Abstract
Prior work has revealed that mnemonic strategy training (MST) can enhance memory for specific content and engages regions in the frontoparietal cognitive control network. Evidence of transfer to novel content is less clear. Here, we provide secondary analysis of functional magnetic resonance imaging (fMRI) data acquired during a randomized controlled trial that compared MST to an active education control condition in patients with amnestic mild cognitive impairment (a-MCI). In the trial, thirty participants with a-MCI were randomized to the education program (EP) or MST, where they learned to apply the technique to face-name associations during four intervening hour long training sessions. Participants underwent pre- and post-training fMRI scans, during which they encoded both the trained (i.e., those used during the four training sessions) and untrained (‘novel’) face-name associations. The primary cognitive outcome measures revealed significantly improved memory for both trained and novel stimuli – effects supporting near transfer of MST. Relative to pre-training, there were significant and highly similar increases in activation for both trained and novel stimuli, especially in regions associated with the frontoparietal cognitive control network bilaterally, but also in temporal areas related to social cognition and emotional processing. Critically, this pattern of activation was notably different from the EP group. Thus, the changes in activation were consistent with the strategies trained and, combined with the cognitively-based near transfer effects, suggest that MST focused on face-name association enhances performance by engaging cognitive control and social/emotional processing. Finally, our data indicated that our MST is a relevant and efficient intervention to a-MCI.
Keywords: Cognitive rehabilitation, memory training, mnemonic strategy, aging, mild cognitive impairment, MCI, neuroimaging, functional magnetic resonance imaging (fMRI), restoration, compensation
1. Introduction
Mild cognitive impairment (MCI) refers to a clinical condition characterized by cognitive decline that exceeds that of normal aging, in the absence of impaired daily functioning (Petersen et al., 2014, Petersen et al., 2001). Thus, the MCI individuals, especially those with the amnestic subtype (a-MCI), are at high risk of converting to dementia (Espinosa et al., 2013). Although memory deficits in a-MCI are typically attributed to medial temporal lobe (MTL) dysfunction (Dickerson and Sperling, 2008, Gomar et al., 2017), there is evidence that frontoparietal dysfunction may be also implicated in the memory deficits observed in this population. For instance, when compared to controls, a-MCI patients present hypoactivation in temporal and lateral frontoparietal regions during memory task performance (Hampstead et al., 2011a; Machula et al., 2009). Also, there is evidence that frontoparietal regions are critical for successful memory formation (Spaniol et al., 2009), which is reinforced by the findings that cognitively intact older adults engage the left frontoparietal network during the successful encoding of relevant ecological associative memory task (Hampstead et al., 2016), a pattern not observed in MCI patients. The relevance of frontoparietal areas for successful memory performance is likely due to their role in mediating cognitive control and working memory (Hampstead et al., 2016; Gills et al., 2016). Thus, the interaction between medial temporal and frontoparietal brain regions may underlie the characteristic memory deficits in MCI and present a viable option for intervention.
The lack of effective pharmacological treatments for a-MCI (McGhee et al., 2016) has led to increased interest in cognitively oriented treatments as a mechanism for enhancing and prolonging cognitive functioning in this population (Sherman et al., 2017, Hill et al., 2017, Hampstead et al., 2014, Reijnders et al., 2013, Simon et al., 2012, Belleville et al., 2011, Bahar-Fuchs et al., 2017, Vermeij et al., 2016). The current report focuses on mnemonic strategy training (MST), which has shown to be a promising approach (Chandler et al., 2016, Hampstead et al., 2014, Mewborn et al., 2017, Huckans et al., 2013, Simon et al., 2012). Mnemonic strategies can be broadly conceptualized as cognitive “tools” that facilitate the organization and association of new information, thereby enhancing depth of processing (Hampstead et al., 2014). In addition, a few studies have used functional magnetic resonance imaging (fMRI) and have shown that MST not only improves memory performance but consistently engages the frontoparietal network in those with a-MCI (Belleville et al., 2011, Hampstead et al., 2011b, Balardin et al., 2015).
Face-name associations are a functionally meaningful target of MST since forgetting names is a frequent cognitive complaint from individuals with a-MCI (James et al., 2008, Papp et al., 2014, Weaver Cargin et al., 2008). Additionally, poor face-name memory is sensitive to Alzheimer’s pathology (Rentz et al., 2011). A small number of prior studies have shown the benefits of various cognitively oriented treatments for face-name associations (Belleville et al., 2011, Clare et al., 2009, Jean et al., 2010). We previously reported that MST enhanced memory for trained face-name associations in an uncontrolled pilot study (Hampstead et al., 2008); improvements that were accompanied by increased activation and effective connectivity in the lateral frontoparietal and temporal cortices (Hampstead et al., 2011b). These findings are consistent with other neuroimaging works showing that MST (re)engages brain regions associated with executive functions and memory (Balardin et al., 2015, Belleville et al., 2011, Belleville and Bherer, 2012, Hampstead et al., 2011b, Hosseini et al., 2014), suggesting compensatory and/or restorative effects of MST.
The present study is the first, to our knowledge, to use fMRI to examine the effects of MST on face-name associations using a randomized, controlled, single blind design in those with a-MCI. In this study, Brazilian patients were randomized to either MST or an education program (EP), which served as an active control condition. Our primary outcome measures focused on the between-group cognitive effects and differences in blood oxygen level dependent (BOLD) signal as well as the maintenance of such effects over the time (Simon et al., 2018). Briefly, there was evidence of near transfer effects and of greater activation in the lateral temporal cortex in the MST group relative to EP, in regions implicated in semantic memory, face processing, theory of mind and social cognition. Here, we present secondary analyses of within-group changes in whole-brain BOLD signal as they relate to 1) training specific gains (i.e., stimuli learned during four training sessions) and 2) untrained (i.e., novel) stimuli that were only seen during the pre- and post-intervention fMRI scans. We also investigated the relationship between BOLD signal changes and MST-induced memory improvement. Our approach replicates previous methodology (Hampstead et al., 2011b) but also critically extends those preliminary findings in a cohort with different cultural background and with a greater sample size. Based on similar previous reports (Hampstead et al., 2011b, Hampstead et al., 2012a, Simon et al., 2018), we hypothesized that MST would enhance lateral temporal and frontoparietal activation and that such changes would be related to memory improvement, whereas no such findings would be evident in the control (EP) condition.
2. Material and Methods
2.1. Participants
A total of 30 older adults with a-MCI (mean age = 72.1, range 62 – 82; mean education = 12.1 years of education, range 4 – 18 years) were included in the study. All participants were volunteers and provided written informed consent, in accordance to the Declaration of Helsinki. The study was approved by the Ethics Committee of the Medical School of University of São Paulo and registered at ClinicalTrials.gov (NCT01978353).
During pre-training visit, participants completed detailed evaluations that included a structured interview to obtain medical, neurological, and psychiatric history as well as a neuropsychological evaluation. To be included in the study, participants had to be 60 years or older, Portuguese speaking, right handed, have at least four years of education, normal or corrected vision and hearing, and diagnosis of a-MCI, according to Petersen’s criteria (Petersen et al., 2001). As detailed previously (Simon et al., 2018), the a-MCI diagnosis included: 1) presence of subjective memory complaint corroborated by an informant; 2) performance of at least 1.5 standard deviation (SD) below the age norms on one memory test or 1.0 SD below norms on more than one memory test; 3) normal general cognitive function; 4) essentially normal functional of daily living (e) absence of dementia. Participants were excluded in they had any MRI contraindication, history of central nervous system disease (not related to MCI), or major ongoing psychiatric condition. Medications were evaluated to exclude those who were using agents that affect cognition.
2.2. Initial Assessment
Participants performed clinical and neuropsychological evaluations. The questionnaires and tests were described elsewhere (Simon et al., 2018), and are listed here: Cambridge Examination for Mental Disorders of Older People (CAMDEX), Hamilton Anxiety Rating Scale (HAMA), Montgomery–Åsberg Depression Rating Scale (MADRS), Beck Depression Inventory (BDI), Beck Anxiety Inventory (BAI), Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE), Bayer Activities of Daily Living Scale (B-ADL), Multifactorial Memory Questionnaire (MMQ), Montreal Cognitive Assessment (MoCA), Vocabulary, Matrix Reasoning and Digit Span from the Wechsler Adult Intelligence Scale Third edition (WAIS-III), Short Cognitive Performance Test (SKT), Stroop Test, Phonemic Fluency (letters FAS), Semantic Fluency (animal category), Boston Naming Test, Rey–Osterrieth Complex Figure Test, Hopkins Verbal Learning Test Revised (HVLT-R), Logical Memory and Faces from the Wechsler Memory Scale Third Edition (WMS-III). We calculated the Intellectual Quotient (IQ) measure based on Vocabulary and Matrix Reasoning’s scores (Ringe et al., 2002).
2.3. Outcome Measures
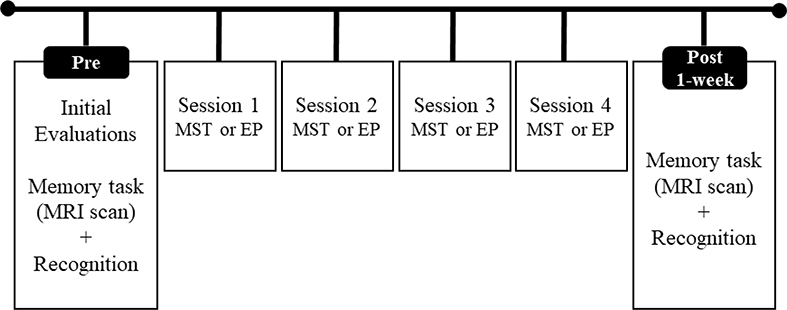
Figure 1 shows the structure of the study (for details on participant’s selection see Simon et al., 2018). To assess training effects, participants were evaluated approximately 1-week after finishing the programs (average of 5.6 days).
Figure 1.
Structure of the study design.
2.3.1. fMRI Task
The stimuli and paradigm were previously described in detail (Hampstead et al., 2008, Hampstead et al., 2011b; Simon et al., 2018). Briefly, stimuli included a total of 74 faces used in an earlier study (Kirwan and Stark, 2004) that were transformed to grayscale images and randomly paired with gender-appropriate names in Portuguese (5–7 letters) (Simon et al., 2018). Seventy-two face-name pairs were divided into two lists, which were matched for sex, race, approximate age, and emotional valence (positive, negative, and neutral), and the remaining two faces (one of each sex) served as control stimuli. We used a block design fMRI paradigm comprised of alternating 20 second (s) rest and 24 s active blocks, the latter of which involved three types of blocks: (1) stimuli from the trained list, (2) stimuli from an untrained (i.e., novel) list, and (3) the two control stimuli repeated in alternation. During active blocks, 4 face-name pairs were shown for 5s each, with a 1s interstimulus interval. Each block type occurred three times during each of the three functional runs. In total, participants were instructed to remember 36 stimuli from the trained and untrained lists; the repeated stimuli were shown 36 times (18 times each). Block order within a run was initially randomized within each run but held constant across participants. The three different stimulus-set orders were randomized for each participant, thereby mitigating confounding factors.
MRI scanning was performed on a 3T Philips Achieva machine MR system. Image acquisition parameters were: echo planar sequence (EPI GRE) encompassing whole brain (TR=2000ms, TE=30ms, flip angle 901/2, 3 dummy scans, 40 slices axial oblique AC-PÇ oriented with 3mm isotropic voxels). Three runs were acquired in the fMRI experiment, each with 199 volumes. A structural 3D T1-weighted scan was acquired immediately after the fMRI acquisition for volumetry brain morphometry (VBM), activation map-coregistration, and normalization procedures described ahead (TR=7ms, TE=3.2ms, flip angle 81/2, 180 slices, 1mm isotropic voxels). An Axial Fluid-Attenuated Inversion Recovery (FLAIR) scan and susceptbility-weighted (Principles of Echo-Shifting with a Train of Observations - PRESTO) acquisitions - together with T1 acquisition - were used to identify brain alterations. All images followed a quality control protocol and were inspected by a neuroradiologist for safety purposes (e.g., to rule out confounding factors like tumors or stroke).
Data processing and statistical analyses were conducted using FMRIB Software Library FSL version 6.0 (Smith et al., 2004). As described in Simon et al. (2018), functional volumes were processed by motion correction (MCFLIRT), slice-timing correction using Fourier-space time-series phase-shifiting, non-brain removal using BET (Smith, 2002), spatial smoothing (FWHM = 5mm) and highpass temporal filtering (sigma = 50.0s), to remove signal drift and low-frequency noise. Time-series statistical analysis was carried out using the general linear model (FILM) with local autocorrelation correction (Woolrich et al., 2004). Then, the functional images were registered with high resolution structural and standard space images using FLIRT (Jenkinson et al., 2002, Jenkinson and Smith, 2001). For higher-level analysis of multiple sessions (mean of three runs and time effect) we used a fixed effect model, and for multiple subjects (group mean) the FLAME (FMRIB’S Local Analysis of Mixed Effects) was used (Beckmann et al., 2003, Woolrich et al., 2004, Woolrich, 2008). Z statistic images (Gaussianised T/F) were thresholded using clusters determined by Z-score > 3.09 and a (corrected) cluster significance threshold of p < 0.05 (Worsley, 2003).
Separate activation maps were generated for trained and untrained stimuli in each intervention condition (MST or EP). For each analysis we used paired t-tests to compare sessions (pre x post-training), in the contrast: POST [stimuli type > repeated stimuli] > PRE [stimuli type > repeated stimuli] to assess increase of activation after the interventions; and PRE [stimuli type > repeated stimuli] > POST [stimuli type > repeated stimuli] to investigate decrease of activation after interventions. In order to identify changes in brain activation, and posteriorly calculate correlation between behavior performance and brain activation, we extracted the beta values for each of the areas of peak of activation and calculated the percentage of change [((time 2 – time 1) / 100) * 100].
2.3.2. Face-Name Recognition Task
Thirty minutes after the fMRI task, participants completed the Face-Name Recognition Task (FNRT) outside the scanner. The FNRT included the 74 stimuli and involved a four-alternative recognition format (Hampstead et al., 2008) that included: 1) the target name, 2) a sex-appropriate name from trained list, 3) a sex-appropriate name from the untrained list, and 4) a sex-appropriate novel name. We measure three aspects of the FNRT for both trained and untrained stimuli: 1) accuracy (percent correct), 2) confidence ratings for each response using an anchored 4-point scale (1 = not confident at all, 4 = extremely confident), and 3) reaction time (RT).
2.4. Interventions
2.4.1. Mnemonic Strategy Training
Full details of the training procedures can be found elsewhere (Simon et al., 2018; also see Hampstead et al., 2008). Briefly, MST was administered individually, with participants attending four 1-hour sessions, twice a week, over a 2-week period. Participants were trained to apply MST using 36 face-name pairs (12 new pairs each session). Our MST approach followed the “feature-reason-image” process in which all cues were provided. First, participants were directed to a salient facial feature (“feature”), learned a “nickname” linking the facial feature to the name (“reason”), and were instructed to create a mental image that integrated the visual and verbal cues in a detailed manner (“image”). The reason cues were phonologically similar to, or rhymed with, the actual name. On each training trial, participants were required to first recall the feature, then the reason, and finally the corresponding name on up to ten training trials. A stimulus was removed if participants correctly recalled this information on three consecutive trials. Following completion the training, the 12 pairs trained during the session were reviewed using the same step-by-step process (same day review). The next training session began with the review of all 12 pairs trained in the previous session in a different order (delayed review), and then a new set of 12 pairs was trained. The last session was a review of all 36 pairs in a random order. Thus, the goal of training was to alter the manner in which participants attempted to learn and subsequently recall the targeted information.
At the end of each session, participants completed an ecologically-oriented “generalization step” in order to enhance comprehension and transfer to their everyday life (Simon et al., 2018). This step required participants to apply the methodology to real people they knew but whose name they had trouble recalling or had forgotten at least once. To do so, participants were asked to imagine the person’s face, describe it out loud, and then apply the FRI methodology, which allowed them to create their own associations. In each session, one real-life example was trained and participants were actively encouraged to use the associative methodology in their everyday lives.
2.4.2. Education Program (EP)
The EP was also delivered individually and was designed to control for non-specific factors (e.g., comparable session length, participant-staff interactions) as in the MST group. In each session, age-relevant topics were discussed, such as: healthy aging, memory functioning, aspects that can interfere in memory (e.g., depression, sleep, and stress), risks and protective factors related to dementia, and information regarding MCI phenotype and Alzheimer’s disease. Review periods were integrated at the same time points as in the MST group during which the main points of the instructional content were reinforced, and in the last session, participants underwent an overall content review.
2.5. Statistical Analysis
In order to evaluate intervention-related changes, we performed paired t-tests for each stimuli type separately in each experimental condition (MST and EP). We also calculated effect sizes (Cohen dz) appropriated to within-subject design as recommended (Lakens, 2013), which are interpreted according to Cohen’s (1988) conventions (i.e., 0.2 = small, 0.5 = medium, 0.8 = large). Last, we performed an exploratory analysis evaluating the relationship between behavioral (i.e., percent change) and activation (i.e., beta-weight) change using Pearson r. In order to reduce false discovery rate (FDR), the significant p-values were considered adjusted based on the Benjamini-Hochberg (BH) procedure (Benjamini and Hochberg, 1995). The BH is defined as p ≤ (i/m)Q, where p represents the individual p-value, i= the individual p-value’s rank, m = total number of tests, and Q = the FDR (which was set at 0.05). All analyses were performed using the Statistical Package for Social Sciences (SPSS) version 23 with results considered significant at p < 0.05.
3. Results
3.1. Group demographics and baseline neuropsychological functioning
Thirty participants were equally allocated in the two groups, and their characteristics are described in Table 1. There were no demographic, clinical or neuropsychological differences between groups at baseline, except for the performance in immediate recall of SKT-Memory [t(28) = 2.16, p = 0.04], indicating that the MST group performed slightly better than the control group.
Table 1.
Baseline characteristics of the sample
| MST (n = 15) M (SD) |
Education (n = 15) M (SD) |
p-Value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 73.3 (5.9) | 71.0 (6.5) | 0.31 |
| Education (years) | 11.7 (3.6) | 12.5 (4.5) | 0.57 |
| Gender (females %) | 73.3% | 80% | 1 |
| Ethnicity, caucasian (%) | 53% | 60% | 1 |
| Clinical Characteristics | |||
| MADRS | 3.6 (3.6) | 2.7 (2.9) | 0.44 |
| HAMA | 3.5 (4.2) | 2.4 (2.7) | 0.41 |
| IQCODE | 3.2 (0.2) | 3.0 (0.6) | 0.22 |
| B-ADL | 1.6 (0.6) | 1.7 (0.7) | 0.81 |
| Neuropsychological Performance | |||
| MoCA | 24.3 (2.2) | 23.9 (2.7) | 0.71 |
| Estimated IQ | 97.9 (7.7) | 97.3 (10.7) | 0.86 |
| Digit Span forward (WAIS-III) | 7.4 (1.6) | 8.0 (2.0) | 0.37 |
| Digit Span backward (WAIS-III) | 4.2 (1.3) | 4.8 (1.5) | 0.25 |
| SKT-Attention Score | 1.6 (2.0) | 2.3 (2.2) | 0.36 |
| SKT-Memory Immediate Recall | 6.7 (0.9) | 5.7 (1.5) | 0.04 |
| SKT-Memory Delayed Recall | 6.2 (1.7) | 6.2 (2.0) | 1 |
| Stroop test (time on third plate) | 34.7 (9.6) | 41.1 (15.7) | 0.23 |
| HVLT-R Immediate recall | 20.8 (2.9) | 20.5 (3.8) | 0.83 |
| HVLT-R delayed recall | 3.5 (2.8) | 2.7 (2.4) | 0.41 |
| Faces Immediate recall (WMS-III) | 33.2 (4.8) | 34.2 (3.7) | 0.53 |
| Faces delayed recall (WMS-III) | 31.2 (4.7) | 33.1 (3.2) | 0.20 |
| LM Immediate recall (WMS-III) | 19.5 (5.7) | 22.3 (5.8) | 0.20 |
| LM delayed recall (WMS-III) | 16.9 (5.3) | 15.8 (8.0) | 0.67 |
| ROCF | 29.8 (3.4) | 27.1 (5.0) | 0.10 |
| COWAT (FAS) | 35.2 (10.0) | 33.5 (11.7) | 0.67 |
| Semantic Fluency (Animal) | 15.8 (3.6) | 13.6 (3.3) | 0.10 |
| Boston Naming Test | 54.9 (4.8) | 50.9 (7.2) | 0.09 |
Note: B-ADL, Bayer Activities of Daily Living Scale; COWAT, Controlled Oral Word Association Test; ED, Education; HAMA, Hamilton Anxiety Rating Scale; HVLT-R Hopkins Verbal Learning Test Revised; IQ, Intelligence Quotient; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly; LM, Logical Memory; M, mean; MADRS, Montgomery-Åsberg; Depression Rating Scale; MoCA, Montreal Cognitive Assessment; MST, Memory Strategy Training; ROCF, Rey- Osterrieth Complex Figure Test; SKT, Short Cognitive Performance Test; SD, Standard Deviation; WAIS, Wechsler Adult Intelligence; WMS, Wechsler Memory Scale.
3.2. Behavioral data
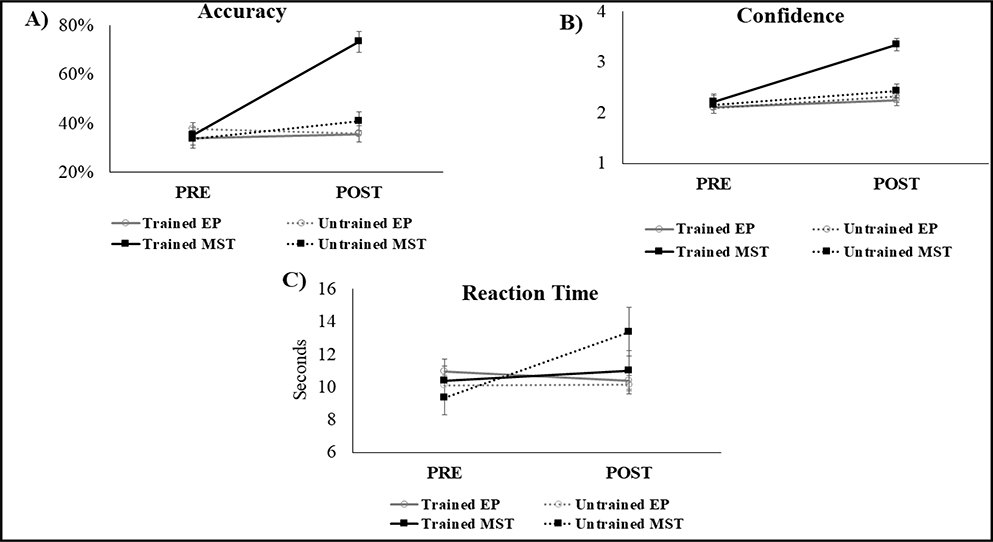
The behavioral results revealed that after MST participants showed significant improvement in accuracy for both trained [t(14) = −9.91, corrected p = 0.008; dz = 2.9] and untrained stimuli [t(14) = −2.27, corrected p = 0.03; dz = 0.6 ]. In contrast, there was no significant change after the EP for either stimuli type (trained stimuli: [t(14) = −0.53, p = 0.60; dz = 0.1]; untrained stimuli: [t(14) = 0.77, p = 0.45; dz = 0.2]) (Figure 2A).
Figure 2.
Behavioral performance by stimuli type and group condition. Performance on A) Accuracy (percent of hits); B) Confidence level of the response; C) Reaction time (seconds). EP = Education; MST = Mnemonic strategy training. Error bars represents standard error.
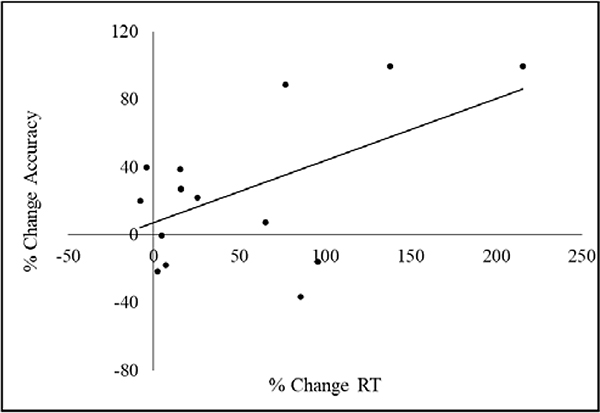
Regarding confidence of the response, the MST group reported increased confidence in memory of the trained stimuli [t(14) = −8.27, corrected p = 0.01; dz = 2.1], but not of the untrained stimuli [t(14) = −1.54, p = 0.14; dz = 0.4]. In contrast, the EP group showed no confidence changes for either stimulus type (trained stimuli: [t(14) = −1.31, p = 0.20; dz = 0.2]; untrained stimuli: [t(14) = −1.83, p = 0.08; dz = 0.4]) (Figure 2B). We observed a significant increase of RT for the untrained stimuli in the MST group [t(14) = −3.14, corrected p = 0.02; dz = 0.8], but no other RT change for the other analysis (MST trained stimuli: t(14) = −0.58, p = 0.57; dz = 0.1; EP trained: t(14) = 0.89, p = 0.38; dz = 0.1; EP untrained stimuli: t(14) = −.10, p = 0.92; dz = 0.0) (Figure 2C). There was a significant positive relationship between the percent change in accuracy and percent of change in RT for the untrained stimuli in the MST group (r = 0.536, p = 0.04), indicating that greater accuracy was associated with slower response time (Figure 3).
Figure 3.
Scatterplot representing the correlation between percent of change in RT (X axis) and percent change in Accuracy (Y axis) for the untrained stimuli.
3.3. fMRI
Brain activations related to the interventions were investigated by comparing activation prior and after intervention, and determining whether there was increase (post > pre) or decrease (pre > post) of activation after the programs. This procedure was conducted for both trained and untrained stimuli, separately.
3.3.1. Activation Changes for the Trained Stimuli
Post > Pre
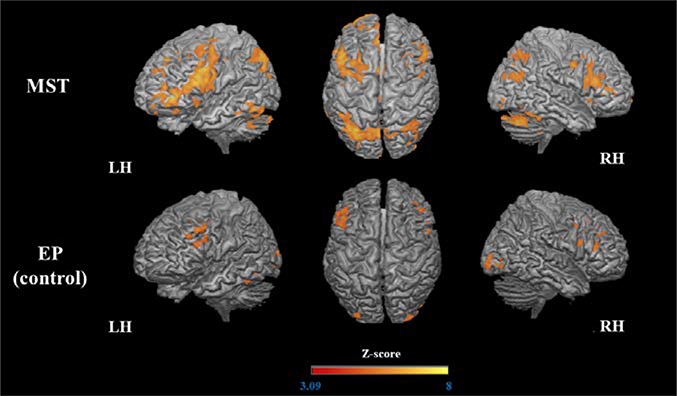
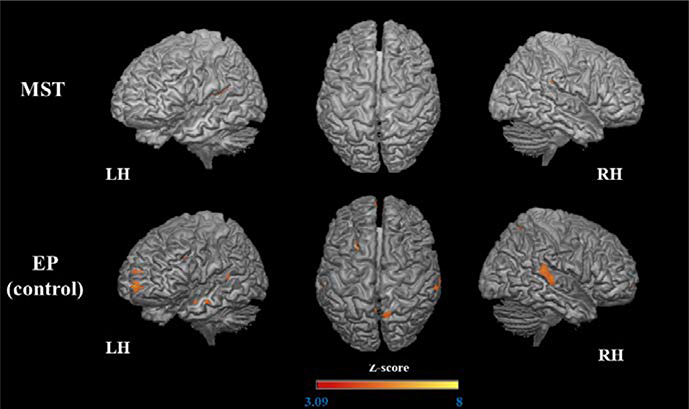
After MST, increased activation was observed in a large number of brain regions bilaterally. Specifically, a large parietal-occipital cluster spanned the bilateral precuneus, posterior cingulate cortex (PCC), inferior parietal lobule, regions of cerebellum and occipital lobe, and the right fusiform gyrus. Frontal clusters were also observed in the left orbital gyri (anterior, medial, and lateral), bilateral inferior frontal gyrus (IFG), middle frontal gyrus (MFG), left superior frontal gyrus (SFG), bilateral precentral gyrus, and left anterior cingulate cortex (ACC). We observed increased activation in additional left regions, such as temporal pole, fusiform gyrus, hippocampus and midbrain (mesencephalon), basal ganglia (including globus pallidus and caudate), and right areas, as thalamus and anterior insula. Regarding the EP group, there was increased activation in the bilateral IFG and MFG, left anterior insula, left fusiform gyrus, and bilateral occipital regions (right occipital pole, right inferior occipital gyrus, and left middle occipital gyrus) but to a far lesser extent than in the MST group (Table 2, Figure 4).
Table 2.
Increase of brain activation (post > pre) related to trained stimuli
| Comparison | Region Peak of Activation (Brodmann Area) | Side | MNI coordinates |
Cluster Size | Z | Cluster p-value1 | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| A) | ||||||||
| Precuneus (7) | L/R | 0 | −72 | 42 | 13486 | 8.17 | <0.0001 | |
| Orbital gyrus (6) | L | −40 | 54 | −4 | 8739 | 8.41 | <0.0001 | |
| MST group | IFG (44) | R | 44 | −2 | 38 | 2048 | 6.55 | <0.0001 |
| Basal ganglia | L | −10 | 18 | −2 | 375 | 4.90 | <0.0001 | |
| Temporal pole (20) | L | −58 | 2 | −36 | 216 | 4.98 | 0.0002 | |
| Insula (anterior) | R | 32 | 20 | −6 | 108 | 4.97 | 0.0017 | |
| Midbrain (mesencephalon) | L | −14 | −20 | −12 | 93 | 5.77 | 0.034 | |
| B) | ||||||||
| IFG (44/45) | R | 38 | 32 | 14 | 689 | 5.24 | <0.0001 | |
| L | −50 | 14 | 40 | 593 | 5.20 | <0.0001 | ||
| EP group | Occipital pole (18) | R | 20 | −94 | −6 | 373 | 5.25 | <0.0001 |
| Fusiform gyrus (20/37) | L | −38 | −44 | −24 | 192 | 4.68 | 0.0010 | |
| Middle occipital gyrus (18/19) | L | −30 | −92 | 12 | 158 | 5.11 | 0.0036 | |
| Inferior occipital gyrus (19) | R | 46 | −82 | 0 | 118 | 4.52 | 0.017 | |
Note.
Values corrected for multiple comparisons.
IFG: inferior frontal gyrus, PCC: posterior cingulate cortex
Figure 4.
Increased activation (post > pre) during encoding of the trained stimuli.
The 3D maps illustrated the areas that were significantly more activated after MST and EP.
Pre > Post
The MST group demonstrated decreased activation in the left posterior insula, right PCC, and superior parietal lobe. In contrast, the EP group demonstrated reduced activation in the bilateral precuneus, STG (posterior segment), paracingulate gyrus, medial frontal gyri, and ACC as well as the left superior frontal sulcus, frontal pole, IFG, anterior insula, MTG, and PCC (Table 3, Figure 5).
Table 3.
Decrease of brain activation (pre > post) related to trained stimuli
| Comparison | Region Peak of Activation (Brodmann Area) | Side | MNI coordinates |
Cluster Size | Z | Cluster p-value1 | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| A) | ||||||||
| Insula (posterior) (22) | L | −40 | −26 | 16 | 207 | 4.79 | 0.0002 | |
| MST group | PCC (31) | R | 4 | −36 | 46 | 130 | 5.12 | 0.0064 |
| STG (40) | R | 46 | −32 | 20 | 88 | 4.66 | 0.043 | |
| B) | ||||||||
| Precuneus (31) | L | −6 | −58 | 34 | 1166 | 4.97 | <0.0001 | |
| R | 10 | −60 | 60 | 200 | 6.11 | 0.0008 | ||
| STG (8/38/21/22/42) | R | 62 | −32 | 14 | 527 | 5.19 | <0.0001 | |
| L | −64 | −28 | 8. | 95 | 5.45 | 0.046 | ||
| L | −52 | 8 | −22 | 110 | 4.69 | 0.024 | ||
| EP group | Paracingulate gyrus (10) | L | −6 | 52 | 12 | 319 | 4.38 | <0.0001 |
| ACC (24) | L/R | 0 | 36 | 20 | 284 | 5.85 | <0.0001 | |
| Superior frontal sulcus (9/46) | L | −22 | 12 | 34 | 204 | 4.51 | 0.0007 | |
| Frontal pole (10/11) | L | −6 | 62 | 0 | 177 | 6.77 | 0.0018 | |
| IFG / insula (45) | L | −38 | 16 | 14 | 147 | 5.02 | 0.005 | |
Note.
Values corrected for multiple comparisons.
ACC: anterior cingulate cortex, IFG: inferior frontal gyrus, STG: superior temporal gyrus.
Figure 5.
Decreased activation (pre > post) during encoding of the trained stimuli.
The 3D maps illustrated the areas that were significantly less activated after MST and EP.
3.3.2. Activation Changes for the Untrained Stimuli
Post > Pre
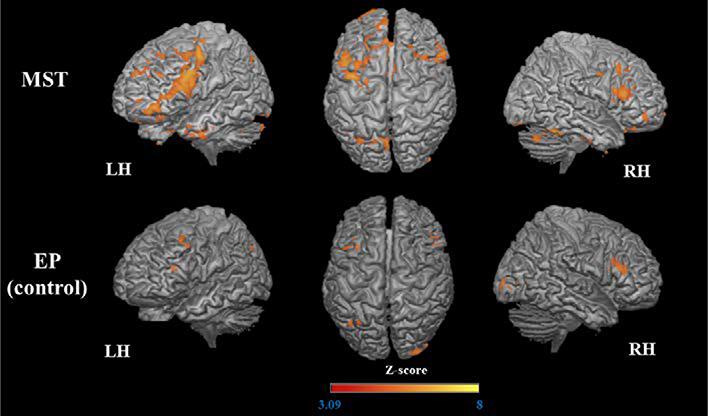
The MST group again demonstrated increased activation in a large frontal cluster that spanned the left IFG, MFG, precentral gyrus, and frontal pole. Other frontal clusters involved right IFG and MFG, bilateral ACC, bilateral medial SFG, orbital gyrus (medial, anterior and lateral portions), left border of precentral sulcus, and right precentral gyrus. There were also increases in the left parietal cortex (precuneus, PCC, and inferior parietal lobule), bilateral fusiform gyrus, cerebellum, occipital pole, and right amygdala. The EP group demonstrated increased activation in the bilateral IFG, in left MFG, anterior insula, and angular gyrus, and in the right occipital pole and orbital gyrus (Table 4, Figure 6).
Table 4.
Increase of brain activation (post > pre) related to untrained stimuli
| Comparison | Region Peak of Activation (Brodmann Area) | Side | MNI coordinates |
Cluster Size | Z | Cluster p-value1 | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| A) | ||||||||
| IFG (6) | L | −40 | 0 | 46 | 4145 | 7.12 | <0.0001 | |
| R | 56 | 24 | 16 | 427 | 5.69 | <0.0001 | ||
| Precuneus (7) | L | −8 | −70 | 38 | 949 | 5.37 | <0.0001 | |
| Fusiform gyrus (37/20) | R | 46 | −50 | −26 | 517 | 7.50 | <0.0001 | |
| L | −42 | −2 | −34 | 474 | 5.41 | <0.0001 | ||
| R | 34 | −20 | −30 | 155 | 4.70 | 0.0024 | ||
| Cerebellum | L | −46 | −74 | −34 | 446 | 5.27 | <0.0001 | |
| R | 16 | −70 | −34 | 149 | 5.08 | 0.0031 | ||
| MST Group | MFG (46/9) | R | 34 | 18 | 44 | 250 | 4.69 | <0.0001 |
| ACC (24/32) | R | 8 | 42 | −6 | 248 | 5.42 | <0.0001 | |
| Temporal pole (20) | R | 44 | 16 | −46 | 199 | 6.73 | 0.0004 | |
| Occipital pole (18/19) | R | 28 | −94 | −20 | 182 | 4.72 | 0.0008 | |
| L | −14 | −90 | −16 | 162 | 4.11 | 0.0018 | ||
| Medial SFG (6) | R/L | 0 | 4 | 60 | 179 | 5.62 | 0.0009 | |
| Inferior parietal lobule (7) | L | −32 | −70 | 44 | 167 | 4.71 | 0.0015 | |
| Amygdala | R | 14 | −2 | −16 | 127 | 4.92 | 0.0079 | |
| Medial orbital gyrus (11) | R | 22 | 34 | −22 | 123 | 4.66 | 0.0094 | |
| L | −18 | 38 | −24 | 90 | 4.35 | 0.042 | ||
| Precentral sulcus (44/6) | L | −32 | −10 | 64 | 115 | 5.34 | 0.013 | |
| R | 44 | −4 | 38 | 89 | 5.15 | 0.044 | ||
| Anterior and lateral orbital (47) | R | 38 | 46 | −8 | 110 | 4.23 | 0.016 | |
| B) | ||||||||
| IFG (44/45) | R | 44 | 30 | 16 | 325 | 5.01 | <0.0001 | |
| Occipital lobe (18/19) | R | 32 | −94 | 4 | 235 | 5.02 | 0.0003 | |
| EP group | Angular gyrus (39) | L | −38 | −64 | 38 | 173 | 4.18 | 0.0026 |
| MFG (9) | L | −34 | 18 | 46 | 117 | 4.76 | 0.021 | |
| IFG / insula (45) | L | −32 | 26 | 14 | 111 | 5.25 | 0.027 | |
| Orbial gyrus (11) | R | 2 | 44 | −24 | 103 | 5.27 | 0.037 | |
Note.
Values corrected for multiple comparisons.
ACC: anterior cingulate cortex, IFG: inferior frontal gyrus, MFG: middle frontal gyrus; SFG: superior frontal gyrus.
Figure 6.
Increased activation (post > pre) during encoding of the untrained stimuli.
The 3D maps illustrated the areas that were significantly more activated after MST and EP.
Pre > Post
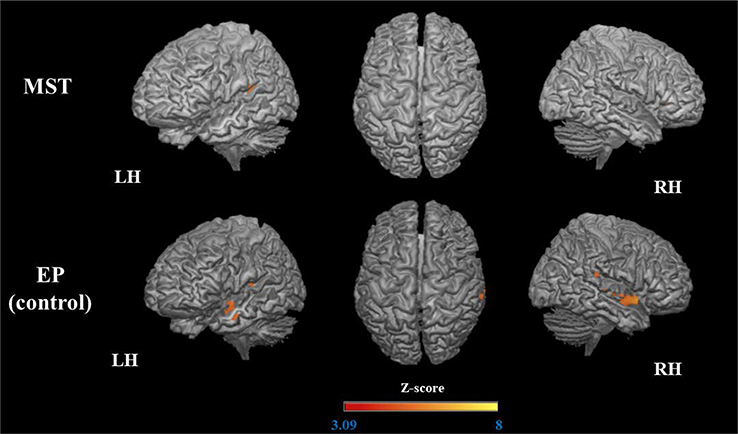
The MST group showed decreased activation in a small portion of the left medial and superior temporal gyrus (STG) (posterior segment) and right IFG. The EP group showed decreased activation in the bilateral STG (posterior, right ACC, left MTG, and posterior insula; Table 5, Figure 7).
Table 5.
Decrease of brain activation (pre > post) related to untrained stimuli
| Comparison | Region Peak of Activation (Brodmann Area) | Side | MNI coordinates |
Cluster Size | Z | Cluster p-value1 | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| A) | ||||||||
| STG (40/22) | L | −42 | −32 | 16 | 207 | 5.42 | 0.0003 | |
| MST group | ||||||||
| IFG (47) | R | 32 | 30 | 4 | 89 | 5.79 | 0.044 | |
| B) | ||||||||
| STG (21/22/38) | R | 48 | 12 | −10 | 360 | 6.74 | <0.0001 | |
| L | −48 | 2 | −10 | 193 | 4.50 | 0.0013 | ||
| R | 12 | 6 | 30 | 167 | 4.76 | 0.0034 | ||
| EP group | Insula (42) | L | −36 | −26 | 6 | 206 | 5.03 | 0.0008 |
| ACC (24) | R | 64 | −4 | −4 | 166 | 4.51 | 0.003 | |
Note.
Values corrected for multiple comparisons.
ACC: anterior cingulate cortex, IFG: inferior frontal gyrus, MFG: middle frontal gyrus; STG: superior temporal gyrus.
Figure 7.
Decreased activation (pre > post) during encoding of the untrained stimuli.
The 3D maps illustrated the areas that were significantly less activated after MST and EP.
3.4. Exploratory Analysis: Correlation between Behavior and Activation Changes
We performed an exploratory correlation analysis using all of the regions listed in Tables 1–4 by calculating percent change in the associated beta values and change in cognitive performance (only using the variables that showed significant change after intervention). We identified three significant correlations: 1) the IFG (MNI coordinate 44, −2, 38) was positively related to confidence change for the trained stimuli (r = 0.517, p = 0.04); 2) the right PCC (MNI coordinate 4, −36, 46) was positively related to accuracy for the trained stimuli (r = 0.519, p = 0.04); and 3) the left precuneus (MNI coordinate −8,−70, 38) was positively related to RT change for the untrained stimuli (r = 0.532, p = 0.04). The relationships between cognition and the PCC (r = 0.517, p = 0.06) and precuneus (r = 0.517, p = 0.06) remained significant after excluding a potential outlier (the IFG relationship was no longer significant - r = −0.33, p = 0.23).
4. Discussion
The current report on MST in individuals with a-MCI demonstrated training-induced effects in both behavior (i.e., memory test performance) and fMRI-based activation. Specifically, primary findings demonstrated 1) robust improvement in accuracy and confidence for the trained face-name associations; 2) near-transfer effects suggested by improved recognition accuracy and slower reaction times for the untrained pairs (measures that were correlated), and 3) increased activation in distributed brain regions particularly in the frontoparietal network that were highly similar for trained and untrained stimuli. Each of these findings is discussed in detail below. Critically, these changes were either absent or nominally evident in the EP control group, thereby supporting MST specific effects. Although the results reported herein are based on within-group analysis, they extend the between-group findings described in our previous report that showed MST was superior to EP (Simon et al., 2018).
4.1. Behavioral changes: training gains and transfer effects
Similar to Hampstead and colleagues’ pilot study (2008), participants demonstrated robust improvement in memory recognition accuracy and confidence in the recognition of the trained face-name pairs following MST, but not after the control (EP) intervention. The magnitude of the improvement (large effect size) may be attributed to the systematic and focused nature of our intervention, which was designed to alter the manner in which MCI individuals learn and remember face-name associations. Such findings could have meaningful functional impacts as MST could be used to help individuals with a-MCI overcome uncomfortable social interactions arising from difficulty remembering people’s names.
We provided participants with the cues for each face-name pair during training to standardize the procedures; however, real-world utility of the strategies depends on the ability to spontaneously generate cues (Hampstead et al., 2014), which we tried to address in the “generalization step” of our training. In this respect, the significant post-training improvements (medium to large effect sizes) on the untrained list are encouraging. Specifically, our findings that participants frequently reported attempting to use MST and that they demonstrated slower RT, which is consistent with the time-intensive nature of MST. Moreover, we found a significant positive correlation between accuracy and RT for the untrained stimuli in the MST but not the EP group.
4.2. Increase of activation after interventions
We identified several similarities in the patterns of activation after MST for the trained and untrained material (that were not observed after EP), which support the behavioral findings and clarify the neuroanatomical correlates of transfer effects. Particularly, we observed increase of activation in the frontoparietal network, suggested as a neural basis for cognitive control (Niendam et al., 2012, Miller and Cohen, 2001, Breukelaar et al., 2017). We posit that our MST approach engages cognitive control (i.e., the ability to regulate, coordinate, and sequence thought/actions in accordance with the current task goals; Koechlin et al., 2003) given the intentional and strategic use of the 3-step process.
For example, we observed increased MFG activation as have prior works investigating other types of strategies like the method of loci (Kondo et al., 2005) and semantic organization (Balardin et al., 2015, Miotto et al., 2006). Other frontal regions showing change, like the orbital gyrus and IFG, have also been implicated in attentional control processes, inhibitory control, and semantic processing (Badre and Wagner, 2007, Chong et al., 2008, Hampshire et al., 2010, Ridderinkhof et al., 2004). Moreover, our findings are reinforced by highly similar results of another study in this same edition in which lateral frontoparietal increases were found after MST in both patients with MCI and cognitively intact older adults (Hampstead et al., in press). Nevertheless, the EP group showed some increased activation in a subset of these regions, which raises the possibility of practice effects and/or that the EP group engaged some type of strategic processing after gaining familiarity with the task. However, there are clear differences between the randomized intervention groups that cannot be easily attributed to mere practice.
In addition and comparable to Hampstead et al., (2011), the precuneus again showed significantly increased activation after MST. This region is known to be the “mind’s eye” and critically involved in mental imagery and episodic memory retrieval (Fletcher et al., 1995, Cavanna and Trimble, 2006). It’s involvement is reasonable given our use of mental imagery and supported by the positive correlation between the regional beta-weight and RT. These results again suggest that the participants were trying to use the more time-consuming MST process, including with the untrained (i.e., novel) stimuli.
Another notables frontoparietal regions more activated after MST for both trained and untrained stimuli included the ACC, which mediates metacognitive control over multiple cognitive processes (Stuss, 2011) relevant to mnemonic strategy use, such sustained attention (Wu et al., 2017), conflict monitoring (Shapira-Lichter et al., 2018) and working memory (Lenartowicz and McIntosh, 2005). The PCC is known to play a relevant role in attention and memory retrieval (Leech and Sharp, 2014), and it was positive correlated with accuracy for the trained stimuli. Our finding is in line with the recent evidence that MST led to greater increase of activation in the PCC, in comparison to a matched exposure group (Hampstead et al., in press). The inferior parietal lobule is critically implicated in sustained attention and detection of salient new items (Singh-Curry and Husain, 2009) and has been previously reported as a critical area of activation after MST (Belleville et al., 2011). The cerebellum has been proposed as a “general-purpose co-processor” (D’Angelo and Casali, 2012) and are implicated in several cognitive tasks (Buckner, 2013), such as attention, working memory, and procedural or skill learning (Tirapu-Ustarroz et al., 2011, D’Angelo and Casali, 2012). In addition, other areas also support working memory process, such as the precentral sulcus (Kaas et al., 2007) and SFG (Li et al., 2013). Taken together these findings suggest that teaching different strategies to associate facial features to names may recruit additional attentional resources and detection of salience. Also, it is likely that keeping in mind new strategies or procedures to associate information may recruit additional working memory processes (e.g., maintenance and manipulation).
Beyond the frontoparietal regions described above, there was increase of activation in the anterior temporal pole in both stimuli type, a region that has been previously linked to face-name associations, specifically to the retrieval of newly learned people names (Tsukiura et al., 2006, Tsukiura et al., 2002). Neuropsychological studies have consistently reported that patients with right anterior temporal lobe lesions showed a loss of “person-related semantics”, or “person-identity node” (for a review see Tsukiura et al., 2006), a critical aspect of our MST. In addition, the temporal pole play a role in the social and emotional processing, including face recognition and theory of mind (Olson et al., 2007). It is relevant to highlight that our training methodology required the patients to use a verbally-based explanation (i.e., semantic organization / elaboration) that explicitly linked the feature, the reason, and the name, and sometimes rely on emotional / theory of mind aspects (e.g., the emotion expressed by face). The finding in this temporal region is in line with our previously reported results regarding between-group comparisons (Simon et al., 2018), as we found that the MST group showed greater increase of activation than EP in the left anterior temporal lobe, involving structures known to be relevant for semantic memory, face processing, social cognition, and theory of mind.
Despite these areas of overlap, we observed unique areas of increase of activation only during the trained stimuli encoding such left basal ganglia (including globus pallidus and caudate), and left mesencephalon along with hippocampus (same cluster). The increase of activation in the basal ganglia can be understood by our systematic approach in the same procedure (i.e., FRI methodology), which may contribute to activate areas relevant to non-declarative procedural memory and learning, such as basal ganglia (Foerde and Shohamy, 2011), suggesting that the MST facilitated the learning process. This finding shows the procedural learning process involved in our training, and suggests that skill learning may play a role in more complex learning, which has been previously found after MST (Belleville et al., 2011; Hampstead et al., 2011b). In addition, the activation in the midbrain (mesencephalon) area along with the left hippocampus is convergent with evidence that midbrain regions participate selectively in hippocampus-dependent process of explicit memory formation (Schott et al., 2004). Critically, our finding in line with a previous report of increased left hippocampal activation in MST following MST (Hampstead et al., 2012a).
In contrast to the MST group, the more restricted changes found following EP (for both stimuli types) likely reflect non-specific changes associated with task performance (e.g., areas involved in face and visual processing, such as occipital and fusiform cortices, respectively). The far more extensive within-group findings reported above following MST highlight the neural substrate underlying the intervention-specific effects.
4.3. Decrease of activation after interventions
Both groups showed areas of reduced activation relative to baseline, including the insula, a region frequently linked to somatomotor functions (Deen et al., 2011, Olausson et al., 2002) and the posterior segment of the STG, which is critical for auditory and language processing (Orlov et al., 2018). We hypothesize that the habituation to the task requirements (i.e. pressing the button), environment (i.e., noises during the MRI exam), and language component of the task (i.e., read the names) may have contributed to the reduced in the BOLD signal in these regions. In addition, the EP group showed reduced activation in several frontoparietal regions relevant to attention, working memory and memory retrieval, suggesting that the repetition of the task (in the absence of the training) may have facilitated the performance, and therefore recruited less cognitive resources. Moreover, the importance of the IFG for novel learning is highlighted by the reduction for trained stimuli but increased activation for untrained (novel) stimuli following MST. Reductions were also evident in the right PCC and superior parietal lobe for the untrained stimuli, regions linked to attention resources and working memory (Badre and Wagner, 2007, Leech and Sharp, 2014, Koenigs et al., 2009), which may suggest the habituation to the task, re-encoding of the stimuli, and/or increased processing efficiency via MST.
4.4. Limitations and future research
Despite the strengths of our study (e.g., randomized controlled design and presence of an active control condition) we acknowledge some limitations. It is possible that the training gains and the pattern of increase of activation observed for the trained stimuli were partially influenced by the repeated exposure of the trained stimuli, or the specific cues provided, though the general lack of repetition suppression effects combined with evidence of transfer effects suggest the active use of MST to be more likely. We cannot rule out the possibility that practice effects also contributed to improvement on the untrained list, though the group-specific differences in behavior and BOLD signal again argue against this possibility. In addition, although we cannot separate a general training effect from a specific MST effect, due to the nature of our control group, there is evidence supporting that our findings arise following MST as opposed to a general training effect. For instance, in comparison to a tightly matched active control group, MST was more effective for improving associative memory (Hampstead et al., 2012b), and led to greater increase of activation in prefrontal and parietal regions (Hampstead et al., in press) as well as the hippocampus (Hampstead et al., 2012a). However, computerized training focused in processing speed and auditory memory did not enhance frontoparietal activation in those with MCI, suggesting that our findings reflect a MST signature, rather than a “drill-practice” training approach (Rosen et al., 2012). It is relevant to highlight that the potential neural mechanisms observed are based in reverse inference (Hutzler et al., 2013) and is critical studies specifically designed to address the neural mechanism underlying the observed changes. In the current study we were not able to include biomarkers, however its inclusion are essential to reassure the underlying pathological process related to the a-MCI, and investigate specific training effects to this population. The findings reported herein are based on a relatively modest sample size, which limits conclusions and generalization to other populations requiring replication with larger groups. Despite that, we were able to replicate in a Brazilian cohort previous findings from an American sample. Finally, future studies need to further examine patients’ ability to generalize MST to everyday life.
4.5. Conclusions
In summary, the current randomized, controlled, single blind study showed that MST effectively improved memory for specific content in patients with a-MCI, with near-transfer effects to untrained stimuli. The fMRI data showed a similar pattern of brain activation after MST for both trained and untrained (‘novel’) stimuli, suggesting that the participants attempt to generalize the mnemonic strategies to new stimuli. The pattern of increase of activation observed were consistent with our MST protocol, including bilateral multiple frontoparietal regions and temporal pole, brain areas involved in cognitive control, memory retrieval, semantic organization, mental imagery, social and emotional processing / theory of mind. Critically, this pattern of activation was notably different from the pattern emerged after EP. Overall, our results add to the body of literature that MST is a valid approach to improve cognitive functioning and indicate that older adults with a-MCI show highly plastic brains that respond to cognitive oriented treatments. In addition, we extend previous findings (Hampstead et al., 2008, 2011b) in a larger sample, and in a different culture (Brazilian cohort), suggesting a good clinical applicability of this MST protocol. Finally, our data indicated that MST focused on face-name association is a relevant and efficient treatment for people with a-MCI.
Acknowledgements
The study was supported by São Paulo Research Foundation - FAPESP (2012/51699-1) and the first author (SSS) received scholarships from the Coordination for the Improvement of Higher Education Personnel/Brazil (CAPES-PROEX), Science without Borders (CAPES - number 99999.002392/2015-01), and Lemann Foundation. GFB was supported by FAPESP (2012/50329-6, 2012/50010-0) and CNPQ-Brazil during the development of this work. BMH also acknowledges VA Merit Review Award (IRX001534) and the NIH/NIA funded Michigan Alzheimer’s Disease Center (5P30AG053760) as well as R01AG058724.
We dedicate this article to Prof. Dr. Cássio M. C. Bottino (in memoriam) and are grateful to the participants who gave their time so generously to this study.
Footnotes
Conflicts of Interest
No authors has any conflict of interest.
References
- BADRE D & WAGNER AD 2007. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia, 45, 2883–901. [DOI] [PubMed] [Google Scholar]
- BAHAR-FUCHS A, WEBB S, BARTSCH L, CLARE L, REBOK G, CHERBUIN N & ANSTEY KJ 2017. Tailored and Adaptive Computerized Cognitive Training in Older Adults at Risk for Dementia: A Randomized Controlled Trial. J Alzheimers Dis, 60, 889–911. [DOI] [PubMed] [Google Scholar]
- BALARDIN JB, BATISTUZZO MC, MARTIN MAG, SATO JR, SMID J, PORTO C, SAVAGE CR, NITRINI R, AMARO E & MIOTTO EC 2015. Differences in prefrontal cortex activation and deactivation during strategic episodic verbal memory encoding in mild cognitive impairment. Front Aging Neurosci, 7, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKMANN CF, JENKINSON M & SMITH SM 2003. General multilevel linear modeling for group analysis in FMRI. Neuroimage, 20, 1052–63. [DOI] [PubMed] [Google Scholar]
- BELLEVILLE S & BHERER L 2012. Biomarkers of Cognitive Training Effects in Aging. Curr Transl Geriatr Exp Gerontol Rep, 1, 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELLEVILLE S, CLÉMENT F, MELLAH S, GILBERT B, FONTAINE F & GAUTHIER S 2011. Training-related brain plasticity in subjects at risk of developing Alzheimer’s disease. Brain, 134, 1623–34. [DOI] [PubMed] [Google Scholar]
- BENJAMINI Y & HOCHBERG Y 1995. Controlling the false discovery rate: Apractical and powerful approach to multiple testing.. Journal of the Royal Statistical Society Series B, 57, 289–300. [Google Scholar]
- BERLOT R, METZLER-BADDELEY C, IKRAM MA, JONES DK & O’SULLIVAN MJ 2016. Global Efficiency of Structural Networks Mediates Cognitive Control in Mild Cognitive Impairment. Front Aging Neurosci, 8, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREUKELAAR IA, ANTEES C, GRIEVE SM, FOSTER SL, GOMES L, WILLIAMS LM & KORGAONKAR MS 2017. Cognitive control network anatomy correlates with neurocognitive behavior: A longitudinal study. Hum Brain Mapp, 38, 631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCKNER RL 2013. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron, 80, 807–15. [DOI] [PubMed] [Google Scholar]
- CAVANNA AE & TRIMBLE MR 2006. The precuneus: a review of its functional anatomy and behavioural correlates. Brain, 129, 564–83. [DOI] [PubMed] [Google Scholar]
- CHANDLER MJ, PARKS AC, MARSISKE M, ROTBLATT LJ & SMITH GE 2016. Everyday Impact of Cognitive Interventions in Mild Cognitive Impairment: a Systematic Review and Meta-Analysis. Neuropsychol Rev, 26, 225–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHONG TT, WILLIAMS MA, CUNNINGTON R & MATTINGLEY JB 2008. Selective attention modulates inferior frontal gyrus activity during action observation. Neuroimage, 40, 298–307. [DOI] [PubMed] [Google Scholar]
- CLARE L, VAN PAASSCHEN J, EVANS SJ, PARKINSON C, WOODS RT & LINDEN DE 2009. Goal-oriented cognitive rehabilitation for an individual with Mild Cognitive Impairment: behavioural and neuroimaging outcomes. Neurocase, 15, 318–31. [DOI] [PubMed] [Google Scholar]
- D’ANGELO E & CASALI S 2012. Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Front Neural Circuits, 6, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEEN B, PITSKEL NB & PELPHREY KA 2011. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex, 21, 1498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESPINOSA A, ALEGRET M, VALERO S, VINYES-JUNQUE G, HERNANDEZ I, MAULEON A, ROSENDE-ROCA M, RUIZ A, LOPEZ O, TARRAGA L & BOADA M 2013. A longitudinal follow-up of 550 mild cognitive impairment patients: evidence for large conversion to dementia rates and detection of major risk factors involved. J Alzheimers Dis, 34, 769–80. [DOI] [PubMed] [Google Scholar]
- FLETCHER PC, FRITH CD, BAKER SC, SHALLICE T, FRACKOWIAK RS & DOLAN RJ 1995. The mind’s eye--precuneus activation in memory-related imagery. Neuroimage, 2, 195–200. [DOI] [PubMed] [Google Scholar]
- FOERDE K & SHOHAMY D 2011. The role of the basal ganglia in learning and memory: insight from Parkinson’s disease. Neurobiol Learn Mem, 96, 624–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLIS MM, GARCIA S, HAMPSTEAD BM 2016. Working memory contributes to the encoding of object location associations: Support for a 3-part model of object location memory. Behav Brain Res, 311, 192–200. [DOI] [PubMed] [Google Scholar]
- HAMPSHIRE A, CHAMBERLAIN SR, MONTI MM, DUNCAN J & OWEN AM 2010. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage, 50, 1313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMPSTEAD BM, STRINGER AY, STILLA R, SATHIAN K Mnemonic strategy training increases neocortical activation in healthy older adults and patients with mild cognitive impairment. International Psychophysiology, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMPSTEAD BM, KHOSHNOODI WY, DESHPANDE G, SATHIAN K 2016. Patterns of effective connectivity during memory encoding and retrieval differ between patients with mild cognitive impairment and healthy older adults. Neuroimage, 124, 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMPSTEAD BM, GILLIS MM & STRINGER AY 2014. Cognitive rehabilitation of memory for mild cognitive impairment: a methodological review and model for future research. J Int Neuropsychol Soc, 20, 135–51. [DOI] [PubMed] [Google Scholar]
- HAMPSTEAD BM, STRINGER AY, STILLA RF, GIDDENS M, SATHIAN K 2012a. Mnemonic strategy training partially restores hippocampal activity in patients with mild cognitive impairment. Hippocampus, 22, 1652–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMPSTEAD BM, SATHIAN K, PHILLIPS PA, AMARANENI A, DELAUNE WR, STRINGER A 2012b. Mnemonic strategy training improves memory for object location assiciations in both healthy elderly and patients with amnestic mild cognitive impairment: A randomized single-blind study. Neuropsychology, 26, 385–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMPSTEAD BM, STRINGER AY, STILLA RF, AMARANENI A 2011a. Where did I put that? Patients with amnestic mild cognitive impairment demonstrate widespread reductions in activity during the encoding of ecologically relevant object location associations. Neuropsychologia, 49, 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMPSTEAD BM, STRINGER AY, STILLA RF, DESHPANDE G, HU X, MOORE AB & SATHIAN K 2011b. Activation and effective connectivity changes following explicit-memory training for face-name pairs in patients with mild cognitive impairment: a pilot study. Neurorehabil Neural Repair, 25, 210–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMPSTEAD BM, SATHIAN K, MOORE AB, NALISNICK C & STRINGER AY 2008. Explicit memory training leads to improved memory for face-name pairs in patients with mild cognitive impairment: results of a pilot investigation. J Int Neuropsychol Soc, 14, 883–889. [DOI] [PubMed] [Google Scholar]
- HILL NT, MOWSZOWSKI L, NAISMITH SL, CHADWICK VL, VALENZUELA M & LAMPIT A 2017. Computerized Cognitive Training in Older Adults With Mild Cognitive Impairment or Dementia: A Systematic Review and Meta-Analysis. Am J Psychiatry, 174, 329–340. [DOI] [PubMed] [Google Scholar]
- HOSSEINI SM, KRAMER JH & KESLER SR 2014. Neural correlates of cognitive intervention in persons at risk of developing Alzheimer’s disease. Front Aging Neurosci, 6, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUCKANS M, HUTSON L, TWAMLEY E, JAK A, KAYE J & STORZBACH D 2013. Efficacy of cognitive rehabilitation therapies for mild cognitive impairment (MCI) in older adults: working toward a theoretical model and evidence-based interventions. Neuropsychol Rev, 23, 63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMES LE, FOGLER KA & TAUBER SK 2008. Recognition memory measures yield disproportionate effects of aging on learning face-name associations. Psychol Aging, 23, 657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEAN L, SIMARD M, WIEDERKEHR S, BERGERON ME, TURGEON Y, HUDON C, TREMBLAY I & VAN REEKUM R 2010. Efficacy of a cognitive training programme for mild cognitive impairment: results of a randomised controlled study. Neuropsychol Rehabil, 20, 377–405. [DOI] [PubMed] [Google Scholar]
- JENKINSON M, BANNISTER P, BRADY M & SMITH S 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage, 17, 825–41. [DOI] [PubMed] [Google Scholar]
- JENKINSON M & SMITH S 2001. A global optimisation method for robust affine registration of brain images. Med Image Anal, 5, 143–56. [DOI] [PubMed] [Google Scholar]
- KAAS AL, VAN MIER H & GOEBEL R 2007. The neural correlates of human working memory for haptically explored object orientations. Cereb Cortex, 17, 1637–49. [DOI] [PubMed] [Google Scholar]
- KIRWAN CB & STARK CE 2004. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus, 14, 919–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOECHLIN E, ODY C & KOUNEIHER F 2003. The architecture of cognitive control in the human prefrontal cortex. Science, 302, 1181–5. [DOI] [PubMed] [Google Scholar]
- KOENIGS M, BARBEY AK, POSTLE BR & GRAFMAN J 2009. Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci, 29, 14980–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONDO Y, SUZUKI M, MUGIKURA S, ABE N, TAKAHASHI S, IIJIMA T & FUJII T 2005. Changes in brain activation associated with use of a memory strategy: a functional MRI study. Neuroimage, 24, 1154–63. [DOI] [PubMed] [Google Scholar]
- LAKENS D 2013. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol, 4, 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEECH R & SHARP DJ 2014. The role of the posterior cingulate cortex in cognition and disease. Brain, 137, 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENARTOWICZ A & MCINTOSH AR 2005. The role of anterior cingulate cortex in working memory is shaped by functional connectivity. J Cogn Neurosci, 17, 1026–42. [DOI] [PubMed] [Google Scholar]
- LI W, QIN W, LIU H, FAN L, WANG J, JIANG T & YU C 2013. Subregions of the human superior frontal gyrus and their connections. Neuroimage, 78, 46–58. [DOI] [PubMed] [Google Scholar]
- MACHULDA MM, SENJEM ML, WEIGAND SD, SMITH GE, IVNIK RJ, BOEVE BF, KNOPMAN DS, PETERSEN RC, JACK CR 2009. Functional magnetic resonance imaging changes in amnestic and nonamnestic mild cognitive impairment during encoding and recognition tasks. J Int Neuropsychol Soc, 15(3), 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGHEE DJ, RITCHIE CW, ZAJICEK JP & COUNSELL CE 2016. A review of clinical trial designs used to detect a disease-modifying effect of drug therapy in Alzheimer’s disease and Parkinson’s disease. BMC Neurol, 16, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEWBORN CM, LINDBERGH CA & STEPHEN MILLER L 2017. Cognitive Interventions for Cognitively Healthy, Mildly Impaired, and Mixed Samples of Older Adults: A Systematic Review and Meta-Analysis of Randomized-Controlled Trials. Neuropsychol Rev. [DOI] [PubMed] [Google Scholar]
- MILLER EK & COHEN JD 2001. An integrative theory of prefrontal cortex function. Annu Rev Neurosci, 24, 167–202. [DOI] [PubMed] [Google Scholar]
- MIOTTO EC, SAVAGE CR, EVANS JJ, WILSON BA, MARTINS MG, IAKI S & AMARO E JR. 2006. Bilateral activation of the prefrontal cortex after strategic semantic cognitive training. Hum Brain Mapp, 27, 288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIENDAM TA, LAIRD AR, RAY KL, DEAN YM, GLAHN DC & CARTER CS 2012. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci, 12, 241–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLAUSSON H, LAMARRE Y, BACKLUND H, MORIN C, WALLIN BG, STARCK G, EKHOLM S, STRIGO I, WORSLEY K, VALLBO AB & BUSHNELL MC 2002. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci, 5, 900–4. [DOI] [PubMed] [Google Scholar]
- OLSON IR, PLOTZKER A & EZZYAT Y 2007. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain, 130, 1718–31. [DOI] [PubMed] [Google Scholar]
- ORLOV ND, GIAMPIETRO V, O’DALY O, LAM SL, BARKER GJ, RUBIA K, MCGUIRE P, SHERGILL SS & ALLEN P 2018. Real-time fMRI neurofeedback to down-regulate superior temporal gyrus activity in patients with schizophrenia and auditory hallucinations: a proof-of-concept study. Transl Psychiatry, 8, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPP KV, AMARIGLIO RE, DEKHTYAR M, ROY K, WIGMAN S, BAMFO R, SHERMAN J, SPERLING RA & RENTZ DM 2014. Development of a psychometrically equivalent short form of the Face-Name Associative Memory Exam for use along the early Alzheimer’s disease trajectory. Clin Neuropsychol, 28, 771–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSEN RC, CARACCIOLO B, BRAYNE C, GAUTHIER S, JELIC V & FRATIGLIONI L 2014. Mild cognitive impairment: a concept in evolution. J Intern Med, 275, 214–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSEN RC, DOODY R, KURZ A, MOHS RC, MORRIS JC, RABINS PV, RITCHIE K, ROSSOR M, THAL L & WINBLAD B 2001. Current concepts in mild cognitive impairment. Arch Neurol, 58, 1985–92. [DOI] [PubMed] [Google Scholar]
- REIJNDERS J, VAN HEUGTEN C & VAN BOXTEL M 2013. Cognitive interventions in healthy older adults and people with mild cognitive impairment: a systematic review. Ageing Res Rev, 12, 263–75. [DOI] [PubMed] [Google Scholar]
- RENTZ DM, AMARIGLIO RE, BECKER JA, FREY M, OLSON LE, FRISHE K, CARMASIN J, MAYE JE, JOHNSON KA & SPERLING RA 2011. Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia, 49, 2776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIDDERINKHOF KR, VAN DEN WILDENBERG WP, SEGALOWITZ SJ & CARTER CS 2004. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn, 56, 129–40. [DOI] [PubMed] [Google Scholar]
- RINGE WK, SAINE KC, LACRITZ LH, HYNAN LS & CULLUM CM 2002. Dyadic short forms of the Wechsler Adult Intelligence Scale-III. Assessment, 9, 254–60. [DOI] [PubMed] [Google Scholar]
- ROSEN AC, SUGIURA L, KRAMER JH, WHITFIELD-GABRIELI S, GABRIELI JD 2012. Cognitive Training Changes Hippocampal Function in Mild Cognitive Impairment: A Pilot Study. J Alzheimer Dis. 26, 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOTT BH, SELLNER DB, LAUER CJ, HABIB R, FREY JU, GUDERIAN S, HEINZE HJ & DUZEL E 2004. Activation of midbrain structures by associative novelty and the formation of explicit memory in humans. Learn Mem, 11, 383–7. [DOI] [PubMed] [Google Scholar]
- SHAPIRA-LICHTER I, STRAUSS I, OREN N, GAZIT T, SAMMARTINO F, GIACOBBE P, KENNEDY S, HUTCHISON WD, FRIED I, HENDLER T & LOZANO AM 2018. Conflict monitoring mechanism at the single-neuron level in the human ventral anterior cingulate cortex. Neuroimage, 175, 45–55. [DOI] [PubMed] [Google Scholar]
- SHERMAN DS, MAUSER J, NUNO M & SHERZAI D 2017. The Efficacy of Cognitive Intervention in Mild Cognitive Impairment (MCI): a Meta-Analysis of Outcomes on Neuropsychological Measures. Neuropsychol Rev, 27, 440–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON SS, HAMPSTEAD BM, NUCCI MP, DURAN FLS, FONSECA LM, MARTIN MGM, ÁVILA R, PORTO FHG, BRUCKI SMD, MARTINS CB, TASCONE LS, AMARO E, BUSATTO GF, BOTTINO CMC 2018. Amnestic Mild Cognitive Impairment: Evidence From a randomized Controlled Trial. Front Aging Neurosci, 10, 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON SS, YOKOMIZO JE & BOTTINO CM 2012. Cognitive intervention in amnestic Mild Cognitive Impairment: a systematic review. Neurosci Biobehav Rev, 36, 1163–78. [DOI] [PubMed] [Google Scholar]
- SINGH-CURRY V & HUSAIN M 2009. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia, 47, 1434–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH SM 2002. Fast robust automated brain extraction. Hum Brain Mapp, 17, 143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH SM, JENKINSON M, WOOLRICH MW, BECKMANN CF, BEHRENS TE, JOHANSEN-BERG H, BANNISTER PR, DE LUCA M, DROBNJAK I, FLITNEY DE, NIAZY RK, SAUNDERS J, VICKERS J, ZHANG Y, DE STEFANO N, BRADY JM & MATTHEWS PM 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23 Suppl 1, S208–19. [DOI] [PubMed] [Google Scholar]
- SPANIOL J, DAVIDSON PD, KIM AS, MOSCOVITCH M, GRADY CL 2009. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likehood estimation. Neuropsychologia, 47(8–9), 1765–1779. [DOI] [PubMed] [Google Scholar]
- STUSS DT 2011. Functions of the frontal lobes: relation to executive functions. J Int Neuropsychol Soc, 17, 759–65. [DOI] [PubMed] [Google Scholar]
- TIRAPU-USTARROZ J, LUNA-LARIO P, IGLESIAS-FERNANDEZ MD & HERNAEZ-GONI P 2011. [Cerebellar contribution to cognitive process: current advances]. Rev Neurol, 53, 301–15. [PubMed] [Google Scholar]
- TSUKIURA T, FUJII T, TAKAHASHI T, XIAO R, SUGIURA M, OKUDA J, IIJIMA T & YAMADORI A 2002. Medial temporal lobe activation during context-dependent relational processes in episodic retrieval: an fMRI study. Functional magnetic resonance imaging. Hum Brain Mapp, 17, 203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUKIURA T, MOCHIZUKI-KAWAI H & FUJII T 2006. Dissociable roles of the bilateral anterior temporal lobe in face-name associations: an event-related fMRI study. Neuroimage, 30, 617–26. [DOI] [PubMed] [Google Scholar]
- VERMEIJ A, CLAASSEN JA, DAUTZENBERG PL & KESSELS RP 2016. Transfer and maintenance effects of online working-memory training in normal ageing and mild cognitive impairment. Neuropsychol Rehabil, 26, 783–809. [DOI] [PubMed] [Google Scholar]
- WEAVER CARGIN J, COLLIE A, MASTERS C & MARUFF P 2008. The nature of cognitive complaints in healthy older adults with and without objective memory decline. J Clin Exp Neuropsychol, 30, 245–57. [DOI] [PubMed] [Google Scholar]
- WOOLRICH M 2008. Robust group analysis using outlier inference. Neuroimage, 41, 286–301. [DOI] [PubMed] [Google Scholar]
- WOOLRICH MW, BEHRENS TE, BECKMANN CF, JENKINSON M & SMITH SM 2004. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage, 21, 1732–47. [DOI] [PubMed] [Google Scholar]
- WORSLEY K 2003. Statistical analysis of activation images In: JEZZARD P, MATTHEWS P & SMITH S (eds.) Functional MRI: an Introduction to Methods. New York: Oxford University Press. [Google Scholar]
- WU D, DENG H, XIAO X, ZUO Y, SUN J & WANG Z 2017. Persistent Neuronal Activity in Anterior Cingulate Cortex Correlates with Sustained Attention in Rats Regardless of Sensory Modality. Sci Rep, 7, 43101. [DOI] [PMC free article] [PubMed] [Google Scholar]