Abstract
Background:
Current availability of several biologic treatments for severe asthma makes it possible to choose the most appropriate for each patient. Sometimes a good percentage of patients with severe asthma may be eligible for biologics that target either the allergic phenotype or the eosinophilic one, but not all respond to that selected as first choice.
The aim of our real-life study was to assess whether, for patients with severe eosinophilic allergic asthma, not previously controlled by the anti-IgE omalizumab, the shift to another biologic targeting interleukin-5, such as mepolizumab, may represent a good therapeutic choice.
Methods:
A total of 41 consecutive patients with severe, persistent allergic, eosinophilic asthma, uncontrolled despite treatment with omalizumab, were enrolled in seven certified Clinical Respiratory Units of Southern Italy (Catania, Catanzaro, Foggia, Bari, Palermo, and two University Respiratory Units of Naples) and shifted to mepolizumab without a wash-out period. Data at baseline, after at least 12 months of therapy with omalizumab, and after at least 12 months of treatment with mepolizumab were collected.
Results:
After at least 12 months of therapy with mepolizumab, patients experienced a significant decrease in the number of exacerbations/year (5.8 ± 1.8 versus 0.7 ± 0.9, p < 0.0001), an increment of asthma control test score (12 ± 2.7 versus 21.9 ± 2.7, p < 0.0001), an increase in pre-bronchodilator forced expiratory volume in 1 s (1.56 ± 0.45 l versus 1.86 ± 0.52 l, p < 0.0001), and a reduction of blood eosinophils (584 ± 196 cells/µl versus 82 ± 56 cells/µl, p < 0.0001). The percentage of patients who were dependent on corticosteroids significantly decreased from 46% at baseline to 5% during treatment with mepolizumab.
Conclusion:
Results of our real-life multicentric experience confirms that the shift to mepolizumab could be a good therapeutic strategy in severe eosinophilic allergic asthma not previously controlled by omalizumab.
The reviews of this paper are available via the supplemental material section.
Keywords: severe asthma, omalizumab, mepolizumab, switching, real life
Introduction
More than 300 million people are affected by asthma worldwide.1 The majority of patients with asthma can be effectively treated with currently available medications, but a substantial subgroup exists who remains difficult to treat. These patients are often candidates for biological therapies; however, not all people with severe asthma can benefit from the same biological drug, and physicians should carefully stratify patients for the selection of an appropriate biologic agent. The goal would be to implement a personalized approach, but we can currently only phenotype/endotype patients with asthma and use a targeted therapy that could result as a winning, but also sometimes as a losing strategy.
At the moment, few biologics directed to the T2 endotype are available for severe asthma in Italy, such as those that target immunoglobulin (Ig)E (omalizumab), and those that target interleukin (IL)-5 (mepolizumab) or its receptor (benralizumab). In addition, some patients may be treated with allergen-specific immunotherapy (SIT), which is the only causal treatment option for allergic asthma. According to Global Initiative for Asthma guidelines, a forced expiratory volume (FEV1) > 70% is required for specific immunotherapy.2 It has been recently reported that SIT can be combined with biological treatments, especially after asthma control has been achieved.3
Although it appears easy to choose among the available biologics, some patients with severe asthma present overlapping features that may benefit from different treatments. Hence, in such situations, physicians have difficulty in prioritizing the optimal therapy for a given patient. Furthermore, for patients who meet prescribing criteria for several biologics, being allergic and with high blood eosinophil levels, there are no clear indications on how to make a given choice. To help clinicians in the choice of the most suitable add-on treatment for these patients, Bousquet et al.4 proposed to consider omalizumab as first-line therapy because of its efficacy and safety assessed by a large body of real-life data and over a decade of post-marketing surveillance; however, not all patients receiving omalizumab seem to respond well to treatment.
A post hoc meta-analysis performed in patients from two phase III studies5 evaluated the efficacy of the licensed dose of mepolizumab versus placebo in severe eosinophilic asthma and associated allergic characteristics, who had previously received omalizumab treatment. The reduced rate of clinically significant exacerbations associated with mepolizumab treatment was similar irrespective of omalizumab eligibility, IgE levels, or atopic phenotype. Moreover, the impact of mepolizumab versus placebo on the improvement in lung function, health-related quality of life, and asthma control was observed at the end of the study in both omalizumab-eligible and ineligible patients. Thus, mepolizumab treatment is efficacious and may provide clinically important benefits to a broad range of patients with severe eosinophilic asthma, regardless of their omalizumab therapy history.
The most important multicenter clinical trial that evaluated the effectiveness of mepolizumab in uncontrolled patients with severe eosinophilic asthma, after a switch from omalizumab, is the OSMO (omalizumab switch to mepolizumab) study.6 This study demonstrated that mepolizumab is a well-tolerated and relevant treatment option in patients with severe eosinophilic asthma that is unresponsive to high-dose inhaled corticosteroids (ICSs) and omalizumab, and that in clinical practice it is possible to switch patients safely and without side effects from one biologic to another with no wash-out period. Consistent results with those of OSMO have been reported by the authors of a real-life study performed in several centers of Northern-Central Italy, where patients with previous omalizumab treatment failure were shifted to mepolizumab.7
Within such a context, the aim of this study was to investigate our real-life experience in several University Clinical Centers of Southern Italy, regarding the shift to mepolizumab of patients with a phenotype of severe allergic eosinophilic asthma, uncontrolled by the previous anti-IgE treatment with omalizumab.
Methods
We carried out a retrospective, multicenter, single-group, self-controlled, real-life study in which 41 patients with severe persistent allergic asthma, uncontrolled by a biologic treatment with omalizumab, were shifted to mepolizumab without a wash-out period. This study was carried out according to the principles of the Declaration of Helsinki.
These patients were managed by seven certified Clinical Respiratory Units of Southern Italy (Catania, Catanzaro, Foggia, Bari, Palermo, and two University Respiratory Units of Naples). All patients had been firstly selected for treatment with omalizumab, and were then shifted to mepolizumab according to clinical practice and on the basis of all the criteria for eligibility approved by the European Medicines Agency. Data at baseline (i.e. before any biological treatment), as well as after at least 12 months of treatment with omalizumab, were collected in a retrospective manner. Data after at least 12 months of mepolizumab therapy were collected at each patient’s last visit.
The main aim of the study was to determine whether treatment with subcutaneous mepolizumab at the licensed dose of 100 mg significantly reduced the number of hospitalizations, the rate of exacerbations, and the need for chronic oral corticosteroid (OCS) therapy over an observational period of at least 1 year. Secondary objectives were to evaluate the eventual improvement of symptoms, change in pre-bronchodilator FEV in 1 s (FEV1), reduction of blood eosinophil count and decrease in fractional exhaled nitric oxide (FeNO). In a small group of enrolled subjects, we were also able to collect sputum samples that were used to analyze airway eosinophil counts.
Patients
Eligible patients were ⩾18 years of age and met the European Respiratory Society/American Thoracic Society (ERS/ATS) guideline criteria for a diagnosis of severe refractory asthma8 (that is, patients with not well controlled asthma, despite taking daily maximal inhaled treatment plus another controller or OCS therapy for at least 6 months during the previous year). In addition, they had total serum IgE levels ranging from 30 to 1500 IU/ml, evidence of sensitization to at least one perennial allergen (by prick test or Radio-AllergoSorbent Test), blood eosinophil counts of at least 150 cells/µl before starting mepolizumab and at least 300 cells/µl during the previous 12 months, and also had a history of omalizumab failure defined as lack of effectiveness (i.e. frequent severe exacerbations and/or uncontrolled symptoms in patients with a poor or moderate response in the global evaluation of treatment effectiveness scale) after at least 12 months of treatment. Patients were excluded if they had an uncontrolled asthma due to inadequate or inappropriate treatment, poor compliance to inhaled therapy or persistent uncontrolled comorbidities, or if they were diagnosed with asthma/chronic obstructive pulmonary disease overlap, or also if any other severe disease was likely to interfere with the conduct of the study.
End-points and assessments
The primary endpoint was the mean change with respect to baseline in frequency of exacerbations requiring hospitalization, as well as in total exacerbations and need for chronic OCS therapy, occurring after at least 1 year of therapy with mepolizumab. Additional end-points included the percentage of patients achieving a clinically important increment from baseline in asthma control test (ACT) score, and the mean change from baseline in pre-bronchodilator FEV1, recorded after at least 1 year of therapy with mepolizumab. An eventual improvement or worsening in symptoms and asthma control was calculated on the basis of the assumption that a 3-point change in ACT score from baseline to the final visit expressed a clinically meaningful difference. Levels of inflammatory biomarkers (FeNO levels and blood/sputum eosinophil counts) were also assessed. The cytological analysis of sputum samples was performed only in a small group of enrolled subjects (10 patients).
Statistical analysis
Data at baseline and after therapy with each asthma biological medication were expressed as mean ± standard deviation (SD) for continuous variables (i.e. age, body mass index (BMI), total IgE count, number of exacerbations, ACT, lung function, and inflammatory biomarkers). Patients falling within each clinical category of interest (i.e. sex, positive skin-prick tests, type of concurrent asthma therapy, OCS-dependent patients) were computed in terms of number and percent. Efficacy end-points were assessed by means of a Student’s t test for continuous variables and Fisher’s exact test for categorical variables. A p < 0.05 was considered as statistically significant.
Ordered categorical data were analyzed with the use of proportional-odds regression. For frequent exacerbators and OCS users, an odds ratio <1 indicated a lower proportion of participants falling within these categories in the mepolizumab period, when compared with post-omalizumab and baseline periods. For decrease in annual exacerbation rate and daily OCS use, hazard ratios were also calculated. A hazard ratio <1 indicated a reduction in the risk of experiencing exacerbations or need of OCS use.
Data were analyzed using GraphPad for Windows.
Results
Demographic characteristics
A total of 41 patients were included in the study. Demographic characteristics are presented in Table 1. In the above population, 10 (24%) had one comorbidity; prevalence of multimorbidity (two or more conditions) was 73%. Only one patient had no comorbidity (Figure 1).
Table 1.
Characteristics of patients at baseline. Values for continuous variables are expressed as mean ± SD (min–max). Clinical category of interest are computed in terms of number and percent (n, %).
| Demographic characteristic | Value |
|---|---|
| Age (years, mean ± SD) | 56.75 ± 9 (26–79) |
| Sex, female (n, %) | 33 (80%) |
| Sex, male (n, %) | 8 (20%) |
| BMI (kg/m2, mean ± SD) | 27.7 ± 3.3 |
| Current smokers (n, %) | 1 (2%) |
| Former smokers (n, %) | 14 (34%) |
| Familiarity for asthma (n, %) | 19 (46%) |
| Age of asthma onset (mean ± SD) | 34.6 ± 12.3 (11–55) |
| Positive skin-prick test to a perennial aeroallergen (n, %) | 41 (100%) |
| Positive skin-prick test to a seasonal aeroallergen (n, %) | 30 (73%) |
| Total IgE count (mean ± SD, min–max) | 332 ± 179 (66–1237) |
| Concurrent therapy use at baseline | |
| ICS (n, %) | 41 (100%) |
| ICS (µg/day BDP equivalent, mean ± SD) | 1404.39 ± 585.75 |
| LABA (n, %) | 41 (100%) |
| SABA (n, %) | 36 (88%) |
| LAMA (n, %) | 37 (90%) |
| LTRA (n, %) | 35 (85%) |
| Comorbidities | n (%) |
| Nasal polyposis | 25 (61%) |
| GER | 21 (51%) |
| Rhinitis | 18 (44%) |
| Bronchiectasis | 9 (22%) |
| OSAS | 3 (7%) |
| AERD | 3 (7%) |
| Urticaria | 1 (2%) |
| Dermatitis | 1 (2%) |
| No comorbidities | 1 (2%) |
AERD, aspirin-exacerbated respiratory disease; BDP, budesonide dipropionate; BMI, body mass index; GER, gastro-esophageal reflux; ICS, inhaled corticosteroid; IG, immunoglobulin; LABA, long-acting beta-agonists; LAMA, long-acting muscarinic antagonists; OSAS, obstructive sleep apnea syndrome; SABA, short-acting beta-agonists; SD, standard deviation.
Figure 1.
Patients’ comorbidities distribution.
Clinical, functional and biological data collected in the course of the study are shown in Table 2.
Table 2.
Clinical, functional and biological data over study time. Values for continuous variables are expressed as mean ± SD (min–max). Clinical category of interest are computed in terms of number and percent (n, %).
| Baseline | Post-omalizumab (pre-shift) | Post-mepolizumab |
p value (baseline versus post-OMA) |
p value (baseline versus post-MEPO) |
|
|---|---|---|---|---|---|
| ACT score; mean ± SD (min–max) |
12.0 ± 2.7 (5–19) |
14.2 ± 1.9 (5–24) |
21.9 ± 2.7 (8–25) |
0.0003 | <0.0001 |
| ACQ-7 score; mean ± SD (min–max) |
3.3 ± 0.3 (2.6–3.8) |
2.8 ± 0.7 (1.5–3.8) |
1.6 ± 0.6 (0.5–3.3) |
NS | <0.0001 |
| AQLQ score; mean ± SD (min–max) |
3.7 ± 0.6 (3–4.6) |
4.6 ± 1.0 (3.1–6.3) |
4.8 ± 0.8 (3.6–6) |
NS | 0.0475 |
| FEV1 (pre-bd l); mean ± SD (min–max) |
1.56 ± 0.45 (0.61–3.21) | 1.56 ± 0.47 (0.69–3.73) |
1.86 ± 0.52 (0.68–3.49) | NS | <0.0001 |
| FEV1 (pre-bd %); mean ± SD (min–max) |
64.0 ± 13.2 (22–96) |
68.3 ± 13.5 (26–102) |
75.7 ± 12.7 (33–109) |
NS | <0.0001 |
| Patients who experienced severe exacerbations in the previous 12 months (requiring a hospital admission); n (%) |
16 (39%) | 5 (12%) | 1 (2%) | <0.0001 | <0.0001 |
| Severe exacerbations in the previous 12 months (requiring a hospital admission); n |
19 | 5 | 1 | <0.0001 | <0.0001 |
| Severe exacerbations in the previous 12 months (requiring a hospital admission); mean ± SD (min–max) |
0.5 ± 0.6 (0–2) |
0.1 ± 0.2 (0–1) |
0.03 ± 0.05 (0–1) |
<0.0001 | <0.0001 |
| Patients who experienced exacerbations in the previous 12 months; n (%) |
41 (100%) |
41 (100%) |
15 (37%) |
NS | <0.0001 |
| Exacerbations in the previous 12 months; N |
200 | 164 | 25 | NS | <0.0001 |
| Exacerbations in the previous 12 months; mean ± SD (min–max) |
5.8 ± 1.8 (2–12) |
4.2 ± 1.4 (1–12) |
0.7 ± 0.9 (0–6) |
NS | <0.0001 |
| Corticosteroid-dependent patients; n (%) |
19 (46%) |
10 (24%) |
2 (5%) |
0.0018 | <0.0001 |
| Blood eosinophils cells/µl; mean ± SD (min–max) |
584 ± 196 (139–1220) |
579 ± 208 (240–1310) |
82 ± 56 (0–419) |
NS | <0.0001 |
| Blood eosinophils%; mean ± SD (min–max) |
8.2 ± 3.5 (2.1–19.1) |
8.3 ± 3.5 (2.4–18.2) |
1.8 ± 1.2 (0.6–8.6) |
NS | <0.0001 |
| Sputum eosinophils%; mean ± SD (min–max) |
48.7 ± 28.5 (9–90) |
43.1 ± 16.3 (9–80) |
9.6 ± 6.2 (0–25) |
NS | 0.0083 |
| FENO50 ppb; mean ± SD (min–max) |
37.3 ± 17.8 (10–79) |
40.1 ± 16 (7.8–88) |
34 ± 15 (12.2–86.4) |
NS | NS |
ACQ-7, Asthma Control Questionnaire (7 items); ACT, asthma control test; AQLQ, Asthma Quality of Life Questionnaire; FENO50, fraction of exhaled nitric oxide at 50 ml/s; FEV1 pre-bd, forced expiratory volume in 1 s pre-bronchodilator; NS, not significant; SD, standard deviation.
Primary end-points: hospitalization, exacerbations and systemic corticosteroids.
The number of patients experiencing exacerbations decreased only during treatment with mepolizumab, and in a statistically highly significant manner [odds ratio, 0.019; 95% confidence interval (CI), 0.001–0.320; hazard ratio p < 0.0001].
Only 1 of 41 patients developed a severe exacerbation, requiring hospitalization, after starting treatment with mepolizumab and during the follow-up period.
A total of 15 patients (37%) experienced a total of 25 moderate exacerbations during treatment with mepolizumab, of whom only 6 required therapy with systemic corticosteroids. Overall, 26 of 41 patients (63%) were considered as being controlled (composite of ACT score of > 20 and no exacerbation).
The mean ± SD number of yearly exacerbations in the overall study population was 5.8 ± 1.8 during the 12 months prior to screening, 4.2 ± 1.4 after treatment with omalizumab, and 0.7 ± 0.9 after therapy with mepolizumab (Figure 2). The decrease in asthma exacerbation rate during treatment with mepolizumab was statistically highly significant, with respect to both the pre-screening period (hazard ratio, 0.125; 95% CI, 0.096–0.162; p < 0.0001) and the period of treatment with omalizumab (hazard ratio, 0.150; 95% CI, 0.115–0.162; p < 0.0001).
Figure 2.
Rate of exacerbations/year in asthmatic patients at baseline, after omalizumab and after mepolizumab.
In the subgroup analysis of patients who experienced >1 exacerbation during the on-treatment period (n = 7), the mean ± SD number of exacerbations during the 12 months prior to screening was 7.4 ± 3.0 (3–12), compared with 2.8 ± 1.0 (2–6) recorded after treatment with mepolizumab. The decrease in asthma exacerbation rate was statistically significant also in this subgroup analysis (p = 0.0057).
The percentage of patients who were receiving daily OCS therapy significantly decreased from 46% at baseline (19 patients) and from 24% after omalizumab treatment (10 patients) to 5% (only two patients) during treatment with mepolizumab (odds ratio, 0.795; 95% CI, 0.420–1.51; p < 0.0001 and odds ratio, 0.564; 95% CI, 0.286–1.112; p < 0.0001 respectively). Therefore, 17 patients who were OCS-dependent prior to receiving any biologic treatment, were able to discontinue this medication after treatment with mepolizumab.
In the same way, the daily OCS use significantly decreased (hazard ratio, 0.024; 95% CI, 0.023–0.025; p < 0.0001 and hazard ratio, 0.096; 95% CI, 0.087–0.106; p < 0.0001 respectively).
Secondary end-points: effect on ACT and lung function
The mean ACT score significantly increased from 12.0 (5–19) at baseline to 21.9 (8–25) during treatment with mepolizumab (p < 0.0001). A worsening in ACT score was detected in only 1 out of 41 patients (2%) (Figure 3).
Figure 3.
ACT score in asthmatic patients at baseline, after omalizumab and after mepolizumab.
ACT, asthma control test.
The mean pre-bronchodilator FEV1 increased from 1.56 ± 0.45 (0.61–3.21) l at baseline to 1.86 ± 0.52 (0.68–3.49) l after mepolizumab treatment (p < 0.0001). The mean pre-bronchodilator FEV1 increased in a statistically significant manner also with respect to the pre-shift period (after at least 16 weeks of treatment with omalizumab) [mean pre-bronchodilator FEV1: 1.56 ± 0.47 (0.69–3.73) l] (Figure 4).
Figure 4.
Pre-bronchodilator FEV1 (l) in asthmatic patients at baseline, after omalizumab and after mepolizumab.
FEV1, forced expiratory volume in 1 s.
On the contrary, treatment with omalizumab was not effective in improving FEV1 from baseline in these severe asthmatic patients (p = not significant).
Additional end-points: inflammatory biomarkers
The mean total blood eosinophil number decreased from 584 ± 196 (139–1220) cells/µl, counted at baseline, to 82 ± 56 (0–419) cells/µl after at least 1 year of therapy with mepolizumab (p < 0.0001). The reduction of mean blood eosinophil count was statistically significant also with respect to the pre-shift period (after treatment with omalizumab) (p < 0.0001) (Figure 5).
Figure 5.
Mean blood eosinophils (cells/µl) in asthmatic patients at baseline, after omalizumab and after mepolizumab.
The differences in fractional exhaled nitric oxide measurement at constant expiratory flow of 50 ml/s (FeNO50) were not statistically significant when comparing pre-screening evaluation (baseline) with post-mepolizumab treatment (37.3 ± 17.8 versus 34 ± 15; p = 0.367), as well as when comparing the pre-shift period (post-omalizumab therapy) to post-mepolizumab period (34 ± 15 versus 40.1 ± 16; p = 0.069).
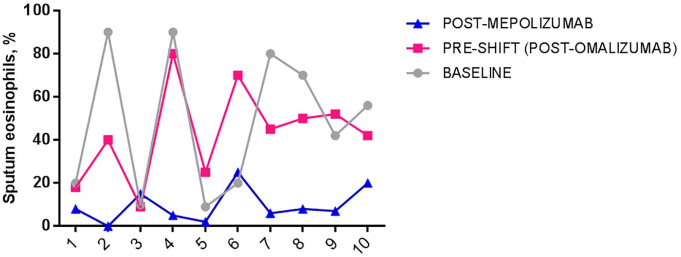
Finally, in a small group of 10 patients we also performed the cytological analysis of sputum samples, and we found a statistically significant reduction in the percentage of eosinophils when comparing baseline values with post-mepolizumab levels (48.7 ± 28.5% versus 9.6 ± 6.2%, p = 0.0083) (Figure 6).
Figure 6.
Mean sputum eosinophils (%) in asthmatic patients at baseline, after omalizumab and after mepolizumab.
Discussion
Treatment of severe asthma has become efficaciously practicable in recent years, probably because of the availability of new biological drugs that allow us a modern approach, such as one of phenotype/endotype-driven therapy. Some patients with severe asthma, however, notwithstanding the biological therapy prescribed as first line, still present uncontrolled asthma and experience exacerbations, leaving us unsatisfied with the clinical response. This problem could be faced in approximately one-third of patients with severe asthma, who exhibit both allergic and eosinophilic phenotypes,9 thus being eligible for either omalizumab, mepolizumab or benralizumab. Consequently, choosing the most appropriate therapy can be challenging for physicians, and the first-line selection can be unsatisfactory.
After the OSMO study, other trials and case reports evaluated the effectiveness of mepolizumab in patients who were not well controlled during a previous period of treatment with omalizumab.7,10,11 These studies ensue from the clinical need of finding an adequate and effective alternative treatment for patients with severe allergic and eosinophilic asthma, who exhibit a poor response to a previous treatment with omalizumab. In this context, we wanted to give our contribution referring to the real-life experience of several University Clinical Centers located in Southern Italy.
The main result of our study, highly consistent with OSMO6 and the similar trial performed by Bagnasco et al.,7 is that patients with severe eosinophilic asthma, not optimally controlled by omalizumab, displayed a significant improvement in asthma control following a direct switch to mepolizumab, as reflected by the important reduction in the rate of both severe and mild-to-moderate exacerbations. In the OSMO study, among 145 patients who were switched to mepolizumab, the rate of clinically significant exacerbations during the 32-week study period decreased by 64% compared with the year prior to study enrollment6. After 1 year of mepolizumab treatment, Bagnasco et al.7 registered a decrease in the mean exacerbation rate of 81% in their 27 patients. Similarly to what was found by Bagnasco et al.,7 in our 41 patients the reduction in the mean exacerbation rate was 83%.
In our population, after switching to mepolizumab only 1 out of 41 patients developed at least one severe exacerbation, requiring hospitalization, compared with 5 patients during treatment with omalizumab, with a decrease in the hospitalization rate of 70%, which represents a higher percentage than that reported by the OSMO study.6 The reduction of overall hospitalization number, also recorded after treatment with omalizumab despite its failure to reach an optimal disease control, could be explained by the fact that patients on treatment with biologics undergo a close follow up, which could help to prevent the outbreak of serious worsening in clinical conditions. Nevertheless, differently from the other two studies,6,7 only six moderate exacerbations required treatment with systemic corticosteroids, thus confirming the efficacy of mepolizumab in maintaining the control of asthma over time. In addition, a remarkable proportion of patients (89%) who were OCS-dependent prior to receiving any biologic treatment, were able to discontinue this medication, similarly to what was found by Bagnasco et al.7 It has been estimated that about 64% of patients with severe asthma in Italy are chronically treated with OCS.12 These patients are at a greater risk of complications due to OCS therapy, such as osteoporosis and fractures, digestive diseases, diabetes, obesity and kidney failure, with an estimated cost of 243 million EUR each year for the management of side effects.12 Though being more expensive than other controller medications for asthma, these new biological drugs are very effective in sparing OCS, thus significantly reducing the cost of the management of OCS damage, which may exceed the estimated economic burden of biologics.13 Therefore, it seems reasonable to encourage the choice of a biologic therapy in suitable patients.
Moreover, in our patients we found relevant improvements in ACT score, Asthma Control Questionnaire at seven items (ACQ-7), and Asthma Quality of Life Questionnaire. These data, consistently with what has been reported in literature,5–7 support the validity of mepolizumab treatment also in terms of benefits perceived by patients as improvements of symptoms and quality of life.
In our study, the increase in pre-bronchodilator FEV1 from baseline to the post-mepolizumab period amounted to 19%, whereas Bagnasco et al. recorded a greater improvement. Although the improvement in pre-bronchodilator FEV1 in our study was statistically significant and exceeded the minimal clinically important difference of 100 ml, we may have expected greater FEV1 increases, given the marked improvements detected with regard to other parameters. However, FEV1 measurements may not reflect improvements in symptoms and asthma control that are perceived by patients with severe/refractory asthma, thereby creating a dissociation between lung function and asthma symptom control.14 Furthermore, we agree with the suggestion of the OSMO study that patients with such severe and long-standing asthma might have undergone a remodeling process that limits their capacity to improve spirometry.
The effectiveness of a given biological drug for asthma in clinical practice mainly depends on the correct patient selection. Most patients enrolled in this study started omalizumab when it was the only biological option for severe asthma, so that they did not have the chance of receiving mepolizumab as first biologic treatment. The current availability of many biologics for severe asthma gives us the opportunity to choose the one that is appropriate for each specific phenotype, and switch to another when the first chosen is not effective.
Omalizumab prescription is currently indicated for patients with severe IgE-mediated asthma, allergic to perennial allergens. In some studies this drug also demonstrated efficacy in patients with severe asthma allergic to seasonal allergens,15,16 and in patients with allergic asthma with the comorbid condition of seasonal allergic rhinitis.17 All the enrolled patients in our study had a positive skin-prick test to perennial aeroallergens. In addition, 73% showed a positive skin-prick test to seasonal aeroallergens. These data seem to suggest that the typology of allergy (i.e. to both perennial and seasonal allergens) has no value in predicting the response to treatment with omalizumab.
Add-on treatment with omalizumab has to be administered every 2 or 4 weeks at a determined dose, based on body weight and total serum IgE level prior to treatment. A sub-analysis of data from a large multicenter study demonstrated that omalizumab is less beneficial for patients with a total serum IgE level of 0–75 (IU/ml), even for those testing positive for perennial allergen-specific IgE.18 On the other hand, in patients with too high IgE levels, especially if they are associated with a high body weight, the therapy may result ineffective because omalizumab cannot decrease IgE levels below a certain necessary threshold (i.e. 90%).19 In our study the mean total serum IgE level was 332 ± 179 IU/ml, with a modal value of 336 IU/ml. Since these values seem perfectly in the average of those considered in the main studies that have ascertained the therapeutic efficacy of omalizumab, we can conclude, also according to what has been previously reported in the literature, that serum IgE levels guide treatment choice towards the use of omalizumab, but are not predictive of the response to this anti-IgE drug.20
In accordance with already published data,5–7 our study showed a significant decrease in peripheral blood eosinophil count after switching from omalizumab to mepolizumab. This was an expected result when considering the direct action of mepolizumab on IL-5, namely the main eosinophil survival factor. Blood eosinophil counts are reliable predictors of the response to treatment with mepolizumab, as previous reports have convincingly demonstrated.21–25 In particular, a post hoc meta-analysis of the two phase III studies MENSA and MUSCA,26 referring to patients who had previously received an unsuccessful treatment with omalizumab, has shown that, although mepolizumab reduced the rate of clinically significant exacerbations across all patient groupings, the effect was lower in patients with a baseline blood eosinophil count <300 cells/µl. In the OSMO study 66% of population had, at baseline, a blood eosinophil count >300 cells/µl.6 Interestingly, in our population the mean eosinophil count at baseline was 595 cells/µl, with only two patients presenting an eosinophil count <300 cells/µl. These data could therefore suggest that mepolizumab provides clinically important benefits for patients with overlapping allergic and eosinophilic asthma phenotypes, who have quite high blood eosinophil counts. In addition, the most common comorbidity condition in patients enrolled in our study was nasal polyposis (61%). In another meta-analysis of MUSCA and MENSA studies,21 and in a post hoc analysis of MUSCA study,27 patients with severe eosinophilic asthma and nasal polyposis seemed to experience greater benefit in terms of quality of life and exacerbation decrease with mepolizumab, compared with patients with eosinophilic asthma and without nasal polyps. A suggestive explanation for this finding is that the local generation of IL-5 within both upper and lower airways in such patients can result in higher circulating blood eosinophil levels, that are predictive biomarkers of a better response to mepolizumab.28 Although this hypothesis needs validation in larger study populations, the prevalence of nasal polyps in our patients may indicate that subjects with severe asthma, nasal polyps and high blood eosinophils experience a better response to mepolizumab rather than to omalizumab.
Another relevant consideration refers to the duration of a biologic treatment for severe asthma. In this regard, phase III trials and other studies have repeatedly shown that after stopping omalizumab treatment, free IgE levels in serum will increase and, in parallel, clinical allergic symptoms will reappear.29 In the same way, cessation of mepolizumab treatment was associated with a rise in blood eosinophil count and loss of asthma control.30 These data suggest that a biologic therapy has to be continued as a maintenance treatment for interminable time after reaching control. Alternatively, it is conceivable to stop treatment only by carefully monitoring biological markers (i.e. IgE, eosinophils) over time, in order to avoid the risk of exacerbations immediately after withdrawal.
In this study we also performed the cytological analysis of sputum samples in a small group of patients, and found a statistically significant reduction in the percentage of airway eosinophils in subjects under treatment with mepolizumab. Performing induced sputum in severe asthmatic patients is often difficult, and sometimes impossible in presence of very low FEV1 values. However, asthma is a respiratory disease, and evaluation of eosinophils in the target organ by analysis of induced sputum could give more reliable pathophysiologic information. The presence of high eosinophils in sputum has been reported to be associated with the propensity to develop frequent exacerbations,31 and further studies evaluating the use in real-life of sputum analysis during the follow up of patients treated with biological drugs targeting IL-5 would be interesting. Finally, in this study we did not observe any statistically significant difference in FeNO50 after biological therapies, whereas Bagnasco et al.7 recorded a significant decrease in FeNO50 levels after treatment with mepolizumab. Such a discordance is probably due to the fact that in our study many patients at baseline and before the shift from omalizumab to mepolizumab were under treatment with high doses of OCS, which lowered FeNO levels.
The main limitations of our study are related to the small number of patients and to its retrospective design. Despite these considerations, the significant decreases in exacerbation rate and OCS intake occurred together with a marked improvement in asthma control and pulmonary function, and favorable changes in biological markers gave consistency to the overall results.
In conclusion, in severe eosinophilic allergic asthma not controlled by omalizumab, the shift from anti-IgE treatment to another biologic therapy targeting IL-5, such as mepolizumab, seems to be the best therapeutic strategy. This approach is favored by the possibility of switching safely patients from a biologic to another without a wash-out period, and is also associated with no risk of side effects due to a chronic use of OCS in case of uncontrolled disease. Hence, physicians should be encouraged to consider a second biological therapy after failure of the first one.
Supplemental Material
Supplemental material, Author_Response_1 for Switching from omalizumab to mepolizumab: real-life experience from Southern Italy by Giovanna Elisiana Carpagnano, Corrado Pelaia, Maria D’Amato, Nunzio Crimi, Nicola Scichilone, Giulia Scioscia, Onofrio Resta, Cecilia Calabrese, Girolamo Pelaia, Carla Maria Irene Quarato and Maria Pia Foschino Barbaro in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Switching from omalizumab to mepolizumab: real-life experience from Southern Italy by Giovanna Elisiana Carpagnano, Corrado Pelaia, Maria D’Amato, Nunzio Crimi, Nicola Scichilone, Giulia Scioscia, Onofrio Resta, Cecilia Calabrese, Girolamo Pelaia, Carla Maria Irene Quarato and Maria Pia Foschino Barbaro in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Switching from omalizumab to mepolizumab: real-life experience from Southern Italy by Giovanna Elisiana Carpagnano, Corrado Pelaia, Maria D’Amato, Nunzio Crimi, Nicola Scichilone, Giulia Scioscia, Onofrio Resta, Cecilia Calabrese, Girolamo Pelaia, Carla Maria Irene Quarato and Maria Pia Foschino Barbaro in Therapeutic Advances in Respiratory Disease
Footnotes
Author contribution(s): Giovanna Elisiana Carpagnano: Conceptualization; Methodology; Supervision; Visualization; Writing-review & editing.
Corrado Pelaia: Data curation; Formal analysis; Methodology; Writing-review & editing.
Maria D’Amato: Data curation; Methodology; Writing-original draft; Writing-review & editing.
Nunzio Crimi: Conceptualization; Data curation; Methodology; Supervision; Writing-review & editing.
Nicola Scichilone: Conceptualization; Data curation; Methodology; Supervision; Writing-review & editing.
Giulia Scioscia: Conceptualization; Data curation; Formal analysis; Methodology; Supervision; Validation; Visualization; Writing-original draft; Writing-review & editing.
Onofrio Resta: Data curation; Project administration; Validation; Visualization; Writing-review & editing.
Cecilia Calabrese: Data curation; Formal analysis; Project administration; Validation; Writing-review & editing.
Girolamo Pelaia: Conceptualization; Formal analysis; Project administration; Validation; Writing-original draft; Writing-review & editing.
Carla Maria Irene Quarato: Data curation; Formal analysis; Investigation; Methodology; Writing-original draft; Writing-review & editing
Maria Pia Foschino Barbaro: Conceptualization; Methodology; Project administration; Validation; Visualization; Writing-review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Giulia Scioscia  https://orcid.org/0000-0002-2667-077X
https://orcid.org/0000-0002-2667-077X
Girolamo Pelaia  https://orcid.org/0000-0001-9288-8913
https://orcid.org/0000-0001-9288-8913
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Giovanna Elisiana Carpagnano, Department of Basic Medical Sciences, Neuroscience and Sense Organs, Section of Respiratory Disease, University “Aldo Moro” of Bari, Bari, Italy.
Corrado Pelaia, Department of Health Sciences, Section of Respiratory Disease, University “Magna Græcia” of Catanzaro, Catanzaro, Italy.
Maria D’Amato, Division of Pneumology, Department of Respiratory Diseases, University of Naples Federico II, AORN dei Colli-Monaldi Hospital, Naples, Italy.
Nunzio Crimi, Department of Internal Medicine and Specialistic Medicine, Section of Respiratory Diseases, University of Catania, Catania, Italy.
Nicola Scichilone, Department of Medicine, Pneumology, Physiology and Nutrition, University of Palermo, Palermo, Italy.
Giulia Scioscia, Department of Medical and Surgical Sciences, Institute of Respiratory Disease, University of Foggia, Policlinico Riuniti di Foggia - Viale Pinto, 1, 71122, Italy.
Onofrio Resta, Department of Basic Medical Sciences, Neuroscience and Sense Organs, Section of Respiratory Disease, University “Aldo Moro” of Bari, Bari, Italy.
Cecilia Calabrese, Department of Translational Medical Sciences, Section of Respiratory Disease, University of Campania “Luigi Vanvitelli”, Naples, Italy.
Girolamo Pelaia, Department of Health Sciences, Section of Respiratory Disease, University “Magna Græcia” of Catanzaro, Catanzaro, Italy.
Carla Maria Irene Quarato, Department of Medical and Surgical Sciences, Institute of Respiratory Diseases, University of Foggia, Policlinico Riuniti di Foggia, Foggia, Italy.
Maria Pia Foschino Barbaro, Department of Medical and Surgical Sciences, Institute of Respiratory Diseases, University of Foggia, Policlinico Riuniti di Foggia, Foggia, Italy.
References
- 1. Mukherjee AB, Zhang Z. Allergic asthma: influence of genetic and environmental factors. J Biol Chem 2011; 286: 32883–32889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global Initiative for Asthma. Global strategy for asthma management and prevention updated 2020, www.ginasthma.org (2020, accessed 3 April 2020).
- 3. Gülsen A, Wallis S, Jappe U. Combination of immunotherapies for severe allergic asthma. J Asthma. Epub ahead of print 5 September 2019. DOI: 10.1080/02770903.2019.1658204. [DOI] [PubMed] [Google Scholar]
- 4. Bousquet J, Brusselle G, Buhl R, et al. Care pathways for the selection of a biologic in severe asthma. Eur Respir J 2017; 50: 1701782. [DOI] [PubMed] [Google Scholar]
- 5. Magnan A, Bourdin A, Prazma CM, et al. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy 2016; 71: 1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Galkin D, Liu MC, Chipps BE, et al. Efficacy and safety of mepolizumab in uncontrolled patients with severe eosinophilic asthma following a switch from omalizumab (OSMO Study): exacerbation and safety outcomes. J Allergy Clin Immunol 2018; 141: AB409. [Google Scholar]
- 7. Bagnasco D, Menzella F, Caminati M, et al. Efficacy of mepolizumab in patients with previous omalizumab treatment failure: real-life observation. Allergy. Epub ahead of print 1 July 2019. DOI: 10.1111/all.13937. [DOI] [PubMed] [Google Scholar]
- 8. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. [DOI] [PubMed] [Google Scholar]
- 9. Tran TN, Zeiger RS, Peters SP, et al. Overlap of atopic, eosinophilic, and TH2-high asthma phenotypes in a general population with current asthma. Ann Allergy Asthma Immunol 2016; 116: 37–42. [DOI] [PubMed] [Google Scholar]
- 10. Imai A, Takizawa T, Sato K, et al. Switching biologics led to good control in severe childhood asthma: a case report. Arerugi 2019; 68: 869–873. [DOI] [PubMed] [Google Scholar]
- 11. Albers FC, Hozawa S, Bratton DJ, et al. Update: mepolizumab treatment in patients with severe eosinophilic asthma and prior omalizumab use. Allergy. Epub ahead of print 2 October 2019. DOI: 10.1111/all.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heffler E, Blasi F, Latorre M, et al. The severe asthma network in Italy: findings and perspectives. J Allergy Clin Immunol Pract 2019; 7: 1462–1468. [DOI] [PubMed] [Google Scholar]
- 13. Canonica GW, Colombo GL, Bruno GM, et al. Shadow cost of oral corticosteroids-related adverse events: a pharmacoeconomic evaluation applied to real-life data from the severe asthma network in Italy (SANI) registry. World Allergy Organ J 2019; 12: 100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 2008; 178: 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Domingo C, Pomares X, Navarro A, et al. Omalizumab is equally effective in persistent allergic oral corticosteroid-dependent asthma caused by either seasonal or perennial allergens: a pilot study. Int J Mol Sci 2017; 18: 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corren J, Casale T, Haselkorn T, et al. Effect of omalizumab on seasonal exacerbations in adolescents and adults with moderate-to-severe allergic asthma. Ann Allergy Asthma Immunol 2018; 121: S6–S7. [Google Scholar]
- 17. Casale TB. Anti-immunoglobulin E (omalizumab) therapy in seasonal allergic rhinitis. Am J Respir Crit Care Med 2001; 164: S18–S21. [DOI] [PubMed] [Google Scholar]
- 18. Bousquet J, Rabe K, Humbert M, et al. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respir Med 2007; 101:1483–1492. [DOI] [PubMed] [Google Scholar]
- 19. Lowe PJ, Renard D. Omalizumab decreases IgE production in patients with allergic (IgE-mediated) asthma: PKPD analysis of a biomarker, total IgE. Br J Clin Pharmacol 2011; 72: 306–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wahn U, Martin C, Freeman P, et al. Relationship between pretreatment specific IgE and the response to omalizumab therapy. Allergy 2009; 64: 1780–1787. [DOI] [PubMed] [Google Scholar]
- 21. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014; 371: 1198–1207. [DOI] [PubMed] [Google Scholar]
- 22. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med 2014; 371: 1189–1197. [DOI] [PubMed] [Google Scholar]
- 23. Yancey SW, Keene ON, Albers FC, et al. Biomarkers for severe eosinophilic asthma. J Allergy Clin Immunol 2017; 140: 1509–1518. [DOI] [PubMed] [Google Scholar]
- 24. Ortega HG, Yancey SW, Mayer B, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med 2016; 4: 549–556. [DOI] [PubMed] [Google Scholar]
- 25. Pelaia C, Busceti MT, Solinas S, et al. Real-life evaluation of the clinical, functional, and hematological effects of mepolizumab in patients with severe eosinophilic asthma: results of a single-centre observational study. Pulm Pharmacol Ther 2018; 53: 1–5. [DOI] [PubMed] [Google Scholar]
- 26. Humbert M, Albers FC, Bratton DJ, et al. Effect of mepolizumab in severe eosinophilic asthma according to omalizumab eligibility. Respir Med 2019; 154: 69–75. [DOI] [PubMed] [Google Scholar]
- 27. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med 2017; 5: 390–400. [DOI] [PubMed] [Google Scholar]
- 28. Howarth P, Chupp G, Nelsen LM, et al. Severe eosinophilic asthma with nasal polyposis: a phenotype for improved sinonasal and asthma outcomes with mepolizumab therapy. J Allergy Clin Immunol. Epub ahead of print 19 February 2020. DOI: 10.1016/j.jaci.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 29. Slavin RG, Ferioli C, Tannenbaum SJ, et al. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol 2009; 123: 107–113.e3. [DOI] [PubMed] [Google Scholar]
- 30. Ortega H, Lemiere C, Llanos JP, et al. Outcomes following mepolizumab treatment discontinuation: real-world experience from an open-label trial. Allergy Asthma Clin Immunol 2019; 15: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sposato B, Latorre M, Scalese M, et al. Blood vs sputum eosinophils as predictors of exacerbations in a cohort of severe asthmatics. Eur Respir J 2018; 52: PA950. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response_1 for Switching from omalizumab to mepolizumab: real-life experience from Southern Italy by Giovanna Elisiana Carpagnano, Corrado Pelaia, Maria D’Amato, Nunzio Crimi, Nicola Scichilone, Giulia Scioscia, Onofrio Resta, Cecilia Calabrese, Girolamo Pelaia, Carla Maria Irene Quarato and Maria Pia Foschino Barbaro in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Switching from omalizumab to mepolizumab: real-life experience from Southern Italy by Giovanna Elisiana Carpagnano, Corrado Pelaia, Maria D’Amato, Nunzio Crimi, Nicola Scichilone, Giulia Scioscia, Onofrio Resta, Cecilia Calabrese, Girolamo Pelaia, Carla Maria Irene Quarato and Maria Pia Foschino Barbaro in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Switching from omalizumab to mepolizumab: real-life experience from Southern Italy by Giovanna Elisiana Carpagnano, Corrado Pelaia, Maria D’Amato, Nunzio Crimi, Nicola Scichilone, Giulia Scioscia, Onofrio Resta, Cecilia Calabrese, Girolamo Pelaia, Carla Maria Irene Quarato and Maria Pia Foschino Barbaro in Therapeutic Advances in Respiratory Disease