Abstract
Exteroceptive and interoceptive signals shape and sustain the bodily self‐awareness. The existence of a set of brain areas, supporting the integration of information coming from the inside and the outside of the body in building the sense of bodily self‐awareness has been postulated, yet the evidence remains limited, a matter of discussion never assessed quantitatively. With the aim of unrevealing where in the brain interoceptive and exteroceptive signals may converge, we performed a meta‐analysis on imaging studies of the sense of body ownership, modulated by external visuotactile stimulation, and studies on interoception, which involves the self‐awareness for internal bodily sensations. Using a multilevel kernel density analysis, we found that processing of stimuli of the two domains converges primarily in the supramarginal gyrus bilaterally. Furthermore, we found a right‐lateralized set of areas, including the precentral and postcentral, and superior temporal gyri. We discuss these results and propose this set of areas as ideal candidates to match multiple body‐related signals contributing to the creation of a multidimensional representation of the bodily self.
Keywords: bodily self, body awareness, body ownership, interoception
1. INTRODUCTION
Bodily self‐awareness has been defined as the feeling that conscious experiences are bound to the self and are experiences of a unitary entity (Berlucchi & Aglioti, 2010; Blanke, 2012; Blanke, Slater, & Serino, 2015; Legrand, 2006, 2010). It is a multidimensional construct, involving several aspects such as the experience of owning a body, the perception of visceral signals coming from our own body, as well as feeling the body in space, or the agency over our actions (Berlucchi & Aglioti, 2010; Blanke, 2012; Blanke et al., 2015). Both exteroceptive and interoceptive information have been considered essential drivers for the sense of self, and most such research has focused on the investigation of one of these two domains in an exclusive manner (Park & Blanke, 2019).
The pivotal role of the exteroceptive information for the bodily self‐awareness has been mainly demonstrated throughout visuotactile experimental paradigms. One such example comes from the famous rubber hand illusion (RHI) paradigm, the visuotactile multisensory conflict generated when a synchronous brushing is administered to someone's hand, occluded from vision, and on a visible nearby rubber hand: under such circumstances, subjects “feel touches on the rubber hand” as if this was belonging their body (Botvinick & Cohen, 1998). This visuotactile mismatch is also effective between the participant's face and another person's face (enfacement illusion; Sforza, Bufalari, Haggard, & Aglioti, 2010), and a virtual body and the own body (full body illusion; Lenggenhager, Tadi, Metzinger, & Blanke, 2007).
On the other hand, interoceptive signals also play a role in the construction of a coherent sense of the self (Craig, 2002; Critchley & Harrison, 2013). Interoception has been mainly studied via paradigms like the heartbeat perception, but there are several other sources of information for different bodily sensations, which have been investigating using self‐evaluated assessments (interoceptive sensibility) and behavioral tests (interoceptive accuracy; Garfinkel, Seth, Barrett, Suzuki, & Critchley, 2015).
Signals coming from the inside and outside of the body may interact, and the interplay between the two domains is currently unclear. From a behavioral point of view, Tsakiris, Jimenez, and Costantini (2011) have shown that interoceptive awareness may modulate the malleability of the sense of the self. They administered the RHI paradigm to a group of subjects showing low or high interoceptive accuracy, as measured by the Heartbeat Perception task, to find the counterintuitive finding that the less interoceptively accurate participants had a stronger incorporation effect of the rubber hand. Later, Suzuki, Garfinkel, Critchley, and Seth (2013) have shown that both subjective and objective measures of virtual‐hand ownership are enhanced by cardio‐visual feedback in time with the actual heartbeat, as compared to asynchronous feedback. They also have shown that these measures, correlated with individual differences in interoceptive sensitivity, were modulated by the integration of proprioceptive signals instantiated using real‐time visual remapping of finger movements of the virtual hand (Suzuki et al., 2013).
Although it is not clear whether a supramodal form of bodily self‐awareness exists, this evidence definitively suggests the existence of forms of integration between interoceptive and exteroceptive signals. While it is possible that integration of these domains might occur by forms of structural and functional connectivity of separate specialized regions, the logic of the human brain organization also allows one to hypothesize the existence of higher‐order neural systems where signals of the different sources are integrated (Zeki & Shipp, 1988).
To date, a quantitative investigation on this hypothesis and differences and commonalities between the two domains is lacking. Moseley, Gallace, and Spence (2012) have proposed a theoretical framework for the interaction between interoceptive and exteroceptive information in the bodily self‐awareness. They proposed the existence of a body matrix, a set of neural structures that “serve to maintain the integrity of the body at both the homeostatic and psychological levels and to adapt to changes in our body structure and orientation” (Moseley et al., 2012). They have also hypothesized that the posterior parietal and insular cortices may play an important role in the body matrix. In a more recent theoretical review (Park & Blanke, 2019), it has been suggested the existence of at least two subnetworks supporting the integration between interoceptive and exteroceptive signals in bodily self‐awareness: a premotor cortex—intraparietal sulcus—insula network preferentially processing signals for self‐identification, and a temporal parietal junction—posterior cingulate cortex—intraparietal sulcus network preferentially processing signals for self‐location. Interestingly, the authors have proposed an overlap of these two subnetworks in the intraparietal sulcus (Park & Blanke, 2019). However, explicit evidence on the anatomical localization of cortical regions linking exteroceptive and interoceptive signals is limited and discrepant to what previously hypothesized. Recently, Blefari et al. (2017) in an fMRI experiment administered the full body illusion paradigm coupled with cardiovascular response and heartbeat awareness task to a group of healthy volunteers: they found and proposed that the Rolandic operculi, in coordinates falling inside ventral premotor cortex, may be the critical regions subserving the integration of external and internal bodily signals. This interesting finding, in line with early fMRI reports on the RHI paradigm (Ehrsson, Spence, & Passingham, 2004), is clearly in need of replication as it would move the emphasis on a premotor view of bodily self‐awareness.
1.1. Aim of the study
In the current study, we aimed at unveiling the neural networks underpinning exteroceptive and interoceptive signals, seeking for modality‐specific and shared systems. As said, the overarching aim of this work was to test the existence of a shared neural system subtending the making of bodily self‐awareness. To this scope, we submitted to a quantitative meta‐analysis, the available neuroimaging studies concerning body ownership (external visuotactile information) and interoception (internal physiological information), which included psychological illusions involving diverse body parts and different approaches to assess interoception. We first performed two separate meta‐analyses investigating which brain regions are associated with the sense of body ownership and the interoceptive awareness according to these paradigms. We then examined whether body ownership and interoception share common neural substrates. Based on previous findings, we hypothesized to find evidence for a role of high‐order associative areas, for example, the parietal regions, where the merging of external and internal information is made possible by the convergence of different inputs. Because of the specific choice made on the to‐be meta‐analyzed materials, we will argue that any such regions may contribute to the building of a bodily self‐awareness.
2. METHODS
2.1. Study selection
To select relevant neuroimaging studies concerned with brain activity related to interoception or body awareness, an extensive database search of peer‐reviewed functional neuroimaging studies with no initial restrictions (regarding the type of publication or publication date) was conducted. We relied on the following sources: MEDLINE library, life science journals, and online books indexed in PubMed. Also, we reviewed the reference lists of these articles to find additional reports and used the Sleuth Software (BrainMap Development Team, Version 2.0.3, Research Imaging Center, University of Texas Health Science Center at San Antonio) to search the BrainMap database for published functional neuroimaging experiments.
The data sets and associated contrasts included in this meta‐analysis, met the following inclusion criteria: (a) description of standard Talairach and Tournoux (1988), or MNI coordinates (to enable comparison of reported peak activation across studies); (b) samples composed of unmedicated and untrained healthy adults; (c) corrected thresholds of significance established at a whole‐brain level; (d) measurement of regional cerebral blood flow (e.g., through PET) or blood oxygenation (e.g., through fMRI); and (e) tasks tapping core processes of interoception or body ownership without assessing related high‐level processes or pursuing more specific goals (such as correlations and/or interactions with other psychological constructs or demographic features).
Unlike a recent meta‐analysis on interoception (Schulz, 2016), the present one was not limited to cardioception and included studies with a variety of tasks. We adopted this inclusive criterion because our study's aim was to compare the awareness of bodily signals (without limiting these to cardiac activity) and the ability to self‐recognize bodily information. Likewise, the meta‐analysis on body ownership included a variety of tasks manipulating the sense of body ownership.
For body ownership, the key words used to search the databases were as follows: “fMRI” <OR>“functional neuroimaging” <OR>“PET” <OR>“neural basis” <OR>neural correlate <AND>“body ownership” <OR>“body disownership” <OR> “rubber hand illusion” <OR>“virtual hand illusion.”
Following this search, our data set included 286 participants drawn from 39 contrasts derived from 16 studies.
The key words for interoception were as follows: “fMRI” <OR>“functional neuroimaging” <OR>“PET” <OR>“neural basis” <OR>neural correlate <AND>“interoception” <OR>“cardioception” <OR>“visceral perception” <OR>“body emotions” <OR>“bodily emotions” <OR>“heartbeat” <OR>“heartbeat detection.” These criteria resulted in a total of 770 participants and 73 contrasts across 40 studies (see Table 1 for lists of included studies).
Table 1.
List of studies included on Body Ownership and Interoception included in the meta‐analysis
| First author (year) | Title | Modality | Participants number | Total contrasts | Contrasts used |
|---|---|---|---|---|---|
| Body ownership | |||||
| Bekrater‐Bodmann et al. (2014) | The importance of synchrony and temporal order of visual and tactile input for illusory limb ownership experiences—An fMRI study applying virtual reality | fMRI | 25 | 9 | 1 |
| Brozzoli, Gentile, and Ehrsson (2012) | That's near my hand! Parietal and premotor coding of hand‐centered space contributes to localization and self‐attribution of the hand | fMRI | 16 | 10 | 3 |
| Ehrsson et al. (2004) | That's my hand! Activity in premotor cortex reflects feeling of ownership of a limb | fMRI | 18 | 15 | 4 |
| Ehrsson, Holmes, and Passingham (2005) | Touching a rubber hand: Feeling of body ownership is associated with activity in multisensory brain areas | fMRI | 15 | 6 | 1 |
| Gentile, Guterstam, Brozzoli, and Ehrsson (2013) | Disintegration of multisensory signals from the real hand reduces default limb self‐attribution: An fMRI study | fMRI | 15 | 37 | 3 |
| Gentile, Björnsdotter, Petkova, Abdulkarim, and Ehrsson (2015) | Patterns of neural activity in the human ventral premotor cortex reflect a whole‐body multisensory percept | fMRI | 16 | 4 | 1 |
| Guterstam, Gentile, and Ehrsson (2013) | The invisible hand illusion: multisensory integration leads to the embodiment of a discrete volume of empty space | fMRI | 14 | 18 | 1 |
| Guterstam, Björnsdotter, Gentile, and Ehrsson (2015) | Posterior cingulate cortex integrates the senses of self‐location and body ownership | fMRI | 15 | 4 | 1 |
| Ionta et al. (2011) | Multisensory mechanisms in temporo‐parietal cortex support self‐location and first‐person perspective | fMRI | 22 | 1 | 1 |
| Limanowski and Blankenburg (2016) | That's not quite me: limb ownership encoding in the brain | fMRI | 13 | 33 | 3 |
| Limanowski and Blankenburg (2015) | Network activity underlying the illusory self‐attribution of a dummy arm | fMRI | 20 | 24 | 6 |
| Limanowski and Blankenburg (2018) | Fronto‐parietal brain responses to visuotactile congruence in an anatomical reference frame. | fMRI | 20 | 13 | 1 |
| Petkova et al. (2011) | From part‐to whole‐body ownership in the multisensory brain | fMRI | |||
| Exp 1 | 26 | 13 | 2 | ||
| Exp 2 | 20 | 29 | 2 | ||
| Exp 3 | 20 | 48 | 1 | ||
| Preston and Ehrsson (2016) | Illusory obesity triggers body dissatisfaction responses in the insula and anterior cingulate cortex | fMRI | 32 | 17 | 2 |
| Tsakiris, Hesse, Boy, Haggard, and Fink (2007) | Neural signatures of body ownership: A sensory network for bodily self‐consciousness | PET | 10 | 10 | 4 |
| Tsakiris, Longo, and Haggard (2010) | Having a body versus moving your body: Neural signatures of agency and body‐ownership | fMRI | 19 | 18 | 2 |
| Interoception | |||||
| Araujo, Kaplan, Damasio, and Damasio (2015) | Neural correlates of different self‐domains | fMRI | 19 | 6 | 1 |
| Avery et al. (2015) | A common gustatory and interoceptive representation in the human mid‐insula | fMRI | 20 | 3 | 1 |
| Bauer, Díaz, Concha, and Barrios (2014) | Sustained attention to spontaneous thumb sensations activates brain somatosensory and other proprioceptive areas | fMRI | 34 | 6 | 1 |
| Becker, Schmälzle, Flaisch, Renner, and Schupp (2014) | Thirst and the state‐dependent representation of incentive stimulus value in human motive circuitry | fMRI | 24 | 2 | 1 |
| Binks, Evans, Reed, Moosavi, and Banzett (2014) | The time‐course of cortico‐limbic neural responses to air hunger | fMRI | 8 | 2 | 1 |
| Blefari et al. (2017) | Bilateral Rolandic operculum processing underlying heartbeat awareness reflects changes in bodily self‐consciousness | fMRI | 16 | 5 | 1 |
| Brannan et al. (2001) | Neuroimaging of cerebral activations and deactivations associated with hypercapnia and hunger for air | fMRI | 9 | 3 | 3 |
| Cameron and Minoshima (2002) | Regional brain activation due to pharmacologically induced adrenergic interoceptive stimulation in humans | PET | 24 | 1 | 1 |
| Caseras et al. (2013) | Anatomical and functional overlap within the insula and anterior cingulate cortex during Interoception and phobic symptom provocation | fMRI | 46 | 2 | 1 |
| Coen et al. (2009) | Negative mood affects brain processing of visceral sensation | fMRI | 12 | 4 | 1 |
| Critchley (2004) | Neural systems supporting interoceptive awareness | fMRI | 17 | 2 | 1 |
| Denton et al. (1999a) | Correlation of regional cerebral blood flow and change of plasma sodium concentration during genesis and satiation of thirst | PET | 10 | 1 | 1 |
| Denton et al. (1999b) | Neuroimaging of genesis and satiation of thirst and an interoceptor‐driven theory of origins of primary consciousness | PET | 10 | 4 | 4 |
| Egan et al. (2003) | Neural correlates of the emergence of consciousness of thirst | fMRI and PET | 10 | 5 | 5 |
| Ernst, Northoff, Böker, Seifritz, and Grimm (2013) | Interoceptive awareness enhances neural activity during empathy | fMRI | 18 | 3 | 1 |
| Evans et al., 2002 | BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger | fMRI | 6 | 1 | 1 |
| Farb, Segal, and Anderson (2013) | Mindfulness meditation training alters cortical representations of interoceptive attention | fMRI | 16 | 3 | 1 |
| Farrell et al. (2006) | Unique, common, and interacting cortical correlates of thirst and pain | PET | 10 | 3 | 3 |
| Haase et al. (2015) | A pilot study investigating changes in neural processing after mindfulness training in elite athletes | fMRI | 7 | 3 | 3 |
| Haase et al. (2016) | When the brain does not adequately feel the body: Links between low resilience and interoception | fMRI | 46 | 4 | 1 |
| Hassanpour et al. (2018) | The insular cortex dynamically maps changes in cardiorespiratory interoception | fMRI | 23 | 2 | 1 |
| Immordino‐Yang, Yang, and Damasio (2014) | Correlations between social–emotional feelings and anterior insula activity are independent from visceral states but influenced by culture | fMRI | 15 | 3 | 3 |
| Isaev, Murphy, Guz, and Adams (2002) | Areas of the brain concerned with ventilatory load compensation in awake men | PET | 10 | 1 | 1 |
| Kuehn, Mueller, Lohmann, and Schuetz‐Bosbach (2016) | Interoceptive awareness changes the posterior insula functional connectivity profile | fMRI | 12 | 7 | 2 |
| Liotti et al. (2001) | Brain responses associated with consciousness of breathlessness (air hunger) | PET | 9 | 2 | 2 |
| May, Stewart, Migliorini, Tapert, and Paulus (2013) | Methamphetamine dependent individuals show attenuated brain response to pleasant interoceptive stimuli | fMRI | 17 | 2 | 1 |
| May, Stewart, Tapert, and Paulus (2014) | The effect of age on neural processing of pleasant soft touch stimuli | fMRI | 58 | 2 | 1 |
| Oberndorfer et al. (2013) | Greater anterior insula activation during anticipation of food images in women recovered from anorexia nervosa versus controls | fMRI | 12 | 4 | 1 |
| Perini, Morrison, and Olausson (2015) | Seeking pleasant touch: Neural correlates of behavioral preferences for skin stroking | fMRI | 18 | 8 | 4 |
| Pollatos, Schandry, Auer, and Kaufmann (2007) | Brain structures mediating cardiovascular arousal and interoception | fMRI | 20 | 4 | 3 |
| Simmons et al. (2013) | Keeping the body in mind: Insula functional organization and functional connectivity integrate interoceptive. Exteroceptive, and emotional awareness | fMRI | 14 | 3 | 1 |
| Stern et al. (2017) | Neural correlates of Interoception: Effects of interoceptive focus and relationship to dimensional measures of body awareness | fMRI | 19 | 7 | 3 |
| Stewart, Parnass, May, Davenport, and Paulus (2013) | Altered frontocingulate activation during aversive interoceptive processing in young adults transitioning to problem stimulant use | fMRI | 29 | 6 | 1 |
| Stewart et al. (2014) | You are the danger: Attenuated insula response in methamphetamineusers during aversive interoceptive decision‐making | fMRI | 22 | 3 | 1 |
| Stewart, Juavinett, May, Davenport, and Paulus (2015) | Do you feel alright? Attenuated neural processing of aversive interoceptive stimuli in current stimulant users | fMRI | 15 | 4 | 2 |
| Strigo et al. (2013) | Altered insula activation during pain anticipation in individuals recovered from anorexia nervosa: Evidence of interoceptive Dysregulation | fMRI | 22 | 3 | 2 |
| Terasawa, Fukushima, and Umeda (2013) | How does interoceptive awareness interact with the subjective experience of emotion | fMRI | 18 | 6 | 4 |
| Tracy et al. (2007) | Functional magnetic resonance imaging analysis of attention to one's heartbeat | fMRI | 17 | 5 | 5 |
| Wang et al. (2008) | Gastric distention activates satiety circuitry in the human brain | fMRI | 18 | 1 | 1 |
| Zaki, Davis, and Ochsner (2012) | Overlapping activity in anterior insula during | fMRI | 16 | 4 | 1 |
2.2. Multilevel kernel density analysis
For this study, we used the multilevel kernel density analysis (MKDA; Kober et al., 2008; Kober & Wager, 2010; Wager, Lindquist, & Kaplan, 2007; Wager, Lindquist, Nichols, Kober, & Van Snellenberg, 2009), which allows one to detect robust effects and to establish the level of consistency across findings (Costafreda, 2009; Lindquist, Satpute, Wager, Weber, & Barrett, 2016; Schurz, Radua, Aichhorn, Richlan, & Perner, 2014). The technique reduces variability between studies in search of convergent findings across a set of studies, to average results to identify brain areas that show consistent activity (Liang, Zou, He, & Yang, 2013; van den Heuvel & Sporns, 2013). The MKDA emphasizes the multilevel hierarchy of the meta‐analytic input data by treating activation peaks as nested within a given contrast. The resulting contrast maps—instead of individual peaks—are the unit of analysis and through this, two levels of analysis (within‐contrasts and between contrasts) are created. Therefore, the MKDA summarizes consistency across studies rather than across peak coordinates. An important consequence of this approach is that any single study that reports a large number of nearby peaks (due to differences in style of how regional effects are reported, voxel size, thresholding, or low spatial smoothness in the data) cannot have an excessive weight on the final results (Wager et al., 2009; for further explanations see also Supporting Information).
More specifically, the MKDA performs multiple nested analyses on individual peaks by: (a) nesting peak activation coordinates within contrasts, and contrasts within studies; (b) modeling the variability across peaks within a contrast, rather than just counting all the peaks without taking into account from which contrast or study the peaks come from—so that true effect sizes are assumed to vary between studies; (c) assessing statistical significance density maps by comparing against the null hypothesis whereby activated regions are randomly distributed throughout the whole brain (Kober & Wager, 2010; Wager et al., 2009).
To this end, one contrast indicator map (CIM) is created for each contrast, by convolving a 10‐mm spherical kernel around each peak reported in this contrast. The CIMs are averaged and weighted by the square root of the sample size of each contrast so that studies with fewer participants are given less weight, while reports with a larger number of participants are given more weight. Additionally, CIMs are weighted with 0.75 for fixed‐effects versus 1.00 for random effects analysis to reduce the impact of fixed‐effect studies (Wager et al., 2007, 2009; see also Supporting Information).
The resulting Summary Density Map built up of the individual CIMs reflects the proportion of contrasts yielding activations near each voxel. Hence, the crucial measure of interest is the number of contrasts that produced activation near a voxel, rather than the number of individual activation peaks.
The general null hypothesis states that peak coordinates of activated regions are randomly distributed across the gray matter of the standard brain. To identify voxels with activations that exceed the frequency expected by chance, a threshold derived from a Monte Carlo Simulation with 5,000 iterations per analysis was used. Building on this, MKDA identified maps of activated clusters according to a “height” or “extent” based threshold. The height‐based threshold encloses voxels that have proportions of contrasts inside the 10 mm kernel regions that exceed the maximum expected over the entire brain by chance (p < .05, family wise error rate—FWER corrected). To determine a cluster extent‐based threshold, the largest cluster of contiguous voxels was saved after each Monte Carlo iteration (with primary alpha levels of .001, .01, and .05) and secondary FWER‐corrected for spatial extent at p < .05. Beyond that, a combined map of voxels meeting both criteria (height [p < .05] and extent [primary alpha level: p < .05]) was computed using SPM8 contiguity assessment procedures (see Figure 1; Kober et al., 2008; Schulz, 2016). In conclusion, the statistics reported in text and tables as the “z‐value” (Kober et al., 2008) correspond to the proportion of contrasts that activated within 10 mm (kernel region) of that voxel, weighted by the sample size and study design analysis (fixed or random effects; Buhle et al., 2014; Denny, Kober, Wager, & Ochsner, 2012; Kober et al., 2008; Mende‐Siedlecki, Said, & Todorov, 2013). Thus, z‐values (maximum statistic values) are based on weighted CIMs rather than studies or peaks and represent the weighted percentage of CIMs that reported activation in each cluster (Kober et al., 2008; see also Supporting Information). The results were visualized using Caret Software (version 5.65, Van Essen Laboratory, Saint Louis; http://brainvis.wustl.edu). Activation maps for both domains containing the combined results from extent‐ and height‐based thresholds (p < .05, FWER‐corrected) were projected onto the left and right hemispheres of the PALS atlas (Population‐Average, Landmark and Surface‐based atlas, Van Essen Laboratory, Saint Louis; http://sumsdb.wustl.edu/sums/humanpalsmore.do) using MNI coordinate space. Axial cut planes of the overlap areas on a template brain image were created using the Mango software (version 4.0.1, Research Imaging Institute, UTHSCSA).
Figure 1.
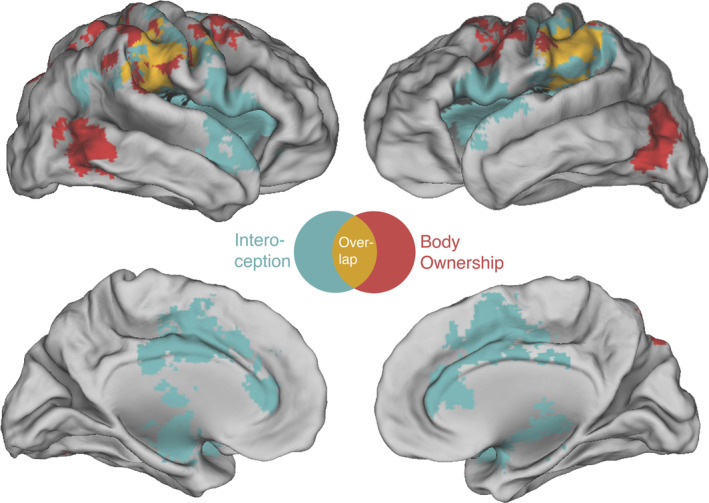
Results of the conjunction analysis. The upper panel shows the body ownership (red) and interoception (light blue) areas of activations. The voxels of overlap between the two functions are shown in yellow. Voxels are family‐wise error rate (FWER) corrected at p < .05 with primary alpha levels of .05 for spatial extent. The lower figure quantifies overlapping areas following the AAL atlas. Z‐scores indicate the maximal weighted percentage of contrasts in an area, separately for both meta‐analyses. Only conjoint activity resulting in a cluster size with a minimum of 10 voxels is reported. Voxel‐size is 2 x 2 x 2 mm; SMG, supramarginal gyrus; R, right; L, left [Color figure can be viewed at http://wileyonlinelibrary.com]
2.3. Statistical analyses to test specific effects
To extract the areas activated by both domains (interoception and body ownership), a conjunction analysis was conducted on the two combined (height‐ and extend‐based) MKDA maps (at 0.05 FWER‐corrected), as described by Nichols, Brett, Andersson, Wager, and Poline (2005) using MATLAB (The MathWorks, Inc., Natick, MA), and SPM8 (Statistical Parametric Maps, Wellcome Department of Cognitive Neurology, http://www.fil.ion.ucl.ac.uk/spm). Only conjoint activity resulting in a cluster‐size with a minimum of 10 voxels is reported. The clusters were analyzed using the xjView toolbox (http://www.alivelearn.net/xjview/).
Furthermore, we compared the activation of body ownership and interoception by subtraction analysis in MKDA: separate maps constructed for both domains were subtracted to yield difference maps. The same procedure was employed during the Monte Carlo randomization to establish a threshold for significant differences.
3. RESULTS
3.1. Body ownership
For studies employing tasks addressing the concept of body ownership, peak areas of high concordance were detected in the inferior temporal gyri spanning to the inferior occipital lobes (right, z = 0.2; left, z = 0.18). Further, we found bilateral clusters in the inferior parietal lobes also involving the postcentral gyri with cluster centers in the supramarginal gyri (right, z = 0.14; left, z = 0.17). Additional cluster centers were located in the precentral gyri (right, z = 0.15; left, z = 0.15), the cerebellum (right cerebellar tonsil, z = 0.16; right declive, z = 0.09; left declive = 0.08), the right parietal inferior lobe (z = 0.15), the right superior parietal lobe (z = 0.12), and the insular cortices (right, z = 0.08; left, z = 0.06; see Table 2 and Figure 1).
Table 2.
Results of the meta‐analysis on body ownership
| Area | x | y | z | Brodmann area | Voxels | Volume (mm3) | Maxstat (z) |
|---|---|---|---|---|---|---|---|
| Height threshold (p < .05, family wise error rate—FWE corrected) | |||||||
| Right precentral gyrus | 48 | 4 | 40 | 6 | 1 | 8 | 0.15 |
| Left inferior parietal lobe | −60 | −30 | 28 | 40 | 1 | 8 | 0.17 |
| Right inferior temporal gyrus | 50 | −56 | −6 | 19 | 28 | 224 | 0.20 |
| Left inferior temporal gyrus | −46 | −70 | −2 | 37 | 21 | 168 | 0.18 |
| Left fusiform gyrus | −40 | −70 | −10 | 19 | 46 | 368 | 0.18 |
| Right cerebellum (cerebellar tonsil) | 28 | −72 | −36 | 1 | 8 | 0.16 | |
| Extent threshold (primary alpha level of p < .001) | |||||||
| Left precentral gyrus | −50 | 0 | 34 | 6 | 236 | 1,888 | 0.15 |
| Right inferior parietal lobe | 54 | −22 | 26 | 40 | 210 | 1,680 | 0.14 |
| Right inferior parietal lobe | 54 | −28 | 46 | 40 | 399 | 3,192 | 0.15 |
| Extent threshold (primary alpha level of p < .01) | |||||||
| Left cerebellum (Declive) | −32 | −70 | −16 | 13 | 104 | 0.08 | |
| Extent threshold (primary alpha level of p < .05) | |||||||
| Right insula | 42 | −14 | 20 | 13 | 25 | 200 | 0.07 |
| Left insula | −42 | −24 | 20 | 13 | 61 | 488 | 0.06 |
| Left inferior parietal lobe | −44 | −28 | 38 | 40 | 43 | 344 | 0.05 |
| Right insula | 42 | −32 | 20 | 13 | 77 | 616 | 0.05 |
| Left inferior parietal lobe | −58 | −34 | 44 | 40 | 49 | 392 | 0.05 |
| Right insula | 58 | −36 | 24 | 13 | 213 | 1,704 | 0.08 |
| Right superior parietal lobe | 34 | −52 | 52 | 7 | 1,366 | 10,928 | 0.12 |
| Right cerebellum (Declive) | 34 | −62 | −24 | 2,275 | 18,200 | 0.09 | |
Note: Stereotactic coordinates for the most consistent clusters across all body ownership studies according to a “height” and “extent” based thresholds. Height‐based threshold encloses voxels that have proportions of contrasts inside the 10 mm kernel regions that exceed the maximum expected over the entire brain by chance (p < .05, family wise error Rate—FWER corrected). Extent‐based threshold encloses contiguous voxels outside the 10 mm of the clusters for the height‐based threshold that showed greater activation than would be expected at a given level of chance (p < .001), and which are secondary FWER‐corrected for spatial extent at p < .05.
3.2. Interoception
The meta‐analysis of interoception showed the most significant cluster in the right insula (z = 0.26) followed by the left insula (z = 0.22), the left inferior parietal gyrus (z = 0.17), the left medial frontal gyrus (z = 0.17), the right thalamus (pulvinar, z = 0.15; medial dorsal nucleus, z = 0.09), the right postcentral (z = 0.14), and precentral gyrus (z = 0.11), the cingulate gyrus (midcingulate, z = 0.14; anterior cingulate, z = 0.13), the right midbrain (z = 0.1), and the right medial temporal gyrus (z = 0.06; see Table 3 and Figure 1).
Table 3.
Results of the meta‐analysis on interoception
| Area | x | y | z | Brodmann area | Voxels | Volume (mm3) | Maxstat (z) |
|---|---|---|---|---|---|---|---|
| Height threshold (p < .05, family wise error rate—FWE corrected) | |||||||
| Right insula | 40 | 8 | 4 | 13 | 755 | 6,040 | 0.26 |
| 40 | 10 | 4 | 559 | 0.26 | |||
| 40 | −6 | 8 | 150 | 0.19 | |||
| 36 | 6 | 12 | 46 | 0.19 | |||
| Mid cingulate gyrus | 2 | 8 | 42 | 24 | 1 | 8 | 0.14 |
| Mid cingulate gyrus | −4 | 6 | 40 | 24 | 1 | 8 | 0.14 |
| Mid cingulate gyrus | 0 | 6 | 38 | 24 | 1 | 8 | 0.14 |
| Left insula | −40 | 4 | 4 | 13 | 269 | 2,152 | 0.22 |
| Left medial frontal gyrus | 2 | 0 | 48 | 6 | 132 | 1,056 | 0.17 |
| −2 | −2 | 44 | 32 | 0.17 | |||
| 4 | 2 | 48 | 100 | 0.16 | |||
| Mid cingulate gyrus | −4 | −10 | 40 | 24 | 1 | 8 | 0.14 |
| Right thalamus (Pulvinar) | 18 | −24 | 8 | 14 | 112 | 0.15 | |
| Right postcentral gyrus | 52 | −24 | 34 | 2 | 1 | 8 | 0.14 |
| Left inferior parietal lobe | −56 | −26 | 24 | 40 | 2 | 16 | 0.14 |
| Left inferior parietal lobe | −58 | −34 | 30 | 40 | 36 | 288 | 0.17 |
| Left inferior parietal lobe | −52 | −34 | 26 | 40 | 3 | 24 | 0.14 |
| Extent threshold (primary alpha level of p < .001) | |||||||
| Anterior cingulate | 0 | 30 | 16 | 24 | 163 | 1,304 | 0.13 |
| Right insula | 44 | −2 | 10 | 13 | 1,823 | 14,584 | 0.13 |
| Right precentral gyrus | 22 | −30 | 62 | 4 | 191 | 1,528 | 0.11 |
| Extent threshold (primary alpha level of p < .01) | |||||||
| Right midbrain (substantia nigra) | 14 | −20 | −8 | 423 | 3,384 | 0.10 | |
| Extent threshold (primary alpha level of p < .05) | |||||||
| Thalamus (medial dorsal Ncl.) | 2 | −10 | 16 | 9,618 | 76,944 | 0.09 | |
| Right mid temporal gyrus | 52 | −62 | 20 | 19 | 28 | 224 | 0.06 |
Note: Stereotactic coordinates for the most consistent clusters across all interoception studies according to a “height” and “extent” based thresholds. Height‐based threshold encloses voxels that have proportions of contrasts inside the 10 mm kernel regions that exceed the maximum expected over the entire brain by chance (p < .05, family wise error Rate—FWER corrected). Extent‐based threshold encloses contiguous voxels outside the 10 mm of the clusters for the height‐based threshold, which showed greater activation than would be expected at a given level of chance (p < .001), and which are secondary FWER‐corrected for spatial extent at p < .05.).
3.3. Contrast analyses between body ownership and interoception
The analysis of the contrast map Body Ownership > Interoception revealed that the left inferior occipital gyrus (z = 0.17) and the right middle occipital gyrus (z = 0.17) were associated with body ownership (see Supporting Information). These regions were in stereotactic locations consistent with the extrastriate visual areas involved in the visual processing of human bodies.
Furthermore, the contrast map Interoception > Body Ownership revealed that several deep cortical and subcortical regions, such as the left and right insula (z = 0.19; z = 0.23), the right (z = 0.13), and left (z = 0.1) thalamus, the left globus pallidus (z = 0.13), and the mid‐cingulate gyrus (z = 0.15) as well as the anterior cingulate gyrus (z = 0.12), were more likely associated with interoception (see Supporting Information).
3.4. Areas shared by the sense of body ownership and interoception
Figure 1 illustrates the MKDA maps (conjoint of height and extent threshold) of body ownership and interoception tasks on a single template (PALS atlas; Van Essen, 2005): the largest areas of overlap were seen bilaterally in the inferior parietal lobules, in particular in the supramarginal gyri (SMG) spanning to the postcentral gyri. Further areas significantly activated by both meta‐analyses are the rolandic opercula, the right precentral gyrus, the superior temporal lobule, and the inferior parietal lobe.
A cluster‐based analysis of such convergence revealed by the conjunction analysis (p < .05, FWER‐corrected, Figure 2) unveiled two large clusters with their cluster centers in the right and left SMG, respectively (left SMG: 1,077 voxels; z = 0.14; right SMG: 1,003 voxels; z = 0.12).1 Additionally, three smaller clusters were found in the right hemisphere. The maximum activation of the respective cluster was hereby found in the precentral gyrus (182 voxels; z = 0.11), the superior temporal gyrus (28 voxels; z = 0.07), and the postcentral gyrus (12 voxels; z = 0.08; see Table 4).
Figure 2.
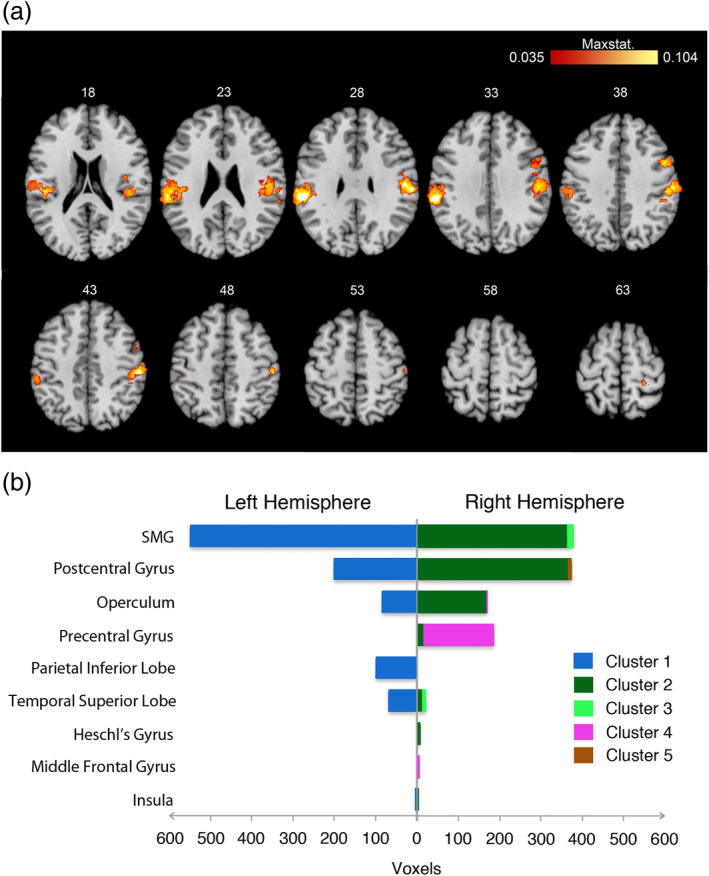
Results of the cluster analysis on the overlap. Panel A shows axial brain slices of the overlapping areas mapped on a standard brain template. Numbers above the slices indicate z‐coordinates. Maxstat is the mean z‐value of both meta‐analyses. Panel B shows the brain areas (following AAL atlas) covered by the respective clusters. Voxel‐size is 2 x 2 x 2 mm; SMG, supramarginal gyrus [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 4.
Results of the cluster analysis
| Area | x | y | z | Brodmann area | Cluster size (voxels) | Maxstat (z) |
|---|---|---|---|---|---|---|
| Left supramarginal gyrus (Cluster 1) | −60 | −30 | 28 | 40 | 1,077 | 0.14 |
| Right supramarginal gyrus (Cluster 2) | 56 | −24 | 42 | 2 | 1,003 | 0.12 |
| Right superior temporal gyrus (Cluster 3) | 62 | −40 | 22 | 13 | 28 | 0.07 |
| Right precentral gyrus (Cluster 4) | 48 | 4 | 40 | 6 | 182 | 0.11 |
| Right postcentral gyrus (Cluster 5) | 24 | −38 | 62 | 5 | 12 | 0.08 |
Note: Stereotactic coordinates for the most consistent clusters of convergence between interoception and body ownership activation studies (p < .05, family wise error rate—FWER corrected.
4. DISCUSSION
We continuously receive different signals from our body, coming from both the inside and outside multisensory channels. Are there specific cerebral areas permitting the convergence of the two domains? Evidence on the topic was scanty with suggestions, based on qualitative reviews of the available evidence, that these levels of multisensory integration might be supported by the insular, posterior parietal cortex (Moseley et al., 2012; Park & Blanke, 2019), while one explicit experiment on the matter pointed to ventral premotor cortex (Blefari et al., 2017).
Here, we instead quantified the available evidence on where the internal and external signals may be integrated and demonstrated the specific importance of the inferior parietal cortex of both hemispheres, comprising the parietal portion of the Rolandic operculum and insula, together with right‐lateralized areas such as the postcentral, precentral, and superior temporal gyri. In what follows, we will discuss in an orderly manner the brain regions mainly associated with body ownership, interoception, and the areas of convergence between the two domains.
4.1. Body ownership
Our meta‐analysis identified a compound set of occipitotemporal, premotor, parietal, and insular regions contributing to the sense of body ownership, consistent with the nature of the triggering paradigms. In keeping with previous studies and a very recent meta‐analysis, this network most likely integrates bodily signals across diverse sensory channels (Grivaz, Blanke, & Serino, 2017).
The areas with the most significant clusters were the right inferior temporal gyrus and the left inferior occipital lobe. These occipitotemporal regions have been identified as part of the body self‐recognition network. The activity of this network, and in particular of the Fusiform Body Area together with the Extrastriate Body Area, has been associated with the perception and identification of bodies and their parts. More specifically, these occipital areas would be involved in the implicit processing of morphological features peculiar of a person's body, which are further processed by more anterior regions to explicitly define who a person might be (De Bellis, Trojano, Errico, Grossi, & Conson, 2017).
Another area with high convergent activation across studies was the left SMG. This parietal region has been associated with the decoding of self‐location (Guterstam et al., 2015) and perceiving limbs in space in a body‐centered reference (Brozzoli et al., 2012). In particular, during the RHI paradigm, being the real hand position remapped onto a prosthetic hand, such remapping associated with activity in the posterior parietal cortex closely reflects changes in the position sense of the arm (Brozzoli et al., 2012). Furthermore, the SMG is a part of the neuronal network integrating visuotactile input applied to the hand (Gentile et al., 2013), and other findings indicate that this region also receives proprioceptive inputs (Berlucchi & Vallar, 2018; Freedman & Ibos, 2018; Paulesu, Frackowiak, & Bottini, 1997; Whitlock, 2017).
Our results further suggest that the right precentral gyrus plays a role in body ownership. Such an area is supposed to be implicated in the experimental manipulation of the sense of ownership concerning peripersonal space remapping (Brozzoli et al., 2012), and the multisensory integration of tactile‐proprioceptive and visual inputs (Ehrsson et al., 2005).
Lastly, our meta‐analysis also highlights the importance of cerebellum in body ownership. In the RHI paradigm, it has been shown that the activity of the cerebellum correlates with the perceived strength of the illusion (Ehrsson et al., 2004; Gentile et al., 2013; Petkova et al., 2011). Furthermore, it also contributes to distinguishing sensory signals generated by the self or by others, as in the case of the sense of touch (Blakemore, Frith, & Wolpert, 2001). In a recent fMRI study on anosognosia for hemiplegia, right cerebellar activation was the only distinctive finding between the veridical appreciations of voluntary motion of the nonparalyzed hand, and the delusional belief of having moved the paralyzed hand (Gandola et al., 2014). This suggests that the cerebellum might have a role in “closing the loop” in a body and action monitoring system. Moreover, the cerebellum is involved in the processing of synchrony and congruence among the senses (Blakemore et al., 2001; Ito, 2000; Miall, Weir, Wolpert, & Stein, 1993), integrating signals coming from different multisensory channels. Indeed, it has been suggested that the cerebellum is crucial for the detection of corresponding multisensory signals and the formation of cross‐modal predictions (Gentile et al., 2013).
Interestingly enough, unlike a recent meta‐analysis on body ownership (Grivaz et al., 2017), we did not find the insula as an area of convergent activation of body ownership paradigms, except when using a less conservative threshold (see Table 2). Of course, the insula is part of the somatosensory network (see review in Bottini et al., 1995; Paulesu et al., 1997) and of the pain matrix and it may participate to the motor awareness (Karnath, 2005). One may speculate that, because the insula is involved in somatosensory perception from ground up, the subtle body ownership paradigms (e.g., the RHI) are not sufficient to give a consistent activation of the structure. Indeed, the difference sought in the paradigm if between the integration of synchronous touches compared to asynchronous touches to the subjects' hand and visual stimuli given to the rubber hand. However, one may wonder why other meta‐analyses found body‐awareness clusters in that region; the only other reason, we can invoke is a methodological difference behind our studies. Unlike Grivaz et al. (2018), we did not consider peaks resulting from small‐volume corrections or ROIs, and we used a more conservative statistical threshold (FWER p < .05).
4.2. Interoception
The meta‐analysis of the studies on interoception confirmed the involvement of brain areas that have entered into common neuroscientific discourse when referring to brain foundations of bodily feelings (Craig, 2002). The area with the clusters of maximal significance in terms of peak height and spatial extent was in the bilateral insulae, widely considered the primary interoceptive cortical area (Craig, 2002; Critchley, 2004; Pollatos et al., 2007). It has been suggested that the right (anterior) insula mediates explicit awareness of internal bodily processes (Craig, 2002; Critchley, Wiens, Rotshtein, Öhman, & Dolan, 2004), the laterality being associated with the nature of afferents. Stimuli such as air hunger, pain, cardiac perception, and visceral perception are primarily projected to the right anterior insula via vagal pathways (Craig, 2002). Instead, stimuli like the subjective sense of fullness (Stephan et al., 2003) are said to provide input primarily to the left anterior insula (Craig, 2002; Kelly et al., 2012). This is in part consistent with a recent cardiac interoception meta‐analysis showing a right hemispheric dominance for cardioception (Schulz, 2016). Our meta‐analysis was not limited to cardioception and included studies with a variety of tasks (e.g., thirst, air‐hunger, attention to spontaneous sensations, soft touch, and gastric balloon distension), possibly explaining the bilateral distribution of the clusters.
We also found highly convergent activation in the cingulate cortex (mainly the middle and anterior portions), which is—along with the insula—involved in emotional, homeostatic/allostatic, sensorimotor, and cognitive functioning (Craig, 2002; Critchley et al., 2003; Critchley, Tang, Glaser, Butterworth, & Dolan, 2005; Devinsky, Morrell, & Vogt, 1995). Resting‐state imaging showed the anterior insula to be functionally connected with the anterior cingulate cortex and the middle cingulate cortex, while the mid/posterior insula seems to be connected with the middle cingulate cortex (Taylor, Seminowicz, & Davis, 2009). Notably, in patients suffering from irritable bowel syndrome abnormal rectal‐evoked, fMRI responses in both, the insula and cingulate cortex have been identified (Davis et al., 2008).
Furthermore, the insula and the cingulate cortex are part of the so‐called salience system, which is said to detect relevant environmental changes regardless of the sensory modality employed in the task (Downar, Crawley, Mikulis, & Davis, 2002). This evidence points in the direction that conjoint activation of the cingulate cortex and the insula contributes to salience detection (Seeley et al., 2007) through task‐set maintenance (Dosenbach et al., 2007; Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008) and sustained focal attention (Nelson et al., 2010). Thus, anterior cingulate cortex may act as an integrative hub for interoceptive perception (Kleckner et al., 2017). As different interoceptive dimensions have different neuroanatomical correlates (García‐Cordero et al., 2016), while the insula seems to be a critical region for interoception in general, anterior cingulate cortex involvement could be dependent on the type of interoceptive processing (Couto et al., 2015). Also related to interoception, we found clusters in the thalamus, a relay of the lamina‐1‐spino‐thalamo‐cortical as well as the vagal pathway projecting physiological signals to the interoceptive network (Craig, 2002).
4.3. Body ownership versus interoception and vice‐versa
4.3.1. Body ownership > interoception
It is worth emphasizing that the occipitotemporal areas discussed above were more frequently associated with the body‐ownership paradigms rather than with the interoception ones. This distinction survived a formal statistical assessment. Prima facie, this may merely reflect the visual nature of the body ownership illusion triggering paradigms: however, the spatial consistency of these foci with visual regions specialized for the processing of body parts makes this observation less than trivial to instead suggest a crucial contribution of these cortices to the illusory perception of ownership of the RHI or the Full Body Illusion. Crucially, TMS over these areas can modulate to what extent the body and its parts are experienced as part of one's own body (De Bellis et al., 2017; Urgesi, Calvo‐Merino, Haggard, & Aglioti, 2007).
4.3.2. Interoception > body ownership
As for body ownership, we also found specific associations in the case of interoception. These localized primarily in the insulae bilaterally. Interestingly, the insular cortices also receive somatosensory stimuli for exteroceptive stimulation (Bottini et al., 1995; Burton, Videen, & Raichle, 1993). Their modulation through caloric vestibular stimulation can reverse attentional hemianaesthesia (Bottini et al., 1995). The same stimulation can revert the pathological sense of disownership observed in somatoparaphrenia (Bisiach, Jisa, Oni, & Vallak, 1991; Rode et al., 1992; Salvato et al., 2016, 2018). The receptive fields of the somatosensory insular or retro‐insular neurons are vast with responses for stimuli to either side of the body or both sides of the body (Paulesu et al., 1997). These anatomo‐physiological considerations suggest a noncomplete separation between interoceptive and exteroceptive signals for the sake of the generation of a sense of ownership instead, they suggest a gradient from regions more, but not exclusively, concerned with interoception, like the insulae, to regions more concerned with exteroceptive stimulation and body ownership (see below).
A close look to Figure 1 suggests the existence of a functional anatomical pattern, with a latero‐medial gradient for the processing of exteroceptive rather than interoceptive stimuli: medial activations were mainly linked to interoception, whereas lateral activations were shared, or dominated by exteroception. This pattern reminds a similar gradient for the default mode network (DMN) and dorsal attentional network (DAN), which are both part of the brain networks observed at rest. Interestingly, the DMN has been associated with self‐referential mental activity and internally oriented emotional processing (Buckner, Andrews‐Hanna, & Schacter, 2008; Gusnard, Akbudak, Shulman, & Raichle, 2001), whereas the DAN is active during any externally directed cognitive process (Corbetta & Shulman, 2002). This scenario fits well with our findings indicating a possible implication of distinctive resting‐state networks in interoceptive (DMN internally driven activity) and the exteroceptive (DAN externally driven activity) processing.
These networks are typically anticorrelated at rest (Gao & Lin, 2012). However, the convergence of the two networks in task‐based activation patterns suggests that their anticorrelation is not an inevitable rule; the same observation suggests that indeed the two networks, or part of them, that is, the areas of convergence, may move in the same functional direction when a sense bodily self‐awareness is generated. Admittedly, this remains a suggestion, though, as a meta‐analysis, by its nature, can only define areas of anatomical convergence but not the timing of such convergence and functional coherence.
4.4. Convergence between body ownership and interoception
The largest areas of shared “meta‐analytical activations” were found in a bilateral cluster centered in the SMG. The most likely homolog of the human SMG is the monkey area 7b, which, to our knowledge, has never been associated with interoception. However, the same area has connections with the granular insula and more generally with the limbic system (Friedman, Murray, O'Neill, & Mishkin, 1986; Hyvärinen, 1982; Mesulam, Van Hoesen, Pandya, & Geschwind, 1977), the main region that we associated with interoception. Accordingly, it becomes less surprising our identification of the SMG as the cortical region for both body ownership and interoception. In humans, it has been demonstrated that the SMG is activated by tasks in which both ownership and interoception are required. Kashkouli Nejad et al. (2015) asked participants to direct their awareness to certain parts of their body during the fMRI scan. They found bilateral activations in SMG, which was modulated by the participants' level of experience concerning the meditative practice involving the body (Kashkouli Nejad et al., 2015). Furthermore, Heydrich et al. (2018) measured brain activity during the “cardio‐visual full body illusion” which combines interoceptive and exteroceptive signals, by providing participants with visual exteroceptive information about their heartbeat in the form of a periodically illuminated silhouette outlining a video image of the participant's body and flashing in synchrony with their heartbeat (Heydrich et al., 2018). They found that a late somatosensory evoked potential component (P45) reflected the illusory self‐identification with a virtual body, demonstrating that the combination of interoceptive and exteroceptive signals modulate activity in the parietal somatosensory cortex (Heydrich et al., 2018).
The parietal cortex also seems to play a role in integrating physiological signals, such as body temperature. Recent studies have suggested the existence of a link between the sense of body ownership and body temperature (Kammers, Rose, & Haggard, 2011; Moseley et al., 2008; Salvato, Gandola, et al., 2018; Sedda, Tonin, Salvato, Gandola, & Bottini, 2016; Tieri, Gioia, Scandola, Pavone, & Aglioti, 2017). In healthy subjects, the transient, illusory incorporation of the fake hand in RHI paradigm has been associated with a decrease of the real hand temperature (Moseley et al., 2008). Furthermore, limb temperature modifications in healthy subjects may affect the strength of the illusion of ownership toward the rubber hand (Kammers et al., 2011).
We also found a set of right‐lateralized clusters centered in the precentral, postcentral, and superior temporal gyri. Interestingly, the precentral gyrus cluster coordinates overlap with the right ventral Premotor cortex (PMv; Mayka, Corcos, Leurgans, & Vaillancourt, 2006), which supports the perception of the self in space (Grivaz et al., 2017; Serino et al., 2013). This area is also essential for sensorimotor integration (Cooke, 2004; Iacoboni, Woods, Lenzi, & Mazziotta, 1997). In the same fashion, the right superior temporal gyrus is a hot‐zone for body representation as well. Indeed, our cluster center is very adjacent to the right temporoparietal junction (rTPJ; from http://www.neurosynth.org Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011; uniformity test and posterior probability = 0.71). It is largely recognized that the rTPJ integrates different multisensory bodily signals (visual, tactile, spatial, vestibular; Bottini et al., 2001; Ionta et al., 2011; Lopez, Halje, & Blanke, 2008). Further, the interference with the rTPJ activity through TMS made the distinction between what may or may not be part of one's body, decreasing the incorporation of the rubber hand while it increased the incorporation of the neutral object in the RHI paradigm (Tsakiris, Costantini, & Haggard, 2008).
Taken together, our results are also coherent with findings on brain‐damaged patients. Neuropsychological investigations are very relevant in supporting the idea that the regions found in the current study contribute to the emergence of a bodily self‐awareness. For instance, lesions to the right‐lateralized set of areas here identified could induce Unilateral Spatial Neglect (USN), a syndrome characterized by the inability to attend to stimuli from the contralesional side of space frequently associated with complex body representation disorders, such as somatoparaphrenia (Gandola et al., 2012; Invernizzi et al., 2013; Vallar & Ronchi, 2009). Damage to the inferior parietal lobule, in particular, is more frequently associated with the personal, bodily components of USN, such as personal neglect (Committeri et al., 2007; Vallar & Calzolari, 2018).
Furthermore, it has been recently suggested that the subjective experience of an external event (e.g., spatial perception) would result from the neural responses to visceral activity such as heartbeats. The integration of visceral input with visual perception would provide visual content with a first‐person perspective (Tallon‐Baudry, Campana, Park, & Babo‐Rebelo, 2018). Tallon‐Baudry et al. (2018) identified a brain network that may be responsible for the interplay between neural signals generated by heartbeats and visual awareness: this includes the rTPJ, the same area found by us. Its damage or disconnection can lead to USN (see Chelazzi, Bisley, & Bartolomeo, 2018) but also to the so‐called “out‐of‐body experience,” a situation in which patients perceive themselves as observing their body from an extracorporeal perspective (Blanke, Landis, Spinelli, & Seeck, 2004). Also, lesions or structural alterations to insular regions have been associated with both body ownership and interoceptive disorders (ereda, Ghika, Maeder, & Bogousslavsky, 2002; Ibañez et al., 2010; Karnath, 2005; Moro et al., 2016; Ronchi et al., 2015; Salvato, Mercurio, Sberna, Paulesu, & Bottini, 2018).
Interestingly, left caloric vestibular stimulation (CVS), associated with insular activations, may induce a temporary remission of both symptoms, also modulating body temperature, as it was previously found in a single right brain‐damaged patient suffering from chronic somatoparaphrenia: the restored sense of limb ownership following CVS was associated with an increase of the body temperature (Salvato, Gandola, et al., 2018). Notably, left CVS acts on the brain areas found here (Lopez et al., 2012; Zu Eulenburg, Caspers, Roski, & Eickhoff 2012), a fact that fits well with a role of these areas in creating a point of contact between a sense of body ownership and interoception.
Finally, it is also important to note that our results partially confirmed the hypotheses suggested in two theoretical papers on the topic (Moseley et al., 2012; Park & Blanke, 2019), in which an integrated system processing exteroceptive and interoceptive signals has been proposed. Such system would involve the parietal cortex and the insula (Moseley et al., 2012), or two subnetworks involving the premotor cortex, TPJ, intraparietal sulcus, posterior cingulate cortex, and the insula, overlapping in the intraparietal sulcus (Park & Blanke, 2019). If on the one hand, we confirmed the role of the parietal regions, precentral and postcentral gyri, and TPJ, on the other hand, we did not find any direct cluster of convergence in the insular cortex. Yet, our SMGs clusters also expand to the Rolandic operculi and posterior insular cortices. Functional activity of the posterior insula and nearby regions is associated with changes in somatosensory processing linked to altered states of bodily self‐awareness (e.g., illusory self‐identification with an avatar; Allison, Wood, McCarthy, & Spencer, 1991; Bufalari, Aprile, Avenanti, Di Russo, & Aglioti, 2007). These regions are strongly multimodal: activity within the posterior insula and parietal operculum were found to be increased by several bodily related stimuli such as touch, noxious heat, and CVS (Zu Eulenburg, Baumgärtner, Treede, & Dieterich, 2013).
5. CONCLUSIONS AND FUTURE DIRECTIONS
In conclusion, our data indicated that external and internal signals might converge in the SMG bilaterally together with a right‐lateralized set of areas such as the precentral, postcentral, and superior temporal gyri. These higher‐order brain areas are involved in integrating multisensory signals, and in recalibrating information from different incoming channels and spatial frames of reference.
Although disorders of body ownership are on record since 1942 (Gerstmann, 1942), and disorders of interoception have been repeatedly described (Critchley & Garfinkel, 2018; Salvato, Mercurio, et al., 2018), it remains to be investigated whether a higher order deficit of the bodily self‐awareness exists with specific deficits due to a perturbed integration of the two dimensions. Our results set the rationale for future neuropsychological and brain stimulation studies that may explore the contribution and weight of each area found in this meta‐analysis in the integration of the bodily self‐awareness.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix S1: Supporting information
Salvato G, Richter F, Sedeño L, Bottini G, Paulesu E. Building the bodily self‐awareness: Evidence for the convergence between interoceptive and exteroceptive information in a multilevel kernel density analysis study. Hum Brain Mapp. 2020;41:401–418. 10.1002/hbm.24810
Gerardo Salvato and Fabian Richter contributed equally to this study.
Endnote
The regional quantification of the spatial extent of the clusters was performed by comparison with AAL atlas (Tzourio‐Mazoyer et al., 2002).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available (see Supporting Information).
REFERENCES
- Allison, T. , Wood, C. C. , McCarthy, G. , & Spencer, D. D. (1991). Cortical somatosensory evoked potentials. II. Effects of excision of somatosensory or motor cortex in humans and monkeys. J Neurophysiol, 66, 64–82. [DOI] [PubMed] [Google Scholar]
- Araujo, H. F. , Kaplan, J. , Damasio, H. , & Damasio, A. (2015). Neural correlates of different self domains. Brain and Behavior: A Cognitive Neuroscience Perspective, 5, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery, J. A. , Kerr, K. L. , Ingeholm, J. E. , Burrows, K. , Bodurka, J. , & Simmons, W. K. (2015). A common gustatory and interoceptive representation in the human mid‐insula. Human Brain Mapping, 36, 2996–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, C. C. C. , Díaz, J. L. , Concha, L. , & Barrios, F. A. (2014). Sustained attention to spontaneous thumb sensations activates brain somatosensory and other proprioceptive areas. Brain and Cognition, 87, 86–96. [DOI] [PubMed] [Google Scholar]
- Becker, C. A. , Schmälzle, R. , Flaisch, T. , Renner, B. , & Schupp, H. T. (2014). Thirst and the state‐dependent representation of incentive stimulus value in human motive circuitry. Social Cognitive and Affective Neuroscience, 10, 1722–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekrater‐Bodmann, R. , Foell, J. , Diers, M. , Kamping, S. , Rance, M. , Kirsch, P. , … Flor, H. (2014). The importance of synchrony and temporal order of visual and tactile input for illusory limb ownership experiences—An fMRI study applying virtual reality. PLoS One, 9, e87013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlucchi, G. , & Aglioti, S. M. (2010). The body in the brain revisited. Experimental Brain Research, 200, 25–35. [DOI] [PubMed] [Google Scholar]
- Berlucchi, G. , & Vallar, G. (2018). The history of the neurophysiology and neurology of the parietal lobe. Handbook of Clinical Neurology, 151, 3–30. [DOI] [PubMed] [Google Scholar]
- Binks, A. P. , Evans, K. C. , Reed, J. D. , Moosavi, S. H. , & Banzett, R. B. (2014). The time‐course of cortico‐limbic neural responses to air hunger. Respiratory Physiology & Neurobiology, 204, 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisiach, E. , Jisa, M. L. , Oni, R. U. S. , & Vallak, G. (1991). Note remission of somatoparaphrenic delusion stimulation through. Neuropsychologia, 29, 1029–1031. [DOI] [PubMed] [Google Scholar]
- Blakemore, S. J. , Frith, C. D. , & Wolpert, D. M. (2001). The cerebellum is involved in predicting the sensory consequences of action. Neuroreport, 12, 1879–1884. [DOI] [PubMed] [Google Scholar]
- Blanke, O. , Landis, T. , Spinelli, L. , & Seeck, M. (2004). Out‐of‐body experience and autoscopy of neurological origin. Brain. [DOI] [PubMed]
- Blanke, O. (2012). Multisensory brain mechanisms of bodily self‐consciousness. Nature Reviews Neuroscience, 13, 556–571. [DOI] [PubMed] [Google Scholar]
- Blanke, O. , Slater, M. , & Serino, A. (2015). Behavioral, neural, and computational principles of bodily self‐consciousness. Neuron, 88, 145–166. [DOI] [PubMed] [Google Scholar]
- Blefari, M. L. , Martuzzi, R. , Salomon, R. , Bello‐Ruiz, J. , Herbelin, B. , Serino, A. , & Blanke, O. (2017). Bilateral Rolandic operculum processing underlying heartbeat awareness reflects changes in bodily self‐consciousness. The European Journal of Neuroscience, 45, 1300–1312. [DOI] [PubMed] [Google Scholar]
- Bottini, G. , Karnath, H. O. , Vallar, G. , Sterzi, R. , Frith, C. D. , Frackowiak, R. S. J. , … Scienze, D. (2001). Cerebral representations for egocentric space functional—Anatomical evidence from caloric vestibular stimulation and neck vibration. Brain, 124, 1182–1196. [DOI] [PubMed] [Google Scholar]
- Bottini, G. , Paulesu, E. , Sterzi, R. , Warburton, E. , Wise, R. J. , Vallar, G. , … Frith, C. D. (1995). Modulation of conscious experience by peripheral sensory stimuli. Nature, 376, 778–781. [DOI] [PubMed] [Google Scholar]
- Botvinick, M. , & Cohen, J. (1998). Rubber hands “feel” touch that eyes see. Nature, 391, 756. [DOI] [PubMed] [Google Scholar]
- Brannan, S. , Liotti, M. , Egan, G. , Shade, R. , Madden, L. , Robillard, R. , … Fox, P. T. (2001). Neuroimaging of cerebral activations and deactivations associated with hypercapnia and hunger for air. Proceedings of the National Academy of Sciences of the United States of America, 98, 2029–2034 Retreived from http://www.pnas.org/cgi/doi/10.1073/pnas.98.4.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozzoli, C. , Gentile, G. , & Ehrsson, H. H. (2012). That's near my hand! Parietal and premotor coding of hand‐centered space contributes to localization and self‐attribution of the hand. The Journal of Neuroscience, 32, 14573–14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R. L., Andrews‐Hanna J. R., & Schacter D. L. (2008). The brain's default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Bufalari, I. , Aprile, T. , Avenanti, A. , Di Russo, F. , & Aglioti, S. M. (2007). Empathy for pain and touch in the human somatosensory cortex. Cerebral Cortex, 17, 2553–2561. [DOI] [PubMed] [Google Scholar]
- Buhle, J. T. , Silvers, J. A. , Wage, T. D. , Lopez, R. , Onyemekwu, C. , Kober, H. , … Ochsner, K. N. (2014). Cognitive reappraisal of emotion: A meta‐analysis of human neuroimaging studies. Cerebral Cortex, 24, 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton, H. , Videen, T. O. , & Raichle, M. E. (1993). Tactile‐vibration‐activated foci in insular and parietal‐opercular cortex studied with positron emission tomography: Mapping the second somatosensory area in humans. Somatosensory & Motor Research, 10, 297–308. [DOI] [PubMed] [Google Scholar]
- Cameron, O. G. , & Minoshima, S. (2002). Regional brain activation due to pharmacologically induced adrenergic interoceptive stimulation in humans. Psychosomatic Medicine, 64, 851–861. [DOI] [PubMed] [Google Scholar]
- Caseras, X. , Murphy, K. , Mataix‐Cols, D. , López‐Solà, M. , Soriano‐Mas, C. , Ortriz, H. , … Torrubia, R. (2013). Anatomical and functional overlap within the insula and anterior cingulate cortex during interoception and phobic symptom provocation. Human Brain Mapping, 34, 1220–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereda, C. , Ghika, J. , Maeder, P. , & Bogousslavsky, J. (2002). Strokes restricted to the insular cortex. Neurology. [DOI] [PubMed]
- Chelazzi, L. , Bisley, J. W. , & Bartolomeo, P. (2018). The unconscious guidance of attention. Cortex. [DOI] [PubMed]
- Coen, S. J. , Yágüez, L. , Aziz, Q. , Mitterschiffthaler, M. T. , Brammer, M. , Williams, S. C. R. , & Gregory, L. J. (2009). Negative mood affects brain processing of visceral sensation. Gastroenterology, 137, 253–261. [DOI] [PubMed] [Google Scholar]
- Committeri, G. , Pitzalis, S. , Galati, G. , Patria, F. , Pelle, G. , Sabatini, U. , … Pizzamiglio, L. (2007). Neural bases of personal and extrapersonal neglect in humans. Brain, 130, 431–441. [DOI] [PubMed] [Google Scholar]
- Cooke, D. F. (2004). Sensorimotor integration in the Precentral Gyrus: Polysensory neurons and defensive movements. Journal of Neurophysiology, 91, 1648–1660. [DOI] [PubMed] [Google Scholar]
- Corbetta, M. , & Shulman, G. L. (2002). Control of goal‐directed and stimulus‐driven attention in the brain. Nature Reviews. Neuroscience, 3, 201–215. [DOI] [PubMed] [Google Scholar]
- Costafreda, S. (2009). Pooling fMRI data: Meta‐analysis, mega‐analysis and multi‐center studies. Frontiers in Neuroinformatics, 3, 33 Retreived from http://journal.frontiersin.org/article/10.3389/neuro.11.033.2009/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto, B. , Adolfi, F. , Sedeño, L. , Salles, A. , Canales‐Johnson, A. , Alvarez‐Abut, P. , … Ibanez, A. (2015). Disentangling interoception: Insights from focal strokes affecting the perception of external and internal milieus. Frontiers in Psychology, 6, 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, A. D. (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews. Neuroscience, 3, 655–666. [DOI] [PubMed] [Google Scholar]
- Critchley, H. D. (2004). The human cortex responds to an interoceptive challenge. Proceedings of the National Academy of Sciences of the United States of America, 101, 6333–6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley, H. D. , & Garfinkel, S. N. (2018). The influence of physiological signals on cognition. Current Opinion in Behavioral Sciences, 19, 13–18. [Google Scholar]
- Critchley, H. D. , & Harrison, N. A. (2013). Visceral influences on brain and behavior. Neuron, 77, 624–638. [DOI] [PubMed] [Google Scholar]
- Critchley, H. D. , Mathias, C. J. , Josephs, O. , O'Doherty, J. , Zanini, S. , Dewar, B. K. , … Dolan, R. J. (2003). Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain, 126, 2139–2152. [DOI] [PubMed] [Google Scholar]
- Critchley, H. D. , Tang, J. , Glaser, D. , Butterworth, B. , & Dolan, R. J. (2005). Anterior cingulate activity during error and autonomic response. NeuroImage, 27, 885–895. [DOI] [PubMed] [Google Scholar]
- Critchley, H. D. , Wiens, S. , Rotshtein, P. , Öhman, A. , & Dolan, R. J. (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7, 189–195. [DOI] [PubMed] [Google Scholar]
- Davis, K. D. , Pope, G. , Chen, J. , Kwan, C. L. , Crawley, A. P. , & Diamant, N. E. (2008). Cortical thinning in IBS: Implications for homeostatic, attention, and pain processing. Neurology, 70, 153–154. [DOI] [PubMed] [Google Scholar]
- De Bellis, F. , Trojano, L. , Errico, D. , Grossi, D. , & Conson, M. (2017). Whose hand is this? Differential responses of right and left extrastriate body areas to visual images of self and others' hands. Cognitive, Affective, & Behavioral Neuroscience, 17, 826–837. [DOI] [PubMed] [Google Scholar]
- Denny, B. T. , Kober, H. , Wager, T. D. , & Ochsner, K. N. (2012). A meta‐analysis of functional neuroimaging studies of self‐and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience, 24, 1742–1752 Retreived from http://www.mitpressjournals.org/doi/abs/10.1162/jocn_a_00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton, D. , Shade, R. , Zamarippa, F. , Egan, G. , Blair‐West, J. , McKinley, M. , … Fox, P. (1999b). Neuroimaging of genesis and satiation of thirst and an interoceptor‐driven theory of origins of primary consciousness. Proceedings of the National Academy of Sciences of the United States of America, 96, 5304–5309 Retreived from http://www.pnas.org/cgi/doi/10.1073/pnas.96.9.5304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton, D. A. , Shade, R. , Zamarippa, F. , Egan, G. , Blair‐West, J. , McKinley, M. J. , & Fox, P. (1999a). Correlation of regional cerebral blood flow and change of plasma sodium concentration during genesis and satiation of thirst. Proceedings of the National Academy of Sciences of the United States of America, 96, 2532–2537 Retreived from http://www.pnas.org/content/96/5/2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky, O. , Morrell, M. J. , & Vogt, B. A. (1995). Contributions of anterior cingulate cortex to behaviour. Brain, 118(Pt 1), 279–306 Retreived from http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&DbFrom=pubmed&Cmd=Link&LinkName=pubmed_pubmed&LinkReadableName=RelatedArticles&IdsFromResult=7895011&ordinalpos=3&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum [DOI] [PubMed] [Google Scholar]
- Dosenbach, N. U. F. , Fair, D. A. , Cohen, A. L. , Schlaggar, B. L. , & Petersen, S. E. (2008). A dual‐networks architecture of top‐down control. Trends in Cognitive Sciences, 12, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach, N. U. F. , Fair, D. A. , Miezin, F. M. , Cohen, A. L. , Wenger, K. K. , Dosenbach, R. A. T. , … Petersen, S. E. (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America, 104, 11073–11078 Retreived from http://www.pnas.org/cgi/doi/10.1073/pnas.0704320104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar, J. , Crawley, A. P. , Mikulis, D. J. , & Davis, K. D. (2002). A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. Journal of Neurophysiology, 87, 615–620 Retreived from http://jn.physiology.org/lookup/doi/10.1152/jn.00636.2001 [DOI] [PubMed] [Google Scholar]
- Egan, G. , Silk, T. , Zamarripa, F. , Williams, J. , Federico, P. , Cunnington, R. , … Denton, D. (2003). Neural correlates of the emergence of consciousness of thirst. Proceedings of the National Academy of Sciences of the United States of America, 100, 15241–15246 Retreived from http://www.pnas.org/cgi/doi/10.1073/pnas.2136650100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson, H. H. , Holmes, N. P. , & Passingham, R. E. (2005). Touching a rubber hand: Feeling of body ownership is associated with activity in multisensory brain areas. The Journal of Neuroscience, 25, 10564–10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson, H. H. , Spence, C. , & Passingham, R. E. (2004). That's my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science, 305, 875–877. [DOI] [PubMed] [Google Scholar]
- Ernst, J. , Northoff, G. , Böker, H. , Seifritz, E. , & Grimm, S. (2013). Interoceptive awareness enhances neural activity during empathy. Human Brain Mapping, 34, 1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, K. C. , Banzett, R. B. , Adams, L. , McKay, L. , Frackowiak, R. S. J. , & Corfield, D. R. (2002). BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. Journal of Neurophysiology, 88, 1500–1511 Retreived from http://jn.physiology.org/content/88/3/1500.full#ref-list-1%5Cnhttp://jn.physiology.org/content/88/3/1500%23cited-by%5Cnhttp://jn.physiology.org/content/88/3/1500.full%5Cnhttp://www.the-aps.org/.%5Cnhttp://jn.physiology.org/%5Cnhttp://www.ncbi.nlm.nih.gov/ [DOI] [PubMed] [Google Scholar]
- Farb, N. A. S. , Segal, Z. V. , & Anderson, A. K. (2013). Mindfulness meditation training alters cortical representations of interoceptive attention. Social Cognitive and Affective Neuroscience, 8, 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell, M. J. , Egan, G. F. , Zamarripa, F. , Shade, R. , Blair‐West, J. , Fox, P. , & Denton, D. A. (2006). Unique, common, and interacting cortical correlates of thirst and pain. Proceedings of the National Academy of Sciences of the United States of America, 103, 2416–2421 Retreived from http://www.pnas.org/cgi/doi/10.1073/pnas.0511019103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman, D. J. , & Ibos, G. (2018). An integrative framework for sensory, motor, and cognitive functions of the posterior parietal cortex. Neuron, 97, 1219–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, D. P. , Murray, E. A. , O'Neill, J. B. , & Mishkin, M. (1986). Cortical connections of the somatosensory fields of the lateral sulcus of macaques: Evidence for a corticolimbic pathway for touch. The Journal of Comparative Neurology, 252, 323–347. [DOI] [PubMed] [Google Scholar]
- Gandola, M. , Invernizzi, P. , Sedda, A. , Ferrè, E. R. , Sterzi, R., Sberna, M., … Bottini, G. (2012). An anatomical account of somatoparaphrenia. Cortex, 48, 1165–78. http://www.ncbi.nlm.nih.gov/pubmed/21774922. [DOI] [PubMed] [Google Scholar]
- Gandola, M. , Bottini, G. , Zapparoli, L. , Invernizzi, P. , Verardi, M. , Sterzi, R. , … Paulesu, E. (2014). The physiology of motor delusions in anosognosia for hemiplegia: Implications for current models of motor awareness. Consciousness and Cognition, 24, 98–112. [DOI] [PubMed] [Google Scholar]
- Gao, W. , & Lin, W. (2012). Frontal parietal control network regulates the anti‐correlated default and dorsal attention networks. Human Brain Mapping, 33, 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Cordero, I. , Sedeño, L. , de la Fuente, L. , Slachevsky, A. , Forno, G. , Klein, F. , … Ibañez, A. (2016). Feeling, learning from and being aware of inner states: Interoceptive dimensions in neurodegeneration and stroke. Philosophical Transactions of the Royal Society B: Biological Sciences, 371, 20160006 Retreived from http://rstb.royalsocietypublishing.org/lookup/doi/10.1098/rstb.2016.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel, S. N. , Seth, A. K. , Barrett, A. B. , Suzuki, K. , & Critchley, H. D. (2015). Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biological Psychology, 104, 65–74. [DOI] [PubMed] [Google Scholar]
- Gentile, G. , Björnsdotter, M. , Petkova, V. I. , Abdulkarim, Z. , & Ehrsson, H. H. (2015). Patterns of neural activity in the human ventral premotor cortex reflect a whole‐body multisensory percept. NeuroImage, 109, 328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile, G. , Guterstam, A. , Brozzoli, C. , & Ehrsson, H. H. (2013). Disintegration of multisensory signals from the real hand reduces default limb self‐attribution: An fMRI study. The Journal of Neuroscience, 33, 13350–13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstmann, J. (1942). Problem of imperception of disease and of impaired body territories with organic lesions. Archives of Neurology & Psychiatry, 48, 890–913. [Google Scholar]
- Grivaz, P. , Blanke, O. , & Serino, A. (2017). Common and distinct brain regions processing multisensory bodily signals for peripersonal space and body ownership. NeuroImage, 147, 602–618. [DOI] [PubMed] [Google Scholar]
- Gusnard, D. A. , Akbudak, E. , Shulman, G. L. , & Raichle, M. E. (2001). Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98, 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterstam, A. , Björnsdotter, M. , Gentile, G. , & Ehrsson, H. H. (2015). Posterior cingulate cortex integrates the senses of self‐location and body ownership. Current Biology, 25, 1416–1425. [DOI] [PubMed] [Google Scholar]
- Guterstam, A. , Gentile, G. , & Ehrsson, H. H. (2013). The invisible hand illusion: Multisensory integration leads to the embodiment of a discrete volume of empty space. Journal of Cognitive Neuroscience, 25, 1078–1099. [DOI] [PubMed] [Google Scholar]
- Haase, L. , May, A. C. , Falahpour, M. , Isakovic, S. , Simmons, A. N. , Hickman, S. D. , … Paulus, M. P. (2015). A pilot study investigating changes in neural processing after mindfulness training in elite athletes. Frontiers in Behavioral Neuroscience, 9, 229 Retreived from http://journal.frontiersin.org/Article/10.3389/fnbeh.2015.00229/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase, L. , Stewart, J. L. , Youssef, B. , May, A. C. , Isakovic, S. , Simmons, A. N. , … Paulus, M. P. (2016). When the brain does not adequately feel the body: Links between low resilience and interoception. Biological Psychology, 113, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanpour, M. S. , Simmons, W. K. , Feinstein, J. S. , Luo, Q. , Lapidus, R. C. , Bodurka, J. , … Khalsa, S. S. (2018). The insular cortex dynamically maps changes in cardiorespiratory interoception. Neuropsychopharmacology, 43, 426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydrich, L. , Aspell, J. E. , Marillier, G. , Lavanchy, T. , Herbelin, B. , & Blanke, O. (2018). Cardio‐visual full body illusion alters bodily self‐consciousness and tactile processing in somatosensory cortex. Scientific Reports, 8(1), 9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvärinen, J. (1982). Posterior parietal lobe of the primate brain. Physiological Reviews, 62, 1060–1129. [DOI] [PubMed] [Google Scholar]
- Iacoboni, M. , Woods, R. P. , Lenzi, G. L. , & Mazziotta, J. C. (1997). Merging of oculomotor and somatomotor space coding in the human right precentral gyrus. Brain, 120, 1635–1645. [DOI] [PubMed] [Google Scholar]
- Ibañez, A. , Gleichgerrcht, E. , & Manes, F. (2010). Clinical effects of insular damage in humans. Brain structure & function. [DOI] [PubMed]
- Immordino‐Yang, M. H. , Yang, X.‐F. , & Damasio, H. (2014). Correlations between social‐emotional feelings and anterior insula activity are independent from visceral states but influenced by culture. Frontiers in Human Neuroscience, 8, 728. Retreived from http://journal.frontiersin.org/article/10.3389/fnhum.2014.00728/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Invernizzi, P. , Gandola, M. , Romano, D. , Zapparoli, L. , Bottini, G. , & Paulesu, E. (2013). What is mine? Behavioral and anatomical dissociations between somatoparaphrenia and anosognosia for hemiplegia. Behavioural Neurology, 26, 139–150 Retreived from http://www.ncbi.nlm.nih.gov/pubmed/22713395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionta, S. , Heydrich, L. , Lenggenhager, B. , Mouthon, M. , Fornari, E. , Chapuis, D. , … Blanke, O. (2011). Multisensory mechanisms in temporo‐parietal cortex support self‐location and first‐person perspective. Neuron, 70, 363–374. [DOI] [PubMed] [Google Scholar]
- Isaev, G. , Murphy, K. , Guz, A. , & Adams, L. (2002). Areas of the brain concerned with ventilatory load compensation in awake man. The Journal of Physiology, 539, 935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, M. (2000). Mechanisms of motor learning in the cerebellum. Brain Research., 886, 237–245. [DOI] [PubMed] [Google Scholar]
- Kammers, M. P. M. , Rose, K. , & Haggard, P. (2011). Feeling numb: Temperature, but not thermal pain. Modulates feeling of body ownership. Neuropsychologia, 49, 1316–1321. [DOI] [PubMed] [Google Scholar]
- Karnath, H.‐O. (2005). Awareness of the functioning of one's own limbs mediated by the insular cortex? Journal of Neuroscience, 25, 7134–7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashkouli Nejad, K. , Sugiura, M. , Nozawa, T. , Kotozaki, Y. , Furusawa, Y. , Nishino, K. , … Kawashima, R. (2015). Supramarginal activity in interoceptive attention tasks. Neuroscience Letters, 589, 42–46. [DOI] [PubMed] [Google Scholar]
- Kelly, C. , Toro, R. , Di Martino, A. , Cox, C. L. , Bellec, P. , Castellanos, F. X. , & Milham, M. P. (2012). A convergent functional architecture of the insula emerges across imaging modalities. NeuroImage, 61, 1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner, I. R. , Zhang, J. , Touroutoglou, A. , Chanes, L. , Xia, C. , Simmons, W. K. , … Feldman Barrett, L. (2017). Evidence for a large‐scale brain system supporting allostasis and interoception in humans. Nature Human Behaviour, 1(5), 0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober, H. , Barrett, L. F. , Joseph, J. , Bliss‐Moreau, E. , Lindquist, K. , & Wager, T. D. (2008). Functional grouping and cortical‐subcortical interactions in emotion: A meta‐analysis of neuroimaging studies. NeuroImage, 42, 998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober, H. , & Wager, T. D. (2010). Meta‐analysis of neuroimaging data. Wiley Interdisciplinary Reviews: Cognitive Science, 1, 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn, E. , Mueller, K. , Lohmann, G. , & Schuetz‐Bosbach, S. (2016). Interoceptive awareness changes the posterior insula functional connectivity profile. Brain Structure & Function, 221, 1555–1571. [DOI] [PubMed] [Google Scholar]
- Legrand, D. (2006). The bodily self: The sensori‐motor roots of pre‐reflective self‐consciousness. Phenomenology and the Cognitive Sciences, 5, 89–118. [Google Scholar]
- Legrand, D. (2010). Subjective and physical dimensions of bodily self‐consciousness, and their dis‐integration in anorexia nervosa. Neuropsychologia, 48, 726–737. [DOI] [PubMed] [Google Scholar]
- Lenggenhager, B. , Tadi, T. , Metzinger, T. , & Blanke, O. (2007). Video ergo sum: Manipulating bodily self‐consciousness. Science, 317, 1096–1099. [DOI] [PubMed] [Google Scholar]
- Liang, X. , Zou, Q. , He, Y. , & Yang, Y. (2013). Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proceedings of the National Academy of Sciences of the United States of America, 110, 1929–1934 Retreived from http://www.pnas.org/cgi/doi/10.1073/pnas.1214900110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limanowski, J. , & Blankenburg, F. (2015). Network activity underlying the illusory self‐attribution of a dummy arm. Human Brain Mapping, 36, 2284–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limanowski, J. , & Blankenburg, F. (2016). That's not quite me: Limb ownership encoding in the brain. Social Cognitive and Affective Neuroscience, 11, 1130–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limanowski, J. , & Blankenburg, F. (2018). Fronto‐parietal brain responses to Visuotactile congruence in an anatomical reference frame. Front Hum Neurosci, 12, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist, K. A. , Satpute, A. B. , Wager, T. D. , Weber, J. , & Barrett, L. F. (2016). The brain basis of positive and negative affect: Evidence from a meta‐analysis of the human neuroimaging literature. Cerebral Cortex, 26, 1910–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti, M. , Brannan, S. , Egan, G. , Shade, R. , Madden, L. , Abplanalp, B. , … Denton, D. (2001). Brain responses associated with consciousness of breathlessness (air hunger). Proceedings of the National Academy of Sciences of the United States of America, 98, 2035–2040 Retreived from http://www.pnas.org/cgi/doi/10.1073/pnas.98.4.2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez, C. , Halje, P. , & Blanke, O. (2008). Body ownership and embodiment: Vestibular and multisensory mechanisms. Neurophysiologie Clinique, 38, 149–161. [DOI] [PubMed] [Google Scholar]
- May, A. C. , Stewart, J. L. , Migliorini, R. , Tapert, S. F. , & Paulus, M. P. (2013). Methamphetamine dependent individuals show attenuated brain response to pleasant interoceptive stimuli. Drug and Alcohol Dependence, 131, 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, A. C. , Stewart, J. L. , Tapert, S. F. , & Paulus, M. P. (2014). The effect of age on neural processing of pleasant soft touch stimuli. Frontiers in Behavioral Neuroscience, 8, 52. Retreived from http://journal.frontiersin.org/article/10.3389/fnbeh.2014.00052/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayka, M. A. , Corcos, D. M. , Leurgans, S. E. , & Vaillancourt, D. E. (2006). Three‐dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: A meta‐analysis. NeuroImage, 31, 1453–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mende‐Siedlecki, P. , Said, C. P. , & Todorov, A. (2013). The social evaluation of faces: A meta‐analysis of functional neuroimaging studies. Social Cognitive and Affective Neuroscience, 8, 285–299 Retreived from http://www.ncbi.nlm.nih.gov/pubmed/22287188/nhttp://www.ncbi.nlm.nih.gov/pmc/articles/PMC3594716/pdf/nsr090.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam, M. M. , Van Hoesen, G. W. , Pandya, D. N. , & Geschwind, N. (1977). Limbic and sensory connections of the inferior parietal lobule (area PG) in the rhesus monkey: A study with a new method for horseradish peroxidase histochemistry. Brain Research, 136, 393–414. [DOI] [PubMed] [Google Scholar]
- Miall, R. C. , Weir, D. J. , Wolpert, D. M. , & Stein, J. F. (1993). Is the cerebellum a smith predictor? Journal of Motor Behavior, 25, 203–216. [DOI] [PubMed] [Google Scholar]
- Moro, V. , Pernigo, S. , Tsakiris, M. , Avesani, R. , Edelstyn, N. M. J., Jenkinson, P. M., & Fotopoulou, A. (2016). Motor versus body awareness: Voxel‐based lesion analysis in anosognosia for hemiplegia and somatoparaphrenia following right hemisphere stroke. Cortex. [DOI] [PubMed]
- Moseley, G. L. , Gallace, A. , & Spence, C. (2012). Bodily illusions in health and disease: Physiological and clinical perspectives and the concept of a cortical “body matrix”. Neuroscience and Biobehavioral Reviews, 36, 34–46. [DOI] [PubMed] [Google Scholar]
- Moseley, G. L. , Olthof, N. , Venema, A. , Don, S. , Wijers, M. , Gallace, A. , & Spence, C. (2008). Psychologically induced cooling of a specific body part caused by the illusory ownership of an artificial counterpart. Proceedings of the National Academy of Sciences of the United States of America, 105, 13169–13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, S. M. , Cohen, A. L. , Power, J. D. , Wig, G. S. , Miezin, F. M. , Wheeler, M. E. , … Petersen, S. E. (2010). A parcellation scheme for human left lateral parietal cortex. Neuron, 67, 156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, T. , Brett, M. , Andersson, J. , Wager, T. , & Poline, J. B. (2005). Valid conjunction inference with the minimum statistic. NeuroImage, 25, 653–660. [DOI] [PubMed] [Google Scholar]
- Oberndorfer, T. , Simmons, A. , McCurdy, D. , Strigo, I. , Matthews, S. , Yang, T. , … Kaye, W. (2013). Greater anterior insula activation during anticipation of food images in women recovered from anorexia nervosa versus controls. Psychiatry Research: Neuroimaging, 214, 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H.‐D. , & Blanke, O. (2019). Coupling inner and outer body for self‐consciousness. Trends in Cognitive Sciences, 23, 377–388. [DOI] [PubMed] [Google Scholar]
- Paulesu, E. , Frackowiak, R. S. J. , & Bottini, G. (1997). Maps of somatosensory systems. In R. S. J. Frackowiak, K. J. Friston, C. D. Frith, R. J. Roland, & J. C. Mazziotta (Eds.), Human brain function (pp. 183–242). San Diego: Academic Press.
- Perini, I. , Morrison, I. , & Olausson, H. (2015). Seeking pleasant touch: Neural correlates of behavioral preferences for skin stroking. Frontiers in Behavioral Neuroscience, 9, 8. Retreived from http://journal.frontiersin.org/Article/10.3389/fnbeh.2015.00008/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova, V. I. , Björnsdotter, M. , Gentile, G. , Jonsson, T. , Li, T. Q. , & Ehrsson, H. H. (2011). From part‐ to whole‐body ownership in the multisensory brain. Current Biology, 21, 1118–1122. [DOI] [PubMed] [Google Scholar]
- Pollatos, O. , Schandry, R. , Auer, D. P. , & Kaufmann, C. (2007). Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Research, 1141, 178–187. [DOI] [PubMed] [Google Scholar]
- Preston, C. , & Ehrsson, H. H. (2016). Illusory obesity triggers body dissatisfaction responses in the insula and anterior cingulate cortex. Cerebral Cortex, 26, 4450–4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode, G. , Charles, N. , Perenin, M.‐T. , Vighetto, A. , Trillet, M. , & Aimard, G. (1992). Partial remission of hemiplegia and Somatoparaphrenia through vestibular stimulation in a case of unilateral neglect. Cortex, 28, 203–208. [DOI] [PubMed] [Google Scholar]
- Salvato, G. , Gandola, M. , Veronelli, L. , Agostoni, E. C. , Sberna, M. , Corbo, M. , & Bottini, G. (2016). The spatial side of somatoparaphrenia: A case study. Neurocase, 22, 154–160. [DOI] [PubMed] [Google Scholar]
- Salvato, G. , Gandola, M. , Veronelli, L. , Berlingeri, M. , Corbo, M. , & Bottini, G. (2018). The vestibular system, body temperature and sense of body ownership: A potential link? Insights from a single case study. Physiology & Behavior, 194(2018), 522–526. [DOI] [PubMed] [Google Scholar]
- Salvato, G. , Mercurio, M. , Sberna, M. , Paulesu, E. , & Bottini, G. (2018). A very light lunch: Interoceptive deficits and food aversion at onset in a case of behavioral variant frontotemporal dementia. Alzheimer's & Dementia, 10, 750–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, S. M. (2016). Neural correlates of heart‐focused interoception: A functional magnetic resonance imaging meta‐analysis. Philosophical Transactions of the Royal Society B: Biological Sciences, 371, 2016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz, M. , Radua, J. , Aichhorn, M. , Richlan, F. , & Perner, J. (2014). Fractionating theory of mind: A meta‐analysis of functional brain imaging studies. Neuroscience and Biobehavioral Reviews., 42, 9–34. [DOI] [PubMed] [Google Scholar]
- Sedda, A. , Tonin, D. , Salvato, G. , Gandola, M. , & Bottini, G. (2016). Left caloric vestibular stimulation as a tool to reveal implicit and explicit parameters of body representation. Consciousness and Cognition, 41, 1–9. [DOI] [PubMed] [Google Scholar]
- Seeley, W. W. , Menon, V. , Schatzberg, A. F. , Keller, J. , Glover, G. H. , Kenna, H. , … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience, 27, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serino, A. , Alsmith, A. , Costantini, M. , Mandrigin, A. , Tajadura‐Jimenez, A. , & Lopez, C. (2013). Bodily ownership and self‐location: Components of bodily self‐consciousness. Consciousness and Cognition., 22, 1239–1252. [DOI] [PubMed] [Google Scholar]
- Sforza, A. , Bufalari, I. , Haggard, P. , & Aglioti, S. M. (2010). My face in yours: Visuo‐tactile facial stimulation influences sense of identity. Social Neuroscience, 5, 148–162. [DOI] [PubMed] [Google Scholar]
- Simmons, W. K. , Avery, J. A. , Barcalow, J. C. , Bodurka, J. , Drevets, W. C. , & Bellgowan, P. (2013). Keeping the body in mind: Insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Human Brain Mapping, 34, 2944–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan, E. , Pardo, J. V. , Faris, P. L. , Hartman, B. K. , Kim, S. W. , Ivanov, E. H. , … Goodale, R. L. (2003). Functional neuroimaging of gastric distention. Journal of Gastrointestinal Surgery, 7, 740–749. [DOI] [PubMed] [Google Scholar]
- Stern, E. R. , Grimaldi, S. J. , Muratore, A. , Murrough, J. , Leibu, E. , Fleysher, L. , … Burdick, K. E. (2017). Neural correlates of interoception: Effects of interoceptive focus and relationship to dimensional measures of body awareness. Human Brain Mapping, 38, 6068–6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, J. L. , Juavinett, A. L. , May, A. C. , Davenport, P. W. , & Paulus, M. P. (2015). Do you feel alright? Attenuated neural processing of aversive interoceptive stimuli in current stimulant users. Psychophysiology, 52, 249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, J. L. , May, A. C. , Poppa, T. , Davenport, P. W. , Tapert, S. F. , & Paulus, M. P. (2014). You are the danger: Attenuated insula response in methamphetamine users during aversive interoceptive decision‐making. Drug and Alcohol Dependence, 142, 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, J. L. , Parnass, J. M. , May, A. C. , Davenport, P. W. , & Paulus, M. P. (2013). Altered frontocingulate activation during aversive interoceptive processing in young adults transitioning to problem stimulant use. Frontiers in Systems Neuroscience, 7, 89. Retreived from http://journal.frontiersin.org/article/10.3389/fnsys.2013.00089/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigo, I. A. , Matthews, S. C. , Simmons, A. N. , Oberndorfer, T. , Klabunde, M. , Reinhardt, L. E. , & Kaye, W. H. (2013). Altered insula activation during pain anticipation in individuals recovered from anorexia nervosa: Evidence of interoceptive dysregulation. The International Journal of Eating Disorders, 46, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, K. , Garfinkel, S. N. , Critchley, H. D. , & Seth, A. K. (2013). Multisensory integration across exteroceptive and interoceptive domains modulates self‐experience in the rubber‐hand illusion. Neuropsychologia, 51, 2909–2917. [DOI] [PubMed] [Google Scholar]
- Talairach, J. , & Tournoux, P. (1988). Co‐planar stereotaxic atlas of the human brain, 1988. Theime, Stuttgart, Ger, 270, 132 Retrieved from http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Co-Planar+Stereotaxis+Atlas+of+the+Human+Brain#1 [Google Scholar]
- Tallon‐Baudry, C. , Campana, F. , Park, H. D. , & Babo‐Rebelo, M. (2018). The neural monitoring of visceral inputs, rather than attention, accounts for first‐person perspective in conscious vision. Cortex. [DOI] [PubMed]
- Taylor, K. S. , Seminowicz, D. A. , & Davis, K. D. (2009). Two systems of resting state connectivity between the insula and cingulate cortex. Human Brain Mapping, 30, 2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa, Y. , Fukushima, H. , & Umeda, S. (2013). How does interoceptive awareness interact with the subjective experience of emotion? An fMRI study. Human Brain Mapping, 34, 598–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieri, G. , Gioia, A. , Scandola, M. , Pavone, E. F. , & Aglioti, S. M. (2017). Visual appearance of a virtual upper limb modulates the temperature of the real hand: A thermal imaging study in immersive virtual reality. The European Journal of Neuroscience, 45, 1141–1151. [DOI] [PubMed] [Google Scholar]
- Tracy, J. , Goyal, N. , Flanders, A. , Weening, R. , Laskas, J. , Natale, P. , & Waldron, B. (2007). Functional magnetic resonance imaging analysis of attention to one's heartbeat. Psychosomatic medicine, 69, 952–960. [DOI] [PubMed] [Google Scholar]
- Tsakiris, M. , Costantini, M. , & Haggard, P. (2008). The role of the right temporo‐parietal junction in maintaining a coherent sense of one's body. Neuropsychologia, 46, 3014–3018. [DOI] [PubMed] [Google Scholar]
- Tsakiris, M. , Hesse, M. D. , Boy, C. , Haggard, P. , & Fink, G. R. (2007). Neural signatures of body ownership: A sensory network for bodily self‐consciousness. Cerebral Cortex, 17, 2235–2244. [DOI] [PubMed] [Google Scholar]
- Tsakiris, M. , Jimenez, A. T. , & Costantini, M. (2011). Just a heartbeat away from one's body: Interoceptive sensitivity predicts malleability of body‐representations. Proceedings of the Royal Society B: Biological Sciences, 278, 2470–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris, M. , Longo, M. R. , & Haggard, P. (2010). Having a body versus moving your body: Neural signatures of agency and body‐ownership. Neuropsychologia, 48, 2740–2749. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15, 273–289. [DOI] [PubMed] [Google Scholar]
- Urgesi, C. , Calvo‐Merino, B. , Haggard, P. , & Aglioti, S. M. (2007). Transcranial magnetic stimulation reveals two cortical pathways for visual body processing. The Journal of Neuroscience, 27, 8023–8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar, G. , & Ronchi, R. (2009). Somatoparaphrenia: A body delusion. A review of the neuropsychological literature. Experimental Brain Research, 192, 533–51. http://www.ncbi.nlm.nih.gov/pubmed/18813916. [DOI] [PubMed] [Google Scholar]
- Vallar, G. , & Calzolari, E. (2018). Unilateral spatial neglect after posterior parietal damage. Handbook of Clinical Neurology, 151, 287–312. [DOI] [PubMed] [Google Scholar]
- van den Heuvel, M. P. , & Sporns, O. (2013). An anatomical substrate for integration among functional networks in human cortex. The Journal of Neuroscience, 33, 14489–14500 Retreived from http://www.ncbi.nlm.nih.gov/pubmed/24005300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen, D. C. (2005). A population‐average, landmark‐ and surface‐based (PALS) atlas of human cerebral cortex. NeuroImage, 28, 635–662. [DOI] [PubMed] [Google Scholar]
- Wager, T. D. , Lindquist, M. , & Kaplan, L. (2007). Meta‐analysis of functional neuroimaging data: Current and future directions. Social Cognitive and Affective Neuroscience, 2, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager, T. D. , Lindquist, M. A. , Nichols, T. E. , Kober, H. , & Van Snellenberg, J. X. (2009). Evaluating the consistency and specificity of neuroimaging data using meta‐analysis. NeuroImage, 45, S210–S221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G. J. , Tomasi, D. , Backus, W. , Wang, R. , Telang, F. , Geliebter, A. , … Volkow, N. D. (2008). Gastric distention activates satiety circuitry in the human brain. NeuroImage, 39, 1824–1831. [DOI] [PubMed] [Google Scholar]
- Whitlock, J. R. (2017). Posterior parietal cortex. Current Biology, 27, R691–R695. [DOI] [PubMed] [Google Scholar]
- Yarkoni, T. , Poldrack, R. A. , Nichols, T. E. , Van Essen, D. C. , & Wager, T. D. (2011). Large‐scale automated synthesis of human functional neuroimaging data. Nature Methods, 8, 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki, J. , Davis, J. I. , & Ochsner, K. N. (2012). Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage, 62, 493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki, S. , & Shipp, S. (1988). The functional logic of cortical connections. Nature, 335, 311–317. [DOI] [PubMed] [Google Scholar]
- Zu Eulenburg, P. , Caspers, S. , Roski, C. , & Eickhoff, S. B. (2012). Meta‐analytical definition and functional connectivity of the human vestibular cortex. Neuroimage, 60, 162–9. http://www.ncbi.nlm.nih.gov/pubmed/22209784. [DOI] [PubMed] [Google Scholar]
- Zu Eulenburg, P. , Baumgärtner, U. , Treede, R. D. , & Dieterich, M. (2013). Interoceptive and multimodal functions of the operculo‐insular cortex: Tactile, nociceptive and vestibular representations. NeuroImage, 83, 75–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
The data that support the findings of this study are openly available (see Supporting Information).


