Abstract
The task of learning predefined sequences of interrelated motor actions is of everyday importance and has also strong clinical importance for regaining motor function after brain lesions. A solid understanding of sequence learning in stroke patients can help clinicians to optimize and individualize rehabilitation strategies. Moreover, to investigate the impact of a focal lesion on the ability to successfully perform motor sequence learning can enhance our comprehension of the underlying physiological principles of motor sequence learning. In this article, we will first provide an overview of current concepts related to motor sequence learning in healthy subjects with focus on the involved brain areas and their assumed functions according to the temporal stage model. Subsequently, we will consider the question of what we can learn from studies investigating motor sequence learning in stroke patients. We will first focus on the implications of lesion location. Then, we will analyze whether distinct lesion locations affect specific learning stages. Finally, we will discuss the implications for clinical rehabilitation and suggest directions for further research.
Keywords: motor cortex, motor rehabilitation, plasticity, sequence learning, stroke
1. INTRODUCTION
Most aspects of human behavior are composed of series of interrelated actions (sequences). This applies not only to motor actions but also to sequences of words, thoughts and memories. Motor skill acquisition is a particularly intuitive example of the ability of the human brain to learn sequences by training. Typical examples of motor sequence learning include learning to play piano and learning to dance. Even motor sequences that appear very difficult at first may be carried out effortlessly given enough time and training. Throughout training, movement parameters are optimized, resulting in efficient and accurate performance. Training also leads to a decreasing need for active attention to movement performance. The changes in resource utilization during the course of training have led to the proposition of different, distinguishable stages of sequence learning. In particular, multiple authors have suggested a division into an early learning phase, a consolidation phase and a slow learning or retention phase. In addition to this temporal model, there is also broad consensus that motor sequence learning takes place in two different modes, mainly distinguished by the presence or absence of conscious awareness of the learned sequence (explicit vs. implicit learning).
Due to the simple accessibility of motor performance and the general importance of the motor system for our quality of life, the motor system is often investigated as a representative of principles underlying sequence learning in general. For this purpose, some standardized tests have been established and will be introduced below.
It is important to note that most complex motor skills consist of sequences of movements (Diedrichsen & Kornysheva, 2015; Hikosaka, Nakamura, Sakai, & Nakahara, 2002; Willingham, 1998). To regain a lost motor skill (e.g., lost through a stroke) not only every single movement of this skill, but also its implementation as a sequence has to be relearned. The inseparable connection between learning motor skills and sequences makes the understanding of sequence learning important for clinical rehabilitation. Disabilities resulting from stroke are particularly significant as they constitute the leading cause of long‐term disability in industrial nations. Hence, there is an urgent need for a solid understanding of sequence learning in stroke patients, which could help clinicians optimize and individualize rehabilitation strategies. Moreover, the impact of a focal lesion on the ability to successfully perform motor sequence learning can help us gain insights into—and an improved comprehension of—the basic physiological principles of motor sequence learning.
In the following sections, we will first provide an overview of important concepts related to motor sequence learning, such as explicit and implicit motor learning, and the dissociation between sequential and motor components. We will then briefly introduce the current methods used to investigate sequential motor learning. Next, we will review the current state of knowledge regarding important brain areas and their assumed functions in motor sequence learning as derived from studies in healthy subjects. Subsequently, we will consider the question of what we can learn from studies investigating motor sequence learning in stroke patients. We will first investigate the evidence of whether patients’ capacity for motor sequence learning is preserved after stroke in general. Next, we will review all studies concerning the implications of stroke for different learning stages according to the temporal stage model. Finally, we will discuss the implications for clinical rehabilitation and suggest directions for further research.
2. BASIC PRINCIPLES OF MOTOR SEQUENCE LEARNING
Motor sequence learning refers to the process in which a predetermined ordered list (sequence) of motor actions is performed with increasing spatial and temporal accuracy. This learning process can occur with or without conscious awareness of the learning process itself and the sequential order of elements. The divergences between conscious and unconscious learning have led to the well‐accepted concept of distinguishing two different modes—the explicit and implicit modes of learning.
2.1. Explicit and implicit motor learning
Explicit and implicit motor learning are fundamental concepts for our understanding of sequence learning. The differentiation is mainly based on awareness of the learned skill. While explicit learning requires active attention and some form of rules that can be stated, implicit motor learning takes place without awareness and in the absence of verbal knowledge of the performed motor task (Kleynen, Braun, et al., 2014; Kleynen, Wilson, et al., 2014). Hence, implicit or procedural learning is defined as a learning process that is both incidental and unconscious (Reber, 1993). The classic example of implicit motor learning is to learn to walk as a child or to ride a bicycle. These learning tasks can be accomplished without instructions. Learned skills are retrieved from implicit memory. However, some authors object that implicit learning also comes along with conscious inputs, which then lead to unconscious implications that are learned (Baars, 2010). This conscious input is called explicit or declarative knowledge. Explicit motor learning was defined in a previous consensus paper as follows: “learning which generates verbal knowledge of movement performance (e.g., facts and rules), involves cognitive stages within the learning process and is dependent on working memory involvement” (Kleynen, Braun, et al., 2014; Kleynen, Wilson, et al., 2014). A typical example of explicit learning would be to learn a prescribed sequence of dance steps.
2.2. Dissociation between sequential and motor components
Another important concept in understanding motor sequence learning is the dissociation between the spatial‐sequential order of a movement sequence and its motor control components (Doya, 2000; Ghilardi, Moisello, Silvestri, Ghez, & Krakauer, 2009; Hikosaka et al., 1999; Penhune & Steele, 2012). The spatial‐sequential task component concerns the order of movements in space and time, while the movement dynamics comprise the sensorimotor integration of movements, such as the speed of single movements and the timing of movements.
It has been proposed that these components are learned in parallel, with different contributions by specific brain areas. Accordingly, learning the sequential/spatial order of movement follows a different time course than learning the motor components. It is thought that in the early phase of motor learning, improvements are dominated by learning the sequential/spatial characteristics of the movements, while the implementation and optimization of the required motor components dominate improvements in later stages of learning (Ghilardi et al., 2009; Savion‐Lemieux & Penhune, 2005).
2.3. Methods to investigate motor sequence learning
The most commonly used method to examine motor sequence learning is the serial reaction time task (SRTT). Participants receive visual stimuli (cues) appearing at different locations on a screen. The participants are asked to respond to each cue by pressing the correct button as fast as possible. The time between cue presentation and motor response defines the reaction time. A decrease in reaction time is considered learning. However, this decrease in reaction time can be influenced by multiple contaminating factors, such as motivation and visuomotor associations. Therefore, the reaction time for predetermined sequences is contrasted with the reaction time for random sequences. The difference between sequential and random sequences in the decrease in reaction time is often used as a measure of sequence‐specific motor learning. The SRTT paradigm investigates implicit sequence motor learning if the participants do not become aware of the predefined sequence. If, however, participants gain knowledge about this sequence, this indicates an explicit component of motor sequence learning. The SRTT is used in several variants.
Other paradigms for assessment of motor sequence learning are the continuous tracking task (CTT, tracking a target moving in a specific pattern across a screen), finger tapping tasks (e.g., the manual sequence task, or MST, which consists of typing a predefined numeral sequence on a finger pad; Karni et al., 1995), and sequences of reaching (Ghilardi et al., 2009) or eye movements (Albouy et al., 2008).
3. CURRENT KNOWLEDGE REGARDING THE CEREBRAL NETWORK ENGAGED IN MOTOR SEQUENCE LEARNING
Motor sequence learning is performed by a complex and distributed network of brain regions. Essential parts of this network include the primary motor cortex (M1), the premotor cortex (PMC), the supplementary motor area (SMA), the basal ganglia (BG), the prefrontal cortex (PFC), the posterior parietal cortex (PPC), and the cerebellum (Figure 1).
Figure 1.
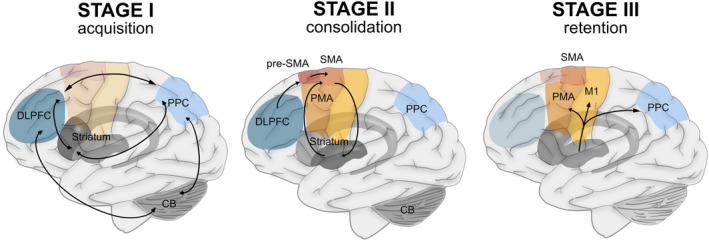
Schematic representation of the locations of areas involved in different stages of motor sequence learning. Abbreviations: CB, cerebellum; DLPFC, dorsolateral prefrontal cortex; M1, primary motor cortex; PMA, premotor area; PPC, posterior parietal cortex; pre‐SMA, pre‐supplementary motor area; SMA, supplementary motor area
In the following section, we will briefly describe the primary functions of these brain areas for motor sequence learning and will then discuss their roles in different phases of the learning process.
The motor cortex, comprising M1, PMC, and SMA, is strongly interconnected with frontoparietal regions as well as the striatum and other parts of the BG and generates significant output to the descending motor system. While M1 stores motor sequence information in a somatotopically organized system, PMC is recognized to play a critical role in visual and sensorimotor integration and movement selection, preparing motor output for M1 (Hardwick, Rottschy, Miall, & Eickhoff, 2013). A major function of the SMA seems to be the planning and self‐initiation of voluntary movements. Input arrives from pre‐SMA, which serves the function of integrating multimodal information by reducing the output of the motor loop when its signals are inconsistent with those of the visual loop (Hikosaka et al., 1999; Nakahara, Doya, & Hikosaka, 2001).
The cerebellum is known to play a crucial role in the fine‐tuning of motor performance via feedback loops with cortical structures which are called cortico‐cerebellar loops. Cerebellar functioning is firmly connected to the parietal, premotor and frontal cortex and accomplishes a central role in movement optimization. By integrating efferent and afferent information, the cerebellum creates an internal predictive model of sensory states that guides learning by the trial‐and‐error principle (Miall, 2010; Penhune & Steele, 2012; Shadmehr, Brandt, & Corkin, 1998). This allows predictive control by a feedforward mechanism in which the current sensory state and cortical motor commands are combined to forecast the future state of the body (Bastian, 2006).
The BG are mainly engaged in the chunking of movements and contribute to the storage of information. It is generally agreed that the BG play a prominent role in implicit learning processes. However, some parts of the BG are also involved in explicit learning. The striatum is considered the most critical BG structure engaged in motor sequence learning. The dorsolateral striatum primarily takes part in sensorimotor circuits by communicating with the parietal and sensorimotor cortices, which supports the idea that it plays a leading role in implicit sequence learning by chunking information (Hikosaka et al., 1999; Penhune & Steele, 2012). Chunking is a process in which a motor sequence is subdivided into shorter segments, known as chunks. By being segmented into these chunks, the information can be retained and restored more easily than unsegmented information. The anterior medial striatum is known to be strongly connected to frontal and premotor areas, which are suggested to play a critical role in explicit learning processes. This connection is also called the “associative” circuit (Janacsek & Nemeth, 2013; Penhune & Steele, 2012). Furthermore, the BG are believed to be involved in reward‐based learning, integrating the motivational component of learning (Hikosaka et al., 1999). The complex bilateral interactions between cortical areas and the BG constitute a circuit known as the corticostriatal loop, which is believed to work in parallel with the corticocerebellar loop.
The PFC and the PPC are mainly engaged in explicit learning processes that are attentionally demanding and require working memory (Eliassen, Souza, & Sanes, 2001; Lewis & Miall, 2003).
Additionally, the parietal cortex is considered to play a crucial role in the integration of somatosensory and visual information and in preparing multimodal sensorimotor input for the PMC (Hardwick et al., 2013; Lewis & Miall, 2003). Furthermore, the parietal cortex is known to be engaged in the delayed recall of learned motor sequences (Doyon, Penhune, & Ungerleider, 2003).
Although this section is intended to be a brief general overview of the functions of different brain areas, the engagement of these areas changes with ongoing practice. This change over time has a strong implication for our understanding of the mechanism underlying motor sequence learning and, furthermore, has implications for practical rehabilitation. Therefore, we will review the current knowledge about learning stages in motor sequence learning in the next section and will then endeavor to discuss the contribution of lesion studies to this topic.
4. TEMPORAL STAGE MODEL OF MOTOR SEQUENCE LEARNING
Current theoretical models suggest that the process of motor sequence learning can be divided into different temporal stages (Doyon, 2008; Doyon et al., 2009; Hikosaka et al., 1999; Karni et al., 1998; Penhune & Steele, 2012). These stages differ in behavioral parameters, such as the amount of learning and the contribution of different components to the learning process (spatial‐sequential or motor component; Doyon et al., 2009; Karni et al., 1995; Karni et al., 1998; Willingham, 1998; Figure 1). Additionally, the contributions of specific brain areas and brain networks to the learning process differ between learning stages (Figure 2).
Figure 2.
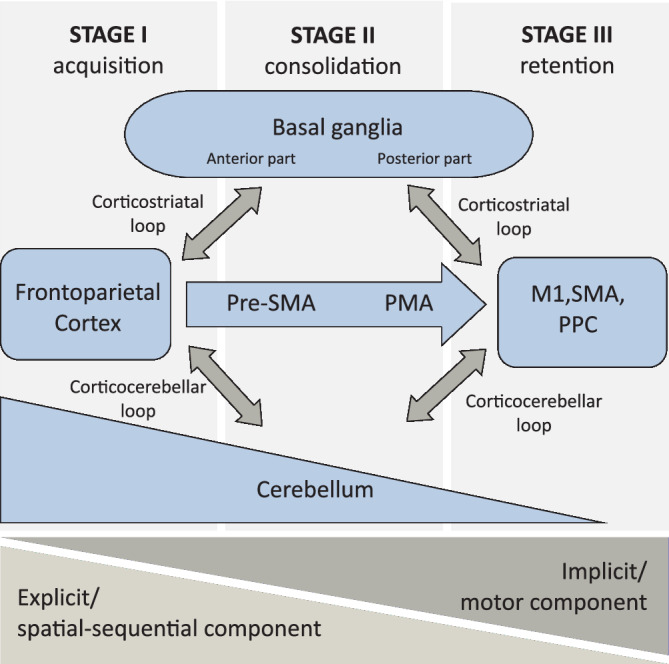
Schematic representation of the involvement and the interaction of brain areas in the three stages of motor sequence learning. Abbreviations: M1, primary motor cortex; PMA, premotor area; PPC, posterior parietal cortex; pre‐SMA, pre‐supplementary motor area; SMA, supplementary motor area)
Several authors propose a differentiation into an early phase, a consolidation phase and a slow learning or retention phase (Censor, Sagi, & Cohen, 2012; Doyon et al., 2003; Karni et al., 1998; Penhune & Steele, 2012), as we will introduce below.
4.1. Stage I—The early learning phase of motor sequence learning
In the early learning phase, a sequence is acquired quickly and flexibly, which allows rapid performance improvement. This performance improvement is mainly caused by improved encoding of the movement in spatial coordinates and therefore affects the spatial‐sequential component of the task (Doya, 2000; Hikosaka et al., 2002).
Essential brain regions involved in this early learning process comprise the DLPFC and the PPC. Both areas maintain strong connections to the anterior part of the BG, especially the head of the caudate nucleus (Hikosaka et al., 1999; Hikosaka et al., 2002; Janacsek & Nemeth, 2013; Penhune & Steele, 2012). This corticostriatal loop is called the “associative” circuit (Penhune & Steele, 2012) and is particularly engaged in early learning and explicit learning tasks, when the spatial‐sequential component is consciously learned and active attention and working memory are needed (Eliassen et al., 2001; Hikosaka et al., 1999; Janacsek & Nemeth, 2013; Jenkins, Brooks, Nixon, Frackowiak, & Passingham, 1994). Accordingly, deficits in learning new sequences were demonstrated in monkeys after excitotoxic lesions of the PFC (Collins, Roberts, Dias, Everitt, & Robbins, 1998) and after reversible blockage of the anterior striatum (Miyachi, Hikosaka, Miyashita, Kárádi, & Rand, 1997).
The corticostriatal loop (or associative circuit) works in parallel with the cortico‐cerebellar loop, which is also of critical importance in early learning. There is evidence that cerebellar connections to the parietal, premotor and frontal cortex are important for successful learning in this stage (Clower, Dum, & Strick, 2005; Diedrichsen, Balsters, Flavell, Cussans, & Ramnani, 2009).
As described above, the corticocerebellar loop allows predictive control by a feedforward mechanism in which the current sensory state and cortical motor commands are combined to forecast the future state of the body (Bastian, 2006). A correct forecast is essential for performing a context‐dependent sequence of movements. The involvement of the corticocerebellar loop and the number of necessary corrections scale with subjective task difficulty and the rate and magnitude of errors. Accordingly, the cerebellum is mainly activated in the early stages of learning, when the internal model to forecast the next body state/movement within the sequence is error‐prone (Doyon et al., 2002; Lehéricy et al., 2005; Wu, Kansaku, & Hallett, 2004).
The early spatial‐sequential part of learning proceeds in an effector‐unspecific manner. Sequential movements are transferred into a spatial coordinate frame, and the sequence is learned mainly independently of the body part that executes it (Censor, 2013; Hikosaka et al., 2002). Storage of motor information in the later learning stages, however, seems to be effector‐specific (Japikse, Negash, Howard, & Howard, 2003). Therefore, the effectiveness of the transfer of learning between the two hands is most significant in this early learning phase and decreases with ongoing learning (Japikse et al., 2003).
4.2. Stage II—The consolidation phase of motor sequence learning
The second phase is characterized by stabilization of the learned motor sequence and is called the “consolidation” phase. Performance improves incrementally by training and gains increased resistance to interference. This process is highly dependent on the ability to chunk sequences into smaller subsequences, which is accomplished by the BG.
The transition from the early learning phase to the consolidation phase is characterized by a slowing down of the learning speed (Doyon & Benali, 2005; Hikosaka et al., 1999).
The pre‐SMA is an important node associated with the transition from Stage I to Stage II. The major function of the pre‐SMA seems to be mediation between spatial sequence processing and sensorimotor movement integration (Nakahara et al., 2001). Hence, the pre‐SMA is active when one must rely on spatial processes and when motor output must be determined and updated by incoming sensory input (Hikosaka et al., 1999; Nakahara et al., 2001), linking conditional rules to actions (Hardwick et al., 2013). This assumption is consistent with anatomical connections projecting from the DLPFC via the pre‐SMA to the SMA (Luppino, Matelli, Camarda, & Rizzolatti, 1993; Tanji, 1994).
The consolidation process primarily includes the encoding of motor associations and the forming of motor chunks and is based mainly on a corticostriatal loop circuit comprising parts of the BG (especially the putamen) and the premotor–motor cortex (especially, the SMA; Hikosaka et al., 1999). In particular, the posterior parts of the putamen and caudate seem to play a central role in the chunking of information. Chunking simplifies the storage and recall of learned sequenced tasks, which is necessary due to the limited capacity of working memory (Fonollosa, Neftci, & Rabinovich, 2015). In addition to the practice‐related consolidation of motor sequences, the BG seem to have a special role in offline consolidation. This concept is supported by imaging studies demonstrating that activation of the BG (especially the putamen) increases after sleep following a motor sequence learning task (Debas et al., 2010). Likewise, it was shown that offline motor consolidation during sleep was associated with increased integration within the corticostriatal network (Albouy et al., 2008; Debas et al., 2014).
During the transition to Phase II, cerebellar activation decreases, while striatal activation increases (Doyon et al., 2002; Lehéricy et al., 2005; Wu et al., 2004). The shift in activity from the cerebellar‐cortical network toward a striatal‐cortical network is thought to correspond to an improved cerebellar model to forecast movements and an associated decrease in the necessity for error correction and model updates.
4.3. Stage III—The retention phase of motor sequence learning
The transition between Stage II and Stage III is very fluent, which makes a clear differentiation difficult. At some point, however, learning enters a late, slow phase, which is called the “automatization” phase. At this point, performance will be optimized with a slow learning rate. The movement execution becomes “automatic” and can be performed with decreasing attention or entirely implicitly (Karni et al., 1998).
In this process, the motor cortex is of particular importance. The M1, the PMC, and the PPC are considered important hubs in the late stage of motor sequence learning. It has been shown that all three areas are engaged in the delayed recall of learned motor sequences (Doyon et al., 2003). While the PMC is associated with the integration of visual and sensorimotor information and the formation of motor routines, the primary motor cortex is thought to regulate and store use‐dependent muscle synergies, creating “motor maps” to optimize and precise sequential movements (Censor et al., 2012; Hardwick et al., 2013; Penhune & Steele, 2012). Hence, the motor cortex is activated when a sequence is learned and a movement becomes “automatic.”. After the motor map is optimized, the information becomes robust and steady.
Furthermore, it was shown in monkeys that neurons of the SMA have come to represent a learned sequence after extensive training (Tanji, 1994). The involvement of the SMA in the representation of well‐learned motor sequences is also supported by studies in humans comparing well‐learned sequences to those that are not well learned (Gordon, Lee, Flament, Ugurbil, & Ebner, 1998; Hund‐Georgiadis & von Cramon, 1999).
Beyond the motor cortex, there is some evidence that even in this late learning stage, cerebellar loop structures might contribute by correcting errors. Despite the well‐established finding that cerebellar activation is mainly present during the fast learning phase and decreases over time (Doyon et al., 2002; Lehéricy et al., 2005; Wu et al., 2004), some studies also demonstrated that the dorsal parts of the dentate nucleus are especially active in the late stage of learning. Hikosaka and coworkers hypothesized a feedback mechanism for fine‐tuning of velocity, force and timing in the late stage of well‐learned movements; this mechanism was hypothesized to be implemented by a loop circuit between the anterior cerebellum and the motor cortices (Hikosaka et al., 1999). However, there seems to be no direct storage of information or motor memory in cerebellar structures (Doyon et al., 2003; Penhune & Steele, 2012).
4.4. Summary
Early stages of sequence learning require a mainly frontoparietal “associative” network (especially, prefrontal and parietal cortex), which is interlinked with the anterior BG on the one hand (corticostriatal loops) and with the cerebellum on the other hand (corticocerebellar loops). While corticostriatal loops are essential in active attention and working‐memory demands, the cerebellar loop is believed to play an important role due to its function in predictive control and adaptive optimization of movement performance.
Over the course of training, the activation shifts to a sensorimotor circuit linking the posterior part of the BG with various motor cortical areas (especially, the SMA). These connections are thought to be mainly responsible for encoding motor associations and forming motor chunks. Hence, BG function is crucial for the consolidation phase of learning. When a sequence is well learned, storage seems to take place in primary motor cortex areas (M1) and the SMA, which are therefore responsible for delayed recall.
The transitions between the different learning stages are fluent, and to a certain extent, the learning processes can occur in parallel. The proportions of activation during the different stages are also dependent on task demands. Even if the learning modes blend together, it can be observed that early and fast learning requires predominantly explicit processes, while the slow or late learning stage is mainly dependent on implicit learning.
5. CONTRIBUTIONS OF STROKE STUDIES TO THE PRESENT UNDERSTANDING OF MOTOR SEQUENCE LEARNING
Based on our current knowledge regarding the functioning and interaction of individual brain areas within the cerebral network, one might expect that localized lesions of specific brain regions might interfere with different aspects of motor sequence learning. However, the question of how a lesion might impair motor sequence learning is highly nontrivial because a lesion affects not only the function of the lesioned area but also the function of all directly or indirectly connected areas. It is therefore impossible to predict the behavioral consequences of brain lesions based purely on our understanding of network functioning in healthy subjects. This question can be answered only by investigating motor sequence learning in patients after structural brain damage. Studying the impact of lesions on network functioning is not only crucial for our pathophysiological understanding but also of immense clinical relevance, especially concerning poststroke rehabilitation strategies.
Multiple studies have investigated the effects of structural lesions on motor sequence learning using the affected and unaffected hands (see Table 1). The benefit of investigating the affected hand is the clinical importance. It allows us to investigate the difficulties that patients encounter in learning a motor sequence task in its full complexity, and it also allows us to test the effectiveness of possible interventions. Most of the available studies have investigated sequence‐specific learning as defined by the difference in reaction time or error rate between random and repeated sequences. A major problem in interpreting the results of such studies is the differentiation of those deficits that are attributable to pure motor impairment and the inability to optimize motor parameters and those deficits that result from an impaired ability to learn the spatial/sequential components. Therefore, multiple studies have investigated motor sequence learning after stroke not in the affected hand but in the unaffected hand. Although primary motor abilities and learning of simple motor responses are highly lateralized functions, learning of spatial/sequential parameters is considered a more global function with a high level of transfer of sequence knowledge from one hand to the other (Japikse et al., 2003). Therefore, utilizing the unaffected hand allows researchers to investigate impairments of motor sequence learning per se (largely independent of pure motor impairments and impairments in the optimization of motor components).
Table 1.
Studies investigating the effects of stroke on motor sequence learning
| Author, year | Lesion location | n | Lesion side | Stroke stage | Method | Tested hand | Endpoint | Learning type | Outcome/conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Boyd & Winstein, 2001 | SMC, pons | 12 | Both, unilateral | Chronic (>6 months) | SRTT, with/ without EI | Unaffected | RT change | Implicit and explicit | Impaired implicit motor‐sequence learning in stroke, EI prior to physical practice benefits implicit learning |
| Boyd & Winstein, 2003 | SMC | 10 | Both, unilateral | Chronic (>6 months) | SRTT, with/ without EI | Unaffected | RT change | Implicit and explicit | Preserved implicit sequence learning, but EI degraded both performance and learning |
| Boyd & Winstein, 2004 | BG | 10 | Both, unilateral | Chronic (>6 months) | CTT, with/ without EI | Unaffected | RMSE changes | Implicit and explicit | No improvement of implicit learning by providing EI |
| Boyd, Quaney, Pohl, & Winstein, 2007 | Cortical / subcortical / both | 28 | Both, unilateral | Chronic (>6 months) | SRTT/ SHMT | Unaffected | RT change | Implicit | Impaired implicit sequence learning in moderate‐stroke group, performance of mild‐stroke and HC dependent on type of task |
| Boyd et al., 2009 | BG | 13 | Both, unilateral | Chronic (>6 months) | SRTT | Unaffected | RT change | Implicit | Impaired implicit sequence learning and chunking in basal ganglia stroke |
| Dirnberger, Novak, & Nasel, 2013 | Cerebellum | 10 | Both, uni‐ or bilateral | Chronic (>6 months) | SRTT | Both, simultaneously | RT change | Implicit | Impaired implicit sequence learning in cerebellar stroke patients |
| Dovern et al., 2015 | MCA (no further information) | 24 | Left | Subacute/chronic (>4 days) | SRTT | Unaffected | RT change | Implicit | Preserved implicit sequence learning only when both spatial and temporal sequence information are provided |
| Dovern et al., 2017 | MCA (no further information) | 12 | Left | Subacute/chronic (129 ± 187d) | SRTT variant | Unaffected | RT change | Implicit | Preserved implicit sequence learning in stroke, even if no predictable temporal information is available, generally slower RT in stroke |
| Exner, Weniger, & Irle, 2001 | Thalamus | 15 | Both, uni‐ or bilateral | Chronic (>6 months) | SRTT variant | “Right or left hand” | RT change | Implicit | Impaired implicit learning in thalamus as well as in BG lesions (esp. lesions located in ventral portions of the thalamus) |
| Fleming, Newham, & Rothwell, 2018 | Cortical / subcortical / infratentoriell | 12 | Both, unilateral | Chronic (>6 months) | Reaching for targets on a monitor | Affected | OT, PL, speed | Explicit | Impaired sequence specific learning in stroke |
| Gomez Beldarrain, 1999 | Prefrontal cortical | 22 | Both, unilateral | Immediately after diagnosis/chronic | SRTT variant | Both, simultaneously | RT change, errors | Implicit | Impaired implicit sequence learning in PFC lesion, larger lesions (>2 cm) significantly more impaired |
| Gomez Beldarrain, Gafman, Ruiz de Velasco, Pascual‐Leone, & Garcia‐Monco, 2002 | Prefrontal cortical | 25 | Both, uni‐ or bilateral | Chronic (>24 months),1x < 12 months | SRTT / PTT | Both, simultaneously | Change of RT (SRTT) / RMSE (PTT) | Implicit and explicit | Impaired implicit and explicit sequence learning in PFC lesioned patients |
| Gomez Beldarrain et al., 2008 | Prefrontal | 14 | Both, uni‐ or bilateral | Chronic (>12 months), 1x < 12 months | SRTT variant before and after sleep | “Preferred” | RT change | Implicit | Impaired sequence learning in PFC and parietal lesion, benefit from a night of sleep only in patients with prefrontal lesions |
| Meehan, Randhawa, Wessel, & Boyd, 2011 | Subcortical | 9 | Right | Chronic (>12 months) | CTT | Affected | RMSE change | Implicit | Preserved implicit sequence motor learning, but more errors in stroke group |
| Orrell, Eves, Masters, & MacMahon, 2007 | “Anterior circulation system” | 7 | Right | Chronic (>12 months) | SRTT variant | Unaffected | RT change | Implicit | Preserved implicit sequence learning but generally slower RT in stroke |
| Pohl, McDowd, Filion, Richards, & Stiers, 2001 | No information given | 47 | Both, unilateral | Chronic (>6 months) | Hand movement sequence | Unaffected | RT change | Implicit | Preserved implicit sequence learning, but generally slower RT in stroke |
| Pohl, McDowd, Filion, Richards, & Stiers, 2006 | No information given | 37 | No information given | Subacute (18–45 d) | Hand movement sequence | Unaffected | RT change | Implicit | Preserved implicit sequence learning in mild and moderate stroke, generally slower RT in moderate stroke group |
| Rösser et al., 2008 | Cortical / subcortical | 18 | No information given | Chronic (>12 months) | SRTT variant | Affected | RT change | Implicit | L‐Dopa treatment improves procedural learning capacity in chronic stroke patients |
| Shin, Aparicio, & Ivry, 2005 | BG | 4 | Both, unilateral | No information given | SRTT | Both, separately | RT learning score (latency and accuracy) | Implicit | Preserved implicit sequence learning (spatial and temporal) in BG stroke for ipsi‐ and contralateral hand |
| Siengsukon & Boyd, 2009 | Cortical / subcortical | 40 | Both, unilateral | Chronic (>12 months) | CTT with / without night of sleep | Unaffected | RMSE change | Implicit and explicit | Sleep improved offline consolidation in stroke patients in explicit and implicit tracking task |
| Vakil, Kahan, Huberman, & Osimani, 2000 | BG | 16 | Both, unilateral | No information given | SRTT (motor + non‐motor) | RH (five affected/11 unaffected) | RT change | Implicit | Impaired implicit sequence learning (motor and non‐motor) in BG stroke while declarative learning is preserved |
| Wadden et al., 2015 | Subcortical | 9 | Right | Chronic (>6 months) | CTT | Affected | RMSE change | Implicit | Preserved implicit sequence learning but higher RMSE in stroke |
| Wadden et al., 2017 | MCA (no further information) | 14 | Right | Chronic (no exact information) | CTT | Affected | RMSE change | Implicit | Impaired implicit sequence learning in stroke, rate of motor skill acquisition during practice predictive for motor learning ability |
| Zimerman et al., 2012 | Subcortical | 12 | Both, unilateral | Chronic (>12 months) | MST with/ without tDCS | Affected | Number of correct sequences | Explicit | tDCS facilitated explicit sequence learning |
Abbreviations: BG, basal ganglia; CTT, continuous tracking task; EI, explicit information; HC, healthy control; MST, manual sequence task; OT, onset time; PL, path length; PTT, pursuit tracking task; RT, response time; RH, right handed; SHMT, serial hand movement task; SMC, sensorimotor cortex; SRTT, serial reaction time task.
5.1. Is motor sequence learning preserved after stroke?—A general overview
As introduced above, several studies have investigated motor sequence learning in the affected or the unaffected hand.
Concerning the affected (contralesional) hand, the results are heterogeneous. Some studies conclude that motor sequence learning is preserved (Meehan et al., 2011; Shin et al., 2005; Wadden et al., 2015), while other studies find an impairment of motor sequence learning for the affected hand after stroke (Dirnberger et al., 2013; Exner et al., 2001; Fleming et al., 2018; Gomez Beldarrain, 1999; Gomez Beldarrain et al., 2002; Vakil et al., 2000; Wadden et al., 2017). All available examples of these studies investigated patients in the chronic phase of stroke.
While two studies mainly addressed explicit learning (Fleming et al., 2018; Zimerman et al., 2012), one study investigated both learning modes (Gomez Beldarrain et al., 2002), and the majority of studies tested implicit learning capacity using the SRTT or the CTT.
Concerning explicit learning, two of the abovementioned studies revealed an impairment in explicit motor learning after stroke (Fleming et al., 2018; Gomez Beldarrain et al., 2002). One study reported a beneficial effect of transcranial direct current stimulation on the impaired capacity for explicit motor learning (Zimerman et al., 2012).
Regarding implicit sequence learning, the results are more divergent than those for explicit learning. Six studies demonstrated an impairment in implicit sequence learning for the affected hand (Dirnberger et al., 2013; Exner et al., 2001; Gomez Beldarrain, 1999; Gomez Beldarrain et al., 2002; Vakil et al., 2000; Wadden et al., 2017), while three others reported that implicit motor learning was preserved after stroke (Meehan et al., 2011; Shin et al., 2005; Wadden et al., 2015). Additionally, a recent meta‐analysis from 2017 investigated the question of whether implicit motor learning is preserved after stroke. The authors found insufficient evidence that stroke patients can implicitly learn with the affected side. No significant difference was found between implicit and explicit sequential motor learning after stroke (Kal, Houdijk, et al., 2016; Kal, Winters, et al., 2016).
Regarding the effect of stroke on the unaffected, ipsilesional hand, there is some evidence that implicit motor sequence learning is possible after stroke (Boyd & Winstein, 2003; Dovern et al., 2015; Dovern et al., 2017; Orrell et al., 2007; Pohl et al., 2001; Shin et al., 2005). This result was also found in the meta‐analysis by Kal et al., who stated that stroke patients are able to learn implicitly on the unaffected side (Kal, Houdijk, et al., 2016; Kal, Winters, et al., 2016). However, multiple studies revealed impairment in implicit motor sequence learning (Boyd et al., 2009, Boyd et al., 2007; Boyd & Winstein, 2001; Dirnberger et al., 2013; Exner et al., 2001; Gomez Beldarrain, 1999; Gomez Beldarrain et al., 2002; Vakil et al., 2000).
With respect to explicit motor learning, the meta‐analysis found no significant difference between implicit and explicit sequential motor learning for the unaffected hand (Kal, Houdijk, et al., 2016; Kal, Winters, et al., 2016).
There are some available data suggesting a correlation between stroke severity and the degree to which motor sequence learning is impaired (Boyd et al., 2007; Gomez Beldarrain, 1999; Pohl et al., 2001). This correlation might also affect the ipsilesional hand. In multiple studies, patients with different degrees of stroke severity (“mild” vs. “moderate”, assessed by scores on the Orpington Prognostic Scale) showed different amounts of absolute improvement in the reaction time of trained, patterned sequences (Boyd et al., 2007; Pohl et al., 2001). In contrast, another study found no difference in sequence‐specific learning between mildly and moderately affected patients, while there was an overall slowing of reaction time in the more severely affected patients (Pohl et al., 2006). Limitations of these studies include the lack of information on the exact location and extent of each lesion. The effects of stroke severity on implicit learning might also be influenced by task context. Mildly affected patients showed greater improvement in the serial hand movement task than in the SRTT, while patients with moderate stroke did not show any difference between the two test modalities (Boyd et al., 2007). The various results of different test modalities emphasize the impact of the type of motor task that is tested, with the tasks possibly assessing different qualities of motor learning that make different demands on working memory.
Despite the heterogeneity of the results, there is a tendency for motor sequence learning to be more impaired in the affected hand than in the unaffected hand, while the unaffected hand also demonstrates at least partly impaired function in some studies. However, a general finding of nearly all studies is a increased reaction time and a permanently increased error rate in stroke patients compared to healthy controls, irrespective of the lesion location, the investigated side or the learning stage (Boyd et al., 2007; Boyd & Winstein, 2003; Dovern et al., 2017; Fleming et al., 2018; Gomez Beldarrain et al., 2002; Meehan et al., 2011; Orrell et al., 2007; Pohl et al., 2006; Siengsukon & Boyd, 2009; Vakil et al., 2000; Wadden et al., 2015).
5.2. Contributions of stroke studies to the temporal stage model of motor sequence learning
In this section, we will analyze the effects of cerebral lesions on motor sequence learning in further detail. We are particularly interested in the relationship between the location of the lesion and its impact on motor sequence learning. However, as described above, the function and importance of a particular brain area for motor sequence learning change across different learning stages. Lesions might, therefore, compromise motor sequence learning differently in different learning stages. It is crucial to differentiate the behavioral consequences of specific lesions in relation to the stages of learning. This differentiation is especially challenging because the available studies do not address the effects of lesion location on learning stages as their primary research questions.
5.2.1. Stage I—Contributions of stroke studies to knowledge of the early learning phase of sequential motor learning
The PFC is known to be one of the core areas involved in early learning. Accordingly, early learning was found to be significantly reduced in patients with prefrontal lesions (Gomez Beldarrain, 1999; Gomez Beldarrain et al., 2002; Gomez Beldarrain, Astorgano, Gonzalez, & Garcia‐Monco, 2008). Prefrontal lesions caused disturbances in explicit learning tasks as measured by a pursuit tracking task (Gomez Beldarrain et al., 2002) as well as in tasks that were mainly implicit (Gomez Beldarrain, 1999). Overall, impairments in motor sequence learning were more severe in the contralateral hand than in the ipsilateral hand and were correlated with lesion size (Gomez Beldarrain, 1999).
Deficits in implicit learning were found to extend beyond the very early learning stage (Gomez Beldarrain, 1999; Gomez Beldarrain et al., 2002). The authors suggested not only that the involvement of the PFC extends beyond the early learning stage but also that different parts of the PFC contribute diversely to the different learning modes and learning stages (Gomez Beldarrain et al., 2002). While the dorsolateral PFC is heavily involved in the network subserving working memory (Manoach et al., 1999), the frontopolar PFC is heavily involved in planning (Koechlin, Basso, Pietrini, Panzer, & Grafman, 1999), suggesting that deficits in early and explicit learning are more pronounced after frontopolar than dorsolateral PFC lesions, while the reverse is true of deficits in implicit learning and during later stages.
Current knowledge regarding lesions in the PFC suggests that every learning task, even if it mainly involves implicit learning, requires prefrontal activation with active attention in the very beginning. If this early learning stage is disturbed, the transition into the consolidation phase may also be impaired and delayed. Accordingly, patients with prefrontal lesions showed greater benefit than healthy controls from a night of sleep (Gomez Beldarrain et al., 2008).
A reduction in early sequence‐specific motor learning was also demonstrated in patients with lesions in the ventral portion of the thalamus, an area that is strongly connected to the PFC (Exner et al., 2001). The authors speculated about the importance of individual thalamic nuclei, suggesting that they might be specific to sequence learning. However, given the importance of thalamic nuclei for cortico‐cortical communication, we suggest instead that these results simply reflect an impairment of the thalamic contribution to driving and modulating cortico‐thalamo‐cortical information transfer.
Concerning lesions of the BG, some available data suggest that early implicit sequence learning is not seriously impaired for the unaffected hand (Dovern et al., 2015; Shin et al., 2005; Vakil et al., 2000) or the affected hand (Shin et al., 2005; Vakil et al., 2000). Accordingly, the administration of levodopa to stroke patients did not improve implicit learning in the very early stages, while improvements were found in later stages (Rösser et al., 2008). These findings are in line with a growing body of literature suggesting that the BG are mainly active in the consolidation of learned sequences. In contrast, one study reported a diminished learning rate in patients with chronic BG lesions, even in the early acquisition phase, as measured by diminished RT changes for repeated sequences (Boyd et al., 2009).
The effect of cerebellar lesions on early learning has not been thoroughly studied. One study found that implicit learning was preserved in the early learning stage, whereas impairments were found in later stages (Dirnberger et al., 2013). This finding seems counterintuitive given what is known about the function of the cerebellum in healthy subjects. One possible explanation might be the diversity of cerebellar lesion locations, possibly mainly affecting regions that are known to be involved in late learning stages, such as the dentate nucleus. The authors further noted that there is a disturbance especially in perception‐based mechanisms (Dirnberger et al., 2013), underlining the role of the cerebellum in the “predictive” mechanism of movement control (Bastian, 2006).
Several studies were unable to find significant impairments of sequential motor learning in the early learning phase after stroke, either for the affected hand (Fleming et al., 2018; Meehan et al., 2011; Siengsukon & Boyd, 2009) or for the unaffected hand (Dovern et al., 2017; Orrell et al., 2007; Pohl et al., 2001; Pohl et al., 2006). One important reason for this lack of a significant effect might be the selection of patients. Multiple studies have selected patients on the basis of anamnestic information on stroke and clinical impairments. These studies have often used clinical scores such as the Orpington Prognostic Scale (Pohl et al., 2001, Pohl et al., 2006), the Fugl‐Meyer Assessment (Fleming et al., 2018), or the MRC Scale (Rösser et al., 2008). These scores, however, do not test for difficulties in learning. They are mainly sensitive to lesions in brain areas responsible for gross motor movements and are particularly altered by lesions of the primary motor cortex itself or its downstream connections. Thus, the lesion locations in the cited studies are strongly biased toward lesions of these areas. The negative results therefore suggest that brain lesions of the motor cortex or its downstream connections exert limited influence on early motor learning. This was observed mainly in the chronic stages of stroke but also seemed to apply to patients in the subacute stroke stage (<45 days after stroke), who also showed no difference in sequence‐specific implicit learning compared to healthy controls (Pohl et al., 2006).
Overall, many studies reported fully or at least partly preserved motor sequence learning. Interestingly, there are no reports of patients with a complete inability to achieve motor learning. Hence, it can be assumed that there is no “all‐or‐nothing principle,” even when one important part of the responsible network is destroyed. This further indicates that sequential motor learning in the early stages is performed by a widespread network of brain regions without a central region mandatory for learning success and that lesions to brain areas engaged in this process can be (at least partly) compensated.
5.2.2. Stage II—Contributions of stroke studies to knowledge of the consolidation phase of sequential motor learning
Multiple studies have investigated motor sequence learning after stroke during the consolidation phase using an extended version of a sequence task or by investigating sequence practice over several days.
Impaired implicit and explicit motor sequence learning was consistently found in patients with lesions of the BG (Siengsukon & Boyd, 2009; Vakil et al., 2000). To further elucidate the underlying mechanisms, analyses have focused on the individual organization of learned responses and the transition from one sequence element to the next. The analysis of this transition allows the identification of subsequences (chunks) of movements (Miller, 1994; Povel & Collard, 1982; Rosenbaum, Kenny, & Derr, 1983). Studies have found substantial differences between patient and control groups in organizing sequential responses (Boyd et al., 2009; Dovern et al., 2015). It was demonstrated that the ability to chunk elements of a repeated, implicitly presented sequence into functional subsequences of movements is impaired after BG lesions (Boyd et al., 2009). Patients organized sequences to a lesser extent than healthy controls, and the chunks were also shorter (Boyd et al., 2009). This impairment was demonstrated for the affected as well as the unaffected hand and even if the patients did not differ from healthy controls during the early learning stage (Boyd et al., 2009; Vakil et al., 2000).
A further study augmented these results by investigating the ability to learn a motor sequence in patients after left MCA lesions by giving them implicit multimodal (spatial and temporal) information (Dovern et al., 2015). The learning progress did not differ between patients and controls. However, patients demonstrated an impaired ability to form independent spatial and temporal sequence representations. The underlying subchunks were found to be more rigid and inflexible than those of healthy controls (Dovern et al., 2015). Imaging analyses revealed that diminished learning scores were strongly associated with lesions of the striatum. These studies demonstrate that the chunking of subsequences might depend on the complexity of the given multidimensional information, which stroke patients learn in a more rigid way than healthy controls. The associations between multiple information dimensions were further investigated by a study that demonstrated preserved implicit learning effects in the event of consistently missing temporal information (Dovern et al., 2017). This further suggests that patients with BG lesions do not show decreased learning effects due to missing additional information but that they are particularly susceptible to context disruptions, which then decrease learning effects (Dovern et al., 2017).
The critical role of the BG in chunking is supported by the finding of faster responses on learned sequences (but not random sequences) in the consolidation phase after administration of levodopa than without levodopa in stroke patients (Rösser et al., 2008).
Results similar to those of BG lesions were also found in lesions of the ventral thalamic nuclei (Exner et al., 2001), with decreased implicit learning not only in the early learning stage but also in the consolidation phase (Exner et al., 2001). However, the effects of thalamic lesions are less investigated than those of BG lesions. It is tempting to speculate that this effect is caused by the high level of interconnection between the thalamus and the BG (Alexander, DeLong, & Strick, 1986; Middleton & Strick, 2000).
Interestingly, when additional explicit information is given to patients with lesions affecting the BG or sensorimotor cortical areas, this information seems to have an adverse effect on implicit learning during the consolidation phase (Boyd & Winstein, 2003; Boyd & Winstein, 2004). Contrary to these findings, a further investigation found an improvement in response to additional explicit information in chronic unilateral stroke patients (11 sensorimotor areas, 1 pontine; Boyd & Winstein, 2001). To explain these findings, the authors suggested differences according to the exact lesion location, particularly with respect to M1, the PMC, and the SMA. In particular, the PMC might play a central role in the integration of explicit information into implicit motor performance due to its strong connections to prefrontal areas (e.g., the DLPFC) and to the caudate nucleus. Hence, a lesion that strongly affects the PMC might interfere with the benefit of additional explicit information for an implicitly formed motor plan. It was further suggested that the additional explicit information given to the subjects imposed an unmanageable working memory load that might interfere with the implicitly made movement plan. However, the small sample sizes in the patient groups that received explicit knowledge (n = 4 [Boyd & Winstein, 2001], n = 5 [Boyd & Winstein, 2003], n = 5 [Boyd & Winstein, 2004]) might have also contributed to these divergences.
Several studies have failed to show a significant poststroke impairment of motor sequence learning during the consolidation phase. Preserved learning was mainly found in studies that were not restricted to specific lesion locations. For instance, preserved implicit motor learning with the unaffected hand during the consolidation phase was found for lesions in the “anterior circulation” or “MCA” territory or for “cortical/subcortical” infarcts (Orrell et al., 2007; Pohl et al., 2001; Siengsukon & Boyd, 2009), but implicit learning was also preserved for the affected hand after subcortical lesions (Meehan et al., 2011; Wadden et al., 2015).
However, there are also studies available demonstrating an impairment of motor sequence learning during the consolidation phase without specifying the exact lesion location (Dovern et al., 2015; Wadden et al., 2017). In contrast to studies demonstrating preserved sequence learning, these studies used different methods of analysis or design. Instead of comparing the group difference in RT changes between random and predefined sequences, Wadden and colleagues analyzed group differences by fitting the learning progress with an exponential curve and included the behavioral parameter of individual motor impairment in the model (Wadden et al., 2017). They demonstrated impaired implicit motor sequence learning with the affected hand after subcortical lesions in the right hemisphere. However, a careful inspection of the available data suggests that the main difference between patients and controls is attributable more to the early learning phase than to the consolidation phase.
However, consolidation does not occur exclusively during extended practice. There is general agreement that the consolidation process of learned sequence information can also be improved by sleep. This process is called sleep‐dependent offline consolidation.
Expectably, BG lesions seem to impair offline consolidation (Dovern et al., 2015). This is consistent with the results of neuroimaging studies in healthy people, which showed increased integration in the corticostriatal network after sleep following a motor sequence‐learning task (Debas et al., 2010; Debas et al., 2014). A similar effect of impaired offline consolidation was also found after parietal lesions. In contrast, patients with prefrontal lesions seem to benefit from offline consolidation during sleep more strongly than healthy subjects (Gomez Beldarrain et al., 2008). These findings support the assumption that the parietal cortex is necessary in both the early and late stages of learning, while the PFC is mainly involved in early learning processes.
Without providing exact lesion information (cortical/subcortical), a further study demonstrated improved sleep‐dependent offline learning in stroke patients for explicit and implicit tasks compared to healthy subjects (Siengsukon & Boyd, 2009). No significant difference was found without sleep. The authors hypothesized a change in sleep structure after stroke with an impact on stroke recovery. However, no sleep measurements were performed to test this hypothesis.
5.2.3. Stage III—Contributions of stroke studies to knowledge of the late learning phase of sequential motor learning
Very limited data are available regarding potential impairments of stroke patients in the late learning phase of motor sequence learning. Studies have investigated motor sequence learning for a maximum of 5 days of training (Meehan et al., 2011; Wadden et al., 2015; Wadden et al., 2017). However, the question of whether a late learning stage is reached depends not only on the training time but also on the complexity of the task. All three cited studies investigated a CTT. It remains unclear whether, after 5 days of training on a CTT, the late learning stage is reached. The available data give some indications that the process remains in the consolidation phase, although the boundary between these learning stages is uncertain. However, even if those studies reached the late learning stage, the available data do not allow us to draw conclusions regarding specific effects of stroke on that stage.
One study tested delayed retention of the SRTT on day 15 after 2 days of practice in patients with right hemispheric lesions. No difference in retention was found between the stroke group and the control group (Orrell et al., 2007). Despite the prolonged time window of retention, the late learning phase is presumable not reached in only 2 days of practice.
5.2.4. Conclusions
Despite some heterogeneities, the results of studies that investigated patients with motor impairments due to brain lesions indicate impairments of motor sequence learning in the early learning stage by impaired integration of multimodal information for motor sequence learning, slowed general reaction time and impaired explicit learning. Stroke patients have greater difficulty in optimizing the motor control components of sequential movements than the sequential/spatial order of movements. There is consistent evidence that the consolidation phase is particularly impaired after BG lesions. This effect is probably due to impaired chunking of information. Chunks in patients with BG lesions seem to be more rigid, more sensitive to disruptions and less adaptable than those in healthy controls. There are some indications that MCA infarcts in general cause difficulties in integrating information necessary for building chunks of sequences during the consolidation phase. Decreased learning rates after the offline consolidation phase were found in striatal and parietal lesions but not in prefrontal lesions.
However, the heterogeneity and small sample sizes of the available studies render it difficult to draw clear conclusions.
5.3. Methodological considerations
From a theoretical perspective, it seems obvious that motor sequence learning after stroke must be impaired to some degree if lesions affect brain structures that are necessary for motor sequence learning. However, as outlined above, multiple studies failed to find clear evidence of impaired motor sequence learning after stroke (Meehan et al., 2011; Shin et al., 2005; Wadden et al., 2015). A recent meta‐analysis found insufficient evidence that stroke patients could implicitly learn with the affected side (Kal, Houdijk, et al., 2016; Kal, Winters, et al., 2016). Although the meta‐analysis was thoroughly performed, the underlying studies varied in so many aspects that results are difficult to combine. To better understand the reasons for the divergences in study results, it is necessary to discuss some methodological issues that, in our opinion, affect the interpretation of results and possible conclusions.
First, an important factor contributing to divergent results is the inhomogeneity of lesions across studies. There are multiple studies available that present no information on the method through which lesion location was determined (Dirnberger et al., 2013; Dovern et al., 2017; Fleming et al., 2018; Gomez Beldarrain et al., 2008; Meehan et al., 2011; Pohl et al., 2001; Rösser et al., 2008; Shin et al., 2005; Wadden et al., 2017; Zimerman et al., 2012). Some studies stated that infarctions were located in the “MCA territory” or “anterior circulation system” without further information (Orrell et al., 2007; Pohl et al., 2001; Pohl et al., 2006). Other studies provided some information on lesion location while mixing different lesion locations together (cortical/subcortical/both (Boyd et al., 2007), cortical/subcortical/infratentorial (Fleming et al., 2018), and cortical/subcortical (Rösser et al., 2008; Siengsukon & Boyd, 2009). Some studies restricted the lesions to subcortical locations (Meehan et al., 2011; Wadden et al., 2015; Zimerman et al., 2012). Only a few studies investigated lesion locations in a more specific way by analyzing the relationship between lesion location and performance in motor sequence learning (Wadden et al., 2017).
Aside from lesion locations, the available studies also varied in other characteristics, such as severity of stroke, time since stroke, sample size, task types, and duration of training. Moreover, it remains insufficiently understood how motor impairment of the affected arm interferes with and affects the results of sequence learning.
The chosen method for testing learning outcomes is another problem. Most studies used an SRT task and measured reaction time as the primary outcome parameter. SRTT is generally very susceptible to experimental changes that may explain some of the variability in the results (Willingham, Greenberg, & Thomas, 1997). In addition, there are methodological concerns regarding the interpretation of SRTT results. Stroke patients have been found to have a generally slower reaction time than healthy subjects (Boyd et al., 2007; Orrell et al., 2007; Pohl et al., 2001; Pohl et al., 2006). Patients were able to improve their reaction time by practicing patterned sequences, while the introduction of random sequences increased their reaction time (Pohl et al., 2001, Pohl et al., 2006). Most studies measured motor sequence learning as changes in reaction time for a learned sequence compared to an equal‐length random sequence. However, it remains unclear how an improvement in patients’ reaction time can be compared to the improvement of healthy controls when their baseline reaction time is different. As measured by the absolute decrease in reaction time, some patients improved more than the healthy subjects (Pohl et al., 2001, Pohl et al., 2006). As measured by percentage improvement, patients with longer baseline reaction time must improve by a greater absolute quantity to achieve the same percentage decrease, which further complicates comparisons. With respect to these methodological problems, the question of whether and to what degree sequence learning after stroke is impaired cannot be answered definitively in most studies. An important methodological advance can be found in a recent study that compared performance data between groups by fitting them to an exponential function. This method allows the rate of skill acquisition to be estimated and additionally allows an improved comparison of learning outcome parameters (Wadden et al., 2017). The authors found significantly slower rates of improvement in implicit sequence‐specific motor performance with the affected hand in stroke patients than in healthy controls.
Theoretical considerations and the limited available data suggest that impairments of sequential motor learning are influenced by stroke severity. However, the available stroke studies are biased concerning the severity of stroke, with a preference for mildly to moderately affected patients, while the ability of severely affected patients to learn motor sequences remains unknown. This is because it is quite difficult to investigate severely impaired patients, as the severity of impairment might interfere with the results in many dimensions (compromised execution due to motor impairment as well as additional neurological disabilities such as neglect, aphasia, apraxia, cognitive impairment, and general slowing of movements). There are also logistical concerns to address.
6. PRACTICAL CONCLUSIONS FOR PATIENT REHABILITATION
One of the most important concepts for practical rehabilitation is the differentiation between explicit and implicit motor learning, as these two forms of motor learning require different processing pathways and cerebral resources. Accordingly, cerebral lesions can affect one system more strongly than the other (Reber & Squire, 1998). For example, severe deficits in explicit learning have been reported after medial temporal lobe damage and in patients with amnesia, while implicit learning capabilities remained intact (Reber & Squire, 1998; Shadmehr et al., 1998). With respect to explicit learning deficits, there are multiple available studies demonstrating that the main process in explicit learning (the shift from using declarative knowledge and active attention toward using procedural knowledge) can be disturbed after stroke (Kal, Houdijk, et al., 2016; Kal, Winters, et al., 2016; Kleynen et al., 2013; Orrell, Masters, & Eves, 2009).
This is particularly problematic because deficits in the level of conscious control of movements are associated with the extent of functional impairment after stroke (Orrell et al., 2009; Stapleton, Ashburn, & Stack, 2001). Accordingly, the more severe the impairments, the less they are able to profit from explicit motor learning.
This problem affects motor rehabilitation, as most physical therapists provide mainly verbal or physical instructions on how a movement should be performed to improve motor performance, thereby engaging explicit motor learning (Durham, Van Vliet, Badger, & Sackley, 2009; Johnson, Burridge, & Demain, 2013). A disturbance in explicit motor learning might prevent patients from reaching a high level of automated motor performance for motor tasks that are initially highly dependent on working memory and conscious control.
The current studies on implicit motor learning are promising because they suggest that this process is preserved in most patients. However, a major problem here is that implicit motor learning after stroke was mostly investigated with the SRTT, which has little or no relevance to practical rehabilitation. It remains unclear whether the positive findings for implicit learning can be extended to more complex motor tasks, in which not only the sequence of movements but also the optimization of the movement parameters of the motor sequence must be considered. The quality of the available studies is mostly insufficient to derive precise recommendations for practical rehabilitation. Nevertheless, the available data suggest that rehabilitation strategies should be better adapted to individual needs based on lesion location and consecutive functional impairments.
Patients suffering from infarcts affecting frontoparietal regions with impairment in the early learning phase should be trained by using slow steps of explicit additional information with little attentional demand. Sequences should be simple so that the consolidation phase can be reached as fast as possible. In addition, rehabilitation approaches for these patients should also use learning strategies that involve implicit learning, rather than relying solely on explicit instructions. Possible and promising approaches are dual‐task learning, analogy learning or errorless learning (Kal, Houdijk, et al., 2016; Kal, Winters, et al., 2016; Kleynen, Braun, et al., 2014; Kleynen, Wilson, et al., 2014; Orrell, Eves, & Masters, 2006). There are few studies exploring these implicit approaches in stroke patients. Orrell et al. investigated the errorless learning strategy by using a dynamic balancing task (Orrell et al., 2006) and found a benefit of implicit motor learning techniques compared to the traditional discovery learning based on explicit rules. The analogy learning approach was investigated in a little pilot study by Kleynen et al. who concluded that analogy learning might be a feasible and useful intervention in stroke rehabilitation (Kleynen, Braun, et al., 2014; Kleynen, Wilson, et al., 2014). Nevertheless, further research concerning stroke patients is needed.
Patients suffering from BG lesions are impaired mainly in the consolidation phase. It remains unclear whether the use of the available attentional resources is beneficial or whether these patients should focus on simple sequences for consolidation. Additional time should be given to the chunking of information to avoid the usage of ineffectively short subchunks.
It also remains unclear whether the potential for interhemispheric transfer of sequence knowledge in healthy subjects can be used to improve motor learning in patients. If sequence learning for the affected hand is impaired, the patient can attempt to learn the sequence with the other hand, at least in the early phase of motor learning. Afterward, the training might be further practiced with the affected hand. However, there are no studies available that investigate this hypothesis. There are also no data available that investigate the impact of patient motivation and the functioning of the reward system on motor sequence learning.
7. PERSPECTIVES AND IMPLICATIONS FOR FUTURE RESEARCH
In the last decade, the body of knowledge regarding the effects of structural brain lesions on motor sequence learning has grown substantially. There is increasing understanding of the effects of a nonlocalized stroke with mild to moderate clinical impairments on the capacity for explicit and implicit motor sequence learning. However, as outlined above, we can further increase our understanding through studies that are less heterogeneous than the existing literature. First, there is an urgent need for studies that investigate motor sequence learning in patients with highly localized lesions. In this respect, the most consistent studies are available for BG lesions. These studies have contributed substantially to our understanding of BG function in motor sequence learning and have laid groundwork for our understanding of deficits in the chunking of information. Similar increases in understanding can be expected from studies investigating lesions in other key regions of the brain network responsible for motor sequence learning.
Most studies used tasks in which the complexity of the sequence was contrasted by the simplicity of its motor implementation (e.g., pressing of buttons). It has been shown in healthy subjects that sequence learning is strongly affected by the complexity of the stimulus–response association. The performance of a well‐synchronized but complex task requires the optimization of multiple parameters such as timing, velocity and force that contribute to performance. The effect of increased complexity of the stimulus–response association on motor sequence learning in stroke patients remains unknown but could provide important insights for the practice of rehabilitation.
It is also unknown whether impaired sequence learning with the affected hand can be improved by training with the unaffected hand in the early learning stages.
Another important question that we are only beginning to investigate is the influence of stroke on different learning stages. There are currently no studies available that focus on the role of lesion location on different learning stages. In particular, there are no studies available that use different learning strategies in different learning stages.
The influence of the structural integrity and functioning of the reward system on sequential motor learning in stroke patients is unknown.
Moreover, in the current state of research, studies without clear descriptions of the lesion locations do not contribute substantially to further insights. Instead, there is a desperate need for studies investigating more complex stimulus–response associations with more precise lesion mapping across different learning stages. Such studies have the potential to reveal the mechanisms underlying impaired sequence learning and insufficient rehabilitation results. Such studies might also contribute to the development of new therapeutic strategies that are more individualized than what is currently available.
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interest.
ACKNOWLEDGMENT
The authors received research grants from Bundesministerium für Bildung und Forschung BMBF (JenAge, 0315581), BMBF (Irestra, 16SV7209), Deutsche Forschungsgemeinschaft DFG (HHDP, FO 1738, WI 830/10‐2, and WI 830/11‐1/OrganAge and RTG1715), Else Kröner Foundation (AntiAge and Jena School for Ageing Research) and TMWWDG (ProExellenz, RegenerAging‐FSU‐I‐03/14). The funding sources had no involvement in the collection, analysis and interpretation of data, in the writing of the report or in the decision to submit the article for publication.
Dahms C, Brodoehl S, Witte OW, Klingner CM. The importance of different learning stages for motor sequence learning after stroke. Hum Brain Mapp. 2020;41:270–286. 10.1002/hbm.24793
Funding information Bundesministerium für Bildung und Forschung, Grant/Award Numbers: 16SV7209, 0315581; Deutsche Forschungsgemeinschaft, Grant/Award Numbers: FO 1738, WI 830/10‐2, WI 830/11‐1/OrganAge, RTG1715; Else Kröner‐Fresenius‐Stiftung; TMWWDG
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- Albouy, G. , Sterpenich, V. , Balteau, E. , Vandewalle, G. , Desseilles, M. , Dang‐Vu, T. , … Maquet, P. (2008). Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron, 58, 261–272. 10.1016/j.neuron.2008.02.008 [DOI] [PubMed] [Google Scholar]
- Alexander, G. E. , DeLong, M. R. , & Strick, P. L. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience, 9, 357–381. 10.1146/annurev.ne.09.030186.002041 [DOI] [PubMed] [Google Scholar]
- Baars, B. J. (2010). Cognition, brain, and consciousness. Elsevier, Amsterdam. 10.1016/C2009-0-01556-6 [DOI] [Google Scholar]
- Bastian, A. J. (2006). Learning to predict the future: The cerebellum adapts feedforward movement control. Current Opinion in Neurobiology, 16, 645–649. 10.1016/j.conb.2006.08.016 [DOI] [PubMed] [Google Scholar]
- Boyd, L. A. , Edwards, J. D. , Siengsukon, C. S. , Vidoni, E. D. , Wessel, B. D. , & Linsdell, M. A. (2009). Motor sequence chunking is impaired by basal ganglia stroke. Neurobiology of Learning and Memory, 92, 35–44. 10.1016/j.nlm.2009.02.009 [DOI] [PubMed] [Google Scholar]
- Boyd, L. A. , Quaney, B. M. , Pohl, P. S. , & Winstein, C. J. (2007). Learning implicitly: Effects of task and severity after stroke. Neurorehabilitation and Neural Repair, 21, 444–454. 10.1177/1545968307300438 [DOI] [PubMed] [Google Scholar]
- Boyd, L. A. , & Winstein, C. J. (2001). Implicit motor‐sequence learning in humans following unilateral stroke: The impact of practice and explicit knowledge. Neuroscience Letters, 298, 65–69. 10.1016/S0304-3940(00)01734-1 [DOI] [PubMed] [Google Scholar]
- Boyd, L. A. , & Winstein, C. J. (2003). Impact of explicit information on implicit motor‐sequence learning following middle cerebral artery stroke. Physical Therapy, 83, 976–989. 10.1093/ptj/83.11.976 [DOI] [PubMed] [Google Scholar]
- Boyd, L. A. , & Winstein, C. J. (2004). Providing explicit information disrupts implicit motor learning after basal ganglia stroke. Learning & Memory, 11, 388–396. 10.1101/lm.80104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor, N. (2013). Generalization of perceptual and motor learning: A causal link with memory encoding and consolidation? Neuroscience, 250, 201–207. 10.1016/j.neuroscience.2013.06.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor, N. , Sagi, D. , & Cohen, L. G. (2012). Common mechanisms of human perceptual and motor learning. Nature Reviews Neuroscience, 13, 658–664. 10.1038/nrn3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clower, D. M. , Dum, R. P. , & Strick, P. L. (2005). Basal ganglia and cerebellar inputs to ‘AIP.’. Cerebral Cortex, 15, 913–920. 10.1093/cercor/bhh190 [DOI] [PubMed] [Google Scholar]
- Collins, P. , Roberts, A. C. , Dias, R. , Everitt, B. J. , & Robbins, T. W. (1998). Perseveration and strategy in a novel spatial self‐ordered sequencing task for nonhuman primates: Effects of excitotoxic lesions and dopamine depletions of the prefrontal cortex. Journal of Cognitive Neuroscience, 10, 332–354. [DOI] [PubMed] [Google Scholar]
- Debas, K. , Carrier, J. , Barakat, M. , Marrelec, G. , Bellec, P. , Tahar, A. H. , … Doyon, J. (2014). Off‐line consolidation of motor sequence learning results in greater integration within a cortico‐striatal functional network. NeuroImage, 99, 50–58. 10.1016/j.neuroimage.2014.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debas, K. , Carrier, J. , Orban, P. , Barakat, M. , Lungu, O. , Vandewalle, G. , … Doyon, J. (2010). Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proceedings of the National Academy of Sciences, 107, 17839–17844. 10.1073/pnas.1013176107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen, J. , Balsters, J. H. , Flavell, J. , Cussans, E. , & Ramnani, N. (2009). A probabilistic MR atlas of the human cerebellum. NeuroImage, 46, 39–46. 10.1016/j.neuroimage.2009.01.045 [DOI] [PubMed] [Google Scholar]
- Diedrichsen, J. , & Kornysheva, K. (2015). Motor skill learning between selection and execution. Trends in Cognitive Sciences, 19, 227–233. 10.1016/j.tics.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnberger, G. , Novak, J. , & Nasel, C. (2013). Perceptual sequence learning is more severely impaired than motor sequence learning in patients with chronic cerebellar stroke. Journal of Cognitive Neuroscience, 25, 2207–2215. 10.1162/jocn_a_00444 [DOI] [PubMed] [Google Scholar]
- Dovern, A. , Fink, G. R. , Timpert, D. C. , Saliger, J. , Karbe, H. , Weiss, P. H. , & Koch, I. (2015). Timing matters? Learning of complex spatiotemporal sequences in left‐hemisphere stroke patients. Journal of Cognitive Neuroscience, 28, 223–236. 10.1162/jocn_a_00890 [DOI] [PubMed] [Google Scholar]
- Dovern, A. , Niessen, E. , Ant, J. M. , Saliger, J. , Karbe, H. , Fink, G. R. , … Weiss, P. H. (2017). Timing independent spatial motor sequence learning is preserved in left hemisphere stroke. Neuropsychologia, 106, 322–327. 10.1016/j.neuropsychologia.2017.09.030 [DOI] [PubMed] [Google Scholar]
- Doya, K. (2000). Complementary roles of basal ganglia and cerebellum in learning and motor control. Current Opinion in Neurobiology, 10, 732–739. [DOI] [PubMed] [Google Scholar]
- Doyon, J. (2008). Motor sequence learning and movement disorders. Current Opinion in Neurology, 21, 478–483. 10.1097/WCO.0b013e328304b6a3 [DOI] [PubMed] [Google Scholar]
- Doyon, J. , & Benali, H. (2005). Reorganization and plasticity in the adult brain during learning of motor skills. Current Opinion in Neurobiology, 15, 161–167. [DOI] [PubMed] [Google Scholar]
- Doyon, J. , Korman, M. , Morin, A. , Dostie, V. , Hadj Tahar, A. , Benali, H. , … Carrier, J. (2009). Contribution of night and day sleep vs. simple passage of time to the consolidation of motor sequence and visuomotor adaptation learning. Experimental Brain Research, 195, 15–26. 10.1007/s00221-009-1748-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon, J. , Penhune, V. , & Ungerleider, L. G. (2003). Distinct contribution of the cortico‐striatal and cortico‐cerebellar systems to motor skill learning. Neuropsychologia, 41, 252–262. 10.1016/S0028-3932(02)00158-6 [DOI] [PubMed] [Google Scholar]
- Doyon, J. , Song, A. W. , Karni, A. , Lalonde, F. , Adams, M. M. , & Ungerleider, L. G. (2002). Experience‐dependent changes in cerebellar contributions to motor sequence learning. Proceedings of the National Academy of Sciences, 99, 1017–1022. 10.1073/pnas.022615199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham, K. , Van Vliet, P. M. , Badger, F. , & Sackley, C. (2009). Use of information feedback and attentional focus of feedback in treating the person with a hemiplegic arm. Physiotherapy Research International, 14, 77–90. 10.1002/pri.431 [DOI] [PubMed] [Google Scholar]
- Eliassen, J. , Souza, T. , & Sanes, J. (2001). Human brain activation accompanying explicitly directed movement sequence learning. Experimental Brain Research, 141, 269–280. 10.1007/s002210100822 [DOI] [PubMed] [Google Scholar]
- Exner, C. , Weniger, G. , & Irle, E. (2001). Implicit and explicit memory after focal thalamic lesions. Neurology, 57, 2054–2063. 10.1212/WNL.57.11.2054 [DOI] [PubMed] [Google Scholar]
- Fleming, M. K. , Newham, D. J. , & Rothwell, J. C. (2018). Explicit motor sequence learning with the paretic arm after stroke. Disability and Rehabilitation, 40, 323–328. 10.1080/09638288.2016.1258091 [DOI] [PubMed] [Google Scholar]
- Fonollosa, J. , Neftci, E. , & Rabinovich, M. (2015). Learning of chunking sequences in cognition and behavior. PLoS Computational Biology, 11, e1004592 10.1371/journal.pcbi.1004592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghilardi, M. F. , Moisello, C. , Silvestri, G. , Ghez, C. , & Krakauer, J. W. (2009). Learning of a sequential motor skill comprises explicit and implicit components that consolidate differently. Journal of Neurophysiology, 101, 2218–2229. 10.1152/jn.01138.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Beldarrain, M. (1999). Procedural learning is impaired in patients with prefrontal lesions | Ovid [WWW Document]. Retrieved from https://oce.ovid.com/article/00006114-199906100-00024. [DOI] [PubMed]
- Gomez Beldarrain, M. , Astorgano, A. G. , Gonzalez, A. B. , & Garcia‐Monco, J. C. (2008). Sleep improves sequential motor learning and performance in patients with prefrontal lobe lesions. Clinical Neurology and Neurosurgery, 110, 245–252. 10.1016/j.clineuro.2007.11.004 [DOI] [PubMed] [Google Scholar]
- Gomez Beldarrain, M. , Gafman, J. , Ruiz de Velasco, I. , Pascual‐Leone, A. , & Garcia‐Monco, J. (2002). Prefrontal lesions impair the implicit and explicit learning of sequences on visuomotor tasks. Experimental Brain Research, 142, 529–538. 10.1007/s00221-001-0935-2 [DOI] [PubMed] [Google Scholar]
- Gordon, A. M. , Lee, J. H. , Flament, D. , Ugurbil, K. , & Ebner, T. J. (1998). Functional magnetic resonance imaging of motor, sensory, and posterior parietal cortical areas during performance of sequential typing movements. Experimental Brain Research, 121, 153–166. [DOI] [PubMed] [Google Scholar]
- Hardwick, R. M. , Rottschy, C. , Miall, R. C. , & Eickhoff, S. B. (2013). A quantitative meta‐analysis and review of motor learning in the human brain. NeuroImage, 67, 283–297. 10.1016/j.neuroimage.2012.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka, O. , Nakahara, H. , Rand, M. K. , Sakai, K. , Lu, X. , Nakamura, K. , … Doya, K. (1999). Parallel neural networks for learning sequential procedures. Trends in Neurosciences, 22, 464–471. 10.1016/S0166-2236(99)01439-3 [DOI] [PubMed] [Google Scholar]
- Hikosaka, O. , Nakamura, K. , Sakai, K. , & Nakahara, H. (2002). Central mechanisms of motor skill learning. Current Opinion in Neurobiology, 12, 217–222. 10.1016/S0959-4388(02)00307-0 [DOI] [PubMed] [Google Scholar]
- Hund‐Georgiadis, M. , & von Cramon, D. Y. (1999). Motor‐learning‐related changes in piano players and non‐musicians revealed by functional magnetic‐resonance signals. Experimental Brain Research, 125, 417–425. [DOI] [PubMed] [Google Scholar]
- Janacsek, K. , & Nemeth, D. (2013). Implicit sequence learning and working memory: Correlated or complicated? Cortex, 49, 2001–2006. 10.1016/j.cortex.2013.02.012 [DOI] [PubMed] [Google Scholar]
- Japikse, K. , Negash, S. , Howard, J. , & Howard, D. (2003). Intermanual transfer of procedural learning after extended practice of probabilistic sequences. Experimental Brain Research, 148, 38–49. 10.1007/s00221-002-1264-9 [DOI] [PubMed] [Google Scholar]
- Jenkins, I. H. , Brooks, D. J. , Nixon, P. D. , Frackowiak, R. S. , & Passingham, R. E. (1994). Motor sequence learning: A study with positron emission tomography. The Journal of Neuroscience, 14, 3775–3790. 10.1523/JNEUROSCI.14-06-03775.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, L. , Burridge, J. H. , & Demain, S. H. (2013). Internal and external focus of attention during gait re‐education: An observational study of physical therapist practice in stroke rehabilitation. Physical Therapy, 93, 957–966. 10.2522/ptj.20120300 [DOI] [PubMed] [Google Scholar]
- Kal, E. , Houdijk, H. , Van Der Wurff, P. , Groet, E. , Van Bennekom, C. , Scherder, E. , & Van der Kamp, J. (2016). The inclination for conscious motor control after stroke: Validating the movement‐specific reinvestment scale for use in inpatient stroke patients. Disability and Rehabilitation, 38, 1097–1106. 10.3109/09638288.2015.1091858 [DOI] [PubMed] [Google Scholar]
- Kal, E. , Winters, M. , van der Kamp, J. , Houdijk, H. , Groet, E. , van Bennekom, C. , & Scherder, E. (2016). Is implicit motor learning preserved after stroke? A systematic review with meta‐analysis. PLoS One, 11, e0166376 10.1371/journal.pone.0166376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni, A. , Meyer, G. , Jezzard, P. , Adams, M. M. , Turner, R. , & Ungerleider, L. G. (1995). Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature, 377, 155–158. 10.1038/377155a0 [DOI] [PubMed] [Google Scholar]
- Karni, A. , Meyer, G. , Rey‐Hipolito, C. , Jezzard, P. , Adams, M. M. , Turner, R. , & Ungerleider, L. G. (1998). The acquisition of skilled motor performance: Fast and slow experience‐driven changes in primary motor cortex. Proceedings of the National Academy of Sciences of the United States of America, 95, 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleynen, M. , Braun, S. M. , Beurskens, A. J. H. M. , Verbunt, J. A. , de Bie, R. A. , & Masters, R. S. W. (2013). Investigating the Dutch movement‐specific reinvestment scale in people with stroke. Clinical Rehabilitation, 27, 160–165. 10.1177/0269215512448381 [DOI] [PubMed] [Google Scholar]
- Kleynen, M. , Braun, S. M. , Bleijlevens, M. H. , Lexis, M. A. , Rasquin, S. M. , Halfens, J. , … Masters, R. S. W. (2014). Using a Delphi technique to seek consensus regarding definitions, descriptions and classification of terms related to implicit and explicit forms of motor learning. PLoS One, 9, e100227 10.1371/journal.pone.0100227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleynen, M. , Wilson, M. R. , Jie, L.‐J. , te Lintel Hekkert, F. , Goodwin, V. A. , & Braun, S. M. (2014). Exploring the utility of analogies in motor learning after stroke: A feasibility study. International Journal of Rehabilitation Research, 37, 277–280. 10.1097/MRR.0000000000000058 [DOI] [PubMed] [Google Scholar]
- Koechlin, E. , Basso, G. , Pietrini, P. , Panzer, S. , & Grafman, J. (1999). The role of the anterior prefrontal cortex in human cognition. Nature, 399, 148–151. 10.1038/20178 [DOI] [PubMed] [Google Scholar]
- Lehéricy, S. , Benali, H. , Van de Moortele, P.‐F. , Pélégrini‐Issac, M. , Waechter, T. , Ugurbil, K. , & Doyon, J. (2005). Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proceedings of the National Academy of Sciences of the United States of America, 102, 12566–12571. 10.1073/pnas.0502762102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, P. A. , & Miall, R. C. (2003). Distinct systems for automatic and cognitively controlled time measurement: Evidence from neuroimaging. Current Opinion in Neurobiology, 13, 250–255. 10.1016/S0959-4388(03)00036-9 [DOI] [PubMed] [Google Scholar]
- Luppino, G. , Matelli, M. , Camarda, R. , & Rizzolatti, G. (1993). Corticocortical connections of area F3 (SMA‐proper) and area F6 (pre‐SMA) in the macaque monkey. Journal of Comparative Neurology, 338, 114–140. 10.1002/cne.903380109 [DOI] [PubMed] [Google Scholar]
- Manoach, D. S. , Press, D. Z. , Thangaraj, V. , Searl, M. M. , Goff, D. C. , Halpern, E. , … Warach, S. (1999). Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biological Psychiatry, 45, 1128–1137. 10.1016/S0006-3223(98)00318-7 [DOI] [PubMed] [Google Scholar]
- Meehan, S. K. , Randhawa, B. , Wessel, B. , & Boyd, L. A. (2011). Implicit sequence‐specific motor learning after subcortical stroke is associated with increased prefrontal brain activations: An fMRI study. Human Brain Mapping, 32, 290–303. 10.1002/hbm.21019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall, C. (2010). Motor control: Correcting errors and learning from mistakes. Current Biology, 20, R596–R598. 10.1016/j.cub.2010.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton, F. A. , & Strick, P. L. (2000). Basal ganglia output and cognition: Evidence from anatomical, behavioral, and clinical studies. Brain and Cognition, 42, 183–200. 10.1006/brcg.1999.1099 [DOI] [PubMed] [Google Scholar]
- Miller, G. A. (1994). The magical number seven, plus or minus two: Some limits on our capacity for processing information. Psychological Review, 101, 343–352. [DOI] [PubMed] [Google Scholar]
- Miyachi, S. , Hikosaka, O. , Miyashita, K. , Kárádi, Z. , & Rand, M. K. (1997). Differential roles of monkey striatum in learning of sequential hand movement. Experimental Brain Research, 115, 1–5. [DOI] [PubMed] [Google Scholar]
- Nakahara, H. , Doya, K. , & Hikosaka, O. (2001). Parallel cortico‐basal ganglia mechanisms for acquisition and execution of visuomotor sequences—A computational approach. Journal of Cognitive Neuroscience, 13, 626–647. 10.1162/089892901750363208 [DOI] [PubMed] [Google Scholar]
- Orrell, A. J. , Eves, F. F. , & Masters, R. S. (2006). Motor learning of a dynamic balancing task after stroke: Implicit implications for stroke rehabilitation. Physical Therapy, 86, 369–380. 10.1093/ptj/86.3.369 [DOI] [PubMed] [Google Scholar]
- Orrell, A. J. , Eves, F. F. , Masters, R. S. W. , & MacMahon, K. M. M. (2007). Implicit sequence learning processes after unilateral stroke. Neuropsychological Rehabilitation, 17, 335–354. 10.1080/09602010600832788 [DOI] [PubMed] [Google Scholar]
- Orrell, A. J. , Masters, R. S. W. , & Eves, F. F. (2009). Reinvestment and movement disruption following stroke. Neurorehabilitation and Neural Repair, 23, 177–183. 10.1177/1545968308317752 [DOI] [PubMed] [Google Scholar]
- Penhune, V. B. , & Steele, C. J. (2012). Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behavioural Brain Research, 226, 579–591. 10.1016/j.bbr.2011.09.044 [DOI] [PubMed] [Google Scholar]
- Pohl, P. S. , McDowd, J. M. , Filion, D. , Richards, L. G. , & Stiers, W. (2006). Implicit learning of a motor skill after mild and moderate stroke. Clinical Rehabilitation, 20, 246–253. 10.1191/0269215506cr916oa [DOI] [PubMed] [Google Scholar]
- Pohl, P. S. , McDowd, J. M. , Filion, D. L. , Richards, L. G. , & Stiers, W. (2001). Implicit learning of a perceptual‐motor skill after stroke. Physical Therapy, 81, 1780–1789. 10.1093/ptj/81.11.1780 [DOI] [PubMed] [Google Scholar]
- Povel, D. J. , & Collard, R. (1982). Structural factors in patterned finger tapping. Acta Psychologica, 52, 107–123. [DOI] [PubMed] [Google Scholar]
- Reber, A. (1993). Implicit learning and tacit knowledge: An essay on the cognitive unconscious (Oxford psychology series, no 19). Oxford: Oxford University Press. [Google Scholar]
- Reber, P. J. , & Squire, L. R. (1998). Encapsulation of implicit and explicit memory in sequence learning. Journal of Cognitive Neuroscience, 10, 248–263. [DOI] [PubMed] [Google Scholar]
- Rosenbaum, D. A. , Kenny, S. B. , & Derr, M. A. (1983). Hierarchical control of rapid movement sequences. Journal of Experimental Psychology. Human Perception and Performance, 9, 86–102. [DOI] [PubMed] [Google Scholar]
- Rösser, N. , Heuschmann, P. , Wersching, H. , Breitenstein, C. , Knecht, S. , & Flöel, A. (2008). Levodopa improves procedural motor learning in chronic stroke patients. Archives of Physical Medicine and Rehabilitation, 89, 1633–1641. 10.1016/j.apmr.2008.02.030 [DOI] [PubMed] [Google Scholar]
- Savion‐Lemieux, T. , & Penhune, V. B. (2005). The effects of practice and delay on motor skill learning and retention. Experimental Brain Research, 161, 423–431. 10.1007/s00221-004-2085-9 [DOI] [PubMed] [Google Scholar]
- Shadmehr, R. , Brandt, J. , & Corkin, S. (1998). Time‐dependent motor memory processes in amnesic subjects. Journal of Neurophysiology, 80, 1590–1597. 10.1152/jn.1998.80.3.1590 [DOI] [PubMed] [Google Scholar]
- Shin, J. C. , Aparicio, P. , & Ivry, R. B. (2005). Multidimensional sequence learning in patients with focal basal ganglia lesions. Brain and Cognition, Neuropsychology of Timing and Time Perception, 58, 75–83. 10.1016/j.bandc.2004.09.015 [DOI] [PubMed] [Google Scholar]
- Siengsukon, C. F. , & Boyd, L. A. (2009). Sleep to learn after stroke: Implicit and explicit off‐line motor learning. Neuroscience Letters, 451, 1–5. 10.1016/j.neulet.2008.12.040 [DOI] [PubMed] [Google Scholar]
- Stapleton, T. , Ashburn, A. , & Stack, E. (2001). A pilot study of attention deficits, balance control and falls in the subacute stage following stroke. Clinical Rehabilitation, 15, 437–444. 10.1191/026921501678310243 [DOI] [PubMed] [Google Scholar]
- Tanji, J. (1994). The supplementary motor area in the cerebral cortex. Neuroscience Research, 19, 251–268. 10.1016/0168-0102(94)90038-8 [DOI] [PubMed] [Google Scholar]
- Vakil, E. , Kahan, S. , Huberman, M. , & Osimani, A. (2000). Motor and non‐motor sequence learning in patients with basal ganglia lesions: The case of serial reaction time (SRT). Neuropsychologia, 38, 1–10. 10.1016/S0028-3932(99)00058-5 [DOI] [PubMed] [Google Scholar]
- Wadden, K. P. , De Asis, K. , Mang, C. S. , Neva, J. L. , Peters, S. , Lakhani, B. , & Boyd, L. A. (2017). Predicting motor sequence learning in individuals with chronic stroke. Neurorehabilitation and Neural Repair, 31, 95–104. 10.1177/1545968316662526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden, K. P. , Woodward, T. S. , Metzak, P. D. , Lavigne, K. M. , Lakhani, B. , Auriat, A. M. , & Boyd, L. A. (2015). Compensatory motor network connectivity is associated with motor sequence learning after subcortical stroke. Behavioural Brain Research, 286, 136–145. 10.1016/j.bbr.2015.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham, D. B. (1998). A neuropsychological theory of motor skill learning. Psychological Review, 105, 558–584. [DOI] [PubMed] [Google Scholar]
- Willingham, D. B. , Greenberg, A. R. , & Thomas, R. C. (1997). Response‐to‐stimulus interval does not affect implicit motor sequence learning, but does affect performance. Memory and Cognition, 25, 534–542. 10.3758/BF03201128 [DOI] [PubMed] [Google Scholar]
- Wu, T. , Kansaku, K. , & Hallett, M. (2004). How self‐initiated memorized movements become automatic: A functional MRI study. Journal of Neurophysiology, 91, 1690–1698. 10.1152/jn.01052.2003 [DOI] [PubMed] [Google Scholar]
- Zimerman, M. , Heise, K. F. , Hoppe, J. , Cohen, L. G. , Gerloff, C. , & Hummel, F. C. (2012). Modulation of training by single‐session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke, 43, 2185–2191. 10.1161/STROKEAHA.111.645382 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


