Abstract
Head motion during magnetic resonance imaging (MRI) induces image artifacts that affect virtually every brain measure. In parallel, cross‐sectional observations indicate a correlation of head motion with age, psychiatric disease status and obesity, raising the possibility of a systematic artifact‐induced bias in neuroimaging outcomes in these conditions, due to the differences in head motion. Yet, a causal link between obesity and head motion has not been tested in an experimental design. Here, we show that a change in body mass index (BMI) (i.e., weight loss after bariatric surgery) systematically decreases head motion during MRI. In this setting, reduced imaging artifacts due to lower head motion might result in biased estimates of neural differences induced by changes in BMI. Overall, our finding urges the need to rigorously control for head motion during MRI to enable valid results of neuroimaging outcomes in populations that differ in head motion due to obesity or other conditions.
Keywords: body mass index, head motion, imaging artifact, neuroimaging, obesity, resting state fMRI
1. INTRODUCTION
Head motion is an important confounder in neuroimaging studies of brain structure and function (Power, Schlaggar, & Petersen, 2015; Savalia et al., 2017). Micro‐movements of the head, driven by spontaneous motion or respiration, strongly affect qualitative and quantitative neuroimaging outcomes, even if targeted image processing techniques are used (Parkes, Fulcher, Yücel, & Fornito, 2018). Moreover, head motion during MRI often correlates with the predictors under study, such as age, psychiatric disease status and obesity (Hodgson et al., 2017; Makowski, Lepage, & Evans, 2019; Torres & Denisova, 2016). This raises the possibility of a systematic image artifact‐induced bias due to differences in head motion in these conditions (Pardoe et al., 2016; Satterthwaite et al., 2012).
One of the strongest predictors of head motion is body mass index (BMI) (Beyer et al., 2017; Ekhtiari, Kuplicki, Yeh, & Paulus, 2019; Hodgson et al., 2017). It seems likely that physiological differences associated with higher weight, for example, increased respiratory rate and amplitude, or spontaneous motion due to uncomfortable positioning in the magnet bore may induce this effect (Littleton, 2012). Yet, within‐subject analysis have indicated that differences in head motion may be also driven by a neurobiological trait which shares genetic variance with BMI (Hodgson et al., 2017; Zeng et al., 2014). Along these lines, impulsivity, for example, more rash action tendencies, might explain a proportion of the shared variance between head motion and obesity (Couvy‐Duchesne et al., 2016; Kong et al., 2014).
Until now, mainly cross‐sectional studies report on the association of BMI and head motion and little is known about how BMI changes may affect head micro‐movements. In this pre‐registered analysis (https://osf.io/epsxt), we therefore aimed to test whether a radical weight‐loss intervention (bariatric surgery) compared to a control group induces consistent changes of head motion during magnetic resonance imaging (MRI) in obese individuals. In addition, we aimed to estimate the confounding effects of a potential change in head motion after bariatric surgery on structural brain measures, according to previous literature (Madan, 2018; Reuter et al., 2015; Savalia et al., 2017).
2. METHODS
To test whether a decrease in BMI would reduce head micro‐movements during MRI, we investigated the effects of bariatric surgery on head motion in patients from the Center for Bariatric and Metabolic Surgery at Charité University Medicine Berlin. The local Ethics Committee of the Charité University Medicine Berlin approved the study protocol and the study was carried out in accordance with the principles of the Declaration of Helsinki. All subjects provided written informed consent and received a small reimbursement for their participation.
This analysis was preregistered (https://osf.io/epsxt). At the date of the preregistration, the acquisition of the data had been finished and the authors had seen summary statistics of BMI for both time points and groups, but the main outcomes (mean and maximal framewise displacement (FD) had not been calculated.
From the total study sample, we included all patients who received a MRI (for more details see (Prehn et al., 2020)). We compared 33 obese participants who underwent bariatric surgery (scanned at baseline n = 21, no baseline BMI information for one participant, 7m/26f, aged 43.0 ± 11.9 years, BMI: 46.4 ± 5.9 kg/m2 (mean ± SD)) to 17 obese participants in a waiting list‐control (scanned at baseline n = 16, 6 m/11f, aged: 47.5 ± 10.9 years, BMI 43.7 ± 5.2 kg/m2). Patients in the control group did not differ significantly from the intervention group regarding baseline characteristics. They had also been recommended to undergo bariatric surgery, but could not be scheduled for this procedure as they had to wait for the approval by their health insurances.
Resting state functional MRI (rsfMRI) (T 2*‐weighted EPI sequence, 150 volumes, 34 slices, repetition time 2000 ms, echo time = 30 ms, flip angle = 90°, voxel size = 3.0 × 3.0 × 4.0 mm) was acquired at three time points maximum (baseline, 6 and 12 months after surgery or waiting period) on a 3 Tesla Siemens Trio MRI. 11/10 participants from intervention/control group completed all three assessments and all participants were included into the linear mixed model analysis. Mean and maximal FD were calculated according to (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). We deviated from the preregistration by log‐transforming mean and maximal FD values prior to the analysis.
To test the hypothesis of a significant interaction of time point with group on mean FD, we set up a linear mixed model with group, time point and their interaction as fixed effects and subject as random effect. We used the function lmer of the R‐package lme4 in R version 3.6.1 (R Core Team, 2019) (Full model H1.1 = lmer(mean FD ~ group × time point + (1Isubj))). We checked the assumption of normally distributed residuals by visually inspecting the qq‐plot and saw no obvious deviations. We reported regression coefficients and R 2 of the fixed effects of this model, calculated with the function r.squaredGLMM from the R‐package MuMin. To test the significance of the interaction effect between time point and group, we compared the full model including the interaction of group and time point to a null model including only main effects (Null model H1.0 = lmer(mean FD ~ group + time + (1Isubj))). Here, we deviated from the wrong specification in the preregistration by including the main effect of group, which is necessary to restrict the test to the interaction. We reported Χ 2 and p‐values of the likelihood ratio test (R function ANOVA) of the interaction, as well as T and p‐values of fixed effect estimates calculated with the R‐package lmerTest. Within‐subject error for plotting was calculated using freely available R‐scripts (http://www.cookbook-r.com/Graphs/Plotting_means_and_error_bars_(ggplot2)/#Helper%20functions) based on (Morey, 2008).
To more comprehensively describe the relation of head motion and BMI we tested the hypothesis that within‐subject BMI change did not predict within‐subject head motion change. If we failed to reject this hypothesis, we would not be able to claim that BMI was a strong determinant of head motion. In this preregistered secondary analysis, we calculated within‐ and between‐subject BMI variability as the average and difference of BMI across time points. For example, if patients had all three time points, this resulted in one value for the average BMI and three values for the difference between the time point BMI and the mean. Participants with only one measurement were assigned to zero within‐subject variability. We then used a likelihood ratio test to compare the full model including both within‐ and between‐subject BMI (H2.1 = lmer(Mean FD ~ BMIbetween + BMIwithin + (1|subj))) to a reduced model (Null model H2.0 = lmer(Mean FD ~ BMIbetween + (1|subj))), which included only between‐subject BMI. For this analysis, we reported R2 of the fixed effects of the full model and Χ2 and p‐value of the likelihood ratio test.
We also repeated the above analyses with maximal FD as outcome measure (H3.1 = lmer(Max FD ~ group × time point + (1|subj)), H3.0 = lmer(Max FD ~ group + time point + (1|subj)), H4.1 = lmer(Max FD ~ BMIbetween + BMIwithin + (1|subj)), Null model H4.0 = lmer(Mean FD ~ BMIbetween + (1|subj))).
All code for this analysis is publicly available under https://github.com/fBeyer89/ADI_preproc/tree/master/Project1_headmotion. The data that support the findings of this study are available from the corresponding author upon request.
3. RESULTS
We compared head motion during rsfMRI of 33 obese participants that underwent bariatric surgery to 17 obese participants in a waiting list‐control group at baseline, 6 and 12 months after surgery/waiting period. Bariatric surgery compared to control led to a systematic reduction of head motion, measured using mean FD of 150 individual brain volumes acquired with a 6 min scan (linear mixed models H.1.1 and H1.0 compared with a likelihood‐ratio test, Χ 2 = 10.8, df = 2, p = .0043, R 2 of fixed effects: .15, based on 107 observations from 50 participants).
Average head motion of control participants did not change, while participants in the intervention group had lower mean FD at 6 and 12 month follow‐up (see Table 1 and Figure 1).
Table 1.
Results for the fixed effects from the linear mixed model (H1.1) predicting logarithmized mean FD (log mean FD ~ group × time point). Time point has the levels baseline (reference level), 6 and 12 month follow‐up (FU). Condition has the levels control group (reference level) and intervention group (IG). Shown are the β estimates, standard errors (SE), T‐ and p values for the main effects of time point (6/12 month FU, compared to baseline), the main effect of condition (IG, compared to control group) and the interaction of time point and condition (6 month FU:IG and 12 month FU:IG, difference in change from baseline to 6/12 month FU in IG compared to control group)
| Fixed effects | β estimate | β SE | T value | p Value |
|---|---|---|---|---|
| (Intercept) | 0.54 | 0.07 | −8.2 | <.001 |
| 6 month FU | 0.06 | 0.05 | 1.2 | .22 |
| 12 month FU | 0.0004 | 0.05 | 0.009 | .99 |
| IG | −0.063 | 0.08 | −0.8 | .45 |
| 6 month FU:IG | −0.21 | 0.06 | −3.4 | .0014 |
| 12 month FU:IG | −0.17 | 0.07 | −2.4 | .019 |
Figure 1.
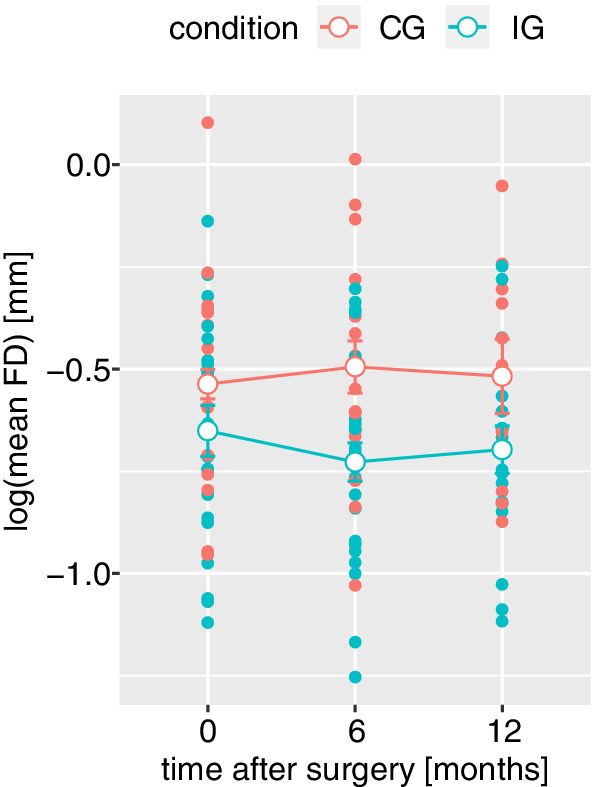
Head motion during resting state fMRI, measured as log‐normalized mean framewise displacement (mean FD), decreases in the intervention (IG) compared to the control group (CG), shown in blue/red, respectively. Open dots represent group and time point averages, error bars represent within‐subject errors
Moreover, the magnitude of weight loss, measured as change in BMI, predicted the decrease in mean FD (linear mixed models H2.1 and H2.0 compared with a likelihood‐ratio test, Χ 2 = 20.6, df = 1, p < .001, R 2 of fixed effects: .053, based on 107 observations from 50 participants, see Figure 2a,b).
Figure 2.
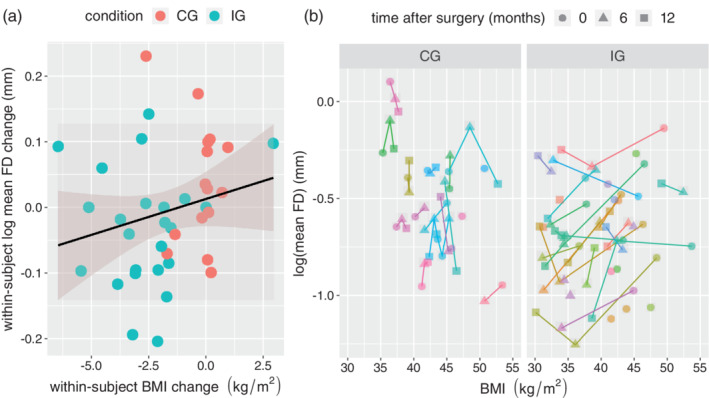
(a) The within‐subject change in BMI (x‐axis) from baseline to 6 month follow up predicts the change in mean FD (y‐axis). Color represents the condition (red: control group (CG), blue: intervention group (IG)). The line shows the regression curve with 95% confidence intervals. (b) Detailed BMI and mean FD trajectories for CG (left) and IG (right). Each color represents one participant. Time after surgery is symbolized by a circle (0 months), triangle (6 months) and square (12 months). BMI, body mass index; FD, framewise displacement
There was no time point by group interaction for maximal FD (linear mixed models H.3.1 and H3.0 compared with a likelihood‐ratio test, Χ 2 = 2.2, df = 2, p = .33, R 2 of fixed effects: .11, based on 107 observations from 50 participants). Yet, similar to the findings for mean FD, the magnitude of weight loss did predict differences in maximal FD (linear mixed models H4.1 and H4.0 compared with a likelihood‐ratio test, Χ 2 = 6, df = 1, p = .014, R 2 of fixed effects: .035, based on 107 observations from 50 participants).
To estimate the impact of head motion changes on structural imaging outcomes, we assumed an average total gray matter volume of ~600 cm3 (based on own data) and 24 cm3 gray matter loss per 0.1 mm of mean FD increase based on results from Alexander‐Bloch et al. (Alexander‐Bloch et al., 2016). Thus, an estimated decrease in mean FD from baseline to follow up in the intervention group of 0.12 mm, as shown in the current analysis, would translate into a false increase in total gray matter volume of 28.8 cm3 (or about 5%) after bariatric surgery, due to the decrease in head motion alone.
4. DISCUSSION
In this preregistered analysis, we showed that a radical change in physiological parameters, in this case body weight loss induced by bariatric surgery, reduced head micro‐movements during MRI. The magnitude of changes in BMI further predicted the magnitude of reduction in head motion. This indicates that head motion strongly depends on body physiology and that BMI differences may result in biased estimates of brain structure and function. Our result highlights the need of rigorous attempts to adjust for and reduce head motion during neuroimaging studies in obesity, as well as in other conditions that may be systematically related to head motion.
Our findings are in line with previous observations in several large imaging cohorts in which BMI accounted for 8–40% of the variance in head motion, making it one of the most important predictors of head motion (Ekhtiari et al., 2019; Hodgson et al., 2017). While our estimate of a weight loss‐induced bias in neuroimaging outcomes (i.e., 5% increase in total gray matter volume after bariatric surgery) relied on between‐subject estimates of the effect of head motion (Alexander‐Bloch et al., 2016) and therefore has to be interpreted with caution, this finding still stresses the importance to control for head motion differences in future MRI analyses. Different techniques, such as multi‐echo sequences (Power et al., 2018), fixation through head molds (Power et al., 2019) and tactile feedback during scanning (Krause et al., 2019) have been proposed to considerably reduce head motion a priori—which is probably the best way to handle this important confound for practically all imaging outcomes (Baum et al., 2018; Beyer et al., 2017; Madan, 2018; Reuter et al., 2015).
Little is known about the mechanisms that may underlie the causal link between body weight and head motion. Possibly, obesity‐related alterations in the respiratory system lead to increased real and apparent head motion in MRI scans (Littleton, 2012). Yet, the association of BMI and head motion was not mediated by respiration rate in previous studies and has been reported for datasets with both short and long imaging repetition times (Beyer et al., 2017; Ekhtiari et al., 2019; Hodgson et al., 2017). More speculatively, alterations in the brain's dopaminergic system could represent a link between BMI and head motion. Evidence suggests that BMI‐related differences in dopamine receptor availability might underlie observed differences in dopamine‐related functions such as reward sensitivity (Tomasi & Volkow, 2013). Given that dopaminergic signaling is crucial for motor inhibition and control (Cools & D'Esposito, 2011; Robertson et al., 2015), alterations in the dopaminergic system might also influence spontaneous head motion.
A limitation of this study is that we did not assess other predictors of head motion such as impulsivity, respiration or dopaminergic signaling. We therefore cannot exclude that these measures mediate the observed effect. Yet, our main conclusion that physiological changes induce changes in head motion, and its implications for future studies, remain valid regardless of the exact mechanisms or other influencing factors. Future studies are encouraged to further investigate the complex interplay of physiological and psychological factors in bariatric surgery (see for example https://osf.io/adnqc).
Taken together, radical weight loss induced by bariatric surgery reduces head motion in a cohort of obese individuals, indicating that the physiological state strongly determines higher head motion in obesity. This urges adequate a‐priori control of head motion in neuroimaging studies of obesity and other conditions with systematically increased head motion to eliminate its confounding effects on measures of brain structure and function.
ACKNOWLEDGMENTS
We thank T. Profitlich, S. Heßler, I. Rangus, C. Reschke, L. Kaiser, A. Winkler, L. Kerti for help in data acquisition. This work was supported by the German Research Foundation, contract grant numbers WI 3342/3‐1, Fl 379‐10/1, Fl 379‐11/1, FL 379‐16/1, DFG‐Exc 257, SFB1315 TP B03 and CRC 1052 “Obesity mechanisms” Project A1.
Beyer F, Prehn K, Wüsten KA, et al. Weight loss reduces head motion: Revisiting a major confound in neuroimaging. Hum Brain Mapp. 2020;41:2490–2494. 10.1002/hbm.24959
Funding information Deutsche Forschungsgemeinschaft, Grant/Award Numbers: 209933838 ‐ SFB 1052, DFG‐Exc 257, FL 379‐16/1, Fl 379‐10/1, Fl 379‐11/1, SFB1315 TP B03, WI 3342/3‐1
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.
REFERENCES
- Alexander‐Bloch, A. , Clasen, L. , Stockman, M. , Ronan, L. , Lalonde, F. , Giedd, J. , & Raznahan, A. (2016). Subtle in‐scanner motion biases automated measurement of brain anatomy from in vivo MRI. Human Brain Mapping, 37, 2385–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum, G. L. , Roalf, D. R. , Cook, P. A. , Ciric, R. , Rosen, A. F. G. , Xia, C. , … Tunç, B. (2018). The impact of in‐scanner head motion on structural connectivity derived from diffusion MRI. NeuroImage, 173, 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer, F. , Kharabian Masouleh, S. , Huntenburg, J. M. , Lampe, L. , Luck, T. , Riedel‐Heller, S. G. , … Witte, A. V. (2017). Higher body mass index is associated with reduced posterior default mode connectivity in older adults. Human Brain Mapping, 38, 3502–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools, R. , & D'Esposito, M. (2011). Inverted‐U–shaped dopamine actions on human working memory and cognitive control. Biological Psychiatry, 69, e113–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvy‐Duchesne, B. , Ebejer, J. L. , Gillespie, N. A. , Duffy, D. L. , Hickie, I. B. , Thompson, P. M. , … Wright, M. J. (2016). Head motion and inattention/hyperactivity share common genetic influences: Implications for fMRI studies of ADHD. PLoS One, 11, e0146271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekhtiari, H. , Kuplicki, R. , Yeh, H.‐w. , & Paulus, M. P. (2019). Physical characteristics not psychological state or trait characteristics predict motion during resting state fMRI. Scientific Reports, 9, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson, K. , Poldrack, R. A. , Curran, J. E. , Knowles, E. E. , Mathias, S. , Göring, H. H. H. , … Almasy, L. (2017). Shared genetic factors influence head motion during MRI and body mass index. Cerebral Cortex, 27(12), 5539–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, X. Z. , Zhen, Z. , Li, X. , Lu, H. H. , Wang, R. , Liu, L. , … Liu, J. (2014). Individual differences in impulsivity predict head motion during magnetic resonance imaging. PLoS One, 9, e104989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause, F. , Benjamins, C. , Eck, J. , Lührs, M. , van Hoof, R. , & Goebel, R. (2019). Active head motion reduction in magnetic resonance imaging using tactile feedback. Human Brain Mapping, 40, 4026–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton, S. W. (2012). Impact of obesity on respiratory function. Respirology, 17, 43–49. [DOI] [PubMed] [Google Scholar]
- Madan, C. R. (2018). Age differences in head motion and estimates of cortical morphology. PeerJ, 6, e5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski, C. , Lepage, M. , & Evans, A. C. (2019). Head motion: The dirty little secret of neuroimaging in psychiatry. Journal of Psychiatry & Neuroscience, 44, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey, R. D. (2008). Confidence intervals from normalized data: A correction to Cousineau (2005), 4, 61–64. [Google Scholar]
- Pardoe, H. R. , Hiess, R. K. , Kuzniecky, R . (2016). Motion and morphometry in clinical and nonclinical populations. Neuroimage, 135, 177–185. [DOI] [PubMed] [Google Scholar]
- Parkes, L. , Fulcher, B. , Yücel, M. , & Fornito, A. (2018). An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting‐state functional MRI. NeuroImage, 171, 415–436. [DOI] [PubMed] [Google Scholar]
- Power, J. , Schlaggar, B. , & Petersen, S. (2015). Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage, 105, 536–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Plitt, M. , Gotts, S. J. , Kundu, P. , Voon, V. , Bandettini, P. A. , & Martin, A. (2018). Ridding fMRI data of motion‐related influences: Removal of signals with distinct spatial and physical bases in multiecho data. Proceedings of the National Academy of Sciences of the United States of America, 115, E2105–E2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Silver, B. M. , Silverman, M. R. , Ajodan, E. L. , Bos, D. J. , & Jones, R. M. (2019). Customized head molds reduce motion during resting state fMRI scans. NeuroImage, 189, 141–149. [DOI] [PubMed] [Google Scholar]
- Prehn, K. , Profitlich, T. , Rangus, I. , Heßler, S. , Witte, A. V. , Grittner, U. , … Flöel, A. (2020). Bariatric surgery and brain health—A longitudinal observational study investigating the effect of surgery on cognitive function and Gray matter volume. Nutrients, 12, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2019). R: A Language and Environment for Statistical Computing.
- Reuter, M. , Tisdall, M. D. , Qureshi, A. , Buckner, R. L. , van der Kouwe, A. J. W. , & Fischl, B. (2015). Head motion during MRI acquisition reduces gray matter volume and thickness estimates. NeuroImage, 107, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, C. L. , Ishibashi, K. , Mandelkern, M. A. , Brown, A. K. , Ghahremani, D. G. , Sabb, F. , … London, E. D. (2015). Striatal D1‐ and D2‐type dopamine receptors are linked to motor response inhibition in human subjects. The Journal of Neuroscience, 35, 5990–5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite, T. D. , Wolf, D. H. , Loughead, J. , Ruparel, K. , Elliott, M. A. , Hakonarson, H. , … C., Gur, R. E. (2012). Impact of in‐scanner head motion on multiple measures of functional connectivity: relevance for studies of neuro development in youth. Neuroimage, 60, 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savalia, N. K. , Agres, P. F. , Chan, M. Y. , Feczko, E. J. , Kennedy, K. M. , & Wig, G. S. (2017). Motion‐related artifacts in structural brain images revealed with independent estimates of in‐scanner head motion. Human Brain Mapping, 38, 472–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. , & Volkow, N. D. (2013). Striatocortical pathway dysfunction in addiction and obesity: Differences and similarities. Critical Reviews in Biochemistry and Molecular Biology, 48, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, E. B. , & Denisova, K. (2016). Motor noise is rich signal in autism research and pharmacological treatments. Scientific Reports, 6, 37422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, L. L. , Wang, D. , Fox, M. D. , Sabuncu, M. , Hu, D. , Ge, M. , … Liu, H. (2014). Neurobiological basis of head motion in brain imaging. Proceedings of the National Academy of Sciences of the United States of America, 111, 6058–6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


