Abstract
Electroconvulsive therapy is regarded as the most effective antidepressant treatment for severe and treatment‐resistant depressive episodes. Despite the efficacy of electroconvulsive therapy, the neurobiological underpinnings and mechanisms underlying electroconvulsive therapy induced antidepressant effects remain unclear. The objective of this investigation was to identify electroconvulsive therapy treatment responsive multimodal biomarkers with the 17‐item Hamilton Depression Rating Scale guided brain structure–function fusion in 118 patients with depressive episodes and 60 healthy controls. Results show that reduced fractional amplitude of low frequency fluctuations in the prefrontal cortex, insula and hippocampus, linked with increased gray matter volume in anterior cingulate, medial temporal cortex, insula, thalamus, caudate and hippocampus represent electroconvulsive therapy responsive covarying functional and structural brain networks. In addition, relative to nonresponders, responder‐specific electroconvulsive therapy related brain networks occur in frontal‐limbic network and are associated with successful therapeutic outcomes. Finally, electroconvulsive therapy responsive brain networks were unrelated to verbal declarative memory. Using a data‐driven, supervised‐learning method, we demonstrated that electroconvulsive therapy produces a remodeling of brain functional and structural covariance that was unique to antidepressant symptom response, but not linked to memory impairment.
Keywords: depressive episodes, electroconvulsive therapy, multimodal fusion, treatment response
1. INTRODUCTION
Depressive episodes (DEP) is a leading cause of disability worldwide (Friedrich, 2017). Though DEP is typically treated with different forms of psychotherapy or pharmacotherapy, electroconvulsive therapy (ECT) is regarded as the most effective treatment for severe and treatment‐resistant depressive episodes for both major depressive and bipolar disorders (Kellner et al., 2006; Medda, Toni, & Perugi, 2014; UK ECT Review Group, 2003). Cumulative studies report that ECT is significantly more effective than pharmacotherapy, with 50–60% of patients achieving rapid remission of depression after an ECT course compared with 10–40% with either pharmacotherapy or psychotherapy (Husain et al., 2004). From a health‐economic standpoint, ECT should be considered after failure of two or more lines of pharmacotherapy and psychotherapy (Ross, Zivin, & Maixner, 2018). But from a clinical point of view, ECT can be a first‐line treatment for patients in urgent clinical situations, including acute suicidality or malnutrition (Jaffe, 2001). ECT is also particularly effective in patients with major depressive disorder (MDD) with psychotic features and in elderly adults with MDD (van Diermen et al., 2018). Despite the efficacy of ECT, the neurobiological underpinnings and the mechanisms underlying symptom improvement of ECT‐induced antidepressant response remains unclear. Identifying ECT responsive biomarkers could clarify the mechanisms of antidepressant action and promote individually tailored and precision treatment.
Although ECT results in significant clinical improvement, patients often show cognitive difficulties during the ECT series (McClintock et al., 2014). Previous investigations have demonstrated that hippocampal dependent cognitive functions, such as declarative memory, are most adversely affected by ECT (Rami‐Gonzalez et al., 2001). In addition, verbal learning and memory, complex visual scanning and cognitive flexibility, and verbal fluency can be adversely impacted for up to 2 weeks following an ECT series (Semkovska & McLoughlin, 2010). Even though the cognitive sequelae are transient and ECT does not increase the risk of dementia (Chu et al., 2018), the possibility of cognitive impairment can be dissuasive to an individual with a depressive episode even if they have found no benefit with other antidepressant modalities, particularly for independent‐living outpatients.
Specific brain regions including the hippocampus (Oltedal et al., 2018), amygdala (Joshi et al., 2016), striatum (Wade et al., 2016), and cerebellum (Depping et al., 2017), as well as the whole‐brain structural changes (Argyelan et al., 2019; Ousdal et al., 2019) were investigated following ECT. In addition, single imaging modality (Abbott et al., 2013; Jiang et al., 2018; Leaver et al., 2019; Redlich et al., 2016; Sun et al., 2019; van Waarde et al., 2015) and multimodal analysis (Cano et al., 2017; Jorgensen et al., 2016; Nickl‐Jockschat et al., 2016) but with a small sample size (n < 25 patients) have been assessed with treatment response. Two recent mega‐analysis of structural imaging investigations confirmed that ECT induced broadly gray matter volume increase (Ousdal et al., 2019), including hippocampus (Oltedal et al., 2018), but neither of them found association with treatment outcome. These results suggest that the efficacy of ECT is unexplained by hippocampal enlargement or single gray matter modality, which alone might not serve as a viable biomarker for treatment outcomes (Zhuo & Yu, 2014). Furthermore, pretranslational (Akers et al., 2014) and translational (van Oostrom et al., 2018) investigations have demonstrated an association between hippocampal neuroplasticity and cognitive impairment. However, the focus on a specific brain region or single imaging modality may limit discovery of structural and functional brain changes in the whole point view that are related to clinical outcomes. Therefore, this investigation aimed to address this limitation by multimodal fusion, which jointly analyzes functional magnetic resonance imaging (fMRI) and structural magnetic resonance imaging (sMRI) data to leverage the cross‐information in the existing data, thereby revealing important relationships that cannot be detected by using a single neuroimaging modality (Qi, Yang, et al., 2018; Sui et al., 2018).
Specifically, we used a depressive symptom‐guided multimodal fusion approach to identify ECT treatment responsive multimodal brain networks. This fusion method allows a data‐driven analysis of ECT treatment responsive networks and the identification of targeted brain regions that exhibited a significant change after ECT treatment. For this investigation, we focused on the following four goals: (a) to identify the brain structural and functional remodeling associated with reduction in depression severity after ECT; (b) to assess the covarying, multimodal treatment responsive‐networks in DEP patients, in contrast with healthy controls (HC); (c) to identify different treatment responsive brain regions between DEP responders and nonresponders; and (d) to evaluate the relationship between treatment responsive brain networks and memory.
2. METHODS AND MATERIALS
2.1. Participants
Patients with a depressive episode (n = 118) and HCs (n = 60) participating in this investigation were recruited from the University of New Mexico (UNM) and University of California Los Angeles (UCLA) after meeting the clinical indication for ECT. Inclusion criteria at both sites included depressive episodes (unipolar depression at UNM (n = 75), unipolar (n = 36), or bipolar (n = 7) depression at UCLA). Two independent psychiatric examinations confirmed diagnosis prior to the initiation of ECT at both sites; in addition, UCLA performed the Mini‐Neuropsychiatric Instrument (Sheehan et al., 1998). Additional inclusion criteria included age (UNM: 50–80 years, UCLA: 18–75 years), treatment resistance (failure of two antidepressants), decisional capacity to consent to research (UNM and UCLA) or assent to research with surrogate decision maker consent (UNM). Exclusion criteria for DEP included the following: (a) defined neurodegenerative or neurological disorder (e.g., Alzheimer's disease, epilepsy or head injury); (b) other psychiatric conditions (e.g., schizoaffective disorder, schizophrenia); (c) current alcohol or drug dependence; (d) pregnancy; and (e) contraindication to magnetic resonance imaging (MRI) (e.g., pacemaker). The clinical assessment was the 17‐item Hamilton Depression Rating Scale (HDRS) at both sites. ECT response was defined as >50% improvement from baseline in HDRS (Heijnen, Birkenhager, Wierdsma, & van den Broek, 2010). Demographically matched HCs were recruited at UNM (n = 27), confirmed with Structured Clinic Interview–Non Patient (First, Spitzer, Gibbon, & Williams, 2002) and UCLA (n = 33, confirmed with MINI; Sheehan et al., 1998).” The healthy controls were scanned twice (PRE and POST ECT) at both sites with a similar 4‐week gap between scan intervals. We performed paired t test on HCs between PRE and POST ECT on both fALFF and GM. Results show that there are no significant (no imaging voxels passed the FDR correction p ≤ 1.0e − 08 for multiple comparison) changes on HC group between two time points.
Depressed subjects completed the cognitive testing on the same date as MRI scans. Pre‐ECT scans were completed within 2 days of ECT start and post‐ECT assessment completed within 7 days of finishing ECT series. UCLA DEP completed a neuropsychological assessment that included the Hopkins Verbal Learning Test‐Revised (HVLT‐R) (Brandt & Benedict, 2001). UNM DEP completed the Repeatable Battery of Neuropsychological Status (RBANS), which included a 10‐word verbal learning and memory task (Randolph, Tierney, Mohr, & Chase, 1998). Percent recall for the HVLT‐R and RBANS was calculated as the percentage of total words recalled during delayed recall trial relative to the maximum of the words from either the second or third learning trials. The percent retention score is a useful measure of hippocampal dependent memory function that reduces the possibility of overestimating memory function from immediate and delayed free recall scores (Clark, Hobson, & O'Bryant, 2010). ECT procedures were similar across both study sites. Subjects started with a right unilateral electrode placement unless bitemporal was clinically indicated (acuity, nonresponsive to right unilateral electrode placement). The average ECT treatments for the entire DEP sample was 11.0 ± 3.4 and the majority of DEP completed the ECT series with a right unilateral electrode placement (n = 81 of 118 total, summarized in Table 1). Earlier investigations at UNM included subjects with concurrent antidepressant medications, but the latter investigations at UNM (n = 48) and all the UCLA subjects discontinued antidepressant medications prior to commencement of the ECT series. The demographic, clinical and medical characteristics of the sample are summarized in Table 1. The UCLA vs. UNM, responder vs. nonresponder and remitter vs. nonremitter (ECT remission was defined as >50% reduction in HDRS and final HDRS‐17 score≤7) information are shown in Tables S1–S3. A correlation analysis between change in HDRS total score (∆HDRS) and clinical measures, as well as t test for ΔHDRS scores with gender, handedness (dichotomous variables) and ANOVA for ΔHDRS with education degree (ordinal variables) are displayed in Tables S4–S5. This study was approved by the institutional review boards of UNM and UCLA and all participants provided written informed consent prior to participation in the study.
Table 1.
Demographic and clinical information of participants
| DEP | HC | T tests | |
|---|---|---|---|
| Demographic characteristics | |||
| Sample size (n) | n = 118 | n = 60 | n/a |
| Age (years) (mean/sd) | 56.2±16.0 | 48.6±14.9 | 0.002 |
| Gender (M/F) | 43/71 | 26/34 | 0.38 |
| Education degreea (mean/sd) | 5.4±1.9 | 6.3±1.7 | 0.0013 |
| Handiness (R/L) | 111/7 | 56/4 | 0.32 |
| Mean frame‐wise displacementb (pre‐ECT) | 0.25/0.14 | 0.17/0.11 | 2.2e−04 |
| Mean frame‐wise displacementb (post‐ECT) | 0.27/0.16 | 0.17/0.11 | 1.5e−05 |
| Clinical characteristics | |||
| Number of major depressive episodes | 6.2±14.4 | n/a | n/a |
| Duration of current depressive episode (months) | 23.3±33.5 | n/a | n/a |
| Total number of ECT treatments | 11.0±3.4 | n/a | n/a |
| Right unilateral (RUL)/mixed RUL‐bitemporal, and BTc | 81/37 | n/a | n/a |
| Pre‐ECT HDRS | 25.4±6.1 | n/a | n/a |
| Post‐ECT HDRS | 11.5±8.9 | n/a | n/a |
| ∆HDRS (pre‐post) | 14.0±10.0 | n/a | n/a |
| Responder (%) | 64 (54%) | n/a | n/a |
| Pre‐ECT % recall (verbal declarative memory) | 73.7±62.0 | n/a | n/a |
| Post‐ECT % recall (verbal declarative memory) | 68.3±30.6 | n/a | n/a |
| ∆ “% recall” of verbal declarative memory (pre‐post) | 5.9±64.5 | n/a | n/a |
| p value of ∆HDRS | p = 2.8e−66 | n/a | n/a |
| p value of ∆ “% recall” | p = .423 | n/a | n/a |
| Medical characteristics | |||
| No medicationd (67%) | 79 | 60 | n/a |
| Antidepressants (33%) | 39 | 0 | n/a |
| SSRI (selective serotonin reuptake inhibitors) | 17 | 0 | n/a |
| SNRI (serotonin–norepinephrine reuptake inhibitors) | 17 | 0 | n/a |
| TCA (tricyclic antidepressants) | 4 | 0 | n/a |
| MAOI (monoamine oxidase inhibitor) | 0 | 0 | n/a |
| Buproprion | 1 | 0 | n/a |
| Antipsychotic (16%) | 20 | 0 | n/a |
“Education degree” details are presented in Supplementary “Education degree” section.
“Mean frame‐wise displacement” reflects head motion in functional imaging data.
Right unilateral (RUL)/mixed RUL‐bitemporal, and BT denote the final electrode placement of the ECT series.
“No medication” denotes tapered off medications before initiation of ECT series (and remained off meds for the duration of the ECT series).
2.2. Multimodal image preprocessing
The fMRI and sMRI imaging parameters have been detailed previously (Abbott et al., 2013; Jiang et al., 2018; Leaver et al., 2016). For fMRI, standard preprocessing in SPM12 included the following: (a) realignment; (b) slice timing correction; (c) normalization to an EPI (3 × 3 × 3 mm3) template; (d) spatial smoothing using a 6‐mm full width half‐maximum Gaussian kernel; (e) regression of parameters including band‐pass temporal filtering (0.01–0.15 Hz), and nuisance variables (six parameters obtained by rigid body head motion correction, cerebrospinal fluid, white matter signals and global signal); and (f) calculation of fractional amplitude of low frequency fluctuations (fALFF) (Zou et al., 2008): the sum of the amplitude values in the 0.01–0.08 Hz low‐frequency power range was divided by the sum of the spectral amplitudes over the entire detectable power spectrum (range: 0–0.25 Hz) (Turner et al., 2013).
The sMRI preprocessing included the following: (a) segmentation into gray matter (GM), white matter (WM) and cerebral spinal fluid (CSF); (b) normalization to Montreal Neurological Institute (MNI) space using the unified segmentation method in SPM12; (c) resliced to 3 × 3 × 3 mm3; and (d) smoothed with an full width‐half maximum 6 mm Gaussian filter. After preprocessing, two representative MRI features (fALFF from fMRI and GM volume from sMRI) were extracted. Next, each modality was reshaped into a feature matrix with columns representing voxels and rows representing subjects. Since ∆HDRS was correlated with age and gender (Table S4), we regressed out age, gender, and site from fALFF and GM prior to fusion analysis. For fALFF, we also regressed out mean frame‐wise displacement (FD). Finally, the obtained two feature matrixes were normalized to have the same average sum of squares (computed across all subjects and all voxels for each modality) to ensure all modalities had the same range of values.
As for head motion, we removed the outlier subjects who have micro motion such as framewise displacements (FD) exceeding 1 mm, as well as head motion exceeding 2.0 mm of maximal translation (in any direction of x, y, or z) or 1.0° of maximal rotation throughout the course of scanning. We also calculated the correlation between mean FD and the brain imaging features (fALFF and GM) before fusion analysis. The results showed that there were no imaging voxels passed p < 1.0e−05 (not FDR corrected for multiple comparisons) for neither PRE nor POST ECT. Moreover, the fusion analysis was conducted on the spatial maps of fALFF, but not the original 4D fMRI data, considering there is no longitudinal difference in head motion, we believe that micromotion is not a major factor affecting the current results.
2.3. Study design
According to the four goals stated in the introduction, we performed the corresponding four analyzes: (a) HDRS total scores were used as a reference to guide a two‐way MRI (fALFF+GM) fusion for all DEP (responders + nonresponders); (b) back‐reconstruction was performed to compare group difference between DEP and HC of the identified multimodal components; (c) HDRS‐guided fusion on responders and nonresponders subgroups separately; and (d) an assessment of the identified treatment‐responsive network and memory (Figure 1).
Figure 1.
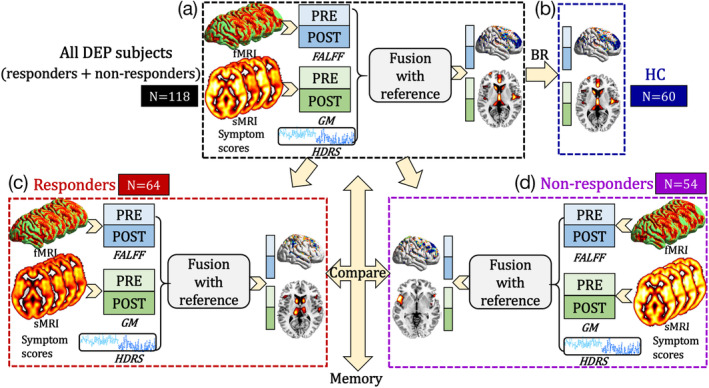
Flowchart of our study design. (a) HDRS total scores were used as a reference to guide a two‐way MRI fusion for all DEP subjects (responders + nonresponders) to identify ECT responsive multimodal brain networks. (b) Back‐reconstruction (BR) was performed on the HC group. Then the same HDRS‐guided fusion was performed on (c) responder and (d) nonresponder subgroups separately to extract specific ECT responsive brain networks. Finally, the correlation between memory scores and the identified ECT responsive multimodal brain networks was examined
Specifically, the preprocessed multimodal MRI features were jointly analyzed by a fusion‐with‐reference model called “MCCAR+jICA” (multisite canonical correlation analysis with reference + joint independent component analysis) (Qi, Calhoun, et al., 2018), as shown in Figure 1a, where subject‐wise HDRS scores were used as a reference to guide joint decomposition of fALFF and GM, in order to investigate the HDRS‐associated, covarying fALFF and GM patterns that may also change between pre‐ and post‐ECT treatment. This supervised fusion model can simultaneously maximize the intermodality covariation and correlations of certain imaging components with a specific measure of interest (HDRS), providing additional control compared to blind N‐way multimodal fusion approaches. As a result, this method enables identification of a joint multimodal component (linked fALFF‐GM components) that is correlated with HDRS. Here, to maximally separate brain regions, we used a higher order ICA (component number = 100). We aimed to identify the joint ICs significantly correlated with HDRS and longitudinally discriminative between Pre‐ECT and Post‐ECT MRI features. To establish how ECT responsive networks vary from normative networks, the back‐reconstruction of ECT responsive brain maps to the HC group (Figure 1b) was performed based on the linear projection model as in Equation (1).
| (1) |
where SDEP,k and ADEP,k denote the brain components and the corresponding mixing matrix derived by MCCAR+jICA for all the DEP subjects. XDEP,k and XHC,k represent the preprocessed imaging feature matrixes as shown in Figure 1. k represents the modality. Consequently, the spatial maps of DEP (SDEP,k) group were used to estimate the mixing matrix of HC group (AHC,k) base on Equation (1). Then, the same HDRS‐guided fusion was performed to identify ECT‐responsive brain network features across all DEP patients, as well as for responder and nonresponder subgroups separately, to extract specific subgroup ECT responsive brain networks (Figure 1c,d). Finally, the correlation between verbal declarative memory scores and the identified ECT responsive multimodal brain networks was examined.
3. RESULTS
3.1. ECT responsive multimodal brain networks
Paired t tests were performed on loading parameters (contribution weight of the corresponding component across subjects) of each component for each modality. One joint (the intermodality correlation is r = 0.58, p = 1.5e−22*) component was identified that longitudinally discriminated pre‐ and post‐ECT (for fALFF, t(117) = −4.6, p = 1.1e−05*, power = 0.91, 117 is the degree of freedom; for GM, t(117) = −5.6, p = 1.5e−07*, power = 0.94) and negatively correlated with the HDRS (for fALFF, r = −0.56, p = 1.5e−20*; for GM, r = −0.57, p = 9.1e−22*, where * signifies false discovery rate correction [FDR] for multiple comparisons). The spatial maps were transformed into Z scores, visualized at |Z| > 2 as in Figure 2a. The positive brain regions (red) indicate higher contribution with the post‐ECT loading coefficients, and the negative brain regions (blue) indicate higher contribution with the pre‐ECT loading coefficients. The identified regions in ECT responsive components are summarized in Table S6 for fALFF and GM (Talairach labels), respectively. Figure 2c shows the correlations between loadings of ECT responsive components and HDRS scores (pre‐ECT: light green dots, post‐ECT: dark green dots). For GM, the lower loadings correspond to higher HDRS scores within the pre‐ (r = −0.58, p = 7.9e−12*), post‐ECT (r = −0.76, p = −3.3e−23*), and the entire DEP datasets (r = −0.57, p = 9.1e−22*) for GM (Figure 2c). Similarly for fALFF, the lower loadings correspond to higher HDRS scores within the pre‐ (r = −0.39, p = 1.6e−5*), post‐ECT (r = −0.66, p = 3.3e−16*), and the whole DEP datasets (r = −0.56, p = 1.5e−20*). The correlations within pre‐ and post‐ECT datasets preserve the same directions. Note that the partial correlations between the identified multimodal brain networks and the HDRS (Figure 2c), as well as the group difference (Figure 2b) still remain significant (almost the same as the original significance level) even after regressing out the number of ECT sessions, or electrode placement. Details can be found in Supplementary “Effect of electrode placement” and “Effect of ECT numbers” sections. After the ECT series, decreased brain activity in the prefrontal cortex (PFC), insula, caudate and hippocampus in fMRI were accompanied with increased GM volume in medial temporal lobe (MTL), insula, caudate, anterior cingulate cortex (ACC), thalamus, and hippocampus in sMRI. Insula and hippocampus were identified within both sMRI and fMRI treatment‐responsive networks.
Figure 2.
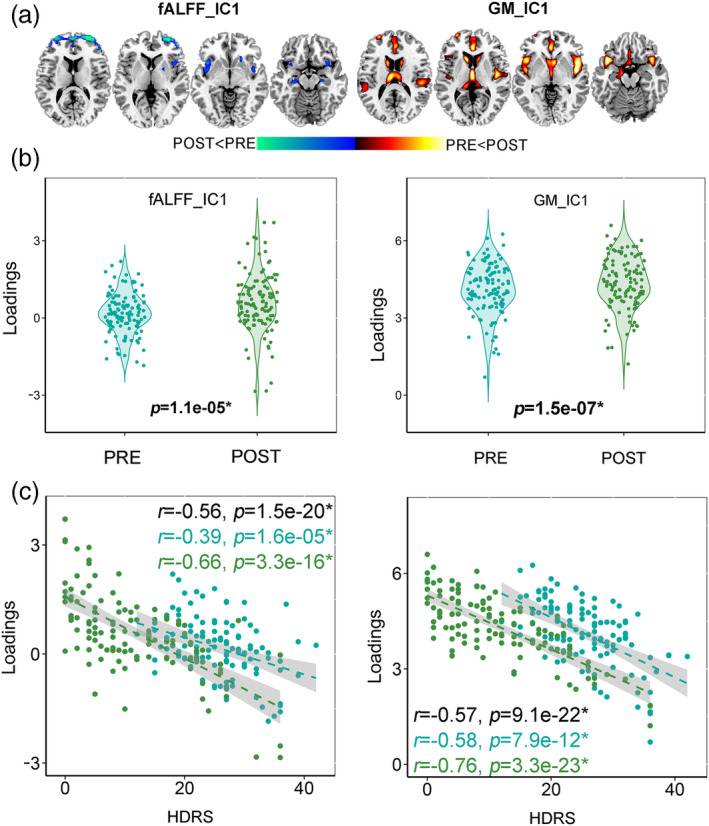
The identified joint components longitudinally discriminative between Pre‐ECT and Post‐ECT and correlated with the HDRS total scores for the entire DEP dataset. (a) The spatial maps visualized at |Z| > 2 thresholds, where the red regions identify the POST > PRE contrast and the blue regions identify the PRE > POST contrast. (b) Longitudinal difference between Pre‐ECT and Post‐ECT of the loading parameters (contribution weight of the corresponding component across subjects) of the target component. (c) Correlation between loadings of the identified components and HDRS (PRE: light green dots, POST: dark green dots). The black, light green and dark green values in each plot represent correlation of whole, Pre‐, and Post‐ECT datasets, respectively
3.2. DEP and HC difference of ECT responsive networks
Using back reconstruction, the mixing matrix of the HC group was obtained for each imaging modality. A two‐sample t test was performed on the loadings between the DEP and HC groups on the identified treatment‐responsive component (IC1). Results (Figure S1) showed that the identified ECT responsive components preserved group difference between the DEP and HC groups for both fMRI and sMRI brain maps in both the pre‐ and post‐ECT groups. The group difference of the sMRI feature (pre‐ECT: t(176) = −20.1, p = 2.1e−95*, post‐ECT: t(176) = −21.6, p = 1.7e−98*) had a more robust difference relative to the fMRI feature (pre‐ECT: t(176) = −4.5, p = 9.4e−06*, post‐ECT: t(176) = −3.2, p = 0.0018).
Note that there was group difference for age, education, and mean FD (Table 1) between DEP and HC, so we also calculated the significance values after regressing out those variables. Results showed that both fALFF (p = 1.1e−04*, 0.03) and GM (p = 2.1e−91*, 4.1e−84*) components still retained significant group differences between DEP and HC for both the PRE and POST ECT groups.
3.3. Comparison between responders and nonresponders
We performed the same HDRS‐guided fALFF‐GM fusion separately on responder and nonresponder subgroups to extract specific ECT responsive brain networks. We identified one joint component for both responder and nonresponder subgroups that longitudinally discriminated between time points (Pre‐ vs. Post‐ECT) and correlated with the HDRS, as displayed in Figures S2–S3. The identified regions in ECT responsive components are summarized in Tables S7–S8 for responder and nonresponder subgroups respectively. The ECT responsive fALFF‐GM brain networks for all DEP subjects, responder and nonresponder subgroups are displayed in Figure 3. The responder and nonresponder groups had unique treatment‐responsive networks. For responders (Figure 3b), the ECT series was associated with decreased brain activity in the PFC (fMRI: t[63] = −6.2, p = 4.8e−08*) and increased GM volume in caudate, thalamus and hippocampus (sMRI: t[63] = −5.1, p = 3.5e−06*). For nonresponders (Figure 3c), the ECT series was associate with decreased brain activity in the temporal cortex and the thalamus (fMRI: t[53] = −3.0, p = 0.004) and increased GM volume in left insula (sMRI: t[53] = −7.4, p = 9.7e‐10). Note that the identified responder and nonresponder specific fALFF‐GM covaried brain networks can be replicated based on more restrictive responder and nonresponder separation criteria (the upper 80% and lower 80% quartiles [Table S9] in terms of >50% change in HDRS pre/post‐ECT, Figure 3d,e). The similarity of the HDRS associated multimodal brain maps for the two different separation criteria are r = 0.72, 0.84 for fALFF and GM in responders, and r = 0.62, 0.78 for fALFF and GM in nonresponders, respectively, all with a significance level of p < 1.0e−04 (permutation test), demonstrating high spatial consistency in the stricter separation criteria validation. Details can be found in Supplementary “Stricter criteria replication” section. Furthermore, linked fALFF and GM components were identified for remitter and nonremitter subgroups respectively. Interestingly, the identified responder vs. nonresponder specific, covarying fALFF‐GM brain networks can be replicated under remitter vs. nonremitter separation criteria (Figure S4d‐e), in which the most replicated brain regions between remitters and responders are PFC in fALFF, as well as caudate and hippocampus in GM. While for nonresponders and nonremitters, middle temporal gyrus in fALFF linked with insula in GM are replicated.
Figure 3.
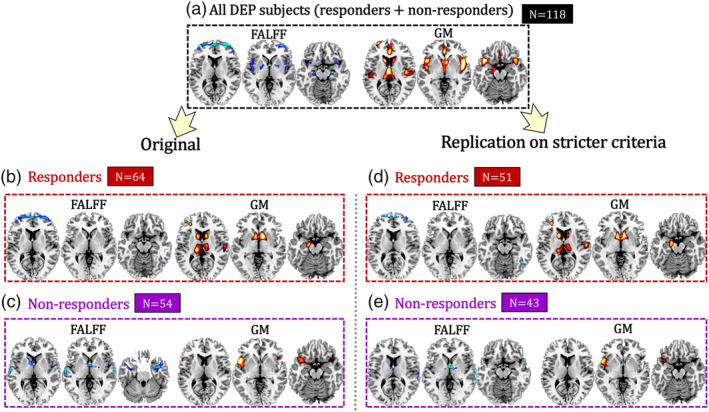
ECT treatment responsive multimodal brain networks for (a) all DEP subjects (responders + nonresponders, n = 118), separated into (b) responder (n = 64), and (c) nonresponder (n = 54) groups based on >50% change in HDRS pre/post‐ECT, and replication in (d) responder (n = 51), and (e) nonresponder (n = 43) groups using stricter response cut‐offs (the upper 80% and lower 80% quartiles of originally defined response)
3.4. Association with memory
We assessed the correlation between the identified ECT responsive multimodal brain networks with verbal memory (percent recall). No relationships were observed for the ECT responsive networks and verbal memory in all DEP subjects, and the responder/nonresponder subgroups (all p‐values > .05, uncorrected, Table S10).
4. DISCUSSION
To the best of our knowledge, this is the first attempt to estimate ECT treatment responsive biomarkers by jointly analyzing two types of MRI data under the guidance of the HDRS total scores. As summarized in Figure 4, our investigation demonstrated the following results. First, ECT treatment response was associated with brain alterations at the multimodal level with reduced fALFF (PFC, insula and hippocampus) and increased GM volume (ACC, insula, thalamus, caudate, medial temporal cortex, and hippocampus). Second, when the subgroups were analyzed separately, ECT responsive brain networks have different patterns in responders (frontal‐limbic) and nonresponders (insula and medial temporal lobe). Third, the identified ECT responsive brain networks in all DEP subjects, responders, and nonresponders were unrelated with memory performance. The above identified ECT responsive multimodal brain networks are related with HDRS even after regressing out the number of ECT sessions, or electrode placement.
Figure 4.
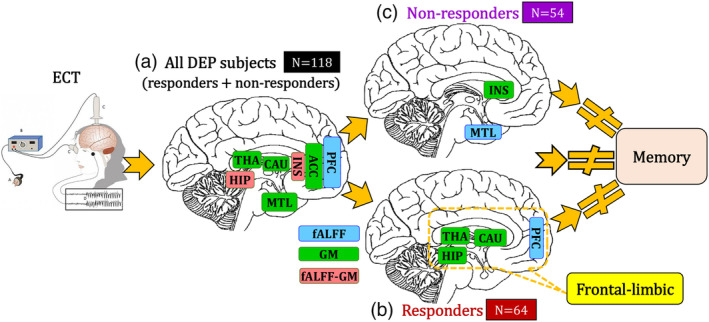
Summary of our findings on ECT responsive multimodal brain networks. ECT responsive fMRI (blue)‐sMRI (green) brain networks identified in the whole DEP dataset (a), responders (b) and nonresponders (c). ECT responsive brain networks have different patterns in responders (b, frontal‐limbic) and nonresponders (c, insula and medial temporal lobe). fALFF‐GM (light red) means these brain areas identified in both fMRI and sMRI. “≠” denotes no correlation. MTL is medial temporal lobe; THA is thalamus; INS is insula; HIP is hippocampus; CAU is caudate; ACC is anterior cingulate cortex; PFC is prefrontal cortex
A major finding was the identification of multimodal treatment responsive networks. The brain regions identified in fALFF and GM components include frontal (superior, inferior, middle), insula, MTL, and subcortical (ACC, caudate, hippocampus, and thalamus) regions all previously identified in depression related circuitry (Pandya, Altinay, Malone Jr., & Anand, 2012). Both functional and structural biomarkers identified the hippocampus and insula (Figure 4a). The hippocampus is one of the most widely implicated brain regions in depression and is impacted by clinical state (Schmaal et al., 2016). However, a recent mega‐analysis did not find relationships between hippocampal volume enlargements observed post ECT and clinical response, suggesting enlargement is masked or at least in part driven by processes related to seizure therapy itself (Oltedal et al., 2018). At the same time, smaller sample size studies have found ECT‐induced volume increases in the hippocampal dentate gyrus to associate with treatment outcome specifically (Nuninga et al., 2019; Takamiya et al., 2019), and ECT response is shown to positively relate to greater structural connectivity between hippocampus and fronto‐limbic regions (Kubicki et al., 2019). Notably, though seemingly disparate, the above findings are in line with our results indicating that a more complex multimodal brain network, including the hippocampus and connected regions, underlies depressive symptom improvement at the brain systems level. Via its connections with fronto‐limbic and association areas, the anterior insula plays a role in emotion regulation, and changes in insula activity and connectivity have been observed in a number of prior depression studies (Sliz & Hayley, 2012). Importantly, the pattern of decreased functional and increased structural loadings may have implications for the restoration of cortical and limbic depression‐related dysregulation after ECT (Mayberg, 2003). This network, as opposed to change in a specific imaging modality or singular region, may thus serve as a more sensitive and viable biomarker of ECT treatment outcome.
ECT responsive and nonresponsive patients have different patterns of functional and structural brain changes during the ECT series. Responder‐specific ECT related brain networks occur in a network of frontal‐limbic regions and are associated with successful therapeutic outcomes. The frontal‐limbic system is the most commonly identified dysfunctional network among existing studies of depression (Mayberg, 2003; Seminowicz et al., 2004). Decreased activity in the PFC after ECT represents the most consistent finding reviewed by a PET study (Schmidt et al., 2008). In contrast, decreased activity in the PFC has also been considered as a state marker for depressed state, suggesting that ECT may not normalize abnormal patterns in depression, which is consistent with that group differences (i.e., patients vs. controls) in the ECT responsive fronto‐limbic network remained post‐ECT. For nonresponders, brain changes are limited to the medial temporal lobe (fALFF) and insula (GM). The MTL and insula, which are affected only in nonresponders in this study, may not be associated with ECT‐mediated treatment response. The differences between responders and nonresponders after ECT treatment suggest that this frontal‐limbic ECT responsive circuit may serve as a potential biomarker of recovery from a depressed episode.
Extensive hippocampal remodeling is one possible hypothesis of ECT‐mediated cognitive impairment (Akers et al., 2014). Consistent with this hypothesis, a recent study demonstrated that increased hippocampal volume was related to decreased cognitive performance (van Oostrom et al., 2018). In contrast, our results demonstrate that ECT responsive brain networks had no contribution to memory impairment from multimodal and network perspectives. However, we only assessed the relationship between the identified treatment‐responsive network and cognitive performance. Another limitation was that we assessed only one cognitive domain, memory function that was based on a summary metric (percent recall) derived from two different neuropsychological tests (RBANS and HVLT‐R). In the future, our analysis methods can identify multimodal biomarkers with a focus on networks that discriminate the pre‐ and post‐ECT time points that may also have a relationship with cognitive performance. A more definitive analysis on ECT‐related neurocognitive impairment would also involve multiple cognitive domains (only memory assessed with our results) that can be impacted by ECT (Semkovska & McLoughlin, 2010).
A possible limitation of this work is that some subjects with DEP (33%) were receiving concurrent pharmacotherapy (antidepressants and/or antipsychotics), but antidepressant dose was held constant during the ECT series. Other investigations have found no association with ECT‐mediated neuroplasticity and concurrent pharmacotherapy or differences in medication‐comparison groups (Dukart et al., 2014; Redlich et al., 2016). Second, there is no longitudinal difference (pre/post ECT) of the verbal declarative memory task (p = .423) scores (Table 1). The median of the final ECT assessment for both UNM and UCLA datasets is 7 days. Furthermore, the final assessment was completed a minimum of 2 days after completion of the ECT series to minimize the acute effects of the procedure. In addition, we only assessed static brain function (fALFF). Dynamic functional network connectivity matrices (Qi et al., 2019) can also be used as fusion input for functional MRI to capture both temporal and spatial coalterations.
In summary, this is the first study to demonstrate that ECT causes a remodeling of both brain function and structure, which differs between responders and nonresponders and is unassociated with memory performance. This data‐driven investigation indicates that our identified ECT responsive multimodal brain regions may have broad impact on translational medicine, by providing opportunities for more effective and timely interventions. Specifically, our results demonstrate that (a) treatment responsive and ECT‐mediated neurocognitive impairment may be related to distinct circuitry, and (b) the treatment responsive frontal‐limbic network, although broadly distributed, could be optimal for targeted engagement by neurostimulation therapies. Future replications on medication‐free depressive subjects can help to exclude the influence of medication in identifying ECT treatment responsive specific brain networks. Similar analysis focused on cognitive‐guided fusion could also identify ECT neurocognitive specific brain networks. From a clinical perspective, we conclude that the treatment‐responsive biomarkers may be separate from cognitive biomarkers and could guide future refinements and precision of ECT stimulus delivery to improve clinical outcomes.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGMENTS
This work is supported in part by the Strategic Priority Research Program of the Chinese Academy of Sciences (grant No. XDBS03040100), China Natural Science Foundation (No. 61773380), Beijing Municipal Science and Technology Commission (No. Z181100001518005), the National Institute of Health (1R56MH117107, R01EB020407, 1R01EB005846, 1R01MH094524, P20GM103472, P30GM122734), the National Science Foundation (1539067), and the National Institute of Mental Health (U01MH111826).
Qi S, Abbott CC, Narr KL, et al. Electroconvulsive therapy treatment responsive multimodal brain networks. Hum Brain Mapp. 2020;41:1775–1785. 10.1002/hbm.24910
Funding information Beijing Municipal Science and Technology Commission, Grant/Award Number: Z181100001518005; China Natural Science Foundation, Grant/Award Number: 61773380; National Institute of Health, Grant/Award Numbers: 1R01EB005846, 1R01MH094524, 1R56MH117107, P20GM103472, P30GM122734, R01EB020407; Strategic Priority Research Program of the Chinese Academy of Sciences, Grant/Award Number: XDBS03040100; National Institute of Mental Health, Grant/Award Number: U01MH111826; National Science Foundation, Grant/Award Number: 1539067
Contributor Information
Christopher C. Abbott, Email: cabbott@salud.unm.edu.
Jing Sui, Email: kittysj@gmail.com.
DATA AVAILABILITY STATEMENT
The supervised fusion code has been released and integrated in the Fusion ICA Toolbox (FIT, https://trendscenter.org/software/fit), which can be downloaded and used directly by users worldwide. The ECT multimodal data used in the present study can be accessed upon request to the corresponding authors.
REFERENCES
- Abbott, C. C. , Lemke, N. T. , Gopal, S. , Thoma, R. J. , Bustillo, J. , Calhoun, V. D. , & Turner, J. A. (2013). Electroconvulsive therapy response in major depressive disorder: A pilot functional network connectivity resting state FMRI investigation. Frontiers in Psychiatry, 4, 10 10.3389/fpsyt.2013.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers, K. G. , Martinez‐Canabal, A. , Restivo, L. , Yiu, A. P. , De Cristofaro, A. , Hsiang, H. L. , … Frankland, P. W. (2014). Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science, 344(6184), 598–602. 10.1126/science.1248903 [DOI] [PubMed] [Google Scholar]
- Argyelan, M. , Oltedal, L. , Deng, Z. D. , Wade, B. , Bikson, M. , Joanlanne, A. , … Abbott, C. (2019). Electric field causes volumetric changes in the human brain. eLife, 8, 1–20. 10.7554/eLife.49115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt, J. , & Benedict, R. (2001). Hopkins verbal learning test‐revised: Professional manual. Florida: PAR. [Google Scholar]
- Cano, M. , Martinez‐Zalacain, I. , Bernabeu‐Sanz, A. , Contreras‐Rodriguez, O. , Hernandez‐Ribas, R. , Via, E. , … Soriano‐Mas, C. (2017). Brain volumetric and metabolic correlates of electroconvulsive therapy for treatment‐resistant depression: A longitudinal neuroimaging study. Translational Psychiatry, 7(2), e1023 10.1038/tp.2016.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, C. W. , Chien, W. C. , Chung, C. H. , Chao, P. C. , Chang, H. A. , Kao, Y. C. , … Tzeng, N. S. (2018). Electroconvulsive therapy and risk of dementia—A nationwide cohort study in Taiwan. Frontiers in Psychiatry, 9, 397 10.3389/fpsyt.2018.00397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, J. H. , Hobson, V. L. , & O'Bryant, S. E. (2010). Diagnostic accuracy of percent retention scores on RBANS verbal memory subtests for the diagnosis of Alzheimer's disease and mild cognitive impairment. Archives of Clinical Neuropsychology, 25(4), 318–326. 10.1093/arclin/acq023 [DOI] [PubMed] [Google Scholar]
- Depping, M. S. , Nolte, H. M. , Hirjak, D. , Palm, E. , Hofer, S. , Stieltjes, B. , … Thomann, P. A. (2017). Cerebellar volume change in response to electroconvulsive therapy in patients with major depression. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 73, 31–35. 10.1016/j.pnpbp.2016.09.007 [DOI] [PubMed] [Google Scholar]
- Dukart, J. , Regen, F. , Kherif, F. , Colla, M. , Bajbouj, M. , Heuser, I. , … Draganski, B. (2014). Electroconvulsive therapy‐induced brain plasticity determines therapeutic outcome in mood disorders. Proceedings of the National Academy of Sciences of the United States of America, 111(3), 1156–1161. 10.1073/pnas.1321399111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M. B. , Spitzer, R. L. , Gibbon, M. , & Williams, J. B. W. (2002). Structured clinical interview for DSM‐IV‐TR axis I disorders, research version, non‐patient edition. New York: New York State Psychiatric Institute, Biomedical Research. [Google Scholar]
- Friedrich, M. J. (2017). Depression is the leading cause of disability around the world. JAMA, 317, 1517. [DOI] [PubMed] [Google Scholar]
- Heijnen, W. T. , Birkenhager, T. K. , Wierdsma, A. I. , & van den Broek, W. W. (2010). Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: A meta‐analysis. Journal of Clinical Psychopharmacology, 30(5), 616–619. 10.1097/JCP.0b013e3181ee0f5f [DOI] [PubMed] [Google Scholar]
- Husain, M. M. , Rush, A. J. , Fink, M. , Knapp, R. , Petrides, G. , Rummans, T. , … Kellner, C. H. (2004). Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): A consortium for research in ECT (CORE) report. Journal of Clinical Psychiatry, 65(4), 485–491. [DOI] [PubMed] [Google Scholar]
- Jaffe, R. (2001). American Psychiatric Association: The practice of electroconvulsive therapy: Recommendations for treatment, training, and privileging (a task force report of the American Psychiatric Association) (2nd ed.). Washington D.C: American Psychiatric Publishing. [Google Scholar]
- Jiang, R. T. , Abbott, C. C. , Jiang, T. Z. , Du, Y. H. , Espinoza, R. , Narr, K. L. , … Calhoun, V. D. (2018). SMRI biomarkers predict electroconvulsive treatment outcomes: Accuracy with independent data sets. Neuropsychopharmacology, 43(5), 1078–1087. 10.1038/npp.2017.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen, A. , Magnusson, P. , Hanson, L. G. , Kirkegaard, T. , Benveniste, H. , Lee, H. , … Jorgensen, M. B. (2016). Regional brain volumes, diffusivity, and metabolite changes after electroconvulsive therapy for severe depression. Acta Psychiatrica Scandinavica, 133(2), 154–164. 10.1111/acps.12462 [DOI] [PubMed] [Google Scholar]
- Joshi, S. H. , Espinoza, R. T. , Pirnia, T. , Shi, J. , Wang, Y. , Ayers, B. , … Narr, K. L. (2016). Structural plasticity of the Hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biological Psychiatry, 79(4), 282–292. 10.1016/j.biopsych.2015.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner, C. H. , Knapp, R. G. , Petrides, G. , Rummans, T. A. , Husain, M. M. , Rasmussen, K. , … Fink, M. (2006). Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: A multisite study from the consortium for research in electroconvulsive therapy (CORE). Archives of General Psychiatry, 63(12), 1337–1344. 10.1001/archpsyc.63.12.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki, A. , Leaver, A. M. , Vasavada, M. , Njau, S. , Wade, B. , Joshi, S. H. , … Narr, K. L. (2019). Variations in hippocampal white matter diffusivity differentiate response to electroconvulsive therapy in major depression. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging, 4(3), 300–309. 10.1016/j.bpsc.2018.11.003 [DOI] [PubMed] [Google Scholar]
- Leaver, A. M. , Espinoza, R. , Pirnia, T. , Joshi, S. H. , Woods, R. P. , & Narr, K. L. (2016). Modulation of intrinsic brain activity by electroconvulsive therapy in major depression. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging, 1(1), 77–86. 10.1016/j.bpsc.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver, A. M. , Vasavada, M. , Joshi, S. H. , Wade, B. , Woods, R. P. , Espinoza, R. , & Narr, K. L. (2019). Mechanisms of antidepressant response to electroconvulsive therapy studied with perfusion magnetic resonance imaging. Biological Psychiatry, 85(6), 466–476. 10.1016/j.biopsych.2018.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg, H. S. (2003). Modulating dysfunctional limbic‐cortical circuits in depression: Towards development of brain‐based algorithms for diagnosis and optimised treatment. British Medical Bulletin, 65, 193–207. [DOI] [PubMed] [Google Scholar]
- McClintock, S. M. , Choi, J. , Deng, Z. D. , Appelbaum, L. G. , Krystal, A. D. , & Lisanby, S. H. (2014). Multifactorial determinants of the neurocognitive effects of electroconvulsive therapy. The Journal of ECT, 30(2), 165–176. 10.1097/YCT.0000000000000137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medda, P. , Toni, C. , & Perugi, G. (2014). The mood‐stabilizing effects of electroconvulsive therapy. The Journal of ECT, 30(4), 275–282. 10.1097/YCT.0000000000000160 [DOI] [PubMed] [Google Scholar]
- Nickl‐Jockschat, T. , Palomero Gallagher, N. , Kumar, V. , Hoffstaedter, F. , Brugmann, E. , Habel, U. , … Grozinger, M. (2016). Are morphological changes necessary to mediate the therapeutic effects of electroconvulsive therapy? European Archives of Psychiatry and Clinical Neuroscience, 266(3), 261–267. 10.1007/s00406-015-0631-z [DOI] [PubMed] [Google Scholar]
- Nuninga, J. O. , Mandl, R. C. W. , Boks, M. P. , Bakker, S. , Somers, M. , Heringa, S. M. , … Sommer, I. E. C. (2019). Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Molecular Psychiatry. 10.1038/s41380-019-0392-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Oltedal, L. , Narr, K. L. , Abbott, C. , Anand, A. , Argyelan, M. , Bartsch, H. , … Dale, A. M. (2018). Volume of the human Hippocampus and clinical response following electroconvulsive therapy. Biological Psychiatry, 84(8), 574–581. 10.1016/j.biopsych.2018.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousdal, O. T. , Argyelan, M. , Narr, K. L. , Abbott, C. , Wade, B. , Vandenbulcke, M. , … GEMRIC . (2019). Brain changes induced by electroconvulsive therapy are broadly distributed. Biological Psychiatry. 10.1016/j.biopsych.2019.07.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Pandya, M. , Altinay, M. , Malone, D. A., Jr. , & Anand, A. (2012). Where in the brain is depression? Current Psychiatry Reports, 14(6), 634–642. 10.1007/s11920-012-0322-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, S. , Calhoun, V. D. , van Erp, T. G. M. , Bustillo, J. , Damaraju, E. , Turner, J. A. , … Sui, J. (2018). Multimodal fusion with reference: Searching for joint neuromarkers of working memory deficits in schizophrenia. IEEE Transactions on Medical Imaging, 37(1), 93–105. 10.1109/TMI.2017.2725306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, S. , Sui, J. , Chen, J. , Liu, J. , Jiang, R. , Silva, R. , … Calhoun, V. D. (2019). Parallel group ICA+ICA: Joint estimation of linked functional network variability and structural covariation with application to schizophrenia. Human Brain Mapping, 40(13), 3795–3809. 10.1002/hbm.24632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, S. , Yang, X. , Zhao, L. , Calhoun, V. D. , Perrone‐Bizzozero, N. , Liu, S. , … Ma, X. (2018). MicroRNA132 associated multimodal neuroimaging patterns in unmedicated major depressive disorder. Brain, 141(3), 916–926. 10.1093/brain/awx366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rami‐Gonzalez, L. , Bernardo, M. , Boget, T. , Salamero, M. , Gil‐Verona, J. A. , & Junque, C. (2001). Subtypes of memory dysfunction associated with ECT: Characteristics and neurobiological bases. The Journal of ECT, 17(2), 129–135. [DOI] [PubMed] [Google Scholar]
- Randolph, C. , Tierney, M. C. , Mohr, E. , & Chase, T. N. (1998). The repeatable battery for the assessment of neuropsychological status (RBANS): Preliminary clinical validity. Journal of Clinical and Experimental Neuropsychology, 20(3), 310–319. 10.1076/jcen.20.3.310.823 [DOI] [PubMed] [Google Scholar]
- Redlich, R. , Opel, N. , Grotegerd, D. , Dohm, K. , Zaremba, D. , Burger, C. , … Dannlowski, U. (2016). Prediction of individual response to electroconvulsive therapy via machine learning on structural magnetic resonance imaging data. JAMA Psychiatry, 73(6), 557–564. 10.1001/jamapsychiatry.2016.0316 [DOI] [PubMed] [Google Scholar]
- Ross, E. L. , Zivin, K. , & Maixner, D. F. (2018). Cost‐effectiveness of electroconvulsive therapy vs pharmacotherapy/psychotherapy for treatment‐resistant depression in the United States. JAMA Psychiatry, 75(7), 713–722. 10.1001/jamapsychiatry.2018.0768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal, L. , Veltman, D. J. , van Erp, T. G. , Samann, P. G. , Frodl, T. , Jahanshad, N. , … Hibar, D. P. (2016). Subcortical brain alterations in major depressive disorder: Findings from the ENIGMA major depressive disorder working group. Molecular Psychiatry, 21(6), 806–812. 10.1038/mp.2015.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, E. Z. , Reininghaus, B. , Enzinger, C. , Ebner, C. , Hofmann, P. , & Kapfhammer, H. P. (2008). Changes in brain metabolism after ECT‐positron emission tomography in the assessment of changes in glucose metabolism subsequent to electroconvulsive therapy—Lessons, limitations and future applications. Journal of Affective Disorders, 106(1–2), 203–208. 10.1016/j.jad.2007.06.009 [DOI] [PubMed] [Google Scholar]
- Seminowicz, D. A. , Mayberg, H. S. , McIntosh, A. R. , Goldapple, K. , Kennedy, S. , Segal, Z. , & Rafi‐Tari, S. (2004). Limbic‐frontal circuitry in major depression: A path modeling metanalysis. NeuroImage, 22(1), 409–418. 10.1016/j.neuroimage.2004.01.015 [DOI] [PubMed] [Google Scholar]
- Semkovska, M. , & McLoughlin, D. M. (2010). Objective cognitive performance associated with electroconvulsive therapy for depression: A systematic review and meta‐analysis. Biological Psychiatry, 68(6), 568–577. 10.1016/j.biopsych.2010.06.009 [DOI] [PubMed] [Google Scholar]
- Sheehan, D. V. , Lecrubier, Y. , Sheehan, K. H. , Amorim, P. , Janavs, J. , Weiller, E. , … Dunbar, G. C. (1998). The mini‐international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. The Journal of Clinical Psychiatry, 59(Suppl. 20), 22–33. [PubMed] [Google Scholar]
- Sliz, D. , & Hayley, S. (2012). Major depressive disorder and alterations in insular cortical activity: A review of current functional magnetic imaging research. Frontiers in Human Neuroscience, 6, 323 10.3389/fnhum.2012.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui, J. , Qi, S. , van Erp, T. G. M. , Bustillo, J. , Jiang, R. , Lin, D. , … Calhoun, V. D. (2018). Multimodal neuromarkers in schizophrenia via cognition‐guided MRI fusion. Nature Communications, 9(1), 3028 10.1038/s41467-018-05432-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, H. , Jiang, R. , Qi, S. , Narr, K. L. , Wade, B. S. C. , Upston, J. , … Sui, J. (2019). Preliminary prediction of individual response to electroconvulsive therapy using whole‐brain functional magnetic resonance imaging data. NeuroImage: Clinical, 102080 10.1016/j.nicl.2019.102080. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamiya, A. , Plitman, E. , Chung, J. K. , Chakravarty, M. , Graff‐Guerrero, A. , Mimura, M. , & Kishimoto, T. (2019). Acute and long‐term effects of electroconvulsive therapy on human dentate gyrus. Neuropsychopharmacology, 44, 1805–1811. 10.1038/s41386-019-0312-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, J. A. , Damaraju, E. , van Erp, T. G. , Mathalon, D. H. , Ford, J. M. , Voyvodic, J. , … Calhoun, V. D. (2013). A multi‐site resting state fMRI study on the amplitude of low frequency fluctuations in schizophrenia. Frontiers in Neuroscience, 7, 137 10.3389/fnins.2013.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK ECT Review Group . (2003). Efficacy and safety of electroconvulsive therapy in depressive disorder: A systematic review and meta‐analysis. Lancet, 361, 799–808. [DOI] [PubMed] [Google Scholar]
- van Diermen, L. , van den Ameele, S. , Kamperman, A. M. , Sabbe, B. C. G. , Vermeulen, T. , Schrijvers, D. , & Birkenhager, T. K. (2018). Prediction of electroconvulsive therapy response and remission in major depression: Meta‐analysis. British Journal of Psychiatry, 212(2), 71–80. 10.1192/bjp.2017.28 [DOI] [PubMed] [Google Scholar]
- van Oostrom, I. , van Eijndhoven, P. , Butterbrod, E. , van Beek, M. H. , Janzing, J. , Donders, R. , … Tendolkar, I. (2018). Decreased cognitive functioning after electroconvulsive therapy is related to increased hippocampal volume: Exploring the role of brain plasticity. The Journal of ECT, 34(2), 117–123. 10.1097/YCT.0000000000000483 [DOI] [PubMed] [Google Scholar]
- van Waarde, J. A. , Scholte, H. S. , van Oudheusden, L. J. B. , Verwey, B. , Denys, D. , & van Wingen, G. A. (2015). A functional MRI marker may predict the outcome of electroconvulsive therapy in severe and treatment‐resistant depression. Molecular Psychiatry, 20(5), 609–614. 10.1038/mp.2014.78 [DOI] [PubMed] [Google Scholar]
- Wade, B. S. , Joshi, S. H. , Njau, S. , Leaver, A. M. , Vasavada, M. , Woods, R. P. , … Narr, K. L. (2016). Effect of electroconvulsive therapy on striatal morphometry in major depressive disorder. Neuropsychopharmacology, 41(10), 2481–2491. 10.1038/npp.2016.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo, C. J. , & Yu, C. S. (2014). Functional neuroimaging changes subsequent to electroconvulsive therapy in unipolar depression a review of the literature. Journal of ECT, 30(4), 265–274. 10.1097/Yct.0000000000000114 [DOI] [PubMed] [Google Scholar]
- Zou, Q. H. , Zhu, C. Z. , Yang, Y. , Zuo, X. N. , Long, X. Y. , Cao, Q. J. , … Zang, Y. F. (2008). An improved approach to detection of amplitude of low‐frequency fluctuation (ALFF) for resting‐state fMRI: Fractional ALFF. Journal of Neuroscience Methods, 172(1), 137–141. 10.1016/j.jneumeth.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Data Availability Statement
The supervised fusion code has been released and integrated in the Fusion ICA Toolbox (FIT, https://trendscenter.org/software/fit), which can be downloaded and used directly by users worldwide. The ECT multimodal data used in the present study can be accessed upon request to the corresponding authors.


