Abstract
Background:
In the PACIFIC study, progression-free survival (PFS) and overall survival (OS) of patients with unresectable, locally advanced, stage III non-small cell lung cancer (NSCLC) were prolonged by durvalumab as maintenance therapy after radical concurrent chemoradiotherapy using platinum-based antitumor agents. However, no data were obtained to reveal the efficacy of durvalumab after radiation monotherapy in patients unsuitable for chemoradiotherapy. Here, we describe an ongoing single-arm, prospective, open-label, multicenter phase II trial of durvalumab in patients with NSCLC ineligible for stage III chemoradiotherapy following radiation monotherapy (SPIRAL-RT study).
Methods:
Durvalumab at 10 mg/kg body weight is administered every 2 weeks after radiation therapy until individual patients meet the discontinuation criteria. The treatment duration is up to 12 months. The primary endpoint is the 1-year PFS rate. Secondary endpoints are response rate, PFS, OS, and safety. Durvalumab treatment after radiation monotherapy is expected to prolong 1-year PFS rate and have acceptable adverse events.
Discussion:
We are conducting an intervention study to investigate the safety and efficacy of durvalumab treatment in patients with NSCLC ineligible for stage III chemoradiotherapy following radiation monotherapy.
Keywords: 1-year progression-free survival rate, anti-programmed death-ligand 1 antibody, maintenance therapy, non-small cell lung cancer, radiotherapy
Introduction
The current standard therapy for unresectable, locally advanced, stage III non-small cell lung cancer (NSCLC) is concurrent chemoradiotherapy.1–5 However, in elderly patients or patients with poor general condition, chemoradiotherapy is likely to cause hazardous toxicity owing to medical complications and deteriorated organ functions.6,7 In a previous meta-analysis, the authors reported that concurrent chemoradiotherapy might moderately improve survival, but the evidence for its benefit was weak.8 Therefore, radiation monotherapy is recommended as the standard therapy for stage III NSCLC that cannot be treated with concurrent chemoradiotherapy based on a randomized comparative study.
In contrast, chemotherapy and radiotherapy increase the expression of the immunosuppression checkpoint programmed death-ligand 1 (PD-L1) and enable cancer cells to evade anticancer immune response.9 Furthermore, the abscopal effect, which causes immune-mediated death of cancer cells at sites distant from the irradiated site, has been demonstrated by a combination therapy with ipilimumab and radiation therapy in a patient with malignant melanoma.10 Many studies of immunoradiotherapy have been conducted in patients with advanced NSCLC, which have established the apparent benefit of immune checkpoint inhibitors.11–13
The PACIFIC study is a randomized, multicenter, double-blind, placebo-controlled study of durvalumab as maintenance therapy in patients with unresectable, locally advanced (stage III) NSCLC who did not have progressive disease after radical concurrent chemoradiotherapy using platinum-based antitumor agents. At the European Society for Medical Oncology in 2017, it was reported that durvalumab achieved a statistically significant and clinically meaningful prolongation of progression-free survival (PFS), which was the primary endpoint, in all enrolled patients with stage III NSCLC in a preplanned interim analysis.14 However, no data were obtained from the PACIFIC study to determine the efficacy of durvalumab after radiation monotherapy in patients ineligible for chemoradiotherapy. The PACIFIC study was only for patients who had undergone chemoradiotherapy and not for those who had undergone radiotherapy alone. Therefore, we planned a clinical study to investigate the efficacy and safety of durvalumab when administered in patients with stage III NSCLC who are ineligible for chemoradiotherapy and do not have progressive disease after radiation monotherapy (Trial registration number: JMA-IIA00434).
Methods
Study design
This was a single-arm, prospective, open-label, multicenter, phase II trial (SPIRAL-RT study).
Ethical consideration and registration
The study obtained ethical approval from the trial review committee in the Kyoto Prefectural University of Medicine on 16 May 2019. The trial is supervised and managed by the Ethics Committee of Kyoto Prefectural University of Medicine, Kyoto, Japan (number: 2019-006). In accordance with the Declaration of Helsinki, written informed consent will be obtained from all patients before registration. Results of the study will be disseminated via publications in peer-reviewed journals.
Eligibility criteria
The inclusion and exclusion criteria are shown in Table 1.
Table 1.
Eligibility criteria.
| Inclusion criteria |
|---|
| (1) Patients aged ⩾20 years at the time of informed consent (regardless of gender and inpatient/outpatient). |
| (2) Patients cytologically or histologically diagnosed with unresectable locally advanced stage III NSCLC (as per General Rule for Clinical and Pathological Record of Lung Cancer 8th edition). |
| (3) Patients ineligible for chemoradiotherapy (e.g. poor PS cases or elderly patients). |
| (4) Patients who have completed radiotherapy, however, the total radiation dose to the patient should be 60 Gy ± 10% (54–66 Gy), and the average radiation dose per organ should be: – average total lung dose: <20 Gy or V20 <35% – average total dose to the esophagus: <34 Gy – average total dose to the heart: V45 <35% or V30 <30% |
| (5) Patients who did not have progressive disease after radiotherapy. |
| (6) Performance status (ECOG) 0–2. |
| (7) Patients with appropriate visceral and bone marrow function who meet the following criteria: – absolute neutrophil count: ⩾1500/mm3 – platelet count: ⩾100,000/mm3 – hemoglobin: ⩾5.6 mmol/L – serum CrCl: ⩾50 ml/min (value estimated by Cockcroft–Gault formula) men: CrCl (ml/min) = [weight (kg) × (140–age)]/[72 × serum creatinine (μmol/L x 0.011)] women: CrCl (ml/min) = [weight (kg) × (140–age)]/[72 × serum creatinine (μmol/L x 0.011)] × 0.85 – total bilirubin: ⩽1.5 times ULN This criterion does not apply to patients with a definitive diagnosis of Gilbert’s syndrome (not presenting with hemolysis or pathological findings of liver disease but mainly with unconjugated persistent or recurrent hyperbilirubinemia). Participation in the study depends on the determination of the investigator (sub-investigator). – aspartic aminotransferase and alanine aminotransferase: ⩽2.5 times ULN |
| (8) Patients expected to survive for at least 3 months. |
| (9) Patients who provided written informed consent by their own free will. |
| Exclusion criteria |
| (1) Patients who have previously been exposed to the anti-PD-1 antibody or anti-PD-L1 antibody. |
| (2) Patients with autoimmune disease confirmed at present or in the past or with history of immunodeficiency. |
| (3) Patients with severe or uncontrolled systemic diseases (including active infections such as active hemorrhagic diathesis, hepatitis B, hepatitis C, and HIV. |
| (4) Patients who have received treatment for immunosuppression within 14 days of the start of study treatment. Nasal or inhaled corticosteroids or systemic corticosteroids at a physiological dose not exceeding prednisone equivalent dose of 10 mg/day are excluded. Systemic corticosteroids to reduce the toxicity caused by radiotherapy performed as part of chemoradiotherapy for locally advanced NSCLC are excluded. |
| (5) Patients who have received attenuated live vaccines within 30 days of informed consent or within 30 days of the start of study treatment. |
| (6) Patients with a history of tuberculosis. |
| (7) Patients with uncontrolled diseases such as symptomatic congestive heart failure, uncontrolled hypertension, and unstable angina. |
| (8) Patients with continued grade 2 or more toxicity after prior radiotherapy. |
| (9) Patients with grade 2 or more pneumonitis in prior radiotherapy. |
| (10) Patients with an inflammatory intestinal disease (Crohn’s disease or ulcerative colitis) confirmed at present or in the past. |
| (11) Male and female patients who do not use appropriate contraception and female patients who are pregnant or breast-feeding. |
| (12) Patients considered ineligible by the investigator or sub-investigator. |
CrCl, creatinine clearance; ECOG, Eastern Cooperative Oncology Group; NSCLC, non-small cell lung cancer; PD-1, programmed cell death 1; PD-L1, programmed death-ligand 1; PS, performance status; ULN, upper limit of normal.
Objective and endpoints
The present study is underway to prospectively evaluate the efficacy and safety of durvalumab when administered in patients with stage III NSCLC who are ineligible for chemoradiotherapy and do not have progressive disease after radiation monotherapy. The primary endpoint is the 1-year PFS rate. Secondary endpoints are response rate (RR), PFS, overall survival (OS), and safety. The exploratory objective is to evaluate PD-L1 expression on tumor cells using anti-PD-L1 antibodies (SP263 antibody).
Rationale for setting the number of enrolled subjects
The threshold for the 12-month PFS rate after radiation monotherapy in the JCOG0301 study15 was approximately 20%. However, since this study is conducted in patients ineligible for chemoradiotherapy, the threshold was determined as 16% based on the assumption that the PFS will be somewhat reduced. Meanwhile, the expected 12-month PFS rate was determined as 35% based on the results of the PACIFIC study,14 assuming that the PFS will be somewhat reduced because the hazard ratio of PFS was 0.52. On the premise that the one-sided significance level is 5% and the power is 90%, the sample size should be 31. However, taking dropouts into consideration, the target sample size was determined as 33 (sample size determined by the survival function test for a single-arm study16).
Population to be analyzed
Efficacy
The efficacy will be analyzed in the full analysis set (FAS). The FAS is an analysis population based on the idea of intention-to-treat and comprises all enrolled patients after excluding those who meet the following criteria: (a) ineligible patients (patients who meet none of the major registration criteria of this study); (b) patients who have not received the study drug: (c) patients with no data at the baseline or during the treatment period; (d) patients who have withdrawn their consent during the study and refused the use of all data.
In this study, the efficacy will be analyzed mainly in the FAS. The primary endpoint will be analyzed in the per protocol set (PPS) that is appropriate for the protocol, and the stability of the analysis result of FAS will be evaluated. The PPS is a population of patients who are included for efficacy evaluation in accordance with the criteria related to the handling of patients that will be prepared before the data lock.
Safety
The population of patients who have received the study drug at least once will be the safety analysis set.
Dosages and treatment regimen
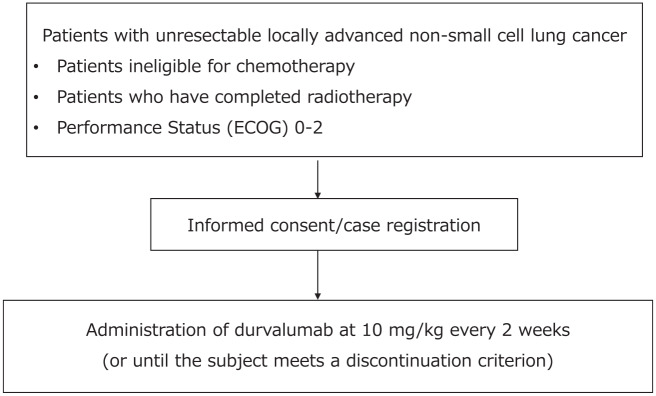
All patients will be treated using conventional fractionation (2 Gy per fraction; one fraction per day, and five fractions per week). The bilateral supraclavicular, mediastinal, ipsilateral hilar, interlobar, lobar, subcarinal, ipsilateral low cervical, and sternal notch lymph node areas are considered regional lymph node areas. Involved field radiation therapy is administered only in those lymph nodes that are proven to be affected by the tumor. The gross tumor volume is expanded to the clinical tumor volume by extending the margin by 0.5–1 cm. The recommended radiation dose as radical treatment is between 54 Gy and 66 Gy in 2 Gy fractions. The dose constraints to adjacent important normal organs/tissues are shown in Table 1. Durvalumab at 10 mg/kg (body weight) is administered every 2 weeks until individual patients meet the discontinuation criteria. The duration of treatment is up to 12 months. The start of durvalumab treatment may be postponed for up to 42 days after completing radiotherapy for patients requiring recovery from toxicity owing to previous treatment. Treatment will be continued until progressive disease or any of the discontinuation criteria specified in the ‘Criteria for discontinuation of protocol treatment’ are met. A schematic illustration of the study is provided in Figure 1.
Figure 1.
Study profile.
ECOG, Eastern Cooperative Oncology Group.
Statistical analysis
The 1-year PFS rate will be estimated by the Kaplan–Meier method, and the confidence interval will be calculated using Greenwood’s method. If the lower limit of the estimated 90% confidence interval exceeds the threshold of 16%, it will be considered statistically significant. As for PFS and OS, the Kaplan–Meier method will be used to estimate the survival curve, median, and annual rate. The Brookmeyer and Crowley method and Greenwood’s method will be used to estimate the median confidence interval and standard error of the annual rate, respectively. RR and the 95% confidence interval (two-sided) will be estimated by the Wilson method. The severity and incidence of each adverse event will be recorded.
Anticipated results
In patients with stage III NSCLC ineligible for chemoradiotherapy, durvalumab treatment after radiation monotherapy is expected to prolong the 1-year PFS rate and have acceptable adverse events.
Footnotes
Conflict of interest statement: TY obtained grants from Nippon Boehringer Ingelheim and Ono Pharmaceutical for different work. JU obtained grants from Eli Lilly Japan K.K. for different work. KT obtained grants from Chugai-Roche and Ono Pharmaceutical, personal fees from AstraZeneca K.K., Chugai-Roche, MSD-Merck, Eli Lilly, Boehringer Ingelheim, and Daiichi-Sankyo for different work.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by ‘Externally sponsored scientific research’ from AstraZeneca (ESR-18-13635).
ORCID iDs: Tadaaki Yamada  https://orcid.org/0000-0002-6945-281X
https://orcid.org/0000-0002-6945-281X
Junji Uchino  https://orcid.org/0000-0003-0651-7767
https://orcid.org/0000-0003-0651-7767
Contributor Information
Tadaaki Yamada, Department of Pulmonary Medicine, Kyoto Prefectural University of Medicine, Kyoto, Japan.
Junji Uchino, Department of Pulmonary Medicine, Kyoto Prefectural University of Medicine, 465 Kajii-cho, Hirokoji-agaru, Kawaramachi-dori, Kamigyo-ku, Kyoto 602-0857, Japan.
Yusuke Chihara, Department of Pulmonary Medicine, Kyoto Prefectural University of Medicine, Kyoto, Japan.
Takayuki Shimamoto, Department of Pulmonary Medicine, Kyoto Prefectural University of Medicine, Kyoto, Japan.
Masahiro Iwasaku, Department of Pulmonary Medicine, Kyoto Prefectural University of Medicine, Kyoto, Japan.
Nobuyo Tamiya, Department of Pulmonary Medicine, Kyoto Prefectural University of Medicine, Kyoto, Japan.
Yoshiko Kaneko, Department of Pulmonary Medicine, Kyoto Prefectural University of Medicine, Kyoto, Japan.
Fumiaki Kiyomi, Statistics and Data Center, Clinical Research Support Center Kyushu, Fukuoka, Japan.
Koichi Takayama, Department of Pulmonary Medicine, Kyoto Prefectural University of Medicine, Kyoto, Japan.
References
- 1. Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 1999; 17: 2692–2699. [DOI] [PubMed] [Google Scholar]
- 2. Curran WJ, Jr, Paulus R, Langer CJ, et al. Sequential vs concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011; 103: 1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995; 311: 899–909. [PMC free article] [PubMed] [Google Scholar]
- 4. Marino P, Preatoni A, Cantoni A. Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stages IIIa and IIIb nonsmall cell lung cancer. A meta-analysis. Cancer 1995; 76: 593–601. [DOI] [PubMed] [Google Scholar]
- 5. Pritchard RS, Anthony SP. Chemotherapy plus radiotherapy compared with radiotherapy alone in the treatment of locally advanced, unresectable, non-small-cell lung cancer. A meta-analysis. Ann Intern Med 1996; 125: 723–729. [DOI] [PubMed] [Google Scholar]
- 6. Repetto L, Balducci L. A case for geriatric oncology. Lancet Oncol 2002; 3: 289–297. [DOI] [PubMed] [Google Scholar]
- 7. Havlik RJ, Yancik R, Long S, et al. The national institute on aging and the national cancer institute SEER collaborative study on comorbidity and early diagnosis of cancer in the elderly. Cancer 1994; 74: 2101–2106. [DOI] [PubMed] [Google Scholar]
- 8. Aupérin A, Le Péchoux C, Pignon JP, et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol 2006; 17: 473–483. [DOI] [PubMed] [Google Scholar]
- 9. Zhang P, Ma Y, Lv C, et al. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci 2016; 107: 1563–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366: 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ko EC, Raben D, Formenti SC. The integration of radiotherapy with immunotherapy for the treatment of non-small cell lung cancer. Clin Cancer Res 2018; 24: 5792–5806. [DOI] [PubMed] [Google Scholar]
- 12. Bhalla N, Brooker R, Brada M. Combining immunotherapy and radiotherapy in lung cancer. J Thorac Dis 2018; 10(Suppl. 13): S1447–S1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bockel S, Durand B, Deutsch E. Combining radiation therapy and cancer immune therapies: from preclinical findings to clinical applications. Cancer Radiother 2018; 22: 567–580. [DOI] [PubMed] [Google Scholar]
- 14. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017; 377: 1919–1929. [DOI] [PubMed] [Google Scholar]
- 15. Atagi S, Kawahara M, Yokoyama A, et al. Thoracic radiotherapy with or without daily low-dose carboplatin in elderly patients with non-small-cell lung cancer: a randomised, controlled, phase 3 trial by the Japan clinical oncology group (JCOG0301). Lancet Oncol 2012; 13: 671–678. [DOI] [PubMed] [Google Scholar]
- 16. Lawless JF. Statistical models and methods for lifetime data, 2nd ed. Hoboken, NJ: John Wiley & Sons, 2003. [Google Scholar]