Abstract
In the human brain, self‐generated auditory stimuli elicit smaller cortical responses compared to externally generated sounds. This sensory attenuation is thought to result from predictions about the sensory consequences of self‐generated actions that rely on motor commands. Previous research has implicated brain oscillations in this process. However, the specific role of these oscillations in motor–auditory interactions during sensory attenuation is still unclear. In this study, we aimed at addressing this question by using magnetoencephalography (MEG). We recorded MEG in 20 healthy participants during listening to passively presented and self‐generated tones. Our results show that the magnitude of sensory attenuation in bilateral auditory areas is significantly correlated with the modulation of beta‐band (15–30 Hz) amplitude in the motor cortex. Moreover, we observed a significant directional coupling (Granger causality) in beta‐band originating from the motor cortex toward bilateral auditory areas. Our findings indicate that beta‐band oscillations play an important role in mediating top–down interactions between motor and auditory cortex and, in our paradigm, suppress cortical responses to predicted sensory input.
Keywords: auditory perception, beta‐band oscillation, MEG, motor–auditory interactions, prediction, sensory attenuation
1. INTRODUCTION
In our everyday life, we receive a mixture of sounds arising from different sources. These sounds could be either due to self‐generated actions such as singing a song, playing a musical instrument, or knocking on a door, or they can come from external sources. The principles underlying the generation of brain responses to self‐generated auditory input are different from external stimuli. Typically, self‐generated sounds elicit smaller cortical responses in auditory cortices compared to externally generated sounds. This phenomenon is known as sensory attenuation (Martikainen, Kaneko, & Hari, 2005; Schafer & Marcus, 1973) and its absence or reduction has been associated with schizophrenia and other neuropathologies (Ford & Mathalon, 2005). This attenuation is likely mediated by direct or indirect interactions between motor and auditory cortices. Indeed, previous research has demonstrated close interactions between both systems in humans and animals (Nelson et al., 2013; Zatorre, Chen, & Penhune, 2007). Typically, these interactions are conceptualised within the framework of a forward model (Friston, 2005; Keller & Mrsic‐Flogel, 2018; Pickering & Clark, 2014). When executing a movement, a copy of the motor command is used by this forward model to predict the resulting sensory consequences. The predicted and real sensory input are compared constantly and the resulting prediction error refines and alters new predictions.
Recently, several studies provided converging evidence that brain oscillations at different frequencies are differentially involved in prediction processes. In a magnetoencephalography (MEG) study of spatial attention in the visual system, Bauer, Stenner, Friston, and Dolan (2014) showed that the suppression of alpha oscillations (7–13 Hz) before stimulus presentation correlated with prediction precision. On the other hand, the increase of oscillations in the gamma‐band (40–90 Hz) following stimulus presentation reflected the mismatch between prediction and sensory stimulus. Similar frequency‐specific results where alpha/beta oscillations are related to predictions and gamma oscillations are related to prediction errors were also observed in the auditory cortex in monkeys (Chao, Takaura, Wang, Fujii, & Dehaene, 2018) and humans (Fontolan, Morillon, Liegeois‐Chauvel, & Giraud, 2014; Sedley et al., 2016). Recently, this model has been tested in the context of sensory attenuation. Cao et al. demonstrated a correlation between the prestimulus alpha increase in auditory cortex and the magnitude of sensory attenuation indicating a downregulation of auditory excitability when predicted self‐generated sensory input is expected (Cao, Thut, & Gross, 2017; Cao, Veniero, Thut, & Gross, 2017). In line with the above‐mentioned functional differentiation between different oscillations, gamma‐band changes in response to self‐generated stimuli seem to reflect prediction errors (Buchholz, David, Sengelmann, & Engel, 2019; Cao, Thut, & Gross, 2017; Cao, Veniero, et al., 2017; Flinker et al., 2010). These prediction errors are computed by comparing real sensory input and the sensory input that is predicted on the basis of the motor command by the internal forward model. This computation, therefore, requires information from both the motor cortex and auditory cortex. The mechanism underlying these motor–auditory interactions is unclear.
Morillon et al. reported that temporal predictions are encoded in beta oscillations originating from the sensorimotor cortex toward auditory regions (Morillon & Baillet, 2017). More recently, Buchholz et al. also found that the motor cortex increased its beta‐band connectivity with middle temporal gyrus and inferior parietal lobe in a continuous visuomotor tapping task (Buchholz et al., 2019). However, the interactions between the motor cortex and auditory cortex in mediating sensory attenuation are still unclear. Neural connections between motor and auditory areas have been assessed from an anatomical perspective in rodents. Animal studies have identified neurons in the secondary motor area (M2) that project directly to the auditory areas and can exert suppressive effects. These neurons convey motor‐related information during self‐generated movements (Nelson et al., 2013; Schneider, Sundararajan, & Mooney, 2018). Although the neural projection from the motor region to auditory areas is well documented, less is known about the role of oscillatory activity in the motor cortex in sensory attenuation.
In this study, we aimed at investigating the relationship between neuronal oscillations in the involved motor area and auditory evoked responses in the bilateral auditory cortices using MEG. We hypothesised that motor oscillatory activity plays a major role in mediating sensory attenuation. To evaluate our hypothesis, we conducted an MEG experiment. We recorded MEG data while participants processed passively presented and self‐generated tones. Using correlations and Granger causality analysis, we found beta‐band oscillations in the motor cortex to be directionally coupled to the auditory cortices and modulate the degree of sensory attenuation.
2. METHODS
2.1. Participants
We recruited 20 healthy right‐handed volunteers (10 males, mean age 26.3 ± 3.7 years, median 25.5, range [21 33]) for this experiment from a local participant pool. The study was approved by the local ethics committee (University of Münster) and conducted in accordance with the Declaration of Helsinki. Prior written informed consent was obtained before the measurement and participants received monetary compensation after the experiment. Data from two participants had to be excluded from data analysis due to data distortion caused by movement artefacts. Therefore, we analysed data from 18 participants (nine males, mean age 26.6 ± 3.9 years, median 26.5, range [21 33]).
2.2. Recording
MEG and electrocardiogram (ECG) were acquired simultaneously. All recordings were carried out with a 275 whole‐head sensor system (CTF Systems) with a sampling rate of 600 Hz. A low‐pass online filter with a 150 Hz cutoff was applied to the recorded data.
2.3. Paradigm
The participants were asked to sit relaxed on the MEG chair and keep their eyes open while performing tasks. There were two different conditions (125 trials each). In the passive condition, the auditory stimulus was presented with random interstimulus intervals between 2,000 and 3,500 ms. The stimulus was a pure tone (1,000 Hz, 50 ms in duration, sound intensity level: 60 dB above the hearing threshold) delivered through plastic tubes binaurally. In the active condition, participants were asked to press a button on a response box with their right index finger every 3 s without inner counting. The auditory stimulus was presented immediately after each button press. The two conditions were presented in a block‐wise random order.
2.4. Preprocessing and data analysis
Prior to data analysis, MEG data were visually inspected for motion‐related artefacts. In order to remove drifts and high‐frequency noise, a zero‐phase Butterworth forth‐order IIR band‐pass filter between 1 and 80 Hz was applied to the data. Heart artefact distorted MEG signals in one of the subjects (Subject #13). The combination of independent component analysis and mutual information (mi) was used based on the method introduced in (Abbasi et al., 2015; Abbasi, Hirschmann, Schmitz, Schnitzler, & Butz, 2016) to identify artefactual components (mi between ICs and ECG signal; two artefactual components were detected for each condition). Finally, MEG data were segmented time locked to sound onset from −2 to 2 s. In the preprocessing and data analysis steps, custom‐made scripts in MATLAB R2018 (The MathWorks, Natick, MA) in combination with the MATLAB‐based FieldTrip toolbox (Oostenveld, Fries, Maris, & Schoffelen, 2011) were used in accordance with current MEG guidelines (Gross et al., 2013).
2.5. Source analysis
Coregistration of T1‐weighted structural magnetic resonance images (MRIs) to the MEG coordinate system was done for each participant by initial identification of three anatomical landmarks (nasion, left, and right preauricular points) in the individual's MRI. Using the implemented segmentation algorithms in Fieldtrip/SPM12, individual head models were constructed from anatomical MRIs. A solution of the forward model was computed using the realistically shaped single‐shell volume conductor model (Nolte, 2003) using a 5 mm grid defined in the template (MNI) brain after linear transformation to the individual MRI.
The eLoreta algorithm, implemented in Fieldtrip, was used to localise the auditory evoked components. The voxels in the left and right auditory areas with the strongest M100 amplitude for the passive condition were selected as representative voxels from the left and right auditory cortex (LAC and RAC).
To study interactions between the motor cortex and auditory cortex, we functionally localised left motor cortex (LMC) in individual participants based on the voxel showing strongest modulation of beta‐band activity (Engel & Fries, 2010; Pfurtscheller & Lopes da Silva, 1999). The beta frequency band and time window for localisation were individually optimised based on time–frequency analysis of sensor data from the active condition above the LMC. The time–frequency spectrograms of groups of MEG sensors above the LMC were inspected visually in order to determine the individual frequency bands and time windows with strongest beta suppression and rebound. Time–frequency power was normalised by computing relative changes with reference to the mean power from −2 to 2 s. Time–frequency spectrograms were plotted from −2 to 2 s around the movement onset. Therefore, negative components (represented by blue contour) indicate beta suppression pattern and positive components (red contours) indicate beta rebound. The individual optimised frequency band, beta suppression, and beta rebound time windows ranged from 12–33 Hz, −0.3–0.5 s, and 0.4–1.7 s, respectively, relative to button press onset. Detailed information about the selected frequency ranges and time periods are presented in Figure S1, Supplementary section.
We used dynamic imaging of coherent sources (Gross et al., 2001), a frequency‐domain beamformer, to identify the voxel showing strongest beta‐band modulation in the individually optimised time and frequency window in the LMC. We used both beta suppression and rebound time range because this peak‐to‐peak measure could help us to find the most relevant voxel representing the motor area. Finally, the eLoreta algorithm was used to compute time series of representative voxels in auditory and motor areas using the linear mixture of spatial filters multiplied by their corresponding orientation vectors. The extracted time series were used for all further analysis steps.
2.6. Evoked response analysis
Single‐trial responses to the auditory stimuli were averaged for the three selected voxels (LAC, RAC, and LMC) for both active and passive conditions. The extracted event‐related fields time locked to tone onset were computed for each condition with baseline (−500 to 0 ms) correction. To find the M100 amplitudes in both, active and passive conditions, we searched for the maximum values within the LAC and RAC time series in the range between 75 and 125 ms. Sensory attenuation values were obtained by computing M100 amplitude relative changes in active condition with reference to passive condition from the extracted auditory voxels time series, that is, (active–passive)/passive. We used a paired t test to compare M100 component amplitudes and sensory attenuation values between the passive and active conditions and also across hemispheres.
2.7. Time–frequency analysis
We applied time–frequency analysis on the extracted time series from the selected voxels for each participant and experimental condition. Time–frequency analysis was performed on time series by a multitaper approach with a smoothing of 2 Hz on a 400 ms long sliding window. The step size of the sliding window was 25 ms and the spectral resolution was interpolated to 1 Hz.
Cluster‐based permutation test was used to assess whether there are differences between the individual time–frequency maps of different conditions (active vs. passive). Time–frequency power was normalised by computing relative changes with reference to the mean power from −2 to 2 s. Monte‐Carlo randomisation was performed using a dependent samples t test for the contrast active versus passive. In 2,000 iterations, the assignment of values to conditions (active, passive) was randomly changed before cluster computation. Briefly, the resulting p‐values were stored in a new p‐value map (2,000 × time × frequency) in each iteration. Adjacent significant samples (p‐value <.05) either in frequency or in time in this map were grouped in clusters. The sum of all the t statistics within each cluster was computed and considered as the cluster level statistics. The largest cluster was detected and its cluster level statistics were used for the permutation distribution. After 2,000 permutations, we estimated the cluster level significance for each observed cluster by computing the proportion of elements of the permutation distribution greater than the observed cluster‐level statistics resulting from the real comparison between the two conditions. The resulting time–frequency clusters with cluster‐level p‐values below an alpha level of α = .05 are considered significant (Maris & Oostenveld, 2007). In Section 3, only significant results are presented (p < .05).
2.8. Correlation analysis
We assessed whether there is a relationship between the power of modulated beta in the motor area and the amount of sensory attenuation in the auditory area using correlation analysis. The individual power of beta suppression and rebound was calculated for the motor voxels in the active condition. To compute the individual beta suppression and rebound average power, we used the frequency ranges and time windows determined by visual inspection in Section 2.5. We defined sensory attenuation as M100 amplitude relative changes in the active condition with reference to the passive condition from the extracted auditory voxels time series. To assess the correlation and the effects of outliers on the correlation results, we computed skipped correlations using an open‐source MATLAB toolbox (Pernet, Wilcox, & Rousselet, 2012).
2.9. Connectivity analysis
We performed connectivity analysis by using a nonparametric variant of spectral Granger causality (Dhamala, Rangarajan, & Ding, 2008). We computed bivariate granger causality to determine the directionality of functional coupling between motor and auditory cortices in the active condition. The computation was based on time series from LAC, RAC, and LMC from −0.5 to 1.5 s relative to stimulus onset. Connectivity measures were computed based on multitaper spectral analysis with a smoothing of 3 Hz on 600 ms long sliding window with 10 ms time resolution. Granger causality is sensitive to power differences between both time series which can lead to spurious connectivity that is not due to genuine time lagged interaction. To detect only genuine interactions, we controlled for this effect by computing time‐reversed Granger causality as suggested by Haufe, Nikulin, Müller, and Nolte (2013). We compared the original Granger causality with the time‐reversed Granger causality using the cluster analysis by Maris and Oostenveld (2007) based on a paired t test and 2,000 permutations to determine the significant directionality between time series from the motor and auditory voxels. In Section 3, only significant results are presented (p < .05).
3. RESULTS
3.1. Sensory attenuation in auditory cortices
The first set of analyses examined the sensory attenuation in the auditory cortex. Our results demonstrate significant M100 attenuation for self‐generated tones (active) as compared to externally generated tones (passive). Source localization of the M100 component for both active and passive conditions showed the strongest M100 attenuation in bilateral temporal auditory areas (Figure 1a). Voxels with the strongest evoked responses in passive conditions were selected as the representative voxels from the right and left auditory cortices (RAC and LAC). RAC and LAC voxels time series were extracted for further analysis. Significant M100 attenuation was observed on the RAC and LAC time series (Figure 1b; RAC: t(17) = 10.64, p < .001; LAC: t(17) = 10.48, p < .001). We also compared M100 amplitude and attenuation between hemispheres. However, no significant difference was observed between the left and right hemisphere.
Figure 1.
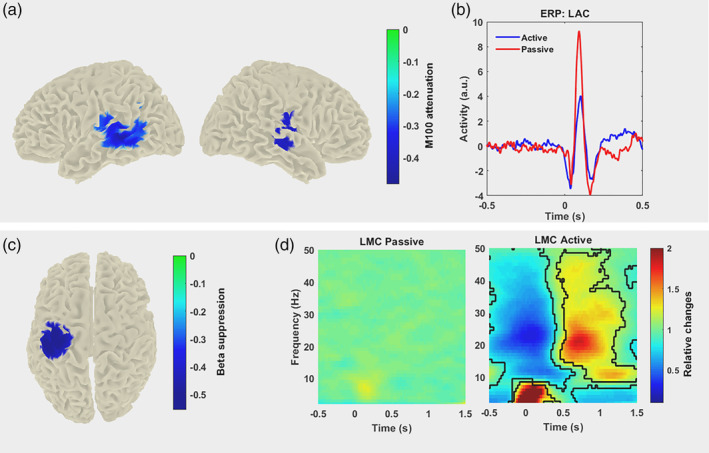
Sensory attenuation in the auditory cortex (top grey panel) and beta‐band modulation in the motor cortex (bottom grey panel). (a) Localization of group average M100 attenuation demonstrates the strongest M100 attenuation in bilateral auditory areas (sensory attenuation value: [active–passive]/passive). (b) Grand averaged of the extracted time series from left auditory cortex (LAC) voxel shows stronger auditory evoked field (M100 component) in passive condition as compared to active condition. (c) Group average beta modulation was localised over the left motor cortex. Colour codes beta power changes relative to beta rebound induced by the right index finger button press. (d) Grand averaged time–frequency spectrogram for left motor cortex (LMC) voxel in passive (left panel) and active (right panel) conditions. The low‐frequency power increase around 0 ms is caused by motor evoked components. Significant differences are designated by contour lines on the active map. The statistical test revealed significant changes in beta‐band power in LMC voxel in the active condition as compared to LMC voxel in the passive condition. Colour codes relative changes. Time point 0 s marks tone presentation onset
3.2. Cortical beta‐band modulation during sensory attenuation
Time–frequency analysis of the acquired MEG data for the active condition revealed strong beta‐band modulation in the LMC—contralateral to the button press side. The beta‐band modulation is characterised by a beta suppression before and during button press followed by a transient increase (rebound). Figure 1c illustrates the source reconstruction results indicating that the beta modulation was most prominent in the LMC.
To examine the differences between modulated oscillatory neural activity in motor and auditory areas, we focus next on the comparisons of the calculated time–frequency maps for LAC, RAC, and LMC. The statistical test revealed significant changes in beta‐band power in the LMC voxel in the active condition as compared to LMC voxel in the passive condition (Figure 1d) as well as compared to LAC and RAC voxels (Figure 2, first and second rows) in the active condition. Our statistical analysis also revealed a significant beta suppression in LAC and RAC for the active condition as compared to passive condition (Figure 2, third and fourth rows).
Figure 2.
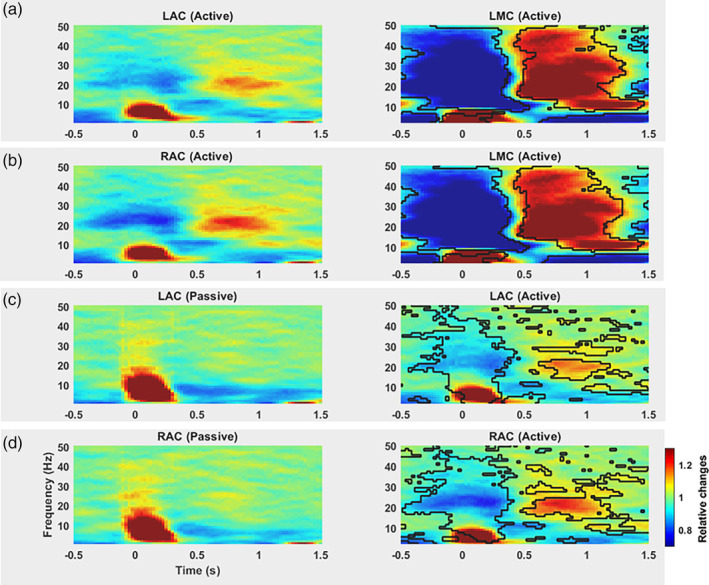
Grand averaged time–frequency spectrogram and statistical comparisons of oscillatory power in the motor cortex and auditory cortices. Rows 1 and 2: Time–frequency spectrograms show beta modulation in LMC, LAC, and RAC voxels in the active condition. The statistical test revealed significant changes in beta‐band power in LMC voxel in the active condition as compared to LAC and RAC voxels in the active condition (a and b, right panels). The resulting time–frequency clusters with cluster‐level p‐values below an alpha level of .05 (obtained from the statistical analysis) are considered significant and designated by black contour on the right spectrograms. Rows 3 and 4: Only left and right time–frequency maps of auditory cortices in the active conditions show beta modulations. Significant changes (p < .05) were found in beta‐band activity in LAC and RAC voxels (c and d, right panel) for the active condition as compared to passive condition. Colour codes the normalised power by computing relative changes with reference to the mean power from −2 to 2 s. Time point 0 s marks tone presentation onset. LAC and RAC, left auditory cortex, LMC, left motor cortex; RAC, right auditory cortex
3.3. Interaction between motor cortex and auditory cortex
Correlation analysis examined whether there was a significant relationship between the modulated beta‐band power in the motor area and the magnitude of the attenuation in auditory evoked responses across participants. We determined for each participant the individual frequency range and time periods for beta suppression and rebound in the motor cortex and calculated the mean beta power changes. Because no significant difference was observed between left and right sensory attenuations values, we combine data from both hemispheres. Robust skipped‐correlation tests demonstrated that the increase of beta rebound in the LMC was significantly negatively correlated with increased sensory attenuation in both auditory cortices (r = −.44 CI = [−0.68–0.14]). This negative correlation means that the increase of the beta rebound (induced by the self‐generated movements in the motor area) is significantly related to the attenuation of the M100 amplitude in the active condition. Figure 3 illustrates the results of the correlation analysis.
Figure 3.
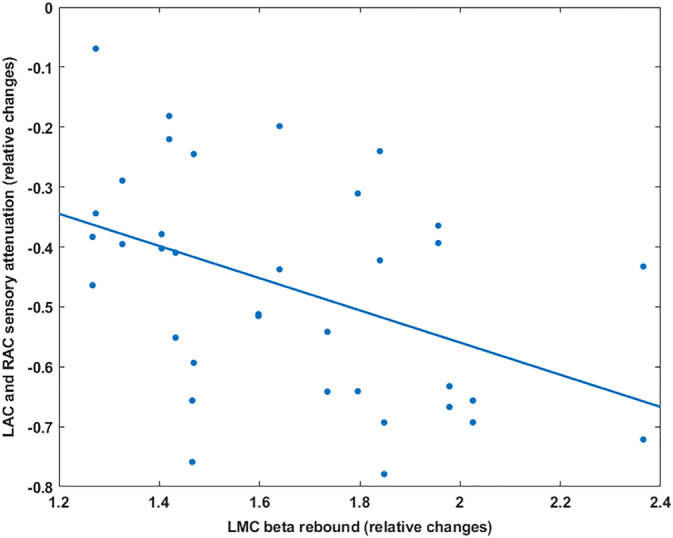
Scatterplot of the LMC beta rebound (x‐axis) and the LAC and RAC sensory attenuation (y‐axis). The blue line indicates the fitted linear regression. The increase of beta rebound in the left motor cortex leads to stronger sensory attenuation in the auditory cortices. LAC and RAC, left auditory cortex, LMC, left motor cortex; RAC, right auditory cortex
3.4. The directionality of motor–auditory cortices interaction
The correlation indicates a significant interaction between motor cortex and auditory cortices during sensory attenuation. However, it is based on intersubject correlations of local effects in the motor and auditory cortex. To assess directly motor–auditory interactions in each participant we computed Granger causality. Figure 4 displays the Granger causality differences between the original and time‐reversed data in LAC, RAC, and LMC for the active condition. We find significant Granger causality in the alpha/beta range from the left motor area to both left and right auditory areas. No meaningful Granger causality was observed from the right motor cortices to LAC and RAC voxels (see Figure S2 in the Supplementary section).
Figure 4.
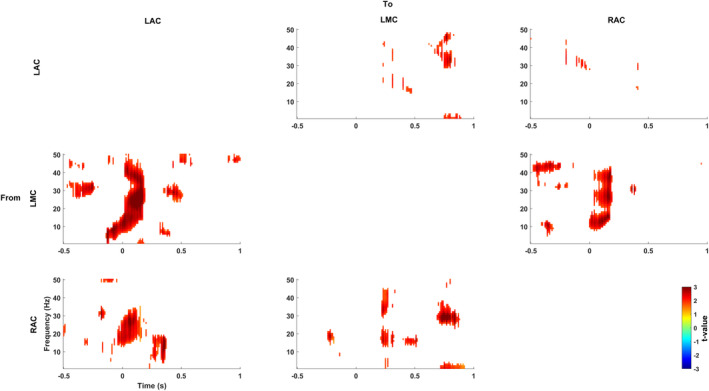
Results of Granger causality analysis. Granger causality differences between the original and time‐reversed data in motor and auditory areas for the active condition. There is significant Granger causality in the alpha/beta range from the motor area to both left and right auditory areas. Colour codes t values. Time point 0 s marks tone presentation onset
4. DISCUSSION
This study set out with the aim of assessing the interactions between motor and auditory areas during sensory attenuation based on the hypothesis that this interaction plays a major role in attenuating responses to self‐generated sensory input (Keller & Mrsic‐Flogel, 2018). Specifically, we tested whether the neuronal responses in the auditory area are modulated by beta‐band oscillations in the motor cortex during the processing of self‐generated stimuli. The results of this study support our hypothesis. Typical sensory attenuation was observed in the auditory evoked responses to self‐generated sounds as compared to sounds generated externally. This result is consistent with previous studies and demonstrates how actions can modulate auditory processing (Cao, Thut, & Gross, 2017; Cao, Veniero, et al., 2017; Hughes, Desantis, & Waszak, 2013; Martikainen et al., 2005; Schneider & Mooney, 2018). The term sensory attenuation has been used for the M100 amplitude suppression for a long time (Cao, Thut, & Gross, 2017; Cao, Veniero, et al., 2017; Hughes et al., 2013; Wolpe et al., 2018). One possible function of neurons generating M100 is to call attention to the availability of stimulus information (Näätänen & Picton, 1987). Therefore, the M100 might represent the initial readout of information from the sensory analysis. Alternatively, as previous studies suggested, neurons in the secondary motor area (M2) that project directly to the auditory areas can exert suppressive effects (Nelson et al., 2013; Schneider et al., 2018). These neurons convey motor‐related information during self‐generated movements. This could support M100 amplitude changes between self‐generated and externally generated sounds. However, further investigations are needed to clarify its detailed mechanism.
Moreover, our results also reveal a significant correlation between beta power changes in the motor cortex and the magnitude of sensory attenuation in auditory cortices. Finally, we report that the motor cortex is functionally connected to bilateral auditory cortices in beta‐band with direction from motor to auditory cortices in the active condition. Taken together, these findings strongly corroborate the essential role of motor oscillations in predicting the sensory consequences of self‐generated auditory stimuli.
In the following, we discuss the main results in more detail. We tested the hypothesis that motor oscillations are involved in sensory attenuation. Our results showed modulation of beta oscillations in the primary motor cortex due to the button press for the active condition in comparison to the passive condition. This is a well‐known and often reported finding. The amplitude of beta oscillations in sensorimotor areas is suppressed before and shortly after movements and is then followed by a transient increase (rebound) (Jenkinson & Brown, 2011; Kilavik, Zaepffel, Brovelli, MacKay, & Riehle, 2013). While the exact functional role of the premovement suppression is unknown, it likely reflects the preparation of the motor system for action. Tan et al. analysed the lateralisation of beta oscillations in the sensorimotor cortex while participants observed pointing movements made by an actor (Tan, Leuthold, & Gross, 2013). Interestingly, this beta lateralisation dynamically tracked the actor's movement within and across visual hemifields. Specifically, while the actor's finger was in the observers' left hemifield, beta lateralisation indicated a bias of observers to perform a left motor response. Once the actor's finger crossed the midline into the other hemifield, the observer's beta lateralisation changed to reflect a bias for responding with the other hand. The amount of beta lateralisation before response correlated with subsequent reaction time.
This indicates that beta amplitude in sensorimotor cortex reflects ongoing predictions about upcoming movements in a way that shapes the execution of this movement. Overall, this is consistent with previous reports showing that high levels of beta‐band power inhibit movement (Jenkinson & Brown, 2011) and preserve the status‐quo (Engel & Fries, 2010). Beta suppression and rebound instead seem to reflect dynamically changing predictions related to upcoming movements. Some studies indicate potentially different roles for suppression and rebound. Beta suppression might be more related to efferent motor control while the postmovement beta rebound might indicate recalibration of the motor system possibly associated with an updating of the internal forward model (Kilavik et al., 2013; Kühn et al., 2006).
However, beta modulation is not restricted to the motor cortex. We report significant beta power decrease in left and right auditory cortex following stimulus onset in the active condition compared to the passive condition. This replicates a similar finding in right auditory cortex in a similar task (Cao, Thut, & Gross, 2017; Cao, Veniero, et al., 2017) and suggests an interaction between motor and auditory cortex in this frequency band. Indeed, we observed two interactions. First, the power change in beta‐band in motor cortex was correlated with the suppression of stimulus‐evoked activity in left and right auditory cortex when the stimulus was self‐generated compared to when it was passively presented. Second, our results also revealed a significant connectivity in the beta‐band originating from motor cortex toward auditory cortices lasting for around 250 ms after the stimulus onset. Our results add to the growing literature that postulates a role of beta oscillations in reflecting top–down processes in auditory and motor–auditory processing. Sedley et al. analysed invasively recorded data from human auditory cortex and found that beta oscillations reflect the process of updating sensory predictions (Sedley et al., 2016). Similarly, an MEG study demonstrated that the modulation of beta oscillations in auditory cortex reflects temporal predictions about upcoming sensory stimuli that likely originate from motor–auditory interactions (Fujioka, Trainor, Large, & Ross, 2012). Morillon et al. reported that temporal predictions are encoded in beta oscillations that originated from sensorimotor cortex toward auditory regions (Morillon & Baillet, 2017). Another study demonstrated increased beta connectivity between motor cortex and inferior parietal lobe and middle temporal gyrus in a visuomotor tapping task and suggested that this is related to the belief of agency (Buchholz et al., 2019). This is relevant in the context of our study because the absence of sensory attenuation in schizophrenia is thought to reflect the reduced sense of agency (Roach et al., 2019). These reports together with our results are in line with previous studies indicating the role of beta oscillations in facilitating long‐range interactions on cortical networks and distant communication in the brain (Buchholz et al., 2019; Kilavik et al., 2013; Spitzer & Haegens, 2017).
Further evidence for the role of beta oscillations in top–down processing comes from invasive and non‐invasive studies that quantify directed connectivity (often through the use of Granger causality as in our study) in cortical hierarchies. The typical finding in these studies is that higher order areas show stronger directed connectivity in the alpha/beta‐band to lower order areas (top–down) as compared to the opposite (bottom‐up) direction (Bastos et al., 2015; Chao et al., 2018; Fontolan et al., 2014; Michalareas et al., 2016). Motor cortex is an important node in the auditory dorsal stream that has been extensively studied in speech perception and production (Hickok, 2012; Hickok & Poeppel, 2015). Indeed, in speech perception, motor areas have been shown to functionally interact with auditory cortex in a way that is behaviourally relevant (Keitel, Gross, & Kayser, 2018; Park, Ince, Schyns, Thut, & Gross, 2015). Overall, a number of recent studies converge on the notion that beta oscillations play an important role in predictive top–down processing along the auditory–motor axis (Spitzer & Haegens, 2017). Here, beta oscillations may carry information that goes beyond a simple preparation signal. Recently, it has been reported that movement and force are encoded in beta oscillations (Tan et al., 2016). In fact, previous studies suggested that beta oscillations can be content specific, enabling them to carry information about currently processed tasks. Beta rhythm can reflect scalar magnitudes (Spitzer & Haegens, 2017; Spitzer, Wacker, & Blankenburg, 2010), the speed of movement (Parkes, Bastiaansen, & Norris, 2006), and also be correlated with electromyogram activity during movement (Demandt et al., 2012; Kilavik et al., 2013; Parkes et al., 2006). Interestingly, content‐specific synchronisation in beta‐band has been observed not only within but also across the brain areas (Spitzer & Haegens, 2017). Our results show that while beta power changes are correlated with the magnitude of sensory attenuation, beta connectivity originating from motor area might indicate the transfer of movement‐related information. However, this needs to be tested in further studies. Movement‐specific information, however, is needed to accurately predict sensory consequences of any movement and reduce the prediction error. This also accords with previous observations which showed that the efference copy (motor command copy) conveys the details of auditory properties in inner speech scenario (Whitford et al., 2017). Similarly, it has been shown that beta oscillations before a movement contain information about future movement (Pape & Siegel, 2016; Tan et al., 2013). For instance, Pape and Siegel reported that beta modulation can predict the sensorimotor decision of the next movement (Pape & Siegel, 2016).
Further supporting evidence regarding the functional role of beta oscillations in the context of motor‐sensory interactions comes from computational models. Dynamic causal modelling of simulations (Brown, Adams, Parees, Edwards, & Friston, 2013) and MEG data (Bhatt et al., 2016) employ predictive coding theory to suggest that the suppression of beta oscillations is an index of reduced precision of sensory evidence associated with a movement.
Finally, investigations on the neurological disorders associated with the abnormal beta activity in cortico‐basal ganglia loop such as Parkinson's disease indicated the absence of sensory attenuation (Oswal, Brown, & Litvak, 2013; Wolpe et al., 2018). Our findings together with the previous studies support the major role of beta oscillation in transferring movement information from the motor cortex to the auditory areas in order to cancel the sensory consequences of a self‐generated movement.
Interestingly, animal studies report that these motor‐sensory interactions can indeed cause the type of sensory attenuation observed in our studies (Schneider & Mooney, 2018). Movement‐related neuronal activity in motor cortex was found to lead to a suppression of neuronal activity in auditory cortex (Nelson et al., 2013; Schneider, Nelson, & Mooney, 2014). Moreover, Schneider et al. also suggested that auditory interneurons are capable of integrating auditory inputs and motor signals and form a dynamic sensory filter that can suppress brain responses to the predictable auditory inputs (Schneider et al., 2018). This finding together with our correlation and connectivity results can support the hypothesis that motor cortex interacts with auditory areas during sensory attenuation.
Our results also demonstrate functional connectivity from motor to auditory cortex in alpha range before the stimulus onset for active condition. This result extends previous observations that prestimulus changes in alpha oscillations in auditory cortex during movement preparation (Cao, Thut, & Gross, 2017; Cao, Veniero, et al., 2017; Müller, Leske, Hartmann, Szebényi, & Weisz, 2015; Stenner, Bauer, Haggard, Heinze, & Dolan, 2014) shape the processing of incoming sensory stimuli. In fact, Granger causality from motor to LAC (Figure 4) suggests a spectral connectivity pattern that shifts from alpha frequencies before tone onset to beta frequencies just after tone onset. While further work is needed to corroborate this finding, it might indicate different stages of motor–auditory interactions. Prestimulus motor–auditory alpha connectivity could represent an unspecific downregulations of auditory excitability (consistent with the prestimulus alpha increase observed in Cao, Thut, and Gross (2017) and Cao, Veniero, et al. (2017)) followed by motor–auditory beta connectivity that could represent more specific information based on the motor efference copy.
5. CONCLUSION
Our finding of functional connectivity between motor and auditory areas shows the important role of motor commands in sensory attenuation. We hypothesise that the neural interactions between these two areas reflect the working of the internal forward model related to the prediction of the sensory consequences of one's own action. Our findings support the hypothesis that temporally coupled movement and auditory inputs form a sensory filter that involves movement information via motor cortical inputs to the auditory cortices that suppress auditory cortical responses to self‐generated sounds.
Supporting information
Appendix S1 Supplementary Information
Abbasi O, Gross J. Beta‐band oscillations play an essential role in motor–auditory interactions. Hum Brain Mapp. 2020;41:656–665. 10.1002/hbm.24830
DATA AVAILABILITY STATEMENT
Data are freely available upon request from the author.
REFERENCES
- Abbasi, O. , Dammers, J. , Arrubla, J. , Warbrick, T. , Butz, M. , Neuner, I. , & Shah, N. J. (2015). Time‐frequency analysis of resting state and evoked EEG data recorded at higher magnetic fields up to 9.4 T. Journal of Neuroscience Methods, 255, 1–11. 10.1016/j.jneumeth.2015.07.011 [DOI] [PubMed] [Google Scholar]
- Abbasi, O. , Hirschmann, J. , Schmitz, G. , Schnitzler, A. , & Butz, M. (2016). Rejecting deep brain stimulation artefacts from MEG data using ICA and mutual information. Journal of Neuroscience Methods, 268, 131–141. 10.1016/j.jneumeth.2016.04.010 [DOI] [PubMed] [Google Scholar]
- Bastos, A. M. , Vezoli, J. , Bosman, C. A. , Schoffelen, J.‐M. , Oostenveld, R. , Dowdall, J. R. , … Fries, P. (2015). Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron, 85, 390–401. 10.1016/j.neuron.2014.12.018 [DOI] [PubMed] [Google Scholar]
- Bauer, M. , Stenner, M.‐P. , Friston, K. J. , & Dolan, R. J. (2014). Attentional modulation of alpha/beta and gamma oscillations reflect functionally distinct processes. The Journal of Neuroscience, 34, 16117–16125. 10.1523/JNEUROSCI.3474-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt, M. B. , Bowen, S. , Rossiter, H. E. , Dupont‐Hadwen, J. , Moran, R. J. , Friston, K. J. , & Ward, N. S. (2016). Computational modelling of movement‐related beta‐oscillatory dynamics in human motor cortex. NeuroImage, 133, 224–232. 10.1016/j.neuroimage.2016.02.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, H. , Adams, R. A. , Parees, I. , Edwards, M. , & Friston, K. (2013). Active inference, sensory attenuation and illusions. Cognitive Processing, 14, 411–427. 10.1007/s10339-013-0571-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz, V. N. , David, N. , Sengelmann, M. , & Engel, A. K. (2019). Belief of agency changes dynamics in sensorimotor networks. Scientific Reports, 9, 1995 10.1038/s41598-018-37912-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, L. , Thut, G. , & Gross, J. (2017). The role of brain oscillations in predicting self‐generated sounds. NeuroImage, 147, 895–903. 10.1016/j.neuroimage.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, L. , Veniero, D. , Thut, G. , & Gross, J. (2017). Role of the cerebellum in adaptation to delayed action effects. Current Biology, 27(16), 2442–2451.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao, Z. C. , Takaura, K. , Wang, L. , Fujii, N. , & Dehaene, S. (2018). Large‐scale cortical networks for hierarchical prediction and prediction error in the primate brain. Neuron, 100, 1252–1266.e3. 10.1016/j.neuron.2018.10.004 [DOI] [PubMed] [Google Scholar]
- Demandt, E. , Mehring, C. , Vogt, K. , Schulze‐Bonhage, A. , Aertsen, A. , & Ball, T. (2012). Reaching movement onset‐ and end‐related characteristics of EEG spectral power modulations. Frontiers in Neuroscience, 6, 65 10.3389/fnins.2012.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamala, M. , Rangarajan, G. , & Ding, M. (2008). Estimating granger causality from fourier and wavelet transforms of time series data. Physical Review Letters, 100, 018701 10.1103/PhysRevLett.100.018701 [DOI] [PubMed] [Google Scholar]
- Engel, A. K. , & Fries, P. (2010). Beta‐band oscillations—Signalling the status quo? Current Opinion in Neurobiology, 20, 156–165. 10.1016/j.conb.2010.02.015 [DOI] [PubMed] [Google Scholar]
- Flinker, A. , Chang, E. F. , Kirsch, H. E. , Barbaro, N. M. , Crone, N. E. , & Knight, R. T. (2010). Single‐trial speech suppression of auditory cortex activity in humans. The Journal of Neuroscience, 30, 16643–16650. 10.1523/JNEUROSCI.1809-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontolan, L. , Morillon, B. , Liegeois‐Chauvel, C. , & Giraud, A.‐L. (2014). The contribution of frequency‐specific activity to hierarchical information processing in the human auditory cortex. Nature Communications, 5, 4694 10.1038/ncomms5694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford, J. M. , & Mathalon, D. H. (2005). Corollary discharge dysfunction in schizophrenia: Can it explain auditory hallucinations? International Journal of Psychophysiology, 58, 179–189. 10.1016/j.ijpsycho.2005.01.014 [DOI] [PubMed] [Google Scholar]
- Friston, K. (2005). A theory of cortical responses. Philosophical Transactions of the Royal Society B: Biological Sciences, 360, 815–836. 10.1098/rstb.2005.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka, T. , Trainor, L. J. , Large, E. W. , & Ross, B. (2012). Internalized timing of isochronous sounds is represented in neuromagnetic β oscillations. The Journal of Neuroscience, 32, 1791–1802. 10.1523/JNEUROSCI.4107-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, J. , Baillet, S. , Barnes, G. R. , Henson, R. N. , Hillebrand, A. , Jensen, O. , … Schoffelen, J.‐M. (2013). Good practice for conducting and reporting MEG research. NeuroImage, 65, 349–363. 10.1016/j.neuroimage.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, J. , Kujala, J. , Hamalainen, M. , Timmermann, L. , Schnitzler, A. , & Salmelin, R. (2001). Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proceedings of the National Academy of Sciences of the United States of America, 98, 694–699. 10.1073/pnas.98.2.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haufe, S. , Nikulin, V. V. , Müller, K.‐R. , & Nolte, G. (2013). A critical assessment of connectivity measures for EEG data: A simulation study. NeuroImage, 64, 120–133. 10.1016/j.neuroimage.2012.09.036 [DOI] [PubMed] [Google Scholar]
- Hickok, G. (2012). Computational neuroanatomy of speech production. Nature Reviews. Neuroscience, 13, 135–145. 10.1038/nrn3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok, G. , & Poeppel, D. (2015). Neural basis of speech perception. Handbook of Clinical Neurology, 129, 149–160. 10.1016/B978-0-12-407794-2.00025-0 [DOI] [PubMed] [Google Scholar]
- Hughes, G. , Desantis, A. , & Waszak, F. (2013). Mechanisms of intentional binding and sensory attenuation: The role of temporal prediction, temporal control, identity prediction, and motor prediction. Psychological Bulletin, 139, 133–151. 10.1037/a0028566 [DOI] [PubMed] [Google Scholar]
- Jenkinson, N. , & Brown, P. (2011). New insights into the relationship between dopamine, beta oscillations and motor function. Trends in Neurosciences, 34, 611–618. 10.1016/j.tins.2011.09.003 [DOI] [PubMed] [Google Scholar]
- Keitel, A. , Gross, J. , & Kayser, C. (2018). Perceptually relevant speech tracking in auditory and motor cortex reflects distinct linguistic features. PLoS Biology, 16, e2004473 10.1371/journal.pbio.2004473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, G. B. , & Mrsic‐Flogel, T. D. (2018). Predictive processing: A canonical cortical computation. Neuron, 100, 424–435. 10.1016/j.neuron.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilavik, B. E. , Zaepffel, M. , Brovelli, A. , MacKay, W. A. , & Riehle, A. (2013). The ups and downs of β oscillations in sensorimotor cortex. Experimental Neurology, 245, 15–26. 10.1016/j.expneurol.2012.09.014 [DOI] [PubMed] [Google Scholar]
- Kühn, A. A. , Doyle, L. , Pogosyan, A. , Yarrow, K. , Kupsch, A. , Schneider, G.‐H. , … Brown, P. (2006). Modulation of beta oscillations in the subthalamic area during motor imagery in Parkinson's disease. Brain, 129, 695–706. 10.1093/brain/awh715 [DOI] [PubMed] [Google Scholar]
- Maris, E. , & Oostenveld, R. (2007). Nonparametric statistical testing of EEG‐ and MEG‐data. Journal of Neuroscience Methods, 164, 177–190. 10.1016/j.jneumeth.2007.03.024 [DOI] [PubMed] [Google Scholar]
- Martikainen, M. H. , Kaneko, K. , & Hari, R. (2005). Suppressed responses to self‐triggered sounds in the human auditory cortex. Cerebral Cortex, 15, 299–302. 10.1093/cercor/bhh131 [DOI] [PubMed] [Google Scholar]
- Michalareas, G. , Vezoli, J. , van Pelt, S. , Schoffelen, J.‐M. , Kennedy, H. , & Fries, P. (2016). Alpha‐beta and gamma rhythms subserve feedback and feedforward influences among human visual cortical areas. Neuron, 89, 384–397. 10.1016/j.neuron.2015.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillon, B. , & Baillet, S. (2017). Motor origin of temporal predictions in auditory attention. Proceedings of the National Academy of Sciences of the United States of America, 114, E8913–E8921. 10.1073/pnas.1705373114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, N. , Leske, S. , Hartmann, T. , Szebényi, S. , & Weisz, N. (2015). Listen to yourself: The medial prefrontal cortex modulates auditory alpha power during speech preparation. Cerebral Cortex, 25, 4029–4037. 10.1093/cercor/bhu117 [DOI] [PubMed] [Google Scholar]
- Näätänen, R. , & Picton, T. (1987). The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology, 24(4), 375–425. [DOI] [PubMed] [Google Scholar]
- Nelson, A. , Schneider, D. M. , Takatoh, J. , Sakurai, K. , Wang, F. , & Mooney, R. (2013). A circuit for motor cortical modulation of auditory cortical activity. The Journal of Neuroscience, 33, 14342–14353. 10.1523/JNEUROSCI.2275-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte, G. (2003). The magnetic lead field theorem in the quasi‐static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Physics in Medicine and Biology, 48, 3637–3652. 10.1088/0031-9155/48/22/002 [DOI] [PubMed] [Google Scholar]
- Oostenveld, R. , Fries, P. , Maris, E. , & Schoffelen, J.‐M. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011, 156869 10.1155/2011/156869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswal, A. , Brown, P. , & Litvak, V. (2013). Synchronized neural oscillations and the pathophysiology of Parkinson's disease. Current Opinion in Neurology, 26, 662–670. 10.1097/WCO.0000000000000034 [DOI] [PubMed] [Google Scholar]
- Pape, A.‐A. , & Siegel, M. (2016). Motor cortex activity predicts response alternation during sensorimotor decisions. Nature Communications, 7, 13098 10.1038/ncomms13098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H. , Ince, R. A. A. , Schyns, P. G. , Thut, G. , & Gross, J. (2015). Frontal top–down signals increase coupling of auditory low‐frequency oscillations to continuous speech in human listeners. Current Biology, 25, 1649–1653. 10.1016/j.cub.2015.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes, L. M. , Bastiaansen, M. C. M. , & Norris, D. G. (2006). Combining EEG and fMRI to investigate the post‐movement beta rebound. NeuroImage, 29, 685–696. 10.1016/j.neuroimage.2005.08.018 [DOI] [PubMed] [Google Scholar]
- Pernet, C. R. , Wilcox, R. , & Rousselet, G. A. (2012). Robust correlation analyses: False positive and power validation using a new open source matlab toolbox. Frontiers in Psychology, 3, 606 10.3389/fpsyg.2012.00606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller, G. , & Lopes da Silva, F. H. (1999). Event‐related EEG/MEG synchronization and desynchronization: Basic principles. Clinical Neurophysiology, 110, 1842–1857. 10.1016/S1388-2457(99)00141-8 [DOI] [PubMed] [Google Scholar]
- Pickering, M. J. , & Clark, A. (2014). Getting ahead: Forward models and their place in cognitive architecture. Trends in Cognitive Sciences, 18, 451–456. 10.1016/j.tics.2014.05.006 [DOI] [PubMed] [Google Scholar]
- Roach, B. J. , Ford, J. M. , Biagianti, B. , Hamilton, H. K. , Ramsay, I. S. , Fisher, M. , … Mathalon, D. H. (2019). Efference copy/corollary discharge function and targeted cognitive training in patients with schizophrenia. International Journal of Psychophysiology, 145, 91–98. 10.1016/j.ijpsycho.2018.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, E. W. , & Marcus, M. M. (1973). Self‐stimulation alters human sensory brain responses. Science, 181, 175–177. 10.1126/science.181.4095.175 [DOI] [PubMed] [Google Scholar]
- Schneider, D. M. , & Mooney, R. (2018). How movement modulates hearing. Annual Review of Neuroscience, 41, 553–572. 10.1146/annurev-neuro-072116-031215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, D. M. , Nelson, A. , & Mooney, R. (2014). A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature, 513, 189–194. 10.1038/nature13724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, D. M. , Sundararajan, J. , & Mooney, R. (2018). A cortical filter that learns to suppress the acoustic consequences of movement. Nature, 561, 391–395. 10.1038/s41586-018-0520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedley, W. , Gander, P. E. , Kumar, S. , Kovach, C. K. , Oya, H. , Kawasaki, H. , … Griffiths, T. D. (2016). Neural signatures of perceptual inference. eLife, 5, e11476 10.7554/eLife.11476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer, B. , & Haegens, S. (2017). Beyond the status quo: A role for beta oscillations in endogenous content (re)activation. eNeuro, 4, ENEURO.0170–ENEU17.2017. 10.1523/ENEURO.0170-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer, B. , Wacker, E. , & Blankenburg, F. (2010). Oscillatory correlates of vibrotactile frequency processing in human working memory. The Journal of Neuroscience, 30, 4496–4502. 10.1523/JNEUROSCI.6041-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenner, M.‐P. , Bauer, M. , Haggard, P. , Heinze, H.‐J. , & Dolan, R. (2014). Enhanced alpha‐oscillations in visual cortex during anticipation of self‐generated visual stimulation. Journal of Cognitive Neuroscience, 26, 2540–2551. 10.1162/jocn_a_00658 [DOI] [PubMed] [Google Scholar]
- Tan, H. , Pogosyan, A. , Ashkan, K. , Green, A. L. , Aziz, T. , Foltynie, T. , … Brown, P. (2016). Decoding gripping force based on local field potentials recorded from subthalamic nucleus in humans. eLife, 5, e19089 10.7554/eLife.19089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, H.‐R. M. , Leuthold, H. , & Gross, J. (2013). Gearing up for action: Attentive tracking dynamically tunes sensory and motor oscillations in the alpha and beta band. NeuroImage, 82, 634–644. 10.1016/j.neuroimage.2013.04.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford, T. J. , Jack, B. N. , Pearson, D. , Griffiths, O. , Luque, D. , Harris, A. W. , … Le Pelley, M. E. (2017). Neurophysiological evidence of efference copies to inner speech. eLife, 6, e28197 10.7554/eLife.28197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpe, N. , Zhang, J. , Nombela, C. , Ingram, J. N. , Wolpert, D. M. , Cam‐CAN , & Rowe, J. B. (2018). Sensory attenuation in Parkinson's disease is related to disease severity and dopamine dose. Scientific Reports, 8, 15643 10.1038/s41598-018-33678-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre, R. J. , Chen, J. L. , & Penhune, V. B. (2007). When the brain plays music: Auditory‐motor interactions in music perception and production. Nature Reviews. Neuroscience, 8, 547–558. 10.1038/nrn2152 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supplementary Information
Data Availability Statement
Data are freely available upon request from the author.


