Abstract
Right hemispheric dominance in tonal bilingualism is still controversial. In this study, we investigated hemispheric dominance in 30 simultaneous Bai‐Mandarin tonal bilinguals and 28 Mandarin monolinguals using multimodal neuroimaging. Resting‐state functional connectivity (RSFC) analysis was first performed to reveal the changes of functional connections within the language‐related network. Voxel‐based morphology (VBM) and tract‐based spatial statistics (TBSS) analyses were then used to identify bilinguals' alterations in gray matter volume (GMV) and fractional anisotropy (FA) of white matter, respectively. RSFC analyses revealed significantly increased functional connections of the right pars‐orbital part of the inferior frontal gyrus (IFG) with right caudate, right pars‐opercular part of IFG, and left inferior temporal gyrus in Bai‐Mandarin bilinguals compared to monolinguals. VBM and TBSS analyses further identified significantly greater GMV in right pars‐triangular IFG and increased FA in right superior longitudinal fasciculus (SLF) in bilinguals than in monolinguals. Taken together, these results demonstrate the integrative role of the right IFG in tonal language processing of bilinguals. Our findings suggest that the intrinsic language network in simultaneous tonal bilinguals differs from that of monolinguals in terms of both function and structure.
Keywords: gray matter volume, language network, resting‐state, simultaneous tonal bilingual, white matter
1. INTRODUCTION
Tonal languages, in which pitch variation is used to differentiate grammatical or semantic information at the lexical level, have been intensively investigated around two competing hypotheses (Tan & Li, 2015; Wang, Behne, Jongman, & Sereno, 2004). The neural characterization of speech perception in tonal languages is accordingly dichotomized into a domain‐specific model (i.e., a functional hypothesis) and a cue‐specific model (i.e., an acoustic hypothesis) (Kwok, Matthews, Yakpo, & Tan, 2019; Zatorre & Gandour, 2008). The functional hypothesis assumes that brain activity is lateralized to the left hemisphere (LH) when pitch variance denotes semantic meaning, otherwise to right hemisphere (RH). In contrast, the acoustic hypothesis focuses on the acoustic properties (e.g., the fundamental frequency, F0) that are inherently encompassed in auditory perception regardless of linguistic functions, which would imply RH lateralization (Jia, Tsang, Huang, & Chen, 2013). Yet, the evidence is inconclusive and more factors are thus taken into consideration, such as the distinctive tonal characteristics among tonal languages (Wang, 2004), temporal distribution of tonal processing (Fournier, Gussenhoven, Jensen, & Hagoort, 2010), and developmental asymmetric function of auditory cortices with age (Yamazaki et al., 2018).
Linguistic experience, among other factors, may influence hemispheric lateralization of tonal languages. Ge et al. (2015) reported the equal importance of right anterior temporal cortex in native Chinese speakers, which had been downplayed due to the prevailing models of nontonal speech comprehension. Ramanujan (2019) posited and tested the hypothesis of Relative Language Distance (i.e., the extent of lexical feature similarity) that may have an impact on cross‐linguistic neural activity. Similarly, right‐hemisphere advantage has been documented in bilinguals, especially in tonal bilinguals (Wong, 2002). For example, a greater value of fractional anisotropy (FA) in right superior longitudinal fasciculus (SLF) and right inferior longitudinal fasciculus (ILF) during Mandarin learning could strongly predict Mandarin learning achievement (Qi, Han, Garel, San Chen, & Gabrieli, 2015). They found that FA values in SLF and ILF were positively correlated with learning ability, that is, exam scores. They also found a positive correlation between FA values in SLF and ILF and phonological learning measured by the Pinyin dictation task. Moreover, a greater density in gray and white matter of right anterior temporal lobe and left insula were found in Chinese speakers relative to nontonal multilinguals suggesting that the anterior temporal lobe and insula play an important role in linking the pitch of words to their meaning for a tonal language (Crinion et al., 2009). However, a recent meta‐analysis failed to find hemispheric specialization in processing lexical pitch of a tonal language even though it showed that right anatomical regions were more recruited for tonal language processing (Kwok et al., 2017). To date, few studies have been done on tonal bilinguals where both languages share similar lexical features or on inherent long‐term configuration that is shaped by such two tonal languages.
Methodological issues may also limit our understanding of neural mechanism underlying lexical tone perception observed in native speakers of tonal languages. For instance, the dominant experimental design of event‐related fMRI might outperform the approach of resting‐state fMRI (rs‐fMRI) in capturing the real‐time neural activity in response to language stimuli, but barely reveals the intrinsic functional coupling of the language network when participants are idle (Pliatsikas & Luk, 2016; Van Dijk et al., 2010; Wang et al., 2012; Wang et al., 2017; Xu et al., 2019; Xu et al., 2019). Nemerous rs‐fMRI studies reveal that the intrinsic state of bilinguals could be altered by their linguistic experience (Berken, Chai, Chen, Gracco, & Klein, 2016; Gullifer et al., 2018; Kousaie, Chai, Sander, & Klein, 2017; Li et al., 2015). Alternatively, neuroanatomical studies report that the microstructural development of white matter tracts (e.g., FA value, an indicator of information transmitting efficiency between brain areas obtained with the diffusion tensor imaging technique) or gray matter density of tonal language speakers may be reconfigured in the long run as well (Schlegel, Rudelson, & Tse, 2012; Yang, Gates, Molenaar, & Li, 2015). Nevertheless, these findings are disconnected across neuroimaging data due to the unimodal approach separately applied to distinct cohort of participants in different studies. Indeed, the integration of both structural and functional neuroimaging approaches within a study is conducive to constructing a reliable and complete profile for neural specification of tonal bilinguals.
Therefore, this study aimed to explore the neural multimodal evidence from intrinsic brain activity to structural reconfiguration for hemispheric dominance of Mandarin‐Bai tonal bilinguals. Mandarin, a tonal language with 1.4 billion speakers and four lexical tones (e.g., Pinyin “ma” means “mother” 妈 of high‐level tone, “hemp” 麻 of rising tone, “horse” 马 of rising‐falling tone and “reproach” 骂 of falling tone), has been frequently investigated to compare with nontonal languages that have a high relative language distance (Ramanujan, 2019). The Bai language, primarily used by Bai ethnicity in China (Sun & Jiang, 1999; Wang, 2014), is also a tonal language but has 6 to 8 lexical tones (see Table 1) evolving from the ancient Baimang language (Wang, 2004; Zhao, 2009). It has an incomplete written form primarily derived from Mandarin (Xu & Zhao, 1984). The similar property of lexical tones between Bai and Mandarin provides a prime object of study to explore the neural mechanism of hemispheric lateralization in tonal bilinguals. The resting‐state language processing network that was recently proposed on the basis of large‐sample, multicenter and different cohorts' data (Tomasi & Volkow, 2012) provided a solid reference for the creation of region of interests in our data analysis. The rs‐fMRI was firstly employed to study the changes of intrinsically functional connectivity in Mandarin‐Bai simultaneous bilinguals compared to Mandarin monolinguals within language network. Next, voxel‐based morphological analyses (VBM) and diffusion tensor imaging (DTI) were used to investigate the structural changes of gray matter and white matter.
Table 1.
The pitch contour of Bai language (Xu & Zhao, 1984)
| Tone name | Tone value | Tone mark | Intense or lax vowel | Word examples | |||
|---|---|---|---|---|---|---|---|
| Pa | WFb | Pa | WFb | ||||
| 1 | 33 |
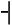
|
Lax | pɑ 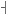
|
泡沫 |
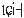
|
拉 |
| 2 | 42 |
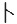
|
Intense | pɑ 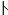
|
奶 |
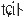
|
追 |
| 3 | 31 |
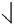
|
Lax | pɑ 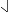
|
闹 |
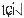
|
田 |
| 4 | 55 |

|
Lax | phɑ 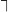
|
扒 |
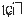
|
多 |
| 5 | 35 |
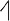
|
Lax | pɑ 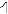
|
八(哥鸟) |
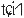
|
急 |
| 6 | 44 |
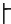
|
Intense | pɑ 
|
倒 |
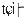
|
蚂蟥 |
| 7 | 21 |
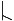
|
Intense | pɑ 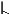
|
蹲 |
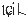
|
赊欠 |
| 8 | 55 |
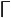
|
Intense | pɑ 
|
(水)坝 |
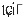
|
寄宿 |
P, pronunciation.
WF, written form.
2. MATERIALS AND METHODS
2.1. Participants
A total of 58 participants including 30 Bai‐Mandarin simultaneous bilinguals who speak both the Mandarin and Bai languages (16 males and 14 females, mean age ± SD = 25.33 ± 4.65 years) and 28 gender‐ and age‐ matched monolinguals who only speak Mandarin (12 males and 16 females, mean age ± SD = 26.32 ± 2.09 years) participated in this cross‐sectional study (Table 2). Relative to the control group who live in a Mandarin culture alone, the group of Bai‐Mandarin bilinguals have been living in both cultures (e.g., Bai culture in ethnic habitation and Mandarin culture in school or work place) in the Yunnan Province of China since birth, thereby acquiring the languages at the same onset age and reporting equivalent language proficiency and frequency of use between the two languages. All the participants were right‐handed healthy college students with no neurological disorders or MRI‐contraindications. The study was in accordance with the latest revision of the Declaration of Helsinki and had full approval from the local ethics committee at the Kunming Medical University. All participants signed written informed consent prior to MRI scanning.
Table 2.
Self‐report demographics of participants
| Bai‐mandarin bilinguals (N = 30, 14 female) | Mandarin monolinguals (N = 28, 16 female) | t | p | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Quantitative description | ||||||
| Age (year) | 25.33 | 4.65 | 26.32 | 2.09 | ||
| Onset AoAa (year) | 0 | 0 | 0 | 0 | ||
| Qualitative description | ||||||
| Handedness | Right | Right | ||||
| Education | College students | College students | ||||
| Language exposure | Equivalent frequency | Frequent | ||||
| Language proficiency | Equal fluency | Fluent | ||||
Onset AoA: onset age of acquisition.
p < .05.
2.2. MRI data acquisition and preprocessing
All the participants were scanned using a 3.0 Tesla Philips MR Scanner. The diffusion weighted imaging (DWI) data included 32 images with non‐colinear diffusion gradients (b = 1,000 s/mm2) and 1 nondiffusion‐weighted images (b = 0 s/mm2). A diffusion MRI for each participant was scanned using the following parameters: 75 slices, acquisition matrix = 96 × 96, flip angle (FA) = 90°, voxel resolution: 2.5 × 2.5 × 2.5 mm3, and no gap. Sagittal 3D T1‐weighted images were also acquired (TR/TE = 8.24/3.78 ms; FA = 7°; FOV = 256 mm × 256 mm; matrix = 256 × 256; slice thickness = 1 mm, no gap; 188 sagittal slices). During the resting‐state fMRI scanning, participants were instructed to close their eyes and lie still and cushions were used to reduce head motion. Two hundred and forty volumes of echo planar images were acquired (repetition time = 2000 ms, echo time = 30 ms; field of view [FOV] =220 × 220 mm2, matrix = 64 × 64, slice thickness = 4 mm, gap = 0.6 mm, flip angle = 90°; 33 axial slices).
Preprocessing of the resting‐state fMRI data was carried out using SPM8 software (Friston, 2007) (https://www.fil.ion.ucl.ac.uk/spm/). The first 10 volumes were discarded to allow for magnetization equilibrium. The slice timing for the remaining images was corrected, and images were realigned to the first volume to account for head motion. All the participants who showed a maximum displacement of less than 2 mm and an angular motion of less than 2° were included in the subsequent analyses. All fMRI images were normalized to the Montreal Neurological Institute (MNI) template and resampled at 3 × 3 × 3 mm3. Finally, the functional images were smoothed using a Gaussian kernel of 6 mm FWHM. Subsequently, the functional images were filtered with a temporal band‐path of 0.01~0.1 Hz and six motion parameters, white matter and cerebrospinal fluid signals were regressed out. To further exclude head motion effects, “scrubbing” was employed to eliminate the bad images captured before two time points and after one time points, which exceeded the pre‐set criteria (frame displacement: FD, FD < 0.5) for excessive motion. Because previous studies have shown that global mean signal regression can lead to spurious resting‐state functional correlations and false inferences on the group level inference (Wang et al., 2017; Wang et al., 2018; Wang et al., 2019), thus, the global mean signal was not regressed during the preprocessing in our current study.
The diffusion MRI data were preprocessed using the FSL software (http://www.fmrib.ox.ac.uk/fsl). First, eddy currents and head motions were corrected by affine transformations to minimize gradient coil eddy current distortions using FMRIB's Diffusion Toolbox. Then, three‐dimensional maps of the diffusion tensor and the FA, mean diffusion (MD), axial diffusion (AD), and radial diffusion (RD) maps were calculated for the following analyses.
2.3. Language network: Resting‐state functional connectivity analyses
The language network, in this study, was defined based on a previous study (Tomasi & Volkow, 2012). The language network included 25 brain areas in frontal, parietal, and temporal lobes, cingulate cortex, and subcortical areas. Finally, the peak MNI coordinates of the 25 areas provided in the previous study were used to create the regions of interests (ROI) with 6 mm radius for subsequent functional connectivity analyses (Table 3).
Table 3.
MNI coordinates were used for definition of language network
| Id | Brain regions | L/R | Abbreviation | MNI coordinates | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| 1 | Wernicke's area | L | WA | −51 | −51 | 30 |
| 2 | Inferior parietal lobule | L | IPL | 57 | −51 | 36 |
| 3 | Broca's area | L | BA | −51 | 27 | 18 |
| 4 | Pars triangularis | R | Pars_tri | 51 | 30 | 18 |
| 5 | Middle frontal gyrus | R | MFG | −39 | 18 | 45 |
| 6 | Pars opercularis | L | Pars_oper | 42 | 21 | 42 |
| 7 | Pars orbitalis | L | Pars_orb | −45 | 39 | −12 |
| 8 | Pars orbitalis | R | Pars_orb | −45 | 39 | −15 |
| 9 | Inferior temporal gyrus | L | ITG | −57 | −30 | −15 |
| 10 | Inferior temporal gyrus | R | ITG | 63 | −30 | −12 |
| 11 | Superior frontal gyrus | L | SFG | −3 | 36 | 45 |
| 12 | Caudate | L | Cau | −12 | 9 | 15 |
| 13 | Caudate | R | Cau | 12 | 12 | 12 |
| 14 | Putamen/globus pallidus | L | Put | −18 | 0 | 9 |
| 15 | Ventral thalamus | L | THAv | −9 | −9 | 0 |
| 16 | Cerebellum crus | R | Cereb | 15 | −81 | −30 |
| 17 | Striate | R | Striate | 6 | −75 | −6 |
| 18 | Extrastriate | R | Extrastriate | 21 | −69 | −15 |
| 19 | Posterior parietal cortex | R | PPC | 6 | −81 | 45 |
| 20 | Superior parietal lobule | R | SPL | 3 | −51 | 57 |
| 21 | Superior temporal gyrus | L | STG | −63 | −18 | 9 |
| 22 | Superior temporal gyrus | R | STG | 60 | −21 | 12 |
| 23 | Cingulate | L/R | Cing | 0 | 0 | 48 |
Abbreviations: MNI, Montreal neurological institute; L, left hemisphere, R, right hemisphere.
The functional connectivity between each pair of the 25 language areas was measured using Pearson's correlation coefficient in both bilinguals and monolinguals, and a 25 × 25 connectivity matrix for each participant was obtained. Finally, the connectivity differences of all the 300 connections were assessed between bilingual and monolingual participants. The significance threshold was set at p < .05 using a network‐based false discovery rate (NB‐FDR) correction method for multiple comparisons. For the NB‐FDR correction, we first used p < .05 to determine the functionally connected sub‐networks. Then, the corrected p was calculated using .05 divided by the number of connections of the sub‐network.
2.4. VBM analyses of inferior frontal cortex
All the 3D T1 structural MRI images were processed using VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html) in SPM8 package. The VBM preprocessing steps include: (a) all images were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF); (b) the segmented images were further spatially normalized to MNI space by applying high dimensional DARTEL normalization; (c) the normalized GM images were modulated to account for volume changes resulting from the normalization process; (d) data quality was checked across the sample, and no participants were excluded for poor quality; and (e) the normalized and modulated images were then smoothed using a Gaussian kernel of 8 mm full‐width at half maximum (FWHM) for statistical analysis.
To validate the right hemispheric dominance of IFG, we defined the bilateral masks of IFG using automatical anatomical label (AAL) atlas in which IFG includes opercular, triangular, and orbital parts. Then, we combined the three subregions into one mask to obtain the IFG mask in both hemispheres. Finally, voxel‐wise two‐sample t test within IFG mask was performed to identify the areas with significant differences in gray matter volume (GMV) between bilingual and monolingual participants. The significance was determined using a cluster‐level Monte Carlo simulation (5,000 times) corrected threshold of p < .05 (cluster‐forming threshold at voxel‐level p < .001).
2.5. Tract‐based spatial statistics analyses of FA images
Diffusion tensor imaging has been widely used to investigate the brain white matter fibers which underlays brain functions (Basser, Mattiello, & LeBihan, 1994; Wang et al., 2019). To identify the structural underlying of the right hemispherical dominance, tract‐based spatial statistics (TBSS) analyses of the FA images were performed using FSL software. First, the FA images of all participants were transformed to standard MNI space. Next, the mean FA image and its skeleton from all participants including both the bilingual and monolingual participants in MNI space were created, and each participant's FA was projected onto the skeleton. Finally, a voxel‐wise two‐sample t test on the skeleton between the FA images of the bilingual and monolingual participants was performed using the threshold‐free cluster enhancement (TFCE) method which can enhance cluster‐like structures without having to define an initial cluster‐forming threshold or carry out a large amount of data smoothing. The significance was determined with p < .01 (TFCE corrected). Moreover, the MD, AD, RD values of the tracts with significantly changed FA were also calculated for comparisons. Two‐sample t tests were performed to identified the significant differences, and the significant level was set at p < .05 corrected with Bonferroni method.
3. RESULTS
A chi‐squared test showed that there was no significant difference in gender between bilinguals and monolinguals (χ2 = 0.085, p = .771). A two‐sample t test did not find a significant difference in age between bilinguals and monolinguals either (p = .307).
Resting‐state functional connectivity (RSFC) analyses identified significantly increased functional connections of right pars‐orbital part of IFG with right caudate, right pars‐opercular part of IFG, and left inferior temporal gyrus in Bai‐Mandarin bilinguals compared to Mandarin monolinguals (Figure 1).
Figure 1.
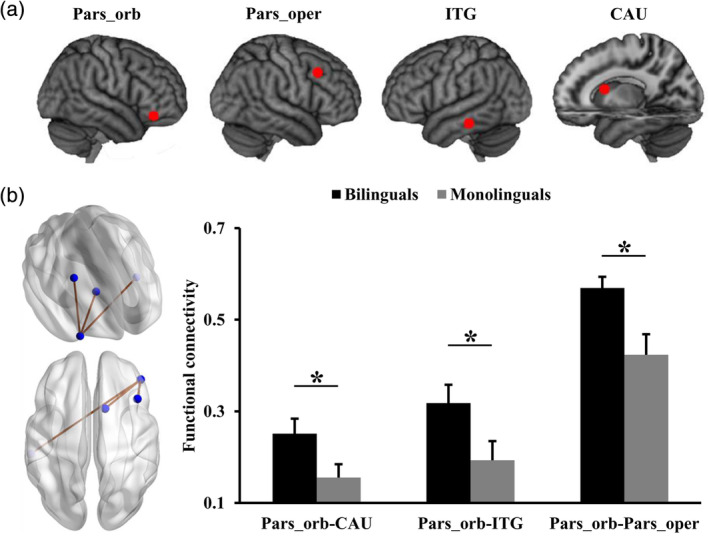
Differences in functional connectivities within language network between bilinguals and monolinguals. (a) Brain areas within language network showed changed functional connectivities in bilinguals compared to monolinguals. (b) Significantly increased functional connections of right pars‐orbital part of IFG (Pars‐orb) with right caudate (CAU), right pars‐opercular part of IFG (Pars‐oper), and left inferior temporal gyrus (ITG) in bilinguals compared to monolinguals
To further validate whether there is right dominance of right IFG with structural differences in Bai‐Mandarin bilinguals compared to Mandarin monolinguals, VBM was used and identified significantly higher GMV in right triangular‐part of IFG in Bai‐Mandarin bilinguals (Figure 2).
Figure 2.
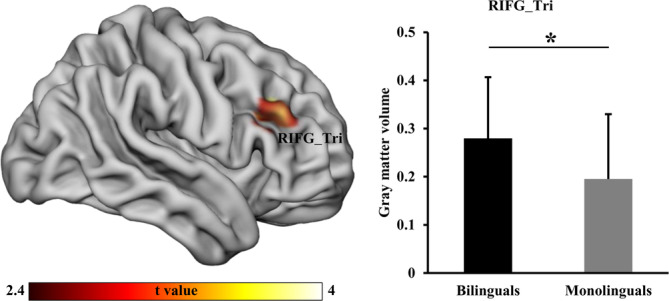
Voxel‐based morphology (VBM) analysis was performed to identify significant alterations in gray matter volume (GMV). VBM analysis revealed significantly increased GMV in the right triangular part of inferior frontal gyrus (IFG_Tri) in bilinguals compared to monolinguals
Finally, TBSS analysis was used to reveal whether the alteration of white matter fasciculus in Bai‐Mandarin bilinguals compared to Chinese monolinguals also reflect the right hemispherical specialization. TBSS analysis detected significantly increased FA values in right SLF (parietal part) in Bai‐Mandarin bilinguals (Figure 3). There were no significant differences in MD, AD, and RD values of SLF.
Figure 3.
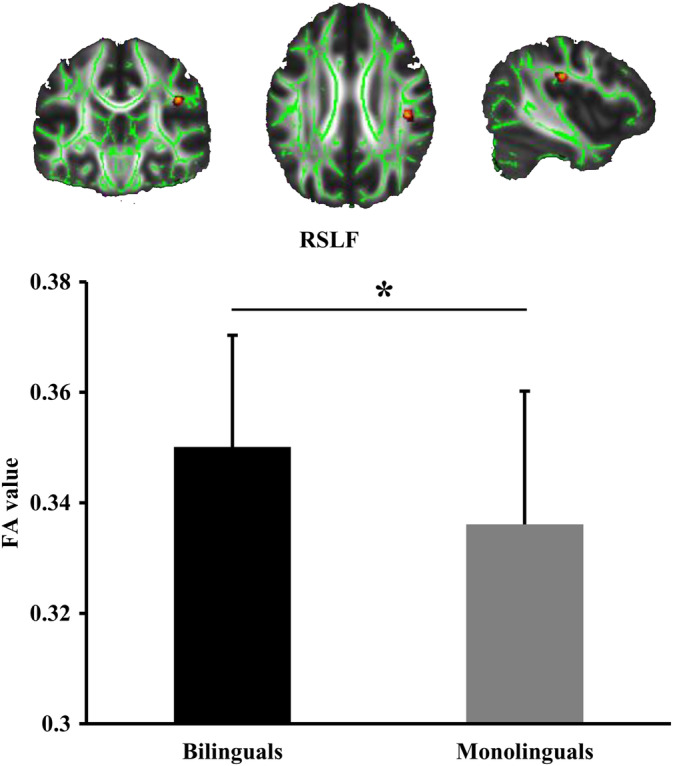
Tract‐based spatial statistics (TBSS) analysis of fractional anisotropy (FA). TBSS was used to detect altered FA and identified significantly greater FA values in the right superior longitudinal fasciculus (SLF) in bilinguals than monolinguals
4. DISCUSSION
Our study revealed that compared with Mandarin monolinguals, Mandarin‐Bai bilinguals exhibited a significant increase in RSFC coupling of the right pars‐orbital part of the IFG (IFGpars‐orb) respectively with right caudate (CAU), right pars‐opercular part of IFG (IFGpars‐oper), and left inferior temporal gyrus (ITG). Furthermore, the analyses of voxel‐based morphology (VBM) and TBSS showed that GMV in the right triangular part of IFG (IFGtri) and FA values in right SLF were also increased in tonal bilinguals relative to tonal monolinguals. Our findings provide preliminary evidence for the brain mechanism of tonal language processing in simultaneous bilinguals and support the hypothesis of right hemispheric specialization of tonal languages.
Increased functional connections between right IFGpars‐orb (BA 47) and each of the three regions—right caudate, right IFGpars‐oper (BA 44) and left ITG (BA 37)—were found in Mandarin‐Bai bilinguals relative to Mandarin monolinguals. This may indicate altered functional connectivity in tonal bilinguals that might have been shaped by their language experiences. These functional connections that repeatedly involved right IFG corroborate previous studies on the importance of this region in bilingualism (Berken et al., 2016; Gullifer et al., 2018), such as response inhibition and attention control (Aron, Robbins, & Poldrack, 2014; Dove, Pollmann, Schubert, Wiggins, & von Cramon, 2000; Hampshire, Chamberlain, Monti, Duncan, & Owen, 2010). Moreover, its synchronous fluctuation intrinsically with caudate and left ITG suggest its central role in integrating information of different modes in that caudate is crucial for language switching and control in both unimodal and bimodal bilinguals (Abutalebi et al., 2008; van Heuven, Schriefers, Dijkstra, & Hagoort, 2008) and the left ITG acts as an interface between sensory input and higher‐level associations (Dehaene & Cohen, 2011; Price & Devlin, 2011). However, the integrative role of the right IFG was not found in the studies of Berken et al. (2016) and Gullifer et al. (2018) who also used the method of RSFC to investigate bilinguals. The difference with the present results may be attributed to different approach in detail, that is, these authors used a predefined seed‐based rather than whole‐language‐network‐based analysis and compared simultaneous with sequential bilinguals. Our findings should thus be interpreted with caution when it is extrapolated to sequential tonal bilinguals.
Alternatively, the intrinsic neural synchrony across regions observed in our study may be interpreted as a product of long‐term tonal perception and differentiation that is associated with semantic meaning (Tong, Choi, & Man, 2018). That is, the contrast between tonal monolinguals and tonal bilinguals in RSFC might demonstrate the higher magnitude of acoustic acuteness that was enhanced by the experience of developing two linguistic systems with tonal features (Bidelman, Hutka, & Moreno, 2013). Belyk and Brown (2014) posited that the right IFGpars‐orb is a hub for semantic content and affective expression, integrating multimodal perceptions and shared by all primates in brain anatomy, thus both emotional and linguistic prosody converging into it. Nevertheless, the present results that were found via resting‐state neuroimaging method deepen our understanding of the theoretical debate on intrinsic laterality of tonal languages when no lexical stimulus is processed. Taken together, the results suggest that the IFG is vital either for language control in bilinguals or acoustic features in tonal languages, which may account for the underlying mechanism of language processing in tonal bilinguals.
Right‐hemispheric specialization in tonal bilinguals was further supported by our evidence concerning the microstructure of white matter tracts and GVM. The increased FA in right SLF and increased GVM in right IFG found in tonal bilinguals relative to monolinguals in our study are consistent with the results of previous studies (Luk, Bialystok, Craik, & Grady, 2011; Pliatsikas, Moschopoulou, & Saddy, 2015). The increased FA of the right parietal bundle of SLF is related to greater white matter integrity and myelination, and suggests faster and more efficient transmission of information across frontal, parietal, and temporal cortices. Its enhancement in the prediction of learning achievement associated with tonal languages (Qi et al., 2015) or contribution to attention processing (Wu et al., 2016) in bilingualism converged to a conclusion that brain plasticity is an outcome of long‐term language experience in tonal bilinguals (Schlegel et al., 2012; Yang et al., 2015). Moreover, compared to Mandarin monolinguals, bilinguals speaking both Mandarin and Bai languages also showed increased right hemispherical functional integration, higher myelination of SLF, and higher GMV suggesting the right hemispherical specialization in tonal processing. The functional specification in tonal processing of the right hemisphere may be related to the cognitive control demands of adapting to new linguistic rules, as well as selecting, switching, and translating between the two languages to recruit areas outside of the network for one's native language, such as prefrontal and parietal areas (Qi et al., 2015).
In contrast to the present study revealing increased GMV in simultaneous tonal bilinguals relative to monolinguals, Klein, Mok, Chen, and Watkins (2014) found no significant change in cortical thickness between simultaneous two such groups. Rather, they only found increased cortical thickness in early and simultaneous bilinguals in contrast to late bilinguals. These inconsistent findings may be caused by different measurements or language families. Klein et al. (2014) used cortical thickness to study English‐French bilinguals whereas we used the GMV which is equal to area multiplied by thickness to investigate Bai‐Mandarin tonal bilinguals. In line with other related findings about the identical cohort of participants with tonal bilingual experience, this finding may demonstrate that GMV is a sensitive method to investigate structural plasticity in tonal bilinguals.
There are some limitations in the present study. First, various control groups of language experience should have been included in order to dissociate the impact of tonal features from that of bilingualism or to control for tonal complexity, for example, Mandarin‐English bilinguals, English monolinguals, and Bai monolinguals. Indeed, it is almost impossible to find a participant who only speaks Bai language because of cultural and linguistic blending with Han Chinese who speak Mandarin in China. Of note, the converging evidence was found from multimodal neuroimaging analyses in the current study. Second, although a within‐subject longitudinal experimental design may be more convincing when arguing for brain plasticity shaped by tonal bilingual experiences than a cross‐sectional design, this was a first preliminary study taking tonal similarity into consideration when bilingualism was studied and, furthermore, we used multimodal neuroimaging methods. As such, the present study paved the way for future investigations on the intrinsic neural specification of tonal bilinguals.
In conclusion, we used a multimodal neuroimaging approach to reveal a hub role of the right IFG in language control and acoustic laterality in tonal bilinguals compared to tonal monolinguals. The right hemisphere specialization that may be shaped longitudinally by language experience was found to be crucial for information integration and processing in tonal bilinguals, and was subserved neuroanatomically by higher GMV and white matter integrity. Our findings further support the right core mechanism of response inhibition in language control in bilinguals and explore neural specification of tonal bilinguals.
Gao Z, Guo X, Liu C, Mo Y, Wang J. Right inferior frontal gyrus: An integrative hub in tonal bilinguals. Hum Brain Mapp. 2020;41:2152–2159. 10.1002/hbm.24936
Funding information National Natural Science Foundation of China, Grant/Award Number: 31600880; Shenzhen Key Basic Research, Grant/Award Number: JCYJ20170818110103216; Guangdong key basic research, Grant/Award Number: 2018B030332001; Guangdong Pearl River Talents Plan, Grant/Award Number: 2016ZT06S220
Contributor Information
Yin Mo, Email: gougou4198625@sina.com.
Jiaojian Wang, Email: jiaojianwang@uestc.edu.cn.
DATA AVAILABILITY STATEMENT
I confirm that my article contains a Data Availability statement even if no data are available (list of sample statements) unless my article type does not require one.
REFERENCES
- Abutalebi, J. , Annoni, J. M. , Zimine, I. , Pegna, A. J. , Seghier, M. L. , Lee‐Jahnke, H. , … Khateb, A. (2008). Language control and lexical competition in bilinguals: An event‐related FMRI study. Cerebral Cortex, 18, 1496–1505. [DOI] [PubMed] [Google Scholar]
- Aron, A. R. , Robbins, T. W. , & Poldrack, R. A. (2014). Inhibition and the right inferior frontal cortex: One decade on. Trends in Cognitive Sciences, 18, 177–185. [DOI] [PubMed] [Google Scholar]
- Basser, P. J. , Mattiello, J. , & LeBihan, D. (1994). Estimation of the effective self‐diffusion tensor from the NMR spin echo. Journal of Magnetic Resonance. Series B, 103, 247–254. [DOI] [PubMed] [Google Scholar]
- Belyk, M. , & Brown, S. (2014). Perception of affective and linguistic prosody: An ALE meta‐analysis of neuroimaging studies. Social Cognitive and Affective Neuroscience, 9, 1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berken, J. A. , Chai, X. , Chen, J. K. , Gracco, V. L. , & Klein, D. (2016). Effects of early and late bilingualism on resting‐state functional connectivity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 36, 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidelman, G. M. , Hutka, S. , & Moreno, S. (2013). Tone language speakers and musicians share enhanced perceptual and cognitive abilities for musical pitch: Evidence for bidirectionality between the domains of language and music. PLoS ONE, 8, e60676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion, J. T. , Green, D. W. , Chung, R. , Ali, N. , Grogan, A. , Price, G. R. , … Price, C. J. (2009). Neuroanatomical markers of speaking Chinese. Human Brain Mapping, 30, 4108–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene, S. , & Cohen, L. (2011). The unique role of the visual word form area in reading. Trends in Cognitive Sciences, 15, 254–262. [DOI] [PubMed] [Google Scholar]
- Dove, A. , Pollmann, S. , Schubert, T. , Wiggins, C. J. , & von Cramon, D. Y. (2000). Prefrontal cortex activation in task switching: An event‐related fMRI study. Brain Research. Cognitive Brain Research, 9, 103–109. [DOI] [PubMed] [Google Scholar]
- Fournier, R. , Gussenhoven, C. , Jensen, O. , & Hagoort, P. (2010). Lateralization of tonal and intonational pitch processing: An MEG study. Brain Research, 1328, 79–88. [DOI] [PubMed] [Google Scholar]
- Friston, K. (2007). Statistical parametric mapping: The analysis of functional brain images. London, United Kingdom: Academic Press. [Google Scholar]
- Ge, J. , Peng, G. , Lyu, B. , Wang, Y. , Zhuo, Y. , Niu, Z. , … Gao, J. H. (2015). Cross‐language differences in the brain network subserving intelligible speech. Proceedings of the National Academy of Sciences of the United States of America, 112, 2972–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullifer, J. W. , Chai, X. J. , Whitford, V. , Pivneva, I. , Baum, S. , Klein, D. , & Titone, D. (2018). Bilingual experience and resting‐state brain connectivity: Impacts of L2 age of acquisition and social diversity of language use on control networks. Neuropsychologia, 117, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire, A. , Chamberlain, S. R. , Monti, M. M. , Duncan, J. , & Owen, A. M. (2010). The role of the right inferior frontal gyrus: Inhibition and attentional control. NeuroImage, 50, 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, S. , Tsang, Y. K. , Huang, J. , & Chen, H. C. (2013). Right hemisphere advantage in processing Cantonese level and contour tones: Evidence from dichotic listening. Neuroscience Letters, 556, 135–139. [DOI] [PubMed] [Google Scholar]
- Klein, D. , Mok, K. , Chen, J. K. , & Watkins, K. E. (2014). Age of language learning shapes brain structure: A cortical thickness study of bilingual and monolingual individuals. Brain and Language, 131, 20–24. [DOI] [PubMed] [Google Scholar]
- Kousaie, S. , Chai, X. J. , Sander, K. M. , & Klein, D. (2017). Simultaneous learning of two languages from birth positively impacts intrinsic functional connectivity and cognitive control. Brain and Cognition, 117, 49–56. [DOI] [PubMed] [Google Scholar]
- Kwok, V. , Matthews, S. , Yakpo, K. , & Tan, L. (2019). Neural correlates and functional connectivity of lexical tone processing in reading. Brain and Language, 196, 104662. [DOI] [PubMed] [Google Scholar]
- Kwok, V. P. Y. , Dan, G. , Yakpo, K. , Matthews, S. , Fox, P. T. , Li, P. , & Tan, L. H. (2017). A meta‐analytic study of the neural Systems for Auditory Processing of lexical tones. Frontiers in Human Neuroscience, 11, 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Abutalebi, J. , Zou, L. , Yan, X. , Liu, L. , Feng, X. , … Ding, G. (2015). Bilingualism alters brain functional connectivity between "control" regions and "language" regions: Evidence from bimodal bilinguals. Neuropsychologia, 71, 236–247. [DOI] [PubMed] [Google Scholar]
- Luk, G. , Bialystok, E. , Craik, F. I. , & Grady, C. L. (2011). Lifelong bilingualism maintains white matter integrity in older adults. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31, 16808–16813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliatsikas, C. , & Luk, G. (2016). Executive control in bilinguals: A concise review on fMRI studies. Bilingualism: Language and Cognition, 19, 699–705. [Google Scholar]
- Pliatsikas, C. , Moschopoulou, E. , & Saddy, J. D. (2015). The effects of bilingualism on the white matter structure of the brain. Proceedings of the National Academy of Sciences of the United States of America, 112, 1334–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, C. J. , & Devlin, J. T. (2011). The interactive account of ventral occipitotemporal contributions to reading. Trends in Cognitive Sciences, 15, 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, Z. , Han, M. , Garel, K. , San Chen, E. , & Gabrieli, J. D. E. (2015). White‐matter structure in the right hemisphere predicts mandarin Chinese learning success. Journal of Neurolinguistics, 33, 14–28. [Google Scholar]
- Ramanujan, K. (2019). The impact of relative language distance on bilingual language control – A functional imaging study. bioRxiv, 771212 10.1101/771212 [DOI] [Google Scholar]
- Schlegel, A. A. , Rudelson, J. J. , & Tse, P. U. (2012). White matter structure changes as adults learn a second language. Journal of Cognitive Neuroscience, 24(8), 1664–1670. [DOI] [PubMed] [Google Scholar]
- Sun, H. , & Jiang, D. (1999). Controversies concerning genealogical classification of Sina‐Tibetan languages ‐ a critical survey. Contemporary LInguistics., 2, 17–32. [Google Scholar]
- Tan, L. H. , & Li, P. (2015). Towards an integrative understanding of the neuroanatomical and genetic bases of language: The Chinese context. Journal of Neurolinguistics, 33, 1–2. [Google Scholar]
- Tomasi, D. , & Volkow, N. D. (2012). Resting functional connectivity of language networks: Characterization and reproducibility. Molecular Psychiatry, 17, 841–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, X. , Choi, W. , & Man, Y. Y. (2018). Tone language experience modulates the effect of long‐term musical training on musical pitch perception. The Journal of the Acoustical Society of America, 144, 690–697. [DOI] [PubMed] [Google Scholar]
- Van Dijk, K. R. , Hedden, T. , Venkataraman, A. , Evans, K. C. , Lazar, S. W. , & Buckner, R. L. (2010). Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. Journal of Neurophysiology, 103, 297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heuven, W. J. , Schriefers, H. , Dijkstra, T. , & Hagoort, P. (2008). Language conflict in the bilingual brain. Cerebral Cortex, 18, 2706–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F. (2004). Language policy for Bai In Zhou M. & Sun H. (Eds.), Language policy in the People's Republic of China: Theory and practice since 1949 (pp. 277–287). Dordrecht: Springer Netherlands. [Google Scholar]
- Wang, F. (2014). Coevolution of language and culture: The method of word group and a comparison of Bai, Chinese and Yi J. Yunan norm. Univ. (Humanitles Soc. Sclences), 5(46), 30–37. [Google Scholar]
- Wang, J. , Becker, B. , Wang, L. , Li, H. , Zhao, X. , & Jiang, T. (2019). Corresponding anatomical and coactivation architecture of the human precuneus showing similar connectivity patterns with macaques. NeuroImage, 200, 562–574. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Fan, L. , Zhang, Y. , Liu, Y. , Jiang, D. , Zhang, Y. , … Jiang, T. (2012). Tractography‐based parcellation of the human left inferior parietal lobule. NeuroImage, 63, 641–652. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Wei, Q. , Bai, T. , Zhou, X. , Sun, H. , Becker, B. , … Kendrick, K. (2017). Electroconvulsive therapy selectively enhanced feedforward connectivity from fusiform face area to amygdala in major depressive disorder. Social Cognitive and Affective Neuroscience, 12, 1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Wei, Q. , Wang, L. , Zhang, H. , Bai, T. , Cheng, L. , … Wang, K. (2018). Functional reorganization of intra‐ and internetwork connectivity in major depressive disorder after electroconvulsive therapy. Human Brain Mapping, 39, 1403–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Xie, S. , Guo, X. , Becker, B. , Fox, P. T. , Eickhoff, S. B. , & Jiang, T. (2017). Correspondent functional topography of the human left inferior parietal lobule at rest and under task revealed using resting‐state fMRI and Coactivation based Parcellation. Human Brain Mapping, 38, 1659–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Wei, Q. , Wang, C. , Xu, J. , Wang, K. , Tian, Y. , & Wang, J. (2019). Altered functional connectivity patterns of insular subregions in major depressive disorder after electroconvulsive therapy. Brain Imaging and Behavior. 10.1007/s11682-018-0013-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Behne, D. M. , Jongman, A. , & Sereno, J. A. (2004). The role of linguistic experience in the hemispheric processing of lexical tone. Applied PsychoLinguistics, 25, 449–466. [Google Scholar]
- Wong, P. C. (2002). Hemispheric specialization of linguistic pitch patterns. Brain Research Bulletin, 59, 83–95. [DOI] [PubMed] [Google Scholar]
- Wu, Y. , Wang, J. , Zhang, Y. , Zheng, D. , Zhang, J. , Rong, M. , … Jiang, T. (2016). The neuroanatomical basis for posterior superior parietal lobule control lateralization of Visuospatial attention. Frontiers in Neuroanatomy, 10, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Lyu, H. , Li, T. , Xu, Z. , Fu, X. , Jia, F. , … Hu, Q. (2019). Delineating functional segregations of the human middle temporal gyrus with resting‐state functional connectivity and coactivation patterns. Human Brain Mapping, 40, 5159–5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Wang, C. , Xu, Z. , Li, T. , Chen, F. , Chen, K. , … Hu, Q. (2019). Specific functional connectivity patterns of middle temporal Gyrus subregions in children and adults with autism spectrum disorder. Autism Research. 10.1002/aur.2239. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Xu, L. , Zhao, Y. (1984) Baiyu jianzhi. Minzu chubanshe.
- Yamazaki, H. , Easwar, V. , Polonenko, M. J. , Jiwani, S. , Wong, D. D. E. , Papsin, B. C. , & Gordon, K. A. (2018). Cortical hemispheric asymmetries are present at young ages and further develop into adolescence. Human Brain Mapping, 39(2), 941–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. , Gates, K. M. , Molenaar, P. , & Li, P. (2015). Neural changes underlying successful second language word learning: An fMRI study. Journal of Neurolinguistics, 33, 29–49. [Google Scholar]
- Zatorre, R. J. , & Gandour, J. T. (2008). Neural specializations for speech and pitch: Moving beyond the dichotomies. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363, 1087–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L. (2009). A brief introduction to the language family category of Bai language. Journal of Minzu University of China (Philosophy and Social Sciences Edition), 36, 114–121. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
I confirm that my article contains a Data Availability statement even if no data are available (list of sample statements) unless my article type does not require one.


