Abstract
Autologous CD19-targeted chimeric antigen receptor-modified T cells (CD19-CART) remarkably improved the outcome of patients with advanced B-cell acute lymphoblastic leukemia (B-ALL). However, the application and outcomes of allogeneic CART cells is still uncertain. Two patients with advanced B-ALL were enrolled to receive a co-infusion of high-dose human leukocyte antigen-haploidentical donor granulocyte colony-stimulating factor mobilized peripheral blood mononuclear cells (GPBMCs; 21.01–25.34 × 108/kg) and the same donor-derived CD19-targeted CART cells (8.44–22.19 × 106/kg) without additional in vitro gene-editing following a reinduction chemotherapy as precondition. They achieved complete remission and full donor chimerism (FDC) with ongoing 20- and 4-month leukemia-free survival. A significant amplification of donor CART cells was detected in peripheral blood and/or cerebrospinal fluid and was associated with the formation of FDC. The highest amount of copies of the donor CART cells reached 4962 per µg of genomic DNA (gDNA) and 2449 per µg of gDNA, and the longest persistence was 20 months associated with B cell aplasia. Two patients experienced Grade II or III cytokine release syndromes and developed controllable Grade II intestinal acute graft-versus-host disease (GVHD) or limited chronic oral GVHD. High-dose donor GPBMC infusion may enhance amplification and persistence of haploidentical CD19-targeted CART cells, suggesting an alternative therapy for advanced B-ALL patients.
Keywords: B-cell acute lymphoblastic leukemia, CD19-targeted chimeric antigen receptor-modified T cells, donor engraftment, graft-versus-host disease, high-dose haploidentical donor cell infusion
Introduction
The application of autologous CD19-targeted chimeric antigen receptor-modified T cells (CD19-CART) has significantly improved outcome of patients with advanced B-cell acute lymphoblastic leukemia (B-ALL).1 However, limitations such as leukemia relapse and application of universal CART cells still exist.2–4 Some researchers utilized bridging allogeneic stem cell transplant (allo-SCT) following CART therapy to prevent or reduce leukemia relapse. Others used transcription activator-like effector nucleases to engineer human leukocyte antigen (HLA)-mismatched donor T cells to produce universal CART19 cells.5 However, although these treatments improved outcome in some patients, those who received CD19-CART to bridge allo-SCT were still subject to sequential toxicities from CART infusion and transplantation, and in vitro CART gene editing is more complicated. Moreover, in vivo outcomes of allogeneic CART cells by non-gene-editing preparation were undetermined. We have previously reported an older patient with advanced ALL who received co-infusion of allogeneic CD19-CART cells and low-dose granulocyte colony-stimulating factor (G-CSF) mobilized peripheral blood mononuclear cells (GPBMCs) and showed a short-term persistence of allogeneic CART cells in vivo, suggesting that co-infusion of allogeneic CART cells and GPBMCs may benefit the expansion and persistence of allogeneic CART cells.6 In addition, some researchers reported that large donor cell doses were able to markedly enhance donor engraftment,7,8 though only in low or high percentages of mixed chimerism rather than in full donor chimerism (FDC). Our recent experiments in H-2 mice further found that if the number of haploidentical G-CSF-mobilized donor splenic cells infused was progressively increased from 1 × 107 to 9 × 107 with 1 Gy total body irradiation and no prevention of graft-versus-host disease (GVHD), recipient mice could establish a high percentage of mixed chimerism or even FDC with mild GVHD. With this in mind, we further designed the study so that two patients with advanced B-ALL received a co-infusion of high dose HLA-haploidentical donor GPBMCs with the same donor-derived CD19-targeted CART cells without additional gene editing following a reinduction chemotherapy. Preliminary results showed that all patients achieved complete remission (CR), stable FDC, and persistence of allogeneic CART cells.
Case presentation
Two patients who had advanced ALL with 57.5–84.5% leukemic cells and who had a failed collection of sufficient autologous T cells were enrolled from March to September 2017. Both of them were lacking an HLA-matched donor but had an available HLA-haploidentical relative donor. Patients received a reinduction chemotherapy (vindesine 4 mg for 1 day, idarubicin 7.5 mg/m2 for 3 days, cyclophosphamide 450 mg/m2 for 2 days, and dexamethasone 5 mg/m2 for 5 days) as a precondition, followed by CD19-CART cell and high-dose GPBMC infusion from the same donor (Table 1). Donor T cells for CART cell preparation were collected ahead of mobilization of peripheral blood cells, and the materials and methods to produce and quantify CD19-CART cells were reported previously.6 Mobilization and apheresis of donor GPBMCs were also described previously.9 CART cells were infused at 48 h after the completion of cyclophosphamide from the second to fifth day with escalating doses, and GPBMCs were infused on the last day of CART cell infusion. No GVHD prophylaxis was used. If patients developed cytokine release syndrome (CRS) after co-infusion, ruxolitinib, tocilizumab, or steroid was given and doses of ruxolitinib were progressively reduced following symptom alleviation. Hematopoietic donor chimerism was assessed with standard cytogenetic, semiquantitative polymerase chain reaction and HLA-typing as previously described.9 Detection of immune function, cytokines, and CD19-CART copy number were performed as previously described.6 Outcome data were determined through March 1st, 2019. Response criteria of ALL, diagnoses, and grading of GVHD and CRS were defined according to published criteria.10–13
Table 1.
Characteristics and outcome of patients.
| Age | Diagnosis | HLA-match | CARTs (106/kg) | MNCs (108/kg) | CRS Degree | GVHD | Peak CART copies in PB (/µg gDNA) | Engraftment (%) | OS(M) | LFS(M) |
|---|---|---|---|---|---|---|---|---|---|---|
| 23/F | ALL-RL2 | 5/10 | 22.19 | 21.01 | II | cGVHD-oral | 4962 (+90d) | 62–100 (+4d to +600d) | 20.3 | 20 |
| 26/M | ALL-RL2 | 5/10 | 8.44 | 25.34 | III | aGVHD-GI | 2499 (+14d) | 87–100 (+7d to +120d) | 4.3 | 4 |
aGVHD, acute GVHD; ALL, acute lymphoblastic leukemia; CART, chimeric antigen receptor-modified T cells; cGVHD, chronic GVHD; CRS, cytokine release syndrome; d, days; gDNA, genomic DNA; GVHD, graft-versus-host disease; HLA, human leukocyte antigen; LFS, leukemia free survival; M, months; MNCs, mononuclear cells; OS, overall survival; PB, peripheral blood; RL, relapse.
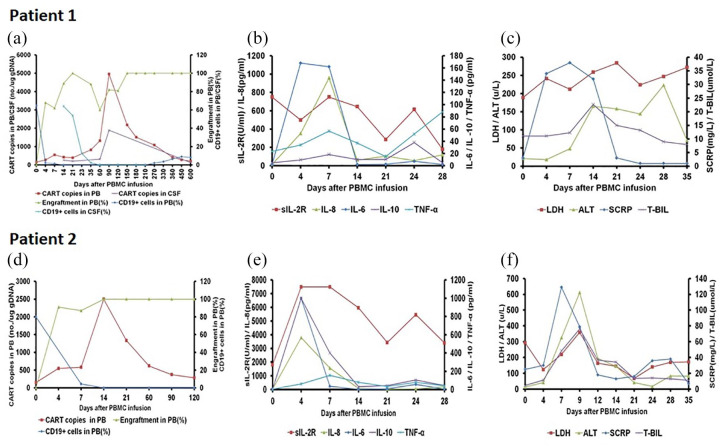
Patient 1 was a 23-year-old female who was diagnosed with BCR-ABL positive ALL. She experienced two relapses and failed to respond to further induction chemotherapy with leukemic cells of 57.5% in the bone marrow before transplant. She was infused with CD19-CART cells (22.19 × 106/kg) and GPBMCs (21.01 × 108/kg) from her HLA 5/10 matched mother (Figure 1 and Table 1) following the reinduction chemotherapy. She developed Grade II CRS accompanied by cytokine elevation (Figure 1b and c) during day 5–14 and had a good response to ruxolitinib treatment. The proportion of donor engraftment was 62%, 89%, and 100% on day 7, 14, and 21, respectively. Copy numbers of CART cells in the peripheral blood were 547, 818, and 4962 per µg of genomic DNA (gDNA) on day 7, 35, and 90, respectively, after transplant. The percentages of leukemic cells in the bone marrow and CD19+ cells in the peripheral blood dropped to 0% and 0.1% respectively on day 14 (Figure 1a). The neutrophil count recovered over 0.5 × 109/L on day 12 and the platelet count recovered over 30 × 109/L on day 15. She developed a limited chronic oral GVHD on day 125. She has maintained FDC and disease-free survival for 20 months accompanied with persistence of CART cells in the peripheral blood.
Figure 1.
Clinical responses of co-infusion of haploidentical CART cells and GPBMCs in two patients with advanced CD19+ ALL. (a) In Patient 1, the proportion of donor engraftment was 62%, 89%, and 100% on day 7, 14, and 21, respectively. The copy numbers of CART cells in the peripheral blood were 547, 818, and 4962 per µg of gDNA on day 7, 35, and 90, respectively, after transplant. The percentages of CD19+ cells in the peripheral blood dropped to 0.1% on day 14. CSF examination on day 14 showed 64% of CD19+ cells and 263 per µg of gDNA of CART cells, respectively. After intrathecal injection, CD19+ cells dropped to 0% and copy numbers of CART cells in the CSF maintained between 207 and 1894 per µg of gDNA. (b) Patient 1 experienced dramatic elevation of cytokine levels, especially IL-6 and IL-8, after treatment. (c) Patient 1 developed a significant elevation of C-reaction protein, alanine transaminase, and total bilirubin after treatment. (d) In Patient 2, donor engraftment in the peripheral blood was 87% on day 7 and 100% on day 14. CART copy numbers in the peripheral blood were 550 per µg of gDNA on day 4, 2499 per µg of gDNA on day 14, and fluctuated between 286 and 1334 per µg of gDNA 21–120 days after treatment. CD19+ cells in the peripheral blood dropped to 0% on day 14 and persisted from 0% to 0.25% 21–120 days after treatment. (e) Patient 2 experienced significant elevations of sIL-2R, IL-6, IL-8, and IL-10 after treatment. (f) Patient 2 developed a significant elevation of C-reaction protein, alanine transaminase, and total bilirubin after treatment.
ALT, alanine transaminase; CSF, cerebrospinal fluid; gDNA, genomic DNA; LDH, lactate dehydrogenase; PB, peripheral blood; SCRP, high sensitivity C-reaction protein; T-BIL, total bilirubin.
Patient 2 was a 26-year-old male who was diagnosed with B-cell ALL. He relapsed twice and had no responses to further reinduction chemotherapy with leukemic cells and CD19+ cells of 84.5% and 94.9%, respectively, in the bone marrow. He received co-infusion of CD19-CART cells (8.44 × 106/kg) and GPBMCs (25.34 × 108/kg) from his HLA 5/10 matched mother (Table 1) following the reinduction chemotherapy. He developed Grade III CRS associated with dramatic cytokine elevation during days 4–14 and had a good response to treatment from ruxolitinib and tocilizumab (Figure 1e and 1f). Donor chimerism in peripheral blood was 87% and 100% on day 7 and 14, respectively. CD19-CART copy numbers were 550 per µg of gDNA on day 4, increased to 2499 per µg of gDNA on day 14, and fluctuated between 286 and 1334 per µg of gDNA during 21–120 days. Leukemic cells in the bone marrow dropped to 0% on day 14 and CD19+ cells in the peripheral blood also dropped to 0% on day 14 and persisted from 0% to 0.25% for 21–120 days after treatment (Figure 1d). The neutrophil and platelet counts were recovered on day 18 and day 30, respectively. He developed Grade II acute intestinal GVHD on day 27 and had a good response to anti-GVHD treatment but was associated with an incomplete hematological recovery. During day 100–125, he developed hemorrhagic cystitis, severe drug-resistant bacteria infection, viremia of cytomegalovirus, and died of multiple organ failure on day 132.
Discussion
Autologous CD19-targeted CART cells could efficiently eliminate leukemic cells with significant amplification in advanced ALL.1 However, it is often difficult to collect enough autologous T cells in patients with high leukemia burdens and the clinical outcome of universal CART cells is still unclear. In this study, two patients had high leukemia burdens and had failed to collect enough autologous T cells. Allogeneic CART cells from haploid donors were collected at a dose of approximately 8.44–22.19 × 106/kg, which is similar to the dosage of autologous CD19-targeted CART cells. Results showed that two patients had a rapid decrease of leukemic cells, achieved CR within 7–14 days, and remained in leukemia-free survival for 4 months or over 20 months. Furthermore, the highest amount of copies of the donor CART cells in the peripheral blood reached 4962 and 2449 per µg of gDNA, respectively, and the longest persistence was 20 months associated with B cell aplasia. These results indicated that donor CART cells without additional gene editing also possessed a strong anti-leukemic activity and a significant amplification and long-term persistence in vivo.
Standard allo-SCT employs myeloablative or non-myeloablative conditioning to establish FDC.14 In this study, two patients only received a high-dose GPBMC infusion (21.01–25.34 × 108/kg) from a HLA-mismatched donor, which was 2–3 folds of the dose in standard transplants, following a reinduction chemotherapy as a precondition and established a stable FDC at day 21 or 14. These results suggested that high-dose GPBMCs may play an important role in establishing FDC except for cyclophosphamide-containing reinduction chemotherapy, consisting with results from our previous animal studies (Supplemental material). In addition, we found that two patients successfully established FDC associated with high amounts of copies of donor CART cells in the peripheral blood, suggesting that the formation of FDC may also contribute to enhancing expansion and persistence of allogeneic CART cells.
Severe CRS and GVHD are major concerns in CART treatment and allo-SCT, respectively.2,10 In this study, two patients presented with Grade II or III CRS and Grade II acute intestinal GVHD or limited chronic GVHD, which was the same as – or at least not more severe than – the CRS/GVHD of the autologous CD19-targeted CART treatment or standard transplantation, suggesting that the toxicity of allogeneic CD19-targeted CART cells is controllable and relatively safe.
Collectively, co-infusion of donor CD19-targeted CART and high-dose HLA-haploidentical GPBMC cells may maintain expansion and persistence of allogeneic CART cells, suggesting an alternative therapy for advanced CD19+ ALL patients.
Supplemental Material
Supplemental material, Supplementary_figure_and_table for Co-infusion of high-dose haploidentical donor cells and CD19-targeted CART cells achieves complete remission, successful donor engraftment and significant CART amplification in advanced ALL by Changlin Yu, Bo Cai, Yao Wang, Zhiqiang Wu, Kaixun Hu, Qiyun Sun, Jianhui Qiao, Yanhong Fang, Hongli Zuo, Yi Wang, Zheng Dong, Zechuan Zhang, Yajing Huang, Zhiqing Liu, Tieqiang Liu, Huisheng Ai, Weidong Han and Mei Guo in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors thank the patients, donors, and their kin for agreement to publication of the report.
Footnotes
Author contributions: HA, MG, and WH conceived the study, analyzed and interpreted the data, have full access to all of the data, and assume responsibility for the integrity of the data and the accuracy of the data analysis. HA, BC, MG, and CY co-wrote the manuscript. YW, ZW, and WH designed and prepared CART cells and performed CART cell copy number detection. CY, MG, BC, YF, KH, QS, JQ, HZ, ZD, ZZ, and YH were responsible for the clinical care of patients. YW, ZL, and TL performed experiments and laboratory tests.
Availability of data and materials: The datasets generated and/or analyzed during this study are available from the corresponding author upon reasonable request.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (No. 31500732 to Kaixun Hu, No. 81830002 and 31870873 to Weidong Han, No. 81800150 to Bo Cai, and No. 81670110 to Yi Wang), Foundation for Young Scientists of Chinese PLA General Hospital (No. QNF19043 to Bo Cai) and the National Key Research and Development Program of China (No. 2016YFC1303501 and 2016YFC1303504 to Weidong Han, and No. 2017YFC0909803 to Yao Wang).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Consent for publication: Written informed consent was obtained from the patients and donors for publication of case reports.
Ethics approval and consent to participate: The protocol was approved by the Human Ethics Committee of Chinese PLA General Hospital (Approval No. S2013-085-02) and was in accordance with the Declaration of Helsinki. All patients and their donors provided written informed consent before the study.
ORCID iD: Huisheng Ai  https://orcid.org/0000-0001-7002-1702
https://orcid.org/0000-0001-7002-1702
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Changlin Yu, Department of Hematology and Transplantation, the Fifth Medical Center, Chinese PLA General Hospital, Beijing, China.
Bo Cai, Department of Hematology and Transplantation, the Fifth Medical Center, Chinese PLA General Hospital, Beijing, China.
Yao Wang, Molecular & Immunological Department, Bio-therapeutic Department, Chinese PLA General Hospital, Beijing, China.
Zhiqiang Wu, Molecular & Immunological Department, Bio-therapeutic Department, Chinese PLA General Hospital, Beijing, China.
Kaixun Hu, Department of Hematology and Transplantation, the Fifth Medical Center, Chinese PLA General Hospital, Beijing, China.
Qiyun Sun, Department of Hematology and Transplantation, the Fifth Medical Center, Chinese PLA General Hospital, Beijing, China.
Jianhui Qiao, Department of Hematology and Transplantation, the Fifth Medical Center, Chinese PLA General Hospital, Beijing, China.
Yanhong Fang, Department of Hematology and Transplantation, the Fifth Medical Center, Chinese PLA General Hospital, Beijing, China.
Hongli Zuo, Department of Hematology and Transplantation, the Fifth Medical Center, Chinese PLA General Hospital, Beijing, China.
Yi Wang, Department of Hematology and Transplantation, the Fifth Medical Center, Chinese PLA General Hospital, Beijing, China.
Zheng Dong, Department of Hematology and Transplantation, the Fifth Medical Center, Chinese PLA General Hospital, Beijing, China.
Zechuan Zhang, Department of Hematology and Transplantation, the Fifth Medical Center, Chinese PLA General Hospital, Beijing, China.
Yajing Huang, Department of Hematology and Transplantation, the Fifth Medical Center, Chinese PLA General Hospital, Beijing, China.
Zhiqing Liu, Department of Hematology and Transplantation, the Fifth Medical Center, Chinese PLA General Hospital, Beijing, China.
Tieqiang Liu, Department of Hematology and Transplantation, the Fifth Medical Center, Chinese PLA General Hospital, Beijing, China.
Huisheng Ai, Department of Hematology and Transplantation, the Fifth Medical Center, Chinese PLA General Hospital, 8 Dongdajie Street, Fengtai District, Beijing, 100071, China.
Weidong Han, Molecular & Immunological Department, Bio-therapeutic Department, Chinese PLA General Hospital, Beijing, China.
Mei Guo, Department of Hematology and Transplantation, the Fifth Medical Center, Chinese PLA General Hospital, Beijing, China.
References
- 1. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371: 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol 2018; 15: 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Srivastava S, Riddell SR. Chimeric antigen receptor T cell therapy: challenges to bench-to-bedside efficacy. J Immunol 2018; 200: 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruella M, Kenderian SS. Next-generation chimeric antigen receptor T-cell therapy: going off the shelf. BioDrugs 2017; 31: 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qasim W, Zhan H, Samarasinghe S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med 2017; 9: pii: eaaj2013. [DOI] [PubMed] [Google Scholar]
- 6. Cai B, Guo M, Wang Y, et al. Co-infusion of haplo-identical CD19-chimeric antigen receptor T cells and stem cells achieved full donor engraftment in refractory acute lymphoblastic leukemia. J Hematol Oncol 2016; 9: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reisner Y, Martelli MF. Bone marrow transplantation across HLA barriers by increasing the number of transplanted cells. Immunol Today 1995; 16: 437–440. [DOI] [PubMed] [Google Scholar]
- 8. Stewart FM, Crittenden RB, Lowry PA, et al. Long-term engraftment of normal and post-5-fluorouracil murine marrow into normal nonmyeloablated mice. Blood 1993; 81: 2566–2571. [PubMed] [Google Scholar]
- 9. Guo M, Hu KX, Yu CL, et al. Infusion of HLA-mismatched peripheral blood stem cells improves the outcome of chemotherapy for acute myeloid leukemia in elderly patients. Blood 2011; 117: 936–941. [DOI] [PubMed] [Google Scholar]
- 10. Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828. [PubMed] [Google Scholar]
- 11. Filipovich AH, Weisdorf D, Pavletic S, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956. [DOI] [PubMed] [Google Scholar]
- 12. Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014; 124: 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alvarnas JC, Brown PA, Aoun P, et al. Acute lymphoblastic leukemia, version 2.2015. J Natl Compr Canc Netw 2015; 13: 1240–1279. [DOI] [PubMed] [Google Scholar]
- 14. Bishop MR, Alyea EP, Cairo MS, et al. National cancer institute’s first international workshop on the biology, prevention, and treatment of relapse after allogeneic hematopoietic stem cell transplantation: summary and recommendations from the organizing committee. Biol Blood Marrow Transplant 2010; 17: 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_figure_and_table for Co-infusion of high-dose haploidentical donor cells and CD19-targeted CART cells achieves complete remission, successful donor engraftment and significant CART amplification in advanced ALL by Changlin Yu, Bo Cai, Yao Wang, Zhiqiang Wu, Kaixun Hu, Qiyun Sun, Jianhui Qiao, Yanhong Fang, Hongli Zuo, Yi Wang, Zheng Dong, Zechuan Zhang, Yajing Huang, Zhiqing Liu, Tieqiang Liu, Huisheng Ai, Weidong Han and Mei Guo in Therapeutic Advances in Medical Oncology