Abstract
Healthy aging is accompanied by changes in brain activation patterns in the motor system. In older subjects, unilateral hand movements typically rely on increased recruitment of ipsilateral frontoparietal areas. While the two central concepts of aging‐related brain activity changes, “Hemispheric Asymmetry Reduction in Older Adults” (HAROLD), and “Posterior to Anterior Shift in Aging” (PASA), have initially been suggested in the context of cognitive tasks and were attributed to compensation, current knowledge regarding the functional significance of increased motor system activity remains scarce. We, therefore, used online interference transcranial magnetic stimulation in young and older subjects to investigate the role of key regions of the ipsilateral frontoparietal cortex, that is, (a) primary motor cortex (M1), (b) dorsal premotor cortex (dPMC), and (c) anterior intraparietal sulcus (IPS) in the control of hand movements of different motor demands. Our data suggest a change of the functional roles of ipsilateral brain areas in healthy age with a reduced relevance of ipsilateral M1 and a shift of importance toward dPMC for repetitive high‐frequency movements. These results support the notion that mechanisms conceptualized in the models of “PASA” and “HAROLD” also apply to the motor system.
Keywords: aging, dorsal premotor cortex, movement kinematics, online TMS interference
1. INTRODUCTION
Unilateral hand movements are primarily driven by a lateralized network of cortical and subcortical areas. However, besides contralateral sensorimotor areas (Porter & Lemon, 1995), also ipsilateral brain regions contribute to the coordination of upper limb movements (Cramer, Finklestein, Schaechter, Bush, & Rosen, 1999; Tinazzi & Zanette, 1998). Bilateral recruitment patterns have particularly been observed in young healthy subjects for more complex motor tasks with higher behavioral demands (Buetefisch, Revill, Shuster, Hines, & Parsons, 2014; Catalan, Honda, Weeks, Cohen, & Hallett, 1998; Hummel, Kirsammer, & Gerloff, 2003; Verstynen, 2004).
As in more complex tasks, the enhanced brain activity of the ipsilateral motor system has frequently been encountered in association with age. Older subjects show more extensive, as well as more bilateral activation patterns (Heuninckx, 2005; Hutchinson, 2002;Mattay et al., 2002 ; Michely et al., 2018 ; Riecker et al., 2006). While at the behavioral level motor performance typically declines with increasing age (Hackel, Wolfe, Bang, & Canfield, 1992; Kolb, Forgie, Gibb, Gorny, & Rowntree, 1998; Salthouse, 2000), ipsilateral brain activity increases, comprising especially the primary motor cortex, premotor and parietal areas (Mattay et al., 2002; Michely et al., 2018; Riecker et al., 2006).
Based on neuroimaging findings which have consistently shown altered activation patterns during aging, two main models have been conceptualized: a decrease in lateralization with increasing age (“Hemispheric Asymmetry Reduction in Older Adults” (HAROLD) theory, (Cabeza et al., 1997; Cabeza, Anderson, Locantore, & McIntosh, 2002)) and a shift of activation from posterior to anterior regions (“Posterior to Anterior Shift in Aging” (PASA) model, (Davis, Dennis, Daselaar, Fleck, & Cabeza, 2008). While initially put forward in the context of cognitive tasks, these models have been readily transferred to the motor system (Heuninckx, 2005; Hutchinson, 2002; Mattay et al., 2002; Michely et al., 2018; Riecker et al., 2006; Ward & Frackowiak, 2003).
From a functional perspective, such changes observed during cognitive tasks have mostly been associated with compensatory recruitment in order to counteract aging‐related structural, functional, and metabolic changes (Cabeza et al., 2002; Grady, 2012; Reuter‐Lorenz & Cappell, 2008) with the ultimate goal of maintaining performance.
However, though intuitive, the functional significance of these age‐related activity changes associated with motor tasks remains to be established (Seidler et al., 2010). Although the principle of compensation may also be relevant for the motor system (Mattay et al., 2002), a majority of studies did not support this idea (Hutchinson, 2002; Langan et al., 2010; Riecker et al., 2006), since the authors could not link the degree of over‐activation to improved motor performance. Besides, altered brain activity may also represent dedifferentiation (Bernard & Seidler, 2012; Langan et al., 2010; Seidler et al., 2010).
Yet, the models of age‐related changes and their functional significance were primarily based on BOLD activity changes correlating with behavior. In this context, a significant limitation of functional neuroimaging studies, including analyses of functional or effective connectivity, is that they cannot derive a causal role of a particular brain region for performance. By contrast, transcranial magnetic stimulation (TMS) can be used to transiently interfere with neural activity of a brain area during task performance (Gerloff et al., 1998; Pascual‐Leone, Bartres‐Faz, & Keenan, 1999; Walsh & Cowey, 2000). By directly interfering with the fine‐tuned neural processing and thereby disturbing task performance, online TMS interference allows insights into the causal role of a specific area for a given task of interest.
We thus used neuronavigated, online TMS interference to elucidate the age‐related role of ipsilateral brain regions in healthy aging. As age‐dependent changes have been frequently reported for ipsilateral primary motor cortex (M1), dorsal premotor cortex (dPMC) and intraparietal sulcus (IPS) (Mattay et al., 2002; Riecker et al., 2006; Ward, Swayne, & Newton, 2008), we investigated their task‐related role in young and older healthy subjects while they performed three motor task of different motor demands: (a) rapid alternating pointing between two fixed targets, (b) maximum finger tapping frequency, and (c) maximum hand tapping frequency. Movement kinematics were recorded using a three‐dimensional (3D) motion analyzer system. Given the increases in frontoparietal activity reported for older subjects (Heuninckx, 2005; Mattay et al., 2002; Riecker et al., 2006), we hypothesized that TMS interference with ipsilateral frontoparietal areas would primarily disturb motor performance in older subjects, consistent with a compensatory role of activity changes in these areas. Moreover, the different motor tasks were not only designed to recruit different motor effectors ranging from the entire arm for the pointing tasks to the single index finger during the finger tapping task, but also to differentially probe the involvement of the three brain regions of interest. Accordingly, primary motor cortex was supposed to be particularly relevant for fine‐tuned repetitive finger movements which strongly rely on the modulation of interhemispheric inhibition applicable to our tapping tasks (Hinder, 2012; Liuzzi, Hörniss, Zimerman, Gerloff, & Hummel, 2011), but may be supported by premotor regions in aging. The cortex in anterior intraparietal sulcus was assumed to be particularly involved during the pointing tasks since the IPS is known to be engaged in such visuomotor tasks (Grefkes & Fink, 2005; Wang, Fink, Dafotakis, & Grefkes, 2009).
2. MATERIALS AND METHODS
2.1. Main experiment
2.1.1. Subjects
Thirty‐two healthy subjects participated in this study: 15 young healthy subjects (five females, all subjects reported right‐handedness according to the Edinburgh Handedness Inventory (EHI) (laterality score ≥ 75%), mean age 27.8 ± 3.5 SD years; range: 24–34 years) and 17 older healthy subjects (four women, all right‐handed, mean age 63.4 ± 9.2 SD years; range: 51–89 years) were recruited from our subject database. Due to the procedure explained in the following, the recruitment was challenged and limited to subjects having an individual MRI scan stored in the database. Post hoc calculation of power confirmed that although the given sample size is small, based on the detected effect sizes and given an alpha‐error of 0.05, we achieved an observed power of 0.79 (G*Power 3.1). None of the subjects had a history of neurological, psychiatric, orthopedic or rheumatic disease nor any contraindication to TMS (Rossi, Hallett, Rossini, & Pascual‐Leone, 2009). All participants gave informed written consent before entering the study, which was approved by the local ethics committee (number of ethical vote: 14–141) and the experiment was carried out in accordance with the Declaration of Helsinki.
2.1.2. Experimental design
We used a single‐blinded, pseudorandomized mixed design. Accordingly, the order of stimulation sites was pseudorandomized per subject before the experiment and balanced within subjects. The order of motor tasks was pseudorandomized across subjects as well. Short bursts of repetitive TMS pulses (rTMS) were administered at a frequency of 10 Hz with a stimulation intensity of 90% of resting motor threshold (see below) concurrent to task execution (Davare, Andres, Cosnard, Thonnard, & Olivier, 2006; Gerloff et al., 1998; Volz et al., 2017), starting with a visual go cue and lasting throughout the trial for 2.0–2.5 s (20–25 pulses) depending on the given motor task (see below).
Trials within a given stimulation block were separated by a 3‐s pause to minimize the likelihood of carry‐over and lasting effects (see below) (Rotenberg, Horvath, & Pascual‐Leone, 2014). The software Presentation® (Version 9.9, Neurobehavioral Systems, Berkeley, CA, http://www.neurobs.com) was used for both stimulus presentation and time‐locked triggering of the TMS machine (Volz et al., 2017).
Three motor tasks of different behavioral demands were used to probe the influence of the stimulation sites on motor performance (Figure 1).
Figure 1.
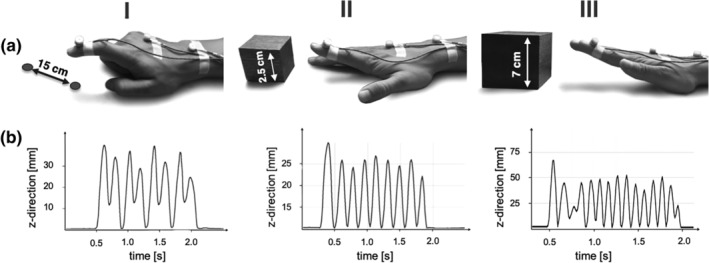
Motor task during online related TMS. (a) (I) Rapid pointing movements touching two spots with a distance of 15 cm as fast as possible. (II) Index finger tapping with a height of 2.5 cm at maximum speed. (III) Hand tapping with a height of 7 cm at maximum speed. All motor tasks were studied using a three‐dimensional motion analyzer system based on ultrasound‐emitting markers that were fixed to the dorsal side of the distal interphalangeal joint of the right index finger (marker I), the dorsal side of the third metacarpophalangeal joint (marker II), and between the styloid processes of ulna and radius (marker III). (b) Illustration of the kinematic data obtained for data analysis: traces of vertical movements (z‐direction) of marker I over time are shown for every motor task (I–III)
2.1.3. Pointing task (I)
The rapid pointing task engaged muscle groups of the entire arm including the shoulder, and particularly required target accuracy. Therefore, it strongly relied on neural systems for action‐space representations (Colby & Goldberg, 1999; Rizzolatti, Fogassi, & Gallese, 1997), sensori‐ and visuomotor coordinate transformations, and visuospatial attention (Grefkes & Fink, 2005; Wang et al., 2009). Subjects were asked to perform repetitive sagittal pointing movements with their right index finger between two targets (Figure 1). For this purpose, we installed an apparatus with two fixed targets. The distance between the pointing targets was 15 cm in the sagittal plane and 3 cm in the vertical plane, which was visible throughout the entire block of pointing movements (Figure 1).
The difference in height ensured that participants lifted their arm, thereby avoiding sliding movements on the table. Each trial lasted 2.5 s followed by a pause of 3 s. To prevent interference with the visual target, movement onsets were indicated by a brief acoustic tone. Subjects were instructed to move as fast and as accurate as possible.
2.1.4. Index finger tapping (II)
The finger tapping task tested repetitive isolated finger movements which strongly rely on the modulation of interhemispheric interactions (Hinder, 2012; Liuzzi et al., 2011). Subjects were instructed to perform repetitive index finger tapping movements as fast and accurate as possible with their right hand upon the appearance of a visual cue which was presented on a video screen controlled by the software Presentation®. Subjects placed their right palm on a defined position on the table and performed repetitive vertical movements at the metacarpophalangeal joint of their index finger (Nowak et al., 2007). A dice with a height of 2.5 cm indicated the target amplitude (Figure 1). Each tapping trial lasted 2 s. As it is assumed that effects of high‐frequency rTMS outlast the stimulation period for about half of the duration of stimulation (Rotenberg et al., 2014), tapping trials were separated with a pause of 3 s to avoid rTMS carry‐over effects and fatigue. Short finger tapping trials were used because the maximum finger tapping frequency is usually highest during the first few seconds of a trial (Wang et al., 2009).
2.1.5. Hand tapping (III)
The hand tapping task was similar to the finger tapping task with respect to its cyclical, repetitive aspect. Moreover, it involved similar motor components and, therefore, additionally served as an internal control condition for effects seen in the finger tapping task. However, the hand tapping task recruited more proximal arm and wrist muscle groups, which are considered to rely somewhat less on interhemispheric interactions compared to isolated index finger movements (Aune, Ettema, & Vereijken, 2016; Harris‐Love, Perez, Chen, & Cohen, 2007; Sohn, Jung, Kaelin‐Lang, & Hallett, 2003). Subjects were asked to place their right hand on the defined position on the table and, in response to the cue, perform repetitive flexion and extension of the wrist with a stretched hand at maximum speed (Nowak et al., 2007). The target movement amplitude was indicated by a cube of 7 cm height (Figure 1). Similarly to (I), one tapping trial lasted 2 s with a pause interval of 3 s (all indicated by visual cues).
The testing battery for each stimulation site consisted of nine blocks, three repetitions of each type of movements. The order of these nine blocks per site was pseudorandomized. One block was composed of five consecutive repetitions of one movement trial (i.e., 5 times of 2.0 s of finger tapping or hand tapping, or 2.5 s of pointing) (Figure 2). This means that we acquired three blocks of five consecutively repetitions per task per stimulation site. Each trial of movement was separated by a 3‐s pause. Each block was separated by 10 s of rest. A break of 30–45 s for switching between the different stimulation sites ensured a reliable location of each region using the neuronavigation system.
Figure 2.
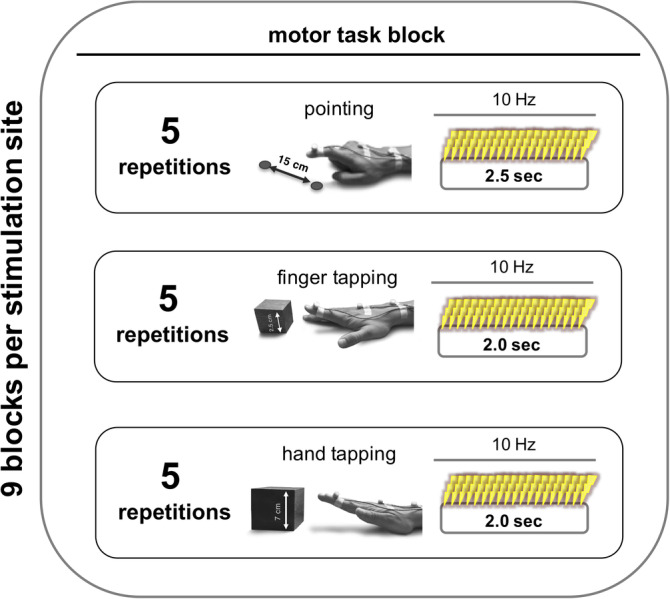
Study design. For each stimulation site, we acquired nine blocks for a given motor task. The order of stimulation sites was pseudorandomized per subject and balanced within subjects. The order of motor tasks was also pseudorandomized across subjects. One block consisted of five consecutive repetitions of one movement trial (i.e., pointing, finger tapping, hand tapping). See methods for more information
Written instructions were displayed for 3 s on a video screen, indicating the upcoming block of motor task (e.g., “pointing,” “finger tapping” or “hand tapping”). Upon the appearance of the visual go cue, instructing the subject to start the requested movements, the software synchronously triggered the TMS machine to apply 10 Hz rTMS throughout the duration of a trial for 2.0–2.5 s (20–25 pulses) depending on the given motor task (see above). A visual stop signal displayed for 3 s indicated the end of a trial. Of note, for the pointing task, start and stop of the trial were also indicated by a brief acoustic tone so that the subjects could focus on the pointing apparatus.
The entire experiment lasted about 60 min (15 min per stimulation site). Before the TMS sessions, subjects were trained in all tasks until they reached a stable performance.
2.1.6. 3D ultrasound movement recording
Motor performance was assessed using the Zebris CMS 20 kinematic motion analyzer system (Zebris Medical Company, Isny, Germany) (Nowak et al., 2007; Wang et al., 2009). The system mainly consists of a measuring sensor, which captures with high spatial (0.1 mm) and temporal (100 Hz) resolution the 3D positions of markers, fixed on the body parts to be examined, emitting ultrasonic pulses (diameter: 5 mm, weight: 1 g) (Wang et al., 2009). For the current experiment, the 3D‐tracking markers were attached to the dorsal side of the distal interphalangeal joint of the right index finger (marker I), the dorsal side of the third metacarpophalangeal joint (marker II), and between the styloid processes of ulna and radius (marker III) (Figure 1). The x‐, y‐, and z‐directions of the position marker coordinates refer to the medio‐lateral, antero‐posterior, and vertical directions with regard to the subject performing the task. Kinematic data were continuously recorded throughout the entire experiment.
2.1.7. Neuronavigated transcranial magnetic stimulation
TMS was performed using a Magstim Super Rapid2 stimulator (The Magstim Co., Ltd, Whitland, United Kingdom) equipped with a 70 mm figure‐of‐eight air film coil. Throughout the TMS sessions, the position of the coil was tracked and recorded using a frameless computerized stereotaxic neuronavigation system (BrainSight V.2.0.7; Rogue Research Ltd; Montreal, Canada). For neuronavigation, the head of the subject was coregistered with the individual high‐resolution anatomical MR image (MDEF sequence; voxel size: 1.0 × 1.0 × 1.0 mm3, FOV 256 mm, 176 sagittal slices, TR 2250 ms, TE 3.93 ms) via anatomical landmarks (see Nettekoven et al., 2014 for further details). The structural MRI images were available in the subject database.
The “motor hotspot” for ipsilateral M1 (i.e., right primary motor cortex) was defined as the coil position eliciting motor evoked potentials (MEP) of the highest amplitude in response to a TMS pulse applied tangentially to the skull in a 45° posterior–anterior current direction, thereby targeting the posterior wall of the precentral gyrus at the hand knob formation (Yousry et al., 1997). MEP amplitudes were assessed with biphasic pulses. In addition, MEP amplitudes of the left first interosseous (FDI) muscle were measured using Ag/AgCl surface electrodes (Tyco Healthcare) in a belly‐to‐tendon montage. The EMG signal was amplified, filtered (0.5 Hz high pass and 30–300 Hz bandpass), and digitized using a Powerlab 26T device and the LabChart software package version 8.0 (AD Instruments, Australia).
The resting motor threshold (RMT) was defined using an algorithm provided by the TMS Motor Threshold Assessment Tool (MTAT) 2.0 (http://www.clinicalresearcher.org/software.html) (Awiszus, 2003; Diekhoff et al., 2011). Using a maximum‐likelihood procedure, the algorithm proposes stimulation intensities that are subsequently tested regarding their ability to induce an EMG response higher than 50 μV, which is accordingly entered by the experimenter. The MTAT has been shown to accurately estimate motor thresholds using less stimuli than the standard 5‐out‐of‐10 rule (Awiszus, 2003).
For motor task interference, 10 Hz rTMS was applied at 90% RMT during task execution (Volz et al., 2017). A subthreshold stimulation intensity was chosen to prevent the induction of MEPs, which may irritate participants and impact on their task performance. Besides, stimulating brain regions in the hemisphere ipsilateral to the moving hand and areas beyond M1 additionally avoid the evocation of distracting muscle activity. Moreover, by using an online TMS approach, we used the immediate effects of TMS interfering with physiological neural activity underneath the stimulation coil while subjects performed a given task. The advantage of online TMS interference compared to the more widely used “offline”‐TMS approach is that by directly disturbing neural activity during task performance stimulation effects are immediately present and less prone to vary with respect to the magnitude and direction of after‐effects as observed for “offline TMS” protocols (Hamada, Murase, Hasan, Balaratnam, & Rothwell, 2013). Of note, TMS interference refers to the neurophysiological mechanisms, that is, disturbing neural activity, rather to the behavioral consequence. Besides, in contrast to the excitatory and inhibitory effects of offline rTMS (Nettekoven et al., 2015), online TMS is considered to invariably interfere with the neural processing, that is, inducing a virtual online lesion, and thereby disturb task performance (Rossi, 2004; Rossini et al., 2015; Walsh & Cowey, 2000). Importantly, there is no evidence that 10 Hz online interventions paradigms have a lasting effect on cortical excitability, which might probably be due to the fact that 10 Hz stimulation applied for increasing cortical excitability in offline‐experiments are administered in a completely different stimulation protocol (longer stimulation trains, higher number of pulses up to 1,000–2,000 in total, and often suprathreshold intensities, for a review see Fitzgerald, Fountain, & Daskalakis, 2006; Rossini et al., 2015).
Furthermore, online TMS paradigms are more flexible with regard to randomization of stimulation sites within a single session. High‐frequency rTMS trains have widely been used before in order to interfere with task performance (Davare, Andres, et al., 2006; Gerloff et al., 1998; Lotze et al., 2006; Volz et al., 2017). In contrast to single pulse TMS, 10 Hz rTMS trains increase the likelihood to effectively and temporally accurately interfere with task performance.
For online interference, the stimulator was controlled and triggered by an in‐house script using the software Presentation® to ensure reliable timing of TMS pulse applications (for further technical details see Volz et al., 2017). The stimulation was triggered by the computer synchronously with the presentation of the go cue, and was administered throughout the trial for 2.0–2.5 s (20–25 pulses) depending on the motor task (see above). Applying TMS throughout the entire duration of the motor task implicated the possibility to intervene not only with movement execution, but also preparation or sensory integration. However, it ensured an effective and comparable interference for all motor tasks, all brain regions and both groups. Of note, the in‐house script simultaneously presented the visual cues and triggered the TMS machine.
The following stimulation sites were investigated all ipsilateral to the right (dominant) hand (Figure 3): (a) right ipsilateral M1, (b) right ipsilateral dPMC, and (c) right ipsilateral IPS. Ipsilateral M1 was equivalent to the motor hotspot as defined above.
Figure 3.
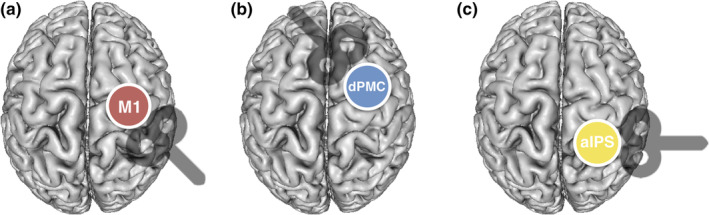
Ipsilateral stimulation sites with coil orientations. (a) Right ipsilateral primary motor cortex (M1), (b) right ipsilateral dorsal premotor cortex (dPMC), and (c) right ipsilateral anterior intraparietal sulcus (IPS)
For targeting ipsilateral dPMC and IPS, we used coordinates based on recent activation likelihood estimation meta‐analyses on hand motor fMRI activity (Hardwick, Rottschy, Miall, & Eickhoff, 2013; Rehme, Eickhoff, Rottschy, Fink, & Grefkes, 2012). For each subject, MNI (X/Y/Z) coordinates (dPMC: 38/6/62; IPS: 42/−40/50) were warped into individual 3D‐space of the anatomical MRI using Statistical Parametric Mapping (SPM12, http://www.fil.ion.ucl.ac.uk/) and MRIcron (Neva, Brown, Mang, Francisco, & Boyd, 2017).
For interference with ipsilateral dPMC activity, the coil was held tangentially to the skull in a 45° anterior–posterior position perpendicular to the course of the precentral sulcus. This position warranted optimal stimulation of dPMC neurons situated in the anterior wall of the precentral gyrus (close to the intersection with the superior frontal sulcus). By contrast, for stimulation of ipsilateral anterior IPS, the coil was held in a 90° latero‐medial position, perpendicular to the intraparietal sulcus, leading to a preferential stimulation of neurons situated in the medial IPS wall, probably equivalent to macaque area MIP which is engaged in reaching movements and visuomotor transformation (Colby, 1998; Grefkes & Fink, 2005; Grefkes, Ritzl, Zilles, & Fink, 2004). Similar to M1, the stimulation intensity for ipsilateral dPMC and IPS was applied at the same intensity, that is, 90% RMT obtained at M1 for a given subject. Using the same stimulation intensity for each site ensures that sensory stimulation effects like acoustic noise or tactile sensations remain comparable between stimulation sites within a given subject. Hence, between‐region differences do not result from differences in sensory stimulation interfering with motor performance, but are most likely of neural origin. Furthermore, it ensured that stimulation effects were comparable between subjects and groups.
Coil positions were logged into the neuronavigation software and maintained throughout the experiment for each stimulation site.
A stimulation over parieto‐occipital vertex with the same intensity served as control (sham) to account for unspecific stimulation effects like tactile and auditory sensation (Herwig, Cardenas‐Morales, Connemann, Kammer, & Schönfeldt‐Lecuona, 2010). Importantly, to reduce possible cortical stimulation effects in the control condition, the coil was angled at 45°, touching the skull not with the center but with the rim opposite to the handle. In this position, the coil to cortex distance is larger such that the electromagnetic field, if at all reaching the cortex, is substantially weaker and far outside the target range while the typical skin sensation is preserved (Herwig et al., 2010). Using this procedure, a recent study reported no difference in the perception of real and sham stimulation (Herwig et al., 2010). Likewise, no significant changes in neural activity and connectivity have been detected using this coil position in other rTMS protocols (Nettekoven et al., 2014).
2.1.8. Data analysis
The kinematic data acquired during the TMS session was analyzed offline using the software 3DAWin (Version 1.2, MedCom, Munich, Germany) and the automated “segment analysis” tool of the 3DAWin software. The researcher performing the analysis was blinded about the stimulated site. First, recording artifacts were identified by visual inspection of the time series and discarded from further analysis. Then, kinematic data were filtered with standard filter bandwidths (for further technical details see (Nowak et al., 2007)), analyzed on a single trial level, and then averaged across trials.
2.1.9. Pointing task
For the pointing task, we analyzed the pointing frequency based on the movements in the sagittal (y‐) direction of marker I. Of note, the length of the pathways (mm) covered by the subject were analyzed in x‐, y‐, and z‐directions and calculated as 3D Euclidean distances. Furthermore, we calculated the deviation from the target by subtracting the actual covered distance from the ideal one (which was 160 mm). We also considered the number of inversion of velocity (NIV), which describes the smoothness and rhythmicity of a repetitive movement (Amengual et al., 2013), along with all three dimensions.
2.1.10. Index finger‐ and hand tapping task
For the tapping conditions, motor performance was quantified by (a) tapping frequency (Hz), (b) vertical movement amplitude (mm), and (c) the number of inversion of velocity (NIV). The data used for all analyses were obtained from the z‐axis of marker I which was attached to the index finger.
2.1.11. Statistical analysis
Statistical analyses were performed using the software package SPSS (Statistical Package for the Social Science, version 23, IBM). To assess age‐related differences between the two groups for the resting motor threshold and motor performance at baseline, we computed independent two‐sided t test. Of note, to isolate region‐specific stimulation effects, we initially calculated the ratio to the difference between verum and sham relative to sham ([VERUM‐SHAM]/SHAM), which were then used for all further analyses. Stimulation effects for each task were evaluated using repeated measures analyses of variance (rm‐ANOVA) including the within‐subject factor STIMULATION SITE (three levels: M1, dPMC, IPS) and the between‐subject factor GROUP (two levels: young and old). Post hoc t tests were used to elucidate significant effects (p < .05). Furthermore, correlation analyses were computed to reveal relationships between significant TMS effects and baseline motor performance. Of note, due to our explicit interests on age‐specific differences, we also computed linear correlations for both age groups separately.
All t tests were Bonferroni‐corrected for multiple comparisons. In addition, we reported the effect sizes.
2.2. Post hoc control experiment
After completing and analyzing all data of the main experiment, we decided to add a control experiment to validate and extend our conclusions drawn from the main experiment concerning the age‐related shift identified for ipsilateral primary motor cortex and dorsal premotor cortex. We were able to reassess a subgroup of subjects which also participated in the main experiment (n = 13; five young healthy subjects (three females), mean age 29.4 ± 4.3 SD years; range: 25–34 years and eight older healthy subjects (one female), mean age 63.1 ± 5.7 SD years; range: 56–70 years) for a follow‐up experiment aiming at measuring interhemispheric inhibition (IHI) exerted by ipsilateral M1 and dPMC on contralateral M1.
To assess IHI, we employed a paired‐pulse stimulation technique using two Magstim 200 machines (Magstim Co., Ltd, Whitland, United Kingdom), each equipped with a 70 mm figure‐of‐eight alpha coil (Ferbert et al., 1992). The test stimulus (TS) was delivered over the left primary motor cortex. The conditioning stimulus (CS) was delivered to right, respectively ipsilateral, primary motor cortex or right dorsal premotor cortex. The interstimulus interval between the conditioning and the test stimulus was 10 ms (Ni et al., 2009; Rossini et al., 2015), which is assumed to probe direct transcallosal connections (Chen, 2004; Chen, Yung, & Li, 2003). The coordinates of the ipsilateral TMS targets (M1, dPMC) were identical to those used in the main experiment. The contralateral M1 coordinate was equivalent to the left motor hotspot as defined above. For dPMC‐M1 stimulation, both coils were orientated as described in the main experiment for the corresponding stimulation sites. By contrast, for M1‐M1 stimulation the coils were orientated in a latero‐medial direction inducing a medially directed current to avoid spatial overlapping of the two coils. Coil positions were logged into the neuronavigation software and maintained throughout the experiment for each stimulation site.
Stimulation intensities were determined separately for each hemisphere and orientation. The test stimuli were applied at the minimum intensity required to evoke a MEP of 1 mV peak‐to‐peak amplitude. The conditioning stimuli were applied at 100% RMT (see above for details on defining the RMT). Although M1‐M1 interhemispheric inhibition is usually assessed with a suprathreshold CS, we decided for this CS‐intensity because we primarily aimed at comparing M1‐M1‐IHI and dPMC‐M1‐IHI, and therefore sought to have comparable stimulation parameters with similar tactile and acoustic effects between stimulation sites (Bäumer et al., 2009; Bestmann et al., 2010; Mochizuki, Huang, & Rothwell, 2004). Importantly, our pre‐experimental tests confirmed that a 100% RMT CS and a latero‐medial coil orientation is sufficient to evoke significant M1‐M1‐IHI.
For the experiment, 15 trials per stimulation site were recorded in an interleaved design. MEPs were recorded using surface EMG from the right FDI as described for the main experiment. IHI was calculated on an individual trial basis for each of the stimulation conditions (dPMC‐M1; M1‐M1) separately, that is, by computing the ratio between the mean peak‐to‐peak amplitude of the conditioned and unconditioned MEPs (Ferbert et al., 1992; Mochizuki et al., 2004). We first eliminated outliers which were attributable to erroneous trials when the EMG signal showed some level of muscle preactivity. Subsequently, we excluded all trials of unconditioned MEPs with EMG response lower than 50 μV, and the corresponding conditioned MEP, from our analyses, since these trials cannot be considered as valid test stimuli. Together, these steps removed 4.3% of the data. Analyses revealed that the data of conditioned and unconditioned MEPs for both stimulation sites were normally distributed (p > .1, Shapiro Wilk test). Therefore, IHI values for each stimulation site were compared using repeated measures analyses of variance (rm‐ANOVA) including the within‐subject factor STIMULATION SITE (two levels: M1, dPMC) and the between‐subject factor GROUP (two levels: young, old). Post hoc two‐sided t tests were used to reveal significant between‐group effects (p < .05). In addition, Pearson correlations were computed to elucidate linear relationships between interhemispheric inhibition and significant TMS effects of ipsilateral M1 and dPMC.
3. RESULTS
3.1. Cortical excitability
Resting motor threshold did not significantly differ between the young (53.1% MSO ± 11.9% SD) and the older subjects (48.7% MSO ± 12.4% SD) (p = .28, d = 0.38, t (30) = 1.09).
3.2. Age difference of motor performance at baseline
We first compared behavioral data between “young” and “old” in the control condition (sham) as an index of motor performance in the absence of a specific neural perturbation (Nettekoven et al., 2014). As expected, for the pointing task, we found a robust between‐group difference concerning the pointing frequency. Older subjects showed a significant slowing compared to younger subjects (p < .001, d = 0.86, t (24.42) = 4.79) (Figure 4). Moreover, a between‐group difference for pointing NIV—a parameter for smoothness and rhythmicity of a movement—was evident with reduced movement smoothness in the older subjects (p = .02, d = 0.65, t (18.14) = 2.99). However, we found no between‐group difference for pointing accuracy, as assessed by absolute deviation from the target (p = 1.0, d = 0.21, t (30) = 0.61).
Figure 4.
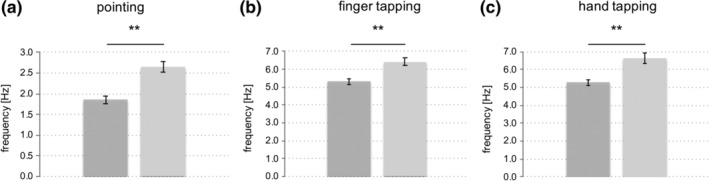
Between‐group differences in motor performance in the sham condition. (a) Pointing frequency, (b) maximum finger tapping frequency, (c) maximum hand tapping frequency, under the control condition (sham stimulation). Importantly, there were no significant differences concerning task accuracy as assessed by NIV (not shown due to no significant effect); (**p ≤ .001, two‐sided t test; error bars: standard error of the mean)
For both tapping conditions (i.e., finger tapping and hand tapping) we found a between‐group difference with respect to tapping frequency, with a significantly slower tapping performance in older subjects compared to young subjects (finger tapping: frequency p = .002, d = 0.67, t (30) = 3.71; hand tapping: frequency p < .001, d = 0.71, t (21.95) = 4.02) (Figure 4). By contrast, tapping smoothness and rhythmicity as assessed by tapping NIV did not significantly differ between groups (finger tapping: p = .22, d = 0.61, t (16.7) = 1.77; hand tapping: p = .40, d = 0.48, t (30) = 1.31). In summary, we found a task‐independent deterioration of movement frequency as well as a reduced rhythmicity during the pointing task in the group of old subjects, consistent with the expected decline in motor performance with higher age.
To rule out that fatigue or learning effects impacted upon motor performance, we compared means of motor performance of the first block to the ones of the last block and found no significant difference neither for young nor for older subjects (young subjects: pointing accuracy: p = .30, d = 0.16, t (14) = 1.09; finger tapping frequency: p = .45, d = 0.07, t (14) = 0.78, hand tapping frequency: p = .47, d = 0.06, t (14) = 1.57; older subjects: pointing accuracy: p = .90, d = 0.02, t (16) = 0.13; finger tapping frequency: p = .52, d = 0.11, t (16) = 0.63; hand tapping frequency: p = .79, d = 0.04, t (16) = 0.43). This also indicates that TMS intervention effects were stable across the entire experiment, with no evidence for after‐effects or additive effects with higher number of administered TMS pulses.
3.3. TMS effects on motor performance during the pointing task
The analysis of the pointing task did not reveal any significant main or interaction effects for pointing frequency (repeated measures ANOVA: main effect STIMULATION SITE: F (2,60) = 0.33, p = .72, η 2 = 0.01; interaction effect STIMULATION SITE × GROUP: F (2,60) = 0.52, p = .6, η 2 = 0.02; main effect GROUP: F (1,30) = 3.34, p = .08, η 2 = 0.1). Hence, younger and older subjects showed no region‐specific task‐effect.
However, for deviation from pointing target, we found a significant main effect involving the factor STIMULATION SITE (F (2,60) = 3.59, p = .03, η 2 = 0.11) (interaction effect STIMULATION SITE × GROUP: F (2,60) = 0.547, p = .58, η 2 = 0.02; main effect GROUP: F (1,30) = 0.002, p = .97, η 2 = 0). Post hoc dependent two‐sample t tests revealed a significant effect for IPS‐stimulation when comparing the three stimulation sites (IPS vs. dPMC: p = .01, d = 0.36, t (31) = −2.97; IPS vs. M1: p = .05, d = 0.22, t (31) = 1.80; M1 vs. dPMC: p = .43, d = 0.14, t (31) = 0.79), indicating that subjects showed a greater deviation from the given target upon TMS interference with ipsilateral IPS. Importantly, this effect also significantly differed from the control condition (IPS: p = .027, d = 0.51, t (31) = 2.90; M1: p = .12, d = 0.38, t (31) = 2.13; dPMC: p = .17, d = 0.35, t (31) = 1.98) (Figure 5).
Figure 5.
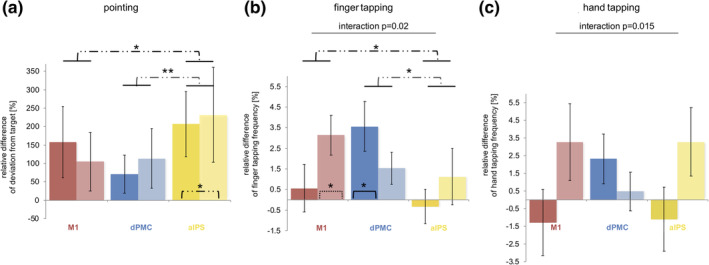
TMS effects on motor performance at different stimulation sites. Dissociation with regard to age and stimulation site for (b) finger tapping frequency: Young subjects (brighter columns and dash lines) only improved their finger tapping frequency upon interference with M1 (p = .018, one‐sample two‐sided t test), while older subjects (darker columns and solid lines) exclusively enhanced it during dPMC‐stimulation (p = .027, one‐sample two‐sided t test) (ANOVA: main effect [STIMULATION SITE] p = .039, interaction effect [STIMULATION SITE × GROUP] p = .02, dependent t test: M1xIPS: p = .04; dPMCxM1: p = .39; dPMCxIPS: p = .03); and for (c) hand tapping frequency: Again, TMS over dPMC improved tapping frequencies of older subjects while young subjects improved hand tapping frequency upon interference with M1 and IPS (ANOVA: interaction effect [STIMULATION SITE × GROUP] p = .015). (a) By contrast, TMS upon IPS reduced all subjects' accuracy as the target deviation increased irrespective of age (dependent t test: IPSxdPMC: p = .01; IPSxM1: p = .05; M1xdPMC: p = .43); (**p < .01, *p ≤ .05, asterisks within columns mark results of one‐sample t tests, error bars: standard error of the mean)
In addition, a similar effect was evident with regard to NIV of the pointing task. We found a significant main effect for STIMULATION SITE (F (2,60) = 4.64, p = .01, η 2 = 0.13) but no main effect for GROUP (F (1,30) = 2.04, p = .16, η 2 = 0.06) or interaction for STIMULATION SITE × GROUP (F (2,60) = 0.243, p = .785, η 2 = 0.01). Although subjects tended to increase the NIV upon interference with IPS and dPMC compared to M1‐stimulation (IPS vs. M1: p = .01, d = 0.51, t (31) = −2.73; M1 vs. dPMC: p = .04, d = 0.42, t (31) = −2.11; IPS vs. dPMC: p = .37, d = 0.16, t (31) = −0.90), only interference with ipsilateral IPS tended to differ from the control condition (ipsilateral M1: p = .23, d = 0.02, t (31) = 1.21; ipsilateral dPMC: p = .21, d = 0.23, t (31) = 1.29; ipsilateral IPS: p = .098, d = 0.30, t (31) = 1.71).
3.4. TMS effects on motor performance during the finger tapping task
Comparing the sham‐normalized TMS data, we found a significant main effect of STIMULATION SITE (F (2,60) = 3.43, p = .039, η 2 = 0.10) and a significant interaction effect for STIMULATION SITE × GROUP (F (2,56) = 3.98, p = .02, η 2 = 0.12) for finger tapping frequency (main effect GROUP: F (1,30) = 0.31, p = .58, η 2 = 0.1), indicating an age‐dependent TMS effect for at least one of the three stimulation sites. Post hoc t tests revealed a double dissociation with respect to region and age which further elucidated this interaction effect: For younger subjects, we found a significant increase in frequency upon ipsilateral M1‐interference (p = .018, d = 0.84, t (14) = 3.25, one‐sample two‐sided t test) but not for interference with ipsilateral dPMC (p = .22, d = 0.50, t (14) = 1.92) and ipsilateral IPS (p = .43, d = 0.20, t (14) = 0.81). By contrast, older subjects showed an increase in tapping frequency during ipsilateral dPMC‐interference (p = .027, d = 0.72, t (16) = 2.97), but did not upon TMS‐induced disturbance of ipsilateral M1 and IPS (M1: p = .63, d = 0.12, t (16) = 0.49; IPS: p = .71, d = 0.09, t (16) = 0.38) (Figure 5).
Importantly, we did not find TMS effects for finger tapping amplitude (main effect of STIMULATION SITE: F (2,60) = 0.31, p = .74, η 2 = 0.01; interaction effect for STIMULATION SITE × GROUP: F (2,60) = 1.01, p = .37, η 2 = 0.03; main effect GROUP: F (1,30) = 0.59, p = .45, η 2 = 0.02) or NIV of finger tapping (main effect of STIMULATION SITE: F (2,60) = .367, p = .649, η 2 = 0.01; interaction effect for STIMULATION SITE × GROUP: F (2,60) = 0.858, p = .429, η 2 = 0.03; main effect GROUP: F (1,30) = 1.97, p = .17, η 2 = 0.06), indicating that the improvement in frequency was not accompanied by reduced tapping amplitude or movement smoothness.
Furthermore, there was no significant correlation between baseline finger tapping frequency and the TMS effect upon ipsilateral M1‐interference (all p > .1). Yet, we found a significant linear relationship between changes of finger tapping frequency evoked by ipsilateral dPMC‐stimulation and baseline finger tapping (r = −.40, p = .04). However, when testing both age groups separately, we found that this correlation was primarily driven by the old subjects (young group: r = .08, p = .76; old group: r = −.66, p = .008). Further correlation analyses, especially with age, did not reveal any significant relationship.
Subsequently, we sought to link our findings for the finger tapping task to interhemispheric inhibition effects. Repeated measures ANOVA indicated a significant main effect for STIMULATION SITE (F (1,11) = 19.94, p = .001, η 2 = 0.64) and a significant interaction effect for STIMULATION SITE × GROUP (F (1,11) = 12.41, p = .005, η 2 = 0.53) (main effect GROUP: F (1,11) = 4.01, p = .07, η 2 = 0.27), thereby pointing to an age‐dependent difference in interhemispheric inhibition. A post hoc t test revealed a double dissociation for region and age, hence resembling TMS effects observed for finger tapping frequency: For interhemispheric inhibition from ipsilateral M1 to contralateral M1, we found a significant group difference between young and old subjects (p = .002, d = 2.27, t (10) = 3.95). While young participants showed strong M1‐M1 IHI with significantly reduced conditioned MEPs (33.98% ± 22.87% SD; p = .016, d = 1.62, t (4) = 4.01, two‐sample t test between unconditioned MEPs and conditioned MEP), IHI in older subjects was diminished compared to young individuals (86.31% ± 23.44% SD; p = .31, d = 0.25, t (7) = 1.10). Consistent with this result, we found a significant linear relationship between individual changes of finger tapping frequency evoked by ipsilateral M1‐stimulation and interhemispheric M1‐M1 inhibition only in young subjects (young group: r = −.997, p = .002; old group: r = −.22, p = .60) (Figure 6). Although, for interhemispheric inhibition from ipsilateral dPMC to contralateral M1, we found a nonsignificant between‐group difference (p = .14, d = 0.54, t (11) = 1.57), young subjects featured on average facilitatory dPMC‐influences in (116.1% ± 23.4% SD), whereas older participants showed a decrease of facilitation compared to young subjects with a trend toward inhibition (94.0% ± 22.0% SD). Plotting the individual data revealed inhibitory influences in a relevant number of older subjects while none of the young subjects showed inhibitory dPMC‐M1 interactions (Figure 7). Moreover, we found a significant correlation between the TMS effects during ipsilateral dPMC‐stimulation in older but not in younger participants (young group: r = .30, p = .59; old group: r = −.71, p = .049), indicating that in particular older individuals featuring stronger interhemispheric inhibition were more susceptible to interference with ipsilateral dPMC (Figure 6). Further correlation analyses between age and IHI did not reveal any significant relationship. Hence, the IHI analyses support the findings for finger tapping performance with a differential effect of age on the contribution of ipsilateral M1 and dPMC.
Figure 6.
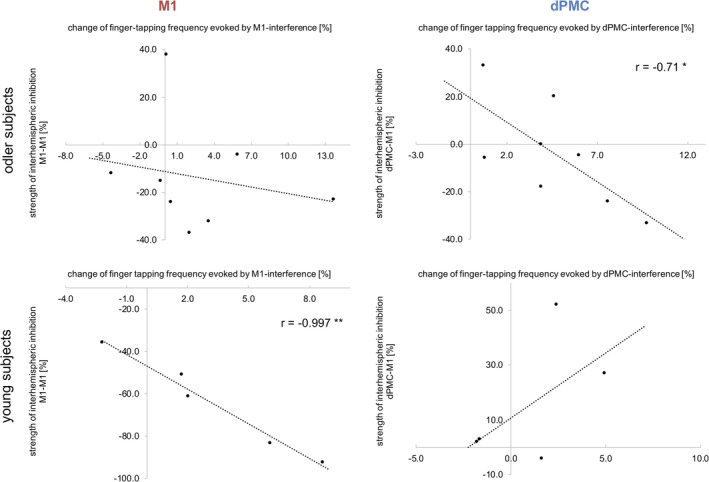
Correlation analyses between strength of IHI and TMS effect. The strength of interhemispheric inhibition between both M1 correlated negatively with the change of finger tapping frequency evoked by TMS interference with ipsilateral M1 only in young healthy individuals. By contrast, in older subjects IHI between ipsilateral dPMC and contralateral M1 correlated negatively with the effect of ipsilateral dPMC‐interference for finger tapping frequency; (*p < .05)
Figure 7.
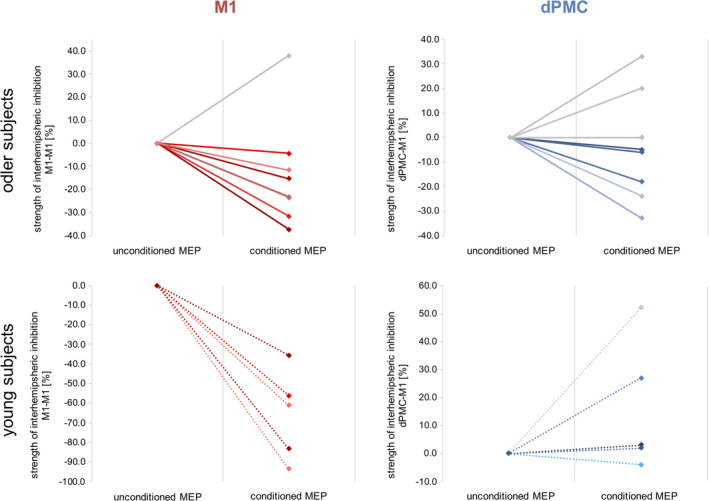
Individual data of inhibition effects. The single subject data of interhemispheric inhibition between M1‐M1 and dPMC‐M1. Please note, that older subjects featuring interhemispheric inhibitory influences are marked with color
3.5. TMS effects on motor performance during the hand tapping task
For hand tapping frequency, no significant main effect was evident for the factor STIMULATION SITE (F (2,60) = 0.06, p = .94, η 2 = 0.002). However, paralleling the findings of finger tapping, we found an interaction effect for STIMULATION SITE × GROUP (F (2,60) = 4.55, p = .015, η 2 = 0.13) (main effect GROUP: F (1,30) = 1.32, p = .26, η 2 = 0.04). Likewise, hand tapping frequencies tended to be faster in younger subjects when interfering with ipsilateral M1 and, additionally, with ipsilateral IPS, while older subjects showed an increase in tapping frequency under ipsilateral dPMC‐stimulation. However, effects were much weaker as indicated by the nonsignificant post hoc one‐sample t tests (young group: ipsilateral M1: p = .16, d = 0.39, t (14) = 1.50; ipsilateral dPMC: p = .68, d = 0.11, t (14) = 0.43, ipsilateral IPS: p = .11, d = 0.44, t (14) = 1.69) (old group: ipsilateral M1: p = .50, d = 0.17, t (16) = 0.69; ipsilateral dPMC: p = .12, d = 0.40, t (16) = 1.63; ipsilateral IPS: p = .55, d = 0.15, t (16) = 0.61) (Figure 5). Likewise, neither M1‐M1 nor dPMC‐M1 IHI correlated with hand tapping effects.
4. DISCUSSION
We here applied online TMS interference to investigate the functional relevance of ipsilateral frontoparietal regions for motor performance in young and older subjects. At the behavioral level, older subjects featured a significant slowing in all investigated motor tasks. Importantly, there was no group difference concerning movement accuracy, indicating that preserved movement accuracy was presumably achieved via reduced movement speed. Interfering with neural activity during task performance yielded differential effects depending on age and stimulation site. A novel finding of our study is the differential relevance of ipsilateral M1 and ipsilateral dPMC on fast repetitive movements depending on age, which was paralleled by age‐related changes of IHI. In particular, the current findings are compatible with an age‐related functional shift from ipsilateral M1 toward ipsilateral dPMC in the attempt to maintain tapping performance at high frequencies in older subjects, while interfering with anterior superior parietal cortex activity affected visuomotor performance independent of age.
4.1. HAROLD and the motor system: The role of ipsilateral primary motor cortex in age
In young adults, there is compelling evidence that ipsilateral M1 contributes to motor tasks of increasing complexity. Neuroimaging‐ as well as TMS studies have revealed that tasks particularly requiring fine‐tuned temporal regulation or high muscle selectivity involve ipsilateral M1 (Chen, Gerloff, Hallett, & Cohen, 1997; Davare, Andres, et al., 2006; Hummel et al., 2003; Kim et al., 1993; Verstynen, 2004). In agreement with these findings, we here observed that interference with ipsilateral M1 significantly impacted upon both finger and hand tapping tasks in young healthy subjects. Specifically, ipsilateral M1‐disruption led to higher tapping frequencies without changes in tapping amplitudes or movement smoothness in both tasks, suggesting an actual improvement of motor performance. Since the majority of previous online TMS studies have reported detrimental behavioral responses for interfering with ipsilateral M1 (Chen et al., 1997; Davare, Andres, et al., 2006; Foltys et al., 2001), our present data—at least at first sight—seems to be at odds with these previous studies. However, the fact that we observed an increase of frequency upon ipsilateral M1 interference not only for the finger tapping but also for the hand tapping task, which served as an additional internal control task, strengthen our data. Moreover, Davare and colleagues (Davare, Duque, Vandermeeren, Thonnard, & Olivier, 2006) have shown that disruption of ipsilateral M1 could either advance or delay muscle recruitment dependent on the timing of TMS interference, adding additional support for differential effects of online TMS interference.
From a mechanistic perspective, online TMS applied to ipsilateral M1 might have modulated interhemispheric inhibition between bilateral M1 which is in accordance with the significant relationship that we found between TMS effects of ipsilateral M1 stimulation and interhemispheric inhibition exerted by ipsilateral M1 in young subjects. In addition, TMS studies as well as connectivity analyses based on task fMRI data have also provided converging evidence that each M1 exerts reciprocal influences onto its contralateral homolog, which seems to be crucial for motor control and muscle recruitment (Di Lazzaro et al., 1999; Ferbert et al., 1992; Meyer et al., 1995).
Accordingly, the interhemispheric influences exerted from ipsilateral M1 onto the contralateral M1 have been shown to be initially inhibitory at rest, to decrease progressively when approaching muscle contraction, and to reverse to facilitation before and during muscle contraction (Davare, Andres, et al., 2006; Duque et al., 2005; Murase, Duque, Mazzocchio, & Cohen, 2004). Therefore, depending on the timing of TMS disruption relative to movement execution, it could either advance or delay muscle recruitment (Davare, Duque, et al., 2006). Accordingly, with respect to the present study, TMS interference seems to have affected the inhibitory role of M1 leading to a more effective disinhibition of contralateral M1, thereby increasing tapping frequency in young subjects (Davare, Andres, et al., 2006; Volz et al., 2017).
For older subjects, neuroimaging studies have typically revealed increased activity in ipsilateral M1 compared to young individuals during hand‐motor tasks (Mattay et al., 2002; Riecker et al., 2006; Ward & Frackowiak, 2003), consistent with an extension of the HAROLD model into the motor domain. We could show that interhemispheric inhibition between both M1 is attenuated in older subjects compared to young individuals, which is supported by a number of studies (Coppi et al., 2014; Talelli, Ewas, Waddingham, Rothwell, & Ward, 2008; Talelli, Waddingham, Ewas, Rothwell, & Ward, 2008). Hence, less hemispheric asymmetry might be a consequence of reduced interhemispheric inhibition effects leading to disinhibition of neural activity in ipsilateral M1 (Langan et al., 2010; Talelli, Greenwood, & Rothwell, 2006; Ward et al., 2008). As several authors could not link over‐activation of ipsilateral M1 to better motor performance, higher activity of this area might also represent unspecific disinhibition rather than compensatory mechanisms (Riecker et al., 2006; Talelli, Ewas, et al., 2008).
Taken together, these findings are relevant for the interpretation of our data, as unlike in younger subjects, we could not find a significant functional involvement of ipsilateral M1 in the employed motor tasks in older subjects.
4.2. PASA and the motor system: Premotor shift in aging
PASA would predict an aging‐associated shift from posterior to anterior regions, not only in the contralateral but also ipsilateral hemisphere (Davis et al., 2008; Michely et al., 2018), which is paralleled by a key finding of our study—an age‐related anterior shift within the motor network. The disruption of ipsilateral dPMC‐activity affected motor performance in older but not younger subjects, indicating a shift from ipsilateral M1 to premotor cortex during repetitive high‐frequency movements.
One might argue that the observed stimulation effects in dPMC in the older group may result from stimulating adjacent M1 neurons, or unspecific facilitation effects due to the sensory input associated with TMS (Duecker & Sack, 2013). However, since we observed a double dissociation between groups and brain areas, this hypothesis appears rather unlikely.
Given that neuroimaging studies have frequently demonstrated a greater activation of ipsilateral premotor regions during motor tasks with advancing age (Heuninckx, Wenderoth, & Swinnen, 2008; Mattay et al., 2002; Riecker et al., 2006; Ward & Frackowiak, 2003) and we here found an improvement of motor performance, one interpretation of the present data is an detrimental influence of ipsilateral premotor cortex activity upon contralateral M1, which is released by TMS interference—similar to what has been described for neural over‐activity in the contralesional hemisphere in stroke patients (Grefkes et al., 2008; Murase et al., 2004; Rehme, Fink, Cramon, Y, & Grefkes, 2011; Volz et al., 2017).
However, we would like to challenge this interpretation, especially since we could also detect an improvement in task performance evoked by TMS in young healthy individuals in whom it seems rather unlikely that ipsilateral areas hold a detrimental role for motor performance. As the effects evoked by the stimulation are thought to be influenced by several factors like timing of stimulation onset or stimulation intensity (Davare, Duque, et al., 2006; Foltys et al., 2001; Jahanshahi & Rothwell, 2000; Silvanto & Cattaneo, 2017; Walsh & Cowey, 2000), the TMS‐induced effects of a distinct region allow to draw conclusions about the causal and functional involvement of this region, but does not necessarily determine a beneficial or maladaptive role.
Highly similar to ipsilateral M1, also ipsilateral dPMC modulates the activity of contralateral M1 through inhibitory and facilitatory influences (Bäumer et al., 2009; Hinder, Fujiyama, & Summers, 2012; Koch et al., 2006; O'Shea, Sebastian, Boorman, Johansen‐Berg, & Rushworth, 2007). Most likely these effects are mediated on the anatomical basis of direct commissural fibers from dorsal premotor cortex to contralateral M1, which have been confirmed in monkeys (Boussaoud, Tanné‐Gariépy, Wannier, & Rouiller, 2005; Jenny, 1979; Marconi, Genovesio, Giannetti, Molinari, & Caminiti, 2003). This notion receives additional support since IHI with short interstimulus intervals is suggested to probe direct transcallosal pathways (Chen, 2004; Chen et al., 2003; Hinder et al., 2012).
Hinder and colleagues found that older subjects particularly rely on a stronger modulation of inhibition toward facilitation to release the motor signal to the contralateral hand (Hinder et al., 2012; Levin, Fujiyama, Boisgontier, Swinnen, & Summers, 2014). Although we were able to assess IHI only in a limited subgroup of the original cohort, we here found hints that, compared to young subjects, interhemispheric interactions between ipsilateral dPMC and contralateral M1 of older participants were shifted toward inhibition. Therefore, a similar modulation stated for ipsilateral M1 in young subjects, namely a TMS‐induced disruption of the inhibition exerted from ipsilateral dPMC onto contralateral M1, may have led to a more effective disinhibition of contralateral M1 and mediated the improvement of motor performance in older subjects. This notion receives further support from the observed relationship between TMS effects of ipsilateral dPMC stimulation and IHI exerted by ipsilateral dPMC in older subjects. Hence, during repetitive high‐frequency movements, ipsilateral dPMC presented a similar TMS profile in older subjects, analogous to the response of younger subjects in ipsilateral M1. Furthermore, the interhemispheric inhibition exerted from ipsilateral dPMC in older subjects revealed a similar relationship to the TMS effect of dPMC interference, paralleling the results of interhemispheric inhibition and TMS effect of ipsilateral M1 in young subjects.
Notably, the IHI experiment bears some limitations such as the small sample size which reduces the generalizability of our data and the fact that both experiments were not conducted on the same day. Nevertheless, the age‐related differential findings fit the results obtained in the main experiment and support our interpretation of an age‐related functional shift for finger tapping performance.
So far, neuroimaging studies have revealed that premotor cortex is more activated during motor tasks in older individuals (Heuninckx, 2005; Heuninckx et al., 2008; Ward et al., 2008) and our data is compatible with a causal involvement of dorsal premotor cortex in motor tasks in older individuals. Given that we found a relationship between baseline motor performance and changes of finger tapping frequency evoked by ipsilateral premotor cortex stimulation, dorsal premotor cortex seems to be particularly relevant in older subjects with poor motor performance. Therefore, the age‐related functional premotor shift, although potentially representing a reorganization due to structural and biochemical changes in the motor system (Seidler et al., 2015; Talelli, Waddingham, et al., 2008; Ward et al., 2008), could not be considered as fully compensatory from a behavioral perspective. Hence, compensation may only be feasible to a limited degree, and those subjects with overt reduction in motor performance, indicating insufficient compensation, are especially sensitive to TMS interference.
4.3. The role of the ipsilateral anterior intraparietal sulcus
For the visuomotor pointing task, we found a similar stimulation effect for TMS interference with ipsilateral IPS for both young and older individuals. There is a large body of literature from both monkeys and humans reporting that anterior intraparietal cortex is firmly engaged in visuospatial aspects of visually guided hand‐motor tasks (Binkofski et al., 1998; Binkofski et al., 1999; Culham et al., 2003; Grefkes et al., 2004; Kalaska, Cisek, & Gosselin‐Kessiby, 2003). Moreover, IPS serves as an interface for the integration of visual, motor, somatosensory, and spatial information (Grefkes & Fink, 2005). Therefore, it seems plausible, that in our study interference with IPS impaired the target accuracy of reaching movements relying on visuomotor integration. These data are compatible with previous findings reported by Davare and colleagues who showed that interfering with both contra‐ and ipsilateral IPS by means of single pulse TMS leads to errors in target‐directed movements, highlighting the functional involvement of bilateral IPS in young controls (Davare, Zénon, Desmurget, & Olivier, 2015). Our data showed comparable effects of ipsilateral IPS‐interference in younger and older subjects, suggesting a similar role in visually guided movements independent of age. Interestingly, despite the evidence that also posterior parietal areas including the IPS show higher task‐related activity in older subjects (Labyt et al., 2004; Ward & Frackowiak, 2003), we could not dissociate between young and older subjects the role of this region in maintaining pointing performance. However, an alternative explanation for the absence of an age‐dependent functional dissociation of IPS is that other areas than tested in the present study like, for example, inferior parietal cortex or more posterior parietal regions might be more relevant for maintaining visuomotor performance specifically in older subjects (Mattay et al., 2002; Ward, 2006).
5. CONCLUSION
In conclusion, we here provide evidence for differential functional roles of ipsilateral M1 and dPMC in age with a decline of relevance of M1 and a functional shift of importance toward dPMC for repetitive high‐frequency movements associated with aging. Our results apply existing models of aging‐associated changes of PASA and HAROLD to the motor system, but extend these accounts regarding the causal relevance of reorganization of neural activity in aging.
ACKNOWLEDGMENTS
C.G. is supported by the German Research Foundation (DFG GR 3285/2‐1). C.G. and G.R.F. are also supported by the Universität zu Köln Emerging Groups Initiative (CONNECT group) implemented into the Institutional Strategy of the Universität zu Köln and the German Excellence Initiative. G.R.F. gratefully acknowledges additional support from the Marga und Walter Boll‐Stiftung.
Tscherpel C, Hensel L, Lemberg K, et al. Age affects the contribution of ipsilateral brain regions to movement kinematics. Hum Brain Mapp. 2020;41:640–655. 10.1002/hbm.24829
Funding information Marga und Walter Boll‐Stiftung; German Excellence Initiative; Universität zu Köln Emerging Groups Initiative; German Research Foundation, Grant/Award Number: DFG GR 3285/2‐1
DATA AVAILABILITY STATEMENT
The data that support the findings of this study and all custom‐written codes are available from the corresponding author upon reasonable request.
REFERENCES
- Amengual, J. L. , Rojo, N. , Veciana de Las Heras, M. , Marco‐Pallares, J. , Grau‐Sanchez, J. , Schneider, S. , … Rodríguez‐Fornells, A. (2013). Sensorimotor plasticity after music‐supported therapy in chronic stroke patients revealed by transcranial magnetic stimulation. PLoS One, 8(4), e61883 10.1371/journal.pone.0061883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune, T. K. , Ettema, G. , & Vereijken, B. (2016). Bilateral asymmetry in upper extremities is more pronounced in distal compared to proximal joints. Journal of Motor Behavior, 48(2), 143–152. 10.1080/00222895.2015.1056766 [DOI] [PubMed] [Google Scholar]
- Awiszus, F. (2003). TMS and threshold hunting. Supplements to Clinical Neurophysiology, 56, 13–23. [DOI] [PubMed] [Google Scholar]
- Bäumer, T. , Schippling, S. , Kroeger, J. , Zittel, S. , Koch, G. , Thomalla, G. , … Münchau, A. (2009). Inhibitory and facilitatory connectivity from ventral premotor to primary motor cortex in healthy humans at rest—A bifocal TMS study. Clinical Neurophysiology, 120(9), 1724–1731. 10.1016/j.clinph.2009.07.035 [DOI] [PubMed] [Google Scholar]
- Bernard, J. A. , & Seidler, R. D. (2012). Evidence for motor cortex dedifferentiation in older adults. Neurobiology of Aging, 33(9), 1890–1899. 10.1016/j.neurobiolaging.2011.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann, S. , Swayne, O. , Blankenburg, F. , Ruff, C. C. , Teo, J. , Weiskopf, N. , … Ward, N. S. (2010). The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS‐fMRI. Journal of Neuroscience, 30(36), 11926–11937. 10.1523/JNEUROSCI.5642-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski, F. , Buccino, G. , Stephan, K. M. , Rizzolatti, G. , Seitz, R. J. , & Freund, H. J. (1999). A parieto‐premotor network for object manipulation: Evidence from neuroimaging. Experimental Brain Research, 128(1–2), 210–213. 10.1007/s002210050838 [DOI] [PubMed] [Google Scholar]
- Binkofski, F. , Dohle, C. , Posse, S. , Stephan, K. M. , Hefter, H. , Seitz, R. J. , & Freund, H. J. (1998). Human anterior intraparietal area subserves prehension—A combined lesion and functional MRI activation study. Neurology, 50(5), 1253–1259. [DOI] [PubMed] [Google Scholar]
- Boussaoud, D. , Tanné‐Gariépy, J. , Wannier, T. , & Rouiller, E. M. (2005). Callosal connections of dorsal versus ventral premotor areas in the macaque monkey: A multiple retrograde tracing study. BMC Neuroscience, 6, 67 10.1186/1471-2202-6-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetefisch, C. M. , Revill, K. P. , Shuster, L. , Hines, B. , & Parsons, M. (2014). Motor demand‐dependent activation of ipsilateral motor cortex. Journal of Neurophysiology, 112(4), 999–1009. 10.1152/jn.00110.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza, R. , Anderson, N. D. , Locantore, J. K. , & McIntosh, A. R. (2002). Aging gracefully: Compensatory brain activity in high‐performing older adults. NeuroImage, 17(3), 1394–1402. 10.1006/nimg.2002.1280 [DOI] [PubMed] [Google Scholar]
- Cabeza, R. , Grady, C. L. , Nyberg, L. , McIntosh, A. R. , Tulving, E. , Kapur, S. , … Craik, F. I. (1997). Age‐related differences in neural activity during memory encoding and retrieval: A positron emission tomography study. The Journal of Neuroscience, 17(1), 391–400. 10.1523/jneurosci.17-01-00391.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalan, M. J. , Honda, M. , Weeks, R. A. , Cohen, L. G. , & Hallett, M. (1998). The functional neuroanatomy of simple and complex sequential finger movements: A PET study. Brain, 121, 253–264. [DOI] [PubMed] [Google Scholar]
- Chen, R. (2004). Interactions between inhibitory and excitatory circuits in the human motor cortex. Experimental Brain Research, 154(1), 1–10. 10.1007/s00221-003-1684-1 [DOI] [PubMed] [Google Scholar]
- Chen, R. , Gerloff, C. , Hallett, M. , & Cohen, L. G. (1997). Involvement of the ipsilateral motor cortex in finger movements of different complexities. Annals of Neurology, 41(2), 247–254. 10.1002/ana.410410216 [DOI] [PubMed] [Google Scholar]
- Chen, R. , Yung, D. , & Li, J.‐Y. (2003). Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. Journal of Neurophysiology, 89(3), 1256–1264. 10.1152/jn.00950.2002 [DOI] [PubMed] [Google Scholar]
- Colby, C. L. (1998). Action‐oriented spatial reference frames in cortex. Neuron, 20(1), 15–24. 10.1016/s0896-6273(00)80429-8 [DOI] [PubMed] [Google Scholar]
- Colby, C. L. , & Goldberg, M. E. (1999). Space and attention in parietal cortex. Annual Review of Neuroscience, 22, 319–349. 10.1146/annurev.neuro.22.1.319 [DOI] [PubMed] [Google Scholar]
- Coppi, E. , Houdayer, E. , Chieffo, R. , Spagnolo, F. , Inuggi, A. , Straffi, L. , … Leocani, L. (2014). Age‐related changes in motor cortical representation and interhemispheric interactions: A transcranial magnetic stimulation study. Frontiers in Aging Neuroscience, 6, 209 10.3389/fnagi.2014.00209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer, S. C. , Finklestein, S. P. , Schaechter, J. D. , Bush, G. , & Rosen, B. R. (1999). Activation of distinct motor cortex regions during ipsilateral and contralateral finger movements. Journal of Neurophysiology, 81(1), 383–387. 10.1152/jn.1999.81.1.383 [DOI] [PubMed] [Google Scholar]
- Culham, J. C. , Danckert, S. L. , De Souza, J. , Gati, J. S. , Menon, R. S. , & Goodale, M. A. (2003). Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Experimental Brain Research, 153(2), 180–189. 10.1007/s00221-003-1591-5 [DOI] [PubMed] [Google Scholar]
- Davare, M. , Andres, M. , Cosnard, G. , Thonnard, J.‐L. , & Olivier, E. (2006). Dissociating the role of ventral and dorsal premotor cortex in precision grasping. Journal of Neuroscience, 26(8), 2260–2268. 10.1523/JNEUROSCI.3386-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare, M. , Duque, J. , Vandermeeren, Y. , Thonnard, J.‐L. , & Olivier, E. (2006). Role of the Ipsilateral primary motor cortex in controlling the timing of hand muscle recruitment. Cerebral Cortex, 17(2), 353–362. 10.1093/cercor/bhj152 [DOI] [PubMed] [Google Scholar]
- Davare, M. , Zénon, A. , Desmurget, M. , & Olivier, E. (2015). Dissociable contribution of the parietal and frontal cortex to coding movement direction and amplitude. Frontiers in Human Neuroscience, 9, 241 10.3389/fnhum.2015.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, S. W. , Dennis, N. A. , Daselaar, S. M. , Fleck, M. S. , & Cabeza, R. (2008). Que PASA? The posterior–anterior shift in aging. Cerebral Cortex, 18(5), 1201–1209. 10.1093/cercor/bhm155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro, V. , Oliviero, A. , Profice, P. , Insola, A. , Mazzone, P. , Tonali, P. , & Rothwell, J. C. (1999). Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Experimental Brain Research, 124(4), 520–524. [DOI] [PubMed] [Google Scholar]
- Diekhoff, S. , Uludağ, K. , Sparing, R. , Tittgemeyer, M. , Cavuşoğlu, M. , Cramon, v. , … Grefkes, C. (2011). Functional localization in the human brain: Gradient‐echo, spin‐echo, and arterial spin‐labeling fMRI compared with neuronavigated TMS. Human Brain Mapping, 32(3), 341–357. 10.1002/hbm.21024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duecker, F. , & Sack, A. T. (2013). Pre‐stimulus sham TMS facilitates target detection. PLoS ONE, 8(3), e57765 10.1371/journal.pone.0057765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque, J. , Hummel, F. , Celnik, P. , Murase, N. , Mazzocchio, R. , & Cohen, L. G. (2005). Transcallosal inhibition in chronic subcortical stroke. NeuroImage, 28(4), 940–946. 10.1016/j.neuroimage.2005.06.033 [DOI] [PubMed] [Google Scholar]
- Ferbert, A. , Priori, A. , Rothwell, J. C. , Day, B. L. , Colebatch, J. G. , & Marsden, C. D. (1992). Interhemispheric inhibition of the human motor cortex. Journal of Physiology, 453, 525–546. 10.1111/(ISSN)1469-7793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, P. B. , Fountain, S. , & Daskalakis, Z. J. (2006). A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clinical Neurophysiology, 117(12), 2584–2596. 10.1016/j.clinph.2006.06.712 [DOI] [PubMed] [Google Scholar]
- Foltys, H. , Sparing, R. , Boroojerdi, B. , Krings, T. , Meister, I. G. , Mottaghy, F. M. , & Töpper, R. (2001). Motor control in simple bimanual movements: A transcranial magnetic stimulation and reaction time study. Clinical Neurophysiology, 112(2), 265–274. [DOI] [PubMed] [Google Scholar]
- Gerloff, C. , Cohen, L. G. , Floeter, M. K. , Chen, R. , Corwell, B. , & Hallett, M. (1998). Inhibitory influence of the ipsilateral motor cortex on responses to stimulation of the human cortex and pyramidal tract. Journal of Physiology, 510(1), 249–259. 10.1111/j.1469-7793.1998.249bz.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady, C. (2012). The cognitive neuroscience of ageing. Nature Reviews Neuroscience, 13(7), 491–505. 10.1038/nrn3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes, C. , & Fink, G. R. (2005). The functional organization of the intraparietal sulcus in humans and monkeys. Journal of Anatomy, 207(1), 3–17. 10.1111/j.1469-7580.2005.00426.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes, C. , Nowak, D. A. , Eickhoff, S. B. , Dafotakis, M. , Küst, J. , Karbe, H. , & Fink, G. R. (2008). Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Annals of Neurology, 63(2), 236–246. 10.1002/ana.21228 [DOI] [PubMed] [Google Scholar]
- Grefkes, C. , Ritzl, A. , Zilles, K. , & Fink, G. R. (2004). Human medial intraparietal cortex subserves visuomotor coordinate transformation. NeuroImage, 23(4), 1494–1506. 10.1016/j.neuroimage.2004.08.031 [DOI] [PubMed] [Google Scholar]
- Hackel, M. E. , Wolfe, G. A. , Bang, S. M. , & Canfield, J. S. (1992). Changes in hand function in the aging adult as determined by the Jebsen test of hand function. Physical Therapy, 72(5), 373–377. [DOI] [PubMed] [Google Scholar]
- Hamada, M. , Murase, N. , Hasan, A. , Balaratnam, M. , & Rothwell, J. C. (2013). The role of interneuron networks in driving human motor cortical plasticity. Cerebral Cortex, 23(7), 1593–1605. 10.1093/cercor/bhs147 [DOI] [PubMed] [Google Scholar]
- Hardwick, R. M. , Rottschy, C. , Miall, R. C. , & Eickhoff, S. B. (2013). A quantitative meta‐analysis and review of motor learning in the human brain. NeuroImage, 67(C), 283–297. 10.1016/j.neuroimage.2012.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris‐Love, M. L. , Perez, M. A. , Chen, R. , & Cohen, L. G. (2007). Interhemispheric inhibition in distal and proximal arm representations in the primary motor cortex. Journal of Neurophysiology, 97(3), 2511–2515. 10.1152/jn.01331.2006 [DOI] [PubMed] [Google Scholar]
- Herwig, U. , Cardenas‐Morales, L. , Connemann, B. J. , Kammer, T. , & Schönfeldt‐Lecuona, C. (2010). Sham or real—Post hoc estimation of stimulation condition in a randomized transcranial magnetic stimulation trial. Neuroscience Letters, 471(1), 30–33. 10.1016/j.neulet.2010.01.003 [DOI] [PubMed] [Google Scholar]
- Heuninckx, S. (2005). Neural basis of aging: The penetration of cognition into action control. Journal of Neuroscience, 25(29), 6787–6796. 10.1523/JNEUROSCI.1263-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx, S. , Wenderoth, N. , & Swinnen, S. P. (2008). Systems neuroplasticity in the aging brain: Recruiting additional neural resources for successful motor performance in elderly persons. Journal of Neuroscience, 28(1), 91–99. 10.1523/JNEUROSCI.3300-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinder, M. R. (2012). Interhemispheric connectivity between distinct motor regions as a window into bimanual coordination. Journal of Neurophysiology, 107(7), 1791–1794. 10.1152/jn.00822.2011 [DOI] [PubMed] [Google Scholar]
- Hinder, M. R. , Fujiyama, H. , & Summers, J. J. (2012). Premotor‐motor interhemispheric inhibition is released during movement initiation in older but not young adults. PLoS ONE, 7(12), e52573 10.1371/journal.pone.0052573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel, F. , Kirsammer, R. , & Gerloff, C. (2003). Ipsilateral cortical activation during finger sequences of increasing complexity: Representation of movement difficulty or memory load? Clinical Neurophysiology, 114(4), 605–613. 10.1016/S1388-2457(02)00417-0 [DOI] [PubMed] [Google Scholar]
- Hutchinson, S. (2002). Age‐related differences in movement representation. NeuroImage, 17(4), 1720–1728. 10.1006/nimg.2002.1309 [DOI] [PubMed] [Google Scholar]
- Jahanshahi, M. , & Rothwell, J. (2000). Transcranial magnetic stimulation studies of cognition: An emerging field. Experimental Brain Research, 131(1), 1–9. 10.1007/s002219900224 [DOI] [PubMed] [Google Scholar]
- Jenny, A. B. (1979). Commissural projections of the cortical hand motor area in monkeys. The Journal of Comparative Neurology, 188(1), 137–145. 10.1002/cne.901880111 [DOI] [PubMed] [Google Scholar]
- Kalaska, J. F. , Cisek, P. , & Gosselin‐Kessiby, N. (2003). Mechanisms of selection and guidance of reaching movements in the parietal lobe. Advances in Neurology, 93, 97–119. [PubMed] [Google Scholar]
- Kim, S. G. , Ashe, J. , Hendrich, K. , Ellermann, J. M. , Merkle, H. , Uğurbil, K. , & Georgopoulos, A. P. (1993). Functional magnetic‐resonance‐imaging of motor cortex—Hemispheric‐asymmetry and handedness. Science, 261(5121), 615–617. [DOI] [PubMed] [Google Scholar]
- Koch, G. , Franca, M. , Fernandez Del Olmo, M. , Cheeran, B. , Milton, R. , Alvarez Sauco, M. , & Rothwell, J. C. (2006). Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. Journal of Neuroscience, 26(28), 7452–7459. 10.1523/JNEUROSCI.1158-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb, B. , Forgie, M. , Gibb, R. , Gorny, G. , & Rowntree, S. (1998). Age, experience and the changing brain. Neuroscience and Biobehavioral Reviews, 22(2), 143–159. [DOI] [PubMed] [Google Scholar]
- Labyt, E. , Szurhaj, W. , Bourriez, J. L. , Cassim, F. , Defebvre, L. , Destee, A. , & Derambure, P. (2004). Influence of aging on cortical activity associated with a visuo‐motor task. Neurobiology of Aging, 25(6), 817–827. 10.1016/j.neurobiolaging.2003.08.010 [DOI] [PubMed] [Google Scholar]
- Langan, J. , Peltier, S. J. , Bo, J. , Fling, B. W. , Welsh, R. C. , & Seidler, R. D. (2010). Functional implications of age differences in motor system connectivity. Frontiers in Systems Neuroscience, 4, 17 10.3389/fnsys.2010.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, O. , Fujiyama, H. , Boisgontier, M. P. , Swinnen, S. P. , & Summers, J. J. (2014). Aging and motor inhibition: A converging perspective provided by brain stimulation and imaging approaches. Neuroscience and Biobehavioral Reviews, 43, 100–117. 10.1016/j.neubiorev.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Liuzzi, G. , Hörniss, V. , Zimerman, M. , Gerloff, C. , & Hummel, F. C. (2011). Coordination of uncoupled bimanual movements by strictly timed interhemispheric connectivity. Journal of Neuroscience, 31(25), 9111–9117. 10.1523/JNEUROSCI.0046-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze, M. , Markert, J. , Sauseng, P. , Hoppe, J. , Plewnia, C. , & Gerloff, C. (2006). The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. Journal of Neuroscience, 26(22), 6096–6102. 10.1523/JNEUROSCI.4564-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi, B. , Genovesio, A. , Giannetti, S. , Molinari, M. , & Caminiti, R. (2003). Callosal connections of dorso‐lateral premotor cortex. European Journal of Neuroscience, 18(4), 775–788. 10.1046/j.1460-9568.2003.02807.x [DOI] [PubMed] [Google Scholar]
- Mattay, V. S. , Fera, F. , Tessitore, A. , Hariri, A. R. , Das, S. , Callicott, J. H. , & Weinberger, D. R. (2002). Neurophysiological correlates of age‐related changes in human motor function. Neurology, 58(4), 630–635. [DOI] [PubMed] [Google Scholar]
- Meyer, B. U. , Roricht, S. , Einsiedel, v. , G, H. , Kruggel, F. , & Weindl, A. (1995). Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus‐callosum. Brain, 118, 429–440. [DOI] [PubMed] [Google Scholar]
- Michely, J. , Volz, L. J. , Hoffstaedter, F. , Tittgemeyer, M. , Eickhoff, S. B. , Fink, G. R. , & Grefkes, C. (2018). Network connectivity of motor control in the ageing brain. NeuroImage: Clinical, 18, 443–455. 10.1016/j.nicl.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki, H. , Huang, Y.‐Z. , & Rothwell, J. C. (2004). Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. The Journal of Physiology, 561(1), 331–338. 10.1016/S0959-4388(01)00266-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase, N. , Duque, J. , Mazzocchio, R. , & Cohen, L. G. (2004). Influence of interhemispheric interactions on motor function in chronic stroke. Annals of Neurology, 55(3), 400–409. 10.1017/S0317167100000196 [DOI] [PubMed] [Google Scholar]
- Nettekoven, C. , Volz, L. J. , Kutscha, M. , Pool, E. M. , Rehme, A. K. , Eickhoff, S. B. , … Grefkes, C. (2014). Dose‐dependent effects of theta burst rTMS on cortical excitability and resting‐state connectivity of the human motor system. Journal of Neuroscience, 34(20), 6849–6859. 10.1523/JNEUROSCI.4993-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettekoven, C. , Volz, L. J. , Leimbach, M. , Pool, E.‐M. , Rehme, A. K. , Eickhoff, S. B. , … Grefkes, C. (2015). Inter‐individual variability in cortical excitability and motor network connectivity following multiple blocks of rTMS. NeuroImage, 118, 209–218. 10.1016/j.neuroimage.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neva, J. L. , Brown, K. E. , Mang, C. S. , Francisco, B. A. , & Boyd, L. A. (2017). An acute bout of exercise modulates both intracortical and interhemispheric excitability. The European Journal of Neuroscience, 45(10), 1343–1355. 10.1111/ejn.13569 [DOI] [PubMed] [Google Scholar]
- Ni, Z. , Gunraj, C. , Nelson, A. J. , Yeh, I.‐J. , Castillo, G. , Hoque, T. , & Chen, R. (2009). Two phases of interhemispheric inhibition between motor related cortical areas and the primary motor cortex in human. Cerebral Cortex, 19(7), 1654–1665. 10.1093/cercor/bhn201 [DOI] [PubMed] [Google Scholar]
- Nowak, D. A. , Grefkes, C. , Dafotakis, M. , Küst, J. , Karbe, H. , & Fink, G. R. (2007). Dexterity is impaired at both hands following unilateral subcortical middle cerebral artery stroke. European Journal of Neuroscience, 25(10), 3173–3184. 10.1111/j.1460-9568.2007.05551.x [DOI] [PubMed] [Google Scholar]
- O'Shea, J. , Sebastian, C. , Boorman, E. D. , Johansen‐Berg, H. , & Rushworth, M. F. S. (2007). Functional specificity of human premotor‐motor cortical interactions during action selection. The European Journal of Neuroscience, 26(7), 2085–2095. 10.1111/j.1460-9568.2007.05795.x [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone, A. , Bartres‐Faz, D. , & Keenan, J. P. (1999). Transcranial magnetic stimulation: Studying the brain‐behaviour relationship by induction of 'virtual lesions’. Philosophical Transactions of the Royal Society of London. Biological Sciences, 354(1387), 1229–1238. 10.1098/rstb.1999.0476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter, R. , & Lemon, R. (1995). Corticospinal function and voluntary movement. Oxford, UK: Oxford University Press; 10.1093/acprof:oso/9780198523758.001.0001 [DOI] [Google Scholar]
- Rehme, A. K. , Eickhoff, S. B. , Rottschy, C. , Fink, G. R. , & Grefkes, C. (2012). Activation likelihood estimation meta‐analysis of motor‐related neural activity after stroke. NeuroImage, 59(3), 2771–2782. 10.1016/j.neuroimage.2011.10.023 [DOI] [PubMed] [Google Scholar]
- Rehme, A. K. , Fink, G. R. , Cramon, v. , Y, D. , & Grefkes, C. (2011). The role of the contralesional motor cortex for motor recovery in the early days after stroke assessed with longitudinal FMRI. Cerebral Cortex, 21(4), 756–768. 10.1093/cercor/bhq140 [DOI] [PubMed] [Google Scholar]
- Reuter‐Lorenz, P. A. , & Cappell, K. A. (2008). Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science, 17(3), 177–182. 10.1111/j.1467-8721.2008.00570.x [DOI] [Google Scholar]
- Riecker, A. , Gröschel, K. , Ackermann, H. , Steinbrink, C. , Witte, O. , & Kastrup, A. (2006). Functional significance of age‐related differences in motor activation patterns. NeuroImage, 32(3), 1345–1354. 10.1016/j.neuroimage.2006.05.021 [DOI] [PubMed] [Google Scholar]
- Rizzolatti, G. , Fogassi, L. , & Gallese, V. (1997). Parietal cortex: From sight to action. Current Opinion in Neurobiology, 7(4), 562–567. [DOI] [PubMed] [Google Scholar]
- Rossi, S. (2004). Age‐related functional changes of prefrontal cortex in long‐term memory: A repetitive transcranial magnetic stimulation study. Journal of Neuroscience, 24(36), 7939–7944. 10.1523/JNEUROSCI.0703-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, S. , Hallett, M. , Rossini, P. M. , & Pascual‐Leone, A. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12), 2008–2039. 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini, P. M. , Burke, D. , Chen, R. , Cohen, L. G. , Daskalakis, Z. , Di Iorio, R. , … Ziemann, U. (2015). Non‐invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clinical Neurophysiology, 126(6), 1071–1107. 10.1016/j.clinph.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg, A. , Horvath, J. C. , & Pascual‐Leone, A. (2014). Neuromethods: Transcranial magnetic stimulation (pp. 1–11). New York, USA: Springer; 10.1007/978-1-4939-0879-0 [DOI] [Google Scholar]
- Salthouse, T. A. (2000). Aging and measures of processing speed. Biological Psychology, 54(1–3), 35–54. [DOI] [PubMed] [Google Scholar]
- Seidler, R. , Bernard, J. A. , Burutolu, T. B. , Fling, B. W. , Gordon, M. T. , Gwin, J. T. , … Lipps, D. B. (2010). Motor control and aging: Links to age‐related brain structural, functional, and biochemical effects. Neuroscience and Biobehavioral Reviews, 34(5), 721–733. 10.1016/j.neubiorev.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler, R. , Erdeniz, B. , Koppelmans, V. , Hirsiger, S. , Mérillat, S. , & Jäncke, L. (2015). Associations between age, motor function, and resting state sensorimotor network connectivity in healthy older adults. NeuroImage, 108(C), 47–59. 10.1016/j.neuroimage.2014.12.023 [DOI] [PubMed] [Google Scholar]
- Silvanto, J. , & Cattaneo, Z. (2017). Brain and cognition. Brain and Cognition, 119, 32–38. 10.1016/j.bandc.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn, Y. H. , Jung, H. Y. , Kaelin‐Lang, A. , & Hallett, M. (2003). Excitability of the ipsilateral motor cortex during phasic voluntary hand movement. Experimental Brain Research, 148(2), 176–185. 10.1007/s00221-002-1292-5 [DOI] [PubMed] [Google Scholar]
- Talelli, P. , Ewas, A. , Waddingham, W. , Rothwell, J. C. , & Ward, N. S. (2008). Neural correlates of age‐related changes in cortical neurophysiology. NeuroImage, 40(4), 1772–1781. 10.1016/j.neuroimage.2008.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talelli, P. , Greenwood, R. J. , & Rothwell, J. C. (2006). Arm function after stroke: Neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clinical Neurophysiology, 117(8), 1641–1659. 10.1016/j.clinph.2006.01.016 [DOI] [PubMed] [Google Scholar]
- Talelli, P. , Waddingham, W. , Ewas, A. , Rothwell, J. C. , & Ward, N. S. (2008). The effect of age on task‐related modulation of interhemispheric balance. Experimental Brain Research, 186(1), 59–66. 10.1007/s00221-007-1205-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinazzi, M. , & Zanette, G. (1998). Modulation of ipsilateral motor cortex in man during unimanual finger movements of different complexities. Neuroscience Letters, 244(3), 121–124. [DOI] [PubMed] [Google Scholar]
- Verstynen, T. (2004). Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. Journal of Neurophysiology, 93(3), 1209–1222. 10.1152/jn.00720.2004 [DOI] [PubMed] [Google Scholar]
- Volz, L. J. , Vollmer, M. , Michely, J. , Fink, G. R. , Rothwell, J. C. , & Grefkes, C. (2017). Time‐dependent functional role of the contralesional motor cortex after stroke. NeuroImage: Clinical, 16, 165–174. 10.1016/j.nicl.2017.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, V. , & Cowey, A. (2000). Transcranial magnetic stimulation and cognitive neuroscience. Nature Reviews Neuroscience, 1(1), 73–79. 10.1038/35036239 [DOI] [PubMed] [Google Scholar]
- Wang, L. E. , Fink, G. R. , Dafotakis, M. , & Grefkes, C. (2009). Noradrenergic stimulation and motor performance: Differential effects of reboxetine on movement kinematics and visuomotor abilities in healthy human subjects. Neuropsychologia, 47(5), 1302–1312. 10.1016/j.neuropsychologia.2009.01.024 [DOI] [PubMed] [Google Scholar]
- Ward, N. S. (2006). Compensatory mechanisms in the aging motor system. Ageing Research Reviews, 5(3), 239–254. 10.1016/j.arr.2006.04.003 [DOI] [PubMed] [Google Scholar]
- Ward, N. S. , & Frackowiak, R. (2003). Age‐related changes in the neural correlates of motor performance. Brain, 126(Pt. 4), 873–888. 10.1093/brain/awg071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, N. S. , Swayne, O. B. C. , & Newton, J. M. (2008). Age‐dependent changes in the neural correlates of force modulation: An fMRI study. Neurobiology of Aging, 29(9), 1434–1446. 10.1016/j.neurobiolaging.2007.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry, T. A. , Schmid, U. D. , Alkadhi, H. , Schmidt, D. , Peraud, A. , Buettner, A. , & Winkler, P. (1997). Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain, 120(1), 141–157. 10.1093/brain/120.1.141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study and all custom‐written codes are available from the corresponding author upon reasonable request.


