Abstract
Background:
Exercise tolerance is an important endpoint in chronic obstructive pulmonary disease (COPD) clinical trials. Little is known about the comparative measurement properties of constant work rate cycle ergometry (CWRCE) and the endurance shuttle walking test (ESWT). The objective of this sub-analysis of the TORRACTO® study was to directly compare the endurance measurement properties of CWRCE and ESWT in patients with COPD in a multicentre, multinational setting. We predicted that both tests would be similarly reliable, but that the ESWT would be more responsive to bronchodilation than CWRCE.
Methods:
This analysis included 151 patients who performed CWRCE and ESWT at baseline and week 6 after receiving once-daily placebo, tiotropium/olodaterol (T/O) 2.5/5 μg or T/O 5/5 μg. Reproducibility was assessed by comparing their respective performance at baseline and week 6 in the placebo group. Responsiveness to bronchodilation was assessed by comparing endurance time at week 6 with T/O with baseline values and placebo. The locus of symptom limitation and end-exercise Borg scales for breathing and leg discomfort for both tests were also analysed.
Results:
The intraclass correlation coefficients for CWRCE and ESWT were 0.56 [95% confidence interval (CI) 0.37–0.71] and 0.75 (95% CI 0.63–0.84). More patients were limited by breathing discomfort during the ESWT than during CWRCE, whereas more patients were limited by leg discomfort or breathing/leg discomfort during CWRCE than the ESWT (p <0.0001). Both tests were responsive to bronchodilator treatment: there was a 19% increase in endurance time from baseline at week 6 (p = 0.0006) assessed with CWRCE, and a 20% increase in endurance time assessed with ESWT (p = 0.0013).
Conclusions:
Both exercise tests performed well in a multicentre clinical trial. Although the locus of symptom limitation differed between the two tests, both were reliable and responsive to bronchodilation. For future clinical trials, the choice of test should depend on the study requirements.
ClinicalTrials.gov identifier:
The reviews of this paper are available via the supplemental material section.
Keywords: clinical trials, COPD pharmacology, exercise, exercise testing, physical activity
Introduction
Evaluating exercise tolerance is considered to be an essential component of disease assessment in chronic obstructive pulmonary disease (COPD).1,2 Of the available exercise-testing protocols, constant work rate cycle ergometry (CWRCE) and the endurance shuttle walking test (ESWT) are gaining popularity, and are shown to be reliable and responsive to pharmacological and non-pharmacological intervention.3–5
In a single-centre study, the ESWT was found to be more sensitive to bronchodilation than CWRCE in patients with COPD; differences in the occurrence of quadriceps muscle fatigue were offered by the authors as a potential explanation of the different responsiveness to change between cycling and walking.6 However, although several multicentre and multinational bronchodilator studies have been conducted using CWRCE,7–15 there is currently only limited information for the ESWT in similar settings.16–18 Furthermore, a direct comparison of ESWT and CWRCE in a multicentre setting is lacking. This knowledge could be important in the planning and design of future multicentre clinical trials assessing the impact of pharmacotherapy on exercise tolerance in COPD.
The TORRACTO® study evaluated the effect of tiotropium/olodaterol (T/O) compared with placebo on endurance during CWRCE in patients with COPD after 12 weeks of treatment.19 Endurance during the ESWT was evaluated in a subset of patients.19 The objective of this analysis was to compare, for the first time, the measurement properties of CWRCE and ESWT in patients with COPD in a multicentre, multinational setting at baseline and after 6 weeks of treatment. Specifically, we compared the test–retest reproducibility and the responsiveness of CWRCE and the ESWT, testing the hypotheses that both tests would be similarly reliable, but that the ESWT would be more responsive to bronchodilation than CWRCE. We also compared the relative intensity of breathing discomfort and leg discomfort, as well as the locus of symptom limitation, during these two exercise testing modalities.
Methods
Study design
TORRACTO® (1237.15) [ClinicalTrials.gov identifier: NCT01525615] was a multicentre, multinational, randomised, double-blind, placebo-controlled, parallel-group trial to evaluate the effects of once-daily T/O (2.5/5 μg and 5/5 μg) compared with placebo on exercise tolerance after 12 weeks of treatment in patients with COPD (methods detailed in the work by Maltais et al.19). This secondary analysis of TORRACTO® involved patients in whom both the ESWT and the CWRCE tests were performed. In total, 25 centres participated in the ESWT sub-study (Supplemental Figure S1 and Table S1), and they received ethics approval for both the main study and the ESWT sub-study.
Patients
Detailed description of the study population is reported elsewhere.19 Patients aged 40–75 years with a clinical diagnosis of COPD, a post-bronchodilator forced expiratory volume in 1 s (FEV1) between ⩾30% and <80% predicted normal and a post-bronchodilator FEV1/forced vital capacity (FVC) <70% were recruited. Key exclusion criteria were significant disease other than COPD, a history of asthma, myocardial infarction in the previous year, unstable or life-threatening cardiac arrhythmia, or hospitalisation for heart failure within the previous year.
The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Tripartite Guideline for Good Clinical Practice. Before the study started, the protocol was reviewed and approved by the institutional review boards, and all patients were required to provide written informed consent.
Exercise testing
The specific details about exercise testing procedures have been previously reported19 and are included in the online supplement. Exercise tests were performed at screening and baseline, and 2 h after dosing at weeks 6 and 12. The CWRCE was always performed first, and the ESWT 2–4 days later, because the CWRCE was the study primary endpoint.
During both exercise testing procedures, patients rated the intensity of breathing discomfort and leg discomfort using the modified Borg scale at rest, at 2-min intervals during exercise and at the end of exercise. Immediately after completing exercise, subjects were asked to identify the primary reason for stopping exercise using a standardised questionnaire.20
CWRCE
Patients performed incremental cycle ergometry to symptom limitation to determine peak work rate (Wpeak) at an initial screening visit. During the run-in period, two CWRCE tests to symptom limitation at 75% Wpeak were conducted: the first test was used to familiarise the patient with the exercise protocol, whereas the second test, conducted at least 4 days later, was a priori defined as the pre-treatment baseline. To avoid unduly long exercise tests that could lead to non-physiological exercise limitation (e.g. discomfort or tiredness), patients who cycled for >25 min at either of the two run-in CWRCE tests were not eligible for randomisation.
For CWRCE, subjects performed 1 min of unloaded pedalling before the work rate was immediately increased to 75% Wpeak; subjects were encouraged to cycle to the point of symptom limitation. For all exercise tests, patients were instructed to self-select a comfortable pedalling rate from 50 to 90 revolutions per minute, and then to maintain that self-selected pedalling rate throughout exercise. Exercise endurance time (EET) was recorded as the time from the increase in work rate to the point of symptom limitation.
ESWT
An incremental shuttle walking test (ISWT)21 was conducted 2–4 days after the initial screening visit to estimate volume of oxygen (VO2) peak.
Training and baseline ESWTs were performed at a walking speed corresponding to 85% of estimated VO2 peak from ISWT.22 Patients with an EET ⩾15 min in the training or baseline ESWT were excluded from further participation in this sub-study. This 15-min limit was set to allow some space for improvement given that the recorded audio signal for the walking pace of the ESWT has a maximum duration of 20 min.
The ESWT was performed in an enclosed corridor on a flat 10 m course. After a 90 s warm-up at a slow pace, walking speed was set at the pace corresponding to 85% of estimated VO2 peak (see online supplement for more detail). Subjects were instructed to walk for as long as possible at the speed dictated by the auditory signal. The EET, as well as the distance covered in that time, were recorded. The warm-up period was excluded from this analysis.
Statistical analysis
Although the comparison of tests was a secondary objective of the trial, the analyses presented herein are post hoc. Owing to the skewness of baseline endurance time during CWRCE and ESWT,4,19,23 we transformed the endurance time in seconds to the logarithm to base 10 scale and also present results in the original units (EET in seconds). The reproducibility of the CWRCE and ESWT endurance times was evaluated by comparing values obtained at baseline and week 6 in the placebo group. The presence of a systematic bias and the agreement between the baseline and week 6 exercise tests were assessed using Bland–Altman plots,24 whereas the linear correlation between exercise time during both CWRCEs and ESWTs was evaluated by the Pearson product-moment correlation. The CWRCE and ESWT performance reproducibility was also assessed with determination of the intraclass correlation coefficient (ICC).25 The ability of the two exercise modalities to detect changes (responsiveness) with both doses of T/O versus baseline and placebo at week 6 was examined. Week 6 data are used in this analysis rather than week 12 data to enhance the chances that patients in the placebo group would have relatively stable measurements for the reproducibility analysis.
We compared the locus of symptom limitation between the two tests using a Bhapkar’s test for marginal homogeneity.
As per study design, patients involved in TORRACTO® could have a CWRCE EET up to 25 min and an ESWT EET up to 15 min at baseline. To take this difference in test duration into account, we excluded from the present analysis the small number of patients with a CWRCE EET at baseline between 15 and 25 min (n = 14). At week 6, the duration of the ESWT was limited at 20 min, and there was no limit on CWRCE.
For these analyses, we pooled the two T/O arms (2.5/5 µg and 5/5 µg). This was done to optimise the statistical power of this analysis, and is justified, considering that both doses of T/O improve CWRCE and ESWT EETs to a similar extent.19,23
Results
Patient disposition and baseline characteristics
From the 165 patients who performed both CWRCE and ESWT in the TORRACTO® study, 14 were excluded because their CWRCE EET at baseline was ⩾15 min, leaving 151 patients for this analysis (Supplemental Figure S1). Of these, 47 were treated with placebo and 104 with T/O (pooled 2.5/5 µg and 5/5 µg arms). Baseline characteristics were generally balanced across treatment groups (Table 1).
Table 1.
Baseline patient characteristics.
| Placebo (n = 47) | T/O (doses combined) (n = 104) | |
|---|---|---|
| Male, n (%) | 29 (61.7) | 73 (70.2) |
| Mean (SD) age, years | 61.1 (6.4) | 64.9 (7.4) |
| Pre-bronchodilator | ||
| Mean (SD) FEV1, l | 1.471 (0.434) | 1.351 (0.420) |
| Post-bronchodilator (albuterol) | ||
| Mean (SD) FEV1, l | 1.662 (0.419) | 1.564 (0.440) |
| Mean (SD) predicted normal FEV1, % | 60.4 (11.6) | 57.0 (13.4) |
| Mean (SD) FEV1 change from pre-bronchodilator, l | 0.191 (0.155) | 0.213 (0.143) |
| Mean (SD) FEV1/FVC, % | 53.2 (9.8) | 50.9 (10.8) |
| GOLD, n (%) | ||
| 2 | 37 (78.7) | 74 (71.2) |
| 3 | 10 (21.3) | 27 (26.0) |
| Mean (SD) body mass index, kg/m2 | 28.3 (5.2) | 27.6 (5.0) |
| Current smoker, n (%) | 22 (46.8) | 36 (34.6) |
| Ex-smoker, n (%) | 25 (53.2) | 68 (65.4) |
| Mean (SD) smoking history, pack-years | 45.8 (23.4) | 50.9 (28.3) |
FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SD, standard deviation; T/O, tiotropium/olodaterol.
Five patients (all in the T/O groups) reached test termination criteria on the ESWT at week 6 (i.e. EET = 20 min); their EET was thus recorded as 20 min. For comparison, two patients had an EET during CWRCE of ⩾20 min at week 6; for these individuals, the actual EET was used for analysis.
Distribution and comparison of exercise duration for CWRCE and ESWT at baseline and week 6
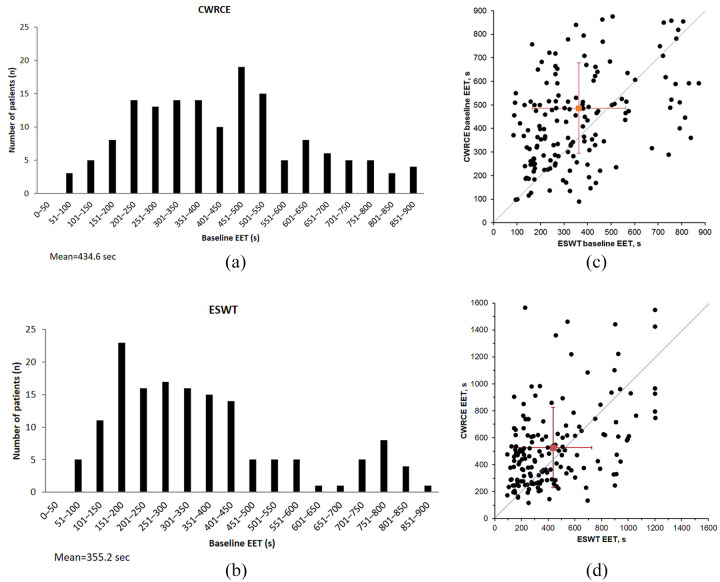
The distributions of the CWRCE and ESWT endurance times at baseline are presented in Figure 1(a,b). In both cases, the distribution of exercise duration showed a rightward skew toward long exercise duration. A scatter plot of exercise duration during cycling and walking at baseline and at week 6 is provided in Figure 1(c,d). The majority of patients exhibited a longer EET during cycling compared with walking.
Figure 1.
Distribution of CWRCE and ESWT endurance times at baseline, as well as exercise duration of cycling and walking at baseline and at 6 weeks.
(a) Distribution of CWRCE endurance time at baseline; (b) distribution of ESWT endurance time at baseline; (c) baseline exercise duration during cycling and walking; (d) week 6 exercise duration during cycling and walking.
CWRCE, constant work rate cycle ergometry; EET, exercise endurance time; ESWT, endurance shuttle walking test; s, second.
Mean endurance time at baseline was 435 s during the CWRCE and 355 s during the ESWT.
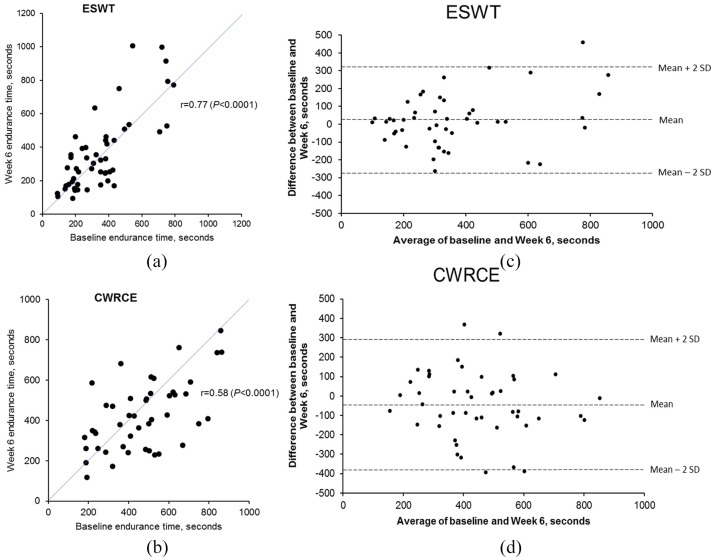
Test–retest reproducibility of the CWRCE and ESWT
Mean values for the CWRCE and ESWT times obtained at baseline and week 6 in the placebo group are reported in Table 2. Scatter plots showing baseline and week 6 endurance time values for CWRCE and the ESWT in the placebo group are shown in Figure 2. Strong linear relationships were found for endurance times obtained at baseline and week 6 for both exercise modalities. Pearson correlation coefficients were 0.77 (p <0.0001) and 0.58 (p <0.0001) for walking and cycling, respectively [Figure 2(a,b)]. The reproducibility of ESWT and CWRCE performance was also confirmed with Bland–Altman plots [Figure 2(c,d)]. The variability of endurance time seems to increase over time during both ESWT and CWRCE; mean (SE) endurance time in the T/O arms was 471.49 (28.74) s on day 46 and 472.73 (30.50) s on day 88 during ESWT, and 561.50 (31.16) s on day 43 and 576.69 (36.28) s on day 85 for CWRCE.
Table 2.
Reproducibility: endurance time measured during CWRCE and ESWT at baseline and week 6 in the placebo group.
| Mean (SE) endurance time, s |
Pearson correlation coefficient (p value) | Intraclass correlation coefficient (95% CI) | |||
|---|---|---|---|---|---|
| Baseline | Week 6 | Change from baseline | |||
| CWRCE (n = 46) | 468.9 (28.5) | 425.2 (25.3) | −43.7 (24.8) | 0.58 (<0.0001) | 0.56 (0.37–0.71) |
| ESWT (n = 47) | 352.6 (27.7) | 375.6 (34.0) | 23.0 (21.8) | 0.77 (<0.0001) | 0.75 (0.63–0.84) |
CI, confidence interval; CWRCE, constant work rate cycle ergometry; ESWT, endurance shuttle walking test; s, seconds; SE, standard error of the mean.
Figure 2.
Baseline and week 6 endurance time values in the placebo group for ESWT and CWRCE, as well as Bland–Altman plots showing reproducibility of the ESWT and CWRCE.
(a) Baseline and week 6 endurance time values in the placebo group; (b) baseline and week 6 endurance time values in the placebo group; (c) Bland–Altman plot showing reproducibility of the ESWT; (d) Bland–Altman plot showing reproducibility of CWRCE.
CWRCE, constant work rate cycle ergometry; ESWT, endurance shuttle walking test; SD, standard deviation.
The reproducibility of both exercise modalities assessed using ICC was 0.75 for the ESWT and 0.56 for CWRCE (Table 2). Although the ICC for CWRCE was numerically smaller, the difference did not reach statistical significance (there was an overlap in ICC 95% CIs between the tests).
Responsiveness of CWRCE and the ESWT to bronchodilation
The responsiveness of both exercise tests was addressed by pooling the data of the two T/O treatment groups. The arithmetic and geometric mean values for the endurance time at baseline and at week 6 for the two exercise modalities are presented in Table 3. Both tests were responsive to bronchodilator treatment; the improvement from baseline in exercise duration with T/O was similar for the two exercise testing modalities at week 6 (19% for cycling and 20% for walking, based on the geometric mean). There were also improvements with T/O compared with placebo at week 6 (22% for cycling and 17% for walking, based on the geometric means; Table 3).
Table 3.
EET in the tiotropium/olodaterol pooled group at week 6 versus baseline and in the tiotropium/olodaterol pooled group versus placebo group at week 6.
| T/O at week 6 versus baseline | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arithmetic mean |
Geometric mean |
|||||||||
| EET, s (SE) |
Change from baseline |
EET, s (SE) |
Ratio of EET at week 6 versus baseline |
|||||||
| Baseline | Week 6 | Mean | 95% CI | p value | Baseline | Week 6 | Mean | 95% CI | p value | |
| CWRCE (n = 103) | 418.6 (18.8) | 507.0 (27.0) | 88.4 | 44.0–132.8 | 0.0001 | 372.0 (19.0) | 441.7 (23.2) | 1.187 | 1.078–1.308 | 0.0006 |
| ESWT (n = 103) | 358.6 (20.2) | 457.2 (30.3) | 98.6 | 48.7–148.6 | 0.0002 | 305.9 (17.3) | 368.3 (24.1) | 1.204 | 1.077–1.346 | 0.0013 |
| T/O versus placebo at week 6 | ||||||||||
| Arithmetic mean |
Geometric mean |
|||||||||
| EET, s (SE) |
Change from placebo |
EET, s (SE) |
Ratio of EET with T/O at week 6 versus placebo |
|||||||
| Placebo | T/O | Mean | 95% CI | p value | Placebo | T/O | Mean | 95% CI | p value | |
| CWRCE | 425.2 (25.3) | 507.0 (27.0) | 118.3 | 45.9–190.8 | 0.0015 | 390.3 (24.9) | 441.7 (23.2) | 1.223 | 1.054–1.420 | 0.0083 |
| ESWT | 375.6 (34.0) | 457.2 (30.3) | 76.3 | −2.8–155.4 | 0.0585 | 316.0 (27.4) | 368.3 (24.1) | 1.165 | 0.979–1.387 | 0.0856 |
CI, confidence interval; CWRCE, constant work rate cycle ergometry; EET, exercise endurance time; ESWT, endurance shuttle walk test; s, seconds; SE, standard error of the mean; T/O, tiotropium/olodaterol.
Locus of symptom limitation
Figure 3 shows the locus of symptom limitation at baseline for all treated patients, that is, whether it was leg discomfort or breathing discomfort, or both, that caused a patient to stop cycling/walking. This differed between the two tests (Bhapkar’s test for marginal homogeneity p <0.0001); a higher proportion of patients were limited by breathing discomfort during the ESWT (57%) than during CWRCE (39%). Moreover, a higher proportion of patients during the CWRCE than during the ESWT were limited by either leg or leg and breathing discomfort.
Figure 3.
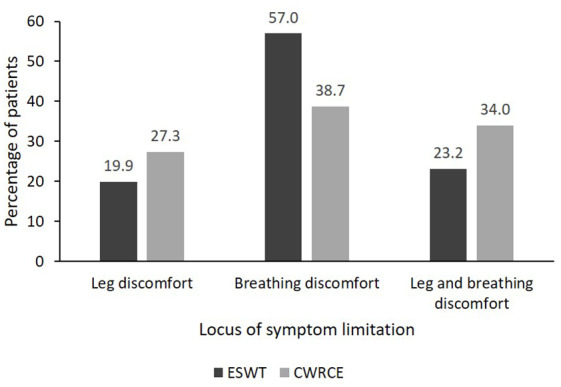
Locus of symptom limitation.
CWRCE, constant work rate cycle ergometry; ESWT, endurance shuttle walking test.
Furthermore, the mean (SD) Borg scale for leg discomfort at the end of exercise at baseline was numerically greater with CWRCE [6.54 (2.97)] than with the ESWT [5.23 (3.31)], whereas the Borg scale for breathing discomfort was similar after both tests [6.59 (2.54) for CWRCE; 6.52 (2.56) for the ESWT].
In an analysis of whether there was a difference in response to bronchodilators by the locus of symptom limitation at baseline, the greatest improvement from baseline with T/O in the ESWT was in patients with breathing discomfort at baseline, and there was no significant difference in patients with leg discomfort (Table 4). The same was true in the CWRCE: the greatest improvement from baseline with T/O occurred in patients who were limited by breathing discomfort at baseline, although CWRCE EET also improved significantly with T/O in patients limited by leg fatigue (Table 5).
Table 4.
Improvement in ESWT EET with T/O by baseline locus of symptom limitation.
| Arithmetic mean |
Geometric mean |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EET, s (SE) |
Change from baseline |
EET, s (SE) |
Ratio of EET at week 6 versus baseline |
|||||||
| Baseline | Week 6 | Mean | 95% CI | p value | Baseline | Week 6 | Mean | 95% CI | p value | |
| Leg discomfort (n = 21) | 409.0 (48.2) | 394.1 (56.2) | −15.0 | −83.7 to 53.8 | 0.6551 | 351.9 (44.7) | 326.2 (44.4) | 0.927 | 0.762–1.128 | 0.4300 |
| Breathing discomfort (n = 60) | 363.1 (28.0) | 526.7 (44.0) | 163.6 | 85.9–241.3 | <0.0001 | 303.0 (24.3) | 420.4 (38.0) | 1.387 | 1.178–1.634 | 0.0002 |
| Leg/breathing discomfort (n = 22) | 298.2 (29.2) | 328.0 (40.3) | 29.8 | −17.5 to 77.1 | 0.2048 | 274.7 (23.7) | 288.0 (31.7) | 1.048 | 0.894–1.229 | 0.5425 |
CI, confidence interval; EET, exercise endurance time; ESWT, endurance shuttle walking test; SE, standard error of the mean; T/O, tiotropium/olodaterol.
Table 5.
Improvement in CWRCE EET with T/O by baseline locus of symptom limitation.
| Arithmetic mean |
Geometric mean |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EET, s (SE) |
Change from baseline |
EET, s (SE) |
Ratio of EET at week 6 versus baseline |
|||||||
| Baseline | Week 6 | Mean | 95% CI | p value | Baseline | Week 6 | Mean | 95% CI | p value | |
| Leg discomfort (n = 27) | 432.7 (34.0) | 492.6 (40.8) | 59.9 | 0.7–119.0 | 0.0474 | 398.3 (32.6) | 448.5 (39.5) | 1.126 | 1.007–1.258 | 0.0376 |
| Breathing discomfort (n = 40) | 378.7 (30.4) | 515.7 (53.8) | 137.0 | 45.7–228.2 | 0.0043 | 324.1 (31.0) | 425.4 (42.0) | 1.313 | 1.074–1.604 | 0.0091 |
| Leg/breathing discomfort (n = 36) | 452.3 (32.6) | 508.1 (39.5) | 55.8 | −10.8 to 122.4 | 0.0978 | 411.9 (30.7) | 455.2 (36.7) | 1.105 | 0.950–1.285 | 0.1876 |
CI, confidence interval; CWRCE, constant work rate cycle ergometry; EET, exercise endurance time; SE, standard error of the mean; T/O, tiotropium/olodaterol.
Discussion
The TORRACTO® study provided an opportunity to directly compare the measurement properties of the ESWT and CWRCE in a multicentre trial. More patients stopped the ESWT due to breathing discomfort than those in CWRCE, although both tests were similarly responsive to bronchodilator treatment. Notably, there were large improvements in walking and cycling duration in individuals in whom breathing discomfort was the primary reason for exercise termination. Overall, both tests were reliable and responsive, and are suitable for use in a multicentre trial setting.
The reproducibility of the CWRCE was lower in our study (ICC = 0.56) compared with previous studies that considered shorter intervals in assessing the reproducibility (ICC = 0.844 and ICC = 0.8526). Reasons for this discrepancy between studies are unclear but a number of factors may have contributed. Implementing exercise testing procedures across several clinical research centres is challenging, and despite efforts to standardise procedures, it is conceivable that variation in EET reproducibility across studies may be related to technical issues associated with the measurements. In the present study, repeated tests were separated by a 6-week period, whereas previous studies looked at variability between two tests with a shorter interval (5 days4 and within 2 weeks26). Differences in study population may also contribute to different reproducibility between studies. For example, in TORRACTO®, there was no requirement for resting hyperinflation to be present, whereas in a combined exercise data set of two large clinical trials,4 patients had resting hyperinflation. Pre-bronchodilator FEV1 % predicted in the current study was 52% predicted, compared with 42% and 40% predicted in previous studies. Finally, the previous analysis had a larger sample size (n = 463)4 than in the present analysis.
Both CWRCE and the ESWT are constant work-rate tests and are therefore subject to a similar profile of physiological demands. The CWRCE is shown to be responsive and reproducible,5,26 and has become a standard method for evaluating the efficacy of long-acting bronchodilators in patients with COPD. It allows the measurement of physiological variables using standard cardiopulmonary test equipment. However, CWRCE may not reflect typical daily activity of patients with COPD, so the ESWT22,27 may have value in this regard. Ambulatory activities such as climbing stairs and walking are affected earlier in the disease progression in patients with COPD.28,29
The ESWT is increasingly used in COPD studies.6,17 The ESWT has been shown to be a more sensitive test than CWRCE to assess bronchodilation in a single-centre study in patients with COPD.6 In this study, Pepin et al. showed that the ESWT was a sensitive test to detect changes in exercise tolerance after bronchodilation, whereas CWRCE time did not improve significantly after bronchodilation despite a significant increase in FEV1.6 The occurrence and extent of quadriceps muscle fatigue was proposed as a potential explanation for the difference in sensitivity between the ESWT and CWRCE in detecting improvements following bronchodilator therapy, as it was more pronounced and frequent after cycling than after walking.6,30 Similarly, we found that the greatest improvement from baseline with T/O occurred in patients who were limited by breathlessness with both exercise methodologies. This may have implications for the selection of participants in clinical trials evaluating the impact of bronchodilation on exercise duration. For example, selecting study participants who are primarily limited by dyspnoea would be an ideal experimental set-up to highlight the potential of bronchodilators to enhance exercise endurance.
Considering that the greatest improvement in exercise duration with T/O occurred in patients limited by dyspnoea, and that this symptom was also a more frequent locus of symptom limitation during the ESWT, one would expect this exercise modality to be more responsive than CWRCE but this was not the case in the present clinical trial. This is because, in contrast to what was seen during the ESWT, patients limited by leg fatigue also improved their cycling exercise duration with T/O. The reason for this disparity in exercise response to T/O in those limited by leg fatigue between the two exercise modalities is uncertain but it contributed to the overall similar responsiveness of the two exercise modalities in the present investigation.
A potential weakness of using this study to compare the two exercise tests is that different upper limits were used for the two different tests; we have accounted for this by excluding all patients with EET ⩾15 min at baseline. Unlike a previous comparison of walking and cycling exercise tests,6 cardiopulmonary monitoring data are not reported here because it was not done in the ESWTs. The European Respiratory Society recommends that the target duration for endurance time should be between 3 and 8 min; this was not true of the testing protocols used here, but this should be considered in the design of future trials.31
Another issue to consider is that there was no predetermined upper limit of exercise duration at week 6 with the CWRCE, but the ESWT was capped at 20 min. In theory, this could have influenced both the reproducibility and responsiveness data for the ESWT. For example, having participants in the placebo group reaching the maximum allowable limit of 20 min at week 6 would tend to decrease the difference between the baseline and week 6 ESWT duration, thus artificially improving the reproducibility data. Only one individual in the placebo group reached the ESWT exercise duration upper limit at week 6. Conversely, fixing an upper limit for the ESWT exercise duration could reduce the magnitude of responsiveness of the test following bronchodilator treatment. In the study, five patients allocated to T/O reached the 20 min ESWT maximum duration after 6 weeks of treatment, and the test was therefore terminated by the investigators. Had we allowed these individuals to continue the walking test, the magnitude of improvement in ESWT seen with T/O would have been larger. Our estimate of the responsiveness of the ESWT is thus conservative.
CWRCE and the ESWT both performed well in the context of a multicentre clinical trial. The locus of symptom limitation differed between the two tests; however, both tests were reliable and responsive to bronchodilation. For future trial design, the choice of test should depend upon the requirements of the study, including the need to collect physiological response data, which can be done more conveniently during stationary cycling.
Supplemental Material
Supplemental material, Author_Response_1 for Comparative measurement properties of constant work rate cycling and the endurance shuttle walking test in COPD: the TORRACTO® clinical trial by François Maltais, Denis E. O’Donnell, Alan Hamilton, Yihua Zhao and Richard Casaburi in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Comparative measurement properties of constant work rate cycling and the endurance shuttle walking test in COPD: the TORRACTO® clinical trial by François Maltais, Denis E. O’Donnell, Alan Hamilton, Yihua Zhao and Richard Casaburi in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Comparative measurement properties of constant work rate cycling and the endurance shuttle walking test in COPD: the TORRACTO® clinical trial by François Maltais, Denis E. O’Donnell, Alan Hamilton, Yihua Zhao and Richard Casaburi in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.2 for Comparative measurement properties of constant work rate cycling and the endurance shuttle walking test in COPD: the TORRACTO® clinical trial by François Maltais, Denis E. O’Donnell, Alan Hamilton, Yihua Zhao and Richard Casaburi in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_3_v.1 for Comparative measurement properties of constant work rate cycling and the endurance shuttle walking test in COPD: the TORRACTO® clinical trial by François Maltais, Denis E. O’Donnell, Alan Hamilton, Yihua Zhao and Richard Casaburi in Therapeutic Advances in Respiratory Disease
Supplemental material, Supplement for Comparative measurement properties of constant work rate cycling and the endurance shuttle walking test in COPD: the TORRACTO® clinical trial by François Maltais, Denis E. O’Donnell, Alan Hamilton, Yihua Zhao and Richard Casaburi in Therapeutic Advances in Respiratory Disease
Acknowledgments
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. They take full responsibility for the scope, direction, content of and editorial decisions relating to the manuscript, were involved at all stages of development and have approved the submitted manuscript. The authors received no compensation related to the development of the manuscript.
Footnotes
Author contribution(s): François Maltais: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing-original draft; Writing-review & editing.
Denis E. O’Donnell: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing-original draft; Writing-review & editing.
Alan Hamilton: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing-original draft; Writing-review & editing.
Yihua Zhao: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing-original draft; Writing-review & editing.
Richard Casaburi: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing-original draft; Writing-review & editing.
Conflict of interest statement: FM has received research grants for participating in multicentre trials for AstraZeneca, Boehringer Ingelheim, GSK, Sanofi, and Novartis, and has received unrestricted research grants and personal fees from Boehringer Ingelheim, Grifols, and Novartis. DEOD reports grants from AstraZeneca and grants from Boehringer Ingelheim during the conduct of the study; and personal fees from AstraZeneca, Boehringer Ingelheim and Novartis outside the submitted work. RC reports grants and personal fees from Boehringer Ingelheim Pharma, AstraZeneca Pharma, GlaxoSmithKline Pharma and Astellas, personal fees from Regeneron Pharma, Genentech Pharma, and Theravance, outside the submitted work. AH and YZ are employees of Boehringer Ingelheim.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by Boehringer Ingelheim Pharma GmbH & Co. KG. Medical writing assistance was provided by Rob Kite of Complete HealthVizion and Claire Scofield of MediTech Media, which was contracted and compensated by Boehringer Ingelheim Pharma GmbH & Co. KG.
ORCID iD: François Maltais  https://orcid.org/0000-0002-6809-4651
https://orcid.org/0000-0002-6809-4651
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
François Maltais, Research Centre, Institut universitaire de Cardiologie et de Pneumologie de Québec, Université Laval, 2725 Chemin Sainte-Foy, Québec, G1V 4G5, Canada.
Denis E. O’Donnell, Department of Medicine, Queen’s University and Kingston Health Sciences Centre, Kingston, ON, Canada
Alan Hamilton, Medical Department, Boehringer Ingelheim, Burlington, ON, Canada.
Yihua Zhao, Biostatistics and Data Sciences, Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, CT, USA.
Richard Casaburi, Rehabilitation Clinical Trial Centre, Los Angeles Biomedical Research Institute at Harbour-UCLA Medical Centre, Torrance, CA, USA.
References
- 1. American Thoracic Society and American College of Chest Physicians. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003; 167: 211–277. [DOI] [PubMed] [Google Scholar]
- 2. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (2019. report), https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf (2019, accessed 26 September 2019).
- 3. Borel B, Pepin V, Mahler DA, et al. Prospective validation of the endurance shuttle walking test in the context of bronchodilation in COPD. Eur Respir J 2014; 44: 1166–1176. [DOI] [PubMed] [Google Scholar]
- 4. O’Donnell DE, Travers J, Webb KA, et al. Reliability of ventilatory parameters during cycle ergometry in multicentre trials in COPD. Eur Respir J 2009; 34: 866–874. [DOI] [PubMed] [Google Scholar]
- 5. Borel B, Provencher S, Saey D, et al. Responsiveness of various exercise-testing protocols to therapeutic interventions in COPD. Pulm Med 2013; 2013: 410748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pepin V, Saey D, Whittom F, et al. Walking versus cycling: sensitivity to bronchodilation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172: 1517–1522. [DOI] [PubMed] [Google Scholar]
- 7. Beeh KM, Watz H, Puente-Maestu L, et al. Aclidinium improves exercise endurance, dyspnea, lung hyperinflation, and physical activity in patients with COPD: a randomized, placebo-controlled, crossover trial. BMC Pulm Med 2014; 14: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beeh KM, Wagner F, Khindri S, et al. Effect of indacaterol on dynamic lung hyperinflation and breathlessness in hyperinflated patients with COPD. COPD 2011; 8: 340–345. [DOI] [PubMed] [Google Scholar]
- 9. Beeh KM, Singh D, Di Scala L, et al. Once-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trial. Int J Chron Obstruct Pulmon Dis 2012; 7: 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beeh KM, Korn S, Beier J, et al. Effect of QVA149 on lung volumes and exercise tolerance in COPD patients: the BRIGHT study. Respir Med 2014; 108: 584–592. [DOI] [PubMed] [Google Scholar]
- 11. O’Donnell DE, Fluge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004; 23: 832–840. [DOI] [PubMed] [Google Scholar]
- 12. Maltais F, Hamilton A, Marciniuk D, et al. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest 2005; 128: 1168–1178. [DOI] [PubMed] [Google Scholar]
- 13. O’Donnell DE, Sciurba F, Celli B, et al. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest 2006; 130: 647–656. [DOI] [PubMed] [Google Scholar]
- 14. Maltais F, Celli B, Casaburi R, et al. Aclidinium bromide improves exercise endurance and lung hyperinflation in patients with moderate to severe COPD. Respir Med 2011; 105: 580–587. [DOI] [PubMed] [Google Scholar]
- 15. O’Donnell DE, Bredenbroker D, Brose M, et al. Physiological effects of roflumilast at rest and during exercise in COPD. Eur Respir J 2012; 39: 1104–1112. [DOI] [PubMed] [Google Scholar]
- 16. Bedard ME, Brouillard C, Pepin V, et al. Tiotropium improves walking endurance in COPD. Eur Respir J 2012; 39: 265–271. [DOI] [PubMed] [Google Scholar]
- 17. Brouillard C, Pepin V, Milot J, et al. Endurance shuttle walking test: responsiveness to salmeterol in COPD. Eur Respir J 2008; 31: 579–584. [DOI] [PubMed] [Google Scholar]
- 18. Maltais F, Singh S, Donald AC, et al. Effects of a combination of umeclidinium/vilanterol on exercise endurance in patients with chronic obstructive pulmonary disease: two randomized, double-blind clinical trials. Ther Adv Respir Dis 2014; 8: 169–181. [DOI] [PubMed] [Google Scholar]
- 19. Maltais F, O’Donnell D, Galdiz Iturri JB, et al. Effect of 12 weeks of once-daily tiotropium/olodaterol on exercise endurance during constant work-rate cycling and endurance shuttle walking in chronic obstructive pulmonary disease. Ther Adv Respir Dis 2018; 12: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamilton AL, Killian KJ, Summers E, et al. Symptom intensity and subjective limitation to exercise in patients with cardiorespiratory disorders. Chest 1996; 110: 1255–1263. [DOI] [PubMed] [Google Scholar]
- 21. Singh SJ, Morgan MD, Scott S, et al. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992; 47: 1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Revill SM, Morgan MDL, Singh SJ, et al. The endurance shuttle walk: a new field test for the assessment of endurance capacity in chronic obstructive pulmonary disease. Thorax 1999; 54: 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O’Donnell DE, Casaburi R, Frith P, et al. Effects of combined tiotropium/olodaterol on inspiratory capacity and exercise endurance in COPD. Eur Respir J 2017; 49: pii: 1601348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bland JM, Altman DJ. Regression analysis. Lancet 1986; 1: 908–909. [DOI] [PubMed] [Google Scholar]
- 25. Lee J, Koh D, Ong CN. Statistical evaluation of agreement between two methods for measuring a quantitative variable. Comput Biol Med 1989; 19: 61–70. [DOI] [PubMed] [Google Scholar]
- 26. van ’t Hul A, Gosselink R, Kwakkel G. Constant-load cycle endurance performance: test-retest reliability and validity in patients with COPD. J Cardiopulm Rehabil 2003; 23: 143–150. [DOI] [PubMed] [Google Scholar]
- 27. Pitta F, Troosters T, Spruit MA, et al. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171: 972–977. [DOI] [PubMed] [Google Scholar]
- 28. Polatli M, Bilgin C, Saylan B, et al. A cross sectional observational study on the influence of chronic obstructive pulmonary disease on activities of daily living: the COPD-Life study. Tuberk Toraks 2012; 60: 1–12. [DOI] [PubMed] [Google Scholar]
- 29. Wheaton AG, Cunningham TJ, Ford ES, et al. Employment and activity limitations among adults with chronic obstructive pulmonary disease — United States, 2013. MMWR Morb Mortal Wkly Rep 2015; 64: 289–295. [PMC free article] [PubMed] [Google Scholar]
- 30. Man WD, Soliman MG, Gearing J, et al. Symptoms and quadriceps fatigability after walking and cycling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 168: 562–567. [DOI] [PubMed] [Google Scholar]
- 31. Puente-Maestu L, Palange P, Casaburi R, et al. Use of exercise testing in the evaluation of interventional efficacy: an official ERS statement. Eur Respir J 2016; 47: 429–460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response_1 for Comparative measurement properties of constant work rate cycling and the endurance shuttle walking test in COPD: the TORRACTO® clinical trial by François Maltais, Denis E. O’Donnell, Alan Hamilton, Yihua Zhao and Richard Casaburi in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Comparative measurement properties of constant work rate cycling and the endurance shuttle walking test in COPD: the TORRACTO® clinical trial by François Maltais, Denis E. O’Donnell, Alan Hamilton, Yihua Zhao and Richard Casaburi in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Comparative measurement properties of constant work rate cycling and the endurance shuttle walking test in COPD: the TORRACTO® clinical trial by François Maltais, Denis E. O’Donnell, Alan Hamilton, Yihua Zhao and Richard Casaburi in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.2 for Comparative measurement properties of constant work rate cycling and the endurance shuttle walking test in COPD: the TORRACTO® clinical trial by François Maltais, Denis E. O’Donnell, Alan Hamilton, Yihua Zhao and Richard Casaburi in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_3_v.1 for Comparative measurement properties of constant work rate cycling and the endurance shuttle walking test in COPD: the TORRACTO® clinical trial by François Maltais, Denis E. O’Donnell, Alan Hamilton, Yihua Zhao and Richard Casaburi in Therapeutic Advances in Respiratory Disease
Supplemental material, Supplement for Comparative measurement properties of constant work rate cycling and the endurance shuttle walking test in COPD: the TORRACTO® clinical trial by François Maltais, Denis E. O’Donnell, Alan Hamilton, Yihua Zhao and Richard Casaburi in Therapeutic Advances in Respiratory Disease