Abstract
Background
Abiotic stresses (e.g., heat or limited water and nutrient availability) limit crop production worldwide. With the progression of climate change, the severity and variation of these stresses are expected to increase. Exogenous silicon (Si) has shown beneficial effects on plant growth; however, its role in combating the negative effects of heat stress and their underlying molecular dynamics are not fully understood.
Results
Exogenous Si significantly mitigated the adverse impact of heat stress by improving tomato plant biomass, photosynthetic pigments, and relative water content. Si induced stress tolerance by decreasing the concentrations of superoxide anions and malondialdehyde, as well as mitigating oxidative stress by increasing the gene expression for antioxidant enzymes (peroxidases, catalases, ascorbate peroxidases, superoxide dismutases, and glutathione reductases) under stress conditions. This was attributed to increased Si uptake in the shoots via the upregulation of low silicon (SlLsi1 and SlLsi2) gene expression under heat stress. Interestingly, Si stimulated the expression and transcript accumulation of heat shock proteins by upregulating heat transcription factors (Hsfs) such as SlHsfA1a-b, SlHsfA2-A3, and SlHsfA7 in tomato plants under heat stress. On the other hand, defense and stress signaling-related endogenous phytohormones (salicylic acid [SA]/abscisic acid [ABA]) exhibited a decrease in their concentration and biosynthesis following Si application. Additionally, the mRNA and gene expression levels for SA (SlR1b1, SlPR-P2, SlICS, and SlPAL) and ABA (SlNCEDI) were downregulated after exposure to stress conditions.
Conclusion
Si treatment resulted in greater tolerance to abiotic stress conditions, exhibiting higher plant growth dynamics and molecular physiology by regulating the antioxidant defense system, SA/ABA signaling, and Hsfs during heat stress.
Keywords: Solanum lycopersicum L., Silicon, Heat stress, Antioxidant, Heat shock protein
Background
Abiotic stress (heat, drought, salinity, and heavy metals) can reduce crop productivity by up to 51–82% [1]. Notably, heat stress is recognized as a major adverse condition affecting yield loss, which is particularly relevant given the current context of global warming and climate change, with temperatures expected to increase by 2 °C [2]. It is predicted that a global temperature increase of 3–4 °C would cause a 15–35% reduction in crop productivity [3]. This scenario will subject crops to a larger range of environmental stressors that could occur simultaneously, leading to severe adverse impacts on crop productivity and future global food security [4]. Physiologically, plants have a well-developed heat stress response (HSR), involving numerous signaling pathways in response to heat stress to minimize or prevent damage during short- and long-term heat exposure [5]. When plants experience heat stress, they undergo morpho-physiological, biochemical, phytohormonal, and transcriptional changes such as osmotic imbalance, enzyme inactivation, reactive oxygen species (ROS) overproduction, and organelle damage, which can lead to cell death [2, 6, 7]. With the predicted increase in the frequency and intensity of heat stress, the rate osmotic potential is significantly hindered by the creation of a cell water potential imbalance, causing tissue damages and influencing essential biochemical pathways. To survive under varying temperature conditions, plants have evolved multiple internal tolerance strategies, such as the secretion of heat shock proteins (HSPs), changes in phytohormone levels, and the scavenging of ROS by different oxidation-reduction enzymes [5]. Furthermore, as molecular chaperones, HSPs play a crucial role in facilitating native protein folding and preventing the irreversible aggregation of denatured proteins. In peanuts, the accumulation of small HSPs was shown to improve heat stress resistance [8]. Studies have also shown that elevated levels of antioxidants are associated with increased thermotolerance [2, 9]. Over-emphasis on individual phytohormones or the interplay of phytohormonal regulation, such as abscisic acid (ABA), salicylic acid (SA), and jasmonic acid play a pivotal role in extending tolerance to heat and drought stress [10, 11]. For example, ABA levels increase significantly following exposure to higher temperatures in peas [12]. To counter the adverse effects of stress, the recruitment of essential metabolites and hormones can be compromised to cope with longer stress duration. Therefore, improving crop stress tolerance is deemed an important undertaking in developing eco-friendly agricultural approaches.
Silicon (Si) is the second most abundant element in the earth’s crust and has frequently been reported to be beneficial for plant growth and development [13, 14]. Due to its strong affinity for other ions, Si is commonly found as silicic acid (H4SiO4), silicate (xM12OySiO2), and silica (SiO2) [15]. Si can increase plant tolerance to different abiotic and biotic stresses [16–18], such as salt and drought stress [19, 20], extreme temperature stress [21], nutrient deficiency [22], aluminum toxicity [23–26], disease resistance [22, 27], and pest resistance (e.g., from damage caused by wild rabbits, [28]. During stress, Si stimulates multiple response pathways, thereby activating antioxidants, enhancing mineral uptake and organic acid anions, exuding phenolic compounds, and regulating hormonal production [29–33].
Despite the known benefits of Si for plant growth under stress conditions, its underlying mechanisms in alleviating heat stress have rarely been investigated. To the best of our knowledge, only a few studies discussed the heat stress tolerance mechanisms induced by Si application. This study aimed to understand the mechanisms underlying heat stress tolerance to elucidate whether (i) Si extends stress aversion by maintaining active plant growth, (ii) it triggers the production and expression of mRNA transcripts related to antioxidative stress responses, (iii) it activates HSR by activating signaling cascades of HSP-related transcription factors and (iv) it influences stress-responsive ABA and SA syntheses. We also hypothesized that heat stress induces a reduction of plant growth and metabolism by overproducing ROS, stress-hormones, and osmotic pressure, which in turn can be minimized by exogenous Si application.
Methods
Plant growth treatment and conditions
Tomato seeds (Solanum lycopersicum L.) were obtained from Wadi Al-Lawami International, Oman that did not require any identification procedures and then thoroughly washed and soaked in autoclaved distilled water for 72 h. Uniformly germinated seeds were transplanted into seed trays containing peat moss (moisture content 38.5%, pH 4.5–5.5, electrical conductivity 2.0 dS m− 1, bulk density 0.7–1.0 mg m− 3, grain size 125–250 μm, with 91.1% organic matter: N, 800–2500 mg kg− 1; P, 150–850 mg kg− 1; Na, 340 mg kg− 1; NaCl, 850 mg kg− 1). Seedlings were irrigated with distilled water for 3 weeks to reach three internodes. Tomato seedlings of equal height and leaf number were then transplanted into larger pots (10 × 9 cm) with 200 g of peat moss. Distilled water was applied for another week to acclimatize the plants before the start of the experiment. Four-week-old (third-internode) tomato seedlings were subjected to a completely randomized design with three replicates, each consisting of 15 seedlings subjected to exogenous Si treatment. Two conditions were tested: (i) normal growth and (ii) heat stress with (+Si) and without Si (−Si; only DW). To +Si plants, 50 mL of 1 mM Si was applied in the form of sodium metasilicate (Na2SiO3), adjusted to pH 7 using HCl. Previous experiments on soybean, tomato, and rice showed that using a 1 Mm Si concentration was most beneficial. After 15 days of Si application, plants were subjected to heat stress (43 ± 0.5 °C). For heat stress, growth chamber conditions were adjusted to (12 h light − 30 °C with 6 h heat − 43 ± 0.5 °C; and 12 h dark − 30 °C) and 60% relative humidity. To mimic normal growth conditions, the growth chamber was set to a 12:12-h dark: light cycle at 30 °C and 60% relative humidity). To gradually increase the temperature, hence changing the growth chamber conditions from those optimized for normal growth to a high-temperature cycle, a 60 min timer was used to increase the temperature gradually by 5 ± 0.5 °C from 30 °C to 43 ± 0.5 °C. Once heat stress conditions were achieved, Si was applied to the +Si group of tomato seedlings using the foliar method (10 mL daily), while –Si seedlings were treated with DW (10 mL daily). Both groups of seedlings were then left under heat stress conditions for 10 days, after which plant growth attributes (shoot length, stem diameter, and the number of leaves) were recorded and the shoot and aerial parts of the plant were harvested and stored in liquid nitrogen until further analysis.
Chlorophyll pigments and leaf relative water content (LRWC)
Photosynthetic pigments were extracted from 200 mg of tomato seedling leaves and mixed with 80% acetone. Chlorophyll a (Chl a) and b (Chl b) contents were estimated according to a previously described method [34]. The absorbance of Chl a, Chl b and carotenoids was recorded at 663, 645, and 480 nm respectively. Chlorophyll content was calculated using the following equations:
Where A is the absorbance and FW is the fresh weight of the leaf sample.
The LRWC was measured according to the method described by Cao, et al. [35]. Second leaves were excised and their fresh mass (FM) was also determined. After being left to float on deionized water for 5 h, saturated mass (SM) was recorded. Leaves were then dried at 80 °C to a constant weight and the dry mass (DM) was measured. The LRWC was calculated using the following equation:
Silicon analysis by inductively coupled plasma mass spectrometry (ICP-MS)
Si was quantified with 0.05 g of ground samples of freeze-dried tomato roots and leaves according to the method described by Bilal, et al. [36] using inductively coupled plasma mass spectrometry (ICP-MS; Optima 7900DV, Perkin-Elmer, United States).
RNA extraction and quantitative real-time PCR (qRT-PCR)
The RNA extraction buffer (0.25 M NaCl, 0.05 M Tris–HCl, pH 7.5, 20 mM, EDTA, 1% w/v sodium dodecyl sulfate [SDS], 4% w/v polyvinyl pyrrolidone) was prepared using the protocol described by Liu, et al. [37]. Before adding the sample, 750 μL of the extraction buffer and chloroform: isoamyl alcohol (CI; 24:1 v/v) were placed in a 2-mL RNase-free microcentrifuge tube, then β-mercaptoethanol (40 μL) was added. Next, samples were carefully transferred to the buffer before thawing. The mixture was incubated at 20 °C for 5–8 min followed by centrifugation 12,000×g for 10 min at 4 °C. Approximately 600 μL of supernatant was transferred to a 2-mL tube and the same volume of CI was added to all samples. The solutions were mixed gently and centrifuged at 12000×g for 10 min at 4 °C. The upper layer was carefully transferred to a 1.5-mL microcentrifuge tube and 1/10 volume of 3 M sodium acetate (pH = 5.2) was added. An equal volume of absolute ethanol was added and the samples were incubated for 30 min at 4 °C. After incubation, samples were centrifuged again at 12000×g for 10 min at 4 °C and RNA was recovered. The pellet was dissolved in 200 μL of diethyl pyrocarbonate (DEPC)-treated water and 500 μL of 10 M LiCl was added to the solution. The solutions were then mixed gently and placed on ice for 60 min. Finally, samples were centrifuged once more at 12000×g for 10 min at 4 °C and the pellet was washed with 70% ethanol. After removing the ethanol, the pellet was air-dried, then dissolved in 50 μL DEPC-treated water. The quality of the RNA was assessed via agarose gel electrophoresis and quantified using a Qubit RNA broad range kit.
The extracted RNA (> 100 ng/μL) was used for cDNA synthesis. High-Capacity cDNA Reverse Transcription Kit from Thermofisher was used for cDNA synthesis. Master Mix was prepared using 10X RT Buffer, 25X dNTPs Mix, MultiScribe™ Reverse Transcriptase, 10X RT random primers, and nuclease-free water. RNA was added to Master Mix following the desired concentration (e.g., for each 100 ng/μL RNA, 10 μL was taken for cDNA synthesis). PCR was performed in a thermocycler under specific conditions (25 °C for 10 min, 37 °C for 2 h and 85 °C for 5 min). The synthesized cDNA was refrigerated at − 80 °C until further analysis.
The synthesized cDNA was used for gene amplification (Table 1). In total, 19 genes related to HSR, Si transport, drought tolerance, antioxidant enzymes, and the SA and ABA biosynthesis pathways were identified in each sample. Actin gene was used as a reference. Forward and reverse primers of 10 pM were used for all genes. For each sample, triplicate reactions were performed to minimize errors and contamination. The reaction was performed under the following conditions: 94 °C for 10 min, 35 cycles of PCR reaction at 94 °C for 45 s, 65 °C for 45 s, 72 °C for 1 min. Finally, the extension temperature was set at 72 °C for another 10 min. A threshold of 0.1 was set for gene amplification. Data were obtained in triplicates and was calculated using CT values and housekeeping gene fold expression patterns reported in Schmittgen and Livak [38].
Table 1.
List of the primer used for gene expression by qRT-PCR
| ID | Forward | Reversed | Encoding protein | Reference | Accession Number |
|---|---|---|---|---|---|
| CAT | GTCGATTGGTGTTGAACAGG | AGGACGACAAGGATCAAACC | Catalase | doi:10.4236/ajps.2010.11004 | M93719.1 |
| APX | GACTCTTGGAGCCCATTAGG | AGGGTGAAAGGGAACATCAG | cytosolic ascorbate peroxidase | doi:10.4236/ajps.2010.11004 | DQ099420.1 |
| POD | TTAGGGAGCAGTTTCCCACT | AGGGTGAAAGGGAACATCAG | peroxidase | doi:10.4236/ajps.2010.11004 | DQ099421.1 |
| GR | TTGGTGGAACGTGTGTTCTT | TCTCATTCACTTCCCATCCA | glutathione reductase | doi:10.4236/ajps.2010.11004 | AW033378 |
| Cu/Zn-SOD | GGCCAATCTTTGACCCTTTA | AGTCCAGGAGCAAGTCCAGT | SOD | doi:10.3390/molecules23030535 | Solyc11g066390 |
| GST | TACTCGTTTTTGGGCTCGTT | CACCGATTCAACTCCCTCTG | GST | doi:10.1371/journal.pone.0054880 | olyc01g086680 |
| GPX | ACGGAGCAAGCGACAATTGACAAC | CGATTGATTCACCGCAAAGCTCGT | GPX | doi:10.1371/journal.pone.0054880 | Solyc08g080940 |
| NCED1 | CTTATTTGGCTATCGCTGAACC | CCTCCAACTTCAAACTCATTGC | Synthesis of abscisic acid | Nitsch et al. (2009) | Z97215 |
| ICS | TGCTGCCTCATGGACATACC | TGCGAATGGGGATTTTTCTT | isochorismate synthase | Not reported | XM_019214147.2 |
| PAL | CACTTGTGAATGGCACAGCA | TCCGTTCATCACTTCAGCAAA | phenylalanine ammonia-lyase 1 | Not reported | XM_004234584.3 |
| PR1b1 | GCACTAAACCTAAAGAAAAATGGG | AAGTTGGCATCCCAAGACATA | Signal pathway of salicylic acid | Tucci et al. (2011) | Y08804 |
| PR-P2 | GGAACAGGAACACAAGAAACAGTGA | CCCAATCCATTAGTGTCCAATCG | Signal pathway of salicylic acid | Tucci et al. (2011) | X58548 |
| HsfA1a | GGGATAAATGAGGCAGCAAA | TTGACCTGCAATTGCTGAAG | HsfA1a | doi: 10.1104/pp.15.01913. | Solyc08g005170 |
| HsfA2 | CTCACCCCATTCAGGTGTTT | TGCTGCAATGGACAATGAAT | HsfA2 | 10.1104/pp.15.01913 | LOC101255223 |
| SlHsfA3 | AGATCCCTTGCAGGTAGCTG | TGATGGCAGTATCCCAATGG | Heat Stress Transcription Factor | doi:10.1371/journal.pone.0054880 | |
| HsfA7 | GCTTCTTTTATCCATGGTGTCC | CTTGAACCTGGAAACTCTTC | Heat Stress Transcription Factor | doi:10.1371/journal.pone.0054880 | Solyc09g065660 |
| HsfA1b | GAAAGCTTGCACTGACGCAGG | GGTCCGATATGATAGATAGTG | Heat Stress Transcription Factor | doi:10.1371/journal.pone.0054880 | Solyc03g097120 |
| DREB2 | ATGATAATAATGTCTACAGAGCAA | CTAATGTTGCCATAAAAAACTCTC | dehydration responsive element binding | DOI 10.1007/s13580-011-0125-5 | |
| MAPK1 | ATGCGCTTACAGAGGAACAGATG | CGGACGGAATGCACACATATATAC | enhanced drought tolerance | 10.1007/s11240-017-1358-5 | AJ535702 |
Determination of oxidative stress during heat stress
The extent of lipid peroxidation based on malondialdehyde (MDA) was determined in a previous study [39]. In our assay, 10 mM phosphate buffer (pH 7) was used to prepare the tissue homogenate. The reaction mixture consisted of 1.5 mL of 20% acetic acid (pH 3.5), 0.2 mL of 8.1% SDS, 1.5 mL of 0.81% thiobarbituric acid (TBA), and 0.2 mL of tissue homogenate. The reaction tube was heated for 60 min. The reaction mixture was then placed at room temperature for 15 min, followed by the addition of 5 mL of the butanol: pyridine (15:1 v/v) solution. The upper organic layer (i.e., the pink solution) was collected and the optical density was recorded at 532 nm. Tetramethoxypropane was used as an external standard and the experiments were performed in triplicates.
The generation rate of O2− was measured using the method described in Gajewska and Skłodowska [40]. Fresh plant powder (1 g) was immersed in phosphate buffer (pH 7) containing 10 mM sodium phosphate, 0.05% (w/v) nitrobluetetrazolium (NBT), and 10 mM sodium azide (NaN3). The mixture was kept for 1 h at room temperature, then, 5 mL of the solution was transferred into a new test tube and heated for 15 min at 85 °C in a water bath. The solution was then cooled on ice and vacuum filtered. The absorbance of the sample was read at 580 nm with a spectrophotometer. The experiment was performed in triplicates.
Quantification of antioxidant enzymes
For the quantification of total protein, a protein extract was prepared by grinding 100 mg of leaf sample with potassium phosphate buffer (100 mM; pH 6.8) containing 0.2 mM EDTA. After centrifugation for 30 min at 12,000×g, the supernatant was transferred to a new tube for the determination of total protein content. The protocol described by Bradford [41] was used to quantify total protein content. The assay was conducted at 595 nm on a spectrophotometer. The experiment was performed in triplicates.
The protocol established by Kar and Mishra [42] was slightly modified to determine the activity of the antioxidant enzymes peroxidase (POD), catalase (CAT), polyphenol oxidase (PPO), and ascorbate oxidase (APX). To quantify these enzymes, 100 mg of powdered leaf sample was mixed with 0.1 M phosphate buffer (pH 7). The resulting mixture was centrifuged at 10,000 rpm and 4 °C for 30 min in a refrigerated centrifuge. To quantify POD, 100 μL of the crude extract was combined with 0.1 M potassium phosphate buffer (pH 6.8), 50 μL H2O2 (50 μM), and 50 μL pyrogallol (50 μM). The reaction mixture was incubated at room temperature for 5 min, followed by the addition of H2SO4 (v/v) (5%). The extent of purpurogallin production was measured based on the optical density at 420 nm. To quantify PPO, we used a similar reaction mixture to that used for POD quantification but added H2O2 (50 μM), and the final assay was conducted at 420 nm. CAT activity was determined using the method developed by Aebi [43]: the protein mixture was combined with 10 mM phosphate buffer (pH 7) and supplemented with 0.2 M H2O2. CAT activity was measured by the decrease in absorbance at 240 nm and expressed as μg of H2O2 released/mg protein/min. To assay APX activity, 100 mg of fresh plant powder was immersed in 1 mL of 50 mM phosphate buffer solution (pH 7) containing 1 mM EDTA and 1 mM ascorbic acid, followed by homogenization at 50 Hz for 30 s. The resulting homogenates were centrifuged at 4830×g at 4 °C for 15 min. Subsequently, the supernatant was combined with the phosphate buffer (pH 7) containing 0.3 mM H2O2 and 15 mM ascorbic acid. The reaction mixture was then analyzed spectroscopically at 290 nm. One unit of APX was defined as the variable quantity of absorbance at 290 nm per minute.
SA extraction and quantification
SA was extracted and quantified from freeze-dried tomato samples according to the method developed by Seskar, et al. [44] and described by Shahzad, et al. [45]. The extracted samples were subjected to high-performance liquid chromatography (HPLC) performed using a Shimadzu device outfitted with a fluorescence indicator (Shimadzu RF-10AxL) with excitation at 305 nm and emission at 365 nm, filled with a C18 reverse phase HPLC column (HP Hypersil ODS, particle size 5 μm, pore size 120 Å, Waters). The flow rate was maintained at 1 mL/min. The experiment was repeated three times and each time comprised three replications.
ABA extraction and quantification
Endogenous ABA was extracted and quantified according to the modified protocol described by Shahzad, et al. [46] and Bilal, et al. [36]. Briefly, samples extracted from ground freeze-dried plants were supplemented with [(±)-3,5,5,7,7,7-d6]-ABA as an internal standard and further analyzed using gas chromatography-mass spectroscopy (GCMS; 6890 N network GC system) and a 5973-network mass selective detector (Agilent Technologies, Palo Alto, CA, USA). To expand the affectability of the method, spectra were recorded for the selected ions at m/z 162 and 190 for Me-ABA, and at m/z 166 and 194 for Me-[2H6]-ABA. Moreover, ABA was calculated from the value of the endogenous peak in comparison to the respective standard. The experiment was repeated three times and each time comprised three replications.
Statistical analysis
All experiments were performed in triplicates and data collected from each repetition were pooled together. All values are presented as the mean ± standard deviation (SD). Means were analyzed using Duncan’s multiple range (DMRT) tests, with significance set at P < 0.05. All analyses were conducted using SAS 9.1 software (Cary, NC, USA).
Results
Effects of exogenous Si application on tomato plant growth parameters
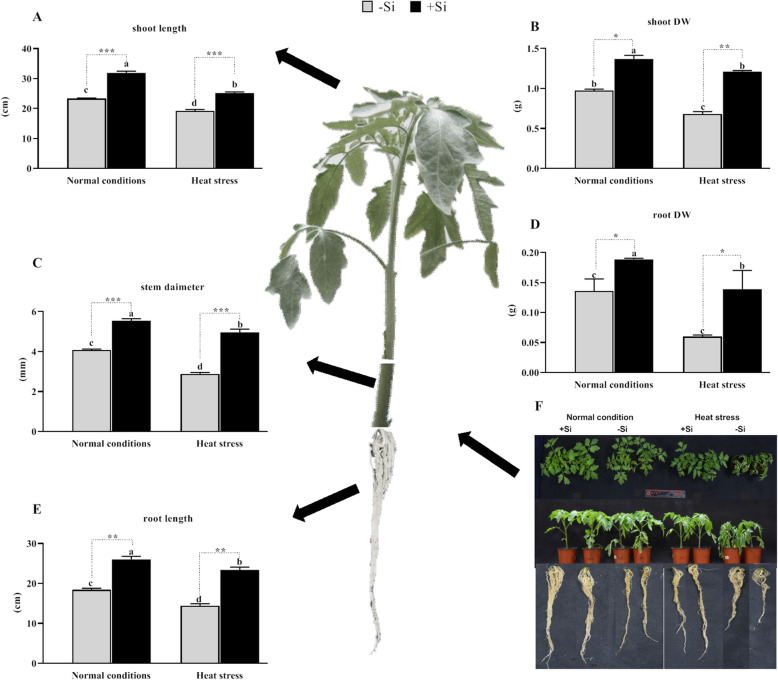
In this study, exogenous Si application increased growth parameters under both normal and heat stress conditions. The results showed that exogenous Si application significantly increased shoot length under normal and heat stress conditions compared to –Si (36 and 31%, respectively; P < 0.001; Fig. 1a). Pronounced wilting of the leaf was prolific in –Si plants compared to the condition in +Si plants. This was further validated by the significant increase in stem diameter in +Si seedlings under both conditions (36 and 72%, respectively; P < 0.001; Fig. 1c). This suggests an ameliorative effect of Si on plant growth under heat stress. Additionally, shoot biomass also significantly increased in +Si plants under normal and heat stress conditions compared to the results for –Si plants (61 and 70%, respectively; P < 0.001; Fig. 1b; Supplementary Fig. S1A). The root length, fresh, and dry biomass decreased under heat stress. However, +Si application significantly improved the root morphological traits and increased root length compared to the levels in –Si plants under heat stress and normal conditions (41 and 62%, respectively; P < 0.001; Fig. 1e), as shown by the secondary and tertiary root development. Similarly, Si application significantly increased the root and fresh root weights in both normal and heat stress conditions (42 and 28%, respectively, P < 0.001; 74 and 62%, respectively, P < 0.001; Fig. 1d; Supplementary Fig. S1B).
Fig. 1.
Effects of silicon (Si) application on the growth parameters of tomato plants grown under normal and heat stress conditions. a Shoot length, b Shoot dry weight, c stem diameter, d root dry weight, e root length, f effect of Si on the phenotype of tomato plants in the presence or absence of heat stress. Values are presented as the mean ± SE (n = 15). Different letters in one measure indicate a statistically significant difference at P < 0.005
Effects of exogenous Si application on chlorophyll pigments and relative water content
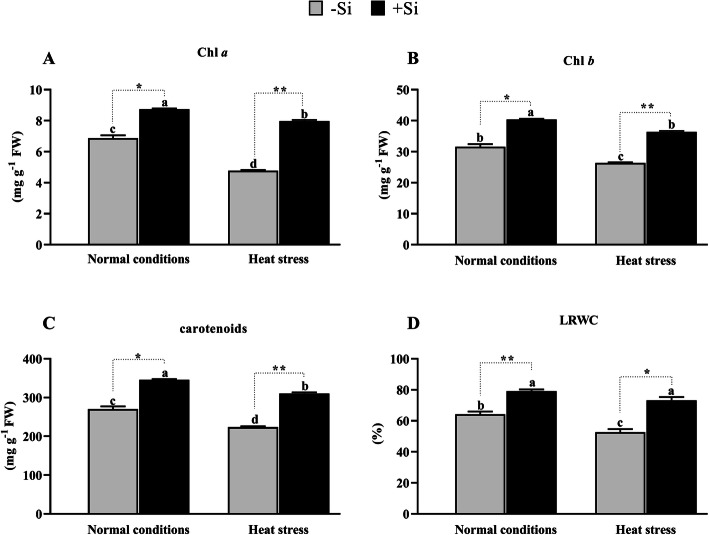
Si application significantly increased Chl a (27 and 38%, P < 0.001), Chl b (27 and 38%, P < 0.001), and carotenoid (27 and 39%, P < 0.001) content in normal and heat-stressed tomato plants. By contrast, significant reductions in Chl a, Chl b, and carotenoid content (19, 19, and 21%) were recorded in heat-stressed –Si plants (Fig. 2a-c). This suggests that Si mitigated the adverse effects of heat stress by improving Chl a, Chl b, and carotenoids. Given the predicted impact of heat stress on plant osmotic potential, we also analyzed LRWC. A significant increase of 13 and 20% (P < 0.001) in the water content was recorded in +Si plants compared to –Si plants under normal and heat-stressed conditions, respectively (Fig. 2d). Heat stress increased the plant’s vulnerability to water stress and plants with an adequate supply of Si were more hydrated than –Si plants.
Fig. 2.
Effects of Si application on photosynthesis. a Chlorophyll a (b) Chlorophyll b (c) Carotenoids (d) leaf relative water content (LRWC) under control and heat stress conditions. Values are presented as the mean ± SE (n = 6). Different letters in one measure indicate a statistically significant difference at P < 0.005
Effects of Si on superoxide anion (O2−) and MDA
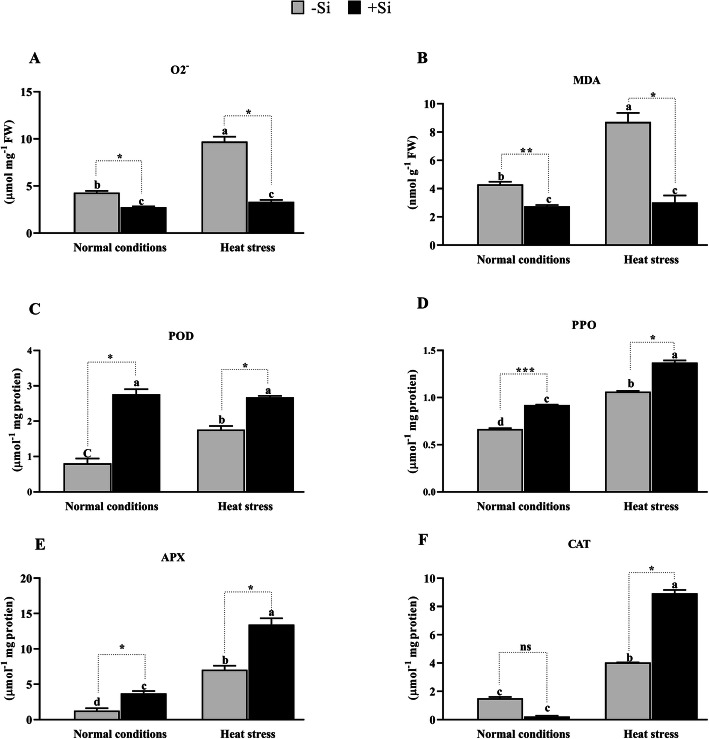
The ROS-induced peroxidation of lipid membranes is a reflection of stress-induced cell damage [47]. One of the main products of ROS is O2−, which causes significant damages to cellular organelles and their respective functions [48]. In this study, we found that the production of O2− increased significantly by 77.9% (P < 0.001) in heat-stressed conditions in –Si plants compared to the production in normal growth conditions (Fig. 3a), while in +Si plants, the production of O2− increased by only 30.2% under heat stress. This increase was significantly lower than that in –Si plants, indicating the regulatory role of +Si during heat stress and the generation of ROS.
Fig. 3.
Effects of Si application on stress-related parameters. a Malondialdehyde (MDA) content and (b) In situ O2−, c Peroxidase (POD), d Polyphenol oxidase (PPO), e ascorbate peroxidase (APX) and (f) catalase (CAT). Each data point presents the mean of three replicates. Means denoted by different letters are significantly different (P < 0.05)
Furthermore, we quantified the level of MDA under –Si and + Si conditions (Fig. 3b). MDA is a by-product of lipid peroxidation and is an indirect indicator of oxidative stress and damage to the lipid bilayer [49]. MDA significantly decreased by 56.0 and 64.5% (P < 0.001) in +Si plants compared to –Si plants under normal and heat-stressed conditions, respectively. This suggests that Si subverted the process of lipid peroxidation compared to –Si during heat stress.
Antioxidant enzyme synthesis and mRNA gene expression profiling in –Si and + Si plants
Our experiments revealed an increased production of POD, CAT, SOD, and PPO in +Si plants compared to –Si plants in both the normal and heat-stressed conditions. However, under normal growth conditions, CAT activity was lower in –Si plants but increased in +Si plants under heat stress. Under normal growth conditions, POD activity was also significantly higher in +Si plants compared to –Si plants (79.2%, P < 0.001); however, POD activity only increased by 51.77% under heat-stressed conditions (Fig. 3c). Furthermore, the activity of POD in –Si and + Si plants under heat stress increased by 35.4 and 14.6%, respectively, compared to POD activity under normal conditions.
Likewise, in +Si tomato seedlings, PPO, APX, and CAT activities increased by 29.24, 91.2, and 121.6% (P < 0.001) under heat-stressed conditions compared to –Si plants (Fig. 3d-f). Under normal growth conditions, however, the activity of PPO and APX increased by 39.4 and 189.1% (P < 0.001), respectively, but the activity of CAT decreased by 86.5% (P < 0.001) in +Si compared to –Si plants. Figure 3d-f show that the activity of PPO, APX, and CAT in –Si and + Si plants under heat stress increased by 60.6, 449.2, and 166.8% (P < 0.001) compared to the normal conditions.
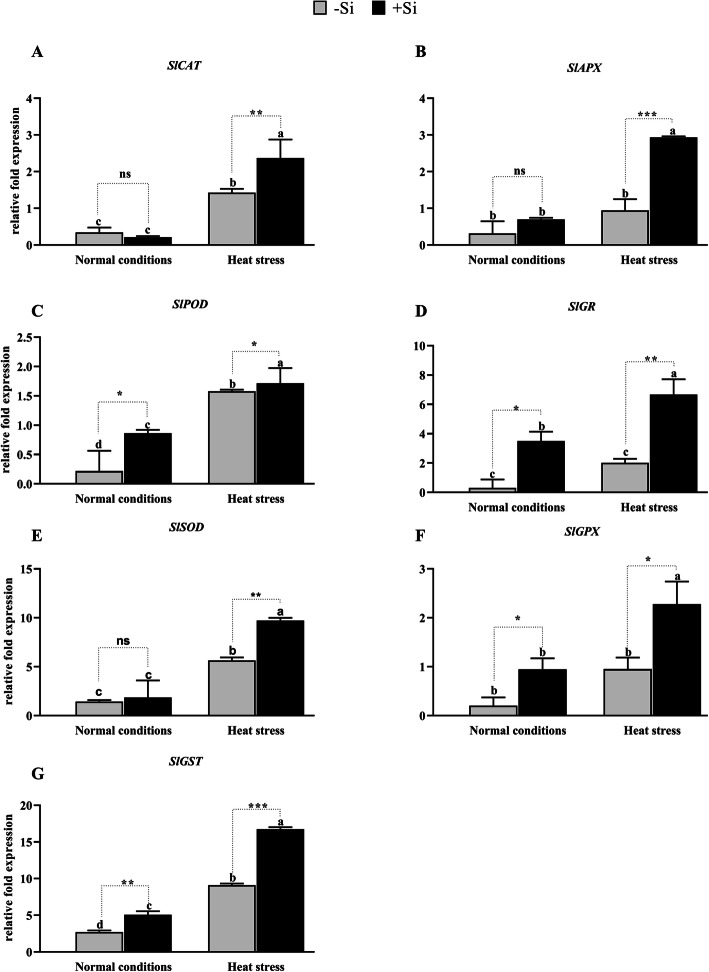
To investigate the molecular mechanisms underlying the effects of Si on antioxidant enzymes in tomato plants during heat-stressed conditions, we evaluated the relative mRNA expression levels of the SlCAT, SlAPX, SlPOD, SlGR, SlSOD, SlGPX, and SlGST genes (Fig. 4). The relative expression levels five of these genes (except SIGR and SIGPX) were significantly upregulated when –Si tomato plants were exposed to heat stress (P < 0.001; Fig. 4a-g). Interestingly, the +Si tomato plants exposed to heat stress showed high relative expression levels of SlCAT (2.3-fold), SlAPX (2.9-fold), SlPOD (1.7-fold), SlGR (6.6-fold), SlSOD (9.7-fold), SlGPX (2.2-fold), and SlGST (16.7-fold) compared to that recorded in –Si plants. Under normal growth conditions, the relative expression levels of SlPOD, SlGR, and SlGST genes increased significantly by 0.86, 3.5, and 5.08-fold (P < 0.001) in +Si plants compared to –Si plants, while SlCAT was downregulated by 0.21-fold. However, no significant increase was noted in the genes SlAPX, SlSOD, and SlGPX (0.69, 1.85, and 0.95-fold) respectively (Fig. 4a-g). The increase in the relative gene expression levels in +Si tomato plants compared to those of –Si plants indicates that Si improved the expression of genes involved in antioxidant enzyme production.
Fig. 4.
Effect of Si application on the transcript of genes encoding antioxidant enzymes. aSlCAT, bSlAPX, cSlPOD, dSLGR, eSlSOD, fSlGPX, and (g) SlGST. Each data point presents the mean of three replicates. Means denoted by different letters are significantly different (P < 0.05)
Effects of Si on relative expression level of genes coding for HSFs and stress-related proteins
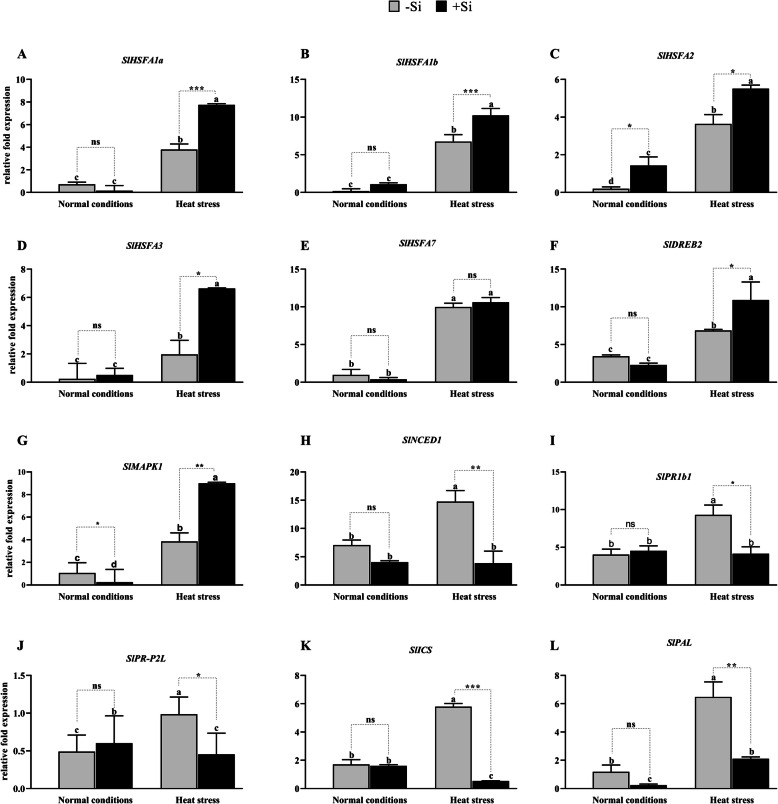
Hsfs regulate the expression of HSPs. In this study, we evaluated the relative mRNA expression level of SlHsfA1a, SlHsfA1b, SlHsfA2, SlHsfA3, and SlHsfA7 under normal and heat-stressed conditions in +Si and –Si plants (Fig. 5a-e). Our results showed that the expression of Hsfs and stress-related genes was upregulated by warm temperatures. In +Si plants, a high relative expression level was recorded for SlHsfA1a (7.7-fold), SlHsfA1b (10-fold), SlHsfA2 (5.5-fold), SlHsfA3 (6.6-fold), and SlHsfA7 (10.62-fold) under heat stress compared to –Si plants. However, under normal growth conditions, there was no significant increase in the relative expression levels of HsfA1b and HsfA3 in +Si plants compared to –Si plants. Under normal growth conditions, HsfA1a and HsfA3 were downregulated in +Si plants compared to –Si plants (Fig. 5a-e). Hence, the exogenous Si-mediated increased the expression of Hsfs and stress-responsive genes under heat stress. This ultimately triggered the HSR in tomato plants by activating Hsps, which consequently led to the prevention of lipid peroxidation and the generation of excessive reactive radicals. Furthermore, the activated HSR further increased the secretion of plant antioxidant enzymes.
Fig. 5.
Effect of Si application on the transcript of genes encoding heat shock transcription factors. aSlHsfA1a, bSlHsfA1b,cSlHsf2A,dSlHsfA3,eSlHsfA7, fSlDREB2, gSlMAPK, hSlNCED1, iSlPRb1, jSlPR-P2l, kSlICS1, and (l) SlPAL. Each data point presents the mean of three replicates. Means denoted by different letters are significantly different (P < 0.05)
The overexpression of transcription factors can modulate a wide range of signaling pathways involved in stress tolerance [50]. The dehydration-responsive element-binding proteins (SlDREB2) transcription factors are regulated by abiotic stress-related genes, thus providing the plant with tolerance to various environmental stimuli [51]. The products of these genes are thought to function both in stress tolerance and in the regulation of gene expression and signal transduction of genes related to stress response [52, 53]. Transcription factor SlDREB2 was weakly expressed in the control plants and was stimulated by heat stress in both –Si and + Si plants by 6.88-fold and 10.91-fold, respectively. These results suggest that Si application increased the expression of SlDREB2, thus improving tomato seedlings tolerance to heat stress (Fig. 5f). Previous studies revealed that protein kinases play a similar role as calcium-dependent protein kinases and mitogen-activated protein kinases (MAPKs) in response to abiotic stress [54]. In this study, we found that the expression of SlMAPKs was induced by heat stress (3.85-fold) in –Si plants, increasing by 9-fold in +Si plants under heat stress (Fig. 5g).
Effects of Si on endogenous phytohormones during heat stress
Plants respond to various abiotic and biotic stressors through alterations in hormonal homeostasis, biosynthesis rate, stability, content, and cell compartmentalization [55]. Phytohormones play an important role in plant growth and development and the current literature suggests these also play a role in the plant’s response to various stresses [56]. In this study, we quantified endogenous ABA and SA hormones in +Si and –Si plants under both normal and heat stress conditions. The results revealed that –Si plants had significantly higher concentrations of ABA (265.91 ng g− 1 FW) and SA (11.34 ng g− 1 FW) compared to +Si plants under normal growth conditions (141.48 and 7.53 ng g− 1 FW, respectively). Furthermore, the application of Si reduced ABA content significantly in tomato seedlings under both the normal and heat stress conditions by 74 and 44%, respectively. Similarly, SA content was reduced by 19 and 32% in plants treated with Si compared to the level in –Si plants under normal and heat stress conditions, respectively (Fig. 6a and b). Our findings indicate that +Si plants produced less ABA and SA, which might be the reason they experienced higher tolerance and a lower amount of heat stress.
Fig. 6.
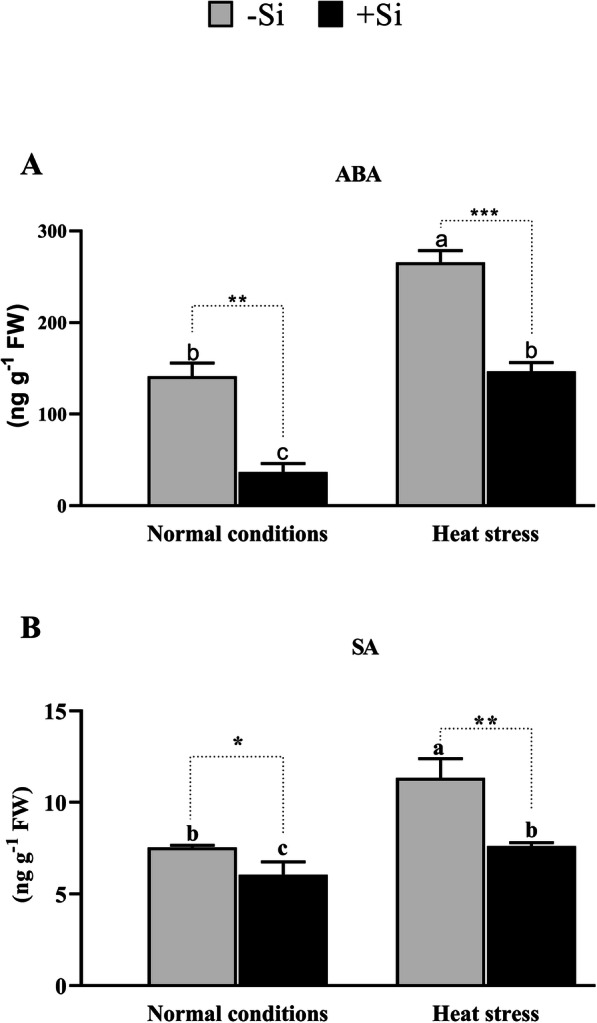
Effect of 1 mM Si application on the level of plant stress tolerance phytohormones (a) Abscisic acid (ABA), and (b) Salicylic acid (SA). Each data point presents the mean of three replicates. Means denoted by different letters are significantly different (P < 0.05)
The effects of heat stress and exogenous Si on SA pathway-related genes (SlR1b1, SlPR-P2, SlICS, and SlPAL) and ABA (SlNCEDI) were examined using qRT-PCR (Fig. 5h-l). The change in expression of SlR1b1, SlPrP2, SlICS, and SlPAL marker genes involved in the SA biosynthesis pathway was strongly and moderately downregulated in the +Si and –Si plants, respectively, under heat stress conditions. Interestingly, these results concur with those observed for the SA levels. However, under normal growth conditions, SlR1b1 and SlPR-P2 were slightly upregulated in +Si plants compared to –Si plants, while SlICS and SlPAL were downregulated, albeit not significantly. The SA signaling pathway appears to be activated under heat stress; however, in the current study, the exogenous application of Si resulted in the downregulation of this pathway. Such results are perfectly consistent with the fact that plant antioxidant capacity is inversely proportional to SA concentration. Furthermore, high concentrations of SA cause cell death or increase vulnerability to abiotic stress. Hence, Si application reduced the biosynthesis of SA, which indirectly enhanced the production of antioxidant enzymes.
Si, Na, and K concentrations in tomato plants under heat stress conditions
Si, Na, and K concentrations were measured in the leaves of +Si and –Si tomato plants after harvest. Unsurprisingly, Si concentration was significantly higher in +Si plants compared to –Si plants in both the normal and heat stress conditions (5.2-fold and 6.1-fold higher, respectively, Table 2). This result was further validated at the gene expression level by investigating the relative expression of the Low Silicon 1 and 2 (SlLsi1 and SlLsi2) genes. As shown in Fig. 7a and b, exogenous Si application improved the expression of SlLsi1 (2.1 and 5.1-fold) and SlLsi2 (1.9 and 2.4-fold) under both the normal and heat stress conditions, respectively. On the other hand, Na concentration in the leaves of the tomato plants was not significantly different between +Si and –Si plants. However, the Na concentration was slightly higher in the +Si plants. This result indicates that exogenous Na application in the form of silicate did not increase Na concentration in the leaves of the tomato plants. However, Si significantly (P < 0.001) improved the uptake of K from the soil by approximately 0.6 to 1-fold (Table 2).
Table 2.
Concentrations of Si, Na and K in the tomato shoot under normal and heat stress conditions
| Treatments | Concentration (μmol g− 1) | ||
|---|---|---|---|
| Si | Na | K | |
| Normal condition (+Si) | 2772.82 ± 10.3 b | 1071.85 ± 17.3a | 32,703.64 ± 165.3 a |
| Normal condition (−Si) | 563.06 ± 17.9 c | 1080.43 ± 55.6 b | 25,561.12 ± 122.8 c |
| Heat stress (+Si) | 3693.89 ± 39.3 a | 1270.98 ± 99.7a | 31,995.73 ± 16.4b |
| Heat stress (−Si) | 601.96 ± 31.1 c | 1210.23 ± 85.1a | 20,002.39 ± 79.4 d |
Mean ± standard error from three replications per treatment. In the column, the same letters indicate No significant difference (P > 0.05) by Duncan’s Multiple Range Test (DMRT)
Fig. 7.
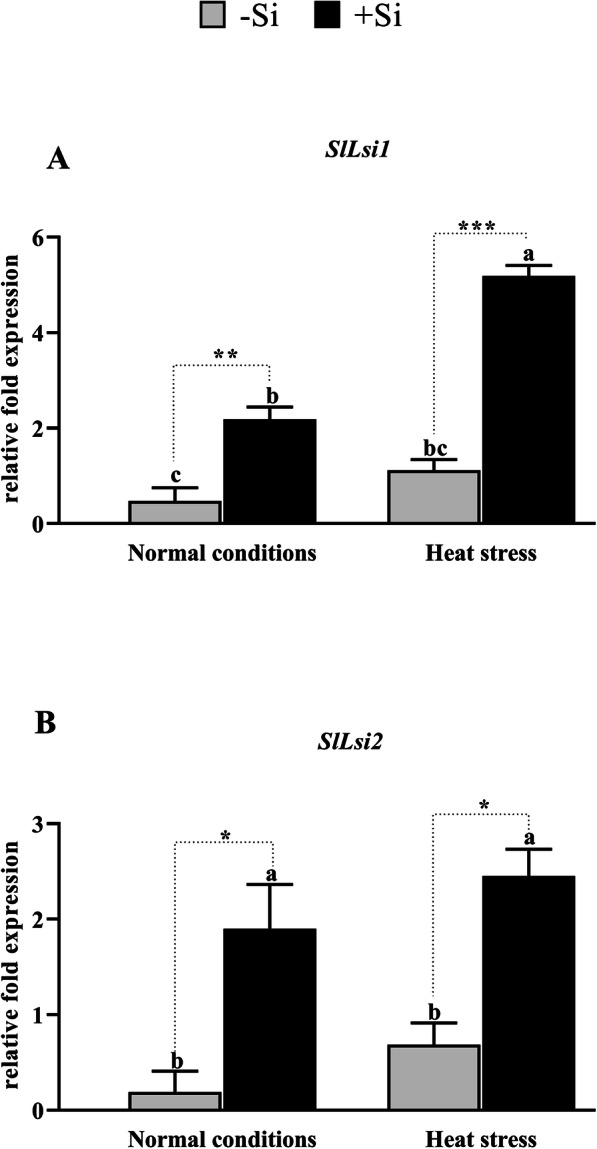
Effect of Si application on the transcript of genes for Si uptake in plants. aSlLsi1,bSlLsi2. Each data point presents the mean of three replicates. Means denoted by different letters are significantly different (P < 0.05)
Discussion
Heat stress severely hinders crop production, causing substantial losses to the economy and endangering global food security [57–60]. Similarly to trends shown in other crops, it also negatively affects the growth and productivity of tomato plants [61–63]. Our findings demonstrate that exogenous Si application can mitigate the adverse effects pf heat stress in tomato plants by improving plant growth attributes, such as shoot length and biomass production. Similar findings were previously reported in Cucumis sativus L. [64], Nephrolepis exaltata L. [65], and Oryza sativa L. [66]. However, little is known about this phenomenon in tomato plants. The results presented in this study concur with those of previous reports by Mahdieh, et al. [67], Abbas, et al. [68], and Chen, et al. [69], where the application of exogenous Si alleviated the negative effects of abiotic stress and restored plant growth. The growth impacts were inferred from the increased levels in the concentration of photosynthetic pigments (Chl a, Chl b, and carotenoids) in Si + heat-stressed plants, while studies by [70], Wang, et al. [71], and Chalanika De Silva and Asaeda [72] showed that these concentrations were reduced by heat stress. These reductions were associated with the reduced production of ROS and thereby indirectly representative of the plants stress level [72, 73]. Furthermore, heat stress drastically decreased LRWC by inducing physiological water-deficit. On the other hand, Si application increased LRWC, which led to the increase in photosynthetic pigments observed in the current study. A similar finding was reached by previous studies following during heat stress [74], salinity [75], drought stress [76], and osmotic stress [77]. Similarly to other abiotic stresses, heat stress increased ROS production that consequently oxidize membrane lipids [78, 79] as high concentrations of MDA and O2− in –Si plants were observed compared to +Si plants. The triggering of both molecules can lead to the disruption of activities of antioxidant enzymes [80]. Our results were similar those observed after Si application on Hordeum vulgare L. [80], Cicer arietinum L. [80], and Nephrolepis exaltata [65].
Plants have evolved an effective antioxidant system using POD, CAT, SOD, PPO, and APX enzymes to eliminate stress-induced excess ROS, thereby protecting the cells from the deleterious effects of oxidation reactions [81]. CAT, APX, and POD antioxidants are known for the dismutation of hydrogen peroxide to water and molecular oxygen in cells [82], as well as the elimination of stress-induced ROS directly or indirectly via the production of ascorbate and glutathione [83]. In this study, we found that the activities of ROS-eliminating enzymes changed significantly. All of the antioxidant enzymes examined in this study, including CAT, POD, PPO, and APX increased significantly in plants exposed to heat stress. However, under normal growth conditions, −Si plants showed higher CAT activity compared to +Si plants. This unusual regulation of CAT activity has been reported in several studies [84, 85]. +Si plants show enhanced CAT activity during heat stress compared to normal conditions (Fig. 8), concurring with previous findings [83, 86, 87]. The roles of Si in genes encoding for antioxidant enzymes have only been studied in a very narrow range. In this study, we elucidated the effects of Si on genes encoding for antioxidant enzymes, such as SlCAT, SlAPX, SlPOD, SlGR, SlSOD, SlGPX, and SlGST genes. Our findings revealed that Si application in heat-stressed tomato plants increased the relative expression of SlCAT, SlAPX, SlPOD, SlGR, SlSOD, SlGPX, and SlGST genes, in line with previous reports by Sahebi, et al. [88] and Alberto, et al. [89]. Ma, et al. [90] reported that Si application induces high stress tolerance in plants by upregulating the antioxidant systems of plants.
Fig. 8.
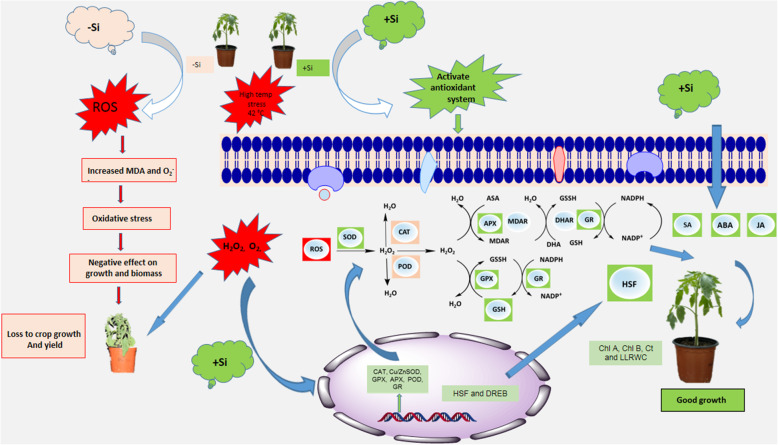
Three strategies are involved in Si enhancement of plant tolerance to high temperatures (43 ± 0.5 °C). In strategy I, Si enhances the antioxidant enzyme activity by both upregulating the expression of antioxidant enzyme genes and reducing the decrease of MDA and O2− caused by Reactive Oxygen Species (ROS). In strategy II, Si activates the heat shock proteins and drought tolerance genes such as SlDREB2 and SlMAPK1. In strategy III, Si downregulates stress-related hormones, such as ABA and SA
Heat stress also influences the translocation of essential nutrients [91] and the resulting imbalance can either increase or decrease them. For example, growth inhibition is correlated with excessive Na+ concentration and K+ deficiency (Fig. 8). Either high Na or low K in the soil are considered as a stress condition that can severely affect plant performance and agricultural productivity (Luan et al., 2009). Give that we used sodium silicate as a Si source in this study, both Si and Na+ were analyzed. We did not observe any difference in the Na+ concentration in plants treated with and without sodium silicate, suggesting that the observed toxicity was not due to Na+ concentration but to high temperatures and induced drought stress. Si uptake was confirmed by measuring the Si concentration in shoots. We also validated the higher uptake of Si in +Si plants at the mRNA level.
The function of SlLsi1 and SlLsi2 genes in Si transport and stress conditions are poorly understood. Previously, Ma, et al. [92] described the role of Oryza sativa OsLsi1 and OsLsi2, present in the plasma membrane of rice plant cells, in Si influx and efflux. OsLsi1 is located on the distal side of the cell while OsLsi2 is located on its proximal side [92]. Our results revealed that both SlLsi1 and SlLsi2 genes were upregulated by 2- and 5-fold and by 1.9- and 2.4-fold in +Si plants under normal and heat stress conditions, respectively. They were also upregulated under heat stress in –Si plants, suggesting they improved Si uptake and played a role in heat stress tolerance (Fig. 8). This role might be attributed to the accumulation of Si in the shoots, providing additional strength to the leaf and stem structure to minimize the adverse effects of heat and drought stress, hence leading to higher tolerance. The role of OsLsi1 and OsLsi2 in heavy metal tolerance was previously reported by Kim, et al. [93].
Hsf, a regulatory protein, plays a central regulatory role in the conversion of heat stress signal perception to the expression of genes involved in the plant’s stress response by interacting with promoters at the cis binding region of HSPs [94]. These in turn activate a cascade of heat stress-responsive genes that work together to improve the plant’s heat stress tolerance. The regulatory role of Hsfs has been studied in Arabidopsis during heat stress conditions and in other crops [95, 96]. Hsfs are conserved in different species, but discrepancies regarding the function of individual Hsfs in HSR have also been reported. HsfA1a is a master regulator for the initiation of HSR in tomato plants, but in Arabidopsis, HsfA1a, HsfA1b, and HsfA1d are responsible for HSR [97, 98]. Furthemore, under heat stress, HsfB1 in tomato shows both coactivator and repressor functions, while the same gene in Arabidopsis acts as a transcriptional repressor [99]. HsfA1a, HsfA2, and HsfB1 are considered essential for the activation of HSR in tomato plants under heat stress conditions [100] HsfA1a and HsfB1 are produced continuously in the cell, while HsfA2 is expressed under normal conditions. It was found that the expression of HsfA2 mRNA and protein increased under heat stress. Consequently, HsfA2 was more abundant when the plant was grown at higher temperatures [101]. In this study, we found that heat stress significantly enhanced the expression of Hsfs, namely SlHsfA1a, SlHsfA1b, SlHsfA2, SlHsfA3, and SlHsfA7, which varied under normal conditions. This finding highlights the role of SlHsfA1a, SlHsfA1b, SlHsfA2, SlHsfA3, and SlHsfA7 in heat stress tolerance. Interestingly, we found a higher expression of Hsfs in +Si plants, leading us to the conclusion that exogenous Si application induced the expression of Hsfs and stress-response genes under heat stress. This ultimately activates HSR in tomato plants by activating Hsps, leading to the prevention of lipid peroxidation and the generation of excessive reactive radicals. Hsfs regulate the transcription changes needed to protect plants from heat stress [8]. Similarly, we found that heat increased the expression of SlDREB2 and SlMAPK1. Wang, et al. [102] previously reported that overexpression of SlMAPK1 enhanced drought and heat stress tolerance in tomato plants. Moreover, the role of MAPK1 in abiotic stresses such as cold, salinity, UV radiation, and wounding has also been reported in Arabidopsis [103, 104].
Stress-related endogenous hormonal regulation and its related pathways (such as ABA and SA) are considered essential in mediating plant growth and physiological response under stress conditions. ABA signaling and ABA-responsive genes have been extensively studied under a wide range of stress conditions. ABA synthesis triggers ABA-inducible gene expression and causes stomatal closure, thereby reducing water loss via transpiration and eventually restricting cellular growth [105–107]. In this study, significantly higher amounts of ABA were observed in heat-stressed –Si plants compared to +Si plants. This difference indicates that tomato plants experienced high levels of stress. In +Si plants, ABA concentration significantly decreased during heat stress and under normal conditions. These results concur with those Kim, et al. [93], who reported that ABA increased significantly when rice plants were treated with Cu, Cd, and a combination of Cd and Cu in the presence of Si. It has been demonstrated in Pisum sativum by Li, et al. [12] that the ABA level increased upon exposure to heat stress, confirming its role in heat stress tolerance. Later, the same group reported that the ABA-deficient mutant tomato genotype is sensitive to heat stress (42 °C for 24 h) [108, 109]. In this study, a significant reduction in ABA was recorded in +Si plants, an indication that +Si plants are less sensitive to heat stress than –Si plants. This was further validated by the gene expression profile, indicating that heat stress upregulated the expression of genes responsible for ABA biosynthesis. We observed higher expression of the NCED1 gene during heat stress and under normal conditions in –Si plants compared to +Si plants, suggesting that Si application reduced the biosynthesis of ABA.
SA is also a naturally-occurring plant hormone involved in the response to abiotic and biotic stresses and in the regulation of pathogenesis-associated protein expression [110]. The SA response under biotic stress has been widely reported [111]. SA also plays an important role in plant growth, ripening, and development [112] and its role in thermotolerance is well-established. Davies [112] and Li, et al. [113] reported that SA-mediated pathways could increase heat stress tolerance in plant species such as mustard, tobacco, bean, potato, tomato, and Arabidopsis thaliana. Similarly, we found that heat stress significantly improved the biosynthesis of SA in our tomato seedlings, suggesting its role in regulating heat stress tolerance. However, the results revealed that exogenous Si application significantly reduced SA concentration. This may be because the plants experienced less stress due to the accumulation of Si in the shoots, forming a protective layer on the leaves. Another possible reason is the interaction with antioxidant systems in the presence of exogenous Si. Li, et al. [113] indicated that high levels of SA caused oxidative stress, while lower levels of SA improved the antioxidative capacity of plants and stimulated the synthesis of protective compounds, leading to enhanced tolerance to abiotic stress. Our results also concur with those of Kim, et al. [93], who reported that exogenous Si application significantly reduced SA biosynthesis in rice plants under heavy metal stress. Under both normal and stress conditions, the synthesis of SA is mediated by two pathways: the phenylalanine ammonia-lyase (PAL) pathway and the isochorismate (IC) pathway. The IC pathway is the major pathway in Solanum lycopersicum and Nicotiana benthamiana [114]. We found similar results at the mRNA level; SA biosynthesis-related genes (SlR1b1, SlPR-P2, SlICS, and SlPAL) were downregulated in +Si plants, but upregulated under heat stress in –Si plants. However, more comprehensive investigations are required to describe the detailed molecular mechanisms underlying the complex roles of SA in abiotic stress tolerance.
Conclusion
This study showed that Si application to tomato seedlings increased the resilience and function of tomato plants under heat stress and induced stress tolerance by modulating oxidative stress, HSP, and endogenous phytohormones, as well as the related mRNA gene expression patterns. Si application reduced the heat-mediated oxidative stress through stimulation of the antioxidant defense mechanism and increased the concentration of photosynthetic pigments in the plant. Thus, using Si for broader field applications with the advent of current changes in global climatic conditions can be an eco-friendly approach to maintain crop growth and productivity.
Supplementary information
Additional file 1: Figure S1. Effects of 1 mM Si application on growth parameters of tomato plants grown under normal and heat stress conditions. (A) shoot fresh weight (B) root fresh weight.
Acknowledgments
The authors acknowledge the support of Ms. Saffia Al Amri and Amna Al Buriaki for assisting in harvesting and treatment of plants.
Abbreviations
- PAL
Phenylalanine ammonia-lyase
- MAPKs
Mitogen-activated protein kinases
- MDA
Malondialdehyde
- HSP
Heat shock proteins
- SA
Salicylic acid
- ABA
Abscisic acids
- ROS
Reactive oxygen species
- POD
Peroxidase
- CAT
Catalase
- SOD
Superoxide dismutase
- PPO
Polyphenol peroxidase
Authors’ contributions
AK, ALK, MN: Designed, planned and wrote the manuscript; MI, YHK, IJL: Performed endogenous phytohormonal analysis; SA, SB: Statistical analysis and graphical representation; ALK, AAH, AAR: Supervised and arranged funding. All authors have read and approved the manuscript.
Funding
The author acknowledges the financial support of University of Nizwa, Internal Funding Program to the corresponding author.
Availability of data and materials
All the data is available within the manuscript.
Ethics approval and consent to participate
No permissions were required to collect plant samples.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abdul Latif Khan, Email: latifepm78@yahoo.co.uk.
Ahmed Al-Harrasi, Email: aharrasi@unizwa.edu.om.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12870-020-02456-7.
References
- 1.Cooke J, Leishman MR. Consistent alleviation of abiotic stress with silicon addition: a meta-analysis. Funct Ecol. 2016;30(8):1340–1357. [Google Scholar]
- 2.Zandalinas SI, Mittler R, Balfagón D, Arbona V, Gómez-Cadenas A. Plant adaptations to the combination of drought and high temperatures. Physiol Plant. 2018;162(1):2–12. doi: 10.1111/ppl.12540. [DOI] [PubMed] [Google Scholar]
- 3.Tayade R, Nguyen T, Oh SA, Hwang YS, Yoon IS, Deshmuk R, Jung K-H, Park SK. Effective strategies for enhancing tolerance to high-temperature stress in Rice during the reproductive and ripening stages. Plant Breed Biotechnol. 2018;6(1):1–18. [Google Scholar]
- 4.Mittler R, Blumwald E. Genetic engineering for modern agriculture: challenges and perspectives. Annu Rev Plant Biol. 2010;61:443–462. doi: 10.1146/annurev-arplant-042809-112116. [DOI] [PubMed] [Google Scholar]
- 5.Hasanuzzaman M, Nahar K, Alam M, Roychowdhury R, Fujita M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci. 2013;14(5):9643–9684. doi: 10.3390/ijms14059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashraf M, Harris P. Abiotic Stresses. Boca Raton: CRC Press; 2005. 10.1201/9781482293609.
- 7.Baxter A, Mittler R, Suzuki N. ROS as key players in plant stress signalling. J Exp Bot. 2013;65(5):1229–1240. doi: 10.1093/jxb/ert375. [DOI] [PubMed] [Google Scholar]
- 8.Fragkostefanakis S, Mesihovic A, Simm S, Paupière MJ, Hu Y, Paul P, Mishra SK, Tschiersch B, Theres K, Bovy A. HsfA2 controls the activity of developmentally and stress-regulated heat stress protection mechanisms in tomato male reproductive tissues. Plant Physiol. 2016;170(4):2461–2477. doi: 10.1104/pp.15.01913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maestri E, Klueva N, Perrotta C, Gulli M, Nguyen HT, Marmiroli N. Molecular genetics of heat tolerance and heat shock proteins in cereals. Plant Mol Biol. 2002;48(5–6):667–681. doi: 10.1023/a:1014826730024. [DOI] [PubMed] [Google Scholar]
- 10.Wasternack C, Stenzel I, Hause B, Hause G, Kutter C, Maucher H, Neumerkel J, Feussner I, Miersch O. The wound response in tomato–role of jasmonic acid. J Plant Physiol. 2006;163(3):297–306. doi: 10.1016/j.jplph.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Zarate SI, Kempema LA, Walling LL. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007;143(2):866–875. doi: 10.1104/pp.106.090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Ahammed G, Zhang Y, Zhang G, Sun Z, Zhou J, Zhou Y, Xia X, Yu J, Shi K. Carbon dioxide enrichment alleviates heat stress by improving cellular redox homeostasis through an ABA-independent process in tomato plants. Plant Biol. 2015;17(1):81–89. doi: 10.1111/plb.12211. [DOI] [PubMed] [Google Scholar]
- 13.Marschner H. Marschner, Mineral Nutrition of Higher Plants. London: Academic; 1995. p. 889. [Google Scholar]
- 14.Yamaji N, Ma JF. Spatial distribution and temporal variation of the Rice silicon transporter Lsi1. Plant Physiol. 2007;143(3):1306–1313. doi: 10.1104/pp.106.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y-H, Khan AL, Lee I-J. Silicon: a duo synergy for regulating crop growth and hormonal signaling under abiotic stress conditions. Crit Rev Biotechnol. 2016;36(6):1099–1109. doi: 10.3109/07388551.2015.1084265. [DOI] [PubMed] [Google Scholar]
- 16.Epstein E. Silicon. Annu Rev Plant Biol. 1999;50(1):641–664. doi: 10.1146/annurev.arplant.50.1.641. [DOI] [PubMed] [Google Scholar]
- 17.Ma JF, Takahashi E. Chapter 3 - Silicon in soil. In: Ma JF, Takahashi E, Editors. Soil, Fertilizer, and Plant Silicon Research in Japan. Amsterdam: Elsevier Science; 2002. p. 27-48. 10.1016/B978-044451166-9/50003-8.
- 18.Ma JF, Yamaji N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006;11(8):392–397. doi: 10.1016/j.tplants.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y, Gong H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron Sustain Dev. 2014;34(2):455–472. [Google Scholar]
- 20.Rizwan M, Ali S, Ibrahim M, Farid M, Adrees M, Bharwana SA, Zia-ur-Rehman M, Qayyum MF, Abbas F. Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: a review. Environ Sci Pollut Res. 2015;22(20):15416–15431. doi: 10.1007/s11356-015-5305-x. [DOI] [PubMed] [Google Scholar]
- 21.Ma JF. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci Plant Nutr. 2004;50(1):11–18. [Google Scholar]
- 22.Marafon AC, Endres L. Embrapa Tabuleiros Costeiros-Artigo em periódico indexado (ALICE) 2013. Silicon: fertilization and nutrition in higher plants. [Google Scholar]
- 23.Shen X, Xiao X, Dong Z, Chen Y. Silicon effects on antioxidative enzymes and lipid peroxidation in leaves and roots of peanut under aluminum stress. Acta Physiol Plant. 2014;36(11):3063–3069. [Google Scholar]
- 24.Pontigo S, Ribera A, Gianfreda L, de la Luz MM, Nikolic M, Cartes P. Silicon in vascular plants: uptake, transport and its influence on mineral stress under acidic conditions. Planta. 2015;242(1):23–37. doi: 10.1007/s00425-015-2333-1. [DOI] [PubMed] [Google Scholar]
- 25.Galvez L, Clark RB. Effects of silicon on growth and mineral composition of sorghum (Sorghum bicolor) grown with toxic levels of aluminium. In: Wright RJ, Baligar VC, Murrmann RP, Editors. Plant-Soil Interactions at Low pH. Developments in Plant and Soil Sciences. vol 45. Dordrecht: Springer; 1991. 10.1007/978-94-011-3438-5_92.
- 26.Pontigo S, Godoy K, Jiménez H, Gutiérrez-Moraga A, Mora MD, Cartes P. Silicon-mediated alleviation of aluminum toxicity by modulation of AL/SI uptake and antioxidant performance in ryegrass plants. Front Plant Sci. 2017;8:642. doi: 10.3389/fpls.2017.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Bockhaven J, De Vleesschauwer D, Höfte M. Towards establishing broad-spectrum disease resistance in plants: silicon leads the way. J Exp Bot. 2012;64(5):1281–1293. doi: 10.1093/jxb/ers329. [DOI] [PubMed] [Google Scholar]
- 28.Cotterill JV, Watkins RW, Brennon CB, Cowan DP. Boosting silica levels in wheat leaves reduces grazing by rabbits. Pest Manag Sci. 2007;63(3):247–253. doi: 10.1002/ps.1302. [DOI] [PubMed] [Google Scholar]
- 29.Kidd P, Llugany M, Poschenrieder C, Gunse B, Barcelo J. The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.) J Exp Bot. 2001;52(359):1339–1352. [PubMed] [Google Scholar]
- 30.Al-aghabary K, Zhu Z, Shi Q. Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. J Plant Nutr. 2005;27(12):2101–2115. [Google Scholar]
- 31.Shahnaz G, Shekoofeh E, Kourosh D, Moohamadbagher B. Interactive effects of silicon and aluminum on the malondialdehyde (MDA), proline, protein and phenolic compounds in Borago officinalis L. J Med Plants Res. 2011;5(24):5818–5827. [Google Scholar]
- 32.Tripathi D, Bashri G, Shweta S, Ahmad P, Singh V. Efficacy of silicon against aluminum toxicity in plants: an overview. Silicon Plants. 2017;1:355–366. [Google Scholar]
- 33.Kim Y-H, Khan AL, Waqas M, Lee I-J. Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: a review. Front Plant Sci. 2017;8:510. doi: 10.3389/fpls.2017.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sumanta N, Haque CI, Nishika J, Suprakash R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res J Chem Sci. 2014;2231:606X. [Google Scholar]
- 35.Cao Y-Y, Yang M-T, Chen S-Y, Zhou Z-Q, Li X, Wang X-J, Bai J-G. Exogenous sucrose influences antioxidant enzyme activities and reduces lipid peroxidation in water-stressed cucumber leaves. Biol Plant. 2015;59(1):147–153. [Google Scholar]
- 36.Bilal S, Shahzad R, Khan AL, Kang SM, Imran QM, Al-Harrasi A, Yun BW, Lee IJ. Endophytic microbial consortia of phytohormones-producing fungus Paecilomyces formosus LHL10 and bacteria Sphingomonas sp. LK11 to Glycine max L. regulates physio-hormonal changes to attenuate aluminum and zinc stresses. Frontiers Plant Sci. 2018;9:1273. doi: 10.3389/fpls.2018.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L, Han R, Yu N, Zhang W, Xing L, Xie D, Peng D. A method for extracting high-quality total RNA from plant rich in polysaccharides and polyphenols using Dendrobium huoshanense. PLoS One. 2018;13(5):e0196592. doi: 10.1371/journal.pone.0196592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C T method. Nat Protoc. 2008;3(6):1101. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 39.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 40.Gajewska E, Skłodowska M. Differential biochemical responses of wheat shoots and roots to nickel stress: antioxidative reactions and proline accumulation. Plant Growth Regul. 2008;54(2):179–188. [Google Scholar]
- 41.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 42.Kar M, Mishra D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 1976;57(2):315–319. doi: 10.1104/pp.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aebi H. Catalase in vitro. In: Methods in enzymology, vol. 105. Academic Press; 1984. p. 121–126. 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed]
- 44.Seskar M, Shulaev V, Raskin I. Endogenous methyl salicylate in pathogen-inoculated tobacco plants. Plant Physiol. 1998;116(1):387–392. [Google Scholar]
- 45.Shahzad R, Waqas M, Khan AL, Asaf S, Khan MA, Kang S-M, Yun B-W, Lee I-J. Seed-borne endophytic bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol Biochem. 2016;106:236–243. doi: 10.1016/j.plaphy.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Shahzad R, Khan AL, Waqas M, Ullah I, Bilal S, Kim Y-H, Asaf S, Kang S-M, Lee I-J. Metabolic and proteomic alteration in phytohormone-producing endophytic bacillus amyloliquefaciens RWL-1 during methanol utilization. Metabolomics. 2019;15(2):16. doi: 10.1007/s11306-018-1467-0. [DOI] [PubMed] [Google Scholar]
- 47.Jain M, Mathur G, Koul S, Sarin N. Ameliorative effects of proline on salt stress-induced lipid peroxidation in cell lines of groundnut (Arachis hypogaea L.) Plant Cell Rep. 2001;20(5):463–468. [Google Scholar]
- 48.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ayala A, Muñoz MF, Argüelles S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Medicine and Cellular Longevity. 2014;2014:360438. 10.1155/2014/360438. [DOI] [PMC free article] [PubMed]
- 50.Chaves M, Oliveira M. Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot. 2004;55(407):2365–2384. doi: 10.1093/jxb/erh269. [DOI] [PubMed] [Google Scholar]
- 51.Agarwal PK, Agarwal P, Reddy M, Sopory SK. Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep. 2006;25(12):1263–1274. doi: 10.1007/s00299-006-0204-8. [DOI] [PubMed] [Google Scholar]
- 52.Shinozaki K, Yamaguchi-Shinozaki K, Seki M. Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol. 2003;6(5):410–417. doi: 10.1016/s1369-5266(03)00092-x. [DOI] [PubMed] [Google Scholar]
- 53.Bartels D, Sunkar R. Drought and salt tolerance in plants. Crit Rev Plant Sci. 2005;24(1):23–58. [Google Scholar]
- 54.Du X, Zhao X, Li X, Guo C, Lu W, Gu J, Xiao K. Overexpression of TaSRK2C1, a wheat SNF1-related protein kinase 2 gene, increases tolerance to dehydration, salt, and low temperature in transgenic tobacco. Plant Mol Biol Report. 2013;31(4):810–821. [Google Scholar]
- 55.Xia X-J, Zhou Y-H, Shi K, Zhou J, Foyer CH, Yu J-Q. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J Exp Bot. 2015;66(10):2839–2856. doi: 10.1093/jxb/erv089. [DOI] [PubMed] [Google Scholar]
- 56.Dobrá J, Černý M, Štorchová H, Dobrev P, Skalák J, Jedelský PL, Lukšanová H, Gaudinová A, Pešek B, Malbeck J. The impact of heat stress targeting on the hormonal and transcriptomic response in Arabidopsis. Plant Sci. 2015;231:52–61. doi: 10.1016/j.plantsci.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 57.Zhou R, Yu X, Ottosen C-O, Rosenqvist E, Zhao L, Wang Y, Yu W, Zhao T, Wu Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017;17(1):24. doi: 10.1186/s12870-017-0974-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen J, Xu W, Velten J, Xin Z, Stout J. Characterization of maize inbred lines for drought and heat tolerance. J Soil Water Conserv. 2012;67(5):354–364. [Google Scholar]
- 59.Mishra KB, Iannacone R, Petrozza A, Mishra A, Armentano N, La Vecchia G, Trtílek M, Cellini F, Nedbal L. Engineered drought tolerance in tomato plants is reflected in chlorophyll fluorescence emission. Plant Sci. 2012;182:79–86. doi: 10.1016/j.plantsci.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 60.Patanè C. Leaf area index, leaf transpiration and stomatal conductance as affected by soil water deficit and VPD in processing tomato in semi arid Mediterranean climate. J Agron Crop Sci. 2011;197(3):165–176. [Google Scholar]
- 61.Abdelmageed A, Gruda N, Geyer B. Effect of high temperature and heat shock on tomato (Lycopersicon esculentum Mill) genotypes under controlled conditions. Göttingen: Deutscher Tropentag 2003; 2003. [Google Scholar]
- 62.Camejo D, Rodríguez P, Morales MA, Dell’Amico JM, Torrecillas A, Alarcón JJ. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J Plant Physiol. 2005;162(3):281–289. doi: 10.1016/j.jplph.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 63.Camejo D, Jiménez A, Alarcón JJ, Torres W, Gómez JM, Sevilla F. Changes in photosynthetic parameters and antioxidant activities following heat-shock treatment in tomato plants. Funct Plant Biol. 2006;33(2):177–187. doi: 10.1071/FP05067. [DOI] [PubMed] [Google Scholar]
- 64.Liu J-J, Lin S-H, Xu P-L, Wang X-J, Bai J-G. Effects of exogenous silicon on the activities of antioxidant enzymes and lipid peroxidation in chilling-stressed cucumber leaves. Agric Sci China. 2009;8(9):1075–1086. [Google Scholar]
- 65.Sivanesan I, Son M, Soundararajan P, Jeong B. Effect of silicon on growth and temperature stress tolerance of Nephrolepis exaltata’Corditas’. Korean J Horticult Sci Technol. 2014;32(2):142–148. [Google Scholar]
- 66.Agarie S, Hanaoka N, Ueno O, Miyazaki A, Kubota F, Agata W, Kaufman PB. Effects of silicon on tolerance to water deficit and heat stress in rice plants (Oryza sativa L.), monitored by electrolyte leakage. Plant Prod Sci. 1998;1(2):96–103. [Google Scholar]
- 67.Mahdieh M, Habibollahi N, Amirjani M, Abnosi M, Ghorbanpour M. Exogenous silicon nutrition ameliorates salt-induced stress by improving growth and efficiency of PSII in Oryza sativa L. cultivars. J Soil Sci Plant Nutr. 2015;15(4):1050–1060. [Google Scholar]
- 68.Abbas T, Sattar A, Ijaz M, Aatif M, Khalid S, Sher A. Exogenous silicon application alleviates salt stress in okra. Hortic Environ Biotechnol. 2017;58(4):342–349. [Google Scholar]
- 69.Chen D, Chen D, Xue R, Long J, Lin X, Lin Y, Jia L, Zeng R, Song Y. Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J Hazard Mater. 2019;367:447–455. doi: 10.1016/j.jhazmat.2018.12.111. [DOI] [PubMed] [Google Scholar]
- 70.Naz N, Durrani F, Shah Z, Khan N, Ullah I. Influence of heat stress on growth and physiological activities of potato (Solanum tuberosum L.) Phyton Int J Exp Botany. 2018;87:225–230. [Google Scholar]
- 71.Wang L-J, Fan L, Loescher W, Duan W, Liu G-J, Cheng J-S, Luo H-B, Li S-H. Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol. 2010;10(1):34. doi: 10.1186/1471-2229-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chalanika De Silva HC. Asaeda T: effects of heat stress on growth, photosynthetic pigments, oxidative damage and competitive capacity of three submerged macrophytes. J Plant Interact. 2017;12(1):228–236. [Google Scholar]
- 73.Li X, Wei J-P, Scott E, Liu J-W, Guo S, Li Y, Zhang L, Han W-Y. Exogenous melatonin alleviates cold stress by promoting antioxidant defense and redox homeostasis in Camellia sinensis L. Molecules. 2018;23(1):165. doi: 10.3390/molecules23010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muneer S, Park YG, Kim S, Jeong BR. Foliar or subirrigation silicon supply mitigates high temperature stress in strawberry by maintaining photosynthetic and stress-responsive proteins. J Plant Growth Regul. 2017;36(4):836–845. [Google Scholar]
- 75.Wang S, Liu P, Chen D, Yin L, Li H, Deng X. Silicon enhanced salt tolerance by improving the root water uptake and decreasing the ion toxicity in cucumber. Front Plant Sci. 2015;6:759. doi: 10.3389/fpls.2015.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi Y, Zhang Y, Han W, Feng R, Hu Y, Guo J, Gong H. Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front Plant Sci. 2016;7:196. doi: 10.3389/fpls.2016.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ali N, Schwarzenberg A, Yvin J-C, Hosseini SA. Regulatory role of silicon in mediating differential stress tolerance responses in two contrasting tomato genotypes under osmotic stress. Front Plant Sci. 2018;9:1475. doi: 10.3389/fpls.2018.01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Merewitz EB, Liu S. Improvement in heat tolerance of creeping Bentgrass with melatonin, Rutin, and silicon. J Am Soc Hortic Sci. 2019;144(2):141–148. [Google Scholar]
- 79.Hussain I, Parveen A, Rasheed R, Ashraf MA, Ibrahim M, Riaz S, Afzaal Z, Iqbal M. Exogenous Silicon Modulates Growth, Physio-Chemicals and Antioxidants in Barley (Hordeum vulgare L.) Exposed to Different Temperature Regimes. Silicon. 2019;11:2753–2762. [Google Scholar]
- 80.Gunes A, Pilbeam DJ, Inal A, Bagci EG, Coban S. Influence of silicon on antioxidant mechanisms and lipid peroxidation in chickpea (Cicer arietinum L.) cultivars under drought stress. J Plant Interact. 2007;2(2):105–113. [Google Scholar]
- 81.Huang F, Wen X-H, Cai Y-X, Cai K-Z. Silicon-mediated enhancement of heavy metal tolerance in Rice at different growth stages. Int J Environ Res Public Health. 2018;15(10):2193. doi: 10.3390/ijerph15102193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. Journal of Botany. 2012;2012:217037. 10.1155/2012/217037.
- 83.Kim YH, Khan AL, Waqas M, Shim JK, Kim DH, Lee KY, Lee IJ. Silicon application to rice root zone influenced the phytohormonal and antioxidant responses under salinity stress. J Plant Growth Regul. 2014;33(2):137–149. [Google Scholar]
- 84.Zhang F-Q, Wang Y-S, Lou Z-P, Dong J-D. Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza) Chemosphere. 2007;67(1):44–50. doi: 10.1016/j.chemosphere.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 85.Zandalinas SI, Balfagón D, Arbona V, Gómez-Cadenas A. Modulation of antioxidant defense system is associated with combined drought and heat stress tolerance in citrus. Front Plant Sci. 2017;8:953. doi: 10.3389/fpls.2017.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu Z, Wei G, Li J, Qian Q, Yu J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.) Plant Sci. 2004;167(3):527–533. [Google Scholar]
- 87.Soundararajan P, Manivannan A, Park YG, Muneer S, Jeong BR. Silicon alleviates salt stress by modulating antioxidant enzyme activities in Dianthus caryophyllus ‘Tula’. Hortic Environ Biotechnol. 2015;56(2):233–239. [Google Scholar]
- 88.Sahebi M, Hanafi MM, Rafii MY, Azizi P, Abiri R, Kalhori N, Atabaki N. Screening and Expression of a Silicon Transporter Gene (Lsi1) in Wild-Type Indica Rice Cultivars. BioMed Research International. 2017;2017:9064129. 10.1155/2017/9064129. [DOI] [PMC free article] [PubMed]
- 89.Alberto CM, Fontão de Lima Filho O, Manuel JC, Gabriela SK, Lia MM, Mui ST. Assessment of the effect of silicon on antioxidant enzymes in cotton plants by multivariate analysis. J Agric Food Chem. 2013;61(47):11243–11249. doi: 10.1021/jf4039088. [DOI] [PubMed] [Google Scholar]
- 90.Ma D, Sun D, Wang C, Qin H, Ding H, Li Y, Guo T. Silicon application alleviates drought stress in wheat through transcriptional regulation of multiple antioxidant defense pathways. J Plant Growth Regul. 2016;35(1):1–10. [Google Scholar]
- 91.Luan S, Lan W, Lee SC. Potassium nutrition, sodium toxicity, and calcium signaling: connections through the CBL–CIPK network. Curr Opin Plant Biol. 2009;12(3):339–346. doi: 10.1016/j.pbi.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 92.Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M. An efflux transporter of silicon in rice. Nature. 2007;448(7150):209. doi: 10.1038/nature05964. [DOI] [PubMed] [Google Scholar]
- 93.Kim Y-H, Khan AL, Kim D-H, Lee S-Y, Kim K-M, Waqas M, Jung H-Y, Shin J-H, Kim J-G, Lee I-J. Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa low silicon genes, and endogenous phytohormones. BMC Plant Biol. 2014;14(1):13. doi: 10.1186/1471-2229-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guo M, Liu J-H, Ma X, Luo D-X, Gong Z-H, Lu M-H. The plant heat stress transcription factors (HSFs): structure, regulation, and function in response to abiotic stresses. Front Plant Sci. 2016;7:114. doi: 10.3389/fpls.2016.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fragkostefanakis S, Roeth S, Schleiff E, SCHARF KD. Prospects of engineering thermotolerance in crops through modulation of heat stress transcription factor and heat shock protein networks. Plant Cell Environ. 2015;38(9):1881–1895. doi: 10.1111/pce.12396. [DOI] [PubMed] [Google Scholar]
- 96.Scharf K-D, Berberich T, Ebersberger I, Nover L. The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochimica et Biophysica Acta. 2012;1819(2):104–119. doi: 10.1016/j.bbagrm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 97.Liu HC, Liao HT, Charng YY. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ. 2011;34(5):738–751. doi: 10.1111/j.1365-3040.2011.02278.x. [DOI] [PubMed] [Google Scholar]
- 98.Yoshida T, Ohama N, Nakajima J, Kidokoro S, Mizoi J, Nakashima K, Maruyama K, Kim J-M, Seki M, Todaka D. Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shock-responsive gene expression. Mol Gen Genomics. 2011;286(5–6):321–332. doi: 10.1007/s00438-011-0647-7. [DOI] [PubMed] [Google Scholar]
- 99.Bharti K, von Koskull-Döring P, Bharti S, Kumar P, Tintschl-Körbitzer A, Treuter E, Nover L. Tomato heat stress transcription factor HsfB1 represents a novel type of general transcription coactivator with a histone-like motif interacting with the plant CREB binding protein ortholog HAC1. Plant Cell. 2004;16(6):1521–1535. doi: 10.1105/tpc.019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hahn A, Bublak D, Schleiff E, Scharf K-D. Crosstalk between Hsp90 and Hsp70 chaperones and heat stress transcription factors in tomato. Plant Cell. 2011;23(2):741–755. doi: 10.1105/tpc.110.076018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scharf K-D, Heider H, Höhfeld I, Lyck R, Schmidt E, Nover L. The tomato Hsf system: HsfA2 needs interaction with HsfA1 for efficient nuclear import and may be localized in cytoplasmic heat stress granules. Mol Cell Biol. 1998;18(4):2240–2251. doi: 10.1128/mcb.18.4.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang L, Zhao R, Li R, Yu W, Yang M, Sheng J, Shen L. Enhanced drought tolerance in tomato plants by overexpression of SlMAPK1. Plant Cell Tissue Org Cult. 2018;133(1):27–38. [Google Scholar]
- 103.Colcombet J, Hirt H. Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. Biochem J. 2008;413(2):217–226. doi: 10.1042/BJ20080625. [DOI] [PubMed] [Google Scholar]
- 104.Mishra NS, Tuteja R, Tuteja N. Signaling through MAP kinase networks in plants. Arch Biochem Biophys. 2006;452(1):55–68. doi: 10.1016/j.abb.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 105.Acharya BR, Assmann SM. Hormone interactions in stomatal function. Plant Mol Biol. 2009;69(4):451–462. doi: 10.1007/s11103-008-9427-0. [DOI] [PubMed] [Google Scholar]
- 106.Yoshida T, Obata T, Feil R, Lunn JE, Fujita Y, Yamaguchi-Shinozaki K, Fernie AR. The role of Abscisic acid signaling in maintaining the metabolic balance required for Arabidopsis growth under nonstress conditions. Plant Cell. 2019;31(1):84–105. doi: 10.1105/tpc.18.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sah SK, Reddy KR, Li J. Abscisic acid and abiotic stress tolerance in crop plants. Front Plant Sci. 2016;7:571. doi: 10.3389/fpls.2016.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Larkindale J, Knight MR. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002;128(2):682–695. doi: 10.1104/pp.010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Larkindale J, Hall JD, Knight MR, Vierling E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005;138(2):882–897. doi: 10.1104/pp.105.062257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hara M, Furukawa J, Sato A, Mizoguchi T, Miura K. Abiotic Stress and Role of Salicylic Acid in Plants. In: Ahmad P, Prasad MNV, Editors. Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability. New York: Springer; 2012. .
- 111.Vlot AC, Dempsey DMA, Klessig DF. Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol. 2009;47:177–206. doi: 10.1146/annurev.phyto.050908.135202. [DOI] [PubMed] [Google Scholar]
- 112.Davies PJ. Plant Hormones Physiology, Biochemistry and Molecular Biology. Dordrecht: Kluwer Academic; 1995. p 1–12.
- 113.Li S, Zhou X, Chen L, Huang W, Yu D. Functional characterization of Arabidopsis thaliana WRKY39 in heat stress. Mol Cells. 2010;29(5):475–483. doi: 10.1007/s10059-010-0059-2. [DOI] [PubMed] [Google Scholar]
- 114.Dempsey DM, Vlot AC, Wildermuth MC, Klessig DF. The Arabidopsis book/American Society of Plant Biologists. 2011. Salicylic acid biosynthesis and metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Effects of 1 mM Si application on growth parameters of tomato plants grown under normal and heat stress conditions. (A) shoot fresh weight (B) root fresh weight.
Data Availability Statement
All the data is available within the manuscript.