been shown to attenuate the proliferation of glial progenitor cells91, and maternal infection can cause glial progenitor cell death in two waves92. Inflammation has been shown to increase expression of Olig2 in mice, and inhibit the expression of other factors important in oligodendrocyte maturation, including the cell cycle factor P27Kip1, resulting in disrupted oligodendrocyte maturation93. Microglial activation during embryogenesis predicts perturbed white matter integrity94, which is particularly vulnerable to inflammatory responses early in the third trimester of gestation, during oligodendrocytic production and maturation94. Neonatal neuroinflammation induced by intracerebral injection of lipopolysaccharide provokes white matter necrosis at P1495. Likewise, neonatal mice treated with IL-1 beta display long lasting hypomyelination at P35, characterised by increased abundance of non-myelinated axons, reduced myelin thickness, reduced white matter fractional anisotropy, higher densities of glial progenitor cells, and cognitive deficits93. In sum, these studies suggest that activated microglial cells contribute to the deficits in macroglial differentiation that are characteristic of schizophrenia.
An integrated model for glial cells in schizophrenia
On the basis of this collection of insights into the four types of glial cells, aberrant glial function might be a central element of schizophrenia pathology. We hypothesise that microglial activation in crucial periods during embryogenesis disrupts glial progenitor cell proliferation and differentiation competence, resulting in delayed oligodendrocyte and astrocyte differentiation, and aberrant maturation of those cells (figure)42. In this scenario, abnormalities in oligodendrocyte numbers and maturation competence lead to structural hypomyelination, which results in cortical and subcortical abnormalities in white matter integrity. Impaired astrocyte differentiation leads to glutamatergic dysfunction, abnormal potassium regulation, and dysregulation of growth factors and neuromodulators, providing a general explanation for the homoeostatic disruptions reported in schizophrenia. The dysfunction of various glial cells in parallel would result in oscillatory abnormalities and dysconnectivity across the brain, and global dyssynchrony, which together account for the hallmark characteristics of schizophrenia: positive and negative symptoms, and symptoms of cognitive impairment.
Since the completion of gliogenesis occurs relatively late in development96, infections occurring during late fetal development would be likely to have selectively greater effects on glial cell development than on resident neurons. The period of final maturation of oligodendrocytes, during which they are vulnerable to microglial activation94, corresponds to that period in which maternal infection and stress have been most strongly associated with schizophrenia97. As discussed in this Review, several of the secondary events in schizophrenia—such as abnormal glutamate and potassium homoeostasis, abnormalities in sleep patterns, or even drug treatment— can lead to later reactive changes in glial cells, which might be quite independent of the initial defects in glial differentiation. These secondary effects might explain the inconsistencies in postmortem analyses of glial cells in the brains of patients with schizophrenia. Importantly, schizophrenia remains a highly heterogeneous disorder in its causation, such that the proportion of schizophrenia cases in which glial cell dysfunction is causally involved is yet to be established. Nonetheless, the studies discussed here suggest that targeting glial pathology offers a new approach towards better understanding and ultimately modifying the complex pathogenesis of schizophrenia.
Key messages
Emerging studies show that microglial cells, oligodendrocytes, and astrocytes are all active participants in higher brain function
Maternal infections can activate microglia cells, which in turn negatively affect the differentiation of glial progenitor cells
Studies of humanised chimeric mice—generated using glial progenitor cells that are derived from the stem cells of patients with schizophrenia—show that glial progenitor cells fail to differentiate into oligodendrocytes and astrocytes, despite normal neuronal architecture
We propose a model in which the failure of oligodendrocytes and astrocytes to differentiate contributes to several of the key characteristics of schizophrenia, including hypomyelination and abnormalities in glutamate and potassium homoeostasis
Search strategy and selection criteria
We identified references for this review through searches of PubMed for articles and reviews published from Feb 1, 1989, to June 30, 2019, using the terms “schizophrenia”, “oligodendrocytes”, “astrocytes”, “NG2 cells”, “glial progenitor cells”, “microglial”, and “macroglial.” Relevant articles published between Feb 2, 1989, and Jun 25, 2019, were identified through searches in Google Scholar with the same terms. We reviewed the articles resulting from these searches and the relevant references cited in those articles, and included articles published in English only.
Footnotes
Declartion of interests: SAG is a co-founder and officer of Oscine, a cell therapy company, and holds relevant patents describing human glial chimeric mice and their use in modelling glial disorders. All other authors declare no competing interests.
References
- 1.Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia,“just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophrenia research. 2008;102(1-3):1–18. [DOI] [PubMed] [Google Scholar]
- 2.Insel TR. Rethinking schizophrenia. Nature. November 11 2010;468(7321):187–193. [DOI] [PubMed] [Google Scholar]
- 3.Kaskie RE, Graziano B, Ferrarelli F. Schizophrenia and sleep disorders: links, risks, and management challenges. Nature and science of sleep. 2017;9:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reichenberg A, Caspi A, Harrington H, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. American Journal of Psychiatry. 2009;167(2):160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angus W. MacDonald C SC, John G. Kerns, Stefan Ursu, Deanna M. Barch, Avram J. Holmes, Andrew Stenger V, Cohen Jonathan D.. Specificity of Prefrontal Dysfunction and Context Processing Deficits to Schizophrenia in Never-Medicated Patients With First-Episode Psychosis. Am J Psychiatry. 2005;162(475-484). [DOI] [PubMed] [Google Scholar]
- 6.Senkowski D, Gallinat J. Dysfunctional prefrontal gamma-band oscillations reflect working memory and other cognitive deficits in schizophrenia. Biological psychiatry. 2015;77(12):1010–1019. [DOI] [PubMed] [Google Scholar]
- 7.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nature reviews neuroscience. 2010;11(2):100–113. [DOI] [PubMed] [Google Scholar]
- 8.Green IW, Glausier JR. Different paths to core pathology: the equifinal model of the schizophrenia syndrome. Schizophrenia bulletin. 2015;42(3):542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilker R, Helenius D, Fagerlund B, et al. Heritability of schizophrenia and schizophrenia spectrum based on the nationwide Danish twin register. Biological psychiatry. 2018;83(6):492–498. [DOI] [PubMed] [Google Scholar]
- 10.Pekka Tienari LCW, Anneli Sorri, Ilpo Lahti, Kristian Läksy, Juha Moring, Mikko Naarala, Pentti Nieminen, Karl-Erik Wahlberg. Genotype-environment interaction in schizophrenia-spectrum disorder. Long-term follow-up study of Finnish adoptees. British Journal of Psychiatry. 2004;184:216–222. [DOI] [PubMed] [Google Scholar]
- 11.Levine SZ, Levav I, Yoffe R, Pugachova I. The effects of pre-natal-, early-life- and indirectly-initiated exposures to maximum adversities on the course of schizophrenia. Schizophrenia research. September 2014;158(1-3):236–240. [DOI] [PubMed] [Google Scholar]
- 12.Trepanier M, Hopperton K, Mizrahi R, Mechawar N, Bazinet R. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Molecular psychiatry. 2016;21(8):1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katsel P, Byne W, Roussos P, Tan W, Siever L, Haroutunian V. Astrocyte and glutamate markers in the superficial, deep, and white matter layers of the anterior cingulate gyrus in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36(6):1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerns D, Vong GS, Barley K, et al. Gene expression abnormalities and oligodendrocyte deficits in the internal capsule in schizophrenia. Schizophr Res. July 2010;120(1-3):150–158. [DOI] [PubMed] [Google Scholar]
- 15.Van Kesteren C, Gremmels H, De Witte L, et al. Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Translational psychiatry. 2017;7(3):e1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hof PR, Haroutunian V, Friedrich VL, et al. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biological Psychiatry. 2003;53(12):1075–1085. [DOI] [PubMed] [Google Scholar]
- 17.Mondelli V, Vernon AC, Turkheimer F, Dazzan P, Pariante CM. Brain microglia in psychiatric disorders. The Lancet Psychiatry. 2017;4(7):563–572. [DOI] [PubMed] [Google Scholar]
- 18.Kelly S, Jahanshad N, Zalesky A, et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Molecular psychiatry. 2018;23(5):1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birnbaum R, Weinberger DR. A Genetics Perspective on the Role of the (Neuro) Immune System in Schizophrenia. Schizophrenia research. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samartzis L, Dima D, Fusar-Poli P, Kyriakopoulos M. White matter alterations in early stages of schizophrenia: a systematic review of diffusion tensor imaging studies. Journal of neuroimaging : official journal of the American Society of Neuroimaging. March-Apr 2014;24(2):101–110. [DOI] [PubMed] [Google Scholar]
- 21.Karlsgodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry. March 1 2008;63(5):512–518. [DOI] [PubMed] [Google Scholar]
- 22.Schlosser RG, Nenadic I, Wagner G, et al. White matter abnormalities and brain activation in schizophrenia: a combined DTI and fMRI study. Schizophrenia research. January 2007;89(1-3):1–11. [DOI] [PubMed] [Google Scholar]
- 23.Sukhwinder S. Shergill R AK, Xavier A. Chitnis, Owen O'Daly, Derek K. Jones, Sophia Frangou, Steven C.R. Williams, Robert J. Howard, Gareth J. Barker, Robin M. Murray, Philip McGuire. A Diffusion Tensor Imaging Study of Fasciculi in Schizophrenia. Am J Psychiatry. 2007;164:467–473. [DOI] [PubMed] [Google Scholar]
- 24.Sim K, Yang GL, Loh D, et al. White matter abnormalities and neurocognitive deficits associated with the passivity phenomenon in schizophrenia: a diffusion tensor imaging study. Psychiatry research. May 15 2009;172(2):121–127. [DOI] [PubMed] [Google Scholar]
- 25.Sun H, Lui S, Yao L, et al. Two Patterns of White Matter Abnormalities in Medication-Naive Patients With First-Episode Schizophrenia Revealed by Diffusion Tensor Imaging and Cluster Analysis. JAMA psychiatry. July 2015;72(7):678–686. [DOI] [PubMed] [Google Scholar]
- 26.Adam M. Brickman M SB, Zlatin Ivanov, Joan C. Borod, Nancy S. Foldi, Eunice Hahn, Serge A. Mitelman, Erin A. Hazlett, Samantha J. Lincoln, Randall E. Newmark, Lina Shihabuddin. Internal Capsule Size in Good-Outcome and Poor-Outcome Schizophrenia. The Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18:364–376. [DOI] [PubMed] [Google Scholar]
- 27.Ozcelik-Eroglu E, Ertugrul A, Oguz KK, Has AC, Karahan S, Yazici MK. Effect of clozapine on white matter integrity in patients with schizophrenia: a diffusion tensor imaging study. Psychiatry research. September 30 2014;223(3):226–235. [DOI] [PubMed] [Google Scholar]
- 28.Mark Walterfang S JW, Dennis Velakoulis, David Copoloc, Christos Pantelis. Diseases of white matter and schizophrenia-like psychosis. Australian and New Zealand Journal of Psychiatry 2005;39:746–756. [DOI] [PubMed] [Google Scholar]
- 29.Du F, Cooper AJ, Thida T, Shinn AK, Cohen BM, Ongur D. Myelin and axon abnormalities in schizophrenia measured with magnetic resonance imaging techniques. Biol Psychiatry. September 15 2013;74(6):451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Hoz L, Simons M. The emerging functions of oligodendrocytes in regulating neuronal network behaviour. Bioessays. January 2015;37(1):60–69. [DOI] [PubMed] [Google Scholar]
- 31.Hill RA, Nishiyama A. NG2 cells (polydendrocytes): listeners to the neural network with diverse properties. Glia. August 2014;62(8):1195–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verkhratsky A, Nedergaard M. Physiology of astroglia. Physiological reviews. 2017;98(1):239–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schafer DP, Lehrman EK, Kautzman AG, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C, Aleksic B, Ozaki N. Glia-related genes and their contribution to schizophrenia. Psychiatry and clinical neurosciences. 2015;69(8):448–461. [DOI] [PubMed] [Google Scholar]
- 35.Bernstein H-G, Steiner J, Guest PC, Dobrowolny H, Bogerts B. Glial cells as key players in schizophrenia pathology: recent insights and concepts of therapy. Schizophrenia research. 2015;161(1):4–18. [DOI] [PubMed] [Google Scholar]
- 36.Katsel P, Davis KL, Li C, et al. Abnormal indices of cell cycle activity in schizophrenia and their potential association with oligodendrocytes. Neuropsychopharmacology. November 2008;33(12):2993–3009. [DOI] [PubMed] [Google Scholar]
- 37.Jaffe AE, Straub RE, Shin JH, et al. Developmental and genetic regulation of the human cortex transcriptome illuminate schizophrenia pathogenesis. Nat Neurosci. 2018;21(8):1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gandal MJ, Zhang P, Hadjimichael E, et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science. 2018;362(6420):eaat8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gandal MJ, Haney JR, Parikshak NN, et al. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science. 2018;359(6376):693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schork AJ, Won H, Appadurai V, et al. A genome-wide association study of shared risk across psychiatric disorders implicates gene regulation during fetal neurodevelopment. Nature neuroscience. 2019:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skene NG, Bryois J, Bakken TE, et al. Genetic identification of brain cell types underlying schizophrenia. Nature genetics. 2018;50(6):825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Windrem MS, Osipovitch M, Liu Z, et al. Human iPSC Glial Mouse Chimeras Reveal Glial Contributions to Schizophrenia. Cell Stem Cell. 2017;21(2):195–208. e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oberheim NA, Takano T, Han X, et al. Uniquely hominid features of adult human astrocytes. Journal of Neuroscience. 2009;29(10):3276–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z, Osipovitch M, Bates J, et al. Dysregulated glial differentiation in schizophrenia may be relieved by suppression of SMAD4 and REST-dependent signaling. Cell Reports 2019;(in press). [DOI] [PMC free article] [PubMed]
- 45.Ian A.McKenzie D O, Huiliang Li, Joana Paes de Faria, Ben Emery, Koujiro Tohyama, William D. Richardson. Motor skill learning requires acitive central myelination. Science. 2014;346(6207):318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J, Dietz K, DeLoyht JM, et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nature neuroscience. December 2012;15(12):1621–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manabu Makinodan K MR, Susumu Ito, Gabriel Corfas. A Critical Period for Social Experience-Dependent Oligodendrocyte Maturation and Myelination. Science. 2012;337:1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomassy GS, Berger DR, Chen HH, et al. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science. April 18 2014;344(6181):319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ford MC, Alexandrova O, Cossell L, et al. Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nature communications. 2015;6:8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lundgaard I, Luzhynskaya A, Stockley JH, et al. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS biology. December 2013;11(12):e1001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brinkmann BG, Agarwal A, Sereda MW, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59(4):581–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mighdoll MI, Tao R, Kleinman JE, Hyde TM. Myelin, myelin-related disorders, and psychosis. Schizophrenia research. January 2015;161(1):85–93. [DOI] [PubMed] [Google Scholar]
- 53.Uranova NA O D, Vikhera OV, Zimina IS, Rakhmanova VI. Morphemetric study of ultrastructural changes in oligodendroglial cells in the postmortem brain in endogenous psychoses. Vestn Ross Akad Med Nauk. 2011;7:42–48. [PubMed] [Google Scholar]
- 54.Byne W, Kidkardnee S, Tatusov A, Yiannoulos G, Buchsbaum MS, Haroutunian V. Schizophrenia-associated reduction of neuronal and oligodendrocyte numbers in the anterior principal thalamic nucleus. Schizophr Res. July 2006;85(1-3):245–253. [DOI] [PubMed] [Google Scholar]
- 55.Aberg K, Saetre P, Jareborg N, Jazin E. Human QKI, a potential regulator of mRNA expression of human oligodendrocyte-related genes involved in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. May 9 2006;103(19):7482–7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shruti NM, Thomas M. Hyde, Radhakrishne Vakkalanka, Bhaskar Kolachana, Danial R. Weinberger, Joel E. Kleinman, Barbara K. Lipska. Expression of Oligodendrocyte-Associated Genes in Dorsolateral Prefrontal Cortex of Patients with Schizophrenia. Schizophrenia research. 2008;98(1-3):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. Journal of Neurochemistry. 2008;107(1):1–19. [DOI] [PubMed] [Google Scholar]
- 58.Geha S, Pallud J, Junier MP, et al. NG2+/Olig2+ cells are the major cycle-related cell population of the adult human normal brain. Brain pathology. March 2010;20(2):399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140(15):3079–3093. [DOI] [PubMed] [Google Scholar]
- 60.Wegener A, Deboux C, Bachelin C, et al. Gain of Olig2 function in oligodendrocyte progenitors promotes remyelination. Brain. 2014;138(1):120–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erin M.Gibson D P, Christopher W. Mount, Andrea K. Goldstein, Grant L. Lin, Lauren S. Wood, Ingrid Inema, Sarah E. Miller, Gregor Bieri, Bradley Zuchero J, Ben A. Barres, Pamelyn J. Woo, Hannes Vogel, Michelle Monje. Neuronal Acitivity Promotes Oligodendrogenesis and Adaptive Myelination in the Mammalian Brain. Science. 2014;344:12523041–125230411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Georgieva L, Moskvina V, Peirce T, et al. Convergent evidence that oligodendrocyte lineage transcription factor 2 (OLIG2) and interacting genes influence susceptibility to schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. August 15 2006;103(33):12469–12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prata DP, Kanaan RA, Barker GJ, et al. Risk variant of oligodendrocyte lineage transcription factor 2 is associated with reduced white matter integrity. Human brain mapping. September 2013;34(9):2025–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chung W-S, Allen NJ, Eroglu C. Astrocytes control synapse formation, function, and elimination. Cold Spring Harbor perspectives in biology. 2015;7(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu W, MacDonald ML, Elswick DE, Sweet RA. The glutamate hypothesis of schizophrenia: evidence from human brain tissue studies. Annals of the New York Academy of Sciences. 2015;1338(1):38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fengfei Ding J OD, Qiwz Xu, Ning Kang, Nanna Goldman, Maiken Nedergaard. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science. 2016;352(6285):550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lima A, Sardinha VM, Oliveira A, et al. Astrocyte pathology in the prefrontal cortex impairs the cognitive function of rats. Molecular psychiatry. 2014;19(7):834–841. [DOI] [PubMed] [Google Scholar]
- 68.Lee HS, Ghetti A, Pinto-Duarte A, et al. Astrocytes contribute to gamma oscillations and recognition memory. Proceedings of the National Academy of Sciences. 2014;111(32):E3343–E3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roder JK, Roder JC, Gerlai R. Conspecific exploration in the T-maze: Abnormalities in S100β transgenic mice. Physiology & behavior. 1996;60(1):31–36. [DOI] [PubMed] [Google Scholar]
- 70.Nishiyama H, Knöpfel T, Endo S, Itohara S. Glial protein S100B modulates long-term neuronal synaptic plasticity. Proceedings of the National Academy of Sciences. 2002;99(6):4037–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bellesi M, Melone M, Gubbini A, Battistacci S, Conti F. GLT-1 upregulation impairs prepulse inhibition of the startle reflex in adult rats. Glia. 2009;57(7):703–713. [DOI] [PubMed] [Google Scholar]
- 72.Williams M, Pearce RK, Hirsch SR, Ansorge O, Thom M, Maier M. Fibrillary astrocytes are decreased in the subgenual cingulate in schizophrenia. European archives of psychiatry and clinical neuroscience. June 2014;264(4):357–362. [DOI] [PubMed] [Google Scholar]
- 73.Lanciotti A, Brignone MS, Bertini E, Petrucci TC, Aloisi F, Ambrosini E. Astrocytes: Emerging Stars in Leukodystrophy Pathogenesis. Translational neuroscience. June 1 2013;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burbaeva GS, Boksha IS, Turishcheva MS, Vorobyeva EA, Savushkina OK, Tereshkina EB. Glutamine synthetase and glutamate dehydrogenase in the prefrontal cortex of patients with schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27(4):675–680. [DOI] [PubMed] [Google Scholar]
- 75.Steffek AE, Haroutunian V, Meador-Woodruff JH. Serine racemase protein expression in cortex and hippocampus in schizophrenia. Neuroreport. 2006;17(11):1181–1185. [DOI] [PubMed] [Google Scholar]
- 76.Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochemical and biophysical research communications. 2004;322(4):1111–1122. [DOI] [PubMed] [Google Scholar]
- 77.Schümberg K, Polyakova M, Steiner J, Schroeter ML. Serum S100B is related to illness duration and clinical symptoms in schizophrenia—a meta-regression analysis. Frontiers in cellular neuroscience. 2016;10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lundgaard I, Osório MJ, Kress B, Sanggaard S, Nedergaard M. White matter astrocytes in health and disease. Neuroscience. 2014;276:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clemens Neusch NR, Russell E. Jacobs, Henry A. Lester, Paulo Kofuji. Kir4.1 Potassium Channel Subunit Is Crucial for Oligodendrocyte Development and In Vivo Myelination. The Journal of Neuroscience. 2001;21(15):5429–5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amanpreet S.Kalsi K G, Graham Wilkin, Arthur M. Butt. Kir4.1 expression by astrocytes and oligodendrocytes in CNS white matter: a developmental study in rat optic nerve. J. Anat. 2004;204:475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sontheimer JT H, Schachner M, Kettenman H. Channel Expression Correlates with Differentiation Stage during the Development of Oligodendrocytes from Their Precursor Cells in Culture. Neuron. 1989;2:1135–1145. [DOI] [PubMed] [Google Scholar]
- 82.Brasko C, Hawkins V, De La Rocha IC, Butt AM. Expression of Kir4.1 and Kir5.1 inwardly rectifying potassium channels in oligodendrocytes, the myelinating cells of the CNS. Brain structure & function. February 15 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McKinnon RD, Waldron S, Kiel ME. PDGF alpha-receptor signal strength controls an RTK rheostat that integrates phosphoinositol 3'-kinase and phospholipase Cgamma pathways during oligodendrocyte maturation. J Neurosci. April 6 2005;25(14):3499–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Norton N, Moskvina V, Morris DW, et al. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. January 5 2006;141B(1):96–101. [DOI] [PubMed] [Google Scholar]
- 85.Roy K, Murtie JC, El-Khodor BF, et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proceedings of the National Academy of Sciences of the United States of America. May 08 2007;104(19):8131–8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Golub MS, Germann SL, Lloyd KK. Behavioral characteristics of a nervous system-specific erbB4 knock-out mouse. Behavioural brain research. 2004;153(1):159–170. [DOI] [PubMed] [Google Scholar]
- 87.Yamamoto K, Hornykiewicz O. Proposal for a noradrenaline hypothesis of schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2004;28(5):913–922. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y, Sloan SA, Clarke LE, et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron. 2016;89(1):37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Han X, Chen M, Wang F, et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell stem cell. 2013;12(3):342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ginhoux F, Lim S, Hoeffel G, Low D, Huber T. Origin and differentiation of microglia. Front Cell Neurosci. 2013;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Taylor DL, Pirianov G, Holland S, et al. Attenuation of proliferation in oligodendrocyte precursor cells by activated microglia. Journal of neuroscience research. June 2010;88(8):1632–1644. [DOI] [PubMed] [Google Scholar]
- 92.Pang Y, Campbell L, Zheng B, Fan L, Cai Z, Rhodes P. Lipopolysaccharide-activated microglia induce death of oligodendrocyte progenitor cells and impede their development. Neuroscience. March 17 2010;166(2):464–475. [DOI] [PubMed] [Google Scholar]
- 93.Favrais G, van de Looij Y, Fleiss B, et al. Systemic inflammation disrupts the developmental program of white matter. Annals of neurology. October 2011;70(4):550–565. [DOI] [PubMed] [Google Scholar]
- 94.Chew LJ, Fusar-Poli P, Schmitz T. Oligodendroglial alterations and the role of microglia in white matter injury: relevance to schizophrenia. Developmental neuroscience. 2013;35(2-3):102–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yi Pang ZC, Philip G. Rhodes. Disturbance of oligodendrocyte development, hypoemyelination and white matter injury in neonatal rat brain after intracerebral injection of lipopolysaccharide. Developmental Brain Research. 2003;140:205–214. [DOI] [PubMed] [Google Scholar]
- 96.Bakken TE, Miller JA, Ding S-L, et al. A comprehensive transcriptional map of primate brain development. Nature. 2016;535(7612):367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zammit S, Lewis S, Gunnell D, Smith GD. Schizophrenia and neural tube defects: comparisons from an epidemiological perspective. Schizophrenia bulletin. 2006;33(4):853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]