Abstract
Impulsivity and sensation seeking are considered to be among the most important personality traits involved in risk‐taking behavior. This study is focused on whether the association of these personality traits and brain functional connectivity depends on individuals' risk proneness. Risk proneness was assessed with the DOSPERT‐30 scale and corroborated with performance in a motorcycle simulator. The associations of impulsivity‐ and sensation seeking‐related traits with the between and within coupling of seven major brain functional networks, estimated from electroencefalograma (EEG) recordings, differ according to whether an individual is risk prone or not. In risk‐prone individuals, (lack of) premeditation enhanced the coupling of the ventral attention and limbic networks. At the same time, emotion seeking increased the coupling of the frontoparietal network and the default mode networks (DMNs). Finally, (lack of) perseverance had a positive impact on the coupling of anterior temporal nodes of the limbic network whilst having a negative impact on some frontal nodes of the frontoparietal network and the DMNs. In general, the results suggest that the predisposition to behave riskily modulates the way in which impulsivity traits are linked to brain functionality, seemingly making the brain networks prepare for an immediate, automatic, and maladaptive response.
Keywords: brain functional coupling, impulsivity, personality traits, risk taking, sensation seeking
1. INTRODUCTION
Taking risks in life is inherent to humans and animals. Almost every human activity (from foraging or finances to science or space exploration) can be regarded as an instance of a game in which the stakes are high. Unsurprisingly, certain personality traits, such as impulsivity and sensation seeking, are inextricably linked to risk taking (Zuckerman & Kuhlman, 2000) and share functional networks in the brain.
One recent influential model regards impulsivity as a construct that includes five dimensions (Cyders et al., 2007): Positive and Negative Urgency, (lack of) Premeditation, (lack of) Perseverance, and Sensation Seeking (UPPS model). In neurobiological studies, UPPS impulsivity factors have been found to be associated with the functional connectivity of brain areas involved in emotional regulation, response suppression, and cognitive control. For instance, Golchert et al. (2017) found that the connectivity of distinct regions of the anterior cingulate cortex (ACC) correlated with three UPPS dimensions. In particular, positive urgency was negatively related to connectivity of subgenual ACC with the bilateral parietal cortex (embracing parts of the precuneus, retrosplenial cortex, and intracalcarine sulcus); (lack of) perseverance was positively related to the connectivity of supragenual ACC with the right middle fontal gyrus (MFG); and (lack of) premeditation was negatively related to the connectivity of supragenual ACC with the bilateral occipital cortex. Thus, it appears that an excessive coupling of prefrontal regions could underlie perseverance difficulties and that (lack of) premeditation could be related to difficulties in the attentional modulation of information processing carried out by sensory regions of the brain (Golchert et al., 2017). Decoupling of subgenual ACC and parietal clusters, particularly retrosplenial, could form the neural basis of problems envisioning the future, which would provoke the impulsive actions that characterize positive urgency (Golchert et al., 2017). Furthermore, urgency has been linked to connectivity within the default mode network (DMN). Using the amplitude of low frequency fluctuations, Zhao et al. (2017) observed that activity in several brain areas of the DMN (such as subgenual ACC, medial frontal gyrus, right dorsolateral prefrontal cortex, left inferior frontal gyrus and MFG, and posterior cingulate/precuneus) is positively associated with mean urgency scores (positive and negative), which suggests that excessive activation of the nodes within the DMN could underpin the urgency trait (Chester et al., 2016).
There is also evidence that negative urgency is associated with brain structural abnormalities. For instance, Muhlert and Lawrence (2015) observed that gray matter volumes in the dorsomedial prefrontal cortex (DMPFC) and the right temporal pole—two areas involved in emotional processing and decision‐making—are negatively related to negative urgency. Sensation seeking was also negatively associated with certain structural characteristics, including cortical thickness (Holmes, Hollinshead, Roffman, Smoller, & Buckner, 2016) and gray matter volume (Wang, Wen, Cheng, & Li, 2017) of brain regions involved in cognitive control and self‐regulation such as the ACC and MFG.
Nonetheless, other factors appear to modulate the differences in the relation between impulsivity and connectivity, such as risk proneness. In a recent study, Barkley‐Levenson et al. (2018), using a Stroop task, observed that, in the congruent condition, risky participants showed greater activation of the ACC, DMPFC, dorsolateral prefrontal cortex, left frontal pole, and right insula in comparison with nonrisky participants. Further analysis suggested that, in the congruent condition, the activation of several of these regions mediates the association between urgency and risk behavior (risky category), which could be interpreted as indirectly supporting the idea that differences in the functioning of the frontoinsular system is responsible for the observed differences in impulse control between risky and nonrisky individuals. Comparing risky with nonrisky adolescents, DeWitt, Aslan, and Filbey (2014) observed that the former group displayed increased connectivity between the amygdala and the right MFG, left cingulate gyrus, left precuneus, and right inferior parietal cortex and between the nucleus accumbens and the right MFG. In a similar vein, Deza Araujo et al. (2018) demonstrated hyperconnectivity between the frontoparietal network and the occipital cortex and between the DMN and medial temporal and frontal regions in high risk‐seeking behavior in losses (observed in people who prefer delayed potential high losses rather than immediate but sure small losses).
However, to the best of our knowledge, no direct information is available on whether the associations between impulsivity or sensation‐seeking traits and brain functional connectivity are different in risk‐prone (RP) and nonrisk‐prone (NRP) individuals or if, on the contrary, these personality traits are linked to connectivity independent of risk proneness. Our aim, therefore, was to test if the associations between functional coupling in large brain networks and impulsivity and sensation‐seeking traits are a function of risk proneness, as derived from the DOSPERT scale (Weber, Blais, & Betz, 2002), in a normal young sample. For this purpose, we used the brain current source density (CSD, determined using sLORETA) estimated from a risk perception task, described in other studies (Megías et al., 2015; Megías, López‐Riañez, & Cándido, 2013). As an a priori approach, we depart from the influential work of Yeo et al. (2011) and estimate both the connectivity between the seven networks described (Visual [VN], Somatomotor [SMN], Dorsal Attention [DAN], Ventral Attention [VAN], Limbic [LN], Frontoparietal [FPN], and Default [DMN]) and between the nodes within these networks.
2. METHOD
2.1. Participants
The participants were selected from a pool of 1,093 students of the University of Granada, who volunteered by responding to the Spanish online version of the Domain‐Specific Risk‐Taking Scale 30 (DOSPERT‐30, Lozano et al., 2017). Only individuals aged between 18 and 25 years and possessing a driver's license were chosen to take part in the study. The intentional risk‐taking subscale was used to identify RP and NRP individuals, using the 75th percentile as high cut‐off value and the 25th percentile as the low cut‐off value. Percentiles were computed separately for men and women given that there are gender differences in the distribution of the scores. A total of 89 individuals (40 women; M = 21.64; SD = 1.99) participated in the study forming two groups according to their propensity to take risks: the RP (N = 45, 21 women, age = 21.67 years) and the NRP group ( N = 44, 19 women, age = 21.61 years).
This study was approved by the Human Research Ethics Committee of the University of Granada (n° 204/CEIH/2016). All participants gave written consent, were informed about their rights according to the Declaration of Helsinki (World Medical Association, 2008), and were paid for their participation.
2.2. Apparatus and stimuli
2.2.1. Questionnaires
First, demographic variables and information about driving experience and behavior (months since obtaining driver's license, km driven per year, number of accidents, and number of fines) were collected. Three questionnaires were administered to collect data regarding personality traits.
Domain‐specific risk‐taking scale 30
The risk‐taking subscale of the Spanish version of the domain‐specific risk‐taking scale 30 (DOSPERT‐30) scale (Lozano et al., 2017) was used to measure the participants' propensity to take risks. This subscale consists of 30 items with a 7‐point Likert scale. To measure risk‐taking propensity, the individual is asked to evaluate the likelihood that he/she would engage in different types of risk‐taking behavior. The items refer to five domains of everyday life (ethical, financial, health/safety, social, and recreational risks). We used a 75–25% cut‐off value to categorize participants as either RP or NRP.
Impulsive behavior scale (UPPS‐P)
The short version of the Spanish UPPS‐P scale (Cándido, Orduña, Perales, Verdejo‐García, & Billieux, 2012) measures five dimensions of the impulsivity trait (Positive and Negative Urgency, (lack of) Premeditation, (lack of) Perseverance, and Sensation seeking). The scale consists of 20 items, with 4 items for every trait, measured with a 4‐point Likert scale.
Sensation‐seeking scale
The short Spanish version of the sensation‐seeking scale (SSS) (Pérez & Torrubia, 1986) consists of 40 dichotomic items (Yes/No), assessing the following four dimensions of the sensation‐seeking trait: thrill and adventure seeking, experience seeking, disinhibition, and boredom susceptibility.
Sensitivity to punishment and sensitivity to reward questionnaire
The sensitivity to punishment and sensitivity to reward questionnaire (SPSRQ‐20) (Aluja & Blanch, 2011) measures the Behavioral Approach System (BAS) and the Behavioral Inhibition System (BIS), which are the two basic motivational systems according to Gray's psychobiological model of personality (Torrubia, Ávila, Moltó, & Caseras, 2001). This instrument consists of 20 dichotomic items (Yes/No) divided into two subscales, sensitivity to reward and sensitivity to punishment, which measure the BAS and BIS, respectively.
2.2.2. Motorcycle riding simulator
The Honda Riding Trainer motorcycle simulator (HRT) consists of a seat, handlebar, pedals, accelerator, brakes, turn indicators, and claxon (Di Stasi et al., 2009; Megías, Cortes, Maldonado, & Cándido, 2017, for more details on the HRT simulator).The road scenarios were projected with a refresh rate of 30 Hz at a distance of 185 cm on the screen (110 × 180 cm, resolution: 1,024 × 768 pixels) in front of the participant seated on the motorcycle simulator. Participants rode through an urban road scenario that includes eight risk situations (e.g., opening doors of parked cars or pedestrians crossing the road). The driving simulation was approximately 5 min long depending on speed, crashes, and variability of the course taken by the participant. From the data measured by the HRT, we computed the following riding indices of risk proneness: average speed (km/h), duration (s) of exceeded speed limits, and average exceeded speed limits (km/hr).
2.2.3. Risk perception task
The SR Research Experiment Builder (SR Research Ltd., Mississauga, ON, Canada) was used to run the risk perception task, consisting of 140 real traffic pictures taken from the driver's perspective. The risk levels of the traffic situations were categorized into 70 high‐risk pictures and 70 low‐risk pictures (see Megías et al., 2015 for more details). All stimuli were projected on the screen using the same parameters as the HRT, with the participant seated on the motorcycle simulator in order to mimic a more realistic environment.
Every trial of the risk perception task began with a 750 ms fixation point that appeared at the center of a white screen followed by a 2,000‐ms traffic scene. The participants were required to indicate whether the traffic scene was risky or not, pulling the front brake only when they perceived risk and not responding at all if they did not perceive the situation as risky. After 2,000 ms, a black screen was displayed for 750 ms. The proportion of affirmative responses of risk perception and correct answers (according to the picture category) were computed for the two picture types (high/low risk) for each subject.
2.2.4. Procedure
As stated previously, DOSPERT‐30 measures were considered online in the participant selection stage. In the experimental session, all participants, after giving written informed consent, completed the risk perception task followed by the riding simulation. EEG recordings were taken during the risk perception task. At the end of the session, participants responded to the remaining questionnaires.
2.2.5. EEG data recording and preprocessing
Brain electrical activity (EEG) was recorded during the risk perception task using a 64 active channel system (Brain Products, Inc.), mounted on an elastic cap and arranged according to the extended 10–20 system. Data were sampled at 1,000 Hz, amplified using a 0.016–1,000 Hz band‐pass filter, and referenced online to FCz. Electrode impedances were below 25 kΩ as recommended by the manufacturer.
Offline signal preprocessing was conducted using EEGLAB (Delorme & Makeig, 2004; freely available at http://sccn.ucsd.edu/eeglab). All EEG recordings were downsampled to 250 Hz, rereferenced offline to average reference, and FCz activity was recovered. Channels with a flatline duration of more than 50 s or with more line noise relative to its signal (4SD) were interpolated using the spherical spline interpolation method, included in EEGLAB software. Recordings were then band‐pass filtered using a .1–30 Hz, 36 dB/octave filter, segmented from −200 to 2.000 ms time‐locked to the stimulus onset, and baseline corrected. Independent Component Analysis was computed using the Second‐Order Blind Identification algorithm (Tang, Sutherland, & McKinney, 2005), and ocular and electromyographic artifacts were removed using MARA's EEGLAB plug‐in (Winkler et al., 2014; Winkler, Haufe, & Tangermann, 2011, freely available at https://irenne.github.io/artifacts). Averaged segments for each participant were submitted to standardized low‐resolution brain electromagnetic tomography software (sLORETA; Pascual‐Marqui, 2002; freely available at http://www.uzh.ch/keyinst/loreta.htm) to determine activity for each voxel of the sLORETA brain template. sLORETA computed CSD using the Montreal Neurological Institute template as the solution space.
2.3. Data analysis
2.3.1. DOSPERT prediction of risky driving behavior
To validate our categorization of DOSPERT‐based risk proneness, we analyzed between‐group differences on performance in the risk perception task and the variables considered by the course driven on the HRT simulator, measuring risky driving behavior.
2.3.2. Personality traits and risk taking (questionnaire data)
To determine the personality trait profile of RP individuals, we aimed to predict the DOSPERT‐based grouping using the scores of all other questionnaires (UPPS‐P, SSS‐V, and SPSRQ‐20). We used a Partial Least‐Squares Discriminant Analysis, as implemented in the Classification Toolbox of the Milano Chemometrics and QSAR Research Group (http://michem.disat.unimib.it/chm/). Following a cross‐validation approach, we used 75% of the sample, selected randomly, to estimate the model parameters (teaching sample) that were tested in the remaining 25% of the sample (test sample). The number of optimal components was estimated using Venetian blind cross‐validation. A single component produced the lowest classification error on the training sample. This model was fitted using variable autoscaling and Bayes assignation criterion.
2.3.3. Brain network of personality traits based on the risk level (EEG data)
We used the Brainnetome atlas coordinates (Fan et al., 2016, http://atlas.brainnetome.org) to compute functional connectivity between and within the seven brain networks described in the work of Yeo et al. (2011). The atlas provides 210 cortical nodes, distributed for each network as follows: 34 for VN, 33 for SMN, 30 for DAN, 22 for VAN, 26 for LN, 26 for FPN, and 36 for DMN (see Fan et al., 2016, and Figure S1 for more detail). Coordinates were translated to the sLORETA template, with each node embracing all the voxels located in a sphere of 10 mm radius, centered at the Brainnetome atlas node coordinates. The time series for each network and each node in each network were spatially averaged using the first eigenvariate of the singular value decomposition of the corresponding cluster of voxels. We used the FSLnets (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLNets) with default regularization parameter (lambda = 0.1) to compute between‐ and within‐network functional connectivity using ridge correlation coefficients with L2‐norm regularization. We then computed multivariate multiple stepwise linear regression, with group (RP and NRP), personality traits, age, and gender as the set of predictors. Afterward, for each significant trait, partial correlations between functional coupling and this trait were performed separately for each group, controlling for age and gender. Correlations were transformed to z‐scores that were then used for the between‐group comparisons. Statistical decisions were made using the Bonferroni correction to hold the corrected p‐value below .05.
3. RESULTS
3.1. Prediction of risky driving behavior
Bonferroni‐corrected comparisons indicated that RP individuals (measured by DOSPERT) perceived less risk in the depicted traffic scenes (42.89 vs. 51.23%, t(87) = 3.54, p < .001), drove at a higher average speed (30.64 vs. 27.28 km/hr, t(87) = 3.97, p < .001) and exceeded speed limits for a longer time (44.22 s vs. 27.34 s, t(87) = 4.59, p < .001) and by a greater amount (6.65 vs. 4.37 km/hr, t(87) = 2.56, p = .012) than NRP individuals.
3.2. Personality traits and risky driving behavior
Analysis using the personality trait scores to predict our grouping of risk proneness showed that overall classification accuracy for the teaching sample was 89.53% (31/34 and 29/33 individuals of the training sample were correctly classified as RP and NRP, respectively). For the test sample, overall classification accuracy was 86.36% (11/11 and 8/11 participants were correctly classified as RP and NRP, respectively). Figure 1a displays scores on the latent variable for the participants in the teaching sample (circles) and in the test sample (squares). Figure 1b shows that loadings were negative for UPPS scores on (lack of) premeditation and (lack of) perseverance and for SPSRQ scores on punishment sensitivity. RP individuals scored higher than NRP individuals with the exception of the three above‐mentioned traits.
Figure 1.
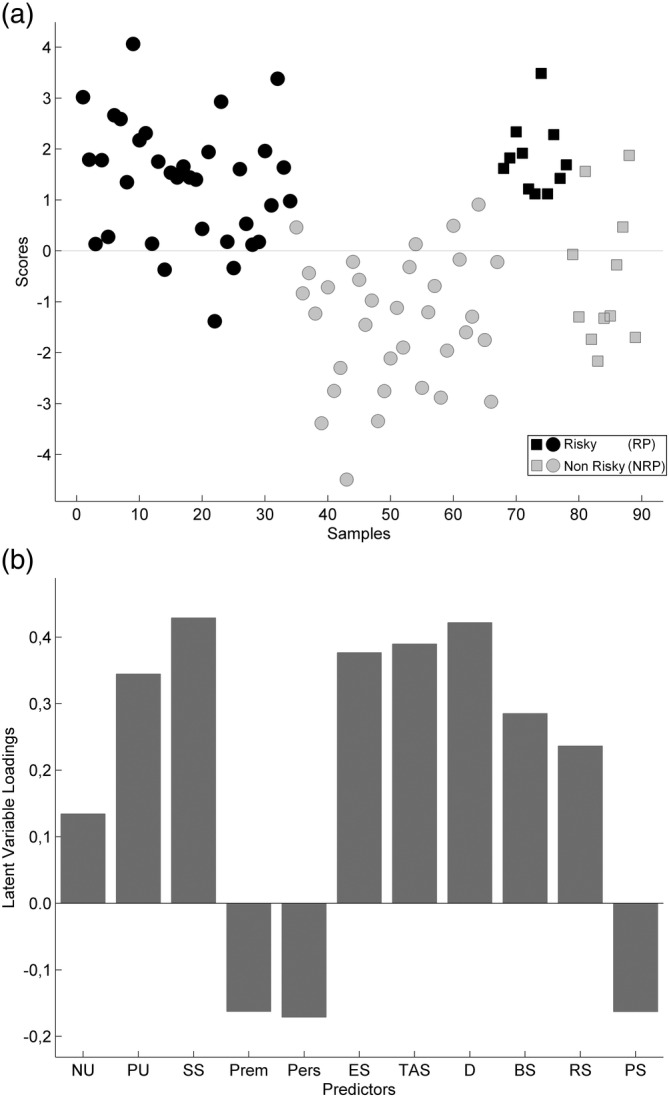
Prediction of risk level by personality traits. Classification analysis showed (a) latent variable scores as a function of risk group (black: risk prone; grey: nonrisk prone). Circles indicate the training sample, and squares indicate the testing sample. (b) Loadings of the predictor variables. BS, boredom susceptibility; D, disinhibition; ES, emotion seeking; NU, negative urgency; Pers, (lack of) perseverance; Prem, (lack of) premeditation; PS, punishment sensitivity; PU, positive urgency; RS, reward sensitivity; SS, sensation seeking; TAS, thrill and adventure seeking
3.3. Personality traits related to brain network connectivity based on risk proneness
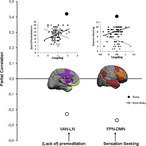
The multivariate multiple stepwise linear regression yielded significant effects of the set of predictors, Λ = 0.52, p < .05. Detailed analyses of this effect indicate that correlations between the functional connectivity of the networks and dimensions of personality traits vary as a function of risk proneness (Figure 2).
Figure 2.
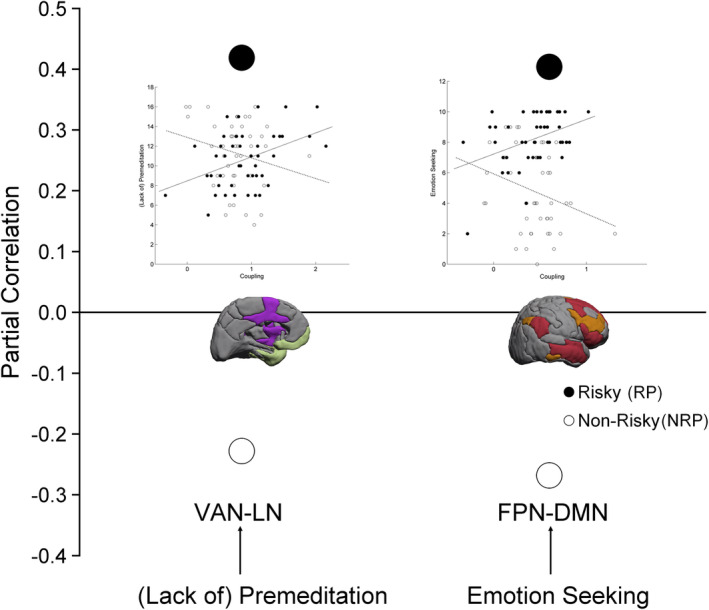
Influence of personality traits on functional coupling of the ventral attention network—limbic network (VAN–LN) and frontoparietal network–default mode network (FPN–DMN) as a function of risk proneness. Colors in the schematic brains indicate the functional networks: violet (VAN), green (LN), orange (FPN), and red (DMN). The dots display the strength of the personality trait–brain coupling association for the risk‐ and nonrisk‐prone group. The insets display scatterplots of these correlations
Independent of risk proneness, (lack of) premeditation modulated the coupling of the VAN with the DAN (r = −40, corrected p < .05), the FPN (r = −40, corrected p < .05), and the DMN (r = 0.52), plus that of VN and the LN (r = 0.49, corrected p < .05). The (lack of) perseverance modulated the VAN–DMN coupling (r = 0.43, corrected p < .05). Interestingly, the SSS scores for emotion seeking showed a stronger correlation with FPN–DMN coupling in RP (r = 0.40) than NRP participants (r = −0.27) (corrected p = .02). Thus, FPN–DMN coupling appears to be modulated by emotion seeking, so it tends to be enhanced in RP emotion seekers but depleted in their NRP counterparts. Moreover, a similar result was observed for the correlation between (lack of) premeditation and the VAN–LN coupling, being stronger for RP (r = 0.42) than for NRP individuals (r = −0.23) (corrected p = .02).
Detailed analysis of these interactions at the node level indicated that (lack of) premeditation differentially modulates the coupling of the right area 13 (LN) and left areas 1/2/3 (lower limb region) (VAN) (rRiskprone = 0.39, rNonriskprone = −0.45, corrected p = .013). Emotion seeking differentially modulates, on the one hand, the coupling of the left area 11 (LN) and right dorsal dysgranular insula (VAN) (rRiskprone = −0.39, rNonriskprone = 0.39, corrected p = .05) and, on the other hand, that of the right medial area 10 (DMN) and right ventral area 9/46 (FPN) (rRiskprone = −0.33, rNonriskprone = 0.45, corrected p = .057).
Personality traits also modulate the coupling between nodes within the same network as a function of risk proneness (Table 1), considering only the networks our previous analysis identified as affected by traits. The multivariate stepwise multiple regression on within‐networks node couplings yielded significant effects of personality traits on each of the couplings (max Λ = 0.03, all p < .05). Detailed analysis of these effects indicated that (lack of) perseverance modulates coupling within the LN and the DMN, so it enhanced coupling between certain nodes for the RP, but not for the NRP, group while depleting the connectivity between areas 9 and 46 (FPN) in the RP group. In stark contrast, the coupling between medial area 9 and caudal area 45 (DMN) is enhanced in the NRP but not in the RP group. (Lack of) Premeditation increased the coupling of rostral area 35/36 and the temporal agranular insula (LN) in the RP but not in the NRP group. Emotion seeking affects the FPN, so it tends to deplete the coupling of ventral area 9/46 and lateral area 10 in the RP, but not in the NRP, group while the reverse pattern is observed for the coupling of lateral area 10 and medial area 7.
Table 1.
Significant paired within‐network couplings associated with personality traits for risk‐prone and nonrisk‐prone individuals
| Questionnaire | Trait | Network | Nodes | r (RP) | r (NRP) | z | p |
|---|---|---|---|---|---|---|---|
| UPPS | (Lack of) perseverance | LN | R A38m–L A35/36r | 0.57 | −0.23 | 4.03 | .01 |
| (Lack of) perseverance | LN | R A20cv–R TI | 0.67 | −0.13 | 4.34 | .00 | |
| (Lack of) premeditation | LN | L A35/36r–R TI | 0.59 | −0.15 | 3.74 | .03 | |
| (Lack of) perseverance | FPN | L A46 – L A9/46v | −0.57 | 0.15 | −3.67 | .04 | |
| (Lack of) perseverance | DMN | L A9m–L A45c | −0.25 | 0.55 | −3.95 | .02 | |
| SSS | Emotion seeking | FPN | R A9/46v–R A10l | −0.50 | 0.35 | −4.16 | .01 |
| Emotion seeking | FPN | R A10l–R A7m | 0.18 | −0.57 | 3.74 | .03 |
Note: The p column displays the Bonferroni‐corrected p‐value. A, area; c, caudal; DMN, default mode network; FPN, frontoparietal network; L, left hemisphere; l, lateral; LN, limbic network; m, medial; NRP, nonrisk‐prone group; R, right hemisphere; r, rostral; RP, risk‐prone group; SSS, sensation seeking scale; TI, temporal agranular insula; v, ventral; UPPS, Positive and Negative Urgency, (lack of) Premeditation, (lack of) Perseverance, and Sensation seeking.
4. DISCUSSION
RP participants show greater impulsivity, sensation seeking, and reward sensitivity but lower punishment sensitivity, in comparison with their NRP counterparts. Moreover, the association between some of these personality traits and functional coupling between brain networks and between nodes within these networks is modulated by risk proneness. At the macroscopic level, the VAN–LN and the FPN–DMN couplings have greater positive correlations with (lack of) premeditation and emotion seeking, respectively, for RP individuals when compared with NRP individuals. These between‐network results appear to be linked to the modulatory effect of these personality traits on the coupling of right area 13 with the left somatosensorial cortex (LN–VAN), the left medial area 11 with the right dorsal dysgranular insula (LN–VAN), and right medial area 10 with right ventral area 9/46 (DMN–FPN). Moreover, the coupling between nodes within the same brain network is also differentially associated with personality traits in RP and NRP individuals. In particular, the positive correlations between (lack of) perseverance and coupling between nodes in the LN (right medial area 38–left rostral area 35/36; right caudoventral area 20–right temporal agranular insula) are higher for the RP than for the NRP group. However, a higher negative correlation was found for the FPN (left area 46–left ventral area 9/46) for this trait in the RP group in comparison with the NRP group. (Lack of) Perseverance was highly correlated with coupling of the DMN (left medial area 9 with left caudal area 45) in the NRP group, and to a lesser extent in the RP group. In addition, there are negative correlations between the emotion‐seeking trait and the coupling of right ventral area 9/46 with right lateral area 10 (FPN) for RP individuals and the coupling of right lateral area 10 and right medial area 7 for NRP individuals.
The results of our personality profile analysis are consistent with previous findings relating risk‐taking behavior to impulsivity and sensation‐seeking traits (Zuckerman & Kuhlman, 2000). Previous studies have found these traits to be positively associated with risk‐taking behavior such as drug use, sexual risk behavior (Donohew, Zimmerman, Cupp, & Novak, 2000), and financial risktaking (Wong & Carducci, 1991), as well as imprudent driving behavior (Beanland, Sellbom, & Johnson, 2014). In addition, lower sensitivity to punishment and greater sensitivity to reward were also associated with risk‐taking behavior, including behavior observed in driving environments (Scott‐parker, Watson, King, & Hyde, 2012). Here, we showed that these personality traits are highly predictive of risk proneness (Figure 1). Thus, a higher risk propensity is predicted by a more impulsive and emotion‐seeking personality profile, being more sensitive to reward and less sensitive to punishment. This is worth taking into account when designing intervention programs for these youths as they might not be responsive to punishments and are instead reinforced by the emotions and sensations evoked by the risk behavior itself, with little control over their impulses.
Regarding the brain connectivity data, we observed that, in the RP group, the emotion‐seeking facet of the sensation‐seeking trait was positively related to the DMN–FPN coupling (Figure 2a), while the (lack of) premeditation facet of the impulsivity trait was positively related to the VAN–LN coupling (Figure 2b). In the NRP group, both relationships were weaker and negative.
The FPN has been shown to be involved in cognitive flexibility and the control and adaptation to the changing behavioral goals or task demands (Cole et al., 2013; Woolgar, Afshar, Williams, & Rich, 2015). The DMN, on the other hand, has been linked with internal processes such as mind wandering (Christoff, Gordon, Smallwood, Smith, & Schooler, 2009). A reduction in DMN activity has been found to enhance performance in externally driven cognitive tasks, while deficits in the suppression of this network appear to underlie a number of mental illnesses (Anticevic et al., 2012). FPN–DMN coupling has been linked to the suppression of task‐irrelevant information by the reduction of DMN activity and the enhancement of task‐relevant features by increasing FPN activity (Chadick & Gazzaley, 2011). Whether this dynamic is caused by FPN action on DMN or by mutual inhibition is still a matter of debate (Anticevic et al., 2012), but we speculate that this causal mechanism is altered in risk‐prone emotion seekers, who will be looking for internal emotional signals rather than attending to the external stimuli of the task. Our results suggest that the coupling of FPN ventral area 9/46 and DMN medial area 10 could be responsible for this effect and that medial area 10 could be necessary for reducing activation of the DMN. This idea is supported by our within‐networks data, which indicate that, in RP individuals, high emotion seeking appears to affect the coupling of prefrontal nodes (ventral area 9/46 and left area 10) of the FPN but not that of the DMN.
(Lack of) Premeditation is associated with biased attentional modulation of information processing (Golchert et al., 2017). Our data show that this is positively correlated with the LN–VAN coupling, possibly by influencing the connectivity of orbitofrontal area 13 (LN) with the somatosensory areas of the VAN, and also that between the two LN nodes, the perirhinal cortex (Areas 35/36), and the temporal agranular insula, in RP, but not in NRP, individuals (in whom it tends to be negative). Given the role of the agranular insula in establishing internal drives and the valuation of rewards, and that it is the connection between this structure and the orbitofrontal cortex that influences the core affect (Wager, Barrett, & Feldman Barrett, 2004), we believe that the (lack of) premeditation trait biases the way in which external stimuli are valued by RP individuals, accelerating the (most likely inappropriate) responses.
Our within‐networks data suggest that risk proneness also affects the association between (lack of) perseverance, that is, the tendency to give up under distress or boredom, and coupling of nodes in the LN, FPN, and the DMN networks. (Lack of) Perseverance has been linked to abnormal gray matter volume and functionality of the medial prefrontal cortex (Wang et al., 2017). In RP individuals—but not in NRP individuals—we observed that the higher the (lack of) perseverance, the lower the coupling between temporal areas of the LN (right temporal agranular insular cortex with the right caudoventral area 20 and the left rostral areas 35/36 with right medial area 38) but found the reverse pattern of results regarding the coupling of left area 46 with left ventral areas 9/46 in the FPN and left medial area 9 with left caudal area 45 in the DMN in LN. These results suggest that (lack of) perseverance enhances the coupling of areas involved in the processing and valuation of the stimuli and, at the same time, reduces the coupling of prefrontal areas involved in cognitive control, which will again promote the delivery of more rapid and inappropriate responses.
Considered together, our results indicate that risk proneness is not only related to a characteristic personality pattern but also to different brain connectivity patterns associated with these personality traits. RP individuals tend to score high on impulsivity and sensation seeking, showing a higher impact of personality traits on the connectivity of brain networks both at the macro‐ and the microscopic (node) levels. This suggests that (a) personality traits modulate the functional connectivity of brain networks and (b) the tendency to behave in a risk‐prone manner influences how impulsivity‐related personality traits are associated with the functionality of these brain networks.
Supporting information
Figure S1. Localization of the nodes used in the brain connectivity analyses. Node color represents the network they are forming part of. Note: purple, visual network; blue, sematomotor network; green, dorsal attention network; violet, ventral attention network; cream, limbic network; orange, frontoparietal network; red, default mode network.
ACKNOWLEDGMENTS
This work was funded by the Spanish Ministry of Economy, Industry, and Competitiveness PSI2016‐80558‐R grant, awarded to A.C. and a predoctoral fellowship of the Spanish Ministry of Education, Culture and Sports (FPU14/05928) to S.B. We would also like to thank the suppport of the Andalusian Regional Government, and the European Regional Development Fund (ERDF), to the Brain, Behavior and Health, scientific excellence unit (SC2), ref: SOMM17 / 6103 / UGR.
Baltruschat S, Cándido A, Megías A, Maldonado A, Catena A. Risk proneness modulates the impact of impulsivity on brain functional connectivity. Hum Brain Mapp. 2020;41:943–951. 10.1002/hbm.24851
Funding information Spanish Ministry of Economy, Industry, and Competitiveness, Grant/Award Number: PSI2016‐80558‐R; Spanish Ministry of Education, Culture and Sports, Grant/Award Number: FPU14/05928; Andalusian Regional Government, Grant/Award Number: SOMM17/6103/UGR
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- Aluja, A. , & Blanch, A. (2011). Neuropsychological behavioral inhibition system (BIS) and behavioral approach system (BAS) assessment: A shortened sensitivity to punishment and sensitivity to reward questionnaire version (SPSRQ–20). Journal of Personality Assessment, 93(6), 628–636. 10.1080/00223891.2011.608760 [DOI] [PubMed] [Google Scholar]
- Anticevic, A. , Cole, M. W. , Murray, J. D. , Corlett, P. R. , Wang, X. J. , & Krystal, J. H. (2012). The role of default network deactivation in cognition and disease. Trends in Cognitive Sciences, 16(12), 584–592. 10.1016/j.tics.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley‐Levenson, E. , Xue, F. , Droutman, V. , Miller, L. C. , Smith, B. J. , Jeong, D. , … Read, S. J. (2018). Prefrontal cortical activity during the stroop task: New insights into the why and the who of real‐world risky sexual behavior. Annals of Behavioral Medicine, 52(5), 367–379. 10.1093/abm/kax019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beanland, V. , Sellbom, M. , & Johnson, A. K. (2014). Personality domains and traits that predict self‐reported aberrant driving behaviours in a southeastern US university sample. Accident Analysis & Prevention, 72, 184–192. 10.1016/j.aap.2014.06.023 [DOI] [PubMed] [Google Scholar]
- Cándido, A. , Orduña, E. , Perales, J. C. , Verdejo‐García, A. , & Billieux, J. (2012). Validation of a short Spanish version of the UPPS‐P impulsive behaviour scale. Trastornos Adictivos, 14(3), 73–78. 10.1016/S1575-0973(12)70048-X [DOI] [Google Scholar]
- Chadick, J. Z. , & Gazzaley, A. (2011). Differential coupling of visual cortex with default or frontal‐parietal network based on goals. Nature Neuroscience, 14(7), 830–832. 10.1038/nn.2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester, D. S. , Lynam, D. R. , Milich, R. , Powell, D. K. , Andersen, A. H. , & DeWall, C. N. (2016). How do negative emotions impair self‐control? A neural model of negative urgency. NeuroImage, 132, 43–50. 10.1016/j.neuroimage.2016.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff, K. , Gordon, A. M. , Smallwood, J. , Smith, R. , & Schooler, J. W. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences, 106(21), 8719–8724. 10.1073/pnas.0900234106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, M. W. , Reynolds, J. R. , Power, J. D. , Repovs, G. , Anticevic, A. , & Braver, T. S. (2013). Multi‐task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience, 16(9), 1348–1355. 10.1038/nn.3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders, M. A. , Smith, G. T. , Spillane, N. S. , Fischer, S. , Annus, A. M. , & Peterson, C. (2007). Integration of impulsivity and positive mood to predict risky behavior: Development and validation of a measure of positive urgency. Psychological Assessment, 19(1), 107–118. 10.1037/1040-3590.19.1.107 [DOI] [PubMed] [Google Scholar]
- Delorme, A. , & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single‐trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- DeWitt, S. J. , Aslan, S. , & Filbey, F. M. (2014). Adolescent risk‐taking and resting state functional connectivity. Psychiatry Research: Neuroimaging, 222(3), 157–164. 10.1016/j.pscychresns.2014.03.009 [DOI] [PubMed] [Google Scholar]
- Deza Araujo, Y. I. , Nebe, S. , Neukam, P. T. , Pooseh, S. , Sebold, M. , Garbusow, M. , … Smolka, M. N. (2018). Risk seeking for losses modulates the functional connectivity of the default mode and left frontoparietal networks in young males. Cognitive, Affective, & Behavioral Neuroscience, 18(3), 536–549. 10.3758/s13415-018-0586-4 [DOI] [PubMed] [Google Scholar]
- Di Stasi, L. L. , Álvarez‐Valbuena, V. , Cañas, J. J. , Maldonado, A. , Catena, A. , Antolí, A. , & Candido, A. (2009). Risk behaviour and mental workload: Multimodal assessment techniques applied to motorbike riding simulation. Transportation Research Part F: Traffic Psychology and Behaviour, 12(5), 361–370. 10.1016/j.trf.2009.02.004 [DOI] [Google Scholar]
- Donohew, L. , Zimmerman, R. , Cupp, P. S. , & Novak, S. (2000). Sensation seeking, impulsive decision‐making, and risky sex: Implications for risk‐taking and design of interventions. Personality and Individual Differences, 28, 1079–1091. [Google Scholar]
- Fan, L. , Li, H. , Zhuo, J. , Zhang, Y. , Wang, J. , Chen, L. , … Jiang, T. (2016). The human brainnetome atlas: A new brain atlas based on connectional architecture. Cerebral Cortex, 26(8), 3508–3526. 10.1093/cercor/bhw157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golchert, J. , Smallwood, J. , Jefferies, E. , Liem, F. , Huntenburg, J. M. , Falkiewicz, M. , … Margulies, D. S. (2017). In need of constraint: Understanding the role of the cingulate cortex in the impulsive mind. NeuroImage, 146, 804–813. 10.1016/j.neuroimage.2016.10.041 [DOI] [PubMed] [Google Scholar]
- Holmes, A. J. , Hollinshead, M. O. , Roffman, J. L. , Smoller, J. W. , & Buckner, R. L. (2016). Individual differences in cognitive control circuit anatomy link sensation seeking, impulsivity, and substance use. Journal of Neuroscience, 36(14), 4038–4049. 10.1523/JNEUROSCI.3206-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano, L. M. , Megías, A. , Catena, A. , Perales, J. C. , Baltruschat, S. , & Cándido, A. (2017). Spanish validation of the domain‐specific risk‐taking (DOSPERT‐30) scale. Psicothema, 29(1), 111–118. 10.7334/psicothema2016.132 [DOI] [PubMed] [Google Scholar]
- Megías, A. , Cortes, A. , Maldonado, A. , & Cándido, A. (2017). Using negative emotional feedback to modify risky behavior of young moped riders. Traffic Injury Prevention, 18(4), 351–356. 10.1080/15389588.2016.1205189 [DOI] [PubMed] [Google Scholar]
- Megías, A. , López‐Riañez, M. , & Cándido, A. (2013). Conductas urgentes y evaluativas en función del nivel de riesgo en situaciones de conducción. Anales de Psicología, 29(3), 1032‐1037. 10.6018/analesps.29.3.145451 [DOI] [Google Scholar]
- Megías, A. , Navas, J. F. , Petrova, D. , Cándido, A. , Maldonado, A. , Garcia‐Retamero, R. , & Catena, A. (2015). Neural mechanisms underlying urgent and evaluative behaviors: An fMRI study on the interaction of automatic and controlled processes. Human Brain Mapping, 36(8), 2853–2864. 10.1002/hbm.22812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlert, N. , & Lawrence, A. D. (2015). Brain structure correlates of emotion‐based rash impulsivity. NeuroImage, 115, 138–146. 10.1016/j.neuroimage.2015.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual‐Marqui, R. D. (2002). Standardized low‐resolution brain electromagnetic tomography (sLORETA): Technical details. Methods and Findings in Experimental and Clinical Pharmacology, 24(Suppl. D), 5–12. [PubMed] [Google Scholar]
- Pérez, J. , & Torrubia, R. (1986). Fiabilidad y validez de la versión española de la escala de búsqueda de sensaciones. Revista Latinoamericana de Psicología, 18(001), 7–22. [Google Scholar]
- Scott‐parker, B. , Watson, B. , King, M. J. , & Hyde, M. K. (2012). The influence of sensitivity to reward and punishment, propensity for sensation seeking, depression, and anxiety on the risky behaviour of novice drivers : A path model. British Journal of Psychology, 103, 248–267. 10.1111/j.2044-8295.2011.02069.x [DOI] [PubMed] [Google Scholar]
- Tang, A. C. , Sutherland, M. T. , & McKinney, C. J. (2005). Validation of SOBI components from high‐density EEG. NeuroImage, 25(2), 539–553. 10.1016/j.neuroimage.2004.11.027 [DOI] [PubMed] [Google Scholar]
- Torrubia, R. , Ávila, C. , Moltó, J. , & Caseras, X. (2001). The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray's anxiety and impulsivity dimensions. Personality and Individual Differences, 31(6), 837–862. 10.1016/S0191-8869(00)00183-5 [DOI] [Google Scholar]
- Wager, T. D. , Barrett, L. F. , & Feldman Barrett, L. (2004). From affect to control: Functional specialization of the insula in motivation and regulation. PsycExtra, 129, 2865 10.1101/102368 [DOI] [Google Scholar]
- Wang, H. , Wen, B. , Cheng, J. , & Li, H. (2017). Brain structural differences between normal and obese adults and their links with lack of perseverance, negative urgency and sensation seeking. Scientific Reports, 7(1), 40595 10.1038/srep40595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, E. U. , Blais, A.‐R. , & Betz, N. E. (2002). A domain‐specific risk‐attitude scale: Measuring risk perceptions and risk behaviors. Journal of Behavioral Decision Making, 15(4), 263–290. 10.1002/bdm.414 [DOI] [Google Scholar]
- Winkler, I. , Brandl, S. , Horn, F. , Waldburger, E. , Allefeld, C. , & Tangermann, M. (2014). Robust artifactual independent component classification for BCI practitioners. Journal of Neural Engineering, 11(3), 035013 10.1088/1741-2560/11/3/035013 [DOI] [PubMed] [Google Scholar]
- Winkler, I. , Haufe, S. , & Tangermann, M. (2011). Automatic classification of artifactual ICA‐components for artifact removal in EEG signals. Behavioral and Brain Functions, 7(1), 30 10.1186/1744-9081-7-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, A. , & Carducci, B. J. (1991). Sensation seeking and financial risk taking in everyday money matters. Journal of Buisness and Psychology, 5(4), 525–530. [Google Scholar]
- Woolgar, A. , Afshar, S. , Williams, M. A. , & Rich, A. N. (2015). Flexible coding of task rules in frontoparietal cortex: An adaptive system for flexible cognitive control. Journal of Cognitive Neuroscience, 27(10), 1895–1911. 10.1162/jocn_a_00827 [DOI] [PubMed] [Google Scholar]
- World Medical Association . (2008). Declaration of Helsinki: Ethical principles for medical research involving human subjects. Seoul, South Korea: Author; Retrieved from. http://www.wma.net/en/30publications/10policies/b3/17c.pdf [Google Scholar]
- Yeo, B. T. T. , Krienen, F. M. , Sepulcre, J. , Sabuncu, M. R. , Lashkari, D. , Hollinshead, M. , … Buckner, R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J. , Tomasi, D. , Wiers, C. E. , Shokri‐Kojori, E. , Demiral, Ş. B. , Zhang, Y. , … J, G. (2017). Correlation between traits of emotion‐based impulsivity and intrinsic default‐mode network activity. Neural Plasticity, 2017, 1–9. 10.1155/2017/9297621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman, M. , & Kuhlman, D. M. (2000). Personality and risk‐taking: Common biosocial factors. Journal of Personality, 68(6), 999–1029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Localization of the nodes used in the brain connectivity analyses. Node color represents the network they are forming part of. Note: purple, visual network; blue, sematomotor network; green, dorsal attention network; violet, ventral attention network; cream, limbic network; orange, frontoparietal network; red, default mode network.
Data Availability Statement
Data available on request from the authors.


