Abstract
Resting‐state functional magnetic resonance imaging (rs‐fMRI) has the potential to shed light on the pathophysiological mechanisms of Huntington's disease (HD), paving the way to new therapeutic interventions. A systematic literature review was conducted in three online databases according to PRISMA guidelines, using keywords for HD, functional connectivity, and rs‐fMRI. We included studies investigating connectivity in presymptomatic (pre‐HD) and manifest HD gene carriers compared to healthy controls, implementing seed‐based connectivity, independent component analysis, regional property, and graph analysis approaches. Visual network showed reduced connectivity in manifest HD, while network/areas underpinning motor functions were consistently altered in both manifest HD and pre‐HD, showing disease stage‐dependent changes. Cognitive networks underlying executive and attentional functions showed divergent anterior–posterior alterations, possibly reflecting compensatory mechanisms. The involvement of these networks in pre‐HD is still unclear. In conclusion, aberrant connectivity of the sensory‐motor network is observed in the early stage of HD while, as pathology spreads, other networks might be affected, such as the visual and executive/attentional networks. Moreover, sensory‐motor and executive networks exhibit hyper‐ and hypo‐connectivity patterns following different spatiotemporal trajectories. These findings could potentially help to implement future huntingtin‐lowering interventions.
Keywords: functional connectivity, Huntington's disease, resting state networks, systematic review
Abbreviations
- ALFF
amplitude of low frequency fluctuations
- DAN
dorsal attention network
- DMN
default mode network
- ECN
executive network
- FPN
frontoparietal network
- HC
healthy controls
- HD
Huntington's disease
- ICA
independent component analysis
- Pre‐HD
presymptomatic Huntington's disease
- ReHo
regional homogeneity
- rs‐fMRI
resting state functional magnetic resonance imaging
- SMN
sensory‐motor network
- VIS
visual network
1. INTRODUCTION
Resting‐state functional magnetic resonance imaging (rs‐fMRI) investigates spontaneous fluctuations of blood oxygenation level dependent signal, which could serve as a proxy for neural activity (Matsui, Murakami, & Ohki, 2016; Schwalm et al., 2017). Cortical and subcortical anatomically separated brain regions which exhibit low frequency (0.01–0.1 Hz) blood oxygen fluctuations correlated in time are assumed to be functionally connected into resting state networks, linked with specific cognitive and motor functions (Damoiseaux et al., 2006). This methodology is emerging as a promising tool to identify brain functional alterations in neurodegenerative pathologies such as Huntington's disease (HD), and might help to better understand early pathophysiological mechanisms and to assess the effectiveness of HD clinical trials (Bohanna, Georgiou‐Karistianis, Hannan, & Egan, 2008; Ross & Tabrizi, 2011).
HD is a progressive neurodegenerative genetic disease caused by CAG repeat expansion in the huntingtin (HTT) gene, encoding for an abnormal conformation of mutant HTT protein (Ross & Tabrizi, 2011). This mutation exhibits toxic features and is responsible for pathological brain alterations, leading to progressive functional disability in gene carriers (Ross & Tabrizi, 2011). For individuals in the stage preceding the clinical symptomatology onset (referred to as presymptomatic HD; pre‐HD), the disease burden score, that takes into account the number of CAG repeats and mutation gene carrier's age (Penney Jr., Vonsattel, MacDonald, Gusella, & Myers, 1997), estimates the cumulative toxicity of the mutant HTT.
The neuroanatomical hallmark of HD includes early loss of GABAergic inhibitory neurons and atrophy of the striatum (Vonsattel et al., 1985). As pathology proceeds, neuronal degeneration spreads to the cortex and other extrastriatal regions (Nanetti et al., 2018; Rosas et al., 2008). However, the relationship between brain atrophy and clinical symptoms is still unclear.
Emerging findings suggest that functional imaging might identify changes in HD before structural brain damage, accounting for the onset of symptoms (Ross et al., 2014). Specifically, the disruption of resting‐state networks identified through rs‐fMRI might provide an intermediate phenotype between neuropathology and clinical symptoms. This hypothesis has been largely investigated in several brain disorders. In Alzheimer's disease (AD), the pattern of atrophy mirrors the topography of the default mode network (DMN), whereas frontotemporal dementia displays an atrophy pattern coherent with the layout of the salience network (Seeley et al., 2009). Moreover, in AD functional abnormalities of the DMN have been linked with the molecular hallmark (i.e., beta‐amyloid deposition) and memory deficits (Buckner et al., 2005). The salience network, which is involved in detecting and filtering relevant social information (Menon, 2015), shows aberrant connectivity in neurodegenerative and psychiatric disorders sharing deficits in social cognition (Quattrini et al., 2018; Zhou et al., 2010). Preliminary results in Parkinson's disease (PD) seem to point out the involvement of a subcortical network (Guan et al., 2017). Similarly, a strong fit between the source of atrophy and functional connectivity patterns was reported in semantic dementia, progressive nonfluent aphasia and corticobasal syndrome, suggesting a selective vulnerability of specific resting‐state networks to different neurodegenerative diseases (Seeley et al., 2009).
The relation between the disruption of neural networks and HD onset and progression is still poorly understood. Recently, Hohenfeld, Werner, and Reetz (2018) reported an extensive review of rs‐fMRI findings in several neurodegenerative diseases. Their aim was to investigate whether rs‐fMRI can serve as a reliable diagnostic biomarker. They reported connectivity alteration in sensorimotor (SMN), visual (VIS) and subcortical (especially the striatum) networks in HD, although no considerations were reported about the directionality of these alterations. Conversely, the aim of the present review is to examine the brain functional fingerprint within the whole HD spectrum, and the direction of functional changes compared to healthy controls (i.e., aberrant hyper‐ or hypo‐connectivity). Different functional imaging approaches have been applied to HD to enhance our understanding of the pathophysiology and potential compensatory mechanisms at play. These methods include seed‐based connectivity, whole‐brain independent component analysis (ICA), regional property measures and graph analysis. This review examines the contribution of these rs‐fMRI techniques to identify specific alterations in distinctive functional neural‐pathways, and specific mechanisms linked with increased or decreased connectivity (i.e., detrimental or compensatory processes). Results might help to better understand pathophysiological mechanisms of HD, and might be used as targets or surrogate outcomes in new clinical trials.
2. METHODS
2.1. Study selection
A systematic literature search of rs‐fMRI studies was conducted according to The PRISMA Statement, a checklist of items and guidelines to collect, check, and critically appraise data for reporting systematic review and meta‐analysis (Liberati et al., 2009). We searched PubMed, EMBASE, and Web of Science databases for rs‐fMRI articles using different combinations of keywords (Huntington AND “resting state”; Huntington AND “functional connectivity”; Huntington AND “functional network”). Further studies were identified through tracing of the retrieved articles and relevant reviews. Figure 1 shows the PRISMA flowchart. We included studies published in English investigating rs‐fMRI in both pre‐HD individuals and manifest HD patients compared to controls or reporting significant relationship between connectivity and genetic load. Aberrant connectivity of large‐scale functional networks was inferred from the findings of studies implementing seed‐based connectivity, ICA, and network‐of‐interest ICA‐based methods. Additionally, studies implementing amplitude of low frequency fluctuations (ALFF), regional homogeneity analysis (ReHo), and graph approaches were included to investigate regional abnormalities of key brain regions belonging to different functional networks. Exclusion criteria were: (a) non rs‐fMRI studies (e.g., task fMRI, positron emission tomography, electroencephalography studies); (b) reviews and meta‐analyses; (c) animal studies; and (d) conference proceedings or abstracts.
Figure 1.
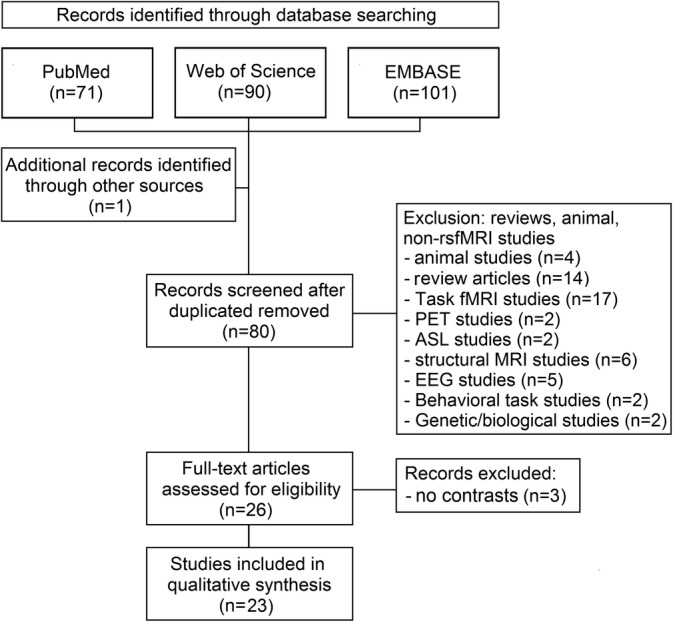
PRISMA flow diagram of the literature search
2.2. Data extraction
From the full articles of the eligible studies, the following variables were recorded: population characteristics (sample size, age, gender, education years, mean age disease, unified Huntington's disease rating scale [UHDRS], and mini‐mental state examination scores), resting state networks investigated, methods for network analysis, acquisition characteristics (scanner field strength, acquisition time), and correlations with disease burden or CAG repeats and cognitive/motor outcomes. Studies reporting overlapping samples or a subset of rs‐fMRI data (Espinoza et al., 2018; Harrington et al., 2015; Koenig et al., 2014; Wolf, Sambataro, Vasic, Baldas, et al., 2014; Wolf, Sambataro, Vasic, Depping, et al., 2014; Wolf et al., 2015) were included.
3. RESULTS
Database searching identified 263 articles, which yielded 80 citations after duplicates were removed. Fifty‐four articles were excluded because reporting analysis in animals (n = 4), reviews (n = 14), or no rs‐fMRI studies (task fMRI, n = 17; positron emission tomography, n = 2; arterial spin labeling, n = 2; structural MRI, n = 6; electroencephalography, n = 5; behavioral task, n = 2; genetic/biological studies n = 2). Twenty‐six full‐text articles were assessed for eligibility. Three of these articles were further excluded because not reporting connectivity contrasts between HD versus healthy controls (HC) or correlation with genetic load. The 23 articles included in the final review encompassed 648 HC (sample range 10–94), 266 manifest HD (sample range 10–34) and 641 pre‐HD (sample range 10–183) individuals (Table 1). Table 2 lists the studies reporting aberrant rs‐fMRI connectivity in pre‐symptomatic Huntington's disease individuals compared to healthy controls, separately for each specific network involved. Twelve articles reported correlations between genetic load (i.e., disease burden score or CAG repeat length) and connectivity (Table 3).
Table 1.
Rs‐fMRI studies reporting aberrant rs‐fMRI connectivity in manifest Huntington's disease patients compared to healthy controls
| Huntington's disease patients | Healthy controls | Main findings | Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| Reference | Sample (% F) | Age (y ± SD) | Education (y ± SD) | Sample (% F) | Age (y ± SD) | Education (y ± SD) | ||
| Visual network | ||||||||
| Wolf, Sambataro, Vasic, Baldas, et al., 2014 | 20 (30%) | 49 ± 9 | 13 ± 2 | 20 (35%) | 47 ± 9 | 14 ± 2 | Both decreased and increased FC | ICA |
| Coppen, Grond, Hafkemeijer, Barkey Wolf, & Roos, 2018 | 20 (45%) | 52 ± 11 | 16 ± 2 | 18 (61%) | 46 ± 11 | 17 ± 2 | Decreased FC | Network of interest |
| Dumas et al., 2013 | 20 (75%) | 47 ± 11 | 1–5b | 28 (54%) | 49 ± 9 | 2–5b | Decreased FC | Network of interest |
| Werner et al., 2014 | 17 (59%) | 45 ± 10 | 3 ± 1b | 19 (58%)a | 48 ± 10 | 4 ± 1b | Increased FC | ICA |
| Poudel et al., 2014 | 23 (43%) | 56 ± 9 | Na | 18 (78%) | 46 ± 14 | Na | No differences | ICA |
| Sensory‐motor network | ||||||||
| Wolf, Sambataro, Vasic, Depping, et al., 2014 | 20 (30%) | 49 ± 9 | 13 ± 2 | 20 (35%) | 47 ± 9 | 14 ± 2 | Increased FC | ICA |
| Werner et al., 2014 | 17 (59%) | 45 ± 10 | 3 ± 1b | 19 (58%)a | 48 ± 10 | 4 ± 1b | Increased FC | ICA |
| Sánchez‐Castañeda et al., 2017 | 10 (40%) | 39 ± 19 | Na | 10 (40%) | 38 ± 16 | Na | Increased FC | Seed |
| Müller et al., 2016 | 34 (29%) | 47 (32–65) | 13 (9–20) | 32 (38%) | 48 (25–62) | 14 (11–19) | Decreased FC | Seed |
| Poudel et al., 2014 | 23 (43%) | 56 ± 9 | Na | 18 (78%) | 46 ± 14 | Na | No differences | ICA |
| Dumas et al., 2013 | 20 (75%) | 47 ± 11 | 1–5b | 28 (54%) | 49 ± 9 | 2–5b | No differences | Network of interest |
| Cerebellum network | ||||||||
| Werner et al., 2014 | 17 (59%) | 45 ± 10 | 3 ± 1b | 19 (58%)a | 48 ± 10 | 4 ± 1b | Increased FC | ICA |
| Poudel et al., 2014 | 23 (43%) | 56 ± 9 | Na | 18 (78%) | 46 ± 14 | Na | No differences | ICA |
| Wolf et al., 2015 | 20 (30%) | 49 ± 9 | 13 ± 2 | 20 (35%) | 47 ± 9 | 14 ± 2 | No differences | Seed |
| Auditory network | ||||||||
| Poudel et al., 2014 | 23 (43%) | 56 ± 9 | Na | 18 (78%) | 46 ± 14 | Na | No differences | ICA |
| Dumas et al., 2013 | 20 (75%) | 47 ± 11 | 1–5b | 28 (54%) | 49 ± 9 | 2–5b | No differences | Network of interest |
| Executive network | ||||||||
| Werner et al., 2014 | 17 (59%) | 45 ± 10 | 3 ± 1b | 19 (58%)a | 48 ± 10 | 4 ± 1b | Frontal increased FC | ICA |
| Wolf, Sambataro, Vasic, Depping, et al., 2014 | 20 (30%) | 49 ± 9 | 13 ± 2 | 20 (35%) | 47 ± 9 | 14 ± 2 | Posterior decreased FC; anterior increased FC | ICA |
| Poudel et al., 2014 | 23 (43%) | 56 ± 9 | Na | 18 (78%) | 46 ± 14 | Na | Posterior/subcortical decreased FC | ICA |
| Dumas et al., 2013 | 20 (75%) | 47 ± 11 | 1–5b | 28 (54%) | 49 ± 9 | 2–5b | Posterior/subcortical decreased FC | Network of interest |
| Frontoparietal network | ||||||||
| Wolf, Sambataro, Vasic, Depping, et al., 2014 | 20 (30%) | 49 ± 9 | 13 ± 2 | 20 (35%) | 47 ± 9 | 14 ± 2 | Both decreased and increased FC | ICA |
| Werner et al., 2014 | 17 (59%) | 45 ± 10 | 3 ± 1b | 19 (58%)a | 48 ± 10 | 4 ± 1b | Increased FC | ICA |
| Poudel et al., 2014 | 23 (43%) | 56 ± 9 | Na | 18 (78%) | 46 ± 14 | Na | Increased FCc | ICA |
| Dumas et al., 2013 | 20 (75%) | 47 ± 11 | 1–5b | 28 (54%) | 49 ± 9 | 2–5b | No differences | Network of interest |
| Dorsal attention network | ||||||||
| Poudel et al., 2014 | 23 (43%) | 56 ± 9 | Na | 18 (78%) | 46 ± 14 | Na | Decreased FC | ICA |
| Werner et al., 2014 | 17 (59%) | 45 ± 10 | 3 ± 1b | 19 (58%)a | 48 ± 10 | 4 ± 1b | Increased FC | ICA |
| Dumas et al., 2013 | 20 (75%) | 47 ± 11 | 1–5b | 28 (54%) | 49 ± 9 | 2–5b | No differences | Network of interest |
| Default mode network | ||||||||
| Quarantelli et al., 2013 | 26 (38%) | 44 ± 12 | Na | 22 (50%) | 39 ± 14 | Na | Both decreased and increased FC | Seed |
| Dumas et al., 2013 | 20 (75%) | 47 ± 11 | 1–5b | 28 (54%) | 49 ± 9 | 2–5b | Decreased FC | Network of interest |
| Sánchez‐Castañeda et al., 2017 | 10 (40%) | 39 ± 19 | Na | 10 (40%) | 38 ± 16 | Na | Increased FC | Seed |
| Poudel et al., 2014 | 23 (43%) | 56 ± 9 | Na | 18 (78%) | 46 ± 14 | Na | No differences | ICA |
| Subcortical network | ||||||||
| Wolf, Sambataro, Vasic, Depping, et al., 2014 | 20 (30%) | 49 ± 9 | 13 ± 2 | 20 (35%) | 47 ± 9 | 14 ± 2 | Both decreased and increased FC | ICA |
| Werner et al., 2014 | 17 (59%) | 45 ± 10 | 3 ± 1b | 19 (58%)a | 48 ± 10 | 4 ± 1b | Increased FC | ICA |
| Müller et al., 2016 | 34 (29%) | 47 (32–65) | 13 (9–20) | 32 (38%) | 48 (25–62) | 14 (11–19) | Decreased FC | Seed |
| Regional properties and graph measures | ||||||||
| Gargouri et al., 2016 | 18 (Na %) | 51 ± 7 | Na | 18 (50%) | 44 ± 10 | Na |
Widespread changes in sensorimotor, associative And limbic networks |
Graph measures |
| Liu et al., 2016 | 10 (90%) | 45 ± 9 | 10 ± 3 | 20 (90%) | 45 ± 8 | Na | Decreased PCC/parietal and increased temporo‐frontal ALFF | ALFF |
| Sarappa et al., 2017 | 28 (39%) | 42 ± 10 | Na | 40 (55%) | 37 ± 14 | Na | Decreased parietal and increased cerebellum ALFF | ALFF |
| Sarappa et al., 2017 | 28 (39%) | 42 ± 10 | Na | 40 (55%) | 37 ± 14 | Na | Decreased cerebellum and increased frontotemporal ReHo | ReHo |
Abbreviations: ALFF, amplitude of low frequency fluctuations; F, female; FC, functional connectivity; Na, not available; PCC, posterior cingulate cortex; ReHo, regional homogeneity; SD; standard deviation; Y, years.
Three HD mutation carriers were presymptomatic.
International Standard Classification of Education.
Compared to presymptomatic Huntington's disease individuals.
Table 2.
Rs‐fMRI studies reporting aberrant rs‐fMRI connectivity in presymptomatic Huntington's disease individuals compared to healthy controls
| Reference | Presymptomatic Huntington's disease | Healthy controls | Main findings | Analysis | ||||
|---|---|---|---|---|---|---|---|---|
| Sample (% F) | Age (y ± SD) | Education (y ± SD) | Sample (% F) | Age (y ± SD) | Education (y ± SD) | |||
| Visual network | ||||||||
| Poudel et al., 2014 | 25 (64%) | 43 ± 9 | Na | 18 (78%) | 46 ± 14 | Na | No differences | ICA |
| Odish et al., 2015 | 22 (55%) | 43 ± 9 | 4 ± 3a | 18 (61%) | 47 ± 7 | 4 ± 3a | No differences | ICA |
| Coppen et al., 2018 | 21 (48%) | 37 ± 9 | 17 ± 3 | 18 (61%) | 46 ± 11 | 17 ± 2 | No differences | Network of interest |
| Dumas et al., 2013 | 28 (61%) | 43 ± 8 | 2–5a | 28 (54%) | 49 ± 9 | 2–5a | Decreased FC | Network of interest |
| Sensory‐motor network | ||||||||
| Poudel et al., 2014 | 25 (64%) | 43 ± 9 | Na | 18 (78%) | 46 ± 14 | Na | Decreased FC | ICA |
| Unschuld et al., 2012 | 10 (40%) | 44 ± 9 | Na | 10 (60%) | 42 ± 11 | Na | Decreased FC | Seed |
| Koenig et al., 2014 | Low 16 (94%) | 33 ± 9 | 14 ± 2 | 16 (75%) | 43 ± 9 | 16 ± 2 | Increased FC | Seed |
| Medium 16 (69%) | 40 ± 10 | 15 ± 3 | No differences | |||||
| High 16 (88%) | 48 ± 13 | 14 ± 2 | Decreased FC | |||||
| Odish et al., 2015 | 22 (55%) | 43 ± 9 | 4 ± 3a | 18 (61%) | 47 ± 7 | 4 ± 3a | No differences | ICA |
| Dumas et al., 2013 | 28 (61%) | 43 ± 8 | 2–5a | 28 (54%) | 49 ± 9 | 2–5a | No differences | Network of interest |
| Gorges et al., 2017 | 12 (75%) | 36 (27–62) | 14 (9–17) | 22 (32%) | 45 (25–51) | 17 (12–19) | No differences | Seed |
| Cerebellum network | ||||||||
| Poudel et al., 2014 | 25 (64%) | 43 ± 9 | Na | 18 (78%) | 46 ± 14 | Na | No differences | ICA |
| Auditory network | ||||||||
| Odish et al., 2015 | 22 (55%) | 43 ± 9 | 4 ± 3a | 18 (61%) | 47 ± 7 | 4 ± 3a | No differences | ICA |
| Poudel et al., 2014 | 25 (64%) | 43 ± 9 | Na | 18 (78%) | 46 ± 14 | Na | No differences | ICA |
| Dumas et al., 2013 | 28 (61%) | 43 ± 8 | 2–5a | 28 (54%) | 49 ± 9 | 2–5a | No differences | Network of interest |
| Executive network | ||||||||
| Odish et al., 2015 | 22 (55%) | 43 ± 9 | 4 ± 3a | 18 (61%) | 47 ± 7 | 4 ± 3a | No differences | ICA |
| Poudel et al., 2014 | 25 (64%) | 43 ± 9 | Na | 18 (78%) | 46 ± 14 | Na | No differences | ICA |
| Dumas et al., 2013 | 28 (61%) | 43 ± 8 | 2–5a | 28 (54%) | 49 ± 9 | 2–5a | No differences | Network of interest |
| Frontoparietal network | ||||||||
| Odish et al., 2015 | 22 (55%) | 43 ± 9 | 4 ± 3a | 18 (61%) | 47 ± 7 | 4 ± 3a | No differences | ICA |
| Dumas et al., 2013 | 28 (61%) | 43 ± 8 | 2–5a | 28 (54%) | 49 ± 9 | 2–5a | No differences | Network of interest |
| Poudel et al., 2014 | 25 (64%) | 43 ± 9 | Na | 18 (78%) | 46 ± 14 | Na | No differences | ICA |
| Dorsal attention network | ||||||||
| Dumas et al., 2013 | 28 (61%) | 43 ± 8 | 2–5a | 28 (54%) | 49 ± 9 | 2–5a | No differences | Network of interest |
| Poudel et al., 2014 | 25 (64%) | 43 ± 9 | Na | 18 (78%) | 46 ± 14 | Na | Decreased FC | ICA |
| Default mode network | ||||||||
| Dumas et al., 2013 | 28 (61%) | 43 ± 8 | 2–5a | 28 (54%) | 49 ± 9 | 2–5a | No differences | Network of interest |
| Odish et al., 2015 | 22 (55%) | 43 ± 9 | 4 ± 3a | 18 (61%) | 47 ± 7 | 4 ± 3a | No differences | ICA |
| Poudel et al., 2014 | 25 (64%) | 43 ± 9 | Na | 18 (78%) | 46 ± 14 | Na | No differences | ICA |
| Seibert et al., 2012 | 34 (59%) | 41 ± 10 | Na | 22 (68%) | 40 ± 12 | Na | No differences | Seed |
| Salience network | ||||||||
| Mason et al., 2018 | 19 (Na) | 46 ± 12 | Na | 21 (Na) | 42 ± 12 | Na | Reduced coupling activity mainly between SN and other sensory and cognitive networks | Network of interest |
| Subcortical network | ||||||||
| Gorges et al., 2017 | 12 (75%) | 36 (27–62) | 14 (9–17) | 22 (32%) | 45 (25–51) | 17 (12–19) | No differences | Seed |
| Seibert et al., 2012 | 34 (59%) | 41 ± 10 | Na | 22 (68%) | 40 ± 12 | Na | No differences | Seed |
| Regional properties and graph measures | ||||||||
| Gargouri et al., 2016 | 24 (Na) | 39 ± 9 | Na | 18 (50%) | 44 ± 10 | Na | Decreasing hub organization in associative and SMN networks | Graph measures |
| Harrington et al., 2015 | Low 16 (94%) | 33 ± 9 | 14 ± 2 | 16 (75%) | 43 ± 9 | 16 ± 2 | No differences | Graph measures |
| Medium 16 (69%) | 39 ± 10 | 15 ± 3 | Weakened connections within ventral attention centers and frontal areas | |||||
| High 16 (88%) | 47 ± 13 | 14 ± 2 | Weakened connections within frontostriatal network and stronger in ventral attention and parietal areas | |||||
| McColgan, Gregory, et al., 2017 | 64 (45%) | 44 ± 8 | Na | 66 (67%) | 46 ± 8 | Na | Increase connectivity in anterior/frontal and decreased in posterior | Graph measures |
| McColgan, Razi, et al., 2017 | 92 (47%) | 42 ± 10 | Na | 94 (60%) | 48 ± 11 | Na | Greater connectivity within parietal‐DMN regions | Graph measures |
| Sarappa et al., 2017 | 11 (55%) | 38 ± 7 | Na | 40 (55%) | 37 ± 14 | Na | Decreased precuneus ALFF | ALFF |
| Sarappa et al., 2017 | 11 (55%) | 38 ± 7 | Na | 40 (55%) | 37 ± 14 | Na | Decreased cerebellum and subcortical and increased frontotemporal ReHo | ReHo |
Abbreviations: ALFF, amplitude of low frequency fluctuations; F, female; FC, functional connectivity; Na, not available; PCC, posterior cingulate cortex; ReHo, regional homogeneity; SD; standard deviation; Y, years.
International Standard Classification of Education.
Table 3.
Rs‐fMRI studies investigating correlation between functional connectivity and genetic burden in manifest and presymptomatic Huntington's disease individuals
| Huntington's disease | Healthy controls | Main findings | Analysis | ||||
|---|---|---|---|---|---|---|---|
| Reference | Sample (% F) | Age (y ± SD) | Disease burden score | Sample (% F) | Age (y ± SD) | ||
| Symptomatic Huntington | |||||||
| Poudel et al., 2014 | 23 (43%) | 56 ± 9 | 390 ± 65 | 18 (78%) | 46 ± 14 | Higher FPN FC correlated with higher disease burden | ICA |
| Wolf, Sambataro, Vasic, Baldas, et al., 2014 | 20 (30%) | 49 ± 9 | 375 ± 69 | 20 (35%) | 47 ± 9 | Lower VIS FC correlated with higher disease burden | ICA |
| Wolf, Sambataro, Vasic, Depping, et al., 2014 | 20 (30%) | 49 ± 9 | 375 ± 69 | 20 (35%) | 47 ± 9 | Lower ECN FC correlated with higher disease burden | ICA |
| Werner et al., 2014 | 19 (58%) | 48 ± 10 | 377 ± 79 | 17 (59%) | 45 ± 10 | No correlations | ICA |
| Wolf et al., 2015 | 20 (30%) | 49 ± 9 | 375 ± 69 | 20 (35%) | 47 ± 9 | Lower cerebellar network FC correlated with higher disease burden | Seed |
| Sánchez‐Castañeda et al., 2017 | 10 (40%) | 39 ± 19 | 51 ± 9a | 10 (40%) | 38 ± 16 | Lower PCC‐DMN nodal degree correlated with higher number of CAG repeats | Graph measures |
| Gargouri et al., 2016 | 18 (Na %) | 51 ± 7 | 345 ± 83 | 18 (50%) | 44 ± 10 | No correlations | Graph measures |
| Presymptomatic Huntington | |||||||
| Poudel et al., 2014 | 25 (64%) | 43 ± 9 | 288 ± 62 | 18 (78%) | 46 ± 14 | No correlations | ICA |
| Espinoza et al., 2018 | 183 (71%) | 42 ± 13 | 42 ± 3a | 78 (68%) | 49 ± 11 | Lower subcortical FC correlated with higher number of CAG repeats | ICA |
| Odish et al., 2015 | 22 (55%) | 43 ± 9 | 43 ± 3a | 18 (61%) | 47 ± 7 | No correlations | Network of interest |
| Mason et al., 2018 | 19 (Na) | 46 ± 12 | 275 ± 49 | 21 (Na) | 42 ± 12 | Negative correlation between overall hypoconnectivity and disease burden | Network of interest |
| Koenig et al., 2014 | 16 (88%) | 47 ± 13 | 439 ± 46 | 16 (75%) | 43 ± 9 | Lower SMN FC was associated with higher disease burden | Seed |
| Gargouri et al., 2016 | 24 (Na %) | 39 ± 9 | 303 ± 40 | 18 (50%) | 44 ± 10 | Correlation between burden score and graph measures in the SMN | Graph measures |
| Harrington et al., 2015 | 48 (83%) | 40 ± 10 | 341 ± 1 | 16 (75%) | 43 ± 9 | Strengthened whole‐brain FC of the inferior parietal lobe correlated with higher disease burden | Graph measures |
DMN, default mode network; ECN, executive network; FC, functional connectivity; SMN, sensory‐motor network; VIS, visual network, FPN, frontoparietal network.
Studies reporting CAG repeat length.
A total of 22 studies reported contrasts between HD and/or pre‐HD versus HC, while only 1 study was considered as reporting significant relationship between connectivity and genetic load. The majority of studies comparing HD to controls involved the SMN (n = 6) and the VIS (n = 5), while the cerebellum and the auditory networks, were investigated only by n = 3 and n = 2 studies, respectively. The DMN, the executive network (ECN), and the frontoparietal network (FPN) were equally investigated (n = 4 studies each network), while n = 3 studies investigated the dorsal attention (DAN) and the subcortical networks. In pre‐HD the SMN was the most investigated (n = 6 studies). The VIS was reported by n = 4 studies, the auditory network by n = 3 studies, while the cerebellar network was poorly investigated (n = 1 study). The number of studies was similar for the DMN (n = 4 studies), the ECN (n = 3 studies), and the FPN (n = 3 studies), while the DAN was reported by only two studies. The subcortical network was reported by two studies, while a single study investigated the salience network. Finally, n = 3 studies and n = 5 studies investigated regional functional properties or whole brain graph measures in manifest and pre‐HD, respectively.
A total of eight studies implemented seed‐based connectivity approaches, three studies used a predefined network of interest approach, and whole brain ICA was performed in six of the included studies. Six studies implemented different metrics, such as graph analysis approaches (n = 4), and ALFF (n = 2), while one study implemented ReHo analysis.
3.1. Sensory and motor networks
In manifest HD the VIS showed abnormal functional connectivity changes in 4 out of 5 independent studies (Coppen et al., 2018; Dumas et al., 2013; Werner et al., 2014; Wolf, Sambataro, Vasic, Baldas, et al., 2014). Reduced connectivity within this network was functionally related to lower attention, visual scanning, and motor speed and with higher disease burden scores (Wolf, Sambataro, Vasic, Baldas, et al., 2014). One study (Wolf, Sambataro, Vasic, Baldas, et al., 2014) reported concomitant increased connectivity, according to the study of Werner et al. (2014). However, no correlations were found between higher connectivity and disease burden, or clinical/motor impairment (Werner et al., 2014; Wolf, Sambataro, Vasic, Baldas, et al., 2014). Therefore, VIS hypoconnectivity might account for worse visuomotor cognition in manifest HD patients, while hyperconnectivity is not related to genotype and clinical symptoms of HD. VIS impairment prior to motor symptom onset is less clear. Most of the studies in pre‐HD did not report changes within this network compared to HC (Coppen et al., 2018; Odish et al., 2015; Poudel et al., 2014). Only Dumas et al. (2013) showed reduced connectivity in the medial VIS, in brain regions positioned outside the canonical visual mask (i.e., left frontal and right parietal lobules), leaving it unknown whether this connectivity pattern could reflect early changes within the VIS.
The SMN showed consistent functional connectivity alterations in both manifest (Müller et al., 2016; Sánchez‐Castañeda et al., 2017; Wolf, Sambataro, Vasic, Depping, et al., 2014; Werner et al., 2014) and pre‐HD (Koenig et al., 2014; Poudel et al., 2014; Unschuld et al., 2012). Specifically, in manifest HD several studies reported aberrant SMN increased connectivity, which correlated with worse motor functions (Werner et al., 2014; Wolf, Sambataro, Vasic, Depping, et al., 2014) and impaired cognitive abilities (Sánchez‐Castañeda et al., 2017). Congruently, the cerebellum, a brain region structurally and functionally linked with motor and cognitive functions (Chen & Desmond, 2005; Laird et al., 2011; Nitschke, Arp, Stavrou, Erdmann, & Heide, 2005), showed aberrant increased connectivity (Werner et al., 2014) in manifest HD. On the opposite, Müller et al. (2016) reported reduced SMN connectivity in a sample of 34 manifest HD, which correlated with worse motor abilities. Several factors may account for these contrasting results, such as methodologies (e.g., ICA vs. seed‐based connectivity) that might be differently sensible to head motion, a factor known to have systematically different effects on functional connectivity, particularly within the SMN (Van Dijk, Sabuncu, & Buckner, 2012). Alternatively, different disease stages may be related to divergent changes in SMN connectivity. Indeed, the cohort of Müller et al. (2016) featured a lower impairment of motor abilities (as assessed with motor UHDRS scale). This assumption seems supported by studies in pre‐HD individuals, reporting reduced functional connectivity within the SMN (Koenig et al., 2014; Poudel et al., 2014; Unschuld et al., 2012). Lower connectivity in these individuals was associated with worse motor (Poudel et al., 2014) and cognitive abilities (Koenig et al., 2014). Moreover, divergent connectivity changes in SMN were also observed between pre‐HD with lower and higher 5‐year probability of a clinical diagnosis (Koenig et al., 2014), which may suggest divergent connectivity abnormalities according to disease stage. Therefore, increased connectivity within the “motor corticocerebellar system” may characterize the manifest HD stage and linked with characteristic motor symptoms, while in the prodromal stage reduced “motor system” connectivity is observed.
Correlations between functional connectivity and disease burden confirmed the involvement of VIS and cortical‐cerebellar motor networks in the whole HD spectrum (Espinoza et al., 2018; Koenig et al., 2014; Wolf, Sambataro, Vasic, Baldas, et al., 2014; Wolf et al., 2015).
The auditory network did not show selective vulnerability at rest in either manifest or pre‐HD (Dumas et al., 2013; Odish et al., 2015; Poudel et al., 2014).
3.2. Executive, frontoparietal, and attentional networks
Several studies investigated functional connectivity within neural networks subserving cognitive domains, such as the ECN, which showed both reduced (Dumas et al., 2013; Poudel et al., 2014; Wolf, Sambataro, Vasic, Depping, et al., 2014) and increased connectivity (Werner et al., 2014; Wolf, Sambataro, Vasic, Depping, et al., 2014) in manifest HD. This divergent pattern (i.e., hypo‐ vs. hyper‐connectivity) seems to follow different spatial trajectories: decreased connectivity mapped mainly to parietal cortex and subcortical structures (Dumas et al., 2013; Poudel et al., 2014; Wolf, Sambataro, Vasic, Depping, et al., 2014); increased connectivity involved instead the frontal cortex (Werner et al., 2014; Wolf, Sambataro, Vasic, Depping, et al., 2014). Posterior ECN connectivity decrease was related with impaired cognitive abilities and higher disease burden (Wolf, Sambataro, Vasic, Depping, et al., 2014). Conversely, no associations were found for anterior hyperconnectivity (Werner et al., 2014; Wolf, Sambataro, Vasic, Depping, et al., 2014), suggesting that only posterior downregulation might be linked with phenotype and genotype of HD. However, studies investigating changes in connectivity within the ECN in pre‐HD implementing ICA approaches failed to report abnormalities (Dumas et al., 2013; Odish et al., 2015; Poudel et al., 2014).
Increased FPN connectivity was reported in manifest HD and was related to motor and clinical deficits (Werner et al., 2014; Wolf, Sambataro, Vasic, Depping, et al., 2014). Similarly, increased synchronization in the right hippocampus and left dorsolateral prefrontal clusters of FPN was reported in manifest HD compared to the presymptomatic phase of the disease, showing positive correlation with disease burden score (Poudel et al., 2014). However, in pre‐HD individuals several studies failed to report changes in FPN (Dumas et al., 2013; Odish et al., 2015; Poudel et al., 2014), leaving the question related to its involvement in early HD stage still unsettled.
Findings within the DAN did not allow to reach univocal interpretations. Preliminary results showed reduced connectivity in pre‐HD and manifest HD, despite this pattern mapped in different cortical regions in the two groups (Poudel et al., 2014). Conversely, Dumas and co‐workers (2013) did not observe neural synchrony changes in the whole clinical spectrum of HD, whereas only one study reported increased connectivity in manifest HD (Werner et al., 2014).
3.3. Default mode and salience networks
In manifest HD, DMN‐key brain regions (i.e., ventromedial prefrontal cortex and angular gyrus) showed reduced functional connectivity (Dumas et al., 2013; Quarantelli et al., 2013). However, increased connectivity was also observed in these patients (Quarantelli et al., 2013; Sánchez‐Castañeda et al., 2017), although only reduction of DMN‐hubs was associated with both genotype and cognitive performance (Quarantelli et al., 2013). Studies investigating DMN in pre‐HD through ICA or seed‐based connectivity approaches did not report connectivity changes (Dumas et al., 2013; Odish et al., 2015; Poudel et al., 2014; Seibert, Majid, Aron, Corey‐Bloom, & Brewer, 2012), leaving open the question about the DMN involvement in the early stage of HD.
To date, no study assessed connectivity changes within the salience network. However, two different studies, investigating long range coupling activity between functional networks in pre‐HD, showed reduced connectivity between salience network and several networks. Espinoza et al. (2018) reported reduced connectivity between insula and subcortical structures related with increasing CAG repeat length. Similarly, Mason and associates (2018) revealed that in pre‐HD participants the majority of the significant hypoconnectivity connections included the salience network.
3.4. Subcortical network
From the literature, we retrieved only three studies investigating connectivity within the subcortical network in manifest HD (Müller et al., 2016; Werner et al., 2014; Wolf, Sambataro, Vasic, Depping, et al., 2014), reporting contrasting results. Müller et al. (2016) reported reduced basal ganglia‐thalamic connectivity, whereas Werner and co‐workers (2014) showed increased connectivity in thalamus, caudate and putamen network. A third independent study reported decreased connectivity in thalamus and temporal gyrus of subcortical network, whereas increased connectivity was observed within the frontal gyrus (Wolf, Sambataro, Vasic, Depping, et al., 2014). In pre‐HD, two independent studies, implementing a seed‐based connectivity approach, failed to report network changes (Gorges et al., 2017; Seibert et al., 2012).
3.5. Regional spontaneous brain activity and graph measures
Studies investigating regional spontaneous brain activity (i.e., ALFF and ReHo) and graph measures corroborated the findings about the involvement of the cortical‐cerebellar motor circuit in both pre‐HD and HD. Sarappa et al. (2017) reported reduced cerebellar ReHo in both HD and pre‐HD, while Gargouri and co‐workers (2016) found a progressive disruption in brain topology within the SMN, which becomes more randomly connected as the clinical diagnosis of HD approaches.
Interestingly, as reported by studies investigating functional connectivity within the ECN, graph measures showed a similar antero‐posterior dissociation in pre‐HD: stronger connections (i.e., upregulation) were reported in anterior/frontal regions, whereas weaker connections (i.e., downregulation) were reported in posterior/parietal brain regions (McColgan, Gregory, et al., 2017). Similarly, a divergent connectivity pattern was reported in pre‐HD individuals stratified according to CAG‐age product (Harrington et al., 2015), a proxy variable for time to diagnosis accounting for the cumulative toxicity of exposure to the CAG expansion (Paulsen et al., 2014). Pre‐HD subjects with lower toxicity exposure to CAG reported no connectivity differences, while medium‐toxicity pre‐HD showed weakened posterior–anterior connections (Harrington et al., 2015). Subjects with higher‐toxicity (i.e., with the highest odds for a clinical diagnosis of HD), reported the greatest number of weakened connections within brain areas roughly corresponding to the ECN, while increased connectivity was observed between ventral posterior, frontal and subcortical regions (Harrington et al., 2015).
Finally, these measures captured functional alterations in brain regions overlapping with the posterior key areas of the DMN in both HD patients and pre‐HD individuals (Liu et al., 2016; McColgan, Razi, et al., 2017), suggesting that regional and graph measures might be more accurate to identify changes within this network.
4. DISCUSSION
The core results of this review are: (a) multiple resting state networks subserving sensory‐motor abilities and cognitive functions are involved in the pathophysiological mechanisms of HD, which could be involved in the triad of symptoms (motor, cognitive and psychiatric) of this pathology; (b) the SMN and the ECN exhibited a divergent spatiotemporal connectivity pattern. Specifically, SMN showed a temporal divergent pattern (pre‐HD vs. HD), while for the ECN was reported a spatial divergence (posterior vs. anterior). These findings could shed light into the spatiotemporal spreading of brain alteration in HD, revealing detrimental and compensatory mechanisms that could drive new interventions.
4.1. Functional networks involved in HD
The VIS showed reduced connectivity in manifest HD, which can account for the worse visuomotor performance in HD patients (Say et al., 2011), although its involvement in the prodromal stage of disease is less clear (Figure 2). A main involvement of SMN was reported in both pre‐HD and manifest HD suggesting early connectivity changes of this network (Figure 2), consistent with reports in the literature on impairment of motor functions (Ross et al., 2014).
Figure 2.
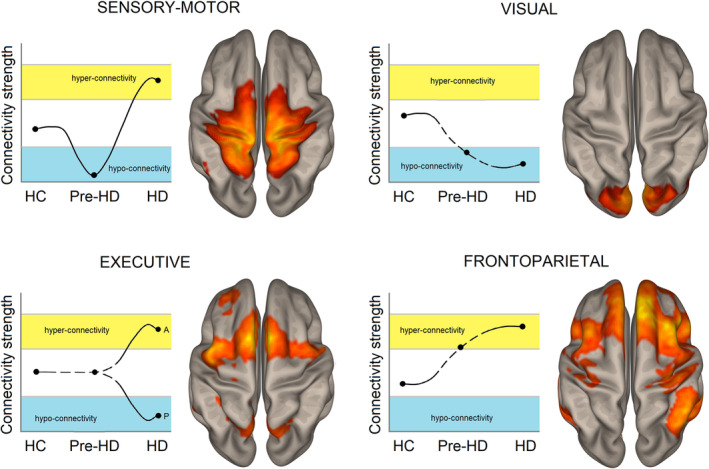
Pattern of aberrant connectivity in the sensory‐motor and visual networks (top row) and executive and frontoparietal networks (bottom row) in the clinical Huntington disease (HD) spectrum. Dashed lines: unclear pattern of connectivity based on preliminary results from literature for presymptomatic HD. A, anterior/frontal regions; HC, healthy controls; P, posterior/parietal regions; pre‐HD, presymptomatic HD
Regarding networks subserving cognitive functions, the ECN showed consistent alterations in manifest HD patients (Figure 2), which might be linked with working memory deficits, one aspect of cognition often impaired in HD (Dumas et al., 2012; Possin et al., 2017; You et al., 2014). Conversely, in pre‐HD individuals, the ECN showed no connectivity changes. The involvement of other networks such as the DAN and the subcortical network is less clear, due to the limited literature and the contrasting results. Even though HD is characterized by subcortical structures dysfunction (Tabrizi et al., 2009), further studies are needed to better clarify the involvement of subcortical changes in connectivity, which may precede subcortical atrophy. Similarly, further studies are needed to understand the involvement of DMN in the physiopathology of HD. In pre‐HD, DMN involvement is even less clear. However, preliminary results in the latter group suggest a divergent connectivity pattern between DMN (increase) and salience network (decrease), which has been observed in other neurodegenerative disorders: in AD, the DMN and the salience network show hypo‐ and hyper‐connectivity, respectively, whereas frontotemporal dementia showed the opposite pattern (Zhou et al., 2010). Further studies should investigate whether these alterations are linked with neuropsychiatric symptoms of HD.
4.2. Divergent connectivity patterns
The main result of this review regards the directionality of connectivity changes within each resting state network (i.e., hyper‐ or hypo‐connectivity) and the association of these changes with the genotype and the clinical phenotype of HD. Interestingly, resting state networks mainly involved in the course of the disease showed divergent connectivity changes (Figure 2).
Specifically, the SMN might follow disease stage‐dependent trajectories: reduced connectivity in pre‐HD switches to aberrant hyperconnectivity pattern in manifest HD (Figure 2). These nonlinear trajectory changes of connectivity have been reported also in healthy aging as a function of age (Staffaroni et al., 2018) and in the AD continuum. Indeed, mild cognitive impairment (MCI) patients who subsequently converted to AD, displayed longitudinal increased connectivity within the DMN (Serra et al., 2016) while, in full‐blown AD dementia, DMN showed consistent functional connectivity reduction (Zhou et al., 2010). This connectivity shift might reflect compensatory functional neural attempts to deal with the emerging motor symptoms. Alternatively, increased connectivity of SMN might represent a maladaptive reorganization of brain functional architecture in HD, as suggested by studies reporting an association between increased SMN connectivity and lower motor performance (Werner et al., 2014; Wolf, Sambataro, Vasic, Depping, et al., 2014). This assumption has been recently reported also in MCI patients, where DMN hyperactivity detrimentally contributes to cognitive impairment (Gardini et al., 2015). To date, only one study investigated longitudinal connectivity changes within several networks, including the SMN, over a period of 3 years, revealing no differences (Odish et al., 2015). However, the low proportion of patients (less than 20% of the sample) converting to manifest HD during the follow‐up might account for a lack of results. Future studies should include larger follow‐up periods, to confirm SMN connectivity shifting from pre‐HD to manifest HD.
A divergent connectivity pattern was also reported within the ECN, in the anterior–posterior axis. In manifest HD, this network showed posterior reduced connectivity, whereas anterior regions displayed aberrant hyperconnectivity (Figure 2). Despite pre‐HD did not show ECN changes, when individuals were stratified according to CAG‐age product, similar dissociations were observed. The functional posterior–anterior dissociation might suggest functional architecture reorganization within the ECN, where increased connectivity may represent a compensatory mechanism to counterbalance downregulation of posterior brain regions as the disease progresses.
This functional shift from posterior to frontal functioning might reflect and emphasize events that have been shown to happen in normal aging (Lövdén, Bäckman, Lindenberger, Schaefer, & Schmiedek, 2010) For example, the PASA model (posterior–anterior shift in aging) suggests that increase in anterior activity, particularly frontal, offsets decrease in posterior cortical activations associated with aging (Davis, Dennis, Daselaar, Fleck, & Cabeza, 2008; Vallesi, 2011). Thus, the compensation is likely to reflect increasing load on frontal executive functions as a first reaction to the loss of computing power in posterior areas. Accordingly, FPN—anchored to the bilateral dorsolateral prefrontal cortex and commonly recruited for top‐down attention processes (Corbetta & Shulman, 2002; Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008)—showed increased connectivity (Figure 2), which might be linked with compensatory mechanisms sustaining emerging cognitive deficits in the presymptomatic stage. This assumption is supported by recent task functional studies reporting increased task‐related brain activity in pre‐HD individuals compared to HC during task performance (Georgiou‐Karistianis et al., 2013; Malejko et al., 2014; Scheller et al., 2013), and by rs‐fMRI studies showing an association between increased frontal connectivity and more preserved cognitive function (Gregory et al., 2018; Klöppel et al., 2015), suggesting compensatory responses.
4.3. Neurobiological and clinical considerations
In HD, neurons in different brain regions accumulate abnormal deposits of misfolded protein, a histopathological hallmark shared with other proteinopathies, such as AD and PD (Braak & Del Tredici, 2016; Saudou & Humbert, 2016). Recent findings suggested that pathogenic proteins, such as beta‐amyloid/Tau in AD and alpha‐synuclein in PD, can spread in a prion‐like fashion via selective functionally connected neural networks, triggering specific clinical phenotypes of brain disorders (Brettschneider, Del Tredici, Lee, & Trojanowski, 2015; Jucker & Walker, 2011; Raj, Kuceyeski, & Weiner, 2012). In AD, neurofibrillary tangles first appear in brain regions overlapping with the DMN (Johnson et al., 2016; Pievani, Filippini, van den Heuvel, Cappa, & Frisoni, 2014), whereas in PD, alpha‐synuclein distribution underlies dysfunction of basal ganglia circuitry responsible for the development of typical motor symptoms (DeLong, 1990; Double, Reyes, Werry, & Halliday, 2010). These observations lead to the “molecular nexopathy” model, which suggested that specific neural networks might represent candidate substrates for the spread of proteinopathies causing neurodegeneration (Warren et al., 2013). This model could be extended to HD, where SMN could represent the macroscopic network fingerprint involved in the pathophysiological mechanisms early on, since the results of the present review consistently point out to an involvement of this network already in the preclinical stage of HD (Figure 3). As the disease progresses, different networks may be affected, such as VIS, FPN, and ECN, accounting for concomitant sensory and cognitive deficits.
Figure 3.
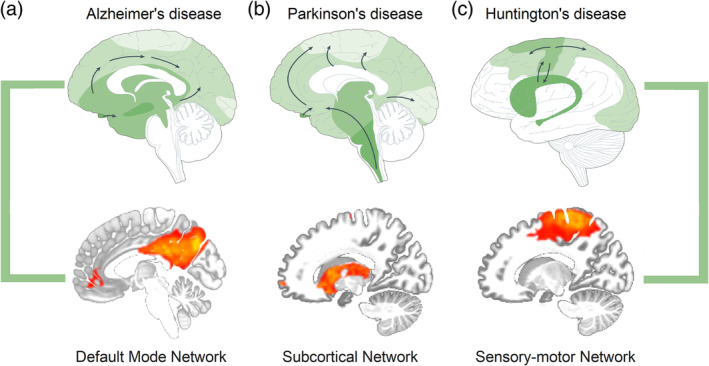
Progression of neuropathological changes in Alzheimer's, Parkinson's, and Huntington's disease (top row) overlapping with specific neural networks (bottom row). Functional connectivity maps were extracted through independent component analysis from a sample of 22 healthy individuals. Figure is adapted from Brundin, Melki, and Kopito (2010) with permission from Springer Nature
Identification of altered brain connectivity will help future huntingtin‐lowering interventions to target the most vulnerable brain regions at the optimal time‐window in order to minimize pathological spread and maximize clinical benefits. For instance, noninvasive brain stimulation interventions may benefit from rs‐fMRI findings, helping to identify disrupted functional networks linked with pathophysiological processes and clinical phenotypes of several neurodegenerative disorders (Pievani et al., 2017; Pievani, Pini, Cappa, & Frisoni, 2016; Pini et al., 2019). Similarly, in HD, brain stimulation interventions targeting vulnerable neural networks in the early stage of the disease might offer other clinical alternatives to slow down both onset and disease progression. Moreover, connectivity markers might be useful as a surrogate outcome to evaluate the effectiveness of clinical interventions in pre‐HD and might pave the way to the development of future personalized preventive treatments.
4.4. Strengths and limitations
The main strength of this review is that we investigated connectivity changes within large scale resting‐state networks, linked, when possible, to disease burden score and to clinical symptoms of HD. This information might shed light on the mechanisms underlying functional change. (i.e., if hyper‐ and hypo‐connectivity is related to compensatory or detrimental processes). To date no effective treatments have been proved to delay disease onset or progression, and classical pharmacological treatments can only provide symptomatic benefit. Identification of reliable biomarkers is currently a research priority, and vulnerable networks linked with early HD pathophysiological processes are optimal candidate which might pave the way to novel therapeutic trials.
Among the limitations, the small number of studies included limited the results, especially regarding cognitive and subcortical networks in HD. This should be taken into account for future meta‐analysis aimed to investigate altered hubs within specific neural networks in both manifest and pre‐HD. A second limitation is the high heterogeneity across studies. We included studies investigating connectivity changes through different methodology, which could account for different results. These limitations highlight the need for further research in this field.
5. CONCLUSION
In summary, the SMN showed consistent connectivity changes in the whole HD spectrum, whereas VIS and executive/attentional network alterations emerged during manifest HD and might account for incoming sensory and cognitive deficits. In addition, we observed divergent connectivity patterns in posterior–anterior axis for the ECN in manifest HD, which could reflect compensatory mechanisms, and a disease stage‐dependent shifting in SMN connectivity alterations.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
All data used for figures were collected according to the Helsinki Declaration and approved by the local ethics committee of the Henri Mondor Hospital, Créteil, France. All participants signed written informed participation consent.
Pini L, Jacquemot C, Cagnin A, et al. Aberrant brain network connectivity in presymptomatic and manifest Huntington's disease: A systematic review. Hum Brain Mapp. 2020;41:256–269. 10.1002/hbm.24790
REFERENCES
- Bohanna, I. , Georgiou‐Karistianis, N. , Hannan, A. J. , & Egan, G. F. (2008). Magnetic resonance imaging as an approach towards identifying neuropathological biomarkers for Huntington's disease. Brain Research Reviews, 58(1), 209–225. [DOI] [PubMed] [Google Scholar]
- Braak, H. , & Del Tredici, K. (2016). Potential pathways of abnormal tau and α‐Synuclein dissemination in sporadic Alzheimer's and Parkinson's diseases. Cold Spring Harbor Perspectives in Biology, 8(11) pii: a023630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettschneider, J. , Del Tredici, K. , Lee, V. M. , & Trojanowski, J. Q. (2015). Spreading of pathology in neurodegenerative diseases: A focus on human studies. Nature Reviews. Neuroscience, 16, 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin, P. , Melki, R. , & Kopito, R. (2010). Prion‐like transmission of protein aggregates in neurodegenerative disease. Nature Reviews. Molecular Cell Biology, 11, 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner, R. L. , Snyder, A. Z. , Shannon, B. J. , LaRossa, G. , Sachs, R. , Fotenos, A. F. , … Mintun, M. A. (2005). Molecular, structural, and functional characterization of Alzheimer's disease: Evidence for a relationship between default activity, amyloid, and memory. The Journal of Neuroscience, 25, 7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. H. , & Desmond, J. E. (2005). Temporal dynamics of cerebro‐cerebellar network recruitment during a cognitive task. Neuropsychologia, 43(9), 1227–1237. [DOI] [PubMed] [Google Scholar]
- Coppen, E. M. , Grond, J. V. , Hafkemeijer, A. , Barkey Wolf, J. J. H. , & Roos, R. A. C. (2018). Structural and functional changes of the visual cortex in early Huntington's disease. Human Brain Mapping, 39(12), 4776–4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta, M. , & Shulman, G. L. (2002). Control of goal‐directed and stimulus‐driven attention in the brain. Nature Reviews. Neuroscience, 3(3), 201–215. [DOI] [PubMed] [Google Scholar]
- Damoiseaux, J. S. , Rombouts, S. A. , Barkhof, F. , Scheltens, P. , Stam, C. J. , Smith, S. M. , & Beckmann, C. F. (2006). Consistent resting‐state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America, 103(37), 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, S. W. , Dennis, N. A. , Daselaar, S. M. , Fleck, M. S. , & Cabeza, R. (2008). Que PASA? The posterior–anterior shift in aging. Cerebral Cortex, 18(5), 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong, M. R. (1990). Primate models of movement disorders of basal ganglia origin. Trends in Neurosciences, 13(7), 281–285. [DOI] [PubMed] [Google Scholar]
- Dosenbach, N. U. , Fair, D. A. , Cohen, A. L. , Schlaggar, B. L. , & Petersen, S. E. (2008). A dual‐networks architecture of top‐down control. Trends in Cognitive Sciences, 12(3), 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Double, K. L. , Reyes, S. , Werry, E. L. , & Halliday, G. M. (2010). Selective cell death in neurodegeneration: Why are some neurons spared in vulnerable regions? Progress in Neurobiology, 92(3), 316–329. [DOI] [PubMed] [Google Scholar]
- Dumas, E. M. , Say, M. J. , Jones, R. , Labuschagne, I. , O'Regan, A. M. , Hart, E. P. , … Stout, J. C. (2012). Visual working memory impairment in premanifest gene‐carriers and early Huntington's disease. Journal of Huntington's Disease, 1, 97–106. [DOI] [PubMed] [Google Scholar]
- Dumas, E. M. , van den Bogaard, S. J. , Hart, E. P. , Soeter, R. P. , van Buchem, M. A. , van der Grond, J. , … Roos, R. A. (2013). TRACK‐HD investigator group. Reduced functional brain connectivity prior to and after disease onset in Huntington's disease. NeuroImage: Clinical, 14(2), 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza, F. A. , Turner, J. A. , Vergara, V. M. , Miller, R. L. , Mennigen, E. , Liu, J. , … Calhoun, V. D. (2018). Whole‐brain connectivity in a large study of Huntington's disease gene mutation carriers and healthy controls. Brain Connectivity, 8(3), 166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardini, S. , Venneri, A. , Sambataro, F. , Cuetos, F. , Fasano, F. , Marchi, M. , … Caffarra, P. (2015). Increased functional connectivity in the default mode network in mild cognitive impairment: A maladaptive compensatory mechanism associated with poor semantic memory performance. Journal of Alzheimer's Disease, 45(2), 457–470. [DOI] [PubMed] [Google Scholar]
- Gargouri, F. , Messé, A. , Perlbarg, V. , Valabregue, R. , McColgan, P. , Yahia‐Cherif, L. , … Lehéricy, S. (2016). Longitudinal changes in functional connectivity of cortico‐basal ganglia networks in manifests and premanifest Huntington's disease. Human Brain Mapping, 37(11), 4112–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou‐Karistianis, N. , Poudel, G. R. , Domínguez, D. J. F. , Langmaid, R. , Gray, M. A. , Churchyard, A. , … Stout, J. C. (2013). Functional and connectivity changes during working memory in Huntington's disease: 18‐month longitudinal data from the IMAGE‐HD study. Brain and Cognition, 83(1), 80–91. [DOI] [PubMed] [Google Scholar]
- Gorges, M. , Müller, H. P. , Mayer, I. M. , Grupe, G. S. , Kammer, T. , Grön, G. , … Orth, M. (2017). Intact sensory‐motor network structure and function in far from onset premanifest Huntington's disease. Scientific Reports, 7, 43841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory, S. , Long, J. D. , Klöppel, S. , Razi, A. , Scheller, E. , Minkova, L. , … Orth, M. (2018). Testing a longitudinal compensation model in premanifest Huntington's disease. Brain, 141(7), 2156–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, X. , Zeng, Q. , Guo, T. , Wang, J. , Xuan, M. , Gu, Q. , … Zhang, M. (2017). Disrupted functional connectivity of basal ganglia across tremor‐dominant and Akinetic/rigid‐dominant Parkinson's disease. Frontiers in Aging Neuroscience, 9, 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington, D. L. , Rubinov, M. , Durgerian, S. , Mourany, L. , Reece, C. , Koenig, K. , … Rao, S. M. (2015). Network topology and functional connectivity disturbances precede the onset of Huntington's disease. Brain, 138(Pt 8), 2332–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenfeld, C. , Werner, C. J. , & Reetz, K. (2018). Resting‐state connectivity in neurodegenerative disorders: Is there potential for an imaging biomarker? NeuroImage: Clinical, 18, 849–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, K. A. , Schultz, A. , Betensky, R. A. , Becker, J. A. , Sepulcre, J. , Rentz, D. , … Sperling, R. (2016). Tau positron emission tomographic imaging in aging and early Alzheimer disease. Annals of Neurology, 79, 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker, M. , & Walker, L. C. (2011). Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Annals of Neurology, 70, 532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöppel, S. , Gregory, S. , Scheller, E. , Minkova, L. , Razi, A. , Durr, A. , … Track‐On Investigators . (2015). Compensation in preclinical Huntington's disease: Evidence from the Track‐on HD study. eBioMedicine, 2(10), 1420–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig, K. A. , Lowe, M. J. , Harrington, D. L. , Lin, J. , Durgerian, S. , Mourany, L. , … PREDICT‐HD Investigators of the Huntington Study Group . (2014). Functional connectivity of primary motor cortex is dependent on genetic burden in prodromal Huntington disease. Brain Connectivity, 4(7), 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, A. R. , Fox, P. M. , Eickhoff, S. B. , Turner, J. A. , Ray, K. L. , McKay, D. R. , & Fox, P. T. (2011). Behavioral interpretations of intrinsic connectivity networks. Journal of Cognitive Neuroscience, 23, 4022–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati, A. , Altman, D. G. , Tetzlaff, J. , Mulrow, C. , Gotzsche, P. C. , Ioannidis, J. P. , … Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: Explanation and elaboration. British Medical Journal, 339, b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W. , Yang, J. , Chen, K. , Luo, C. , Burgunder, J. , Gong, Q. , & Shang, H. (2016). Resting‐state fMRI reveals potential neural correlates of impaired cognition in Huntington's disease. Parkinsonism & Related Disorders, 27, 41–46. [DOI] [PubMed] [Google Scholar]
- Lövdén, M. , Bäckman, L. , Lindenberger, U. , Schaefer, S. , & Schmiedek, F. (2010). A theoretical framework for the study of adult cognitive plasticity. Psychological Bulletin, 136(4), 659–676. [DOI] [PubMed] [Google Scholar]
- Malejko, K. , Weydt, P. , Süßmuth, S. D. , Grön, G. , Landwehrmeyer, G. B. , & Abler, B. (2014). Prodromal Huntington disease as a model for functional compensation of early neurodegeneration. PLoS One, 9(12), e114569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, S. L. , Daws, R. E. , Soreq, E. , Johnson, E. B. , Scahill, R. I. , Tabrizi, S. J. , … Hampshire, A. (2018). Predicting clinical diagnosis in Huntington's disease: An imaging polymarker. Annals of Neurology, 83(3), 532–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui, T. , Murakami, T. , & Ohki, K. (2016). Transient neuronal coactivations embedded in globally propagating waves underlie resting‐state functional connectivity. Proceedings of the National Academy of Sciences of the United States of America, 113, 6556–6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColgan, P. , Gregory, S. , Razi, A. , Seunarine, K. K. , Gargouri, F. , Durr, A. , … Johnson, H. (2017). White matter predicts functional connectivity in premanifest Huntington's disease. Annals of Clinical Translational Neurology, 4(2), 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColgan, P. , Razi, A. , Gregory, S. , Seunarine, K. K. , Durr, A. , Roos, A. C. , … Track On‐HD Investigators . (2017). Structural and functional brain network correlates of depressive symptoms in premanifest Huntington's disease. Human Brain Mapping, 38(6), 2819–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, V. (2015). Salience network In Toga A. W. (Ed.), Brain mapping: An encyclopedic reference, 2 (pp. 597–611). Academic Press: Elsevier. [Google Scholar]
- Müller, H. P. , Gorges, M. , Grön, G. , Kassubek, J. , Landwehrmeyer, G. B. , Süßmuth, S. D. , … Orth, M. (2016). Motor network structure and function are associated with motor performance in Huntington's disease. Journal of Neurology, 263(3), 539–549. [DOI] [PubMed] [Google Scholar]
- Nanetti, L. , Contarino, V. E. , Castaldo, A. , Sarro, L. , Bachoud‐Levi, A. C. , Giavazzi, M. , … Mariotti, C. (2018. Jun). Cortical thickness, stance control, and arithmetic skill: An exploratory study in premanifest Huntington disease. Parkinsonism Relat Disord, 51, 17–23. 10.1016/j.parkreldis.2018.02.033 [DOI] [PubMed] [Google Scholar]
- Nitschke, M. F. , Arp, T. , Stavrou, G. , Erdmann, C. , & Heide, W. (2005). The cerebellum in the cerebro‐cerebellar network for the control of eye and hand movements—An fMRI study. Progress in Brain Research, 148, 151–164. [DOI] [PubMed] [Google Scholar]
- Odish, O. F. , van den Berg‐Huysmans, A. A. , van den Bogaard, S. J. , Dumas, E. M. , Hart, E. P. , Rombouts, S. A. , … TRACK‐HD Investigator Group . (2015). Longitudinal resting state fMRI analysis in healthy controls and premanifest Huntington's disease gene carriers: A three‐year follow‐up study. Human Brain Mapping, 36(1), 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen, J. S. , Long, J. D. , Ross, C. A. , Harrington, D. L. , Erwin, C. J. , Williams, J. K. , … PREDICT‐HD Investigators and Coordinators of the Huntington Study Group . (2014). Prediction of manifest Huntington's disease with clinical and imaging measures: A prospective observational study. Lancet Neurology, 13(12), 1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney, J. B., Jr. , Vonsattel, J. P. , MacDonald, M. E. , Gusella, J. F. , & Myers, R. H. (1997). CAG repeat number governs the development rate of pathology in Huntington's disease. Annals of Neurology, 41, 689–692. [DOI] [PubMed] [Google Scholar]
- Pievani, M. , Filippini, N. , van den Heuvel, M. P. , Cappa, S. F. , & Frisoni, G. B. (2014). Brain connectivity in neurodegenerative diseases—From phenotype to proteinopathy. Nature Reviews. Neurology, 10(11), 620–633. [DOI] [PubMed] [Google Scholar]
- Pievani, M. , Pini, L. , Cappa, S. F. , & Frisoni, G. B. (2016). Brain networks stimulation in dementia: Insights from functional imaging. Current Opinion in Neurology, 29(6), 756–762. [DOI] [PubMed] [Google Scholar]
- Pievani, M. , Pini, L. , Ferrari, C. , Pizzini, F. B. , Boscolo Galazzo, I. , Cobelli, C. , … Frisoni, G. B. (2017). Coordinate‐based meta‐analysis of the default mode and salience network for target identification in non‐invasive brain stimulation of Alzheimer's disease and behavioral variant Frontotemporal dementia networks. Journal of Alzheimer's Disease, 57(3), 825–843. [DOI] [PubMed] [Google Scholar]
- Pini, L. , Manenti, R. , Cotelli, M. , Pizzini, F. B. , Battista, G. B. , & Pievani, M. (2019). Non‐invasive brain stimulation in dementia: A complex network story. Neurodegenerative Diseases, 18(5–6), 281–301. [DOI] [PubMed] [Google Scholar]
- Possin, K. L. , Kim, H. , Geschwind, M. D. , Moskowitz, T. , Johnson, E. T. , Sha, S. J. , … Kramer, J. H. (2017). Egocentric and allocentric visuospatial working memory in premotor Huntington's disease: A double dissociation with caudate and hippocampal volumes. Neuropsychologia, 101, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudel, G. R. , Egan, G. F. , Churchyard, A. , Chua, P. , Stout, J. C. , & Georgiou‐Karistianis, N. (2014). Abnormal synchrony of resting state networks in premanifest and symptomatic Huntington disease: The IMAGE‐HD study. Journal of Psychiatry & Neuroscience, 39(2), 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarantelli, M. , Salvatore, E. , Giorgio, S. M. , Filla, A. , Cervo, A. , Russo, C. V. , … De Michele, G. (2013). Default‐mode network changes in Huntington's disease: An integrated MRI study of functional connectivity and morphometry. PLoS One, 8(8), e72159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrini, G. , Pini, L. , Pievani, M. , Magni, L. R. , Lanfredi, M. , Ferrari, C. , & Rossi, R. (2018). Abnormalities in functional connectivity in borderline personality disorder: Correlations with metacognition and emotion dysregulation. Psychiatry Research. Neuroimaging, 283, 118–124. [DOI] [PubMed] [Google Scholar]
- Raj, A. , Kuceyeski, A. , & Weiner, M. (2012). A network diffusion model of disease progression in dementia. Neuro, 73(6), 1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas, H. D. , Salat, D. H. , Lee, S. Y. , Zaleta, A. K. , Pappu, V. , Fischl, B. , & Hersch, S. M. (2008). Cerebral cortex and the clinical expression of Huntington's disease: Complexity and heterogeneity. Brain, 131(4), 1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, C. A. , Aylward, E. H. , Wild, E. J. , Langbehn, D. R. , Long, J. D. , Warner, J. H. , … Tabrizi, S. J. (2014). Huntington disease: Natural history, biomarkers and prospects for therapeutics. Nature Reviews. Neurology, 10(4), 204–216. [DOI] [PubMed] [Google Scholar]
- Ross, C. A. , & Tabrizi, S. J. (2011). Huntington's disease: From molecular pathogenesis to clinical treatment. Lancet Neurology, 10(1), 83–98. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Castañeda, C. , de Pasquale, F. , Caravasso, C. F. , Marano, M. , Maffi, S. , Migliore, S. , … Squitieri, F. (2017). Resting‐state connectivity and modulated somatomotor and default‐mode networks in Huntington disease. CNS Neuroscience & Therapeutics, 23(6), 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarappa, C. , Salvatore, E. , Filla, A. , Cocozza, S. , Russo, C. V. , Saccà, F. , … Quarantelli, M. (2017). Functional MRI signal fluctuations highlight altered resting brain activity in Huntington's disease. Brain Imaging and Behavior, 11(5), 1459–1469. [DOI] [PubMed] [Google Scholar]
- Saudou, F. , & Humbert, S. (2016). The biology of Huntingtin. Neuron, 89(5), 910–926. [DOI] [PubMed] [Google Scholar]
- Say, M. J. , Jones, R. , Scahill, R. I. , Dumas, E. M. , Coleman, A. , Santos, R. C. , … TRACK‐HD Investigators . (2011). Visuomotor integration deficits precede clinical onset in Huntington's disease. Neuropsychologia, 49(2), 264–270. [DOI] [PubMed] [Google Scholar]
- Scheller, E. , Abdulkadir, A. , Peter, J. , Tabrizi, S. J. , Frackowiak, R. S. J. , & Klöppel, S. (2013). Interregional compensatory mechanisms of motor functioning in progressing preclinical neurodegeneration. NeuroImage, 75, 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalm, M. , Schmid, F. , Wachsmuth, L. , Backhaus, H. , Kronfeld, A. , Aedo Jury, F. , … Stroh, A. (2017). Cortex‐wide BOLD fMRI activity reflects locally‐recorded slow oscillation‐associated calcium waves. Elife, 6 pii: e27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley, W. W. , Crawford, R. K. , Zhou, J. , Miller, B. L. , Greicius, M. D. (2009). Neurodegenerative diseases target large‐scale human brain networks. Neuron, 62(1), 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert, T. M. , Majid, D. S. , Aron, A. R. , Corey‐Bloom, J. , & Brewer, J. B. (2012). Stability of resting fMRI interregional correlations analyzed in subject‐native space: A one‐year longitudinal study in healthy adults and premanifest Huntington's disease. NeuroImage, 59(3), 2452–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra, L. , Cercignani, M. , Mastropasqua, C. , Torso, M. , Spanò, B. , Makovac, E. , … Bozzali, M. (2016). Longitudinal changes in functional brain connectivity predicts conversion to Alzheimer's disease. Journal of Alzheimer's Disease, 51(2), 377–389. [DOI] [PubMed] [Google Scholar]
- Staffaroni, A. M. , Brown, J. A. , Casaletto, K. B. , Elahi, F. M. , Deng, J. , Neuhaus, J. , … Kramer, J. H. (2018). The longitudinal trajectory of default mode network connectivity in healthy older adults varies as a function of age and is associated with changes in episodic memory and processing speed. The Journal of Neuroscience, 38(11), 2809–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi, S. J. , Langbehn, D. R. , Leavitt, B. R. , Roos, R. A. , Durr, A. , Craufurd, D. , … TRACK‐HD Investigators . (2009). Biological and clinical manifestations of Huntington's disease in the longitudinal TRACK‐HD study: Cross‐sectional analysis of baseline data. Lancet Neurology, 8(9), 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unschuld, P. G. , Joel, S. E. , Liu, X. , Shanahan, M. , Margolis, R. L. , Biglan, K. M. , … Ross, C. A. (2012). Impaired cortico‐striatal functional connectivity in prodromal Huntington's disease. Neuroscience Letters, 514(2), 204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallesi, A. (2011). Targets and non‐targets in the aging brain: A go/nogo event‐related potential study. Neuroscience Letters, 487(3), 313–317. [DOI] [PubMed] [Google Scholar]
- Van Dijk, K. R. , Sabuncu, M. R. , & Buckner, R. L. (2012). The influence of head motion on intrinsic functional connectivity MRI. NeuroImage, 59(1), 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonsattel, J. P. , Myers, R. H. , Stevens, T. J. , Ferrante, R. J. , Bird, E. D. , Richardson, E. P. Jr. (1985). Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol, 44(6), 559–577. [DOI] [PubMed] [Google Scholar]
- Warren, J. D. , Rohrer, J. D. , Schott, J. M. , Fox, N. C. , Hardy, J. , & Rossor, M. N. (2013). Molecular nexopathies: A new paradigm of neurodegenerative disease. Trends in Neurosciences, 36, 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner, C. J. , Dogan, I. , Saß, C. , Mirzazade, S. , Schiefer, J. , Shah, N. J. , … Reetz, K. (2014). Altered resting‐state connectivity in Huntington's disease. Human Brain Mapping, 35(6), 2582–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, R. C. , Sambataro, F. , Vasic, N. , Baldas, E. M. , Ratheiser, I. , Bernhard Landwehrmeyer, G. , … Orth, M. (2014). Visual system integrity and cognition in early Huntington's disease. The European Journal of Neuroscience, 40(2), 2417–2426. [DOI] [PubMed] [Google Scholar]
- Wolf, R. C. , Sambataro, F. , Vasic, N. , Depping, M. S. , Thomann, P. A. , Landwehrmeyer, G. B. , … Orth, M. (2014). Abnormal resting‐state connectivity of motor and cognitive networks in early manifest Huntington's disease. Psychological Medicine, 44(15), 3341–3356. [DOI] [PubMed] [Google Scholar]
- Wolf, R. C. , Thomann, P. A. , Sambataro, F. , Wolf, N. D. , Vasic, N. , Landwehrmeyer, G. B. , … Orth, M. (2015). Abnormal cerebellar volume and corticocerebellar dysfunction in early manifest Huntington's disease. Journal of Neurology, 262(4), 859–869. [DOI] [PubMed] [Google Scholar]
- You, S. C. , Geschwind, M. D. , Sha, S. J. , Apple, A. , Satris, G. , Wood, K. A. , … Possin, K. L. (2014). Executive functions in premanifest Huntington's disease. Movement Disorders, 29(3), 405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. , Greicius, M. D. , Gennatas, E. D. , Growdon, M. E. , Jang, J. Y. , Rabinovici, G. D. , … Seeley, W. W. (2010). Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain, 133, 1352–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]


