Abstract
Diffusion tensor imaging is often used to assess white matter (WM) changes following traumatic brain injury (TBI), but is limited in voxels that contain multiple fibre tracts. Fixel‐based analysis (FBA) addresses this limitation by using a novel method of analysing high angular resolution diffusion‐weighted imaging (HARDI) data. FBA examines three aspects of each fibre tract within a voxel: tissue micro‐structure (fibre density [FD]), tissue macro‐structure (fibre‐bundle cross section [FC]) and a combined measure of both (FD and fibre‐bundle cross section [FDC]). This study used FBA to identify the location and extent of micro‐ and macro‐structural changes in WM following TBI. A large TBI sample (N mild = 133, N moderate–severe = 29) and control group (healthy and orthopaedic; N = 107) underwent magnetic resonance imaging with HARDI and completed reaction time tasks approximately 7 months after their injury (range: 98–338 days). The TBI group showed micro‐structural differences (lower FD) in the corpus callosum and forceps minor, compared to controls. Subgroup analyses revealed that the mild TBI group did not differ from controls on any fixel metric, but the moderate to severe TBI group had significantly lower FD, FC and FDC in multiple WM tracts, including the corpus callosum, cerebral peduncle, internal and external capsule. The moderate to severe TBI group also had significantly slower reaction times than controls, but the mild TBI group did not. Reaction time was not related to fixel findings. Thus, the WM damage caused by moderate to severe TBI manifested as fewer axons and a reduction in the cross‐sectional area of key WM tracts.
Keywords: adults, diffusion‐weighted imaging, fixel‐based analysis, neuroimaging, traumatic brain injury
1. INTRODUCTION
Traumatic brain injury (TBI) is a major cause of death and disability, affecting an estimated 69 million people each year (Dewan et al., 2018). Cognitive, physical, psychological, and behavioural problems are all common following TBIs and can vary in both severity and duration (Bigler & Stern, 2015; Cristofori & Levin, 2015; Griffen & Hanks, 2014). Diffuse axonal injury (DAI), which alters white matter (WM) micro‐structure and affects the ability of axons to relay information, is thought to be a primary contributor to these problems (Hill, Coleman, & Menon, 2016; Huisman et al., 2004; Hulkower, Poliak, Rosenbaum, Zimmerman, & Lipton, 2013). Widely available imaging modalities, such as computed tomography and conventional magnetic resonance imaging (MRI), lack the sensitivity to visualise the full extent of this DAI (Shenton et al., 2012; Voelbel, Genova, Chiaravalotti, & Hoptman, 2012). However, with the development of diffusion‐weighted imaging, it is now possible to examine micro‐structural changes to WM (e.g., DAI), even after mild TBI (Shenton et al., 2012; Strauss et al., 2015).
Diffusion‐weighted imaging assesses WM changes by measuring the movement of water molecules, which is constrained by the cellular structure of axons (Niogi & Mukherjee, 2010). Diffusion tensor imaging (DTI) is the most commonly used model for quantifying the data obtained from diffusion‐weighted imaging; providing voxel‐level information regarding the coherence (fractional anisotropy [FA]) and magnitude or amount (mean diffusivity [MD]) of diffusion (Asken, DeKosky, Clugston, Jaffee, & Bauer, 2017; Shenton et al., 2012). FA and MD are often used to examine WM changes following TBI, with high FA (range 0–1) and low MD both thought to indicate intact WM, and low FA and high MD suggesting WM damage in voxels containing single fibre populations (Niogi & Mukherjee, 2010). Many studies use a region of interest (ROI) approach to analyse DTI data, whereby mean (or median) measures (FA, MD) are extracted from pre‐determined regions within the brain (Froeling, Pullens, & Leemans, 2016). Lower FA and higher MD are typically reported following TBI, particularly in the subacute and chronic periods (Asken et al., 2017; Shenton et al., 2012; Wallace, Mathias, & Ward, 2018) and after moderate to severe TBIs (Castano‐Leon et al., 2018; Wallace et al., 2019).
The single tensor model does not take into account the different fibre orientations that are contained within a voxel and are therefore of limited use when voxels contain multiple tracts and/or crossing fibres because any damage that is detected cannot be attributed to a specific tract (Raffelt et al., 2017). Fibres cross when a single fibre tract changes direction/orientation or when multiple fibre tracts are contained within a single voxel (Mori & Tournier, 2014), which is estimated to occur in up to 90% of all voxels (Jeurissen, Leemans, Tournier, Jones, & Sijbers, 2013). This limitation can be overcome using high angular resolution diffusion‐weighted imaging (HARDI), which is a higher order MRI protocol producing data that can be used to differentiate between fibre orientations when fibres cross (Mori & Tournier, 2014). A number of methods have been developed to estimate fibre orientation distributions (FODs) from HARDI data, including constrained spherical deconvolution (Mori & Tournier, 2014; Tournier, Calamante, & Connelly, 2007). The FODs obtained from constrained spherical deconvolution can be analysed using a recently developed statistical method, known as fixel‐based analysis (FBA) (Raffelt et al., 2017), which examines the different fibre orientations within a single voxel in order to provide specific anatomical information about individual WM tracts. A “fixel” refers to a specific fibre population within a single voxel (Raffelt et al., 2015; Raffelt et al., 2017), with most voxels containing multiple fixels.
FBA assesses tissue micro‐ and macro‐structure using three metrics: fibre density (FD, which assesses micro‐structure), fibre‐bundle cross section (FC, which assesses macro‐structure), and a measure that combines the two (FD and fibre‐bundle cross section; FDC) (Raffelt et al., 2017). WM damage that reduces the number of axons within a fibre bundle, but not the area they occupy (i.e., fewer axons less densely packed within the same number of voxels), will lead to a decrease in FD. If the density of axons is not reduced, but the fibre bundle occupies less area/space (fewer voxels), FC will decrease. Finally, if there is both a reduction in the density of axons within a fibre bundle and the area that the fibre bundle occupies, FDC will decrease (Raffelt et al., 2017).
FBA has been used to examine a number of different neurological conditions, including multiple sclerosis (Gajamange et al., 2018), temporal lobe epilepsy (Vaughan et al., 2017), and Alzheimer's disease (Mito et al., 2018), but has yet to be used with a TBI sample. Overall, FBA appears to provide a promising technique for detecting micro‐ and macro‐structural changes that addresses one of the main limitations of DTI (multiple tracts and crossing fibres) and yields more readily interpreted data.
The current study therefore used FBA to examine WM changes following TBI. Specifically, it compared the FD, FC and FDC obtained from a TBI group to those of a control group (orthopaedic and healthy controls) in order to identify which WM tracts of the brain were most damaged. The impact of injury severity was also investigated by separately examining the mild and moderate to severe injuries (the latter being combined due to low participant numbers); the expectation being that more severe injuries would lead to larger and more spatially extensive changes (i.e., lower FD, FC, FDC).
2. METHODS
2.1. Participants
Participants were recruited as a part of a larger research project investigating cognitive, psychological and brain imaging outcomes following TBI, which was conducted at the Royal Adelaide Hospital (Adelaide, Australia). Three samples were recruited on a prospective basis between 2008 and 2012, comprising (a) participants who had sustained a mild, moderate or severe TBI; (b) orthopaedic controls who had sustained injuries that did not involve the face or head; and (c) healthy controls who were friends or family of the TBI group or visitors to the Royal Adelaide Hospital. Participants were eligible for the research project if: (a) they were aged between 18 and 80 years; (b) English was their first language; (c) they did not have a known history of substance abuse, intellectual disabilities, or psychiatric or neurological problems; and (d) they were able to complete the cognitive tests and MRI (no contraindications).
The lowest recorded Glasgow Coma Scale (GCS) scores were used to classify TBIs as mild (GCS: 13–15), moderate (GCS: 9–12), or severe (GCS: ≤8). Where this information was not available, the length of loss of consciousness (mild: <20 min; moderate: 20 min–6 hr; severe: >6 hr) and/or post‐traumatic amnesia (mild: <60 min; moderate: 60 min–24 hr; severe: >24 hr) were used.
A total of 221 people who had sustained a TBI and 168 controls (84 healthy, 84 orthopaedic controls) were initially recruited for the research project. Participants who did not have usable MRI images (e.g., did not complete the MRI, image registration failed, MRI signal dropout, excessive participant movement; N TBI = 45, N healthy = 8, N orthopaedic = 15), had incidental findings on their MRI (N TBI = 11, N healthy = 16, N orthopaedic = 22), or who sustained their TBI more than 400 days before the MRI examination (N mild = 1, N moderate = 1, N severe = 1) were excluded from the current study. Therefore, the current sample comprised 162 people who had a TBI (N mild = 133, N moderate = 15, N severe = 14) and 107 controls (N healthy = 60, N orthopaedic = 47). The healthy and orthopaedic controls did not differ demographically (age: t(105) = −.488, p = .626; education: t(103) = .432, p = .667; proportion of males and females: X 2 (1) = 3.324, p = .068) or in terms of reaction times (compatible reaction time: t(101) = −.107, p = .915; incompatible reaction time: t(101) = −.526, p = .600) or fixel findings (see Supplementary figures); thus, all analyses were completed using a combined control group (Mathias, Dennington, Bowden, & Bigler, 2013; Wallace et al., 2019). The moderate and severe TBI groups were additionally combined for the subgroup analyses because they were too small to examine separately (N moderate–severe = 29).
2.2. Procedure
The original study was approved by the Human Research Ethics Committees at the Royal Adelaide Hospital and the University of Adelaide. All participants provided written informed consent. Hospital records were used to identify potential participants for the TBI and orthopaedic control groups, who were sent a letter from the Royal Adelaide Hospital providing information about the study and inviting them to participate. Recipients were given an opt‐out procedure if they did not want to be contacted by the researchers regarding the study. Healthy controls consisted of friends or family of the TBI group, and visitors to the Royal Adelaide Hospital who responded to flyers promoting the study. All participants were initially screened by phone for study eligibility.
Eligible participants subsequently completed an interview (which collected demographic and medical information), self‐report questionnaires (not examined here), and 2–3 hr of cognitive testing in a single session with a researcher at the University (selected data only examined here). All participants additionally underwent MRI with HARDI in a separate session within a few days of the cognitive assessment, which occurred after an average of around 7 months after the injury (TBI = 209 days, SD = 91.5; orthopaedic controls = 218 days, SD = 41.8). Participants were paid an honorarium of $40 to assist with expenses incurred when travelling for the MRI. All data were collected solely for research purposes and could not be used for litigation.
2.2.1. Image acquisition
Participants underwent MRI using a 3T Siemens scanner (TimTrio, Erlangen, Germany). Importantly, all scans were performed at the same site on the same machine, therefore alleviating the inconsistencies and artefacts that can arise from the use of multiple scanners (e.g., Fortin et al., 2017). An optimised diffusion sequence (Jones, Horsfield, & Simmons, 1999) was used to acquire diffusion data for each participant. The following parameters were used: 64 diffusion‐weighted images (b = 3,000 s/mm2) and one nondiffusion‐weighted image; 60 axial slices; FOV = 25 × 25 cm2; TR/TE = 9,400/116 ms; slice thickness = 2.5 mm; acquisition matrix = 100 × 100; isotropic image resolution = 2.5 mm. The total acquisition time for diffusion imaging was 10:41 min. A field map was acquired (TE1/TE2 4.76/7.22 ms) that assists the correction for susceptibility distortions in diffusion data.
2.2.2. Fixel‐based analysis
The diffusion images underwent preprocessing, including corrections for head motion, eddy‐current distortions, susceptibility distortions and intensity inhomogeneities using the FMRIB Diffusion Toolbox (FMRIB, Oxford, UK) (Andersson & Sotiropoulos, 2016; Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012). Global intensity normalisation was performed across participants, using the median WM b = 0 intensity using tools implemented in MRtrix3 (http://www.mrtrix.org; Tournier, Calamante, & Connelly, 2012; Tournier et al., 2019). Next, a group response function was calculated from all participants' fibre response functions, which reflect the signal that would be expected from a voxel containing a single, typical fibre bundle (Tournier, Calamante, Gadian, & Connelly, 2004). Individual fibre response functions were estimated using the convenient and reliable “tournier” algorithm in MRtrix3 (http://mrtrix.org), and these were subsequently averaged to result in a group response function. Diffusion‐weighted images underwent upsampling by a factor of 2, to improve image resolution. Constrained spherical deconvolution, a technique that uses the response function to estimate the distribution of fibre orientations contained within each voxel (Tournier et al., 2004; Tournier et al., 2007), was used to estimate the FODs (Tournier et al., 2004).
A subset of 40 participants (N TBI = 20, N healthy = 10, N orthopaedic = 10) were used to generate a study‐specific FOD template using both linear and nonlinear registration of FOD images (Raffelt et al., 2011). FOD images from all participants were then nonlinearly registered to this template, and MRtrix3 was used to calculate three fixel metrics: FD, FC, and FDC (Raffelt et al., 2017).
2.2.3. Processing speed
Processing speed, which is frequently impaired following a TBI (e.g., Cristofori & Levin, 2015; Rabinowitz & Levin, 2014), was assessed using four‐choice compatible and incompatible visual reaction time tasks (Mathias, Beall, & Bigler, 2004; Mathias, Bigler, et al., 2004). These tasks formed part of a larger battery of cognitive and self‐report measures that were administered to all participants. Four white rectangles were presented on a computer screen, two either side of a central fixation point. When one of the rectangles turned red (stimulus), participants were required to press a button as quickly and accurately as possible (response). For the four‐choice compatible reaction time task, participants were required to press a button using the hand on the same side as the stimulus (e.g., right side stimulus, right hand response), with either their index (inner rectangle) or middle (outer rectangle) finger. The incompatible task required participants to press a button using the hand on the opposite side of the stimulus (e.g., right side stimulus, left hand response), and thus required interhemispheric processing. Participants completed 60 trials to control for anticipatory responses and attentional lapses, with median reaction times calculated (Mathias, Beall, & Bigler, 2004; Mathias, Bigler, et al., 2004; Wallace et al., 2019).
2.3. Statistical analysis
IBM SPSS Statistics version 21.0 (IBM Corp., 2012) was used to compare the TBI and control groups in terms of their mean age and education (t tests), and the proportion of males and females (chi‐square test), and to determine whether reaction times were slower following a TBI (all TBI vs. controls). Additionally, Welch's F tests (Welch, 1951) and Games–Howell post hoc comparisons (Toothaker, 1993) were used to examine whether reaction times differed depending on the presence and severity of injury (mild TBI, moderate–severe TBI, controls). Standardised mean differences (Hedges' g) were calculated to assess the extent of the group differences, with g = −0.2, −0.5, and −0.8 corresponding to small, medium, and large effects, respectively (Cohen, 1992).
MRtrix3 was used for all fixel‐based statistical analyses. A WM analysis mask was generated, with a threshold of 0.33 applied to the average FOD amplitude. Connectivity‐based fixel enhancement—which identifies fixels that are connected and likely to share anatomy and pathology, using probabilistic tractography—was used to correct for multiple comparisons, with 5,000 permutations (Raffelt et al., 2015). FD, FC, and FDC values from each WM fixel in the TBI and control groups were compared (Raffelt et al., 2017), and any fixels that showed group differences in terms of the specific measure (FD, FC, FDC) were colour coded by the corresponding t‐statistic (thresholded at p < .05). WM integrity is often reduced in older people, and males and females show some differences in WM micro‐structure (Kanaan et al., 2012; Sullivan & Pfefferbaum, 2006), both of which can affect FBAs. Thus, age and sex were controlled for in these analyses. Time post‐injury was also considered as a covariate, given that there was a wide range in the intervals between injury and MRI, and that the progression of WM changes can be affected by time. However, it was found that time post‐injury was not associated with fixel findings; as such, it was not controlled for in subsequent analyses. FC and FDC were additionally corrected for brain volume, which is also known to affect these two measures (see Raffelt et al., 2017). Three group comparisons were performed in order to examine whether FD, FC, and FDC differed depending on injury severity: TBI group (all) versus controls, mild TBI versus controls, and moderate–severe TBI versus controls. The association between reaction time and fixel findings (FD, FC, FDC) in the TBI group (all TBI, mild TBI, moderate–severe TBI) was also investigated.
3. RESULTS
3.1. Participants
Table 1 summarises the demographic and injury information for the TBI (all TBI, mild TBI, moderate–severe TBI) and control groups. Participants in the TBI (all) and control groups were mostly young to middle‐aged adults who had, on average, completed high school (12 years) and one to 2 years post‐secondary training/education. The TBIs and orthopaedic injuries were sustained, on average, 7 months prior to undergoing brain imaging. Consistent with the known risk factors for TBI (Chua, Ng, Yap, & Bok, 2007), there were many more males than females in this sample (79%); however, this was not the case for controls (56%). Also consistent with the epidemiology of TBI (Faul & Coronado, 2015), fewer participants sustained moderate (N = 16) and severe (N = 15) TBIs, thus the TBI group was divided into mild (N = 134) and moderate–severe (N = 31) subgroups when examining the impact of injury severity (see Table 1 for summary subgroup data). GCS scores were not available for 22 TBI participants (N mild = 20, N moderate‐severe = 2). TBIs were largely the result of motor vehicle accidents, falls, bicycle accidents or assaults. Most participants were right‐handed and had not previously sustained a TBI, and very few were involved in litigation regarding their injuries (TBI or orthopaedic).
Table 1.
Summary demographic information for the TBI (all, mild, moderate–severe) and control (combined orthopaedic and healthy) groups
| Variable | All TBI (N = 162) | Mild TBI (N = 133) | Moderate–severe TBI (N = 29) | Combined controlsa (N = 107) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Range | N | Mean | SD | Range | N | Mean | SD | Range | N | Mean | SD | Range | |
| Age (years) | 162 | 43.4 | 16.6 | 19–80 | 133 | 43.6 | 16.9 | 19–80 | 29 | 42.6 | 15.5 | 20–72 | 107 | 39.3 | 16.6 | 18–77 |
| Education (years) | 157 | 13.1 | 2.6 | 7–22 | 128 | 13.3 | 2.5 | 8–20 | 29 | 12.6 | 3.2 | 7–22 | 105 | 14.0 | 2.8 | 7–20 |
| Days since injury | 157 | 199.5 | 41.4 | 98–338 | 128 | 194.4 | 38.3 | 98–338 | 29 | 222.1 | 47.6 | 135–336 | 45b | 218.3 | 41.8 | 136–344 |
| N | % | N | % | N | % | N | % | |||||||||
| Sex | 162 | 133 | 29 | 107 | ||||||||||||
| Females | 34 | 21.0 | 30 | 22.6 | 4 | 13.8 | 47 | 43.9 | ||||||||
| Males | 128 | 79.0 | 103 | 77.4 | 25 | 86.2 | 60 | 56.1 | ||||||||
| TBI severity | 162 | 133 | 29 | |||||||||||||
| Mild | 133 | 82.1 | 133 | 100.0 | 0 | |||||||||||
| Moderate | 15 | 9.3 | 0 | 15 | 51.7 | |||||||||||
| Severe | 14 | 8.6 | 0 | 14 | 48.3 | |||||||||||
| Cause of injury | 162 | 133 | 29 | 47b | ||||||||||||
| Motor vehicle | 39 | 24.1 | 32 | 24.1 | 7 | 24.1 | 1 | 2.1 | ||||||||
| Fall | 38 | 23.5 | 28 | 21.1 | 10 | 34.5 | 11 | 23.4 | ||||||||
| Bicycle | 34 | 21.0 | 28 | 21.1 | 6 | 20.7 | 7 | 14.9 | ||||||||
| Assault | 30 | 18.5 | 26 | 19.5 | 4 | 13.8 | 0 | 0.0 | ||||||||
| Sport | 11 | 6.8 | 11 | 8.3 | 0 | 0 | 18 | 38.3 | ||||||||
| Pedestrian | 4 | 2.5 | 2 | 1.5 | 2 | 6.9 | 1 | 2.1 | ||||||||
| Other | 6 | 3.7 | 6 | 4.5 | 0 | 0 | 9 | 19.1 | ||||||||
| Handedness | 154 | 125 | 29 | 104 | ||||||||||||
| Right | 135 | 87.7 | 110 | 88.0 | 25 | 86.2 | 97 | 93.3 | ||||||||
| Left | 19 | 12.3 | 15 | 12.0 | 4 | 13.8 | 7 | 6.7 | ||||||||
| Previous TBI (self‐report) | 156 | 127 | 29 | 104 | ||||||||||||
| Yes | 44 | 28.2 | 39 | 30.7 | 5 | 17.2 | 1 | 1 | ||||||||
| No | 112 | 71.8 | 88 | 69.3 | 24 | 82.8 | 103 | 99 | ||||||||
| Involved in litigation | 156 | 127 | 29 | 44b | ||||||||||||
| Yes | 30 | 19.2 | 24 | 18.9 | 6 | 20.7 | 2 | 4.5 | ||||||||
| No | 126 | 80.8 | 103 | 88.1 | 23 | 79.3 | 42 | 95.5 | ||||||||
Note: Healthy controls N = 60 and orthopaedic controls N = 47.
Abbreviations: GCS = Glasgow Coma Scale; N, number of participants; SD, standard deviation; TBI, traumatic brain injury.
All controls (orthopaedic + healthy controls).
Orthopaedic controls only.
When the demographic characteristics of the TBI (all TBI) and control groups were compared, the TBI group was found to be significantly older (t(270) = −1.978, p = .049, Hedges' g = .25), had completed slightly less education (t(263) = 2.489, p = .013, Hedges' g = .31), and had more males (χ2 (1) = 15.901, p = .000) than the control group. Not only did the groups differ in terms of age and sex, but these variables are also known to be associated with differences in WM structure (Kanaan et al., 2012; Sullivan & Pfefferbaum, 2006); consequently, they were used as covariates in the statistical analyses. Although significant, the difference in education was small and therefore not entered as a covariate. There was no significant difference between the two groups in the number of volumes rejected for motion (t(267) = −.851, p = .396).
3.2. TBI (all) versus controls: FD, FC, and FDC
The FD, FC, and FDC values for each fixel were compared for the TBI (all TBI: mild, moderate, and severe; N = 162) and control (N = 107) groups in order to determine whether TBI affected tissue micro‐structure and macro‐structure and, if so, what regions were most affected. Figure 1 provides eight axial slices overlaid with the fixels that showed significant group differences in FD (Figure 1a), FC (Figure 1b), and FDC (Figure 1c), with Figure 2 labelling the brain regions that were identified by this analysis. The fixels where FD, FC, and FDC were significantly lower (p < .05) in the TBI group, relative to controls, are colour coded according to the corresponding t‐statistic (blue: t = −5; red: t = 5), thresholded to display only those fixels that are significant at p < .05. Age and sex were covariates in all analyses, and brain volume was additionally included in the FC and FDC analyses.
Figure 1.
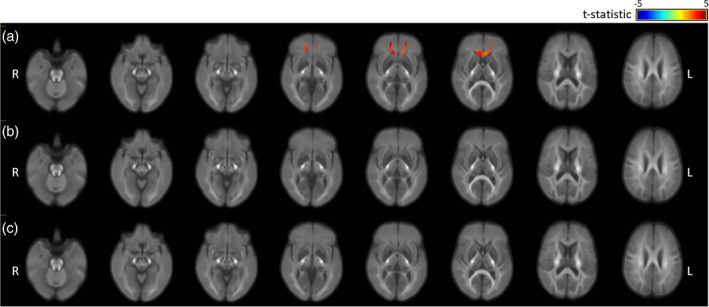
Fixels showing significant differences in the fibre density (FD), fibre‐bundle cross section (FC) and FD and bundle cross section (FDC) of the traumatic brain injury (TBI; all TBI) and control groups, controlling for age and sex (all analyses) and brain volume (FC and FDC), and colour coded by effect size (t‐statistic, thresholded at p < .05): (a) FD, (b) FC, and (c) FDC
Figure 2.
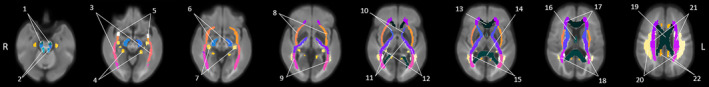
Labels for the brain regions identified by the fixel‐based analyses shown in figures 1, 3, and 4. 1: corticospinal tract, 2: superior cerebellar peduncle, 3: sagittal stratum, 4: cingulum (hippocampus), 5: uncinate fasciculus, 6: cerebral peduncle, 7: fornix (cres)/stria terminalis, 8: external capsule, 9: posterior thalamic radiation, 10: anterior limb of internal capsule, 11: posterior limb of internal capsule, 12: retrolenticular part of internal capsule, 13: genu of corpus callosum, 14: splenium of corpus callosum, 15: tapetum, 16: fornix, 17: anterior corona radiata, 18: posterior corona radiata, 19: body of corpus callosum, 20: superior longitudinal fasciculus, 21: superior corona radiata, 22: cingulum (cingulate gyrus)
As seen in Figure 1a, the TBI group (all TBI) showed significantly lower FD in the corpus callosum (genu, body) and forceps minor (see Figure 2 for labelled regions). Unlike FD, there were no significant group differences in FC (see Figure 1b) or FDC (see Figure 1c). These findings indicate that, approximately 7 months after sustaining a TBI, there were changes to WM micro‐structure that were not attributable age or sex. WM macro‐structure (cross section) was unaffected.
3.3. Mild TBI versus controls: FD, FC, and FDC
Next, the FD, FC, and FDC values of the mild TBI and control groups were compared to determine whether mild injuries caused micro‐structural and/or macro‐structural changes that were detectable using FBA. Supplementary Figure S2 shows eight axial slices overlaid with the fixels that displayed significant group differences (p < .05) in FD, FC, and FDC, after correcting for both age and sex (FC and FDC also corrected for brain volume) (blue: t = −5; red: t = 5), thresholded to display only those fixels that are significant at p < .05. The mild TBI group did not have significantly lower FD, FC, or FDC in any of the fixels, when compared to controls (see Supplementary Figure S2). Therefore, the current sample did not show significantly altered WM micro‐ or macro‐structure approximately 7 months after their mild TBI.
3.4. Moderate–severe TBI versus controls: FD, FC, and FDC
Finally, the moderate–severe TBI and control groups were compared, with Figure 3 showing the brain regions that differed significantly (thresholded at p < .05) in terms of FD (Figure 3a), FC (Figure 3b), and FDC (Figure 3c), after correcting for age and sex (all analyses) and brain volume (FC, FDC analyses). As seen in Figure 3a, FD was significantly lower in multiple regions, including: the corpus callosum, cerebral peduncle, internal and external capsule, corona radiata, cingulum, and tapetum (see Figure 2 for labelled regions). Similar WM structures also showed lower FC, but the affected regions tended to be smaller (Figure 3b). Finally, FDC was reduced in a number of regions, including the corpus callosum, internal and external capsule and cingulum (Figure 3c). Thus, more serious TBIs resulted in altered micro‐ and macro‐structure in multiple important WM tracts approximately 7 months after sustaining an injury: changes that could not be attributed to age, sex or brain volume.
Figure 3.
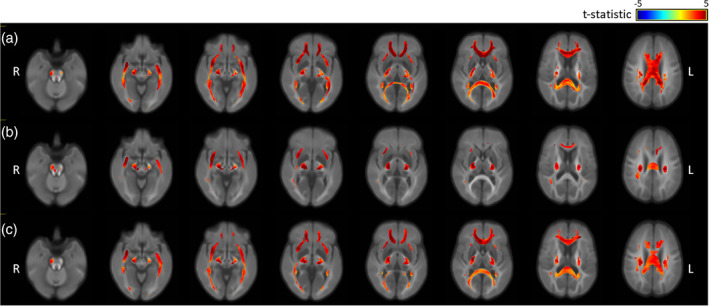
Fixels showing significant differences in the fibre density (FD), fibre‐bundle cross section (FC) and FD and bundle cross section (FDC) of the moderate–severe TBI and control groups, controlling for age and sex (all analyses) and brain volume (FC and FDC), and colour coded by effect size (t‐statistic, thresholded at p < .05): (a) FD, (b) FC, and (c) FDC
When the un‐thresholded effect size maps for both the mild and moderate–severe groups were compared (see Supplementary figures), the pattern of injury appeared to be quite consistent, but with considerably larger effects found following more severe injury. This suggests that similar brain regions are affected by TBIs of all severities.
3.5. Reaction time
Table 2 displays the reaction times for the TBI (all TBI) and control groups. The reaction times for the compatible and incompatible tasks were both significantly slower in the TBI group, with these differences equating to small effects (g = −.30 and −.33, respectively). Subgroup analyses (Table 3) revealed that, although the reaction times of the mild TBI group did not differ from the controls (p > .05, small effects), the moderate–severe TBI group was significantly slower on the both the compatible and incompatible tasks (large effects: g = −.82 and − .72, respectively).
Table 2.
Reaction time data for the traumatic brain injury and control groups
| Cognitive test | TBI | Controls | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | Hedges' g | t | p | |
| Four‐choice compatible RT task | 154 | 464.7 | 94.3 | 103 | 437.20 | 90.63 | −0.30 | −2.33 | .021 |
| Four‐choice incompatible RT task | 152 | 700.4 | 198.9 | 103 | 638.13 | 173.76 | −0.33 | −2.58 | .010 |
Abbreviations: N, number of participants; RT, reaction time; SD, standard deviation; t, t test; TBI, traumatic brain injury.
Table 3.
Reaction time data for the mild TBI, moderate–severe TBI, and control groups
| Cognitive test | Mild TBI | Moderate–severe TBI | Controls | Mild TBI vs. controls | Moderate–severe vs. controls | Mild TBI vs. moderate–severe | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Welch's F | Hedges' g | p | Hedges' g | p | Hedges' g | p | |
| Four‐choice compatible RT task | 453.0 | 83.3 | 517.5 | 121.6 | 437.20 | 90.63 | F(2, 69.91) = 5.36, p = .007 | −0.18 | .365 | −0.82 | .007 | −0.70 | .031 |
| Four‐choice incompatible RT task | 685.8 | 195.2 | 768.1 | 205.6 | 638.13 | 173.76 | F(2, 71.46) = 5.17, p = .008 | −0.26 | .127 | −0.72 | .013 | −0.42 | .152 |
Note: Four‐choice compatible RT task (N mild = 126, N moderate‐severe = 28, N controls = 103) and four‐choice incompatible RT task (N mild = 125, N moderate‐severe = 27, N controls = 103).
Abbreviations: RT, reaction time; SD, standard deviation; TBI, traumatic brain injury.
The association between reaction time and fixel findings was also examined. No statistically significant associations were found, suggesting that reaction time is not related to fixel findings. Further analysis showed that age was strongly and significantly related to both the compatible and incompatible reaction time tasks (r = −.58 and −.57, respectively; see Table 4).
Table 4.
Pearson r correlations (p‐value) between reaction time and age
| Cognitive tests | Age |
|---|---|
| Four‐choice compatible visual RT task | −.58 (.000) |
| Four‐choice incompatible visual RT task | −.57 (.000) |
Note: Four‐choice compatible visual RT task (N = 257) and four‐choice incompatible visual RT task (N = 255).
Abbreviation: RT, reaction time.
4. DISCUSSION
This study undertook a FBA of diffusion‐weighted data to examine micro‐ and macro‐structural changes in the WM of adults who had sustained mild, moderate, and severe TBIs on average 7 months earlier. As a whole, the TBI group (all TBI) showed evidence of altered tissue micro‐structure (lower FD) in the corpus callosum (genu, body) and forceps minor. Subgroup analyses additionally revealed that there was no evidence of altered WM in the mild TBI group: FD, FC, and FDC were all unaffected. However, the WM micro‐ and macro‐structure of the moderate to severe TBI group was altered (lower FD, FC, FDC) in multiple WM tracts, including the corpus callosum, corona radiata, and internal and external capsule. According to Raffelt et al. (2017), these changes indicate that there were fewer axons within these WM tracts and that they occupied a smaller cross‐sectional area. The moderate to severe TBI group also had significantly slower reaction times than the controls, but the mild group did not. There was, however, no significant association between reaction times and fixel findings.
DTI has previously been used to examine WM changes following TBI and has identified many regions where there appears to be damage (for reviews, see Amyot et al., 2015; Niogi & Mukherjee, 2010; Shenton et al., 2012; Wallace et al., 2018). However, DTI is limited by the fact that the measures obtained from it (FA, MD) are averaged across all of the fibre tracts that are contained within a voxel, making interpretation problematic when more than one fibre tract is present (Mori & Tournier, 2014). Although low FA values often occur when WM is damaged following a TBI, damage to a single fibre tract in a voxel that contains multiple tracts/populations may result in null findings if the other fibre tracts are undamaged. This, in turn, may be incorrectly interpreted as indicating a lack of damage because the information provided by FA is not tract‐specific (Mori & Tournier, 2014; Raffelt et al., 2012). FBA provides an alternative method of analysing diffusion data that is able to overcome this considerable limitation. Specifically, changes can be attributed to individual WM fibre tracts in voxels that contain more than one tract (Raffelt et al., 2015). In addition, FBA is able to determine the specific ways in which the WM has been affected: namely, whether there are fewer axons that are less densely packed (FD), the tracts have a reduced cross‐sectional area representing morphometric changes (FC), and/or there is a combination of both changes (FDC) (Mito et al., 2018; Raffelt et al., 2017). FBA may therefore provide more specific anatomical information than DTI.
The TBI group, as a whole, displayed lower FD in the corpus callosum (genu, body) and forceps minor, indicating that there were fewer axons contained within these fibre tracts. There were no differences in WM macro‐structure (FC), or in the combined measure of micro‐ and macro‐structure (FDC). Following mild TBI, there was no evidence of WM changes. These findings contrast with those of previous DTI studies, which report that mild TBI is associated with altered WM in multiple regions, including the corpus callosum, fornix, superior longitudinal fasciculus, thalamic radiations, external and internal capsule, cingulum, and corona radiata (e.g., Grossman et al., 2012; Messe et al., 2012; Wallace et al., 2018; Zhu et al., 2014). Although FBA is designed to be more sensitive to damage to individual WM tracts in areas where fibres cross (Raffelt et al., 2017), it may still be unable to detect the subtle damage that can occur following minor injuries. Alternatively, the mild participants may not have sustained WM damage as a consequence of their injuries, given that most had a GCS of 15 (63%). Indeed, this mild group did not differ significantly from the controls in terms of FA and MD in the five regions that were examined in a recent ROI study (genu, body, and splenium of corpus callosum, fornix, superior longitudinal fasciculus) (see Wallace et al., 2019). There was, however, a weak trend toward lower FA and higher MD in the mild TBI group, relative to controls (Wallace et al., 2019).
As expected, the largest WM alterations were found in people who had sustained more serious injuries. Specifically, moderate to severe TBI led to micro‐ and macro‐structural differences (lower FD, FC, FDC) in a large number of important WM tracts, including commissural fibres that connect equivalent regions in the two hemispheres (e.g., corpus callosum), association fibres that provide within‐hemisphere connections (e.g., superior longitudinal fasciculus) and projection fibres that connect cortical and subcortical regions (e.g., internal capsule) (Aralasmak et al., 2006). People with more severe injuries also had considerably slower reaction times, but reaction times were not associated with fixel findings. It is possible that an association exists; however, no significant relationship was found after the fixel data were corrected for age; it is therefore possible that any effect was confounded by age. Although the physical, psychological, behavioural, and cognitive impairments experienced by people who suffer a TBI (Cristofori & Levin, 2015; Griffen & Hanks, 2014) may be the result of decreased FD in addition to alterations to the broader WM structure (i.e., fewer axons contained within WM tracts that have a reduced cross‐sectional area), further research is needed to determine this.
5. LIMITATIONS
Although FBA was able detect damage to specific WM tracts in our TBI sample, group comparisons fail to consider individual differences in the extent and location of WM changes post‐TBI (Hulkower et al., 2013). Given the heterogeneous nature of TBI damage, injury progression and recovery (Bigler & Stern, 2015; Hulkower et al., 2013), the utility of FBA now needs to be investigated with individual participants. However, a large normative FBA database would be needed in order to investigate individual differences in WM changes.
The current study examined participants on a single occasion, which meant that the progression of WM damage was not assessed, and the range of post‐injury intervals was quite large (i.e., interval between injury and MRI). WM damage may initially manifest as a reduction in tissue micro‐structure (FD), but over time tissue macro‐structure (FC) may be more affected due to WM degeneration and atrophy (Raffelt et al., 2017). WM degeneration can continue for years after a TBI (Hill et al., 2016). Therefore FC, which is thought to reflect accumulated axonal loss (Raffelt et al., 2017), may decrease progressively as this degeneration continues, however FC was not related to time post‐injury in the current study. In addition, thin WM structures (e.g., fornix, anterior commissure) may not be accurately assessed using FBA; although micro‐structural changes (FD) can be detected in small structures, FC can be insensitive and any macro‐structural changes may instead present as micro‐structural changes (FD) (Raffelt et al., 2017; Vaughan et al., 2017). This problem may be exacerbated by the large voxel size used to acquire the images (2.5 mm3), in addition to partial volume effects, which occur when there are two or more different types of tissue present within a single voxel (e.g., WM, grey matter, cerebrospinal fluid) (Raffelt et al., 2017; Vos, Jones, Viergever, & Leemans, 2011). Image resolution may be improved by using smaller voxels, enabling thinner WM structures (e.g., fornix) to be examined more thoroughly (Raffelt et al., 2017), at the cost of increased scan time and reduced signal‐to‐noise.
Additionally, there was a group difference in age and sex and, although these variables were entered as covariates in the fixel analyses, it is possible that this method may not have entirely accounted for these differences. Future studies should endeavour to use more closely matched controls. Finally, the moderate (N = 15) and severe (n = 14) TBI samples were both small, making it necessary to combine them for the subgroup analysis. Given that more serious TBIs generally lead to greater WM damage (e.g., Castano‐Leon et al., 2018), it is likely that the extent and, potentially, location of the changes to the WM may differ for moderate and severe TBIs. Unfortunately, it was not possible to examine whether this was the case.
6. DIRECTIONS FOR FUTURE RESEARCH
The reliability and generalisability of these findings now need to be evaluated in other TBI samples (e.g., different age groups, different post‐injury periods). Most of the mild TBI group had a GCS of 15 (63%); other groups of mild participants with a wider range of GCS scores should be examined to determine whether FBA detects changes following these injuries. Additionally, larger samples of people with moderate and severe injuries are needed to determine whether there are differences in the pattern of WM changes following moderate and severe TBI. Furthermore, large‐scale, longitudinal FBA studies are needed to examine the progression of micro‐ and macro‐structural WM changes following TBI. These studies should assess whether FD, FC, and FDC are differentially affected at earlier and/or later post‐injury intervals, given that WM degeneration can continue for years after an injury (Hill et al., 2016). Finally, although the current study failed to find an association between FBA and reaction times, additional research is needed to determine whether FBA findings are related to other cognitive, behavioural, and psychological outcomes.
7. CONCLUSIONS
This study examined whether micro‐ and/or macro‐structural WM changes were detected using FBA, 7 months after sustaining a TBI. Moderate to severe TBI led to WM damage that manifested as a reduction in the number of axons, together with broader structural changes (lower FD, FC, FDC) in multiple brain regions, including the corpus callosum, corona radiata, cerebral peduncle, and internal and external capsule. People with moderate to severe TBI also had slower reaction times; however, no significant associations were found between reaction time and fixel findings. These findings have shown that moderate to severe TBI leads to a reduction in the number of axons within fibre tracts that have a reduced cross‐sectional area. Although these WM changes may limit the ability of axons to relay information, the impact of these changes needs to be examined further.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Figure S1 Fixels showing significant differences in the fibre density (FD), fibre‐bundle cross‐section (FC) and fibre density and bundle cross‐section (FDC) of the healthy and orthopaedic and control groups, controlling for age and sex (all analyses), and brain volume (FC and FDC) and colour coded by effect size (t‐statistic, thresholded at p < .05): (a) FD; (b) FC; (c) FDC No statistically significant group differences were observed.
Figure S2. Fixels showing significant differences in the fibre density (FD), fibre‐bundle cross‐section (FC) and fibre density and bundle cross‐section (FDC) of the mild TBI and control groups, controlling for age and sex (all analyses), and brain volume (FC and FDC) and colour coded by effect size (t‐statistic, thresholded at p < .05): (a) FD; (b) FC; (c) FDC No statistically significant group differences were observed.
Figure S3. Fixels showing un‐thresholded differences in the fibre density (FD), fibre‐bundle cross‐section (FC) and fibre density and bundle cross‐section (FDC) of the mild TBI and control groups, controlling for age and sex (all analyses), and brain volume (FC and FDC) and colour coded by effect size (t‐statistic) thresholded at p < .05): (a) FD; (b) FC; (c) FDC
Figure S4. Fixels showing un‐thresholded differences in the fibre density (FD), fibre‐bundle cross‐section (FC) and fibre density and bundle cross‐section (FDC) of the moderate–severe TBI and control groups, controlling for age and sex (all analyses), and brain volume (FC and FDC) and colour coded by effect size (t‐statistic) thresholded at p < .05): (a) FD; (b) FC; (c) FDC
ACKNOWLEDGMENT
This work was supported by the National Health and Medical Research Council (Project Grant number 519220).
Wallace EJ, Mathias JL, Ward L, Fripp J, Rose S, Pannek K. A fixel‐based analysis of micro‐ and macro‐structural changes to white matter following adult traumatic brain injury. Hum Brain Mapp. 2020;41:2187–2197. 10.1002/hbm.24939
Funding information National Health and Medical Research Council, Grant/Award Number: 519220
DATA AVAILABILITY STATEMENT
The data that support the findings of this study may be available from the corresponding author upon reasonable request.
REFERENCES
- Amyot, F. , Arciniegas, D. B. , Brazaitis, M. P. , Curley, K. C. , Diaz‐Arrastia, R. , Gandjbakhche, A. , … Stocker, D. (2015). A review of the effectiveness of neuroimaging modalities for the detection of traumatic brain injury. Journal of Neurotrauma, 32(22), 1693–1721. 10.1089/neu.2013.3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, J. L. R. , & Sotiropoulos, S. N. (2016). An integrated approach to correction for off‐resonance effects and subject movement in diffusion MR imaging. NeuroImage, 125, 1063–1078. 10.1016/j.neuroimage.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aralasmak, A. , Ulmer, J. L. , Kocak, M. , Salvan, C. V. , Hillis, A. E. , & Yousem, D. M. (2006). Association, commissural, and projection pathways and their functional deficit reported in literature. Journal of Computer Assisted Tomography, 30(5), 695–715. 10.1097/01.rct.0000226397.43235.8b [DOI] [PubMed] [Google Scholar]
- Asken, B. M. , DeKosky, S. T. , Clugston, J. R. , Jaffee, M. S. , & Bauer, R. M. (2017). Diffusion tensor imaging (DTI) findings in adult civilian, military, and sport‐related mild traumatic brain injury (mTBI): A systematic critical review. Brain Imaging and Behavior, 12, 585–612. 10.1007/s11682-017-9708-9 [DOI] [PubMed] [Google Scholar]
- Bigler, E. D. , & Stern, Y. (2015). Traumatic brain injury and reserve In Handbook of clinical neurology (Vol. 128, pp. 691–710). Elsevier; 10.1016/b978-0-444-63521-1.00043-1 [DOI] [PubMed] [Google Scholar]
- Castano‐Leon, A. M. , Cicuendez, M. , Navarro, B. , Munarriz, P. M. , Cepeda, S. , Paredes, I. , … Lagares, A. (2018). What can we learn from diffusion tensor imaging from a large traumatic brain injury cohort? White matter integrity and its relationship with outcome. Journal of Neurotrauma, 35, 2365–2376. 10.1089/neu.2018.5691 [DOI] [PubMed] [Google Scholar]
- Chua, K. S. , Ng, Y. S. , Yap, S. G. , & Bok, C. W. (2007). A brief review of traumatic brain injury rehabilitation. Annals of the Academy of Medicine, Singapore, 36(1), 31–42. [PubMed] [Google Scholar]
- Cohen, J. (1992). A power primer. Psychological Bulletin, 112(1), 155–159. [DOI] [PubMed] [Google Scholar]
- Cristofori, I. , & Levin, H. S. (2015). Traumatic brain injury and cognition In Handbook of clinical neurology (Vol. 128, pp. 579–611). Elsevier; 10.1016/B978-0-444-63521-1.00037-6 [DOI] [PubMed] [Google Scholar]
- Dewan, M. C. , Rattani, A. , Gupta, S. , Baticulon, R. E. , Hung, Y. C. , Punchak, M. , … Park, K. B. (2018). Estimating the global incidence of traumatic brain injury. Journal of Neurosurgery, 130, 1–18. 10.3171/2017.10.Jns17352 [DOI] [PubMed] [Google Scholar]
- Faul, M. , & Coronado, V. (2015). Epidemiology of traumatic brain injury In Handbook of clinical neurology (Vol. 127, pp. 3–13). Elsevier; 10.1016/b978-0-444-52892-6.00001-5 [DOI] [PubMed] [Google Scholar]
- Fortin, J. P. , Parker, D. , Tunc, B. , Watanabe, T. , Elliott, M. A. , Ruparel, K. , … Shinohara, R. T. (2017). Harmonization of multi‐site diffusion tensor imaging data. NeuroImage, 161, 149–170. 10.1016/j.neuroimage.2017.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froeling, M. , Pullens, P. , & Leemans, A. (2016). DTI analysis methods: Region of interest analysis In Diffusion Tensor Imaging: A Practical Handbook (pp. 175–182). New York: Springer. [Google Scholar]
- Gajamange, S. , Raffelt, D. , Dhollander, T. , Lui, E. , van der Walt, A. , Kilpatrick, T. , … Kolbe, S. (2018). Fibre‐specific white matter changes in multiple sclerosis patients with optic neuritis. NeuroImage: Clinical, 17, 60–68. 10.1016/j.nicl.2017.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffen, J. , & Hanks, R. (2014). Cognitive and behavioral outcomes from traumatic brain injury In Sherer M. & Sander A. M. (Eds.), Handbook on the neuropsychology of traumatic brain injury (pp. 25–45). New York, NY: Springer New York. [Google Scholar]
- Grossman, E. J. , Ge, Y. L. , Jensen, J. H. , Babb, J. S. , Miles, L. , Reaume, J. , … Inglese, M. (2012). Thalamus and cognitive impairment in mild traumatic brain injury: A diffusional kurtosis imaging study. Journal of Neurotrauma, 29(13), 2318 10.1089/neu.2011.1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, C. S. , Coleman, M. P. , & Menon, D. K. (2016). Traumatic axonal injury: Mechanisms and translational opportunities. Trends in Neurosciences, 39(5), 311–324. 10.1016/j.tins.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman, T. A. , Schwamm, L. H. , Schaefer, P. W. , Koroshetz, W. J. , Shetty‐Alva, N. , Ozsunar, Y. , … Sorensen, A. G. (2004). Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR American Journal of Neuroradiology, 25(3), 370–376. [PMC free article] [PubMed] [Google Scholar]
- Hulkower, M. B. , Poliak, D. B. , Rosenbaum, S. B. , Zimmerman, M. E. , & Lipton, M. L. (2013). A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR American Journal of Neuroradiology, 34(11), 2064–2074. 10.3174/ajnr.A3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson, M. , Beckmann, C. F. , Behrens, T. E. , Woolrich, M. W. , & Smith, S. M. (2012). FSL. NeuroImage, 62(2), 782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Jeurissen, B. , Leemans, A. , Tournier, J. D. , Jones, D. K. , & Sijbers, J. (2013). Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Human Brain Mapping, 34(11), 2747–2766. 10.1002/hbm.22099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D. K. , Horsfield, M. A. , & Simmons, A. (1999). Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magnetic Resonance in Medicine, 42(3), 515–525. [PubMed] [Google Scholar]
- Kanaan, R. A. , Allin, M. , Picchioni, M. , Barker, G. J. , Daly, E. , Shergill, S. S. , … McGuire, P. K. (2012). Gender differences in white matter microstructure. PLoS One, 7(6), e38272 10.1371/journal.pone.0038272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias, J. L. , Beall, J. A. , & Bigler, E. D. (2004). Neuropsychological and information processing deficits following mild traumatic brain injury. Journal of the International Neuropsychological Society, 10(2), 286–297. 10.1017/s1355617704102117 [DOI] [PubMed] [Google Scholar]
- Mathias, J. L. , Bigler, E. D. , Jones, N. R. , Bowden, S. C. , Barrett‐Woodbridge, M. , Brown, G. C. , & Taylor, D. J. (2004). Neuropsychological and information processing performance and its relationship to white matter changes following moderate and severe traumatic brain injury: A preliminary study. Applied Neuropsychology, 11(3), 134–152. 10.1207/s15324826an1103_2 [DOI] [PubMed] [Google Scholar]
- Mathias, J. L. , Dennington, V. , Bowden, S. C. , & Bigler, E. D. (2013). Community versus orthopaedic controls in traumatic brain injury research: How comparable are they? Brain Injury, 27(7–8), 887–895. 10.3109/02699052.2013.793398 [DOI] [PubMed] [Google Scholar]
- Messe, A. , Caplain, S. , Pelegrini‐Issac, M. , Blancho, S. , Montreuil, M. , Levy, R. , … Benali, H. (2012). Structural integrity and postconcussion syndrome in mild traumatic brain injury patients. Brain Imaging and Behavior, 6(2), 283–292. 10.1007/s11682-012-9159-2 [DOI] [PubMed] [Google Scholar]
- Mori, S. , & Tournier, J. D. (2014). Chapter 8—Moving beyond DTI: High angular resolution diffusion imaging (HARDI) In Introduction to diffusion tensor imaging (2nd ed., pp. 65–78). San Diego, CA: Academic Press. [Google Scholar]
- Niogi, S. N. , & Mukherjee, P. (2010). Diffusion tensor imaging of mild traumatic brain injury. Journal of Head Trauma Rehabilitation, 25(4), 241–255. 10.1097/HTR.0b013e3181e52c2a [DOI] [PubMed] [Google Scholar]
- Rabinowitz, A. R. , & Levin, H. S. (2014). Cognitive sequelae of traumatic brain injury. Psychiatric Clinics of North America, 37(1), 1–11. 10.1016/j.psc.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffelt, D. A. , Smith, R. E. , Ridgway, G. R. , Tournier, J. D. , Vaughan, D. N. , Rose, S. , … Connelly, A. (2015). Connectivity‐based fixel enhancement: Whole‐brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. NeuroImage, 117, 40–55. 10.1016/j.neuroimage.2015.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffelt, D. A. , Tournier, J. D. , Fripp, J. , Crozier, S. , Connelly, A. , & Salvado, O. (2011). Symmetric diffeomorphic registration of fibre orientation distributions. NeuroImage, 56(3), 1171–1180. 10.1016/j.neuroimage.2011.02.014 [DOI] [PubMed] [Google Scholar]
- Raffelt, D. A. , Tournier, J. D. , Rose, S. , Ridgway, G. R. , Henderson, R. , Crozier, S. , … Connelly, A. (2012). Apparent fibre density: A novel measure for the analysis of diffusion‐weighted magnetic resonance images. NeuroImage, 59(4), 3976–3994. 10.1016/j.neuroimage.2011.10.045 [DOI] [PubMed] [Google Scholar]
- Raffelt, D. A. , Tournier, J. D. , Smith, R. E. , Vaughan, D. N. , Jackson, G. , Ridgway, G. R. , & Connelly, A. (2017). Investigating white matter fibre density and morphology using fixel‐based analysis. NeuroImage, 144, 58–73. 10.1016/j.neuroimage.2016.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton, M. E. , Hamoda, H. M. , Schneiderman, J. S. , Bouix, S. , Pasternak, O. , Rathi, Y. , … Zafonte, R. (2012). A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging and Behavior, 6(2), 137–192. 10.1007/s11682-012-9156-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss, S. , Hulkower, M. , Gulko, E. , Zampolin, R. L. , Gutman, D. , Chitkara, M. , … Lipton, M. L. (2015). Current clinical applications and future potential of diffusion tensor imaging in traumatic brain injury. Topics in Magnetic Resonance Imaging, 24(6), 353–362. 10.1097/rmr.0000000000000071 [DOI] [PubMed] [Google Scholar]
- Sullivan, E. V. , & Pfefferbaum, A. (2006). Diffusion tensor imaging and aging. Neuroscience and Biobehavioral Reviews, 30(6), 749–761. 10.1016/j.neubiorev.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Toothaker, L. E. (1993). Multiple comparison procedures. London, England: Sage Publications. [Google Scholar]
- Tournier, J. D. , Calamante, F. , & Connelly, A. (2007). Robust determination of the fibre orientation distribution in diffusion MRI: Non‐negativity constrained super‐resolved spherical deconvolution. NeuroImage, 35(4), 1459–1472. 10.1016/j.neuroimage.2007.02.016 [DOI] [PubMed] [Google Scholar]
- Tournier, J. D. , Calamante, F. , & Connelly, A. (2012). MRtrix: Diffusion tractography in crossing fiber regions. International Journal of Imaging Systems and Technology, 22(1), 53–66. 10.1002/ima.22005 [DOI] [Google Scholar]
- Tournier, J. D. , Calamante, F. , Gadian, D. G. , & Connelly, A. (2004). Direct estimation of the fiber orientation density function from diffusion‐weighted MRI data using spherical deconvolution. NeuroImage, 23(3), 1176–1185. 10.1016/j.neuroimage.2004.07.037 [DOI] [PubMed] [Google Scholar]
- Tournier, J. D. , Smith, R. E. , Raffelt, D. A. , Tabbara, R. , Dhollander, T. , Pietsch, M. , … Connelly, A. (2019). MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. bioRxiv, 551739 10.1101/551739 [DOI] [PubMed] [Google Scholar]
- Vaughan, D. N. , Raffelt, D. , Curwood, E. , Tsai, M. H. , Tournier, J. D. , Connelly, A. , & Jackson, G. D. (2017). Tract‐specific atrophy in focal epilepsy: Disease, genetics, or seizures? Annals of Neurology, 81(2), 240–250. 10.1002/ana.24848 [DOI] [PubMed] [Google Scholar]
- Voelbel, G. T. , Genova, H. M. , Chiaravalotti, N. D. , & Hoptman, M. J. (2012). Diffusion tensor imaging of traumatic brain injury review: Implications for neurorehabilitation. NeuroRehabilitation, 31(3), 281–293. 10.3233/NRE-2012-0796 [DOI] [PubMed] [Google Scholar]
- Vos, S. B. , Jones, D. K. , Viergever, M. A. , & Leemans, A. (2011). Partial volume effect as a hidden covariate in DTI analyses. NeuroImage, 55(4), 1566–1576. 10.1016/j.neuroimage.2011.01.048 [DOI] [PubMed] [Google Scholar]
- Wallace, E. J. , Mathias, J. L. , & Ward, L. (2018). Diffusion tensor imaging changes following mild, moderate and severe adult traumatic brain injury: A meta‐analysis. Brain Imaging and Behavior, 12, 1607–1621. 10.1007/s11682-018-9823-2 [DOI] [PubMed] [Google Scholar]
- Wallace, E. J. , Mathias, J. L. , Ward, L. , Pannek, K. , Fripp, J. , & Rose, S. (2019). White matter changes detected using diffusion tensor imaging following adult traumatic brain injury and their relationship to cognition. Manuscript submitted for publication. [DOI] [PubMed]
- Welch, B. L. (1951). On the comparison of several mean values: An alternative approach. Biometrika, 38, 330–336. 10.2307/2332579 [DOI] [Google Scholar]
- Zhu, Y. , Li, Z. , Bai, L. , Tao, Y. , Sun, C. , Li, M. , … Zhang, M. (2014). Loss of microstructural integrity in the limbic‐subcortical networks for acute symptomatic traumatic brain injury. BioMed Research International, 2014, 1–7. 10.1155/2014/548392 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Fixels showing significant differences in the fibre density (FD), fibre‐bundle cross‐section (FC) and fibre density and bundle cross‐section (FDC) of the healthy and orthopaedic and control groups, controlling for age and sex (all analyses), and brain volume (FC and FDC) and colour coded by effect size (t‐statistic, thresholded at p < .05): (a) FD; (b) FC; (c) FDC No statistically significant group differences were observed.
Figure S2. Fixels showing significant differences in the fibre density (FD), fibre‐bundle cross‐section (FC) and fibre density and bundle cross‐section (FDC) of the mild TBI and control groups, controlling for age and sex (all analyses), and brain volume (FC and FDC) and colour coded by effect size (t‐statistic, thresholded at p < .05): (a) FD; (b) FC; (c) FDC No statistically significant group differences were observed.
Figure S3. Fixels showing un‐thresholded differences in the fibre density (FD), fibre‐bundle cross‐section (FC) and fibre density and bundle cross‐section (FDC) of the mild TBI and control groups, controlling for age and sex (all analyses), and brain volume (FC and FDC) and colour coded by effect size (t‐statistic) thresholded at p < .05): (a) FD; (b) FC; (c) FDC
Figure S4. Fixels showing un‐thresholded differences in the fibre density (FD), fibre‐bundle cross‐section (FC) and fibre density and bundle cross‐section (FDC) of the moderate–severe TBI and control groups, controlling for age and sex (all analyses), and brain volume (FC and FDC) and colour coded by effect size (t‐statistic) thresholded at p < .05): (a) FD; (b) FC; (c) FDC
Data Availability Statement
The data that support the findings of this study may be available from the corresponding author upon reasonable request.


