Abstract
Objective
To review new devices and drugs relevant to otolaryngology–head and neck surgery that were approved by the US Food and Drug Administration (FDA) in 2019.
Data Sources
Approval notifications for 2019 were extracted from the ENT (ear, nose, and throat) and general and plastic surgery sections of the FDA’s medical devices and therapeutics listings.
Review Methods
New therapeutics and medical devices identified from the query were analyzed by members of the American Academy of Otolaryngology–Head and Neck Surgery’s Medical Devices and Drugs Committee. Technologies were assessed by 2 independent reviewers to ascertain relevance to otolaryngology, prioritized, and classified to subspecialty field with critical review based on extant scientific literature.
Conclusions
Query of the FDA drug and device database returned 105 ENT devices (50 cleared, 55 with premarket approval, and 0 de novo), 543 general and plastic surgery devices (372 cleared, 170 with premarket approval, and 1 de novo), and 46 new otolaryngology-relevant drug approvals that occurred in 2019. Advances spanned all subspecialty areas with otology predominating, primarily due to hearing-related technologies. While scientific evidence was available for all new devices, there was significant heterogeneity in rigor of supporting scientific data.
Implications for Practice
Technological and pharmaceutical innovation is an important catalyst for advances in the surgical specialties. Familiarity with new devices and therapeutics in otolaryngology–head and neck surgery ensures that clinicians keep abreast of developments with potential to improve prevailing standards of care.
Keywords: medical device, therapeutic, drug, FDA
Otolaryngology is one of the few surgical specialties that manages the medical and surgical aspects of the patients whom they treat. Surgeons at times have hindered the innovations in their field, while others have charged themselves with pushing past the dogma of the field to bring changes that improve patient care.1 Because of this, it is imperative for otolaryngologists to remain up to date with the innovations that come into the field so that they are able to evaluate the new innovations themselves and decide how they fit best into their practice. Awareness of new innovations also allows otolaryngologists to be key influencers on how practice is shaped into the future, particularly when there is overlap with our specialties.
The Medical Devices and Drugs Committee of the American Academy of Otolaryngology–Head and Neck Surgery has reviewed the new approvals in calendar year 2019 with the aim of bringing them to the attention of the profession and providing insight into how some of the more innovative new drugs and devices may affect our field.
Methods
All medical devices and drugs approved for human use with a decision date between January 1, 2019, and December 31, 2019, were considered eligible for inclusion in this review. The US Food and Drug Administration’s (FDA’s) publically available approval databases were reviewed. These included medical device databases for 510(k), premarket approval, and de novo devices.2-4 The devices in these databases were scanned within the ENT (ear, nose, and throat) and general and plastic surgery panels or advisory committee list. The list was reviewed by members of the American Academy of Otolaryngology–Head and Neck Surgery’s Medical Devices and Drugs Committee. Drugs and devices were prioritized for detailed review based on relevance and impact to the specialty, as assessed by 2 independent reviewers, at least 1 of whom had fellowship training in the applicable subspecialty or commensurate expertise.
In the FDA databases, there were 50 ENT and 372 general and plastic surgery 510(k) cleared devices, 55 ENT and 170 general and plastic surgery premarket approval devices, and 0 ENT and 1 general and plastic surgery de novo devices during the year 2019. The new drugs were accessed by the FDA’s new therapeutic approvals website.5 According to the FDA database, there were 46 new drug approvals in 2019. This analysis confirmed advances that spanned all subspecialty areas within otolaryngology, with otology predominating, primarily due to hearing-related technologies. The majority of filings related to updates of existing devices or therapeutics, and these established technologies were not further analyzed. While scientific evidence was available in support for all newly FDA-approved devices, there was significant heterogeneity in rigor of supporting scientific data.
Discussion
Otology and Neurotology
Pexidartinib for Tenosynovial Giant Cell Tumor
Pexidartinib (Turalio; Daiichi Sankyo) was approved within the FDA’s orphan drugs program to address advanced tenosynovial giant cell tumor. These very rare tumors are benign but locally aggressive lesions of large joints, mainly the knee and ankle joints. Within otolaryngology, case reports describe involvement of the temporomandibular joint or external auditory canal, with rare extension to the middle fossa.6-8 Surgery remains the preferred method of addressing tenosynovial giant cell tumors, and pexidartinab was designed to address tumors that recur or are present in patients unable to tolerate surgery. The drug is a tyrosine-kinase inhibitor; its primary target is colony-stimulating factor 1.9 Within a phase 3 trial comparing this medication with placebo for 25 weeks, 56% of patients demonstrated a reduction in tumor volume.10 Hepatotoxicity, sometimes severe, is the principal adverse event of concern, and it occurred in 10% of patients receiving the medication in this trial. Research is ongoing with initial promise for treatment of solid tumors in combination with other chemotherapeutics and for plexiform neurofibromas in neurofibromatosis type 1 in monotherapy. Treatment for glioblastomas has also been explored, but no efficacy was found in a phase 2 trial.11
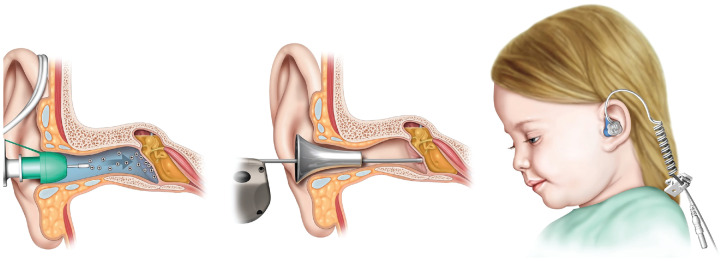
Bonebridge Bone Conduction Implant
The Bonebridge from MED-EL is the first active transcutaneous bone conduction implant system approved in the United States ( Figure 1 ). The device comprises an active bone conduction implant and an externally worn audio processor. The implant lies under intact skin and is coupled to the bone via a stable 2-point fixation. The externally worn audio processor picks up sound and transmits the signal wirelessly to the implant, which in turn generates vibrational stimulation directly to the bone with a floating mass. The first-generation device has been available outside the United States since 2012 and within the United States since 2018. The second-generation device received FDA approval in 2019. Prior bone conduction implant designs were passive systems where the audio processor generated the vibrational stimulation. These older devices were coupled to the head via a percutaneous abutment or magnetically with a pressure-based magnetic coupling, and they have a number of potential disadvantages. Percutaneous abutments take time to osseointegrate, and they protrude from the skull and through the skin, requiring the patient to make a lifelong commitment to the cosmetic appearance as well as to the care and maintenance, with the associated risks of skin breakdown, infection, or instability. Passive transcutaneous systems put local pressure onto the skin, which can be uncomfortable for patients who want wear their audio processors all day long and which can also lead to skin complications related to the chronic application of pressure.12,13 In a recent systematic review and meta-analysis summarizing 39 publications and 487 patients, patients with conductive, mixed, and single-sided deafness received a weighted functional gain of 31 dB.14 Overall weighted mean improvement in word recognition scores was 52% in these groups.14 Significant improvements were found for speech in noise performance; satisfaction rates were high; and complication rates were low.14 Regarding recent efforts in implantable device innovation, there has been a paradigm shift in the area of bone conduction implants, with active devices such as the Bonebridge becoming the dominant design and with active systems potentially replacing passive devices as the standard of care in the near future.
Figure 1.

The Bonebridge (MED-EL): the first active transcutaneous bone conduction implant. Provided courtesy of and with permission from MED-EL.
Osia Bone Conduction Implant
The Osia System (Cochlear) is a new category of bone conduction implant that combines an osseointegrated implant with piezoelectric power transduction. It increases the fitting range up to 55 dB without the need for a percutaneous abutment. The system consists of an implant and a button sound processor. The implant is made up of a receiver coil and a piezoelectric-based actuator, which is anchored to the bone through an osseointegrated implant. The piezoelectric transducer allows an increase in high-frequency amplification beyond what is possible with an electromagnetic transducer. It also circumvents the need for an implanted system of moving or magnetic components, which have implications for reliability and magnetic resonance imaging (MRI) compatibility. The sound processor communicates with the implant via a digital radiofrequency link that has the advantage of no signal loss when transferred across the skin and less interference than analogue links.
The first generation of the system was made available to the market in a limited release and was used to test the performance of the system in clinical studies. Based on the experience from these trials, a second-generation device was launched in 2019 and has an improved form factor ( Figure 2 ). The first generation of the Osia System has been evaluated in a sponsored multicenter clinical investigation, a sponsored pediatric study, and several investigator-initiated research studies over the past 2 years.15 Results of implantation in 150 recipients were presented at the Seventh International Congress on Bone Conduction and Related Technologies (Osseo) in December 2019.16 Studies presented at the Osseo meeting reported improvements in high-frequency power and speech recognition as compared with softband systems and other implantable bone conduction systems.
Figure 2.

The Osia bone conduction implant (Cochlear) combines an implanted receiver coil and piezoelectric-based actuator, which is anchored to the bone through an osseointegrated implant. Generation 1 (panel 1). Generation 2 (panel 2) with an improved form factor. Illustration provided courtesy of and with permission from Cochlear.
Cochlear Implant Innovations
In 2019, MED-EL obtained FDA approval for a cochlear implant (CI) for single-sided deafness in ages ≥5 years. CIs for single-sided deafness have been approved outside the United States for approximately a decade. Evidence of benefits of CIs in single-sided deafness includes data on improvements in tinnitus, sound localization, and listening and speech discrimination in difficult environments.17-21 Cochlear gained approval for changes to the magnets in its CIs, allowing them to be MRI conditional up to 3 T without magnet removal, joining MED-EL and Advanced Bionics. All 3 companies use a similar strategy of allowing the implanted magnet or magnets to move within the plane of the device. This addresses some complications from intentional or inadvertent MRI use, including magnet displacement and pain, and negates the need for a head bandage during MRI. There are theoretical risks of uncomfortable warming during MRI use and demagnetization, but so far, these concerns have not been reported in patients.22 These modifications to CIs reduce the need for surgical magnet removal and postimaging replacement, but the potential for an imaging artifact may still require their removal if the imaging target is near the device. This may be simple with devices with the magnet facing away from the patient (Cochlear or Advanced Bionics) or more challenging and possibly requiring explantation in devices with the magnet on the patient side of the device (MED-EL).
EarLens for Hearing Amplification
The EarLens (EarLens) is a novel contact hearing device, molded (semitranslucent in Figure 3 ) to sit in contact with an individual’s tympanic membrane (TM) and ear canal. It directly drives the TM instead of producing an amplified acoustic signal in the ear canal, as traditional hearing aids do. When the Receive Ring (antenna) on the Lens (blue in Figure 3 ) receives the signal, it extracts power and audio signal to vibrate the umbo of the malleus on the surface of the TM with a small umbo platform that attaches to a motor (silver rectangular shape in Figure 3 ). Only the umbo platform makes contact with the surface of the TM, resulting in minimal damping of hearing while the lens is not powered. Previously, the device was driven by light to a photoactuator on the device.23 The latest approved device has changed to inductive coupling, which solves previous issues of reduced performance when the photoactuator was not aligned, which could occur during jaw opening with eating, laughing, and other facial expressions. The EarLens (EarLens) overcomes some significant limitations of conventional hearing aids. As it directly vibrates the TM, it is able to deliver a much broader bandwidth of audibility, spanning from 125 Hz to 10 kHz for a hearing loss fitting range from mild to severe. This performance significant improves on the range of 400 Hz to 6 kHz, within which acoustic devices can most effectively amplify. A broader frequency range improves perceptions of naturalness of sound for speech and music and enhances speech understanding in the presence of noise.24,25 The EarLens does not use an acoustic signal and therefore does not cause acoustic feedback. Since feedback is not an issue, the EarLens does not need to occlude the ear canal, avoiding the occlusive effects (reverberation of the patient’s voice in one’s ear) seen with traditional hearing aids. It is also able to amplify the lower frequencies that would typically leak out of the ear canal while maintaining an open fit.
Figure 3.

The EarLens (EarLens) directly drives the tympanic membrane to amplify sound via an inductive mechanism. Illustration provided courtesy of and with permission from EarLens.
EQ Balance and ClearEdge Balance Systems for Vestibular Rehabilitation
EQ Balance (Highmark Health) and ClearEdge Balance System (Quadrant Biosciences) received approval as functionally equivalent to a previous system, Sway Balance (Sway Medical). All 3 systems use the accelerometers within a smartphone or mobile device (EQ Balance; Sway Balance) or a separate device that communicates with a smartphone or mobile device (ClearEdge Balance). They measure body tilt (EQ Balance) or sway (Sway Balance, ClearEdge Balance). These systems are integrated into platforms of various cognitive games. They are marketed for athletes and their rehabilitation or for general cognitive health. Research supporting their use on vestibular rehabilitation is limited, but these technologies may have a role in vestibular physical therapy in the future.26
Rhinology
Trikafta (Elexacaftor/Ivacaftor/Tezacaftor) for Cystic Fibrosis
Many patients cared for by otolaryngologists have cystic fibrosis (CF), which can severely affect the sinuses in addition to the upper and lower aerodigestive system. Trikafta (Vertex Pharmaceuticals) is a triple-combination therapy (elexacaftor/ivacaftor/tezacaftor) for patients with the most common CF mutation, F508 deletion. It was approved for CF patients aged ≥12 years with at least 1 F508 deletion mutation in the CF transmembrane conductance regulator gene (CFTR), which corresponds to approximately 90% of the CF patient population. The approval extends access to a number of CF subgroups that previously did not have approved treatment options. CF is caused by abnormal chloride transport through the CF transmembrane conductance regulator protein (CFTR). CFTR modulators such as Trikafta are molecules that bind to the CFTR protein to enhance function and/or structural stability. The efficacy of Trikafta was demonstrated in 2 clinical trials. The first was a randomized double-blind placebo-controlled trial in 403 patients who had 1 F508del mutation and, on the second allele, a mutation resulting in either no CFTR protein or a CFTR protein that is not responsive to ivacaftor (Kalydeco) or tezacaftor/ivacaftor (Symdeko) alone.27 The second trial was a randomized double-blind active-controlled trial in 107 patients who had 2 identical F508del mutations.28 In each trial, Trikafta increased the percentage predicted forced expiratory volume in 1 second. In the first trial, treatment with Trikafta also resulted in fewer pulmonary exacerbations and improved body mass index (weight-to-height ratio) as compared with placebo. The availability of Trikafta represents a significant advance in the medical treatment of patients with CF who are burdened significantly with upper and lower airway disease.
Dupilumab for Chronic Rhinosinusitis With Nasal Polyps
Dupilumab (Sanofi) is a monoclonal antibody targeting the interleukin 4 (IL-4) and IL-13 signaling pathway, critical to type 2 inflammation. In the developed world, type 2 inflammation is known to play a role in allergic diseases, asthma, and certain types of chronic rhinosinusitis (CRS). Dupilumab was initially FDA approved for the treatment of atopic dermatitis in 2017 and then for eosinophilic asthma and corticosteroid-dependent asthma in 2018 before being approved for CRS with nasal polyps (CRSwNP) in 2019. In a multicenter randomized double-blinded placebo-controlled trial comparing Dupilumab plus intranasal mometasone with placebo plus intranasal mometasone in patients with CRSwNP found improvement in nasal polyp score, sinonasal symptomatology, and sense of smell in the group treated with Dupilumab.29 These results were confirmed with 2 sponsored randomized controlled trials in which Dupilumab reduced nasal polyp score, nasal obstruction and congestion scores, and Lund-Mackay computed tomography scores.30 While there are some exceptions, most other treatment options for CRSwNP, including oral corticosteroids, topical corticosteroids, and surgery, broadly decrease inflammation without targeting the specific underlying inflammatory etiology. With the introduction of Dupilumab to the repertoire of treatment for CRS, there is impetus to better understand the underlying endotype that drives a particular individual’s disease, potentially serving as a catalyst to further discovery of CRS pathophysiology. Additional studies are needed to understand how long Dupilumab should be administered and when in the treatment algorithm it should be offered for patients with CRSwNP.
PuraSinus Hemostatic Agent
Adhesion formation is the one of the most common complications following septoplasty, inferior turbinate reduction, and sinus surgery. Additionally, packing material is often placed at the end of these procedures to aid in hemostasis. PuraSinus (3-D Matrix, Ltd) has a self-assembling peptide technology that prevents adhesions from forming and functions as a hemostatic agent. This product reduces scar formation while limiting procedure-related bleeding. There are multiple options for nasal dressings for use after nasal surgery, although these products have not been compared head-to-head.31
Balloon Sinuplasty Adjuncts
The Relieva Ultirra Sinus Balloon Catheter (Acclarent, Johnson & Johnson Medical Devices) serves as a balloon to dilate the sinus ostia and as a tool to irrigate the sinus, allowing a minimally invasive technique as compared with traditional endoscopic sinus surgery. Because balloon sinuplasty is easier for patients to tolerate in an office setting relative to traditional sinus surgery, this device offers a method for treating medical refractory CRS without general anesthesia. This approach is especially relevant in patients with multiple medical comorbidities. Balloon sinus catheters were initially introduced in 2006 as indications for catheter-based technology were rapidly expanding in other specialties.32 Safety and efficacy of the balloon sinus catheters have been confirmed with postmarket trials.33 The ability to provide sinus ostia dilation and deliver irrigations of saline or other medications to the paranasal sinuses in a minimally invasive manner provides an opportunity for research and advances in patient care.
The TruDi Navwire is another product made by Acclarent (Johnson & Johnson Medical Devices). This device is a flexible wire that provides electromagnetic guidance to identify when the device is in the sinus via the TruDi Navigation System. The device is compatible with the Relieva Ultirra Sinus Balloon Catheter and can be used to ensure that the balloon catheter is in the correct sinus prior to dilation of the ostia. Shortly after development of the balloon sinus catheter, surgeons were confirming correct placement with fluoroscopy, which exposed patients to ionizing radiation. In 2007, an image guidance catheter was proposed that could confirm correct placement, and the balloon catheter was then placed over the image guidance catheter to precisely dilate the targeted sinus.34 This idea was later commercially developed. The ability to precisely place sinus balloon catheters increases the safety profile of this minimally invasive technique.
The Relieva Tract Nasal Dilation System (Acclarent, Johnson & Johnson Medical Devices) is a balloon catheter that expands to temporarily displace the inferior turbinate and lower nasal septum to create access for intranasal procedures. When endoscopic nasal procedures are performed, a deviated septum or inferior turbinate hypertrophy may obstruct surgical access to key structures such as the paranasal sinuses, posterior nasal cavity, or nasopharynx. By dilating the balloon and exerting pressure on these structures, one can temporarily increase the intranasal volume to improve access to the nose and paranasal sinuses, as well as make room for multiple instruments that are often required in endoscopic procedures. No specific clinical trials or observational studies have been published on this technology. Future research is required to evaluate the long-term effects of this procedure on outcomes such as nasal obstruction or the persistence of volume increase in the nose.
ClearUP for Sinus Pain in Allergic Rhinitis
ClearUP (Tivic Health Systems, Inc) is available directly to patients who experience sinus pain with allergic rhinitis. It is a noninvasive option that proposes to identify optimal treatment points and then provide paranasal transcutaneous electrical nerve stimulation. ClearUP was recently studied in a randomized double-blinded placebo-controlled trial of 71 patients with facial pain attributed to sinonasal disease. The microcurrent-treated cohort reported a significantly greater reduction in pain scores as compared with that using a placebo device. The trial found that the device was safe and effective in providing rapid relief of nasal/sinus pain.35 The early findings with this product may be of interest to rhinologists whose patients often grapple with sinus pain, which often does not correlate with radiological image or respond reliably to surgery.
Laryngology
Benralizumab for Eosinophilic Esophagitis
Benralizumab (Fasenra; AstraZeneca) was granted orphan drug designation as the first FDA-approved treatment for eosinophilic esophagitis. Patients with eosinophilic esophagitis often present with symptoms of solid food dysphagia. The current treatment is a combination of pneumatic dilation of esophageal strictures, treatment with proton pump inhibitors, elimination diet, and swallowed topical steroids.36 Benralizumab is a monoclonal antibody that binds to the IL-5Rα (interleukin 5 receptor alpha) that is expressed on the surface of eosinophils and basophils. This binding induces apoptosis, thus depleting the number of eosinophils in blood and tissues. Previous clinical trials strongly support a role for IL-5 in eosinophil accumulation in eosinophilic esophagitis, as most patients receiving active treatment (mepolizumab and reslizumab) experience a 50% reduction in eosinophilia. However, only a minority have a complete histologic response, and the effect on eosinophilia appears to reach a plateau within weeks.37,38 The effect of treatment on symptoms remains inconsistent with other IL-5Rα inhibitors, and consequently, it is likely to be variable with benralizumab as well.
Silk Voice for Vocal Fold Augmentation
Silk Voice (Sofregen) is a novel silk-based vocal fold augmentation material. Silk Voice has received 510(k) clearance as an injectable for use during injection laryngoplasty for correction of glottic insufficiency. The main innovation lies in the use of porous silk microparticles crosslinked with hyaluronic acid. These silk microparticles are >65 µm, highly cohesive in suspension, and thus less likely to migrate away from the injection site. Migration from the injection site has been reported with calcium hydroxylapatite (CAHA) in carboxymethyl cellulose. Reports have described migration to distant sites, such as retropharyngeal lymph nodes, which can limit the long-term efficacy of this injectable and lead to complications. CAHA is currently the mainstay for long-lasting injection laryngoplasty for glottic insufficiency. In a canine mode, Silk Voice compared favorably in terms of longevity and immune response to CAHA at 6 months and was shown to remain without migration at the injection site in all animals.39,40
Silk Voice is becoming an important option for injection laryngoplasty given the undesirable tendency for CAHA migration that limits its longevity. Porcine studies suggest that Silk Voice lasts 18 months, equivalent to CAHA when no migration occurs. This new injectable material will be sold with a single-use flexible catheter, allowing otolaryngologists to perform injection laryngoplasty via the channel of a flexible laryngoscope, as opposed to the transoral or percutaneous approaches currently performed during in-office injection laryngoplasty. This would make the injection technically less challenging and may allow for more practitioners to perform the injections.
Head and Neck
Pembrolizumab for Metastatic or Unresectable Recurrent Head and Neck Squamous Cell Carcinoma
Pembrolizumab was originally approved for use in combination with (or after failure with) platinum-based and fluorouracil chemotherapeutics for patients with PD-L1-expressing recurrent or metastatic head and neck squamous cell carcinoma. Pembrolizumab (Keytruda; Merck) is now approved for first-line treatment of patients with metastatic or unresectable recurrent head and neck squamous cell carcinoma. Pembrolizumab is a monoclonal antibody designed to bind to the PD-1 receptor, thus blocking immune suppression ligands PDL-1 and PDL-2 from recognizing PD-1 and thereby increasing T-cell immune response against malignant cells. As pembrolizumab acts to remove an immune system checkpoint, it may also cause T cells to attack healthy cells. It has been approved for the treatment of a multitude of cancers, from melanoma to lung cancer. It continues to be tested in a range of clinical trials, termed KEYNOTE trials. KEYNOTE-048 showed superior overall survival in patients with locally incurable recurrent or metastatic head and neck cancer whose tumors express PD-L1. The study was conducted in patients who had no prior systemic therapy in the recurrent or metastatic setting. Compared with cetuximab, there was noninferiority in the total population.41 These results support pembrolizumab and pembrolizumab/platinum/5-FU as new first-line standards of care for recurrent or metastatic head and neck squamous cell carcinoma. Pembrolizumab has a number of side effects, including immune-mediated pneumonitis, hepatitis, colitis, nephritis, and skin reactions.
New-Generation Da Vinci System for Transoral Robotic Surgery
Da Vinci SP Surgical System, EndoWrist SP Instruments (Intuitive), is the company’s fourth-generation robot, and its improvements in maneuverability have significant implications for otolaryngology surgery. The fourth-generation robot includes 3 multijointed wristed instruments and the first da Vinci fully wristed 3-dimensional HD camera, emerging through a single cannula and allowing a 360° range of motion. The console is unchanged from the prior da Vinci systems. The 2019 clearance includes use of the da Vinci SP system for radical tonsillectomy and transoral tongue base resection. In a prospective phase 2 clinical trial, the da Vinci SP system was used for transoral endoscopic head and neck surgery to access the nasopharynx, oropharynx, larynx, and hypopharynx.42 The flexible robotic arms allow access to the larynx, which was previously inaccessible with the rigid robotic arms.43
Pediatric Otolaryngology
The Tomi Scope for Middle Ear Imaging
The Tomi Scope (PhotoniCare Inc) is a device that uses optical coherence tomography to detect TM and middle ear pathology.44 The primary unmet need that the device addresses is the limitation of the otoscope in detecting middle ear abnormalities, particularly in children. A number of studies have shown that the primary care physician’s diagnosis of middle ear effusion (MEE) with an otoscope has a high false-positive and false-negative rate. Detection of MEE with the Tomi Scope has been reported with 91% accuracy, 91% sensitivity, 90% specificity, and 93% and 87% intra- and interreader agreement, respectively. In evaluating the differentiating MEE type, identification of nonserous MEE had 71% accuracy, 54% sensitivity, 80% specificity, and 83% and 75% intra- and interreader agreement.45
Tula System for Pressure Equalization Tube Insertion
The Tula System (Tusker Medical) is a novel system for anesthetizing the TM and for delivery of a tube into the TM ( Figure 4 ). The Tula Iontophoresis System delivers a combined solution of an amide local anesthetic and an alpha- and beta-adrenergic agonist that results in anesthesia of the TM. The Tula Tube Delivery System is then used to place the ear tube in the TM while the child sits in the office. In a study of 70 pediatric patients (127 ears) aged 6 months to 22 years, the Tula System was successful in 90% (114 of 127 ears).46 The Tula System provides an opportunity to simplify the way that myringotomy and tubes are performed in pediatric patients, who previously needed general anesthesia.
Figure 4.
With the Tula System (Tusker Medical), the tympanic membrane is anesthetized (first panel), and then the tube is placed (second panel). The child wears a device in the office to anesthetize the tympanic membrane (third panel). Illustration provided courtesy of and with permission from Tusker Medical.
Facial Plastics
Latera Implant for Nasal Valve Support
The Latera Absorbable Nasal Implant System (Stryker) is a poly-L-lactide polymer that is inserted with a 16-guage cannula and is designed to support the upper and lower lateral cartilages for correction of the internal and external nasal valve regions ( Figure 5 ). The Latera implant is typically offered as an office-based procedural approach, and it can be performed by otolaryngologists without the invasiveness or recovery time required for conventional surgical approaches to the nasal valve. Part of device’s appeal is the potentially short learning curve, limited downtime, lack of donor site morbidity, and simplicity. The approach allows for longer effect than nasal dilators or nasal sprays and is largely invisible due to subcutaneous insertion. The recommended positioning spans the upper and lower lateral cartilages and anchors to the caudal aspect of the ascending process of the maxilla, achieving a cantilevering effect.
Figure 5.
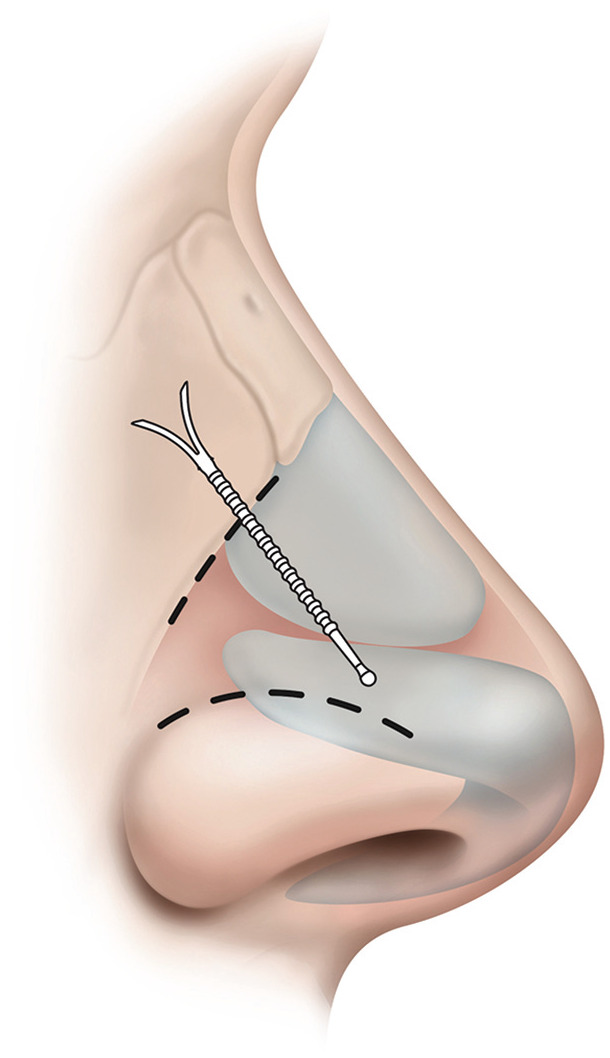
The Latera Absorbable Nasal Implant (Stryker) can be delivered in office to support the upper and lower lateral cartilages. Illustration provided courtesy of and with permission from Stryker.
A defining feature is its targeted approach to a specific anatomic site of nasal blockage. Notably, the product will not mitigate mucosal disease or correct other structural abnormalities that affect the nasal airway. The implant is designed to maintain structural integrity for 12 months but then dissolves, thereby decreasing risk of complications seen with permanent implants. After implantation, the device induces a fibrous capsule formation, stabilizing the implant in position and inducing remodeling of surrounding tissue to provide long-term stability analogous to conventional autologous cartilage grafts.47 Quality-of-life assessments with the device show a mean NOSE score reduction of 56% through 6 months and 52% at 18 months. Symptomatic improvement is reflected in benefit across all 4 parameters of the NOSE instrument (reductions in congestion or blockage, difficulty with breathing, impaired airflow during exertion, and difficulty sleeping).48-50
Sleep Medicine
Solriamfetol for Excessive Daytime Sleepiness Associated With Narcolepsy
Solriamfetol (Sunosi; Jazz Pharmaceuticals) is a selective dopamine and norepinephrine reuptake inhibitor with wake-promoting effects.51 It is indicated to improve wakefulness in adult patients with excessive daytime sleepiness associated with narcolepsy or obstructive sleep apnea. Narcolepsy is a chronic, disabling neurologic disorder characterized by excessive daytime sleepiness and, in up to 60% of patients, cataplexy.52 In narcolepsy, the efficacy of solriamfetol in improving wakefulness and reducing excessive daytime sleepiness was demonstrated in a 12-week double-blind placebo-controlled study in adult patients with a diagnosis of narcolepsy. As compared with the placebo group, patients in this multicenter parallel-group study who were randomized to 150 mg of solriamfetol showed statistically significant improvements on the Maintenance of Wakefulness Test and on the Epworth Sleepiness Scale.53 In obstructive sleep apnea, the efficacy of solriamfetol in improving wakefulness and reducing excessive daytime sleepiness was demonstrated in a 12-week multicenter double-blind placebo-controlled study. As compared with the placebo group, patients randomized to 37.5 mg, 75 mg, and 150 mg of Solriamfetol showed statistically significant improvements on the Maintenance of Wakefulness and Epworth Sleepiness Scale. Patients’ compliance with a primary obstructive sleep apnea therapy device was similar across the placebo and Solriamfetol treatment groups at baseline and did not change during the 12-week study period in any treatment group.54 In patients with narcolepsy and patients for whom sleepiness remains after continuous positive airway pressure, few alternatives are available. Solriamfetol is generally well tolerated, with most common adverse events being mild in nature, including headache (10.1%), nausea (7.9%), decreased appetite (7.6%), anxiety (7.0%), and nasopharyngitis (5.1%); it should be considered as a possible adjunct or primary therapy.
Pitolisant for Excessive Daytime Sleepiness Associated With Narcolepsy
Pitolisant (Wakix; Harmony Bioscience), an N-piperidyl derivative, is a H3R antagonist/inverse agonist with wake-promoting and anticataplectic effects. It blocks the inhibitory effect of histamine (or H3R agonists) on endogenous histamine release, and it enhances histamine release throughout the central nervous system.55 It is approved for the treatment of excessive daytime sleepiness in adult patients with narcolepsy; the recommended dose range is 17.8 to 35.6 mg/d. Pitolisant was compared with modafinil (Provigil; Teva Pharmaceuticals) in a double-blind active placebo and active comparator trial. In this 8-week phase 3 randomized controlled study, adult patients with narcolepsy with or without cataplexy were given pitolisant (10-40 mg/d) or modafinil (100-400 mg/d) as an active comparator. The primary endpoint was the difference between pitolisant and placebo in change in Epworth Sleepiness Scale. Improvements from baseline were found in all groups on the Epworth Sleepiness Scale and Maintenance of Wakefulness Test; the results demonstrated the efficacy of pitolisant over placebo but did not demonstrate noninferiority with respect to modafinil.56 Few options are available for the treatment of Narcolepsy with Cataplexy. Pitolisant is a relatively safe alternative, with the most common adverse effects reported being insomnia (8.4%), headache (7.7%), nausea (4.8%), anxiety (2.1%), irritability (1.8%), dizziness (1.4%), depression (1.3%), tremor (1.2%), sleep disorders (1.1%), fatigue (1.1%), vomiting (1.0%), vertigo (1.0%), dyspepsia (1.0%), weight increase (0.9%), and abdominal pain upper (0.9%). Serious adverse effects were rare and included abnormal weight decrease (0.09%) and abortion spontaneous (0.09%).57
Limitations
This review focused on the medical devices and drugs that had approvals or clearance through FDA filings in 2019, but this analysis does not represent the entirety of medical or technological innovation affecting otolaryngology. There exists significant overlap between otolaryngology and a number of other specialties, ranging from endocrine surgery to oromaxillofacial or neurosurgical procedures. Only ENT and general and plastics were queried in this report. Furthermore, beyond the areas of drugs and medical devices, there are other potential advances that may relate to diagnostics, software, tissue engineering, and emergent fields not directly attributed to our specialty. Incremental advances or updates of established technologies are also of importance, but they were not evaluated in this study. For example, refinements of existing laser technologies, updates in filler products, or enhancements in already available tools were not evaluated in this analysis.
While we sought to include all major new device products, we did not have explicit objective criteria of device or therapeutics that could be applied across specialties to prioritize the impact of devices on the field. There is also potential for publication bias, favoring reporting of positive findings in studies of products that are being brought to market. Most scientific literature emphasizes successful results. Perhaps most significant, most new products have a short history, and long-term effects are not yet known. Postmarketing surveillance plays a critical role in identifying unanticipated effects on patients and potentially new application of novel devices.
Implications for Practice
Given the rapid pace of innovation within the field of otolaryngology, it is important that the otolaryngologist maintain awareness of newly approved drugs and medical devices. Some of these innovations may not have lasting impact on the field, whereas others may rapidly become the new standard of care. In early years after introduction, the approaches of individual clinicians primarily determine how these new innovations are adopted. As technology becomes mainstream, the development of consensus statements and clinical practice guidelines can further inform practice. Dissemination and implementation of new technologies are often driven by marketing efforts of industry, which makes curating of new technology, as presented in this report, of particular value for practitioners seeking to advance the field.
Author Contributions
Anais Rameau, editing, writing and analysis of section; Robert Stephen Hong, editing, writing and analysis of section; Hamid Djalilian, editing, writing and analysis of section; Isaac David Erbele, editing, writing and analysis of section; Katie M. Phillips, editing, writing and analysis of section; Robson Capasso, editing, writing and analysis of section; Austin S. Rose, editing, writing and analysis of section; Michael Joel Brenner, editing, writing and analysis of section; Peter Luke Santa Maria, Conceiving article, editing, analysis.
Disclosures
Competing interests: Peter Luke Santa Maria, Auration Biotech, Flotherm, Stryker (not relevant); Anais Rameau, MyophonX, Corp; Robert Stephen Hong, Olympus; Hamid Djalilian, Beyond Tinnitus, Cactus Medical, Novus; Robson Capasso, Samsung, Bryte; Austin S. Rose, Tivic Health Systems.
Sponsorships: None.
Funding source: None.
References
- 1. Riskin DJ, Longaker MT, Gertner M, Krummel TM. Innovation in surgery: a historical perspective. Ann Surg. 2006;244:686-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Food and Drug Administration. 510(k) premarket notification. Published 2019. Accessed Feburary 11, 2020 https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm
- 3. Food and Drug Administration. Premarket approval (PMA). Published 2019. Accessed Feburary 11, 2020 https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm
- 4. Food and Drug Administration. Device classification under section 513(f)(2)(de novo). Published 2019. Accessed Feburary 11, 2020 https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/denovo.cfm
- 5. Food and Drug Administration. Novel drug approvals for. 2019 https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2019 Accessed Feburary 11, 2020.
- 6. Yan H, Wang F, Xiang L, Zhu W, Liang C. Diffuse giant cell tumors of the tendon sheath in temporomandibular joint: two case reports and review of the literature. Medicine (Baltimore). 2018;97:e11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vassalli G, Vassalli L, Black M, Lim JH. Tenosynovial giant cell tumor of temporomandibular joint and skull base presenting as ear canal mass. Ear Nose Throat J. 2019;7:145561319840543. [DOI] [PubMed] [Google Scholar]
- 8. Liu YK, Chan JY, Chang CJ, Huang JS. Pigmented villonodular synovitis of the temporomandibular joint presenting as a middle cranial fossa tumor. J Oral Maxillofac Surg. 2012;70:367-372. [DOI] [PubMed] [Google Scholar]
- 9. Tap WD, Wainberg ZA, Anthony SP, et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N Engl J Med. 2015;373:428-437. [DOI] [PubMed] [Google Scholar]
- 10. Tap WD, Gelderblom H, Palmerini E, et al. Pexidartinib versus placebo for advanced tenosynovial giant cell tumour (ENLIVEN): a randomised phase 3 trial. Lancet. 2019;394:478-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamb YN. Pexidartinib: first approval. Drugs. 2019;79(16):1805-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Briggs R, Van Hasselt A, Luntz M, et al. Clinical performance of a new magnetic bone conduction hearing implant system: results from a prospective, multicenter, clinical investigation. Otol Neurotol. 2015;36:834-841. [DOI] [PubMed] [Google Scholar]
- 13. Nelissen RC, Agterberg MJ, Hol MK, Snik AF. Three-year experience with the Sophono in children with congenital conductive unilateral hearing loss: tolerability, audiometry, and sound localization compared to a bone-anchored hearing aid. Eur Arch Otorhinolaryngol. 2016;273:3149-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Magele A, Schoerg P, Stanek B, Gradl B, Sprinzl GM. Active transcutaneous bone conduction hearing implants: systematic review and meta-analysis. PLoS One. 2019;14:e0221484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. ClinicalTrials.gov. Clinical performance, safety and patient reported outcomes of an active osseointegrated steady-state implant system. Published. 2019 doi: 10.1097/MAO.0000000000003590. https://clinicaltrials.gov/ct2/show/NCT04041700 Accessed Feburary 18, 2020. [DOI] [PMC free article] [PubMed]
- 16. Nevoux J. Outcomes of the new Osia System compared to Baha Attract. Paper presented at: Seventh International Congress on Bone Conduction and Related Technologies (Osseo); December 11-14, 2019; Miami, FL. [Google Scholar]
- 17. Holder JT, O’Connell B, Hedley-Williams A, Wanna G. Cochlear implantation for single-sided deafness and tinnitus suppression. Am J Otolaryngol. 2017;38:226-229. [DOI] [PubMed] [Google Scholar]
- 18. Prejban DA, Hamzavi JS, Arnoldner C, et al. Single sided deaf cochlear implant users in the difficult listening situation: speech perception and subjective benefit. Otol Neurotol. 2018;39:e803-e809. [DOI] [PubMed] [Google Scholar]
- 19. Sladen DP, Frisch CD, Carlson ML, Driscoll CL, Torres JH, Zeitler DM. Cochlear implantation for single-sided deafness: a multicenter study. Laryngoscope. 2017;127:223-228. [DOI] [PubMed] [Google Scholar]
- 20. Sullivan CB, Al-Qurayshi Z, Zhu V, et al. Long-term audiologic outcomes after cochlear implantation for single-sided deafness. Laryngoscope. 2019;11:28358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zeitler DM, Dorman MF. Cochlear implantation for single-sided deafness: a new treatment paradigm. J Neurol Surg B Skull Base. 2019;80:178-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Srinivasan R, So CW, Amin N, Jaikaransingh D, D’Arco F, Nash R. A review of the safety of MRI in cochlear implant patients with retained magnets. Clin Radiol. 2019;74:972, e979-972, e916. [DOI] [PubMed] [Google Scholar]
- 23. Fay JP, Perkins R, Levy SC, Nilsson M, Puria S. Preliminary evaluation of a light-based contact hearing device for the hearing impaired. Otol Neurotol. 2013;34:912-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arbogast TL, Moore BCJ, Puria S, et al. Achieved gain and subjective outcomes for a wide-bandwidth contact hearing aid fitted using CAM2. Ear Hear. 2019;40:741-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levy SC, Freed DJ, Nilsson M, Moore BC, Puria S. Extended high-frequency bandwidth improves speech reception in the presence of spatially separated masking speech. Ear Hear. 2015;36:e214-e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paul SS, Dibble LE, Walther RG, Shelton C, Gurgel RK, Lester ME. Reduced purposeful head movements during community ambulation following unilateral vestibular loss. Neurorehabil Neural Repair. 2018;32:309-316. [DOI] [PubMed] [Google Scholar]
- 27. Clinicaltrials.gov. A phase 3 study of VX-445 combination therapy in subjects with cystic fibrosis heterozygous for the F508del mutation and a minimal function mutation (F/MF). Published 2019. Accessed Feburary 24, 2020 https://clinicaltrials.gov/ct2/show/NCT03525444
- 28. ClinicalTrials.gov. NCT03525548: a study of V-445 combination therapy in CF subjects homozygous for F508del (F/F). Published 2019. Accessed Feburary 24, 2020 https://clinicaltrials.gov/ct2/show/NCT03525548
- 29. Bachert C, Mannent L, Naclerio RM, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315:469-479. [DOI] [PubMed] [Google Scholar]
- 30. Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394:1638-1650. [DOI] [PubMed] [Google Scholar]
- 31. Valentine R, Wormald PJ. Nasal dressings after endoscopic sinus surgery: what and why? Curr Opin Otolaryngol Head Neck Surg. 2010;18:44-48. [DOI] [PubMed] [Google Scholar]
- 32. Bolger WE, Vaughan WC. Catheter-based dilation of the sinus ostia: initial safety and feasibility analysis in a cadaver model. Am J Rhinol. 2006;20:290-294. [DOI] [PubMed] [Google Scholar]
- 33. Weiss RL, Church CA, Kuhn FA, Levine HL, Sillers MJ, Vaughan WC. Long-term outcome analysis of balloon catheter sinusotomy: two-year follow-up. Otolaryngol Head Neck Surg. 2008;139:S38-S46. [DOI] [PubMed] [Google Scholar]
- 34. Leventhal D, Heffelfinger R, Rosen M. Using image guidance tracking during balloon catheter dilation of sinus ostia. Otolaryngol Head Neck Surg. 2007;137:341-342. [DOI] [PubMed] [Google Scholar]
- 35. Maul XA, Borchard NA, Hwang PH, Nayak JV. Microcurrent technology for rapid relief of sinus pain: a randomized, placebo-controlled, double-blinded clinical trial. Int Forum Allergy Rhinol. 2019;9:352-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Straumann A, Katzka DA. Diagnosis and treatment of eosinophilic esophagitis. Gastroenterology. 2018;154:346-359. [DOI] [PubMed] [Google Scholar]
- 37. Straumann A, Conus S, Grzonka P, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59:21-30. [DOI] [PubMed] [Google Scholar]
- 38. Roufosse F. Targeting the interleukin-5 pathway for treatment of eosinophilic conditions other than asthma. Front Med (Lausanne). 2018;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brown JE, Gulka CP, Giordano JEM, Montero MP, Hoang A, Carroll TL. Injectable silk protein microparticle-based fillers: a novel material for potential use in glottic insufficiency. J Voice. 2019;33:773-780. [DOI] [PubMed] [Google Scholar]
- 40. Gulka CP, Brown JE, Giordano JEM, et al. A novel silk-based vocal fold augmentation material: 6-month evaluation in a canine model. Laryngoscope. 2019;129:1856-1862. [DOI] [PubMed] [Google Scholar]
- 41. Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915-1928. [DOI] [PubMed] [Google Scholar]
- 42. Chan JYK, Tsang RK, Holsinger FC, et al. Prospective clinical trial to evaluate safety and feasibility of using a single port flexible robotic system for transoral head and neck surgery. Oral Oncol. 2019;94:101-105. [DOI] [PubMed] [Google Scholar]
- 43. Smith RV. Transoral robotic surgery for larynx cancer. Otolaryngol Clin North Am. 2014;47:379-395. [DOI] [PubMed] [Google Scholar]
- 44. Preciado D, Nolan RM, Joshi R, et al. Otitis media middle ear effusion identification and characterization using an optical coherence tomography otoscope. Otolaryngol Head Neck Surg. 2020;21:194599819900762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shelton RL, Jung W, Sayegh SI, McCormick DT, Kim J, Boppart SA. Optical coherence tomography for advanced screening in the primary care office. J Biophotonics. 2014;7:525-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zeiders JW, Syms CA, Mitskavich MT, et al. Tympanostomy tube placement in awake, unrestrained pediatric patients: a prospective, multicenter study. Int J Pediatr Otorhinolaryngol. 2015;79:2416-2423. [DOI] [PubMed] [Google Scholar]
- 47. Sanan A, Most SP. A bioabsorbable lateral nasal wall stent for dynamic nasal valve collapse: a review. Facial Plast Surg Clin North Am. 2019;27:367-371. [DOI] [PubMed] [Google Scholar]
- 48. Stolovitzky P, Senior B, Ow RA, Mehendale N, Bikhazi N, Sidle DM. Assessment of bioabsorbable implant treatment for nasal valve collapse compared to a sham group: a randomized control trial. Int Forum Allergy Rhinol. 2019;9:850-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stolovitzky P, Sidle DM, Ow RA, Nachlas NE, Most SP. A prospective study for treatment of nasal valve collapse due to lateral wall insufficiency: outcomes using a bioabsorbable implant. Laryngoscope. 2018;128:2483-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sidle DM, Stolovitzky P, Ow RA, et al. Twelve-month outcomes of a bioabsorbable implant for in-office treatment of dynamic nasal valve collapse. Laryngoscope. 2019;28:28151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Baladi MG, Forster MJ, Gatch MB, et al. Characterization of the neurochemical and behavioral effects of solriamfetol (JZP-110), a selective dopamine and norepinephrine reuptake inhibitor. J Pharmacol Exp Ther. 2018;366:367-376. [DOI] [PubMed] [Google Scholar]
- 52. Scammell TE. Narcolepsy. N Engl J Med. 2015;373(27):2654-2662. [DOI] [PubMed] [Google Scholar]
- 53. Thorpy MJ, Shapiro C, Mayer G, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol. 2019;85:359-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schweitzer PK, Rosenberg R, Zammit GK, et al. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3): a randomized controlled trial. Am J Respir Crit Care Med. 2019;199:1421-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li S, Yang J. Pitolisant for treating patients with narcolepsy. Expert Rev Clin Pharmacol. 2020;23:1-6. [DOI] [PubMed] [Google Scholar]
- 56. Dauvilliers Y, Bassetti C, Lammers GJ, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12:1068-1075. [DOI] [PubMed] [Google Scholar]
- 57. Romigi A, Vitrani G, Lo Giudice T, Centonze D, Franco V. Profile of pitolisant in the management of narcolepsy: design, development, and place in therapy. Drug Des Devel Ther. 2018;12:2665-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]