Abstract
Background:
YYB101, a humanized monoclonal antibody against hepatocyte growth factor (HGF), has shown safety and efficacy in vitro and in vivo. This is a first-in-human trial of this antibody.
Materials and Methods:
YYB101 was administered intravenously to refractory cancer patients once every 4 weeks for 1 month, and then once every 2 weeks until disease progression or intolerable toxicity, at doses of 0.3, 1, 3, 5, 10, 20, 30 mg/kg, according to a 3+3 dose escalation design. Maximum tolerated dose, safety, pharmacokinetics, and pharmacodynamics were studied. HGF, MET, PD-L1, and ERK expression was evaluated for 9 of 17 patients of the expansion cohort (20 mg/kg).
Results:
In 39 patients enrolled, no dose-limiting toxicity was observed at 0.3 mg/kg, and the most commonly detected toxicity was generalized edema (n = 7, 18.9%) followed by pruritis and nausea (n = 5, 13.5%, each), fatigue, anemia, and decreased appetite (n = 4, 10.8%, each). No patient discontinued treatment because of adverse events. YYB101 showed dose-proportional pharmacokinetics up to 30 mg/kg. Partial response in 1 (2.5%) and stable disease in 17 (43.5%) were observed. HGF, MET, PD-L1, and ERK proteins were not significant predictors for treatment response. However, serum HGF level was significantly lowered in responders upon drug administration. RNA sequencing revealed a mesenchymal signature in two long-term responders.
Conclusion:
YYB101 showed favorable safety and efficacy in patients with refractory solid tumors. Based on this phase I trial, a phase II study on the YYB101 + irinotecan combination in refractory metastatic colorectal cancer patients is planned.
Conclusion:
ClinicalTrials.gov Identifier: NCT02499224
Keywords: hepatocyte growth factor, pharmacodynamics, pharmacokinetics, phase I, refractory cancer
Implications for practice
YYB101, a humanized monoclonal antibody against hepatocyte growth factor (HGF), showed favorable safety and efficacy in patients with refractory solid tumors. Our previous preclinical study also showed that a combination of YYB101 plus irinotecan exhibited antitumor activity in a colon cancer xenograft mouse model with irinotecan resistance. Based on this phase I trial, a phase II study on the YYB101 + irinotecan combination as a salvage treatment in refractory metastatic colorectal cancer patients is planned.
Introduction
Hepatocyte growth factor (HGF) and its receptor, the MET transmembrane tyrosine kinase (MET), are overexpressed in various human tumors, including brain, liver, prostate, colon, breast, and skin tumors.1–4 Excessive expression of HGF and MET is known to be highly correlated with patient prognosis and metastasis.5,6 MET is the only identified receptor for HGF. Upon activation, MET induces angiogenesis and cell proliferation, increases the expression of cell surface protease, changes cell shape, increases cell motility, and increases cell resistance to apoptosis.7,8 HGF is also known as a scatter factor (SF) and a multifunctional heterodimer polypeptide generated by mesenchymal cells; it exhibits diverse regulatory functions related to cell proliferation and growth, making it one of the main targets of anticancer agents.9,10
YYB101 is a humanized neutralizing monoclonal antibody with an IgG4 structure against HGF. YYB101 was shown to prevent HGF-induced scattering in MDCK-2 cells, thus blocking ERK phosphorylation, which is a downstream molecule of MET involved in cancer cell proliferation.11 YYB101 specifically binds to human HGF to inhibit its activity. The HGF/MET axis is an important axis involved in metastasis of tumor cells from the primary site to distant organs by promoting epithelial-mesenchymal transition (EMT).4,12 Interestingly, YYB101-sensitive primary glioma stem cells (GSCs) show MET overexpression or amplification and mesenchymal phenotype, compared with YYB101-resistant GSCs in preclinical models.13 Thus far, however, several HGF-targeting agents have failed to show survival benefit in solid tumor patients. Anti-HGF antibodies, including rilotumumab14 and ficlatuzumab,15 have failed to confer survival benefit in gastroesophageal or lung cancer in clinical trials.
Herein, we report a first-in-human phase I study of YYB101 in patients with refractory solid tumors. The primary objective was to determine the maximum tolerated dose (MTD) for a phase II study. The secondary objectives were to define safety, pharmacokinetics (PK), and conduct exploratory biomarker study for YYB101.
Materials and methods
Patients
Patients enrolled in this study had measurable, histologically confirmed metastatic solid cancer. The trial was conducted in accordance with the Declaration of Helsinki and the Guidelines for Good Clinical Practice [ClinicalTrial.gov identifier: NCT02499224]. The trial protocol was approved (#2015-02-027) by the Institutional Review Board of Samsung Medical Center (Seoul, Korea), and all patients provided written informed consent before enrollment. Written informed consent included the disclosure of information, competency of patients to make a decision, and voluntary nature of decision for the purpose, benefit, and potential risk of this study
The following were the inclusion criteria: patients aged at least 19 years; patients with an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2; patients who failed to respond to standard systemic therapies; and patients with adequate hematologic, hepatic, and renal functions. The following were the exclusion criteria: patients who received any palliative chemotherapy or investigational therapies within 28 days of the first administration of YYB101 and patients with symptomatic brain metastases requiring local therapy.
Study procedure and study design
YYB101 was administered intravenously once every 4 weeks for 1 month, followed by once every 2 weeks until disease progression or intolerable toxicity. YYB101 was administered according to a modified Fibonacci design, following the conventional 3+3 design. In phase I of the trial, subjects were assigned sequentially to each of the following dose cohorts (3 or 6 subjects per cohort): level 1–0.3 mg/kg Q.D.; level 2–1 mg/kg Q.D.; level 3–3 mg/kg Q.D.; level 4–5 mg/kg Q.D; level 5–10 mg/kg Q.D; level 6–20 mg/kg Q.D; and level 7–30 mg/kg Q.D.
Dose-limiting toxicity (DLT; defined in the subsequent section) was evaluated during the first cycle of treatment for each patient. Dose escalation continued when at least two subjects developed a DLT in each 3–6 subject cohort (when >33% of total subjects experienced a DLT at the concerned dose level).
Up to 15 subjects were planned to be enrolled in phase Ib of the trial, with the final sample size being dependent on the number of subjects who experienced DLTs as well as the safety data at each dose level based on DLTs and other safety data.
Definition of DLT
Hematologic toxicity was defined as grade 4 neutropenia lasting >8 days, febrile neutropenia of any grade or duration as defined by the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) 4.0, platelets <25 × 103/μl or platelets <50 × 103/μl with bleeding requiring medical intervention. Non-hematologic toxicity was defined as any grade ⩾3 non-hematologic adverse event, grade ⩾3 headache lasting ⩾7 days despite optimal supportive care, grade ⩾3 fatigue, grade ⩾3 edema lasting ⩾7 days despite prophylactic and/or symptomatic treatment, and grade ⩾3 abnormal liver function.
Determination of the initial dose and schedule
According to the US FDA guidelines, when setting the initial dose in humans on the basis of toxicity, a drug having a molecular weight >100 kDa, such as YYB101, can be administered with the no-observed adverse effect level (NOAEL) as the human equivalent dose (HED) without adjusting for the body surface area. However, when there is a difference in binding affinity to the target between humans and animal models, unexpected toxicity may occur when the initial dose is decided based on the NOAEL; this is the case with YYB101. Therefore, it is considered reasonable to calculate HED using the ratio of binding affinity between humans and animals and to set the initial dose by using a safety factor of 1/10.16 The Kd value of YYB101 was about 3.6 pM in humans and approximately 231 pM in monkeys, a value that is approximately 64 times higher.11 Therefore, considering an NOAEL 200 mg/kg (confirmed in a monkey toxicity study) and this difference in binding affinity, the initial dose in this clinical study was calculated to be 0.31 mg/kg and the lower dose of 0.3 mg/kg was set as the starting dose. Through in vitro and in vivo studies of YYB101, the minimum effective concentration expected to show anticancer activity upon human administration was estimated to be 100 μg/ml, based on our preclinical data. Thus, to set the treatment schedule for reaching the minimum effective concentration of YYB101, the half-life in the human body was estimated by species-invariant time method based on the pharmacokinetic data from the monkey study. Based on the simulation results, the dose that can reach the effective steady state concentration at the interval of 2 or 3 weeks was estimated to be ⩾5 mg/kg. Considering that the subject of the clinical trial was a patient with advanced solid cancer, the administration interval was set to 2 weeks to reach the minimum effective concentration within 6 weeks for faster drug efficacy.
Tumor and toxicity assessment
At the first visit, medical history, physical examination, blood tests, urinalysis, electrocardiography, echocardiogram, chest X-ray, and abdomen and pelvis computed tomography (CT) scan results of the patients were reviewed. Physical examinations, chest X-rays, and blood tests were repeated before beginning each cycle of chemotherapy. Tumor responses were evaluated every 2 months according to the RECIST 1.1 criteria. Toxicities were graded based on the NCI-CTCAE 4.03.
Exploratory analysis
Analysis of biomarkers to predict response to YYB101 was planned in parallel. The expression of MET, HGF, PD-1, and pERK in the tumor tissue was evaluated by immunohistochemistry (IHC) analysis according to previously published methods.5 The change in HGF level in the serum was also tested using ELISA (Human HGF Quantikine ELISA Kit; R&D Systems) following the manufacturer’s instructions. The serum was separated from collected blood samples, aliquoted, and stored at –80°C until analysis.
Gene expression profiling: nanostring
In the nanostring assay, we included 584 genes that were previously published to define 4 subtypes, including 15 housekeeping and 14 technical control genes. The nanostring assays were performed following the standard protocol “Setting up 12 nCounter Assays (MAN-C0003-03, 2008-2013).” Hybridization incubations were performed for between 17 and 18 h. Cartridges were either read immediately or stored in the dark (in aluminum foil) at 4°C until reading. All cartridges were read within 2 days of preparation on an AZ GEN2 Digital Analyzer station with high resolution selected. Data were processed using nCounter PanCancer pathways.17 Data were normalized by dividing the raw counts by the geometric mean of the manufacturer-defined housekeeping genes and transformed into a log10 scale.17,18
Immunohistochemistry
Immunohistochemistry (IHC) assay was performed on 3-μm sections of formalin-fixed, paraffin-embedded tissues. For staining, Benchmark XT (Ventana, Tucson, AZ, USA) with OptiView DAB IHC Detection kit (760-700) was used for CONFIRM anti-Total MET (SP44 rabbit monoclonal primary antibody) and Phospho-ERK1/2 (Thr202, Tyr204 monoclonal antibody; 1:500; eBioscience™). For PD-L1 IHC 22C3 pharmDx (SK006: DAKO) and HGFα (H-10: 1:50; Santacruz), DAKO Autostainer Link48 was used. Staining was interpreted as positive when overt brown staining was observed in low power field examinations and the stained areas were also calculated. For MET, only strong simultaneous membranous and cytoplasmic overexpression was defined as positive.5 For PD-L1, combined positive scores were selected as previously described.19
Gene expression cross-platform concordance filter
For each gene, we calculated the correlation between the gene expression level on the nanostring platform and on the microarray platform in the training set (n = 70). Following inspection of the distribution of correlations,17 we chose a cutoff of 0.4 correlation to select genes that were concordant between the two platforms.
Gene signature analysis
We calculated the mesenchymal signature on the nanostring platform using the average of the genes in our previously defined mesenchymal signature, down-selected to genes present on the nanostring platform, and with cross-platform concordance as defined in the previous section.
Results
Patient characteristics
Baseline demographics and characteristics of the 39 enrolled patients are provided in Table 1. All enrolled patients were heavily pretreated for metastatic cancer (number of lines of prior treatment: median, 4; range, 1–9). A total of 22 patients were enrolled for the dose escalation cohort and 17 for the expansion cohort. Patients with the following cancer types were included: colorectal cancer (n = 13), ovarian cancer (n = 10), melanoma (n = 4), sarcoma (n = 4), gastric cancer (n = 3), sebaceous carcinoma (n = 2), and others (n = 3). The most common metastatic sites were the lymph nodes (n = 20), followed by the liver (n = 18) and lung (n = 14) in the order of frequency.
Table 1.
Patients’ characteristics (n = 39).
| Variables | n (%) |
|---|---|
| Age | |
| Median | 57.0 |
| Range | 23.0–74.0 |
| Gender | |
| Male | 16 (41.03) |
| Female | 23 (58.97) |
| Tumor types | |
| Gastric cancer | 3 (7.69) |
| Colon cancer | 7 (17.95) |
| Rectal cancer | 6 (15.38) |
| Hepatocellular carcinoma | 1 (2.56) |
| Ovarian cancer | 10 (25.64) |
| Cervical cancer | 1 (2.56) |
| Lung cancer | 1 (2.56) |
| Melanoma | 4 (10.26) |
| Sebaceous carcinoma | 2 (5.13) |
| Sarcoma | 4 (10.26) |
| Prior lines of chemotherapy | |
| 1 regimen | 1 (2.56) |
| 2 regimens | 7 (17.95) |
| 3 regimens | 6 (15.38) |
| 4 regimens | 9 (23.08) |
| 5–9 regimens | 16 (41.03) |
| Common metastatic site | |
| Liver | 18 (46.15) |
| Lung | 14 (35.90) |
| Lymph nodes | 20 (51.28) |
Safety profile and PK analysis
The most commonly detected toxicity was generalized edema (n = 7, 18.9%), followed by pruritis and nausea (n = 5, 13.5%, each), fatigue, anemia, and decreased appetite (n = 4, 10.8%, each) (Table 2). Other toxicities included rash (n = 3, 8.1%), mucositis, azotemia, and dizziness (n = 1, 2.7%, each). Toxicities of grade 3 or higher were anemia (n = 3, 8.1%), generalized edema, thrombocytopenia, and azotemia (n = 1, 2.7%, each). No patient discontinued treatment because of adverse events. DLTs were evaluated during the first 28-day cycle (the first cycle) after a single intravenous dose of YYB101 on day 1. In the 0.3 mg/kg group, one of three patients experienced disease progression before evaluation of DLT; therefore, this patient was excluded from DLT analysis and an additional patient was enrolled in the same cohort (0.3 mg/kg group). No DLT occurred in the overall study cohort (0.3 mg/kg) and the dose escalation cohorts (1 mg/kg and 30 mg/kg). There was no significant accumulation of YYB101 at steady state in the 0.3, 1, 3, 5, 10, and 20 mg/kg dose groups. In addition, although the capacity proportionality evaluated by Cmax,57 and AUCτ,57 was not confirmed, a significant capacity proportionality was confirmed at Cmax, 1.
Table 2.
Toxicity profiles (n = 39).
| Cohort | Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|---|
| Cohort 1 | Oral mucositis | 0 | 1 | 0 | 0 | 0 |
| (n = 4, 0.3 mg/kg) | Fatigue | 0 | 1 | 0 | 0 | 0 |
| Decreased appetite | 0 | 1 | 0 | 0 | 0 | |
| Azotemia | 0 | 1 | 1 | 0 | 0 | |
| Pruritis | 1 | 0 | 0 | 0 | 0 | |
| Cohort 2 | Nausea | 1 | 0 | 0 | 0 | 0 |
| (n = 3, 1 mg/kg) | Decreased appetite | 1 | 0 | 0 | 0 | 0 |
| Pruritis | 1 | 0 | 0 | 0 | 0 | |
| Cohort 3 | Dizziness | 1 | 0 | 0 | 0 | 0 |
| (n = 3, 3 mg/kg) | ||||||
| Cohort 4 | Anemia | 0 | 0 | 1 | 0 | 0 |
| (n = 3, 5 mg/kg) | Nausea | 1 | 0 | 0 | 0 | 0 |
| Decreased appetite | 1 | 0 | 0 | 0 | 0 | |
| Cohort 5 | Nausea | 1 | 0 | 0 | 0 | 0 |
| (n = 3, 10 mg/kg) | Pruritis | 1 | 0 | 0 | 0 | 0 |
| Rash | 1 | 0 | 0 | 0 | 0 | |
| Cohort 6 | Fatigue | 1 | 0 | 0 | 0 | 0 |
| (n = 3, 20 mg/kg) | Decreased appetite | 1 | 0 | 0 | 0 | 0 |
| Rash | 1 | 0 | 0 | 0 | 0 | |
| Generalized Edema | 1 | 0 | 0 | 0 | 0 | |
| Cohort 7 | Nausea | 0 | 1 | 0 | 0 | 0 |
| (n = 3, 30 mg/kg) | Anemia | 0 | 0 | 1 | 0 | 0 |
| Generalized Edema | 0 | 1 | 0 | 0 | 0 | |
| Thrombocytopenia | 0 | 0 | 1 | 0 | 0 | |
| Expansion Cohort | Nausea | 1 | 0 | 0 | 0 | 0 |
| (n = 17, 20 mg/kg) | Fatigue | 1 | 1 | 0 | 0 | 0 |
| Pruritis | 2 | 0 | 0 | 0 | 0 | |
| Rash | 0 | 1 | 0 | 0 | 0 | |
| Anemia | 0 | 1 | 1 | 0 | 0 | |
| Generalized Edema | 1 | 3 | 1 | 0 | 0 |
In PK analysis for the dose escalation cohort (cohort 1–cohort 7), we confirmed that YYB101 level in the blood was above the predicted minimum effective concentration (100 μg/ml) on day 43 in cohort 5 (10 mg/kg) and cohort 6 (20 mg/kg). HGF level was also less than 200 pg/ml on day 43 in cohort 6 (20 mg/kg). Therefore, based on these data, the recommended dose of YYB101 for the expansion cohort was set as 20 mg/kg.
Efficacy and biomarker analysis
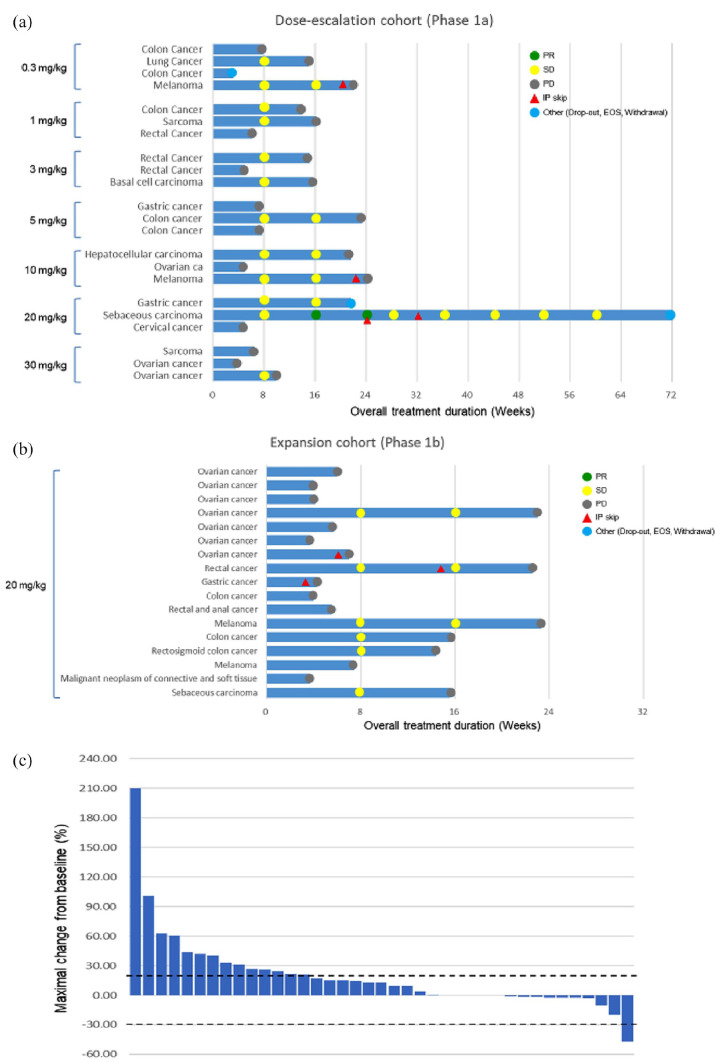
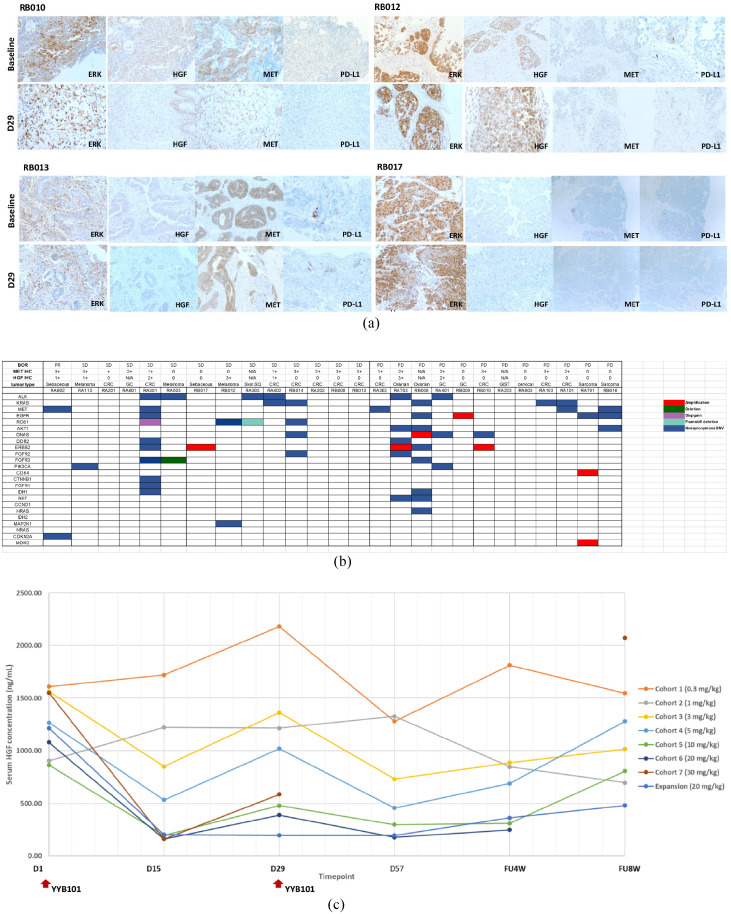
Partial response (PR) for >16.8 months was observed in one patient [(2.5%) of 2 sebaceous carcinoma patients] and stable disease (SD) was observed in 17 patients [43.5%, n = 17; 7 (of 13) colorectal carcinoma, 3 (of 4) melanoma, 1 (of 2) sebaceous carcinoma, 1 (of 3) gastric cancer, 1 (of 1) basal cell carcinoma, 2 (of 10) ovarian cancer, 1 (of 1) hepatoma, 1 (of 1) lung cancer] (Figure 1 and Tables 3 and 4). In Figure 1(c), a waterfall plot showing treatment response is provided. Of note, one sebaceous metastatic carcinoma patient, who failed to respond to prior cytotoxic chemotherapy, was enrolled in cohort 6 (20 mg/kg). The patient’s tumor was responsive to the treatment for 16.8 months. Among 17 patients in the expansion cohort, tumor biopsies for pre-planned biomarker analysis were available in nine patients (Table 5). In the tumor tissue of these nine patients including 5 paired biopsies with on-treatment biopsy on Day 29, HGF, MET, PD-L1, and p-ERK levels were evaluated (Tables 4 and 5). In all, a melanoma patient (RB012) with highly overexpressed HGF protein at baseline biopsy achieved stable disease for 18 weeks. The patient was refractory to multiple regimens, including prior anti-PD-L1 therapy (Table 5). Erk expression levels (both baseline and on-treatment biopsy) did not significantly correlate with treatment response to anti-HGF antibody (Figure 2a). Likewise, MET overexpression at baseline did not seem to predict response to anti-HGF antibody. YYB101 treatment did not alter PD-L1 overexpression at baseline and on-treatment (D29) biopsies (Figure 2a). Patients who had strong MET overexpression (3+) in their tumor did not show MET amplification in tumor genomic profiling (Figure 2b). As an exploratory analysis, we performed focused RNA expression assay to classify tumors according to EMT subtype (non-EMT versus EMT) using pan-cancer panel from NanoString. Owing to the small number of patients, no definitive conclusion can be drawn from the analysis. However, it was interesting to observe that RA113 (melanoma, maximal tumor change –20%), which achieved SD for 18 weeks, had EMT subtype and highly elevated HGF RNA level at tissue immediately before treatment (Table 6).
Figure 1.
a) Swimmer plot for patients in the dose-escalation cohort; b) Swimmer plot for patients in the expansion cohort; and c) Waterfall plot for all enrolled patients.
Table 3.
Treatment outcomes of dose escalation cohort (n = 22).
| Cohort | Subject # | Disease type | MET IHC | HGF IHC | DLT | Best response | Duration of treatment (days) |
|---|---|---|---|---|---|---|---|
| 1 (0.3 mg/kg) |
RA101 | CRC | 2+ | 0 | None | PD | 48.0 |
| RA102 | Lung Cancer | 3+ | 0 | None | SD | 98.0 | |
| RA103 | CRC | 3+ | + | None | PD | 0.0 | |
| RA113 | Melanoma | 3+ | n/a | None | SD | 140.0 | |
| 2 (1 mg/kg) |
RA201 | CRC | 0 | 0 | None | SD | 84.0 |
| RA202 | Sarcoma | 3+ | + | None | SD | 99.0 | |
| RA203 | CRC | 2+ | N/A | None | PD | 26.0 | |
| 3 (3 mg/kg) |
RA301 | CRC | 1+ | 2+ | None | SD | 98.0 |
| RA302 | CRC | N/A | 0 | None | PD | 0.0 | |
| RA303 | Basal cell carcinoma | 2+ | N/A | None | SD | 101.0 | |
| 4 (5 mg/kg) |
RA401 | Gastric cancer | 1+ | 2+ | None | PD | 43.0 |
| RA402 | CRC | 2+ | 2+ | None | SD | 156.0 | |
| RA403 | CRC | N/A | N/A | None | PD | 43.0 | |
|
5 (10 mg/kg) |
RA501 | Hepatocellular carcinoma | N/A | N/A | None | SD | 124.0 |
| RA502 | Ovarian ca | 0 | N/A | None | PD | 29.0 | |
| RA503 | Melanoma | 2+ | 1+ | None | SD | 156.0 | |
| 6 (20 mg/kg) |
RA601 | GC | 0 | N/A | None | SD | 126.0 |
| RA602 | Sebaceous carcinoma | 0 | 1+ | None | PR | 503.0 | |
| RA603 | Cervical cancer | 0 | 0 | None | PD | 26.0 | |
| 7 (30 mg/kg) |
RA701 | Sarcoma | 0 | 0 | None | PD | 42.0 |
| RA702 | Ovarian cancer | n/a | 0 | None | PD | 0.0 | |
| RA703 | Ovarian cancer | 2+ | 3+ | None | SD | 41.0 |
CRC, colorectal cancer; DLT, dose-limiting toxicity; HGF, hepatocyte growth factor; IHC, immunohistochemistry; PD, progressive disease; PR, partial response; SD, stable disease.
Table 4.
Treatment outcomes of expansion cohort (n = 17).
| Cohort | Subject # | Disease type | DLT | Best response | Duration of treatment (days) |
|---|---|---|---|---|---|
| 20 mg/kg | RB001 | Ovarian cancer | None | PD | 28.0 |
| RB002 | Ovarian cancer | None | PD | 14.0 | |
| RB003 | Ovarian cancer | None | PD | 14.0 | |
| RB004 | Ovarian cancer | None | SD | 147.0 | |
| RB005 | Ovarian cancer | None | PD | 30.0 | |
| RB006 | Ovarian cancer | None | PD | 14.0 | |
| RB007 | Ovarian cancer | None | PD | 35.0 | |
| RB008 | CRC | None | SD | 144.0 | |
| RB009 | Gastric cancer | None | PD | 17.0 | |
| RB010 | CRC | None | PD | 14.0 | |
| RB011 | CRC | None | PD | 32.0 | |
| RB012 | Melanoma | None | SD | 156.0 | |
| RB013 | CRC | None | SD | 99.0 | |
| RB014 | CRC | None | SD | 92.0 | |
| RB015 | Melanoma | None | PD | 44.0 | |
| RB016 | Sarcoma | None | PD | 15.0 | |
| RB017 | Sebaceous carcinoma | None | SD | 100.0 |
CRC, colorectal cancer; DLT, dose-limiting toxicity; PD, progressive disease; SD, stable disease.
Table 5.
Exploratory biomarker analysis.
| Enrollment no. | Type of cancer | Best response | Maximal change (%) | Treatment duration (weeks) | Bx points | HGFa | p-ERK | cMET |
PD-L1 |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| + or – | + or – | + or – | Tumor | TIL | CPS, % | ||||||
| RB008 | Rectal cancer | SD | 15.4 | 22.6 | Pre | – | – | + (90%) | 5 | 5 | 10 |
| D29 | – | + (20%) | + (80%) | 15 | 15 | ||||||
| RB012 | Melanoma | SD | 2.3 | 23.3 | Pre | + (90%) | – | – | 2 | 1 | 3 |
| D29 | + (90%) | + (90%) | – | 1 | 0 | 1 | |||||
| RB013 | Colon cancer | SD | 18.3 | 15.7 | Pre | – | – | + (90%) | 10 | 1 | 11 |
| D29 | – | – | + (40%) | 5 | 3 | 8 | |||||
| RB014 | Colon cancer | SD | 12.8 | 14.4 | Pre | – | – | + (80%) | 0 | 0 | 0 |
| RB017 | Sebaceous carcinoma | SD | –1.2 | 15.6 | Pre | – | + (100%) | – | 0 | 0 | 0 |
| D29 | – | + (70%) | – | 0 | 1 | 1 | |||||
| RB009 | Gastric cancer | PD | 21.4 | 4.3 | Pre | – | – | – | 10 | 0 | 10 |
| RB010 | Colon cancer | PD | 26.7 | 4.0 | Pre | – | + (60%) | + (90%) | 0 | 2 | 2 |
| D29_1 | – | + (80%) | – | 0 | 0 | 0 | |||||
| D29_2 | – | – (95%) | – | 0 | 0 | 0 | |||||
| RB015 | Melanoma | PD | 101.0 | 5.6 | Pre | – | + (70%) | – | 1 | 1 | 2 |
| RB016 | Sarcoma | PD | 210.2 | 3.7 | Pre | – | – | – | 8 | 3 | 11 |
Figure 2.
a) Pre- and on-treatment biopsy immunohistochemistry (IHC) assay result. RB010 was a CRC patient who had disease progression after 4 weeks. RB010 IHC profile: baseline ERK 60%/ hepatocyte growth factor (HGF) 0/MET 90%/PD-L1 2% and D29 biopsy with ERK 95%/HGF 0/MET 0%/PD-L1 CPS 0. RB012 was a melanoma patient who achieved stable disease for 23 weeks. RB012 IHC profile: baseline ERK 90% HGF 40% MET 90% PD-L1 2% and D29 biopsy with ERK 90%/HGF 90%/MET 0%/PD-L1 1%. RB013 was a CRC patient who had stable disease for 15.7 weeks. RB013 IHC profile: baseline ERK 0%/HGF 0%/MET 90%/PD-L1 11% and D29 biopsy ERK 0%/HGF 0%/MET 40%/PD-L1 8%. RB017 was a sebaceous carcinoma patient who had stable disease for 15.6 weeks. RB017 IHC profile: ERK 100%/HGF 0/MET 0/PD-L1 0% and D29 biopsy had ERK 70%/HGF 0/MET 0/PD-L1 0%; b) Genomic landscape of enrolled patients. None of the MET overexpressed tumor specimens harbored MET amplification in NGS; and c) Change in blood HGF level before and after YYB101 treatment. YYB101 efficiently decreased serum HGF level. CRC, Colorectal cancer; D, Day; NGS, Next generation sequencing.
Table 6.
Correlation between EMT signature and response to YYB101.
| Patient ID | Best response | EMT score | EMT_signature | HIERI | ERK(MAPK) | HGF(UP) |
|---|---|---|---|---|---|---|
| RA202 | SD | –0.385439394 | nonEMT | 2.281666667 | 1.36666666666667 | 1.826666667 |
| RB013 | SD | –0.250484848 | nonEMT | 1.985 | 1.386666667 | 1.95 |
| RB015 | PD | 0.02730303 | EMT | 2.055 | 0.843333333 | 1.5 |
| RA103 | PD | –0.56580303 | nonEMT | 2.113333333 | 1.051666667 | 1.546666667 |
| RA113 | SD | 0.680606061 | EMT | 1.535 | 1.191666667 | 1.846666667 |
| RB014 | SD | –0.645318182 | nonEMT | 1.91 | 1.256666667 | 2.136666667 |
| RB016 | PD | 0.727121212 | EMT | 1.738333333 | 1.638333333 | 2.236666667 |
| RA102 | SD | –0.445984848 | nonEMT | 1.678333333 | 1.158333333 | 1.396666667 |
| RA101 | PD | –0.396212121 | nonEMT | 2.113333333 | 1.45 | 2.113333333 |
Lastly, we evaluated the correlation between treatment response and serial HGF levels in blood before and during administration of YYB101 (Figure 2c). In all, HGF level decreased as the dose of HGF antibody increased from 0.3 mg/kg to 30 mg/kg. In addition, serum HGF levels were profoundly downregulated in cohorts 3–7 (3.0–30 mg/kg) and the expansion cohort (20 mg/kg). Collectively, the on-target effect of YYB101 was shown with profound decrease in serum HGF levels as the dose of YYB101 increased.
Discussion
YYB101 is a neutralizing antibody that specifically binds to HGF to inhibit its activity. The present phase I study showed that YYB101 is a feasible treatment option with tolerable safety-profiles and moderate antitumor activity in heavily pretreated solid tumor patients. No DLT was observed in all dose-escalation cohorts. The overall response rate (ORR) was 2.5% (n = 1 PR) and there were 17 SDs (43.5%). YYB101 acts by blocking the HGF/MET signaling pathway to inhibit tumor growth, migration, and infiltration. Although HGF, MET, PD-L1, and pERK levels were evaluated in 9 of 17 tumor samples from patients in the expansion cohort, the overexpression levels of these biomarkers did not significantly differentiate responders or non-responders. YYB101 was found to be effective in several preclinical models, including mouse xenograft models of colorectal cancer and glioblastoma, when co-administered with irinotecan or temozolomide.11,20
In the case of Met inhibition, clinical trials have yielded little benefit to patients. The failure of clinical trials raises the common concern to many targeting approaches of whether the appropriate patient population was selected. Of course, the present study was not the biomarker pre-selected trial. However, YYB101 has some merits as compared with other anti-HGF antibodies. One of the reasons that Rilotumumab, a HGF-targeting antibody drug candidate, has not been successful in the clinical trials has been attributed to less optimal efficacy of the antibody resulting from its incomplete blocking of HGF binding to its receptor, MET, as the antibody has its epitope in the beta chain of HGF leaving the high affinity alpha chain still available for the MET binding.21 YYB101, on the contrary, has its epitope in the alpha chain of HGF enabling more efficient blocking of HGF binding to its receptor leading to almost complete inhibition of the signaling events downstream of HGF-ET complex (unpublished data). In addition, YYB101 has 10–100-fold higher affinity for HGF than that of Rilotumumab. These characteristics of YYB101 may implicate potentially better clinical efficacy of YYB101 as compared with Rilotumumab. Another feature of YYB101 is its modified IgG4 framework that has no effector function like antibody dependent cellular cytotoxicity (ADCC) or complement dependent cytotoxicity (CDC) and thus reducing the potential side effects that might arise from off-tumor/on-target binding of the antibody. Taken together, we believe YYB101 has a potential to give clinical benefits to patients that other HGF-targeting antibodies cannot.
In ovarian cancer preclinical models, the efficacy of YYB101 efficiently blocked HGF leading to inhibition of the growth of ovarian cancer cells through downregulation of the MET axis.22 In this phase I trial, we enrolled 10 ovarian cancer patients who failed to respond to at least four previous regimens. Although serum HGF levels were proficiently blocked, as shown in Figure 2c, none of the ovarian cancer patients responded to single-agent YYB101 treatment. A plausible explanation for this discrepancy between preclinical and clinical efficacies would be the existence of heterogeneous tumor cells at the time of enrollment. By the time of failure to multiple chemotherapy regimens, cancer cells may have diversified (i.e. HGF-dependent or -independent) tumor clones, as shown by recent studies on tumor evolution.23,24
Of note, one patient achieved a long-term response (PR) to YYB101 treatment after failing to respond to dacarbazine/cisplatin, etoposide/cisplatin, and radiotherapy. The patient had multiple lymph nodes that were refractory to all regimens available. This patient’s tumor did not show MET overexpression and showed weak HGF expression at baseline. After receiving YYB101 for one year, the patient achieved near complete response (CR) with <5 mm residual lymph node. The patient is in still remission for post-treatment 1 year at the time of this writing. The other patient revealed the stable disease over 18 weeks to YYB101 treatment. The patient had malignant melanoma that was refractory to prior immunotherapy and chemotherapy. The tumor sample of this melanoma patient showed highly overexpressed HGF protein at baseline biopsy. In this study, we measured the total HGF including both the active and inactive forms of HGF. Thus, we cannot evaluate the exact role of HGF as a novel biomarker to YYB101 treatment. The active form of HGF has been known as a key role in the activation of HGF-MET signaling, however, until now there has been the lack of a molecular diagnostic tool able to selectively detect the active form of HGF.25,26 Recently, some studies reported that several anti-HGF monoclonal antibodies could recognize the active form of HGF.25,27 To confirm the role of HGF as biomarker for YYB101 treatment, further investigation with YYB101 using tools that are able to detect an active form of HGF are needed. Furthermore, activated/phosphorylated MET (pMET) using specific antibodies have been used to detect the activation of HGF-MET signaling. In this study, we did not evaluate the status of pMET in tumor tissue because of a deficiency in the samples. The evaluation of pMET as a biomarker for YYB101 treatment will be valuable.
For colorectal cancer (n = 13), seven colorectal cancer patients achieved stable disease for more than 3 months with YYB101 monotherapy (Figure 1a, 1b). The three CRC patients who achieved stable disease for >3 months were RA402 (KRAS mutation CRC), RA301 (KRAS wild-type), and RA201 (KRAS wild-type). RA301 and RA201 KRAS wild-type patients failed to respond to prior cetuximab-based chemotherapy. Our previous preclinical study showed that a combination of YYB101 plus irinotecan exhibited antitumor activity in a colon cancer xenograft mouse model with irinotecan resistance.20 This finding supports that a combination of irinotecan and YYB101 might be effective in refractory CRC patients who fail to respond to standard systemic chemotherapies, including irinotecan. Hence, salvage treatment with YYB101 plus irinotecan combination chemotherapy in irinotecan-refractory CRC patients is being explored as a phase II trial.
Conclusion
We have successfully completed a phase I trial with YYB101, which showed favorable safety and dose-proportional PK. The recommended dose of YYB101 for the expansion cohort was determined to be YYB101 20 mg/kg. Prospective analysis of the mesenchymal signature for predicting response to YYB101 will be pursued.
Footnotes
Author contributions: Conception/design: Jeeyun Lee, Seung Tae Kim, Young Whan Park, Song-Jae Lee, Jung Yong Hong
Provision of study material or patients: Se Hoon Park, Joon Oh Park, Young Suk Park, Ho Yeong Lim, Won Ki Kang, Seung Tae Kim, Jung Yong Hong, Jeeyun Lee, Do-Hyun Nam, Jeong-Won Lee
Collection and/or assembly of data: Se Hoon Park, Joon Oh Park, Young Suk Park, Ho Yeong Lim, Won Ki Kang, Seung Tae Kim, Jung Yong Hong, Jeeyun Lee, Keunchil Park
Data analysis and interpretation: Kyung Kim, Kyoung-Mee Kim, Neunggyu Park, Hukeun Lee, Sung Hee Hong, Seong-Won Song
Manuscript writing: Jeeyun Lee, Seung Tae Kim, Kyung Kim, Kyoung-Mee Kim, Hukeun Lee
Final approval of manuscript: Seung Tae Kim, Jung Yong Hong, Se Hoon Park, Joon Oh Park, Young Whan Park, Neunggyu Park, Hukeun Lee, Sung Hee Hong, Song-Jae Lee, Seong-Won Song, Kyung Kim, Young Suk Park, Ho Yeong Lim, Won Ki Kang, Do-Hyun Nam, Jeong-Won Lee, Keunchil Park, Kyoung-Mee Kim, and Jeeyun Lee
Conflict of interest statement: S-J L and S-W S are employees of CellabMED Inc., Korea. The remaining authors declare no conflicts of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This trial was supported by the National OncoVenture/National Cancer Center funded by Ministry of Health and Welfare, Republic of Korea (No. HI17C2196010019).
Contributor Information
Seung Tae Kim, Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea, Republic of (South).
Jung Yong Hong, Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea, Republic of (South).
Se Hoon Park, Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea, Republic of (South).
Joon Oh Park, Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea, Republic of (South).
Young Whan Park, National OncoVenture, National Cancer Center, Goyang, Korea, Republic of (South).
Neunggyu Park, National OncoVenture, National Cancer Center, Goyang, Korea, Republic of (South).
Hukeun Lee, National OncoVenture, National Cancer Center, Goyang, Korea, Republic of (South).
Sung Hee Hong, National OncoVenture, National Cancer Center, Goyang, Korea, Republic of (South).
Song-Jae Lee, CellabMED Inc, Guro-gu, Seoul, Korea, Republic of (South).
Seong-Won Song, CellabMED Inc, Guro-gu, Seoul, Korea, Republic of (South).
Kyung Kim, Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea, Republic of (South).
Young Suk Park, Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea, Republic of (South).
Ho Yeong Lim, Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea, Republic of (South).
Won Ki Kang, Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea, Republic of (South).
Do-Hyun Nam, Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine Seoul, Korea, Republic of (South).
Jeong-Won Lee, Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea, Republic of (South).
Keunchil Park, Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea, Republic of (South).
Kyoung-Mee Kim, Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine Seoul, Korea, Republic of (South).
Jeeyun Lee, Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea.
References
- 1. Scagliotti GV, Novello S, von Pawel J. The emerging role of MET/HGF inhibitors in oncology. Cancer Treat Rev 2013; 39: 793–801. [DOI] [PubMed] [Google Scholar]
- 2. Lee J, Jain A, Kim P, et al. Activated cMET and IGF1R-driven PI3K signaling predicts poor survival in colorectal cancers independent of KRAS mutational status. PLoS One 2014; 9: e103551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gherardi E, Birchmeier W, Birchmeier C, et al. Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012; 12: 89–103. [DOI] [PubMed] [Google Scholar]
- 4. Bottaro DP, Rubin JS, Faletto DL, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 1991; 251: 802–804. [DOI] [PubMed] [Google Scholar]
- 5. Ha SY, Lee J, Kang SY, et al. MET overexpression assessed by new interpretation method predicts gene amplification and poor survival in advanced gastric carcinomas. Mod Pathol 2013; 26: 1632–1641. [DOI] [PubMed] [Google Scholar]
- 6. Lee SJ, Lee J, Park SH, et al. c-MET overexpression in colorectal cancer: a poor prognostic factor for survival. Clin Colorectal Cancer 2018; 17: 165–169. [DOI] [PubMed] [Google Scholar]
- 7. Parikh RA, Wang P, Beumer JH, et al. The potential roles of hepatocyte growth factor (HGF)-MET pathway inhibitors in cancer treatment. Onco Targets Ther 2014; 7: 969–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stella MC, Trusolino L, Comoglio PM. The Slit/Robo system suppresses hepatocyte growth factor-dependent invasion and morphogenesis. Mol Biol Cell 2009; 20: 642–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bottaro DP, Liotta LA. Cancer: out of air is not out of action. Nature 2003; 423: 593–595. [DOI] [PubMed] [Google Scholar]
- 10. Takayama H, LaRochelle WJ, Sharp R, et al. Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci U S A 1997; 94: 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim H, Hong SH, Kim JY, et al. Preclinical development of a humanized neutralizing antibody targeting HGF. Exp Mol Med 2017; 49: e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Owusu BY, Galemmo R, Janetka J, et al. Hepatocyte growth factor, a key tumor-promoting factor in the tumor microenvironment. Cancers (Basel) 2017; 9: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sa JK, Kim SH, Lee JK, et al. Identification of genomic and molecular traits that present therapeutic vulnerability to HGF-targeted therapy in glioblastoma. Neuro Oncol 2019; 21: 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Catenacci DVT, Tebbutt NC, Davidenko I, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18: 1467–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tan EH, Lim WT, Ahn MJ, et al. Phase 1b trial of ficlatuzumab, a humanized hepatocyte growth factor inhibitory monoclonal antibody, in combination with gefitinib in Asian patients with NSCLC. Clin Pharmacol Drug Dev 2018; 7: 532–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tibbitts J, Cavagnaro JA, Haller CA, et al. Practical approaches to dose selection for first-in-human clinical trials with novel biopharmaceuticals. Regul Toxicol Pharmacol 2010; 58: 243–251. [DOI] [PubMed] [Google Scholar]
- 17. Lee J, Cristescu R, Kim KM, et al. Development of mesenchymal subtype gene signature for clinical application in gastric cancer. Oncotarget 2017; 8: 66305–66315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee J, Kim K. Biomarkers for gastric cancer: molecular classification revisited. Precis Future Med 2017; 1: 59–68. [Google Scholar]
- 19. Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med 2018; 24: 1449–1458. [DOI] [PubMed] [Google Scholar]
- 20. Woo JK, Kang JH, Kim B, et al. Humanized anti-hepatocyte growth factor (HGF) antibody suppresses innate irinotecan (CPT-11) resistance induced by fibroblast-derived HGF. Oncotarget 2015; 6: 24047–24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Greenall SA, Adams TE, Johns TG. Incomplete target neutralization by the anti-cancer antibody rilotumumab. MAbs 2016; 8: 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim HJ, Lee S, Oh YS, et al. Humanized anti-hepatocyte growth factor monoclonal antibody (YYB-101) inhibits ovarian cancer progression. Front Oncol 2019; 9: 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim ST, Banks KC, Pectasides E, et al. Impact of genomic alterations on lapatinib treatment outcome and cell-free genomic landscape during HER2 therapy in HER2+ gastric cancer patients. Ann Oncol 2018; 29: 1037–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pectasides E, Stachler MD, Derks S, et al. Genomic heterogeneity as a barrier to precision medicine in gastroesophageal adenocarcinoma. Cancer Discov 2018; 8: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sakai K, Passioura T, Sato H, et al. Macrocyclic peptide-based inhibition and imaging of hepatocyte growth factor. Nat Chem Biol 2019; 15: 598–606. [DOI] [PubMed] [Google Scholar]
- 26. Kawaguchi M, Kataoka H. Mechanisms of hepatocyte growth factor activation in cancer tissues. Cancers (Basel) 2014; 6: 1890–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jangphattananont N, Sato H, Imamura R, et al. Distinct localization of mature HGF from its precursor form in developing and repairing the stomach. Int J Mol Sci 2019; 20: 2955. [DOI] [PMC free article] [PubMed] [Google Scholar]