Abstract
Aims
The arcuate nucleus is a vital brain region for coursing of pain command. G protein-coupled kinase 6 (GRK6) accommodates signaling through G protein-coupled receptors. Studies have demonstrated that GRK6 is involved in inflammatory pain and neuropathic pain. The present study was designed to explore the role and the underlying mechanism of GRK6 in arcuate nucleus of chronic visceral pain.
Methods
Chronic visceral pain of rats was induced by neonatal maternal deprivation and evaluated by monitoring the threshold of colorectal distension. Western blotting, immunofluorescence, real-time quantitative polymerase chain reaction techniques, and Nissl staining were employed to determine the expression and mutual effect of GRK6 with nuclear factor κB (NF-κB).
Results
Expression of GRK6 in arcuate nucleus was significantly reduced in neonatal maternal deprivation rats when compared with control rats. GRK6 was mainly expressed in arcuate nucleus neurons, but not in astrocytes, and a little in microglial cells. Neonatal maternal deprivation reduced the percentage of GRK6-positive neurons of arcuate nucleus. Overexpression of GRK6 by Lentiviral injection into arcuate nucleus reversed chronic visceral pain in neonatal maternal deprivation rats. Furthermore, the expression of NF-κB in arcuate nucleus was markedly upregulated in neonatal maternal deprivation rats. NF-κB selective inhibitor pyrrolidine dithiocarbamate suppressed chronic visceral pain in neonatal maternal deprivation rats. GRK6 and NF-κB were expressed in the arcuate nucleus neurons. Importantly, overexpression of GRK6 reversed NF-κB expression at the protein level. In contrast, injection of pyrrolidine dithiocarbamate once daily for seven consecutive days did not alter GRK6 expression in arcuate nucleus of neonatal maternal deprivation rats.
Conclusions
Present data suggest that GRK6 might be a pivotal molecule participated in the central mechanisms of chronic visceral pain, which might be mediated by inhibiting NF-κB signal pathway. Overexpression of GRK6 possibly represents a potential strategy for therapy of chronic visceral pain.
Keywords: Chronic visceral pain, neonatal maternal deprivation, arcuate nucleus, GRK6, NF-κB
Introduction
Irritable bowel syndrome (IBS) is a common gastrointestinal dysfunction disease,1,2 which is related to patients’ psychological and genetic factors.3,4 It is mainly manifested as repeated abdominal pain, while the clinical examination is normal. Up to now, there have been no effective drugs to treat chronic visceral pain. It is therefore urgent for researchers and doctors to explore the exact etiology and effective therapeutic targets. Recent studies show that neonatal maternal deprivation (NMD) can induce chronic visceral pain in adult rats.5,6 NMD is an early life stress model in which pups were left alone in a dark and warm compartment for 3 h per day from 2 to 15 days after birth.7 We used this model to imitate IBS in human.8,9 Studies indicate that dysfunction of peripheral nervous system such as dorsal root ganglion (DRG) could induce IBS symptom in NMD rats.7 In addition, the upregulation expression of transient receptor potential vanilloid 1 (TRPV1) in the basolateral amygdala (BLA) region abducted chronic visceral pain in NMD rats.10 The arcuate nucleus (ARC) is located in the medial base of the hypothalamus and plays a significant role in the regulation of nociception.11 We have demonstrated that the upregulation of cystathionine β-synthetase in ARC produces pain hypersensitivity in rats.12 However, whether ARC is involved in chronic visceral pain in IBS remains to be elucidated.
G protein-coupled kinases (GRKs) is a crucial protein that accommodates cell signals by phosphorylating specific amino acid residues in the cytoplasmic domain of G protein-coupled receptor (GPCRs) to desensitize GPCRs. GPCRs contain seven hydrophobic residues, which after being stimulated by external signals can produce a second messenger inside the cell. Thus, it allows the extracellular signal to be delivered to intracellular, impacting the cell behavior.13 Researches show that GRKs participated in the regulation of pain signals in inflammatory environments.14 Moreover, GRK6 can directly act on multiple downstream signaling molecules to regulate cell signaling pathways.15,16 GRK6 is a major member in the GRKs family. Previous reports have increasingly concentrated in the roles of GRK6 in inflammatory diseases.13 For instance, GRK6 is involved in the adjustment of inflammatory pain by inhibiting the interaction of neurons with inflammatory factors.17 Recently studies indicated that the downregulated expression of GRK6 plays a crucial role in neuropathic pain.18,19 It is an elegant example that overexpressed GRK6 eases neuropathic pain of chronic constriction injury rats at DRG level.19 These studies showed that GRK6 might be referred to pain regulation processes at the peripheral level under inflammatory pain conditions. However, whether GRK6 in ARC is involved in the initiation and maintenance of chronic visceral pain remains largely unclear.
NF-κB is identified as a vital transcription factor in the processing of inflammation and pain.20–22 We have manifested that NF-κB could induce chronic visceral pain at peripheral level and that TLR4 upregulates the expression of cystathionine beta-synthetase (CBS) through activating NF-κB signal in IBS rats.23 It also has been shown that the upregulation of CBS expression activated NF-κB signal in diabetic rats, which contributed to chronic visceral pain.24 Previous researches have indicated that GRK6 was linked to tumor necrosis factor (TNF)-α-induced inflammation via activation of NF-κB signaling in peritoneal macrophages.25 However, whether NF-κB in ARC is involved in the development of chronic visceral pain remains unknown.
In the present study, we hypothesized that the downregulated expression of GRK6 in ARC results in the upregulated expression of NF-κB, thus contributing to chronic visceral hypersensitivity. A previously established visceral pain model induced by NMD was used. We showed for the first time that NMD resulted in the downregulation of GRK6 expression in ARC of rats. We also demonstrated that NMD significantly enhanced the expression of NF-κB in ARC of rats. Our results indicated that in the hypothalamic ARC, GRK6 regulating NF-κB signaling pathway plays an important role in the occurrence and maintenance of chronic visceral pain of adult rats with NMD. The present study also presents a potential strategy for the treatment of chronic visceral hypersensitivity in patients with functional gastrointestinal disorders such as IBS.
Materials and methods
Induction of chronic visceral hyperalgesia
Experiments were executed in male Sprague-Dawley rats. The reason why only male rats were used in the present study is because female rats produce estrogen, which may affect the results. To minimize the interference of irrelevant variables, all male rats were used in the present study. Animals were properly fed (room temperature maintained at 25 ± 1°C around the clock; lights on 07:00–19:00). When experiments were accomplished, animals were euthanized, which was accorded with the relevant provisions of the Institutional Animal Care and Use Committee of Soochow University. Besides, we abided by the guidelines of the International Association for the Study of Pain. Chronic visceral pain was established by NMD described previously.7,26 Hyperalgesia of the abdomen were determined by colorectal distension (CRD) and abdominal withdrawal reflex (AWR), as described previously.27,28
Implantation of intracerebral guide cannula and ARC microinjection
Rats weighting from 180 to 220 g were used. The 4% chloral hydrate (Sinopharm Chemical Reagent Co. Ltd, Shanghai, China) was injected into abdominal cavity (1 mL/100 g body weight). Rats were immobilized on a brain stereoscopic locator. After the surgical instruments have been sterilized, an stainless-steel guide cannula (no. 8 steel tube) was settled 2.8 mm dorsally to the ARC (anteroposterior (AP): −4.0, L: 0.5, V: 9.8 mm; AP, preceding (+) or subsequent (−) to Bregma; L, lateral to midline; V, ventral to the surface of skull) and fixed to the skull surface by dental acrylic as described previously.12,29 Drugs in the volume of 0.5 µL were slowly injected into ARC area within 3–4 min and needle was stayed for 5 min. This procedure enabled ARC to fully absorb the drug and also to prevent drugs from returning along the original path. In behavioral experiments, pyrrolidine dithiocarbamate (PDTC, P8765-1G, Sigma, USA), a selective inhibitor of NF-κB, which dissolved in sulfoxide (DMSO), were injected into ARC area by brain stereoscopic localization method at different doses (0, 80, 800, and 8000 ng/kg). Dose selection was based on literature30 and preliminary behavior exploration. Drug was injected once for behavior detection or injected once a day for seven consecutive days for measuring molecule expression.12 Lentiviral vectors production and infection was consistent with previously reported methods.19 The approximate density of negative control lentiviral (LV-NC)/LV-GRK6 obtained were 1.25 × 108 transducing units per milliliter (TU/mL). Similarly, the 0.5 µL drug was injected into the ARC region by microinjection. After the operation, rats were permitted to recover for at least five days.
Measurement of hindpaw withdrawal threshold and latency
All rats were fed in soft padding from birth to death, with the purpose of protecting rats’ feet and reducing the influence of irrelevant factors on the experimental results. Before formal experiment, rats were drilled to acclimate three or four days in advance. The pain threshold was measured in control (CON) and NMD rats before and after injected with LV-GRK6 and LV-NC. Paw withdrawal threshold (PWT) in response to von Frey Filament stimulation and thermal paw withdrawal latency (PWL) in response to radiant heat applied to the hindpaw plantar surface were performed as described previously.31 All behavioral experiments were performed in a double-blinded manner.
Isolation of brain tissues
Under deep anesthesia, the rat brain was rapidly isolated from the cranial cavity. According to the Paxinos and Watson atlas, the coronal sectioning was done and the specific brain areas (ARC, insular cortex (IC), and BLA) were collected as described previously.
Separation of ARC tissues
The arcuate (ARC) nucleus is one of the two major sites in the hypothalamus that are located in its periventricular zone.32,33 After the brain was quickly removed, diencephalon was dissected out by an anterior coronal section, anterior to the optic chiasma, and a posterior coronal cut at the posterior border of the mammillary bodies. For separation of the ARC from the anteroventral periventricular, the third coronal cut was made in middle of the optic tract, just rostral to infundibulum. In addition, the hippocampus, fornix, and mammillothalamic tracts were always used as landmarks for the ARC nucleus (AP: −4.0 mm, L: 0.5 mm, V: 9.8 mm in Paxinos and Watson atlas). ARC was placed in a freezer tube in a liquid nitrogen box, and then the vial was kept in −80°C freezer until use.
Separation of BLA tissues
According to Paxinos and Watson spectrum, the BLA was located at AP = −2.8 mm, L = 5.0 mm, and H = 8.6 mm.32,34 After the rat brain was slowly lifted up out of the skull, the coronal plane was cut vertically in front of its anterior end and behind its posterior end. The anterior and posterior parts of the brain were discarded if there is no need for other uses. The coronal plane is placed flat, keeping the dorsal surface away from the experimenter. Two hemispheres and then the cortex from the contiguous amygdala were separated. Then, a triangular area of BLA tissue was cut with the blade on the left and right sides. BLA was placed in a freezer tube in a liquid nitrogen box and then vial was kept in −80°C freezer until needed.34
Separation of IC tissues
According to Paxinos and Watson atlas, the IC was located at AP = −3.2 mm, L = 4.4 mm, and H = 5.2 mm. The procedures for isolation of IC tissues were very similar to BLA as described above. The IC tissue was taken out and placed in a freezer tube in a liquid nitrogen box. The tissue was stored in a refrigerator at −80°C for later use.
Western blotting
The expressions of GRK6 and NF-κB were detected by Western blot as reported previously.31 The membrane was incubated with anti-GRK6 (1:1000, Santa Cruz Biotechnology, USA), or anti-NF-κB (1:1000, Santa Cruz Biotechnology) and anti-GAPDH (1:1000, Goodhere, China), anti-rabbit secondary antibody (1:2000, Jackson Immuno Research Laboratories, Inc), anti-mouse secondary antibody (1:2000, Jackson Immuno Research Laboratories, Inc). Experimental results were analyzed by ImageJ software (Bio-Rad, USA).
Real-time qPCR
Real-time polymerase chain reaction (PCR) was used to detect the messenger RNA (mRNA) expression of NF-κB and GRK6 as described previously.24,35 In brief, purified total RNA was extracted from the hypothalamus ARC tissue with trizol (Ambion, USA). The samples were mixed according to the system in the reverse transcription kit (Transgen Biotech, China) as described previously. Reverse transcription reaction was performed on the PCR apparatus, and the extracted RNAs were reversely transcribed into cDNA. Then, the mRNA expressions of GRK6 and NF-κB were detected by the real-time quantitative PCR (qPCR) reaction. Relevant conditions were set on ABI-7500 for on-board reaction. It took a total of 129 min and went through two cycles. In the second phase of the second cycle, the program started collecting fluorescent signals. Data were analyzed according to the obtained Ct value. The sequences of the primers for GRK6, NF-κB, and GAPDH are shown in Table 1.
Table 1.
Primer sequences used in the present study.
| Primers | Sequences (5′ to 3′) |
|---|---|
| GRK6-F | ATGTCTTTGGGCTGGATGGG |
| GRK6-R | GGTGGGGAGCTCTTCTTCAC |
| NF-κB-F | CCCAGCCCTATGCCTTTTCA |
| NF-κB-R | ACAGGTGCAGACAGGGATTG |
| GAPDH-F | GATGGCCTTCCGTGTTCCTA |
| GAPDH-R | CTTCACCACCTTCTTGATGTCATC |
GAPDH: glyceraldehyde-3-phosphate dehydrogenase; NF-κB: nuclear factor κB; GRK6: G protein-coupled kinase 6.
Immunofluorescence study
Ten days after lentiviral injection, the CON or NMD rats were intracardially perfused with 500 mL 0.9% normal saline solution followed by 4% paraformaldehyde (Sinopharm Chemical Reagent Co. Ltd, Shanghai, China). The brain was removed and after fixed by paraformaldehyde overnight and followed gradient dehydration by 10%, 20%, and 30% sucrose solution (Sinopharm Chemical Reagent Co. Ltd). What needs special attention was that rats injected with LV-GRK6 needed to avoid light all the way to prevent fluorescence quenching. The ARC area was cut at 14 µm on a freezing microtome (Leica, Germany). The primary antibodies used in the present experiment included anti-GRK6 (1:100, Santa Cruz Biotechnology), anti-NF-κB (1:200; Santa Cruz, CA, USA), anti-neuronal nuclei (NeuN) (1:200, Merck Millipore, USA), anti-CD11b (1:400, Miltenyi Biotec), and anti-glial fibrillary acidic protein (GFAP) (1:200, Cell Signaling Technology). The secondary antibodies included Alexa Fluor 488 (1:500, Molecular Probes, NY, USA), Alexa Fluor 555 (1:200, Molecular Probes), Alexa Fluor 568 (1:300, Invitrogen by Thermo Fisher Scientific, USA), Dylight 488 (1:300, Boster, China), and Cy3 (1:300, Boster, China). Immunofluorescence technology was performed as described previously,19,35 and the difference was blockade with 7% donkey serum at room temperature for 6 h. The brain slices were synchronously incubated with primary antibodies for 36–48 h at 4°C.
Nissl staining
Nissl staining was performed as previously reported.10 Briefly, the frozen sections with thickness 35 µm were rinsed in distilled water for 2 min. Sections were then dyed with Nissl staining solution (Beyotime Institute of Biotechnology, China) for 10 min at 37°C, replaced with fresh distilled water, and washed for another 2 min. Next, they were soaked in 95% ethanol for 5 s and washed twice with 70% alcohol every 5 s.
Data quantification and statistics
All data are shown as the mean ± standard error of the mean. Two-way repeated measures analysis of variance (ANOVA) was used to analyze behavioral data of microinjection of lentivirus and antagonists. Two-tailed two-sample t-test was implemented to analyze data of Western blotting, immunofluorescence, qPCR, and some behavioral outcomes that fit the normal distribution. Mann–Whitney test was applicable to analysis data of behavior results that was not a normally distributed population. A comparison was considered statistically significant when the p value was < 0.05.
Results
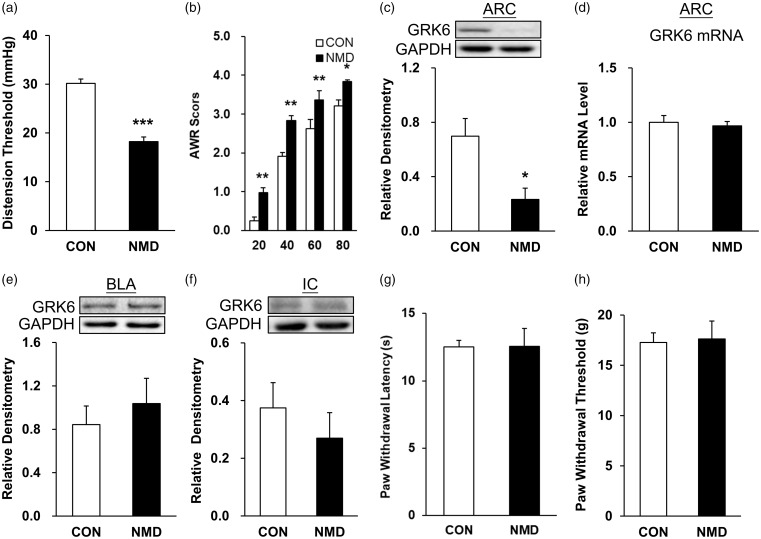
NMD suppressed GRK6 expression in ARC
Consistent with previous studies,10,36 chronic visceral hypersensitivity was induced by NMD rats manifested by the reduced CRD threshold (Figure 1(a), ***p < 0.001, compared with CON group, two-tailed two-sample t-test) and the increased AWR scores (Figure 1(b), *p < 0.05, **p < 0.01, compared with CON group, two-way ANOVA). The value of CRD threshold for CON rats (n = 9) at six weeks was 30.15 ± 0.89 mmHg. The value of CRD threshold for NMD rats (n = 9) at six weeks was 18.21 ± 0.97 mmHg. The values of AWR scores for CON rats (n = 9) at 20, 40, 60, and 80 mmHg were 0.25 ± 0.10, 1.91 ± 0.10, 2.62 ± 0.25, and 3.20 ± 0.15, respectively. The values of AWR scores for NMD rats (n = 9) at 20, 40, 60, and 80 mmHg were 0.98 ± 0.12, 2.83 ± 0.12, 3.37 ± 0.23, 3.83 ± 0.04, respectively. To determine whether GRK6 is involved, Western blotting was used to detect its expression in the ARC, BLA, and IC regions of NMD with CON rats at the age of six weeks (Figure 1(c) to (f)). Statistical analysis showed that the GRK6 level at ARC regions was significantly reduced in NMD when compared with CON rats (Figure 1(c), *p < 0.05, n = 4 rats for each group, two-tailed two-sample t-test). There was no significant difference in GRK6 expression in BLA and IC regions of NMD rats when compared with age-matched CON rats (Figure 1(e) and (f), p > 0.05, two sample t-test). Meanwhile, reverse transcription PCR (RT-PCR) assays were performed to detect the mRNA level of GRK6 in hypothalamic ARC regions of rats. The relative mRNA level of GRK6 was 1.00 ± 0.07 in CON rats (n = 4) and 0.97 ± 0.04 in NMD rats (n = 4). GRK6 expression at the mRNA level was not remarkably altered in ARC regions of rats (Figure 1(d), p > 0.05, two-tailed two-sample t-test). Since previous studies have shown that ARC was an important region for regulating mechanical and thermal pain in rats,29 we next investigated whether NMD induced somatic pain in adult rats. PWL and PWT of the rat hind limbs were tested. No significant changes of PWL were observed in NMD group when compared with CON group (Figure 1(g), p > 0.05, two-tailed two-sample t-test). The values of PWL for CON rats (n = 6) and NMD group (n = 5) were 12.53 ± 0.48 and 12.57 ± 1.32 s, respectively. Similarly, the PWT was not markedly altered in NMD rats (Figure 1(h), p > 0.05, Mann–Whitney test). The values of PWT for CON rats (n = 6) and NMD group (n = 5) were 17.27 ± 0.96 and 17.62 ± 1.78 g, respectively.
Figure 1.
NMD suppressed GRK6 expression in ARC. (a) The colorectal distention (CRD) threshold of NMD rats was significantly lower than that of control rats (n = 9 for each group, ***p < 0.001, two-tailed two- samples t-test). (b) The AWR score of NMD rats was significantly higher than the control rats (n = 9 for each group, *p < 0.05, **p < 0.01, two-way ANOVA). (c) GRK6 expression was significantly decreased in ARC of NMD rats (n = 6 for CON and n = 5 for NMD, *p < 0.05 two sample t-test). (d) The mRNA level of GRK6 was not altered in ARC of NMD rats (n = 4 for each group, p > 0.05, two sample t-test). There was no significant alteration in the expression of GRK6 in BLA (e) and IC (f) brain regions of NMD rats (n = 4 for each group, p > 0.05, two sample t-test). Compared with the age-matched control group (n = 6), there were no significant alterations in the thermal pain threshold ((g), p > 0.05, two-tailed two-sample t-test) and mechanical pain threshold ((h), p > 0.05, Mann–Whitney test) of hind limbs in NMD rats (n = 5). CON: control; NMD: neonatal maternal deprivation; ARC: arcuate nucleus; mRNA: messenger RNA; BLA: basolateral amygdala; IC: insular cortex; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GRK6: G protein-coupled kinase 6.
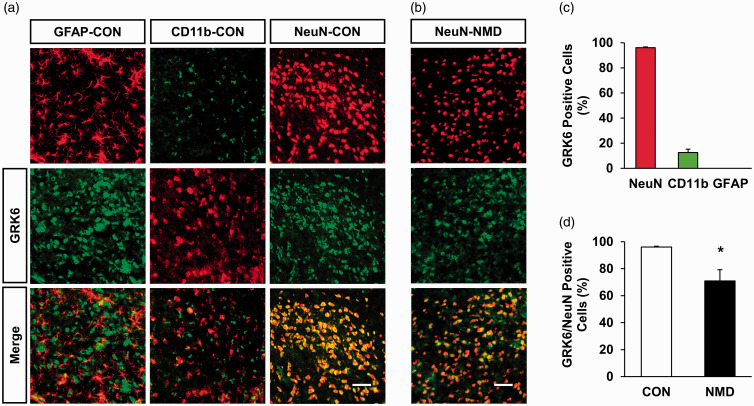
NMD reduced the percentage of GRK6-positive ARC neurons
We then determined the location of GRK6 in the ARC region using immunofluorescence. Antibodies of NeuN (a marker of neurons) or CD11b (a marker of microglial cell) or GFAP (a marker of astrocytes) were used. The results showed that GRK6 was co-expressed predominately in neurons, but not in astrocytes, and a little with microglial cells of control rats (Figure 2(a)). GRK6-positive cells were also co-labeled with the NeuN-positive cells in the ARC of NMD rats (Figure 2(b)). Quantative analysis showed that the percentage of GRK6 localization with NeuN, CD11b, and GFAP was 96.04%, 12.53%, and 0, respectively (Figure 2(c), n = 3 rats). More importantly, the percentage of GRK6-positive neurons was markedly reduced in ARC of NMD rats when compared with control rats (Figure 2(d), n = 3, for each group, *p < 0.05, two-tailed two-sample t-test). GRK6-positive cells of NeuN in CON and NMD rats were 96.04% and 70.90%, respectively.
Figure 2.
NMD reduced the percentage of GRK6-positive ARC neurons. (a) GRK6-positive cells (middle) were co-labeled with the NeuN-positive cells (upper right, red). It was co-expressed with CD11b-positive cells (upper middle, green) in a small amount but not with GFAP-positive cells (upper left, red) in the ARC of CON rats. Scale bar = 50 µm. (b) GRK6-positive cells (middle, green) were co-labeled with the NeuN-positive cells (upper, red) in the ARC of NMD rats. Scale bar = 50 µm. (c) Data analysis showed that GRK6 was mainly present in NeuN-labeled neurons, a small amount in CD11b-labeled microglial cells, and not in GFAP-labeled astrocytes in ARC. (d) Statistic analysis indicated that GRK6-positive cells of NeuN in the ARC region of NMD rats was significantly decreased when compared with the CON rats (n = 3 for each group, *p < 0.05, two-tailed two-sample t-test). GFAP: glial fibrillary acidic protein; CON: control; NMD: neonatal maternal deprivation; NeuN: neuronal nuclei; GRK6: G protein-coupled kinase 6.
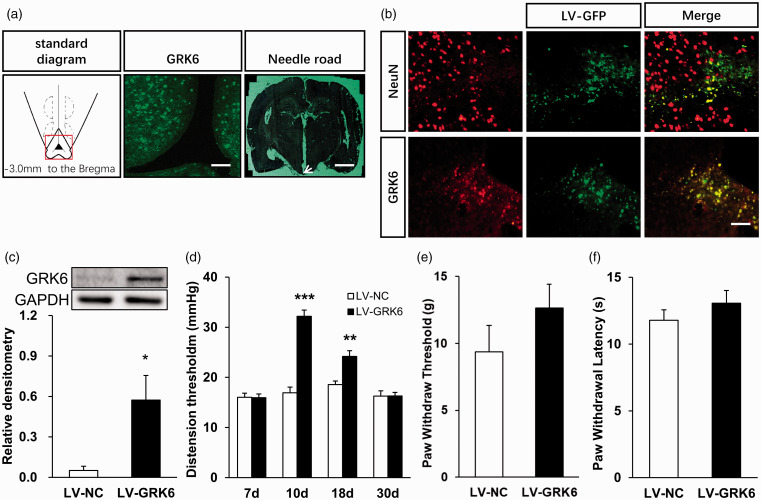
Overexpression of GRK6 attenuated visceral pain in NMD rats
To further explore the role of GRK6 in the regulation of chronic visceral pain, we next examined the effect of microinjection of LV-GRK6 into ARC area of NMD rats. GRK6 LV-NC was used as control group. CRD threshold were detected seven days after LV injection. A standard diagram in Figure 3(a) (left) indicates the coronal section at 3.0 mm posterior to Bregma as described previously.37,38 A fluorescence image of GRK6 in Figure 3(a) (middle) was recorded from the ARC area indicated within the red square on the diagram. A picture of actual track of microinjection in the ARC as pointed out by the white arrow was shown in Figure 3(a) (right). Figure 3(b) also shows that transgene expression could be achieved in the ARC region after injection of lentiviral carrying the green fluorescent protein (LV-GFP) into the ARC (Figure 3(b), middle). Many LV-GFP-positive cells in Figure 3(b) (top middle, green) were associated with NeuN-positive cells (top left, red). Almost all the LV-GFP-positive cells (bottom middle, green) were co-expressed with GRK6-positive cells (bottom left, red). The LV-GRK6 efficiency in ARC was examined by Western blot assay (Figure 3(c)). GRK6 protein expression of NMD-LV-GRK6 rats was significantly enhanced when compared with NMD-LV-NC rats (Figure 3(c), n = 4 for each group, *p < 0.05, two-sample t-test). The relative densitometry values of LV-NC and LV-GRK6 group were 0.05 ± 0.03 and 0.57 ± 0.18, respectively. GRK6 overexpression by lentiviral injection in ARC area significantly attenuated visceral pain sensitivity (Figure 3(d), **p < 0.01, ***p < 0.001, vs. LV-NC, two-way ANOVA) in rats with NMD. Microinjection of GRK6 overexpression lentiviral on ARC area did not significantly change the PWT (Figure 3(e), p >0.05 vs. LV-NC, Mann–Whitney test) and PWL (Figure 3(f), p > 0.05 vs. LV-NC, Mann–Whitney test) in NMD rats. The PWT values of rats in LV-NC (n = 8) and LV-GRK6 (n = 8) group were 9.36 ± 1.99 and 12.63 ± 1.79 g, respectively. The PWL values of rats in LV-NC (n = 8) group and LV-GRK6 (n = 8) group were 11.78 ± 0.78 and 13.06 ± 0.95, respectively.
Figure 3.
Overexpression of GRK6 in ARC region reversed chronic visceral pain in NMD rats. (a) A standard diagram (left) indicates the coronal section at 3.0 mm posterior to Bregma. A fluorescence image of GRK6 (middle, green) was recorded from the ARC area indicated within the red square on the diagram (left). Scale bar = 100 µm. The image (right) was a needle track from cerebral cortex to ARC region dyed by nitrite staining. Scale bar = 1200 µm. (b) Many LV-GFP-positive cells (top middle, green) were associated with NeuN-positive cells (top left, red). Almost all the LV-GFP-positive cells (bottom middle, green) were co-expressed with GRK6-positive cells (bottom left, red). Scale bar = 50 µm. (c) GRK6 protein expression in ARC of LV-GRK6 rats was significantly upregulated when compared with LV-NC rats (n = 4 for each group, *p < 0.05, two sample t-test). (d) Overexpression of GRK6 in ARC significantly attenuated chronic visceral pain sensitivity of adult NMD rats (LV-NC group n = 7 and LV-GRK6 group n = 10, **p < 0.01, ***p < 0.001, vs. LV-NC, two-way ANOVA). Compared with LV-NC rats, the mechanical (e) and thermal (f) pain thresholds of hindpaw of LV-GRK6 rats had no significant change (n = 8 rats for each group, p>0.05, Mann–Whitney test). GRK6: G protein-coupled kinase 6; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; NC: negative control; LV: lentiviral.
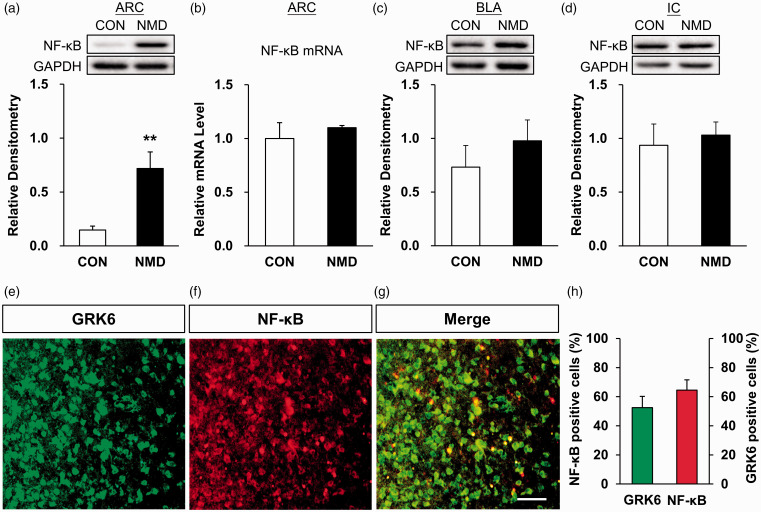
NF-κB expression in ARC was upregulated and was co-expressed in GRK6-positive neurons of CON rats
Existing studies showed that the downregulated expression of NF-κB was participated in the generation and maintenance of inflammatory pain.39,40 To test whether NF-κB is involved in chronic visceral pain, RT-PCR and Western blotting analyses were performed. Compared with CON rats (n = 6), the protein expression of NF-κB in ARC of NMD rats (n = 5) was significantly increased (Figure 4(a), **p < 0.01, two-tailed two-sample t-test). The relative densitometry values of NF-κB expression in CON and NMD rats were 0.15 ± 0.04 and 0.72 ± 0.15, respectively. However, the mRNA expression of NF-κB in hypothalamus ARC region of NMD rats was not significantly altered when compared with the control group (Figure 4(b), n = 4 for each group, p > 0.05, two-tailed two-sample t-test). The relative mRNA expression level of the CON and NMD group was 1 ± 0.08 and 1.01 ± 0.08, respectively. To further confirm whether the upregulated expression of NF-κB in ARC region is tissue specific, the protein expression of NF-κB in BLA and IC areas was also tested. The results showed that there were no significant changes in the expression of NF-κB in NMD rats compared with CON rats (Figure 4(c) and (d), n = 4 for each group, p > 0.05, two-tailed two-sample t-test). In the BLA region, the relative densitometry values of NF-κB in CON and NMD group were 0.73 ± 0.20, 0.98 ± 0.19, respectively. In the IC region, the relative densitometry values of NF-κB in CON and NMD group were 0.94 ± 0.20 and 1.03 ± 0.12, respectively.
Figure 4.
Upregulated expression of NF-κB in ARC and co-expression of GRK6 with NF-κB in ARC neurons of CON rats. (a) Compared with CON rats (n = 6), NF-κB protein expression was significantly increased in ARC of NMD rats (n = 5) (**p < 0.01, two-tailed two-sample t-test). (b) The mRNA expression of NF-κB receptor in ARC region of NMD rats was not significantly altered (n = 4, for each group, p > 0.05, two-tailed two-sample t-test). (c and d) Compared with CON rats, NF-κB protein expression was not significantly altered in the BLA and IC regions of NMD rats (n = 4 for each group, p > 0.05, two sample t-test). (e) GRK6-positive cells were colored with green fluorescent secondary antibody. (f) NF-κB-positive cells were colored with red fluorescent secondary antibody. (g) GRK6 and NF-κB was co-expressed in ARC neurons (kelly). Scale bar = 50 µm. (h) Statistical analysis showed that most of GRK6-positive cells and NF-κB-positive cells were co-expressed in ARC neurons (n = 4 rats for each group). GRK6: G protein-coupled kinase 6; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; CON: control; NMD: neonatal maternal deprivation; ARC: arcuate nucleus; BLA: basolateral amygdala; NF-κB: nuclear factor κB; IC: insular cortex.
A recent study had shown that TNF-α induced GRK6 activation, and GRK6 phosphorylates IκBα at Ser32/Ser36 to induce the NF-κB signaling pathway, which promoted inflammation.25 To further investigate whether there is the same pathway between GRK6 and NF-κB in the hypothalamus ARC region, the localization of GRK6 and NF-κB in CON rats was detected by immunofluorescence analysis. As shown in Figure 4, GRK6-positive cells were colored with green fluorescent secondary antibodies (Figure 4(e)), and NF-κB-positive cells were colored with red fluorescent secondary antibodies (Figure 4(f)). GRK6 and NF-κB were co-expressed in the hypothalamus ARC region (kelly, Figure 4(g)). Statistical analysis showed that majority of GRK6-positive cells was co-expressed with NF-κB in same ARC neurons (Figure 4(h), n = 4 rats). The proportion of GRK6-positive cells in total NF-κB-positive cells was 52.94%. The proportion of NF-κB-positive cells in total GRK6-positive cells was 64.62%.
NF-κB-selective inhibitor PDTC suppressed visceral pain in NMD rats
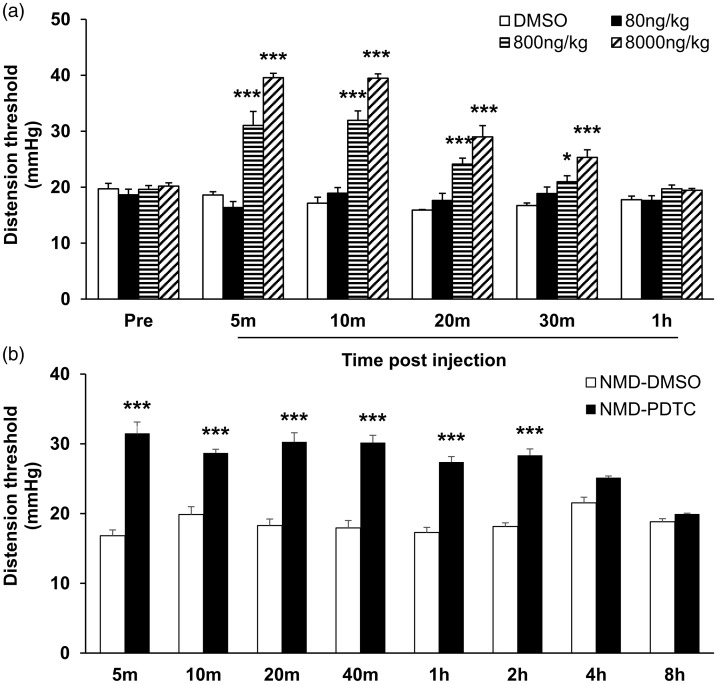
To further confirm the role of NF-κB in chronic visceral pain sensitization, we next determined whether treatment with NF-κB-selective inhibitor PDTC attenuated visceral pain. On the basis of published articles,24 PDTC at the doses of 0, 80, 800, and 8000 ng/kg was administered. As shown in Figure 5(a), single injection of PDTC (800 and 8000 ng/kg) into the ARC area of NMD rats significantly attenuated visceral pain sensitivity of NMD rats (n = 5 for each group, *p < 0.05, ***p < 0.001, two-way ANOVA). Moreover, the effective time of the 800 ng/kg PDTC group lasted from 5 min to 30 min after injection. The effective time of the 8000 ng/kg PDTC group lasted from 5 min to 30 min after injection. A single injection of drugs into the ARC region may induce a stress response in rats. Therefore, to further confirm whether visceral pain relief was caused by stress or the therapeutic effect of drugs, 800 ng/kg PDTC and DMSO solution were repeatedly injected into the ARC area of NMD rats for seven consecutive days. As shown in Figure 6(b), compared with the DMSO group at the same time period, consecutive seven-day injection of 800 ng/kg PDTC into ARC area remarkably attenuated the chronic visceral pain of adult NMD rats (Figure 5(b), n = 6 for each group, ***p < 0.001, two-way ANOVA). This effect lasted from 5 min to 2 h after the drug injection.
Figure 5.
NF-κB-selective inhibitor PDTC suppressed chronic visceral pain in NMD rats. (a) Single injection of PDTC (800 ng/kg and 8000 ng/kg) into the ARC area significantly attenuated chronic visceral pain sensitivity of NMD rats (n=5 for each group, *p<0.05, ***p<0.001, two-way ANOVA). (b) Compared with the DMSO group at the same time period, continuous injection of 800 ng/kg PDTC into ARC area for seven consecutive days remarkably attenuated the chronic visceral pain of adult NMD rats (n=6 for each group, ***p<0.001, two-way ANOVA). NMD: neonatal maternal deprivation; PDTC: pyrrolidine dithiocarbamate.
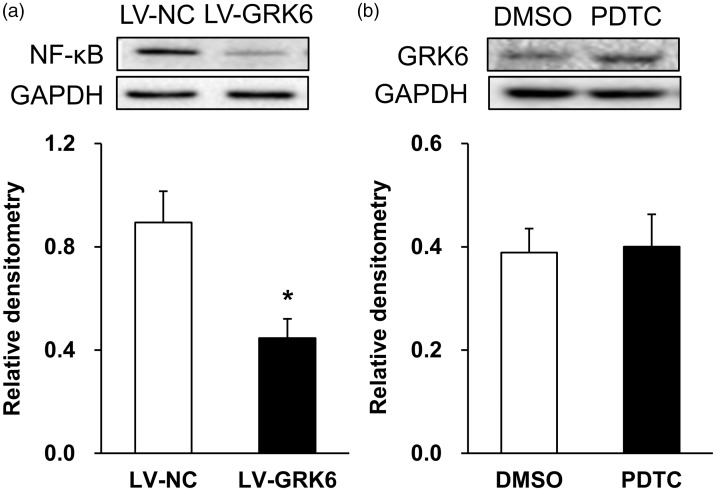
Figure 6.
Overexpression of GRK6 reversed the expression of NF-κB. (a) Compared with LV-NC rats, the protein expression of NF-κB receptor in LV-GRK6 group was significantly reduced (n = 4 for each group, *p < 0.05, two-tailed two-sample t-test). (b) There was no significant alteration in GRK6 protein expression in PDTC-treated group compared with DMSO group (n = 6 for DMSO and n = 5 for PDTC group, p > 0.05, two-tailed two-sample t-test). PDTC: pyrrolidine dithiocarbamate; GRK6: G protein-coupled kinase 6; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; NF-κB: nuclear factor κB; NC: negative control; LV: lentiviral.
Overexpression of GRK6 suppressed NF-κB expression
We next determined whether GRK6 downregulation contributed to the upregulation of NF-κB expression. Western blot was performed in this experiment. As described above, LV-GRK6 or LV-NC (0.5 µL) was injected into the ARC region of NMD rats by intracranial microinjection. Ten days after injection, the samples were taken and the expression of NF-κB was detected. Compared with LV-NC rats, the protein expression of NF-κB receptor in LV-GRK6 group was significantly reduced (Figure 6(a), n = 4 for each group, *p < 0.05, two-tailed two-sample t-test). Relative densitometry values of LV-GRK6 and LV-NC group were 0.89 ± 0.12 and 0.45 ± 0.07, respectively. To further confirm the upstream and downstream relationship between GRK6 and NF-κB. We injected PDTC (800 ng/kg) or DMSO solution into the ARC region of NMD rats for seven consecutive days. Then, the protein expression of GRK6 was detected. There was no significant alteration in GRK6 expression in PDTC-treated group (n = 5) when compared with DMSO group (Figure 6(b), n = 6, p > 0.05, two-tailed two-sample t-test). Relative densitometry values of GRK6 in DMSO and PDTC group were 0.39 ± 0.05 and 0.40 ± 0.06, respectively.
Discussion
It has been shown that hypothalamus ARC participated in pain regulation by releasing beta-endorphin molecules.12,41 ARC’s efferent fibers travel around from its center to many areas of the brain stem. This structure makes ARC to be a key center to promote pain under inflammatory conditions.42,43 However, the detailed molecular mechanisms involved remain largely unknown. Studies have demonstrated that downregulated expression of GRK6 can lower the pain threshold.14,19 In addition, hypothalamic ARC has been reported to play a key role in chronic pancreatitic pain conditions.12 However, whether ARC plays a role in chronic colonic pain sensitivity has not been reported. Our study was the first to demonstrate that hypothalamic ARC plays an important role in chronic visceral pain of IBS animal models. This conclusion is supported by the following observations. GRK6 expression was downregulated in ARC of NMD rats. Overexpression of GRK6 in ARC region attenuated chronic visceral pain in NMD rats. Therefore, we provide new evidence to support the view that the downregulated expression of GRK6 in ARC neurons is involved in the occurrence and maintenance of chronic visceral pain in functional gastrointestinal diseases at the central nervous system level.
Of note is that GRK6 expression at protein level was downregulated, while the GRK6 expression at mRNA level is not significantly altered in ARC region of NMD rats. The possible reasons for this inconsistency are as follows. There is no linear relationship between the mRNA abundance of the gene and its translation product-protein expression because there are many levels of regulation of gene expression.44–46 The regulation at transcription level is only one of the mechanisms. Post-transcriptional regulation, translation, and post-translational regulation also play a role in protein expression. Moreover, factors such as mRNA degradation, protein degradation, and modified folding may also lead to inconsistency between mRNA abundance and protein expression level. However, we did observe that the overexpression of GRK6 by lentiviral system significantly enhanced the protein expression in ARC area. The detailed mechanism by which LV-GRK6 promoted GRK6 protein expression needs to be further investigated in the future. In addition, the expression of GRK6 was not changed in other tissues such as BLA and IC, indicating tissue-specific downregulation of GRK6 expression. Meanwhile, our experiment proved that NMD did not induce somatic mechanical pain sensitivity and heat pain sensitivity in adult rats.
The molecular mechanisms by which GRK6 downregulation in ARC lead to chronic visceral pain remain unknown. Our previous study showed that the upregulated expression of NF-κB at DRG level was involved in the chronic visceral pain sensitivity in NMD rats.47 Was NF-κB in the ARC region involved in NMD-induced chronic visceral pain sensitivity? Was there a signaling pathway between GRK6 and NF-κB in ARC region in NMD rats? To answer these questions, we first examined the expression and function of NF-κB in ARC region in NMD rats. Our results showed that the expression of NF-κB in the ARC region was upregulated. However, the protein expression of NF-κB in BLA and IC regions was not significant altered in NMD model, indicating the tissue-specific upregulation of NF-κB expression. This result was consistent with previous studies.39,40,48 Importantly, a single injection of PDTC into the ARC region significantly reversed the chronic visceral pain sensitivities of NMD rats. The long-time analgesic effect was achieved after the PDTC injection for seven consecutive days. These data suggest that NF-κB in ARC region plays effects in chronic visceral pain in NMD rats. To explore whether there is an interaction between GRK6 and NF-κB in ARC region, the immunofluorescence approaches and pharmacologic methods were employed in the present study. Immunofluorescence results showed that GRK6 and NF-κB were co-expressed in the ARC neurons, indicating a possibility that GRK6 regulates NF-κB expression. Further experiment showed that overexpression of GRK6 significantly reversed the upregulated expression of NF-κB in the ARC region of NMD rats. However, continuous injection of PDTC did not alter the expression of GRK6 in NMD rats. These data strongly suggest that GRK6 regulated the transcription of NF-κB in ARC region of NMD rats. It has been reported that in macrophages, inflammatory factors stimulate GRK6 trafficking into the cell membrane surface and directly acted on IκBα. This interaction promoted NF-κB to enter the nucleus and bound the promoter region of the related genes, thus enhanced the transcription of inflammatory genes.25 Therefore, we speculated that GRK6 in ARC region also regulates the expression of NF-κB, thus causing chronic visceral pain sensitivity in NMD rats.
In summary, our study demonstrated that GRK6 plays an important role in the development and maintenance of the chronic visceral pain by regulating NF-κB at the ARC level in NMD rats. The current results indicate that GRK6 might be a key molecule for the treatment of chronic visceral pain sensitivity at the central level. Overexpression of GRK6 may provide a potential therapeutic target for the treatment of chronic visceral pain sensitivity in patients with functional gastrointestinal disorders.
Author Contributions
XL and Y-CX performed experiments, analyzed data, and prepared figures and the manuscript. Y-QT, P-AZ, S-FH performed experiments and analyzed data. L-HW and X-HJ analyzed data and revised the manuscript. G-YX designed experiments, supervised the experiments, and finalized the manuscript. All the authors have read and approved the paper.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from National Natural Science Foundation of China (31730040, 81920108016 to GYX, 81771187 to X-HJ, and 81801115 to P-AZ) and from the Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
ORCID iD
Guang-Yin Xu https://orcid.org/0000-0002-5495-4120
References
- 1.Brown PW. The irritable bowel syndrome. J Kans Med Soc 1947; 48: 309–312. [PubMed] [Google Scholar]
- 2.Grad S, Dumitrascu DL. Irritable bowel syndrome subtypes: new names for old medical conditions. Dig Dis 2020; 38: 122–126. [DOI] [PubMed] [Google Scholar]
- 3.Whitehead WE, Engel BT, Schuster MM. Irritable bowel syndrome: physiological and psychological differences between diarrhea-predominant and constipation-predominant patients. Dig Dis Sci 1980; 25: 404–413. [DOI] [PubMed] [Google Scholar]
- 4.Sandler RS, Drossman DA, Nathan HP, McKee DC. Symptom complaints and health care seeking behavior in subjects with bowel dysfunction. Gastroenterology 1984; 87: 314–318. [PubMed] [Google Scholar]
- 5.Du WJ, Hu S, Li X, Zhang PA, Jiang X, Yu SP, Xu GY. Neonatal maternal deprivation followed by adult stress enhances adrenergic signaling to advance visceral hypersensitivity. Neurosci Bull 2019; 35: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan F, Tang Y, Dai H, Cao Y, Sun P, Chen Y, Chen A, Lin C. Blockade of BDNF signalling attenuates chronic visceral hypersensitivity in an IBS-like rat model. Eur J Pain 2020; 24: 839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu S, Xiao Y, Zhu L, Li L, Hu CY, Jiang X, Xu GY. Neonatal maternal deprivation sensitizes voltage-gated sodium channel currents in colon-specific dorsal root ganglion neurons in rats. Am J Physiol Gastrointest Liver Physiol 2013; 304: G311–G321. [DOI] [PubMed] [Google Scholar]
- 8.Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol 2002; 282: G307–G316. [DOI] [PubMed] [Google Scholar]
- 9.Gareau MG, Jury J, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation causes colonic dysfunction in rat pups including impaired host resistance. Pediatr Res 2006; 59: 83–88. [DOI] [PubMed] [Google Scholar]
- 10.Xiao Y, Chen X, Zhang PA, Xu Q, Zheng H, Xu GY. TRPV1-mediated presynaptic transmission in basolateral amygdala contributes to visceral hypersensitivity in adult rats with neonatal maternal deprivation. Sci Rep 2016; 6: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun YG, Gu XL, Yu LC. The neural pathway of galanin in the hypothalamic arcuate nucleus of rats: activation of beta-endorphinergic neurons projecting to periaqueductal gray matter. J Neurosci Res 2007; 85: 2400–2406. [DOI] [PubMed] [Google Scholar]
- 12.Zheng H, Zhu HY, Zhang XY, Wang M, Xiao Y, Xu GY, Jiang XH. Upregulation of cystathionine beta-synthetase in the arcuate nucleus produces pain hypersensitivity via PKC upregulation and GluN2B phosphorylation in rats with chronic pancreatitis. Sheng Li Xue Bao 2016; 68: 575–584. [PubMed] [Google Scholar]
- 13.Hamm HE. How activated receptors couple to G proteins. Proc Natl Acad Sci USA 2001; 98: 4819–4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eijkelkamp N, Heijnen CJ, Carbajal AG, Willemen HL, Wang H, Minett MS, Wood JN, Schedlowski M, Dantzer R, Kelley KW, Kavelaars AG. G protein-coupled receptor kinase 6 acts as a critical regulator of cytokine-induced hyperalgesia by promoting phosphatidylinositol 3-kinase and inhibiting p38 signaling. Mol Med 2012; 18: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruiz-Gomez A, Mellström B, Tornero D, Morato E, Savignac M, Holguín H, Aurrekoetxea K, González P, González-García C, Ceña V, Mayor F, Naranjo JR. G protein-coupled receptor kinase 2-mediated phosphorylation of downstream regulatory element antagonist modulator regulates membrane trafficking of Kv4.2 potassium channel. J Biol Chem 2007; 282: 1205–1215. [DOI] [PubMed] [Google Scholar]
- 16.Hall RA, Spurney RF, Premont RT, Rahman N, Blitzer JT, Pitcher JA, Lefkowitz RJ. G protein-coupled receptor kinase 6A phosphorylates the Na(+)/H(+) exchanger regulatory factor via a PDZ domain-mediated interaction. J Biol Chem 1999; 274: 24328–24334. [DOI] [PubMed] [Google Scholar]
- 17.Norbury TA, MacGregor AJ, Urwin J, Spector TD, McMahon SB. Heritability of responses to painful stimuli in women: a classical twin study. Brain 2007; 130: 3041–3049. [DOI] [PubMed] [Google Scholar]
- 18.Lombardi MS, Kavelaars A, Cobelens PM, Schmidt RE, Schedlowski M, Heijnen CJ. Adjuvant arthritis induces down-regulation of G protein-coupled receptor kinases in the immune system. J Immunol 2001; 166: 1635–1640. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Li RJ, Li M, Liu X, Zhu HY, Ju Z, Miao X, Xu GY. Overexpression of GRK6 attenuates neuropathic pain via suppression of CXCR2 in rat dorsal root ganglion. Mol Pain 2016; 12: 1744806916646381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tegeder I, Niederberger E, Schmidt R, Kunz S, Guhring H, Ritzeler O, Michaelis M, Geisslinger G. Specific inhibition of IkappaB kinase reduces hyperalgesia in inflammatory and neuropathic pain models in rats. J Neurosci 2004; 24: 1637–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang ZC, Li LH, Bian C, Yang L, Lv N, Zhang YQ. Involvement of NF-kappaB and the CX3CR1 signaling network in mechanical allodynia induced by tetanic sciatic stimulation. Neurosci Bull 2018; 34: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang K, Shi J, Shi J. Morin alleviates vincristine-induced neuropathic pain via nerve protective effect and inhibition of NF-kappaB pathway in rats. Cell Mol Neurobiol 2019; 39: 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan B, Tang WH, Lu LJ, Zhou Y, Zhu HY, Zhou YL, Zhang HH, Hu CY, Xu GY. TLR4 upregulates CBS expression through NF-kappaB activation in a rat model of irritable bowel syndrome with chronic visceral hypersensitivity. WJG 2015; 21: 8615–8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang HH, Hu J, Zhou YL, Hu S, Wang YM, Chen W, Xiao Y, Huang LY, Jiang X, Xu GY. Promoted interaction of nuclear factor-kappaB with demethylated cystathionine-beta-synthetase gene contributes to gastric hypersensitivity in diabetic rats. J Neurosci 2013; 33: 9028–9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohba Y, Nakaya M, Watari K, Nagasaka A, Kurose H. GRK6 phosphorylates IkappaBalpha at Ser(32)/Ser(36) and enhances TNF-alpha-induced inflammation. Biochem Biophys Res Commun 2015; 461: 307–313. [DOI] [PubMed] [Google Scholar]
- 26.Zhang PA, Xu QY, Xue L, Zheng H, Yan J, Xiao Y, Xu GY. Neonatal maternal deprivation enhances presynaptic P2X7 receptor transmission in insular cortex in an adult rat model of visceral hypersensitivity. CNS Neurosci Ther 2017; 23: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology 2000; 119: 1276–1285. [DOI] [PubMed] [Google Scholar]
- 28.Xu GY, Shenoy M, Winston JH, Mittal S, Pasricha PJ. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut 2008; 57: 1230–1237. [DOI] [PubMed] [Google Scholar]
- 29.Bu F, Tian H, Gong S, Zhu Q, Xu GY, Tao J, Jiang X. Phosphorylation of NR2B NMDA subunits by protein kinase C in arcuate nucleus contributes to inflammatory pain in rats. Sci Rep 2015; 5: 15945–15910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang B, Wang D, Ji TF, Shi L, Yu JL. Overexpression of lncRNA ANRIL up-regulates VEGF expression and promotes angiogenesis of diabetes mellitus combined with cerebral infarction by activating NF-kappaB signaling pathway in a rat model. Oncotarget 2017; 8: 17347–17359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Q, Zhang BY, Zhang PA, Hu J, Zhang HH, Xu GY. Downregulation of glucose-6-phosphate dehydrogenase contributes to diabetic neuropathic pain through upregulation of toll-like receptor 4 in rats. Mol Pain 2019; 15: 1744806919838659–1744806919838603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paxino G, Watson C. The rat brain in stereotaxic coordinates. Samford: Australian Academic Press, 1982, pp. 48–52. [Google Scholar]
- 33.Salehi MS, Namavar MR, Shirazi MRJ, Rahmanifar F, Tamadon A. A simple method for isolation of the anteroventral periventricular and arcuate nuclei of the rat hypothalamus. Anatomy 2013; 6–7: 48–51. [Google Scholar]
- 34.Gilbert K, Godbout R, Rousseau G. Caspase-3 activity in the rat amygdala measured by spectrofluorometry after myocardial infarction. J Vis Exp 2016; 107: e53207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu R, Zhang PA, Liu X, Zhou Y, Xu M, Jiang X, Yan J, Xu GY. Decreased miR-325-5p contributes to visceral hypersensitivity through post-transcriptional upregulation of CCL2 in rat dorsal root ganglia. Neurosci Bull 2019; 35: 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang PA, Zhu HY, Xu QY, Du WJ, Hu S, Xu GY. Sensitization of P2X3 receptors in insular cortex contributes to visceral pain of adult rats with neonatal maternal deprivation. Mol Pain 2018; 14: 1744806918764731–1744806918764703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frantz KJ, O'Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology 2007; 32: 625–637. [DOI] [PubMed] [Google Scholar]
- 38.Ma H, Yao C, Ma P, Zhou J, Gong S, Tao J, Yu XM, Jiang X. Src activation in the hypothalamic arcuate nucleus may play an important role in pain hypersensitivity. Sci Rep 2019; 9: 3827–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun T, Luo J, Jia M, Li H, Li K, Fu Z. Small interfering RNA-mediated knockdown of NF-kappaBp65 attenuates neuropathic pain following peripheral nerve injury in rats. Eur J Pharmacol 2012; 682: 79–85. [DOI] [PubMed] [Google Scholar]
- 40.Luo JG, Zhao XL, Xu WC, Zhao XJ, Wang JN, Lin XW, Sun T, Fu ZJ. Activation of spinal NF-kappaB/p65 contributes to peripheral inflammation and hyperalgesia in rat adjuvant-induced arthritis. Arthritis Rheumatol 2014; 66: 896–906. [DOI] [PubMed] [Google Scholar]
- 41.Yin QH, Duanmu ZX, Guo SY, Yu XM, Zhang YJ. Role of hypothalamic arcuate nucleus in acupuncture analgesia. A review of behavioral and electrophysiological studies. J Tradit Chin Med 1984; 4: 103–110. [PubMed] [Google Scholar]
- 42.Seo YJ, Kwon MS, Choi SS, Han EJ, Jung JS, Choi HW, Park SH, Jang JE, Suh HW. Characterization of the hypothalamic proopiomelanocortin gene and beta-endorphin expression in the hypothalamic arcuate nucleus of mice elicited by inflammatory pain. Neuroscience 2008; 152: 1054–1066. [DOI] [PubMed] [Google Scholar]
- 43.Murga C, Mayor F., Jr. GRK6, a gatekeeper of visceral hyperalgesia. Brain Behav Immun 2009; 23: 16–17. [DOI] [PubMed] [Google Scholar]
- 44.Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol 1999; 19: 1720–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen G, Gharib TG, Huang CC, Taylor JM, Misek DE, Kardia SL, Giordano TJ, Iannettoni MD, Orringer MB, Hanash SM, Beer DG. Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics 2002; 1: 304–313. [DOI] [PubMed] [Google Scholar]
- 46.Tian Q, Stepaniants SB, Mao M, Weng L, Feetham MC, Doyle MJ, Yi EC, Dai H, Thorsson V, Eng J, Goodlett D, Berger JP, Gunter B, Linseley PS, Stoughton RB, Aebersold R, Collins SJ, Hanlon WA, Hood LE. Integrated genomic and proteomic analyses of gene expression in mammalian cells. Mol Cell Proteomics 2004; 3: 960–969. [DOI] [PubMed] [Google Scholar]
- 47.Li L, Xie R, Hu S, Wang Y, Yu T, Xiao Y, Jiang X, Gu J, Hu CY, Xu GY. Upregulation of cystathionine beta-synthetase expression by nuclear factor-kappa B activation contributes to visceral hypersensitivity in adult rats with neonatal maternal deprivation. Mol Pain 2012; 8: 89–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun T, Yu E, Yu L, Luo J, Li H, Fu Z. LipoxinA(4) induced antinociception and decreased expression of NF-kappaB and pro-inflammatory cytokines after chronic dorsal root ganglia compression in rats. Eur J Pain 2012; 16: 18–27. [DOI] [PubMed] [Google Scholar]