Abstract
Background:
This study aimed to investigate the efficiency and toxicities of concurrent chemoradiotherapy (CCRT) and induction chemotherapy (IC) followed by radiotherapy (RT) in different risk locoregionally advanced nasopharyngeal carcinoma (NPC).
Methods:
A total of 1814 eligible patients with stage II–IVB disease treated with CCRT or IC plus RT were included. The overall survival (OS), progression-free survival (PFS) and distant metastasis-free survival (DMFS) were calculated using the Kaplan–Meier method, and the differences were compared using the log-rank test.
Results:
Nomograms were developed to predict OS, PFS and DMFS (C-index: 0.71, 0.70 and 0.71, respectively). Patients were then divided into three different risk groups based on the scores calculated by the nomogram for OS. In the low and intermediate-risk group, no significant survival differences were observed between patients treated with IC plus RT alone and CCRT (5-year OS, 97.3% versus 95.6%, p = 0.642 and 87.6% versus 89.7%, p = 0.381, respectively; PFS, 95.9% versus 95.6%, p = 0.325 and 87.6% versus 89.0%, p = 0.160, respectively; DMFS, 97.2% versus 94.8%, p = 0.339 and 87.2% versus 89.3%, p = 0.628, respectively). However, in the high-risk group, IC plus RT displayed an unfavorable 5-year OS (71.0% versus 77.2%, p = 0.022) and PFS (69.4.0% versus 75.4%, p = 0.019) compared with CCRT. A significantly higher incidence of grade 3 and 4 adverse events was documented in patients treated with CCRT than in those treated with IC plus RT in all risk groups (p = 0.040).
Conclusion:
IC followed by RT represents an alternative treatment strategy to CCRT for patients with low and intermediate-risk NPC, but it is not recommended for patients with high-risk NPC.
Keywords: clinical outcome, concurrent chemotherapy, EBV DNA, induction chemotherapy, nasopharyngeal carcinoma
Introduction
Nasopharyngeal carcinoma (NPC) is a unique malignancy arising from the epithelial tissues of the nasopharynx and associated with Epstein–Barr virus (EBV) infection in most cases.1 Based on the findings of several prospective randomized trials and meta-analyses, radiotherapy (RT) in combination with cisplatin-based concurrent chemotherapy is the standard of care for previously untreated locoregionally advanced NPC.2–5 The National Comprehensive Cancer Network guidelines recommend RT concurrent with cisplatin 100 mg/m² every 3 weeks for patients with stage II–IVB disease. However, concurrent chemotherapy could increase treatment-related toxicities and decrease treatment compliance, leading discontinuation of RT in some patients. Discontinuing or prolonging treatment can reduce RT efficacy.6 Moreover, the rationale of concurrent chemotherapy with RT in the management of NPC has been largely derived from experience with conventional RT. In the intensity-modulated radiation therapy (IMRT) era, the optimal strategy for combining the use of chemotherapy and RT has not been sufficiently addressed.
The addition of induction chemotherapy (IC) to the previously established regimen is an attractive multidisciplinary approach.7,8 Theoretically, changing concurrent chemotherapy to IC may improve treatment tolerance and help in the early eradication of potential micrometastases. Furthermore, early tumor shrinkage could help attain better coverage of the gross tumor and optimize the design of the RT plan.9 Nevertheless, the therapeutic value of IC followed by IMRT alone has not been fully evaluated.
In this study, we established a nomogram model to improve prediction accuracy compared with clinical risk factors for survival in stage II–IVB NPC. Then, applying this nomogram, patients were divided into different risk groups and the efficacy of concurrent chemoradiotherapy (CCRT) versus IC followed by RT was evaluated in patients from different risk groups. The data may provide an additional dimension for risk stratification and individualized therapy.
Patients and methods
Patients
From October 2007 to October 2013, 1824 consecutive previously untreated patients with biopsy-confirmed NPC were identified in our study institute. The eligibility criteria were as follows: (a) age ⩾18 years; (b) stage II–IVB disease according to the 7th edition of the International Union Against Cancer/American Joint Committee on Cancer staging system; (c) score of 0 or 1 with the Eastern Cooperative Oncology Group (ECOG) performance status grade; (d) treatment with IMRT; (e) administration of CCRT or IC plus RT; (f) complete data of pretreatment plasma EBV DNA level; and (g) adequate hematological, liver and renal function. Patients who were administered previous treatment for NPC, the presence of a distant metastasis, pregnancy, lactating women, or with a prior malignancy were excluded from the study. In total, 1814 eligible patients were included for analysis. This study was approved by the Clinical Research Committee of the study institute (approved number, GZR2014-069) and written informed consent was required when the patients were admitted to receive treatment as a general standard procedure for patients treated in our institute.
Pretreatment assessment
Before treatment, all patients underwent complete physical examination, fiberoptic nasopharyngoscopy, and laboratory work-up including complete blood count, biochemical profile, and plasma level of EBV DNA measured by real-time quantitative polymerase chain reaction (PCR).10,11 Magnetic resonance imaging (MRI) of the nasopharynx and neck, chest radiograph, abdominal sonography, electrocardiography and bone scan or 18 F-fluorodeoxyglucose positron emission tomography/computed tomography scans were carried out for accurate disease staging.
Treatment
All patients were treated with IMRT and a simultaneously integrated boost was mandatory in this study. The IMRT plan was designed according to previous studies, and treatment administration was done following the general principle of our institute (see supplemental materials). A total of 1331 (73.4%) patients received concurrent cisplatin (100 mg/m2) chemotherapy on days 1, 22 and 43 of RT; 483 (26.6%) patients received induction TPF (cisplatin (75 mg/m2, day 1) and docetaxel (75 mg/m2, day 1) with 5-fluorouracil (750 mg/m2, 96 h continuous intravenous infusion)) or PF (cisplatin (80 mg/m2, day 1) with 5-fluorouracil (800–1000 mg/m2, 96 h of continuous intravenous infusion)) chemotherapy,10,12 but without concurrent chemotherapy.
Outcome and follow-up
The primary endpoint of the study was overall survival (OS), which was defined as the time from the start of treatment until death from any cause or patient censoring at the last follow-up. Secondary endpoints included progression-free survival (PFS), calculated from the start of treatment to the date of first failure at any site or death from any cause or patient censoring at last follow-up; distant metastasis-free survival (DMFS), calculated from the start of treatment to the date of distant relapse or patient censoring at the date of last follow-up and toxicity. After treatment, patients were followed up at least every 3 months for the first 3 years and every 6 months thereafter or until death. Acute toxicities were classified according to the Common Toxicity Criteria for Adverse Events version 4.0 and late radiotherapy-related toxic effects were assessed and graded based on the Radiation Therapy Oncology Group/European Organization for Research and Treatment of cancer morbidity scoring schema.
Statistical analysis
Statistical analyses were performed using SPSS version 24.0 (SPSS, Chicago, IL, USA) and R version 3.5.0 (www.r-project.org). Categorical variables were compared with the χ2 test or Fisher’s exact test. The Kaplan–Meier method was used to estimate the time-to-event endpoints, and survival curves were compared using the log-rank test. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated with the Cox proportional hazards model. Multivariate analyses with Cox proportional hazards models were performed to evaluate the potential prognostic factors. All statistical testing was two-sided, and a p value less than 0.05 was considered significant. Forest plots were generated to present adjusted HRs and 95% CIs of the potential prognostic factors for OS, PFS and DMFS. In addition, nomograms were formulated based on the results of multivariable Cox regression analyses. The selection of the final prediction model was performed with a backward step-down selection process with the Akaike information criterion.13 The performance of nomograms was assessed by the concordance index (C-index) and evaluated by comparing the nomogram-predicted versus nomogram-observed Kaplan–Meier estimates of survival probability. A larger C-index indicated a greater predictive accuracy. The total points of each patient were calculated according to the established nomograms.
Results
The baseline demographic and clinical characteristics of 1814 patients are listed in Table 1. After a median follow-up of 77 months (range: 1–152 months), 288 patients died, 352 patients developed disease progression, and 253 patients exhibited distant metastasis.
Table 1.
Baseline characteristics.
| Total patients |
Low-risk group |
Intermediate-risk group |
High-risk group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCRT | IC+RT | p value | CCRT | IC+RT | p value | CCRT | IC+RT | p value | CCRT | ICT+CCRT | p value | |
| Characteristic | No. of patients (%) | No. of patients (%) | No. of patients (%) | No. of patients (%) | No. of patients (%) | No. of patients (%) | No. of patients (%) | No. of patients (%) | ||||
| Total | 1331 | 483 | 488 | 115 | 419 | 140 | 424 | 228 | ||||
| Age, years | 0.142 | 0.403 | 0.354 | 0.765 | ||||||||
| 18–29 | 73 (5.5%) | 34 (7.0%) | 49 (10.0%) | 18 (15.7%) | 17 (4.1%) | 9 (6.4%) | 7 (1.7%) | 7 (3.1%) | ||||
| 30–39 | 307 (23.1%) | 98 (20.3%) | 145 (29.7%) | 36 (31.3%) | 122 (29.1%) | 38 (27.1%) | 40 (9.4%) | 24 (10.5%) | ||||
| 40–49 | 496 (37.3%) | 163 (33.7%) | 205 (42.0%) | 43 (37.4%) | 141 (33.7%) | 44 (31.4%) | 150 (35.4%) | 76 (33.3%) | ||||
| 50–59 | 318 (23.9%) | 125 (25.9%) | 84 (17.2%) | 18 (15.7%) | 114 (27.2%) | 45 (32.1%) | 120 (28.3%) | 62 (27.2%) | ||||
| >60 | 137 (10.3%) | 63 (13.0%) | 5 (1.0%) | 0 (0.0%) | 25 (6.0%) | 4 (2.9%) | 107 (25.2%) | 59 (25.9%) | ||||
| Gender | 0.963 | 0.667 | 0.028 | 0.111 | ||||||||
| Female | 360 (27.0%) | 131 (27.2%) | 231 (47.3%) | 57 (49.6%) | 87 (20.8%) | 42 (30.0%) | 42 (9.9%) | 31 (14.1%) | ||||
| Male | 971 (73.0%) | 351 (72.8%) | 257 (52.7%) | 58 (50.4%) | 332 (79.2%) | 98 (70.0%) | 382 (90.1%) | 195 (85.9%) | ||||
| Histology, WHO type | 0.187 | 0.634 | 0.210 | 0.387 | ||||||||
| I | 2 (0.2%) | 3 (0.6%) | 1 (0.2%) | 0 (0.0%) | 0 (0.0%) | 1 (0.7%) | 1 (0.2%) | 2 (0.9%) | ||||
| II | 46 (3.5%) | 20 (4.1%) | 16 (3.3%) | 2 (1.7%) | 15 (3.6%) | 7 (5.0%) | 15 (3.5%) | 11 (4.8%) | ||||
| III | 1283 (96.4%) | 460 (95.2%) | 471 (96.5%) | 113 (98.3%) | 404 (96.4%) | 132 (94.3%) | 408 (96.2%) | 215 (94.3) | ||||
| T stage | <0.001 | 0.064 | 0.343 | 0.008 | ||||||||
| T1 | 86 (6.5%) | 19 (3.9%) | 51 (10.5%) | 13 (11.3%) | 23 (5.5%) | 4 (2.9%) | 12 (2.8%) | 2 (0.9%) | ||||
| T2 | 243 (18.3%) | 87 (18.0%) | 95 (19.5%) | 29 (25.2%) | 84 (20.0%) | 29 (20.7%) | 64 (15.1%) | 29 (12.8%) | ||||
| T3 | 793 (59.6%) | 238 (49.4%) | 323 (66.2%) | 63 (54.8%) | 270 (64.4%) | 87 (62.1%) | 200 (47.2%) | 88 (38.8%) | ||||
| T4 | 209 (15.7%) | 138 (28.6%) | 19 (3.9%) | 10 (8.7%) | 42 (10.0%) | 20 (14.3%) | 148 (34.9%) | 108 (47.6%) | ||||
| N stage | <0.001 | 0.243 | 0.107 | 0.022 | ||||||||
| N0 | 197 (14.8%) | 54 (11.2%) | 140 (28.7%) | 34 (29.6%) | 42 (10.0%) | 13 (9.3%) | 15 (3.5%) | 7 (3.1%) | ||||
| N1 | 582 (43.7%) | 160 (33.1%) | 277 (56.8%) | 57 (49.6%) | 181 (43.2%) | 51 (36.4%) | 124 (29.2%) | 52 (22.8%) | ||||
| N2 | 443 (33.3%) | 187 (38.7%) | 64 (13.1%) | 23 (20.0%) | 170 (40.6%) | 59 (42.1%) | 209 (49.3%) | 105 (46.1%) | ||||
| N3 | 109 (8.2%) | 82 (17.0%) | 7 (1.4%) | 1 (0.9%) | 26 (6.2%) | 17 (12.1%) | 76 (17.9%) | 64 (28.1% | ||||
| Overall stage | <0.001 | 0.076 | 0.056 | <0.001 | ||||||||
| II | 154 (11.6%) | 38 (7.9%) | 108 (22.1%) | 29 (25.2%) | 36 (8.6%) | 9 (6.4%) | 10 (2.4%) | 0 | ||||
| III | 867 (65.1%) | 238 (49.3%) | 353 (72.3%) | 74 (64.3%) | 315 (75.2%) | 94 (67.1%) | 199 (46.9%) | 70 (30.7%) | ||||
| IVa | 201 (15.1%) | 125 (25.9%) | 20 (4.1%) | 11 (9.6%) | 42 (10.0%) | 20 (14.3%) | 139 (32.8%) | 94 (41.2%) | ||||
| IVb | 109 (8.2%) | 82 (17.0%) | 7 (1.4%) | 1 (0.9%) | 26 (6.2%) | 17 (12.1%) | 76 (17.9%) | 64 (28.1%) | ||||
| Smoking | 0.514 | 0.317 | 0.073 | 0.178 | ||||||||
| No | 832 (62.5%) | 310 (64.2%) | 378 (77.5%) | 94 (81.7%) | 258 (61.6%) | 98 (70.0%) | 192 (46.2%) | 118 (51.8%) | ||||
| Yes | 499 (37.5%) | 173 (35.8%) | 110 (22.5%) | 21 (18.3%) | 161 (38.4%) | 42 (30.0%) | 228 (53.8%) | 110 (48.2%) | ||||
| 0.350 | 0.784 | 0.068 | ||||||||||
| No | 1178 (88.5%) | 435 (90.1%) | 433 (88.7%) | 101 (87.8%) | 363 (86.6%) | 130 (92.9%) | 382 (90.1%) | 204 (89.5%) | ||||
| Yes | 153 (11.5%) | 48 (9.9%) | 55 (11.3%) | 14 (12.2%) | 56 (13.4%) | 10 (7.1%) | 42 (9.9%) | 24 (10.5%) | ||||
| EBV DNA level (copies/ml) | <0.001 | 0.233 | 0.395 | 0.844 | ||||||||
| <1000 | 623 (46.8%) | 172 (35.6%) | 392 (80.3%) | 92 (80.0%) | 175 (41.8%) | 55 (39.3%) | 56 (13.2%) | 25 (11.0%) | ||||
| 1000–9999 | 353 (26.5%) | 147 (30.4%) | 73 (15.0%) | 22 (19.1%) | 152 (36.3%) | 52 (37.1%) | 128 (30.2%) | 73 (32.0%) | ||||
| 10000–99999 | 236 (17.7%) | 111 (23.0%) | 20 (4.1%) | 1 (0.9%) | 72 (17.2%) | 30 (21.4%) | 144 (34.0%) | 80 (35.1%) | ||||
| 100000–999999 | 101 (7.6%) | 41 (8.5%) | 3 (0.6%) | 0 (0.0%) | 20 (4.8%) | 3 (2.1%) | 78 (18.4%) | 38 (16.7%) | ||||
| ⩾1000000 | 18 (1.4%) | 12 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 18 (4.2%) | 12 (5.3%) | ||||
CCRT, concurrent chemoradiotherapy; IC, induction chemotherapy; RT, radiotherapy.
Nomogram development
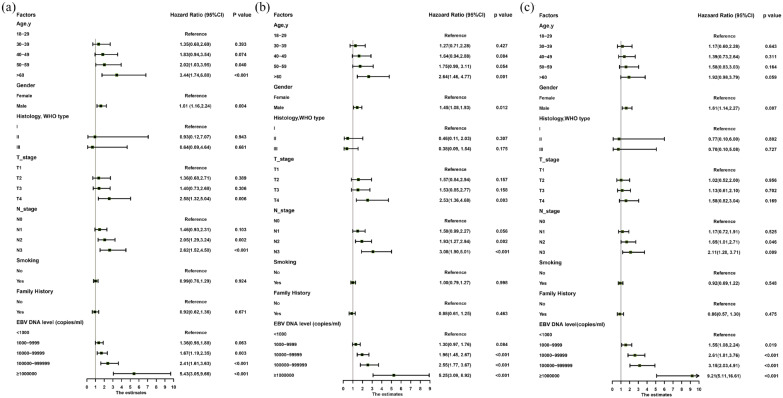
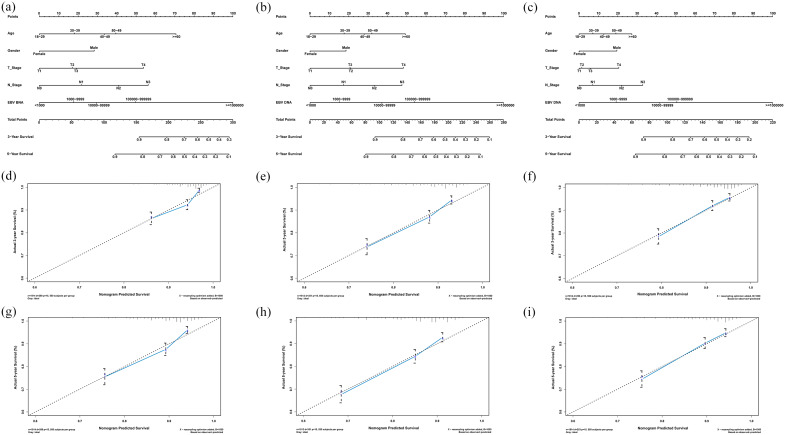
Multivariable analyses demonstrated that age, gender, T stage, N stage and plasma EBV DNA levels were independent prognostic factors for all endpoints (Figure 1 and Supplemental Appendix Table 2). Hence, we built nomograms to predict the 3 and 5-year OS, PFS and DMFS using the aforementioned variables. The prognostic nomograms provided a good accuracy for predicting OS, PFS and DMFS with corresponding C-index values of 0.71 (95% CI 0.67–0.76), 0.70 (95% CI 0.66–0.75) and 0.71 (95% CI 0.66–0.75), respectively. The calibration plots for the probabilities of survival showed a good agreement between prediction by nomogram and actual observation (Figure 2).
Figure 1.
Forest plots of the multivariate association of clinicopathological characteristics with overall survival (A), progression-free survival (B) and distant metastasis-free survival (C).
Figure 2.
Nomograms and calibration curves for predicting the 3 and 5-year overall survival (A, D, G), progression-free survival (B, E, H) and distant metastasis-free survival (C, F, I).
Risk stratification
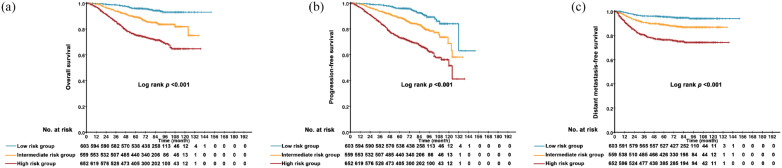
Eligible patients in our study were divided into three different risk groups according to tertiles (105 and 137) of total scores calculated by the nomogram for OS—low-risk group (total scores ⩽105 points), intermediate-risk group (105 < total score ⩽137 points) and high-risk group (total score >137 points). The characteristics of patients treated with different methods in different risk groups are demonstrated in Table 1. Survival curves were significantly segregated among patients in different risk groups for 5-year OS (p < 0.001), PFS (p < 0.001) and DMFS (p < 0.001) (Figure 3 and Supplemental Appendix Table 1).
Figure 3.
Results of comparison among patients in different risk groups with regard to the overall survival (A), progression-free survival (B) and distant metastasis-free survival (C).
Relationship between treatment methods and survival outcome in each risk group
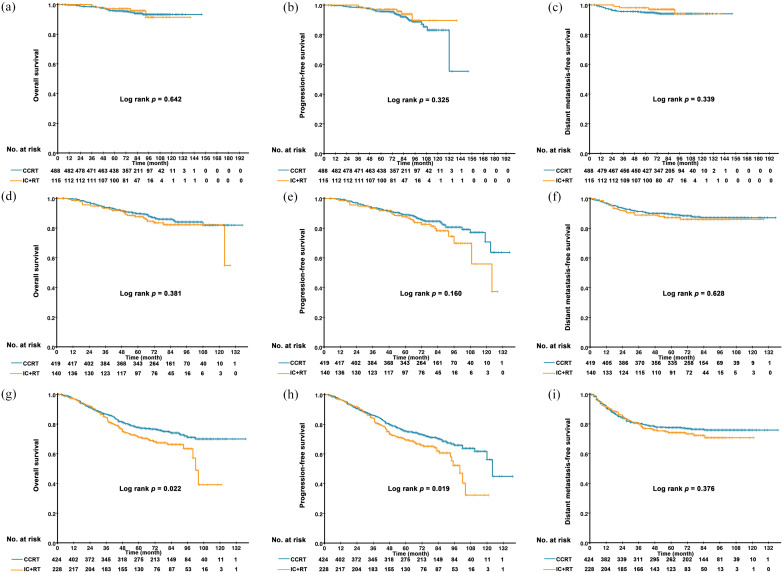
In the low-risk group, no statistically significant survival differences were observed between patients treated with IC plus RT and CCRT (5-year OS, 97.3% versus 95.6%, p = 0.642; 5-year PFS, 95.9% versus 95.6%, p = 0.325; and 5-year DMFS, 97.2% versus 94.8%, p = 0.339) (Figure 4). Similarly, patients treated with IC plus RT in the intermediate-risk group had no significant better survival than those in the CCRT group (5-year OS, 87.6% versus 89.7%, p = 0.381; 5-year PFS, 87.6% versus 89.0%, p = 0.160; and 5-year DMFS, 87.2% versus 89.3%, p = 0.628) (Figure 4). However, in the high-risk group, IC plus RT had an unfavorable 5-year OS (71.0% versus 77.2%, p = 0.022) and PFS (69.4.0% versus 75.4%, p = 0.019) compared with CCRT, except for the 5-year DMFS (74.2% versus 77.6%, p = 0.376) (Figure 4).
Figure 4.
Kaplan–Meier curves of overall survival (A, B, C), progression free-survival (D, E, F) and distant metastasis-free survival (G, H, I) in patients treated with CCRT and IC plus RT in different risk groups.
Toxicities
During and after treatment, 772 (58%) patients from the CCRT group and 254 (53.8%) from the IC plus RT group experienced grade ⩾3 toxicities (p = 0.040). In the low and intermediate-risk groups, we recorded a higher frequency of grade 3 or 4 vomiting (p = 0.037 and p < 0.001, respectively), nausea (p = 0.027 and p = 0.003, respectively) and mucositis (p = 0.005 p = 0.036, respectively) in the CCRT group than in the IC plus RT group, whereas the frequency of grade 3 or 4 leucopenia (p = 0.054 and p = 0.017, respectively) and neutropenia (p = 0.001 and p < 0.001, respectively) was higher in the IC plus RT group than in the CCRT group (Table 2). In the high-risk group, the proportion of patients with grade 3 or 4 vomiting (p = 0.001) and nausea (p = 0.017) was also significantly higher in the CCRT group than in the IC plus RT group, while the occurrence of grade 3 or 4 neutropenia (p < 0.001) was higher in the IC plus RT group (Table 2). After completion of treatment, patients in the CCRT group had a higher frequency of grade 3 or 4 late hearing loss than patients in the IC plus RT group (p = 0.03 in intermediate-risk group) (Table 2).
Table 2.
Acute and late toxicities in patients treated with CCRT and IC plus RT in different risk groups.
| Low-risk group |
Intermediate-risk group |
High-risk group |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCRT group | IC+RT group | p value | CCRT group | IC+RT group | p value | CCRT group | IC+RT group | p value | ||||||||||
| Acute toxicities | All grades | Grade 3–4 | All grades | Grade 3–4 | Grade ⩾1 | Grade ⩾3 | All grades | Grade 3–4 | All grades | Grade 3–4 | Grade ⩾1 | Grade ⩾3 | All grades | Grade 3–4 | All grades | Grade 3–4 | Grade ⩾1 | Grade ⩾3 |
| Leucopenia | 355 (72.7%) | 60 (12.3%) | 90 (78.3%) | 22 (19.1%) | 0.226 | 0.054 | 300 (71.6%) | 50 (11.9%) | 108 (77.1%) | 28 (20.0%) | 0.201 | 0.017 | 313 (73.8%) | 64 (15.1%) | 185 (81.1%) | 38 (16.7%) | 0.036 | 0.598 |
| Neutropenia | 228 (46.7%) | 20 (4.1%) | 61 (53.0%) | 14 (12.2%) | 0.222 | 0.001 | 194 (46.3%) | 29 (6.9%) | 89 (63.6%) | 28 (20.0%) | <0.001 | <0.001 | 214 (50.5%) | 35 (8.3%) | 142 (62.3%) | 45 (19.7%) | 0.004 | <0.001 |
| Anaemia | 204 (41.8%) | 9 (1.8%) | 51 (44.3%) | 0 (0.0%) | 0.619 | 0.219 | 159 (37.9%) | 15 (3.6%) | 59 (42.1%) | 3 (2.1%) | 0.378 | 0.577 | 196 (46.2%) | 17 (4.0%) | 110 (48.2%) | 8 (3.5%) | 0.622 | 0.751 |
| Thrombocytopenia | 96 (19.7%) | 11 (2.3%) | 25 (21.7%) | 4 (3.5%) | 0.619 | 0.670 | 89 (21.2%) | 15 (3.6%) | 31 (22.1%) | 2 (1.4%) | 0.822 | 0.318 | 104 (24.5%) | 26 (6.1%) | 52 (22.8%) | 8 (3.5%) | 0.623 | 0.151 |
| ALT increased | 65 (13.3%) | 6 (1.2%) | 26 (22.6%) | 2 (1.7%) | 0.012 | 1 | 50 (11.9%) | 1 (0.2%) | 25 (17.9%) | 2 (1.4%) | 0.075 | 0.317 | 54 (12.7%) | 4 (0.9%) | 35 (15.4%) | 2 (0.9%) | 0.354 | 1 |
| AST increased | 49 (10.0%) | 4 (0.8%) | 15 (13%) | 0 (0.0%) | 0.347 | 0.594 | 36 (8.6%) | 0 (0.0%) | 16 (11.4%) | 1 (0.7%) | 0.317 | 0.250 | 36 (8.5%) | 1 (0.2%) | 27 (11.8%) | 2 (0.9%) | 0.167 | 0.584 |
| Total bilirubin | 95 (19.5%) | 15 (3.1%) | 8 (7.0%) | 1 (0.9%) | 0.001 | 0.317 | 64 (15.3%) | 10 (2.4%) | 15 (10.7%) | 3 (2.1%) | 0.180 | 1 | 84 (19.8%) | 9 (2.1%) | 16 (7.0%) | 3 (1.3%) | <0.001 | 0.671 |
| Total protein | 24 (4.9%) | 0 (0.0%) | 3 (2.6%) | 0 (0.0%) | 0.408 | * | 18 (4.3%) | 0 (0.0%) | 3 (2.1%) | 0 (0.0%) | 0.366 | * | 34 (8.0%) | 0 (0.0%) | 18 (7.9%) | 0 (0.0%) | 0.956 | * |
| BUN increase | 9 (1.8%) | 1 (0.2%) | 0 (0.0%) | 0 (0.0%) | 0.219 | 1 | 6 (1.4%) | 0 (0.0%) | 1 (0.7%) | 0 (0.0%) | 0.824 | * | 14 (3.3%) | 0 (0.0%) | 3 (1.3%) | 0 (0.0%) | 0.208 | * |
| Creatinine increase | 70 (14.3%) | 1 (0.2%) | 9 (7.8%) | 0 (0.0%) | 0.062 | 1 | 67 (16.0%) | 0 (0.0%) | 14 (10.0%) | 0 (0.0%) | 0.081 | * | 83 (19.6%) | 1 (0.2%) | 17 (7.5%) | 0 (0.0%) | <0.001 | 1 |
| Vomiting | 390 (79.9%) | 80 (16.4%) | 74 (64.3%) | 10 (8.7%) | <0.001 | 0.037 | 347 (82.8%) | 80 (19.1%) | 101 (72.1%) | 6 (4.3%) | 0.006 | <0.001 | 328 (77.4%) | 65 (15.3%) | 144 (63.2%) | 15 (6.6%) | <0.001 | 0.001 |
| Nausea | 368 (75.4%) | 46 (9.4%) | 70 (60.9%) | 3 (2.6%) | 0.002 | 0.027 | 314 (74.9%) | 40 (9.5%) | 82 (58.6%) | 2 (1.4%) | <0.001 | 0.003 | 321 (75.7%) | 33 (7.8%) | 132 (57.9%) | 7 (3.1%) | <0.001 | 0.017 |
| Mucositis | 430 (88.1%) | 113 (23.2%) | 100 (87.0%) | 13 (11.3%) | 0.732 | 0.005 | 376 (89.7%) | 98 (23.4%) | 118 (84.3%) | 21 (15.0%) | 0.081 | 0.036 | 372 (87.7%) | 91 (21.5%) | 194 (85.1%) | 41 (18.0%) | 0.341 | 0.292 |
| Dermatitis | 356 (73.0%) | 17 (3.5%) | 86 (74.8%) | 1 (0.9%) | 0.690 | 0.239 | 314 (74.9%) | 9 (2.1%) | 95 (67.9%) | 4 (2.9%) | 0.102 | 0.874 | 317 (74.8%) | 15 (3.5%) | 153 (67.1%) | 3 (1.3%) | 0.038 | 0.161 |
| Hypokalaemia | 80 (16.4%) | 12 (2.5%) | 10 (8.7%) | 0 (0.0%) | 0.037 | 0.136 | 50 (11.9%) | 8 (1.9%) | 18 (12.9%) | 1 (0.7%) | 0.772 | 0.559 | 61 (14.4%) | 6 (1.4%) | 27 (11.8%) | 4 (1.8%) | 0.364 | 0.998 |
| Hyponatraemia | 79 (16.2%) | 1 (0.2%) | 10 (8.7%) | 0 (0.0%) | 0.042 | 1 | 87 (20.8%) | 2 (0.5%) | 19 (13.6%) | 0 (0.0%) | 0.060 | 0.624 | 107 (25.2%) | 2 (0.5%) | 47 (20.6%) | 0 (0.0%) | 0.185 | 0.544 |
| Hypocalcaemia | 6 (1.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.366 | * | 1 (0.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 | * | 3 (0.7%) | 0 (0.0%) | 1 (0.4%) | 1 (0.4%) | 1 | 0.350 |
| Late toxicities | All grades | Grade 3–4 | All grades | Grade 3–4 | Grade ⩾1 | Grade ⩾3 | All grades | Grade 3–4 | All grades | Grade 3–4 | Grade ⩾1 | Grade ⩾3 | All grades | Grade 3–4 | All grades | Grade 3–4 | Grade ⩾1 | Grade ⩾3 |
| Hearing loss | 109 (22.4%) | 28 (5.7%) | 11 (9.6%) | 6 (5.2%) | 0.002 | 0.828 | 88 (21.0%) | 23 (5.5%) | 18 (12.9%) | 1 (0.7%) | 0.033 | 0.030 | 90 (21.2%) | 20 (4.7%) | 48 (21.1%) | 10 (4.4%) | 0.959 | 0.847 |
| Trismus | 6 (1.2%) | 0 (0.0%) | 1 (0.9%) | 0 (0.0%) | 1 | * | 9 (2.1%) | 0 (0.0%) | 2 (1.4%) | 0 (0.0%) | 0.858 | * | 5 (1.2%) | 0 (0.0%) | 1 (0.4%) | 0 (0.0%) | 0.607 | * |
| Dysphagia | 7 (1.4) | 0 (0.0%) | 1 (0.9%) | 0 (0.0%) | 0.981 | * | 11 (2.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.074 | * | 8 (1.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.056 | * |
| Neck fibrosis | 127 (26.0%) | 9 (1.8%) | 26 (22.6%) | 1 (0.9%) | 0.449 | 0.741 | 121 (28.9%) | 10 (2.4%) | 35 (25.0%) | 2 (1.4%) | 0.376 | 0.734 | 124 (29.4%) | 13 (3.1%) | 52 (22.8%) | 2 (0.9%) | 0.077 | 0.133 |
| Xerostomia | 156 (32.0%) | 18 (3.7%) | 37 (32.2%) | 1 (0.9%) | 0.966 | 0.208 | 157 (37.5%) | 14 (3.3%) | 55 (39.3%) | 4 (2.9%) | 0.701 | 0.996 | 132 (31.1%) | 17 (4.0%) | 74 (32.5%) | 8 (3.5%) | 0.729 | 0.751 |
| Cranial nerve palsy | 31 (6.4%) | 0 (0.0%) | 4 (3.5%) | 0 (0.0%) | 0.335 | * | 18 (4.3%) | 0 (0.0%) | 3 (2.1%) | 0 (0.0%) | 0.366 | * | 20 (4.7%) | 0 (0.0%) | 11 (4.8%) | 0 (0.0%) | 0.951 | * |
| Radiation encephalopathy | 48 (9.8%) | 3 (0.6%) | 10 (9.6%) | 2 (1.7%) | 0.709 | 0.532 | 40 (9.5%) | 3 (0.7%) | 15 (10.7%) | 0 (0.0%) | 0.688 | 0.577 | 41 (9.7%) | 4 (0.9%) | 28 (12.3%) | 3 (1.3%) | 0.301 | 0.967 |
CCRT, concurrent chemoradiotherapy; IC, induction chemotherapy; RT, radiotherapy.
No grade 3 or 4 toxicities were recorded.
In addition, in the low and intermediate-risk group, the incidence of grade 1–2 adverse events of vomiting (p < 0.001 and p = 0.006, respectively), nausea (p = 0.002 and p < 0.001, respectively), hyponatremia (p = 0.042 and p = 0.060, respectively) and late hearing toxicity (p = 0.002 and p = 0.033, respectively) were statistically different in the IC plus RT group and the CCRT group. Besides, in the low-risk group, a higher occurrence of renal (p = 0.062) and liver dysfunction (p = 0.012) was observed in the CCRT group than the IC plus RT group (Table 2). Nevertheless, in the intermediate and high-risk groups, more patients in the IC plus RT group developed grade 1–2 hematological toxicities (leucopenia and neutropenia) than patients in the CCRT group (Table 2).
Discussion
To the best of our knowledge, the efficacy of IC followed by RT has not been fully investigated. In this study, we found that the survival benefit achieved by IC plus RT was comparable to CCRT in the low and intermediate-risk groups. Furthermore, patients in the IC plus RT group suffered from fewer treatment-related adverse events but survival rates in the IC plus RT group yielded to CCRT in the high-risk group.
Since the landmark Intergroup 0099 trial, studies concerning the interaction between the timing of chemotherapy and the effect on various endpoints have been ongoing. Chemoradiotherapy, including concurrent cisplatin chemoradiotherapy combined with/without either IC or adjuvant chemotherapy, has been widely applied to treat locoregionally advanced NPC. Recently, an increasing number of studies have demonstrated that adding IC to CCRT favorably improves the survival rate of patients with NPC. A large multicenter, randomized controlled phase III trial conducted by Zhang et al. has reported that the addition of IC to CCRT significantly improves the 3-year recurrence-free survival (85.3% versus 76.5%) and OS (94.6% versus 90.3%).14 Another trial by Sun et al. has showed that the addition of TPF IC to concurrent chemoradiotherapy significantly improves failure-free survival in patients with locoregionally advanced NPC.15 In daily clinical work, because of the increased treatment-related toxicities accompanying concurrent cisplatin chemotherapy, a certain proportion of patients refused to receive CCRT and underwent IC followed by RT alone. Moreover, the benefits of concurrent chemotherapy in the IMRT era have not been fully explored. A study evaluating the long-term survival outcomes and toxicity of 868 patients with NPC has demonstrated that concurrent chemotherapy does not improve the survival rates of patients with advanced locoregional disease. Compared with IMRT alone, IMRT plus concurrent chemotherapy increases the severity of acute toxicities.16 According to Cao et al., concurrent chemotherapy does not improve survival rates for patients with stage T4 disease.17 However, several meta-analyses and studies have shown that the survival benefit of chemotherapy primarily comes from the concurrent phase.18–21 Thus, the most effective way to combine chemotherapy with radiotherapy still needs further investigation.
Currently, there were only a few studies focusing on patients being administered IC plus RT alone. A retrospective study in 370 patients with locoregionally advanced NPC has reported that IC plus RT produces a superb outcome in terms of local control, regional control, metastasis-free survival, disease-free survival and OS rates.22 Wei et al. also found that IC plus RT achieved favorable survival outcomes and had a lower incidence of toxicity.23 One possible reason for these controversial results was that the therapeutic decisions in the aforementioned studies were simply based on the tumor-node-metastasis (TNM) stage. Given the biological heterogeneity of cancer, the present staging system remains inadequate for predicting NPC patient prognosis. Therefore, we developed three nomograms including age, gender, and the anatomical information on tumors and plasma EBV DNA levels to provide individualized estimates of potential OS, PFS and DMFS for patients with locoregionally advanced NPC. We then divided patients into three different risk groups categorized by the nomograms; this provided excellent discrimination in OS. Moreover, we explored the efficacy of IC plus RT and CCRT in different risk patients. We found that patients in the low and intermediate-risk groups achieved comparable OS, PFS and DMFS from IC plus RT when compared with CCRT. Furthermore, grade 3 or 4 toxicities were significantly less frequent in the IC plus RT group, except for hematological toxicities (leucopenia and neutropenia). We showed that patients treated with IC plus RT had improved treatment tolerability compared to patients treated with CCRT. Patient intolerance to these adverse effects limits the usefulness of CCRT. However, in the high-risk group, patients in the IC plus RT group had a worse OS and PFS. Hence, based on the results of our study, changing concurrent chemotherapy to IC is recommended for patients with a low or intermediate risk of treatment failure, but not for those in high-risk groups.
Our study has several limitations. First, this is a retrospective study in a single center; therefore, these results must be validated by other datasets and prospective studies. Second, the sample size of patients treated with IC plus RT in each risk group was relatively small. A larger sample size of patients is needed to evaluate the long-term outcomes of these patients. Third, the lack of quality of life data for the different treatment methods makes these results underpowered. In the future, a well-designed, multicenter, prospective, randomized study is needed to validate our results.
Conclusion
In conclusion, our study demonstrated that, in the low and intermediate-risk groups, IC plus RT is an alternative treatment strategy to concurrent cisplatin chemoradiotherapy in patients with locoregionally advanced NPC. IC plus RT had no benefits in patients classified as high-risk patients. Thus, IC followed by RT alone was not recommended for patients with a high risk of treatment failure. The results of this study could widen the choice of the timing of chemotherapy offered to patients with NPC. Further investigation is necessary to confirm our findings.
Supplemental Material
Supplemental material, Appendix_materials for Induction chemotherapy followed by radiotherapy versus concurrent chemoradiotherapy in the treatment of different risk locoregionally advanced nasopharyngeal carcinoma by Li-Ting Liu, Yu-Jing Liang, Shan-Shan Guo, Hao-Yuan Mo, Ling Guo, Yue-Feng Wen, Hao-Jun Xie, Qing-Nan Tang, Xue-Song Sun, Sai-Lan Liu, Xiao-Yun Li, Jin-Hao Yang, Zhen-Chong Yang, Lin-Quan Tang, Qiu-Yan Chen and Hai-Qiang Mai in Therapeutic Advances in Medical Oncology
Supplemental material, Appendix_table_1 for Induction chemotherapy followed by radiotherapy versus concurrent chemoradiotherapy in the treatment of different risk locoregionally advanced nasopharyngeal carcinoma by Li-Ting Liu, Yu-Jing Liang, Shan-Shan Guo, Hao-Yuan Mo, Ling Guo, Yue-Feng Wen, Hao-Jun Xie, Qing-Nan Tang, Xue-Song Sun, Sai-Lan Liu, Xiao-Yun Li, Jin-Hao Yang, Zhen-Chong Yang, Lin-Quan Tang, Qiu-Yan Chen and Hai-Qiang Mai in Therapeutic Advances in Medical Oncology
Supplemental material, Appendix_table_2 for Induction chemotherapy followed by radiotherapy versus concurrent chemoradiotherapy in the treatment of different risk locoregionally advanced nasopharyngeal carcinoma by Li-Ting Liu, Yu-Jing Liang, Shan-Shan Guo, Hao-Yuan Mo, Ling Guo, Yue-Feng Wen, Hao-Jun Xie, Qing-Nan Tang, Xue-Song Sun, Sai-Lan Liu, Xiao-Yun Li, Jin-Hao Yang, Zhen-Chong Yang, Lin-Quan Tang, Qiu-Yan Chen and Hai-Qiang Mai in Therapeutic Advances in Medical Oncology
Footnotes
Author contributions: Study concepts: Hai-Qiang Mai, Qiu-Yan Chen and Lin-Quan Tang
Study design: Hai-Qiang Mai, Li-Ting Liu, Qiu-Yan Chen and Lin-Quan Tang
Data acquisition: Li-Ting Liu, Yu-Jing Liang and Shan-Shan Guo
Quality control of data and algorithms: Hai-Qiang Mai, Li-Ting Liu, Yu-Jing Liang and Shan-Shan Guo
Data analysis and interpretation: Li-Ting Liu
Statistical analysis: Li-Ting Liu and Yu-Jing Liang
Manuscript preparation: Li-Ting Liu, Yu-Jing Liang, Shan-Shan Guo and Hao-Yuan Mo
Manuscript editing: Li-Ting Liu, Yu-Jing Liang, Shan-Shan Guo, Hao-Yuan Mo and Ling Guo
Manuscript review: Li-Ting Liu, Yu-Jing Liang, Shan-Shan Guo, Hao-Yuan Mo, Ling Guo, Yue-Feng Wen, Hao-Jun Xie, Qing-Nan Tang, Xue-Song Sun, Sai-Lan Liu, Xiao-Yun Li, Jin-Hao Yang, Zhen-Chong Yang, Hai-Qiang Mai, Qiu-Yan Chen and Lin-Quan Tang
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from: the National Key R&D Program of China (2017YFC1309003, 2017YFC0908500), the National Natural Science Foundation of China (no. 81425018, no. 81672868, no. 81602371), the Sci-Tech Project Foundation of Guangzhou City (201707020039), the Sun Yat-sen University Clinical Research 5010 Program, the Special Support Plan of Guangdong Province (no. 2014TX01R145), the Natural Science Foundation of Guangdong Province (no. 2017A030312003, no. 2018A0303131004), the Natural Science Foundation of Guangdong Province for Distinguished Young Scholar (no. 2018B030306001), the Pearl River S&T Nova Program of Guangzhou (no. 201806010135), the Sci-Tech Project Foundation of Guangdong Province (no. 2014A020212103), the Health & Medical Collaborative Innovation Project of Guangzhou City (no. 201400000001), the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (no. 2014BAI09B10), the PhD Start-up Fund of Natural Science Foundation of Guangdong Province China (no. 2016A030310221), the cultivation foundation for the junior teachers in Sun Yat-sen University (16ykpy28) and the Fundamental Research Funds for the Central Universities.
Conflict of interest: The authors declare that there is no conflict of interest.
ORCID iD: Hai-Qiang Mai  https://orcid.org/0000-0003-3371-634X
https://orcid.org/0000-0003-3371-634X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Li-Ting Liu, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, Guangzhou, China.
Yu-Jing Liang, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, Guangzhou, China.
Shan-Shan Guo, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, Guangzhou, China.
Hao-Yuan Mo, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, Guangzhou, China.
Ling Guo, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, Guangzhou, China.
Yue-Feng Wen, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, Guangzhou, China.
Hao-Jun Xie, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, Guangzhou, China.
Qing-Nan Tang, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, Guangzhou, China.
Xue-Song Sun, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, Guangzhou, China.
Sai-Lan Liu, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, Guangzhou, China.
Xiao-Yun Li, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, Guangzhou, China.
Jin-Hao Yang, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, Guangzhou, China.
Zhen-Chong Yang, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, Guangzhou, China.
Lin-Quan Tang, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, Guangzhou, China.
Qiu-Yan Chen, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, Guangzhou, China.
Hai-Qiang Mai, Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou 510060, P. R. China; Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou 510060, China.
References
- 1. Pathmanathan R, Prasad U, Sadler R, et al. Clonal proliferations of cells infected with Epstein–Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N Engl J Med 1995; 333: 693–698. [DOI] [PubMed] [Google Scholar]
- 2. Chen L, Hu CS, Chen XZ, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol 2012; 13: 163–171. [DOI] [PubMed] [Google Scholar]
- 3. Chen QY, Wen YF, Guo L, et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J Natl Cancer Inst 2011; 103: 1761–1770. [DOI] [PubMed] [Google Scholar]
- 4. Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol 2015; 16: 645–655. [DOI] [PubMed] [Google Scholar]
- 5. Ribassin-Majed L, Marguet S, Lee AWM, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? An individual patient data network meta-analysis. J Clin Oncol 2017; 35: 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kwong DL, Sham JS, Chua DT, et al. The effect of interruptions and prolonged treatment time in radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 1997; 39: 703–710. [DOI] [PubMed] [Google Scholar]
- 7. Hui EP, Ma BB, Leung SF, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol 2009; 27: 242–249. [DOI] [PubMed] [Google Scholar]
- 8. OuYang PY, Xie C, Mao YP, et al. Significant efficacies of neoadjuvant and adjuvant chemotherapy for nasopharyngeal carcinoma by meta-analysis of published literature-based randomized, controlled trials. Ann Oncol 2013; 24: 2136–2146. [DOI] [PubMed] [Google Scholar]
- 9. Lee AW, Lau KY, Hung WM, et al. Potential improvement of tumor control probability by induction chemotherapy for advanced nasopharyngeal carcinoma. Radiother Oncol 2008; 87: 204–210. [DOI] [PubMed] [Google Scholar]
- 10. Bossi P, Orlandi E, Bergamini C, et al. Docetaxel, cisplatin and 5-fluorouracil-based induction chemotherapy followed by intensity-modulated radiotherapy concurrent with cisplatin in locally advanced EBV-related nasopharyngeal cancer. Ann Oncol 2011; 22: 2495–2500. [DOI] [PubMed] [Google Scholar]
- 11. Shao JY, Li YH, Gao HY, et al. Comparison of plasma Epstein–Barr virus (EBV) DNA levels and serum EBV immunoglobulin A/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer 2004; 100: 1162–1170. [DOI] [PubMed] [Google Scholar]
- 12. Lee AW, Ngan RK, Tung SY, et al. Preliminary results of trial NPC-0501 evaluating the therapeutic gain by changing from concurrent-adjuvant to induction-concurrent chemoradiotherapy, changing from fluorouracil to capecitabine, and changing from conventional to accelerated radiotherapy fractionation in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer 2015; 121: 1328–1338. [DOI] [PubMed] [Google Scholar]
- 13. Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996; 15: 361–387. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, Chen L, Hu GQ, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med 2019; 381: 1124–1135. [DOI] [PubMed] [Google Scholar]
- 15. Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 2016; 17: 1509–1520. [DOI] [PubMed] [Google Scholar]
- 16. Sun X, Su S, Chen C, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol 2014; 110: 398–403. [DOI] [PubMed] [Google Scholar]
- 17. Cao CN, Luo JW, Gao L, et al. Update report of T4 classification nasopharyngeal carcinoma after intensity-modulated radiotherapy: an analysis of survival and treatment toxicities. Oral Oncol 2015; 51: 190–194. [DOI] [PubMed] [Google Scholar]
- 18. Baujat B, Audry H, Bourhis J, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys 2006; 64: 47–56. [DOI] [PubMed] [Google Scholar]
- 19. Xie R, Xia B, Zhang X, et al. T4/N2 classification nasopharyngeal carcinoma benefit from concurrent chemotherapy in the era of intensity-modulated radiotherapy. Oncotarget 2016; 7: 81918–81925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Langendijk JA, Leemans CR, Buter J, et al. The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: a meta-analysis of the published literature. J Clin Oncol 2004; 22: 4604–4612. [DOI] [PubMed] [Google Scholar]
- 21. Lee AW, Lau WH, Tung SY, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 trial by the Hong Kong nasopharyngeal cancer study group. J Clin Oncol 2005; 23: 6966–6975. [DOI] [PubMed] [Google Scholar]
- 22. Lin S, Lu JJ, Han L, et al. Sequential chemotherapy and intensity-modulated radiation therapy in the management of locoregionally advanced nasopharyngeal carcinoma: experience of 370 consecutive cases. BMC Cancer 2010; 10: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wei Z, Zhang Z, Luo J, et al. Induction chemotherapy plus IMRT alone versus induction chemotherapy plus IMRT-based concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: a retrospective cohort study. J Cancer Res Clin Oncol 2019; 145: 1857–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendix_materials for Induction chemotherapy followed by radiotherapy versus concurrent chemoradiotherapy in the treatment of different risk locoregionally advanced nasopharyngeal carcinoma by Li-Ting Liu, Yu-Jing Liang, Shan-Shan Guo, Hao-Yuan Mo, Ling Guo, Yue-Feng Wen, Hao-Jun Xie, Qing-Nan Tang, Xue-Song Sun, Sai-Lan Liu, Xiao-Yun Li, Jin-Hao Yang, Zhen-Chong Yang, Lin-Quan Tang, Qiu-Yan Chen and Hai-Qiang Mai in Therapeutic Advances in Medical Oncology
Supplemental material, Appendix_table_1 for Induction chemotherapy followed by radiotherapy versus concurrent chemoradiotherapy in the treatment of different risk locoregionally advanced nasopharyngeal carcinoma by Li-Ting Liu, Yu-Jing Liang, Shan-Shan Guo, Hao-Yuan Mo, Ling Guo, Yue-Feng Wen, Hao-Jun Xie, Qing-Nan Tang, Xue-Song Sun, Sai-Lan Liu, Xiao-Yun Li, Jin-Hao Yang, Zhen-Chong Yang, Lin-Quan Tang, Qiu-Yan Chen and Hai-Qiang Mai in Therapeutic Advances in Medical Oncology
Supplemental material, Appendix_table_2 for Induction chemotherapy followed by radiotherapy versus concurrent chemoradiotherapy in the treatment of different risk locoregionally advanced nasopharyngeal carcinoma by Li-Ting Liu, Yu-Jing Liang, Shan-Shan Guo, Hao-Yuan Mo, Ling Guo, Yue-Feng Wen, Hao-Jun Xie, Qing-Nan Tang, Xue-Song Sun, Sai-Lan Liu, Xiao-Yun Li, Jin-Hao Yang, Zhen-Chong Yang, Lin-Quan Tang, Qiu-Yan Chen and Hai-Qiang Mai in Therapeutic Advances in Medical Oncology