Abstract
Background:
Leflunomide (LEF) has been considered as an alternative treatment for Takayasu arteritis (TA); however, data on its efficacy are still scanty.
Objective:
To investigate the efficacy and safety of LEF versus cyclophosphamide (CYC) for initial-onset TA.
Methods:
Initial-onset TA patients with active disease were enrolled in this research. Patients enrolled from 1 January 2009 to 31 December 2015 were treated with glucocorticoids and CYC, while patients enrolled from 1 January 2016 to 31 October 2018 received glucocorticoids and LEF. Treatment response including complete remission (CR), partial remission (PR), and effectiveness rate (ER) and side effects were evaluated at 6 and 12 months.
Results and conclusion:
In total, 92 patients were enrolled. A total of 47 patients were treated with LEF, while 45 patients were treated with CYC. The CR and ER rates were 75.55%, and 88.89% at 6 months, and 85.37% and 95.12% at 12 months in the LEF group. The CR and ER rates were 39.02% and 70.73% at 6 months, and 56.41% and 82.05% at 12 months in the CYC group. The CR rate was significantly higher in the LEF group than in the CYC group both at 6 months (75.61% versus 38.24%, p < 0.01) and 12 months (77.42% versus 53.33%, p < 0.05) after adjustment for propensity scores. The incidence of side effects in the LEF group was much lower than that in the CYC group (21.28% versus 44.44%). In conclusion, LEF provided a better treatment response, along with lower reproductive toxicity, compared with CYC in initial-onset TA.
Keywords: cyclophosphamide, leflunomide, Takayasu arteritis, treatment response
Introduction
Takayasu arteritis (TA) is a large-vessel vasculitis characterized by granulomatous inflammation of the aorta and its main branches.1 TA is commonly seen in Asian women of childbearing age, with a prevalence of 12.9–40 cases per million.2,3 Persistent inflammation and subsequent fibrosis would lead to stenosis and/or occlusion of involved arteries, which finally results in ischemic manifestations and organ dysfunction.4 Thus, timely and effective treatment against inflammation and fibrosis is essential.
Treatments of glucocorticoids (GC) combined with disease-modifying antirheumatic drugs (DMARDs) have been recommended for TA instead of monotherapy with GC in order to prolong remission and help GC tapering.5–7 In China, cyclophosphamide (CYC) is usually the first choice of DMARDs for active TA, especially in patients with important organs involved.8 The remission rate for CYC is relatively high, ranging from 40.0% to 82.1% reported in different studies.9–13 Nevertheless, gonadal and reproductive toxicity of CYC was commonly seen,12,13 which may limit its use in women of childbearing age.
Leflunomide (LEF) could inhibit cell proliferation, suppress tyrosine kinase signaling and block the production of pro-inflammatory cytokines.14–17 Data on the efficacy and safety of LEF in TA treatment are scanty, although it has been considered as an alternative DMARD for TA. In 2012, a small-sample study of 15 TA patients from South America with a mean follow-up time of 9.1 months showed that LEF (20 mg/day) could yield a favorable clinical response (80%) without discontinuation due to side effects.18 And 4 years later, five patients (41.6%) who had continued LEF treatment still demonstrated sustained remission.19 Our previous study demonstrated that LEF could lead to a quick induction (clinical response of 83.9% at 6 months) and sustained remission (clinical response of 69.6% at 12 months) of TA.20 In patients resistant to CYC, alternative treatment with LEF also yielded a favorable clinical response (86.7% at 6 months and 80.0% at 12 months).20 Thus, the underlying efficacy and good tolerability of LEF in active TA showed promise.
To our knowledge, to date there has been no large-sample controlled study reporting the efficacy and safety of LEF compared with CYC in the treatment of TA. So, we designed this prospective cohort study to investigate the efficacy and safety of LEF versus CYC for initial-onset TA in the Chinese population.
Materials and methods
Study design
This was a prospective cohort study, which was based on an ongoing prospective observational cohort, named East China Takayasu Artery (ECTA). The ECTA cohort was initially established in 2009, centered in Zhongshan Hospital, Fudan University, Shanghai, and it had satellite centers in other areas of East China. A professional and fixed team, composed of rheumatologists and radiologists, was responsible for the disease assessment and follow-up. The database was managed by specially assigned persons. The protocol for the ECTA cohort has been approved by the Ethics Committee of Zhongshan Hospital (B2013-115(3)), Fudan University. It complies with the precepts of the Declaration of Helsinki, and written informed consent was obtained from all the patients.
All patients enrolled into the ECTA cohort met the 1990 American College of Rheumatology classification criteria.21 Up to 1 November 2019, the total number of patients contained in the ECTA cohort was 815. Disease activity was assessed using the National Institutes of Health (NIH) criteria22 as the gold standard. At baseline and every 6 months, whole-body enhanced magnetic resonance angiography (MRA) was performed, instead of conventional angiography, and angiographic findings were classified according to the 1996 Numano classification.23,24 The follow-up frequency was once a month in the active phase and once every 3 months in the remission phase. Symptoms/physical signs, laboratory profiles, and imaging results were collected at each visit.
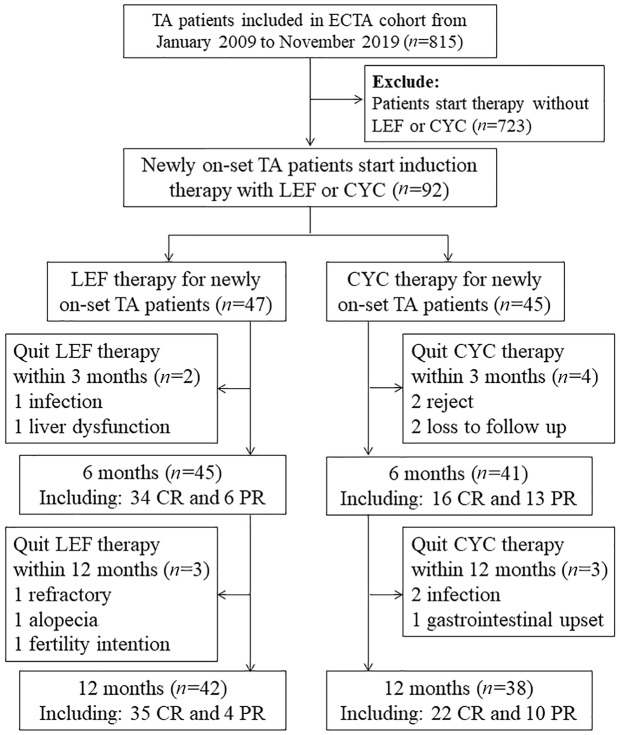
Patients enrolled in the present study had to satisfy all the following criteria: (a) with active disease (NIH score ⩾ 2); (b) initial-onset case with no use of any DMARDs in the past 3 months; (c) with induction therapy of GCs combined with LEF or CYC; (d) with no infections. Patients enrolled from 1 January 2009 to 31 December 2015 were treated with a regimen of GCs and CYC, while patients enrolled from 1 January 2016 to 31 October 2018 received a revised regimen of GCs and LEF. Prednisone was initiated (0.8–1.0 mg/kg/day, orally) for 4 weeks and tapered gradually to a maintenance dose of 0.1–0.2 mg/kg/day within the next 5 months. CYC (0.5–0.75 g/m2, intravenously) was given every 4 weeks. LEF was administered at 20 mg/day, p.o. The duration of induction was 9 months, while the total follow-up duration was 12 months. The follow-up chart is shown in Figure 1.
Figure 1.
Follow-up chart. TA, Takayasu’s arteritis; LEF, leflunomide; CYC, cyclophosphamide. Treatment response was defined as complete remission (CR), partial remission (PR), and effectiveness rate (ER). All the following criteria should be satisfied for CR: (a) no new/worsened systemic symptoms; (b) no new/worsened vascular symptoms or signs; (c) erythrocyte sedimentation rate (ESR) was normal (≤ 40 mm/hour); (d) glucocorticoid dose ≤ 15 mg/day. PR was denoted if item (b) was satisfied combined with at least one of the other three items. ER referred to the total rate of patients receiving CR or PR.
Treatment response
Treatment response was defined as complete remission (CR), partial remission (PR), and effectiveness rate (ER). All the following criteria should be satisfied for CR: (a) no new/worsened systemic symptoms; (b) no new/worsened vascular symptoms or signs; (c) erythrocyte sedimentation rate (ESR) was normal (⩽40 mm/hour); (d) GC dose ⩽15 mg/day. PR was denoted if item (b) was satisfied combined with at least one of the other three items. ER referred to the total rate of patients receiving CR or PR. Treatment response was evaluated at 6 months and 12 months. Side effects were also analyzed.
Imaging assessment
MRA was performed at baseline and every 6 months during the follow-up. Imaging progression was defined as new lesions or vascular stenosis and/or wall thickening progression ⩾20% confirmed by MRA. Imaging improvement in MRA was defined as an increase ⩾20% of the lumen of the original lesion. All of the MRA angiograms were read in a blinded manner by two radiologists who were not aware of the treatment regimen. Dispute was resolved by discussion.
Statistical analyses
All the data were analyzed using SPSS statistical software version 22.0 (IBM Corp., Armonk, NY, USA). Continuous variables are presented as mean and standard deviation for normal distributions or median and interquartile range for non-normal distributions. Between-group differences were analyzed using the t-test or the Wilcoxon rank-sum test, as appropriate. Categorical variables are presented as numbers and percentages and the χ2 test was used to compare differences between groups, or Fisher’s exact test was used when appropriate. A p value < 0.05 was set as statistically significant. To avoid selection bias, propensity score analysis was applied, given the unbalanced baseline characteristics between the LEF and CYC groups. Stata v.11.2 SE (Stata, TX, USA) was used for propensity score matching (PSM). The confounding factors for PSM included age, gender, duration, complication and ESR at baseline. Nearest neighbor matching was carried out at a 1:1 ratio, with the caliper value set at 0.05.
Results
Patients’ characteristics at baseline
Ninety-two initial-onset patients with active disease were enrolled in the present study. The median age at diagnosis was 33.00 years (22.00–43.50 years), with median disease duration of 7.50 months (2.00–48.00 months). Most patients were women (66/92, 71.74%). Headache/dizziness (35/92, 38.04%), chest pain/distress (20/92, 21.74%), and hypertension (22/92 23.91%) were the most commonly seen manifestations and complications. Type V (41/92, 44.57%) was the most common imaging type, followed by type I (19/92, 20.65%) and type IIb (11/92, 11.95%).
Among the total enrolled patients, 47 patients were treated with LEF and the other 45 cases were treated with CYC. There were no significant differences in age, sex, and disease duration between the two groups. Greater disease activity was observed at baseline in the CYC group than in the LEF group, including higher ESR levels (p = 0.01), higher C-reactive protein (CRP) levels (p < 0.01), and higher NIH scores (p = 0.02). A lower initial dose of GCs was observed in the LEF group (p < 0.01). After PSM was performed, there were no significant differences in age, sex duration, complications, imaging type, and disease activity between the two groups. Patient characteristics at baseline are shown in Table 1.
Table 1.
Patient characteristics at baseline.
| Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| LEF group (n = 47) | CYC group (n = 45) | p value | LEF group (n = 41) | CYC group (n = 34) | p value | |
| Demographic data | ||||||
| Age at diagnosis, years | 33.50 (24.50–41.00) | 31.00 (26.50–49.00) | 0.56 | 34.00 (25.00–41.00) | 30.00 (25.25–46.00) | 0.94 |
| Female, n (%) | 36 (80.00%) | 30 (73.20%) | 0.45 | 33 (80.49%) | 26 (76.47%) | 0.67 |
| Disease duration, months | 5.00 (1.00–36.00) | 12.00 (2.50–48.00) | 0.19 | 5.00 (1.00–54.00) | 15 (2.00–48.00) | 0.30 |
| Manifestation and complications, n (%) | ||||||
| Headache/dizziness | 18 (40.00%) | 17 (41.50%) | 0.89 | 18 (43.90%) | 13 (38.24%) | 0.62 |
| Chest pain/distress | 8 (17.80%) | 12 (29.30%) | 0.21 | 8 (19.51%) | 8 (23.53%) | 0.67 |
| Hypertension | 12 (26.70%) | 10 (25.00%) | 0.86 | 12 (29.30%) | 9 (26.47%) | 0.79 |
| Cardiac failure | 5 (11.10%) | 2 (4.90%) | 0.44 | 5 (12.20%) | 1 (2.94%) | 0.21 |
| Renal failure | 1 (2.20%) | 1 (2.40%) | 0.73 | 1 (2.44%) | 1 (2.94%) | 0.89 |
| Cerebral infarction | 5 (11.10%) | 1 (2.40%) | 0.21 | 5 (12.20%) | 1 (2.94%) | 0.21 |
| Imaging type, n (%) | 0.46 | 0.39 | ||||
| Type I | 13 (28.90%) | 6 (14.60%) | 13 (31.71%) | 5 (14.71%) | ||
| Type IIa | 2 (4.40%) | 1 (2.40%) | 2 (4.88%) | 1 (2.94%) | ||
| Type IIb | 5 (11.10%) | 6 (14.60%) | 5 (12.20%) | 5 (14.71%) | ||
| Type III | 3 (6.70%) | 4 (9.80%) | 3 (7.32%) | 4 (11.76%) | ||
| Type IV | 1 (2.2%) | 4 (9.80%) | 0 (0.00%) | 2 (5.89%) | ||
| Type V | 21 (46.70%) | 20 (48.80%) | 18 (43.90%) | 17 (50.00%) | ||
| Disease activity assessment | ||||||
| ESR, mm/H | 24.00 (9.00–54.25) | 45.50 (24.75–69.75) | 0.01* | 25.00 (9.00–54.50) | 41.50 (23.25–57.50) | 0.09 |
| CRP, mg/l | 5.50 (1.40–24.25) | 16.20 (7.50–41.90) | <0.01* | 5.79 (1.40–24.58) | 14.30 (6.50–34.65) | 0.05 |
| IL-6, pg/ml | 5.00 (2.10–9.80) | 6.80 (2.28–11.45) | 0.47 | 5.10 (2.18–9.83) | 5.20 (2.00–12.65) | 0.75 |
| NIH score, n (%) | 0.02* | 0.09 | ||||
| 2 | 21 (46.70%) | 11 (26.80%) | 17 (41.46%) | 9 (26.47%) | ||
| 3 | 19 (42.20%) | 15 (36.60%) | 19 (46.34%) | 14 (41.18%) | ||
| 4 | 5 (11.10%) | 15 (36.60%) | 5 (12.20%) | 11 (32.35%) | ||
| GC dosage, mg/day | 30.00 (20.00–40.00) | 40.00 (40.00–50.00) | <0.01* | 30.00 (22.50–40.00) | 40.00 (40.00–50.00) | <0.01* |
CRP, C-reactive protein; CYC, cyclophosphamide; ESR, erythrocyte sedimentation rate; GC, glucocorticoids; IL-6, interleukin-6; LEF, leflunomide; NIH, National Institutes of Health.
Imaging results: patients were grouped according to the angiographic classification of the International TA Conference in Tokyo (1996) based on lesion distribution: type I, branches of the aortic arch; IIa, ascending aorta, aortic arch, and its branches; IIb, ascending aorta, aortic arch, its branches, and thoracic descending aorta; III, thoracic descending aorta, abdominal aorta, and/or renal arteries; IV, abdominal aorta and/or renal arteries; V, combined features of IIb and IV; p value: comparison between the LEF group and CYC group, p value < 0.05 (*) was considered to indicate statistical significance.
Treatment response at 6 months
At the end of 6 months of treatment, significant improvements in disease activity compared with baseline, including lower ESR levels and lower NIH scores, were observed (p < 0.01) in the LEF group. The dose of GCs was successfully tapered to 15.00 mg/day (10.63–15.00 mg/day) (p < 0.01). In the CYC group, the cumulative dose of CYC was 4.20 g (3.30–4.80 g) at the end of 6 months. Similar improvements in disease activity compared with baseline were also demonstrated in the CYC group (p < 0.01) at the end of 6 months. The daily dose of GCs was decreased to 15 mg (12.50–16.25 mg) (p < 0.01) (Table 2).
Table 2.
Changes from baseline in inflammatory index and disease activity at 6 months and 12 months.
| LEF group |
CYC group |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | p value | Baseline | 6 months | 12 months | p value | |
| (n = 47) | (n = 45) | (n = 42) | (n = 45) | (n = 41) | (n = 38) | |||
| ESR, mm/H | 24.00 | 9.00 | 11.00 | <0.01* | 45.50 | 13.00 | 11.50 | <0.01* |
| (9.00–54.25) | (3.75–17.00) | (4.50–20.50) | (24.75–69.75) | (4.00–21.00) | (6.25–33.25) | |||
| CRP, mg/l | 5.50 | 2.20 | 2.50 | 0.06 | 16.20 | 6.00 | 6.65cc | <0.01* |
| (1.40–24.25) | (0.70–8.30) | (0.65–9.90) | (7.50–41.90) | (0.98–11.25) | (1.10–21.80) | |||
| IL-6, pg/ml | 5.00 | 5.35 | 5.60 | 0.89 | 6.80 | 3.10 | 4.00 | 0.18 |
| (2.10–9.80) | (2.58–6.83) | (2.80–8.10) | (2.28–11.45) | (2.30–5.80) | (2.45–6.95) | |||
| NIH score ⩽1, n (%) | 0 | 42 (93.33%) | 40 (91.5%) | <0.01* | 0 | 35 (85.37%) | 35 (89.74%) | <0.01* |
| GC dosage, mg/day | 30.00 | 15.00 | 10.00 | <0.01* | 40.00 | 15.00 | 10.00 | <0.01* |
| (20.00–40.00) | (10.63–15.00) | (10.00–10.00) | (40.00–50.00) | (12.50–16.25) | (10.00–15.00) | |||
CRP, C-reactive protein; CYC, cyclophosphamide; ESR, erythrocyte sedimentation rate; GC, glucocorticoids; IL-6, interleukin 6; LEF, leflunomide; NIH, National Institutes of Health.
p Value: comparison among baseline, 6 months and 12 months, p value < 0.05 (*) was considered to indicate statistical significance.
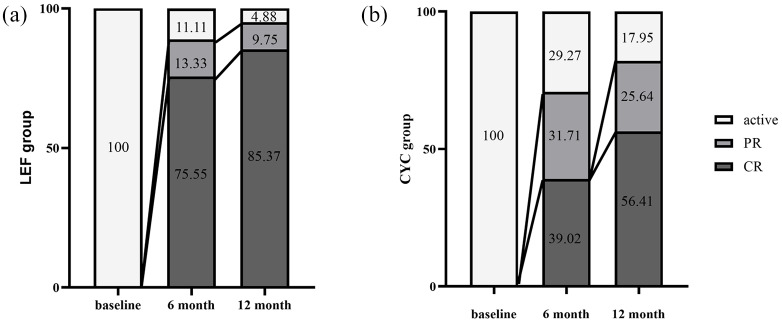
The overall CR, PR, and ER rates at 6 months were 58.14%, 22.09%, and 80.23%, respectively. In the LEF group, the CR, PR, and ER rates were 75.55%, 13.33%, and 88.89%, while in the CYC group, the CR, PR, and ER rates were 39.02%, 31.71%, and 70.73%, respectively. Higher CR (p < 0.01) and ER rates (p = 0.04) were demonstrated in the LEF group compared with the CYC group. Lower PR rates (p = 0.04) were observed in the LEF group. After adjustment for propensity scores, the LEF group still reached a higher CR rate (75.61% versus 38.24%, p < 0.01) and lower PR rate (12.20% versus 32.35%, p = 0.03) compared with the CYC group. There were no significant differences in ER rate between the two groups after PSM (Table 3 and Figure 2).
Table 3.
Relative risks (95% CI) of CR, PR, and ER at 6 months and 12 months.
| 6 Months |
12 Months |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before matching | After matching | Before matching | After matching | |||||||||
| LEF group (n = 45) | CYC group (n = 41) | p value | LEF group (n = 41) | CYC group (n = 34) | p value | LEF group (n = 42) | CYC group (n = 38) | p value | LEF group (n = 31) | CYC group (n = 30) | p value | |
| CR, n (%) | 34 (75.55%) | 16 (39.02%) | <0.01 | 31 (75.61%) | 13 (38.24%) | <0.01 | 35 (85.37%) | 22 (56.41%) | <0.01* | 24 (77.42%) | 16 (53.33%) | <0.05 |
| (95% CI) | (26.2–69.0%) | (48.2–79.6%) | (62.47–88.75%) | (21.90–54.58%) | (42.8–90.6%) | (49.1–82.1%) | (62.70–92.14%) | (35.48–71.18%) | ||||
| RR | 1 | 0.21 | 1 | 0.20 | 1 | 0.22 | 1 | 0.33 | ||||
| (95% CI) | (0.08–052) | (0.07–0.54) | (0.08–0.65) | (0.11–1.01) | ||||||||
| PR, n (%) | 6 (13.33%) | 13 (31.71%) | 0.04 | 5 (12.20%) | 11 (32.35%) | 0.03 | 4 (9.75%) | 10 (25.64%) | 0.06 | 4 (12.90%) | 10 (33.33%) | 0.06 |
| (95% CI) | (9.3–47.9%) | (6.–32.3%) | (2.18–22.22%) | (17.23–48.07%) | (3.1–46.9%) | (5.55–32.85%) | (1.1–24.7%) | (16.44–50.22%) | ||||
| RR | 1 | 3.02 | 1 | 3.44 | 1 | 3.19 | 1 | 3.38 | ||||
| (95% CI) | (1.02–8.91) | (1.06–11.20) | (0.91–11.22) | (0.92–12.33) | ||||||||
| ER, n (%) | 40 (88.89%) | 39 (70.73%) | 0.04 | 36 (87.80%) | 24 (70.59%) | 0.06 | 39 (95.12%) | 32 (82.05%) | 0.08 | 28 (90.32%) | 26 (86.67%) | 0.71 |
| (95% CI) | (58–94.4%) | (71.1–95.5%) | (77.78–97.81%) | (55.27–85.91) | (77.7–105.7%) | (67.8–94.8%) | (79.91–100.73%) | (74.51–98.83%) | ||||
| RR | 1 | 0.3 | 1 | 0.33 | 1 | 0.23 | 1 | 0.70 | ||||
| (95% CI) | (0.10–0.95) | (0.10–1.10) | (0.05–1.21) | (0.14–3.41) | ||||||||
CI, confidence interval; CR, complete remission; CYC, cyclophosphamide; ER, effectiveness rate; LEF, leflunomide; PR, partial remission; RR, relative risk.
p Value: comparison between the LEF group and CYC group, p value < 0.05 (*) was considered to indicate statistical significance.
Figure 2.
Treatment response at 6 months and 12 months. Treatment response was defined as complete remission (CR), partial remission (PR), and effectiveness rate (ER). All the following criteria should be satisfied for CR: (a) no new/worsened systemic symptoms; (b) no new/worsened vascular symptoms or signs; (c) erythrocyte sedimentation rate (ESR) was normal (⩽40 mm/hour); (d) glucocorticoid dose ⩽15 mg/day. PR was denoted if item (b) was satisfied combined with at least one of the other three items. ER referred to the total rate of patients receiving CR or PR.
Treatment response at 12 months
In the LEF group, the disease activity status observed at 12 months, including ESR, CRP, interleukin (IL)-6 levels, and NIH score, was similar to that at 6 months. The daily dose of GCs was 10.00 mg (10.00–10.00 mg) at 12 months, which was significantly lower than that at 6 months (p < 0.01). In the CYC group, the cumulative dose of CYC was 6.20 g (5.55–7.00 g). The levels of ESR and daily dose of GCs were significantly lower than those at 6 months (p < 0.01) (Table 2). In the CYC group, after disease remission was achieved, nine (34.62%) patients changed to azathioprine, eight (30.77%) changed to LEF, six (23.08%) changed to methotrexate, two (7.09%) changed to cyclosporin and one (3.85%) changed to thalidomide.
The overall CR, PR, and ER rates of enrolled patients at 12 months was 71.25%, 17.50%, and 88.75%, respectively. In the LEF group, the CR, PR, and ER rates were 85.37%, 9.75%, and 95.12%, respectively. In the CYC group, the CR, PR, and ER rates were 56.41%, 25.64%, and 82.05%, respectively. The LEF group reached a higher CR rate than the CYC group at the end of 12 months (p < 0.01). After adjustment for propensity scores, the LEF group still reached a higher CR rate (77.42% versus 53.33%, p < 0.05). There were no significant differences in PR and ER rates between the two groups before and after PSM (Table 3 and Figure 2).
Imaging assessment
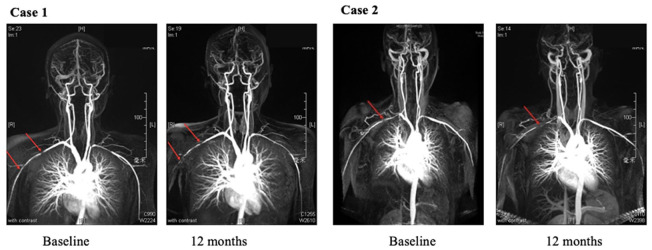
At 6 months, four (8.88%) cases in the LEF group and eight (19.51%) cases in the CYC group demonstrated imaging progression. Stable imaging results were observed in the other patients. At 12 months, imaging progression was observed in three (7.32%) cases in the LEF group and six (15.38%) cases in the CYC group (Figure 3). There were no significant differences in imaging progression rate between the two groups either at 6 months or at 12 months.
Figure 3.
Imaging progressions in different treatment group during the 12 months follow-up. Case 1 was a 19 years old woman treated with leflunomide, imaging progressions were shown at the right subclavian artery (red arrows) at 12 months. Case 2 was a 32 years old woman treated with cyclophosphamide, and suffered from worsen lesion at right subclavian artery (red arrows) at 12 months.
Safety
A total of 10/47 (21.28%) patients in the LEF group had side effects. The most common side effect was infection: there were five cases of infection, including four pulmonary infections and one urinary infection. One patient who suffered from a pulmonary infection at 2 months quit LEF therapy. Hair loss was found in two cases, and one case quit LEF treatment after 6 months and then turned to methotrexate. One patient complained of diarrhea after 6 months of treatment, which was cured in a few days. Mild liver dysfunction (liver enzymes < 3 times normal levels) was observed in one case after 2 months of LEF treatment. After this patient quit LEF treatment, liver enzymes decreased to normal levels. Menstrual disorder was seen in one patient after 6 months of treatment.
In the CYC group, 20/45 (44.44%) patients had side effects. The most commonly seen side effect was gastrointestinal reaction (17/45, 37.78%, mainly nausea, vomiting, loss of appetite in the first 3–5 days post CYC therapy), and menstrual disorder (12/45, 26.67%). Other side effects were malaise (9/45, 20%), myelosuppression (5/45, 11.11%), and infection (9/45, 20%, pulmonary infection in six cases, urinary tract infection in one case and skin infection in one case). Most side effects were tolerable and did not interrupt induction treatment, except that two patients stopped CYC treatment due to pulmonary infection and one patient quit CYC because of intolerable malaise and vomiting. Symptoms were relieved after stopping CYC.
Discussion and conclusion
TA is a rare disease with relatively low incidence. Evidence of TA treatment has come mostly from small-sample open-label studies. To our knowledge, this study was the largest-sample prospective cohort study to evaluate the efficacy of LEF versus CYC for TA treatment. To avoid selection bias, which might have affected conclusions, we only enrolled initial-onset TA for analysis and PSM analysis was also performed. Our data indicated that: (a) LEF had favorable treatment response both at 6 months (CR 75.55%, ER 88.89%) and 12 months (CR 85.37%, ER 95.12%); (b) LEF provided better treatment response compared with CYC (higher CR rate in the LEF group both at 6 months and 12 months after adjustment for propensity scores); (c) LEF had better tolerability and lower reproductive toxicity. Thus, LEF may become an alternative to CYC for initial-onset TA in the Chinese population.
For years CYC has been widely used as the first line DMARD for TA treatment in the Chinese population. The efficacy of CYC for initial TA patients on the present study was 70.73% at 6 months and 82.05% at 12 months, which was in accordance with former reports.12,13 Although hemorrhagic cystitis due to CYC treatment has commonly been seen in western countries,9,11 its incidence is relatively low in the Chinese population. The biggest contraindication was the reproductive toxicity, when CYC was administrated to childbearing women. In the present study, 26.67% patients suffered from menstrual disorder, which was in accord with previous research.12,20 Finding an alternative treatment with equivalent efficacy, but with less reproductive toxicity was very important to improve the quality of life for young female patients.
LEF may be an alternative immunosuppressive agent for TA treatment, as it plays important roles in anti-inflammation and anti-proliferation.14–17 LEF has been successfully used in rheumatoid arthritis, lupus nephritis, and small vessel vasculitis.25–29 In giant cell arteritis, which is also a type of large-vessel vasculitis with similar characteristics to TA, LEF treatment showed promising effects as a steroid-sparing agent and had a lower relapse rate, lower cumulative GC dose, and good safety.30 However, evidence from large-sample, prospective, controlled studies supporting its efficacy and safety in TA is still lacking.
Our study indicated that LEF treatment could significantly improve the inflammatory index and reduce the daily GC dose after 6 months of treatment and have this improvement sustained to 12 months. The CR and ER rates were 75.55% and 88.89% at 6 months and 85.37% and 95.12% at 12 months, which were higher than those in the CYC group. These data supported the efficacy of LEF in TA treatment and were similar to those reported in one South American study.18,19 What is more, the effect of LEF was superior to CYC with a higher CR rate both at 6 months and 12 months before and after PSM analysis. Thus, LEF is a promising alternative DMARD for initial-onset TA in the Chinese population.
The incidence of side effects of LEF was much lower than that of CYC (21.28% versus 44.44%), and most side effects were tolerable. Importantly, only one patient (2.13%) treated with LEF complained of menstrual disorders, while 12/45 (26.67%) patients treated with CYC suffered from menstrual disorders. So LEF showed good tolerability and fewer effects on reproduction in TA treatment, which is very important for women of childbearing age. To date, no evidence has directly shown that LEF increased the risks of adverse pregnancy outcomes and congenital abnormalities in humans,31–33 although it has been confirmed to be teratogenic in rodents.34 LEF has a long half-life of 14–15 days, with its serum metabolite detectable even after 2-year discontinuation.35 Thus, according to the recommendation in rheumatoid arthritis and other rheumatic diseases, LEF should be discontinued 2 years before a planned pregnancy.36–38
There are several limitations of the study. First, the follow-up time was relatively short and a longer-time study was needed in the future to confirm the maintained efficacy of LEF for TA. Second, only initial-onset TA patients were enrolled in the study. In a further study, more patients including those refractory cases should be enrolled to evaluate the efficacy of LEF in the treatment of TA.
In conclusion, the present study was the first prospective cohort study to evaluate the efficacy of LEF versus CYC for TA treatment. Our data indicated that LEF provided a better treatment response, along with better tolerability and lower reproductive toxicity, compared with CYC in initial-onset TA. Thus, LEF may become a promising alternative treatment for initial-onset TA in the Chinese population. A further random, double-blind, controlled study would be appropriate to confirm the conclusion.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was sponsored by the National Natural Science Foundation of China (NSFC 81601398; NSFC 81771730).
ORCID iD: Jiang Lindi  https://orcid.org/0000-0001-7747-7788
https://orcid.org/0000-0001-7747-7788
Contributor Information
Sun Ying, Department of Rheumatology, Zhongshan Hospital, Fudan University, Shanghai, P. R. China.
Cui Xiaomeng, Department of Rheumatology, Zhongshan Hospital, Fudan University, Shanghai, P. R. China.
Dai Xiaomin, Department of Rheumatology, Zhongshan Hospital, Fudan University, Shanghai, P. R. China.
Lin Jiang, Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai, P. R. China.
Lv Peng, Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai, P. R. China.
Ma Lili, Department of Rheumatology, Zhongshan Hospital, Fudan University, Shanghai, P. R. China.
Chen Rongyi, Department of Rheumatology, Zhongshan Hospital, Fudan University, Shanghai, P. R. China.
Ji Zongfei, Department of Rheumatology, Zhongshan Hospital, Fudan University, Shanghai, P. R. China.
Chen Huiyong, Department of Rheumatology, Zhongshan Hospital, Fudan University, Shanghai, P. R. China.
Jiang Lindi, Department of Rheumatology, Zhongshan Hospital, Fudan University, No 180 Fenglin Road Shanghai, 200032, P. R. China Centre of Evidence-based Medicine, Zhongshan Hospital, Fudan University, Shanghai, P. R. China.
References
- 1. Skeik N, Ostertag-Hill CA, Garberich RF, et al. Diagnosis, management, and outcome of aortitis at a single center. Vasc Endovasc Surg 2017; 51: 470–479. [DOI] [PubMed] [Google Scholar]
- 2. Toshihiko N. Current status of large and small vessel vasculitis in Japan. Int J Cardiol 1996; 54: S91–S98. [DOI] [PubMed] [Google Scholar]
- 3. Birlik M, Kucukyavas Y. Epidemiology of Takayasu’s arteritis in Turkey. Clin Exp Rheumatol 2016; 34: S33–S39. [PubMed] [Google Scholar]
- 4. Hallahan CW, Giordano J, Leavitt RY, et al. Takayasu arteritis. Ann Intern Med 1994; 120: 919–929. [DOI] [PubMed] [Google Scholar]
- 5. Makasimowicz-McKinnon K, Clark TM, Hoffman GS. Limitations of therapy and a guarded prognosis in an American cohort of Takayasu arteritis patients. Arthritis Rheum 2007; 56: 1000–1009. [DOI] [PubMed] [Google Scholar]
- 6. Agueda AF, Monti S, Luqmani RA, et al. Management of Takayasu arteritis: a systematic literature of informing the 2018 update of the EULAR recommendation for the management of large vessel vasculitis. RMD Open 2019; 5: e001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hellmich B, Agueda A, Monti S, et al. 2018 update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. Epub ahead of print 3 July 2019. DOI: 10.1136/annrheumdis-2019-215672. [DOI] [PubMed] [Google Scholar]
- 8. Dai X, Dong Z, Chen S, et al. Chinese expert investigation on diagnosis and disease activity evaluation in Takayasu’s arteritis. Fudan Univ J Med Sci 2017; 44: 127–133. [Google Scholar]
- 9. Stern S, Clemente G, Reiff A, et al. Treatment of pediatric Takayasu arteritis with infliximab and cyclophosphamide: experience from an American-Brazilian cohort study. J Clin Rheumatol 2014; 20: 183–187. [DOI] [PubMed] [Google Scholar]
- 10. Hahn D, Thomson PD, Kala U, et al. A review of Takayasu’s arteritis in children in Gauteng. South Africa Periatr Nephrol 1998; 12: 668–675. [DOI] [PubMed] [Google Scholar]
- 11. de Franciscis S, Serra R, Luongo Am, Sabino G, et al. The management of Takayasu’s arteritis: personal experience. Ann Vasc Surg 2007; 21: 754–760. [DOI] [PubMed] [Google Scholar]
- 12. Sun Y, Ma L, Ma L, et al. Cyclophosphamide could be a better choice than methotrexate as induction treatment for patients with Takayasu’s arteritis. Rheumatol Int 2017; 37: 2019–2026. [DOI] [PubMed] [Google Scholar]
- 13. Sun Y, Ma L, Chen H, et al. Analysis of predictive factors for treatment resistance and disease relapse in Takayasu’s arteritis. Clin Rheumatol 2018; 37: 2789–2795. [DOI] [PubMed] [Google Scholar]
- 14. Siemasko KF, Chong AS, Williams JW, et al. Regulation of B cell function by the immunosuppressive agent leflunomide. Transplantation 1996; 61: 635–642. [DOI] [PubMed] [Google Scholar]
- 15. Xu X, Williams JW, Bremer EG, et al. Inhibition of protein tyrosine phosphorylation in T cells by a novel immunosuppressive agent, leflunomide. J Biol Chem 1995; 270: 12398–12403. [DOI] [PubMed] [Google Scholar]
- 16. Manna SK, Mukhopadhyay A, Aggarwal BB. Leflunomide suppresses TNF-induced cellular responses: effects on NF-kappa B, activator protein-1, c-Jun N-terminal protein kinase and apoptosis. J Immunol 2000; 165: 5962–5969. [DOI] [PubMed] [Google Scholar]
- 17. Burger D, Begue-Pastor N, Benavent S, et al. the active metabolite of leflunomide, A77 1726, inhibits the production of prostaglandin E 2, matrix metalloproteinase 1 and interleukin 6 in human fibroblast-like synoviocytes. Rheumatology (Oxford) 2003; 42: 89–96. [DOI] [PubMed] [Google Scholar]
- 18. de Souza AW, da Silva MD, Machado LS, et al. Short-term effect of leflunomide in patients with Takayasu’s arteritis: an observational study. Scand J Rheumatol 2012; 41: 227–230. [DOI] [PubMed] [Google Scholar]
- 19. de Souza AW, de Almeida Agustinelli R, de Cinque Almeida H, et al. Leflunomide in Takayasu arteritis – a long term observational study. Rev Bras Rheumatol 2016; 56: 371–375. [DOI] [PubMed] [Google Scholar]
- 20. Cui X, Dai X, Yang C, et al. Efficacy and safety of leflunomide treatment in Takayasu arteritis: case series form the East China cohort. Semin Arthritis Rheum. Epub ahead of print 13 June 2019. DOI: 10.1016/j.semarthrit.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 21. Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 1990; 33: 129–134. [DOI] [PubMed] [Google Scholar]
- 22. Kerr GS, Hallahan CW, Giordano J, et al. Takayasu arteritis. Ann Intern Med 1994; 120: 919–929. [DOI] [PubMed] [Google Scholar]
- 23. Sun Y, Ma L, Ji Z, et al. Value of whole-body contrast-enhanced magnetic resonance angiography with vessel wall imaging in quantitative assessment of disease activity and follow-up examination in Takayasu’s arteritis. Clin Rheumatol 2016; 35: 685–693. [DOI] [PubMed] [Google Scholar]
- 24. Hata A, Noda M, Moriwaki R, et al. Angiographic findings of Takayasu arteritis: new classification. Int J Cardiol 1996; 54: S155–S163. [DOI] [PubMed] [Google Scholar]
- 25. Calvo Alen J, Perez T, Romero Yuste S, et al. Efficacy and safety of combined therapy with synthetic disease-modifying antirheumatic drugs in rheumatoid arthritis: systematic literature review. Rheumatol Chin. Epub ahead of print 18 September 2018. DOI: 10.1016/j.reuma.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 26. Liu Y, Xiao J, Shi X, et al. Immunosuppressive agents versus steroids in the treatment of IgA nephropathy-induced proteinuria: a meta-analysis. Exp Ther Med 2016; 11: 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Remer CF, Weisman MH, Wallace DJ. Benefits of leflunomide in systemic lupus erythematosus: a pilot observational study. Lupus 2001; 10: 480–483. [DOI] [PubMed] [Google Scholar]
- 28. Stiegler JD, Sami N. Successful treatment of cutaneous small vessel vasculitis with leflunomide. JAMA Dermatol 2017; 153: 940–942. [DOI] [PubMed] [Google Scholar]
- 29. Metzler C, Miehle N, Manger K, et al. Elevated relapse rate under oral methotrexate versus leflunomide for maintenance of remission in Wegener’s granulomatosis. Rheumatology (Oxford) 2007; 46: 1087–1091. [DOI] [PubMed] [Google Scholar]
- 30. Hocevar A, Jese R, Rotar Z, et al. Does leflunomide have a role in giant cell arteritis? An open label study. Clin Rheumatol. Epub ahead of print 6 August 2018. DOI: 10.1007/s10067-018-4232-x. [DOI] [PubMed] [Google Scholar]
- 31. Berard A, Zhao JP, Shui I, et al. Leflunomide use during pregnancy and the risk of adverse pregnancy outcomes. Ann Rheum Dis 2018; 77: 500–509. [DOI] [PubMed] [Google Scholar]
- 32. Chambers CD, Johnson DL, Robinson LK, et al. Birth outcomes in women who have taken leflunomide during pregnancy. Arthritis Rheum 2010; 62: 1494–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cassina M, Johnson DL, Robinson LK, et al. Pregnancy outcome in women exposed to leflunomide before or during pregnancy. Arthritis Rheum 2012; 64: 2085–2094. [DOI] [PubMed] [Google Scholar]
- 34. Fukushima R, Kanamori S, Hirashiba M, et al. Teratogenicity study of the dihydroorotate-dehydrogenase inhibitor and protein tyrosine kinase inhibitor Leflunomide in mice. Reprod Toxicol 2007; 24: 310–316. [DOI] [PubMed] [Google Scholar]
- 35. Prakash A, Jarvis B. Leflunomide: a review of its use in active rheumatoid arthritis. Drugs 1999; 58: 1137–1164. [DOI] [PubMed] [Google Scholar]
- 36. Gerosa M, Schioppo T, Meroni PL. Challenges and treatment options for rheumatoid arthritis during pregnancy. Expert Opin Pharmacother 2016; 17: 1539–1547. [DOI] [PubMed] [Google Scholar]
- 37. Gotestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis 2016; 75: 795–810. [DOI] [PubMed] [Google Scholar]
- 38. Weber-Schoendorfer C, Beck E, Tissen-Diabate T, et al. Leflunomide – a human teratogen? A still not answered question. An evaluation of the German Embryotox pharmacovigilance database. Reprod Toxicol 2017; 71: 101–107. [DOI] [PubMed] [Google Scholar]