Abstract
Background
Persistent bronchodilator response (BDR) following diagnosis of asthma is an underrecognized treatable trait, associated with worse lung function and asthma control. The forced oscillation technique (FOT) measures respiratory system impedance, and BDR cutoffs have been proposed for healthy adults; however, the relevance in asthma is unknown. We compared BDR cutoffs, using FOT and spirometry, in asthma and the relationship with asthma control.
Methods
Data from patients with asthma who withheld bronchodilator medication for at least 8 h before a tertiary airway clinic visit were reviewed. All subjects performed FOT and spirometry before and after salbutamol administration, and completed the Asthma Control Test. FOT parameters examined included respiratory system resistance (R5) and reactance (X5) at 5 Hz, and area under the reactance curve (AX). BDR was defined by standard recommendations for spirometry and based on the 95th percentile of BDR in healthy adults for FOT.
Results
Fifty-two subjects (18 men; mean age, 53 ± 18 years) were included. BDR was identified more frequently by FOT than spirometry (54% vs 27% of subjects). BDR assessed by X5 and AX, but not R5, was associated with spirometric BDR (χ2, P < .01) and correlated with asthma control (X5: rs = –0.36, P < .01; AX: rs = 0.34, P = .01). BDR measured by reactance parameters identified more subjects with poor asthma control than did spirometry (AX, 69% vs spirometry, 41%).
Conclusions
BDR assessed by FOT can identify poor asthma control. Reactance parameters were more sensitive in identifying poor asthma control than spirometry, supporting the use of FOT to complement spirometry in the clinical management of asthma.
Key Words: asthma control, bronchodilator response, forced oscillation technique, symptoms
Abbreviations: ACT, Asthma Control Test; AX, area under the reactance curve, between 5 Hz and resonant frequency; BDR, bronchodilator response; FOT, forced oscillation technique; ICS, inhaled corticosteroid; R5, resistance at 5 Hz; X5, reactance at 5 Hz
Variable expiratory airflow limitation is the hallmark of asthma. The presence of a bronchodilator response (BDR) is useful in establishing the diagnosis of asthma; however, its role in subsequent asthma management is less clear. BDR is associated with more impaired spirometry (lower FEV1 and/or FEV1/FVC) and loss of asthma control in the absence of antiinflammatory use, but also predicts greater spirometric and symptomatic response to antiinflammatory treatment in untreated asthma.1, 2, 3 A persistent BDR despite antiinflammatory treatment has also been associated with greater inhaled corticosteroid doses, lower FEV1, worse asthma control, higher exacerbation rates, and increased mortality.4, 5, 6, 7 These findings suggest that ongoing BDR may be an important but underrecognized therapeutic target in asthma.
The importance of treatable traits and personalized medicine is increasingly discussed in asthma management. This approach has largely focused on inflammatory biomarkers and cellular targets. In this regard, the advent of targeted monoclonal antibody therapy has transformed the treatment for some patients with severe asthma. Yet, there is an unmet need for patients with asthma of any severity to accurately predict the therapeutic intervention that will most likely benefit those individuals. Identifying particular physiologic treatable traits that are easily detected and monitored, such as BDR, could help with the long-term treatment of patients and facilitate the success of precision medicine. Although asthma guidelines emphasize the importance of longitudinal monitoring of lung function and asthma symptom control,8 lung function is often limited to spirometry (FEV1) and commonly discordant with reported symptoms.9 Alternative measures of lung function, such as oscillometry, may provide supplementary and clinically relevant information about BDR in asthma.
The forced oscillation technique (FOT) noninvasively measures respiratory system impedance by the superimposition of oscillatory pressure/flow waves at the mouth during normal tidal breathing. The relationship between pressure and flow is partitioned into respiratory system resistance (R), which is a measure of airway caliber; and reactance (X), which represents the elastic and inertive properties of the respiratory system. FOT has greater sensitivity to detect BDR than does spirometry.10, 11, 12, 13, 14, 15 Pediatric studies have shown that BDR assessed by FOT relates to the diagnosis of asthma independent of spirometry and is associated with lower FEV1 and increased resistance.11, 12, 13,16,17 Furthermore, FOT may better relate to asthma control than spirometry,15 although this relationship has not been evaluated for BDR. Oostveen et al18 published normative values for FOT in adults and proposed cutoffs for a significant BDR for FOT, based on the 95th percentile of BDR in healthy adults. The clinical relevance of these cutoffs is yet to be evaluated or compared with spirometry in airway disease.
The use of FOT in clinical practice has increased, partly facilitated by the advent of commercial devices. Consequently, there is a need for better understanding of the relationship between FOT, spirometry, and symptom control in asthma. We hypothesized that BDR cutoffs for FOT can be used in asthma and are sensitive in identifying poor asthma control.
Materials and Methods
Subject Characteristics and Study Design
Data from patients who attended a tertiary adult airway clinic from 2015 to 2017 with a consensus diagnosis of asthma confirmed by two respiratory physicians were reviewed.8 Subjects were eligible if all bronchodilator medications were withheld for at least 8 h before testing and all study assessments were successfully completed in a single visit. Exclusion criteria included coexisting respiratory disease, inability to complete the study questionnaire because of cognitive impairment or language barrier, and lung function measurements not performed to international recommendations. Subject demographics and smoking history were documented. This study was approved by the Sydney Local Health District Human Ethics Review Board (LNR/14/CRGH/206, NSW, Australia).
Lung function testing was performed before and at least 10 min after the administration of bronchodilator (400 μg of salbutamol delivered by metered dose inhaler and spacer).
Forced Oscillation Technique
FOT was performed during tidal breathing (tremoFlo C-100; Thorasys Thoracic Medical Systems) as per European Respiratory Society (ERS) recommendations.19 FOT was performed immediately before spirometry, both before and after bronchodilator administration. The mean of three 30-s measurements before and after bronchodilator administration (tremoFlo software build 1.0.40.38) was recorded. Acceptability included at least three breaths free from artifact due to occlusion, leak or drift, or extreme (> 5 SD of mean) or negative resistance.19 Reference values and BDR cutoffs were derived from Oostveen et al.18 BDR was defined as an absolute change in R5 (respiratory system resistance at 5 Hz) ≥ –1.40 cmH2O·s/L, X5 (respiratory system reactance at 5 Hz) ≥ cmH2O·s/L, or AX (area under the reactance curve, between 5 Hz and resonant frequency) ≥ –3.98 cmH2O/L after bronchodilator administration.
Spirometry
Spirometry (Masterlab; Jaeger) was performed immediately after FOT, before and after bronchodilator administration, as per American Thoracic Society (ATS)/ERS recommendations.20 Reference values were derived from the Global Lung Initiative.21 BDR was defined as ≥ 200 mL and ≥ 12% improvement in FEV1 and/or FVC after bronchodilator administration as per ATS/ERS criteria.22
Asthma Control
Asthma-related symptoms were assessed with the five-item Asthma Control Test (ACT).23 Asthma control was categorized as either well controlled (ACT score ≥ 20), not well controlled (ACT score 16-19), or very poorly controlled (ACT score ≤ 15).23,24
Statistical Analysis
Statistical analysis was performed with SPSS Statistics version 25 (IBM Corporation) and graphs were prepared with GraphPad Prism 7 (GraphPad Software Inc). Cohen’s κ was used to assess agreement between BDR measured by FOT and spirometry. Cross-tabulation using two-by-two tables was done to compare BDR as identified by FOT vs spirometry, and poor asthma control. Association between categorical variables was assessed by χ2 tests. Univariate correlations were assessed using Spearman correlations. A P value < .05 was considered statistically significant.
Results
Fifty-two subjects met eligibility criteria. Subject demographics and lung function are shown in Tables 1 and 2. There was a spectrum of airflow limitation and symptom burden. Only 20 of 52 subjects (38%) reported good symptom control on current treatment. The majority of subjects were never smokers and there were no current smokers (ex-smokers, 15 of 52; 29%). Most subjects were prescribed both inhaled corticosteroid (ICS) (81%) and a long-acting β-agonist (77%).
Table 1.
Subject Characteristics
| Characteristic | Value |
|---|---|
| Subjects, No. | 52 |
| Sex, male/female | 18/34 |
| Age, y | 53 ± 18 |
| BMI, kg/m2 | 28.6 ± 6.8 |
| Smoking, pack-years | 5 ± 10 |
| Budesonide equivalent, μg/d | 786 |
| ICS, % | 81 |
| LABA, % | 77 |
| LAMA, % | 10 |
| ACT score (5-25) | 17 ± 6 |
| Well/not well/very poorly controlled, No. | 20/16/16 |
Data are presented as mean ± SD, unless otherwise specified. ACT = Asthma Control Test; ICS = inhaled corticosteroid; LABA = long-acting bronchodilator; LAMA = long-acting muscarinic antagonist.
Table 2.
Lung Function and Bronchodilator Response According to Spirometry and Forced Oscillation Technique
| Prebronchodilator | Postbronchodilator | Subjects With Significant BDRa | |
|---|---|---|---|
| Spirometry | |||
| FEV1, L | 2.04 ± 0.80 | 2.20 ± 0.83 | 13 (25%)b |
| FEV1, % predicted | 70 ± 19 | 75 ± 19 | |
| FVC, L | 3.01 ± 0.96 | 3.18 ± 0.98 | 6 (12%)b |
| FVC, % predicted | 86 ± 17 | 89 ± 18 | |
| FEV1/FVC, % | 66 ± 13 | 69 ± 13 | |
| FOT | |||
| R5, cmH2O·s/L | 5.50 ± 1.80 | 4.89 ± 1.87 | 10 (19%) |
| R5, % predicted | 164 ± 66 | 143 ± 62 | |
| X5, cmH2O·s/L | –3.59 ± 2.79 | –2.76 ± 2.15 | 21 (40%) |
| X5, % predicted | 270 ± 182 | 203 ± 133 | |
| AX, cmH2O/L | 35.67 ± 33.10 | 24.75 ± 26.24 | 27 (52%) |
| AX, % predicted | 971 ± 978 | 665 ± 820 |
Data are presented as mean ± SD, unless otherwise specified. AX = area under the reactance curve between 5 Hz and the resonant frequency; BDR = bronchodilator response; FOT = forced oscillation technique; R5 = resistance at 5 Hz; X5 = reactance at 5 Hz.
Of 52 subjects who met eligibility criteria.
Spirometric BDR defined as ≥ 200 mL and ≥ 12% improvement in FEV1 and/or FVC.
FOT and Spirometric BDR
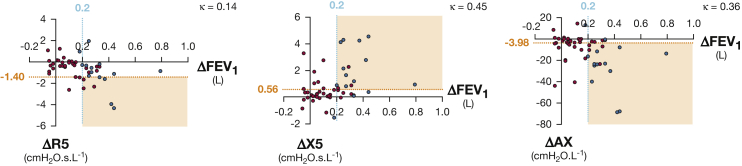
Subjects with spirometric BDR predominantly had a bronchodilator response in FEV1 (with or without a bronchodilator response in FVC). Only one subject had spirometric BDR based on a response in FVC alone. BDR was identified more frequently by FOT (28 of 52, 54%) than spirometry (14 of 52, 27%) (Tables 2 and 3, Fig 1). An additional 15 subjects met BDR criteria by reactance parameters X5 and AX but not spirometry. Only two subjects met BDR criteria by spirometry but not FOT. BDR as assessed by X5 (χ2 = 11.6, P = .001) and AX (χ2 = 8.8, P = .003) was associated with BDR on spirometry. There was weak to moderate agreement between BDR measured by spirometry vs X5 (κ = 0.45) and AX (κ = 0.36) (Fig 1). There was no association or agreement between BDR assessed using R5 and spirometry (P = .3).
Table 3.
Bronchodilator Response Assessed by Forced Oscillation Technique Parameters as Compared With Spirometry
| BDR Assessed by Spirometrya |
|||
|---|---|---|---|
| Present | Absent | ||
| BDR assessed by R5: | Present | 4 | 6 |
| Absent | 10 | 32 | |
| BDR Assessed by Spirometrya |
|||
|---|---|---|---|
| Present | Absent | ||
| BDR assessed by X5: | Present | 11 | 10 |
| Absent | 3 | 28 | |
| BDR Assessed by Spirometrya |
|||
|---|---|---|---|
| Present | Absent | ||
| BDR assessed by AX: | Present | 12 | 15 |
| Absent | 2 | 23 | |
Data presented indicate the number of subjects by cross-tabulation. See Table 2 legend for expansion of abbreviations.
Spirometric BDR defined as ≥ 200 mL and ≥ 12% improvement in FEV1 and/or FVC.
Figure 1.
Comparison between bronchodilator response (BDR) identified by spirometry and forced oscillation technique (FOT). Shown are the following: cutoff for identifying BDR by spirometry (blue line) and FOT (orange line); subjects with (blue circles) or without (red circles) BDR in FEV1 and/or FVC as assessed by spirometry; region of BDR concordance between spirometry and FOT (orange-shaded areas); Cohen’s κ; change in parameter after bronchodilator administration (postbronchodilator minus prebronchodilator) (Δ). Spirometric BDR is defined as ≥ 200 mL and ≥ 12% improvement in FEV1 and/or FVC.
BDR and Asthma Control
The ACT score correlated with BDR measured by X5 (rs = –0.36, P < .01) and AX (rs = 0.34, P = .01) but not R5 or spirometry (Fig 2). When categorized according to asthma control, poor symptom control (ACT < 20) was associated with BDR assessed by spirometry (χ2 = 7.94, P = .005), X5 (χ2 = 8.70, P < .003), and AX (χ2 = 9.44, P = .002) but not R5 (P = .271). There was weak to moderate agreement between poor asthma control and BDR measured by spirometry (κ = 0.31), X5 (κ = 0.37), and AX (κ = 0.42) but not R5 (P = .2). BDR measured by X5 and AX identified an additional 10 subjects with poor asthma control not identified by spirometry. In comparison, spirometry identified only one subject with poor asthma control not detected by FOT. BDR assessed by AX had the greatest sensitivity to detect poor asthma control (22 subjects; sensitivity, 69%; specificity, 75%), followed by X5 (18 subjects; sensitivity, 56%; specificity, 85%) and then spirometry (13 subjects; sensitivity, 41%; specificity, 95%). All subjects with BDR in FVC had poor asthma control.
Figure 2.
Association between the presence of BDR and asthma control. Shown are the following: cutoff for poor asthma control (Asthma Control Test score < 20) (blue line); cutoff for BDR by spirometry or FOT (orange line); subjects with spirometric BDR (blue circles) or no spirometric BDR (red circles) in FEV1 and/or FVC; region of concordance between BDR and poor asthma control (orange-shaded areas); Spearman’s correlation (rs); change in parameter after bronchodilator administration (postbronchodilator minus prebronchodilator) (Δ). Spirometric BDR is defined as ≥ 200 mL and ≥ 12% improvement in FEV1 and/or FVC. ACT = Asthma Control Test; AX = area under the reactance curve, between 5 Hz and resonant frequency; R5 = resistance at 5 Hz; X5 = reactance at 5 Hz. See Figure 1 legend for expansion of other abbreviations.
Discussion
The results from this study show that the bronchodilator response measured by spirometry and FOT is clinically relevant and identifies poor asthma control. The BDR cutoffs previously published for FOT, using normative data, can be used in patients with asthma. Importantly, we demonstrate that BDR is associated with worse asthma control. The presence of BDR as determined by FOT can identify more patients with poorly controlled asthma when compared with spirometry.
The finding in the present study that BDR assessed by FOT is related to poor symptom control is consistent with similar associations with spirometry; in addition, a correlation between the magnitude of BDR and worse asthma symptoms was observed. The importance of identifying and monitoring BDR in the long-term follow-up of patients with asthma has not been extensively studied. Although the presence of BDR at the initial assessment is recognized as a known feature of asthma, it has been assumed that persisting BDR despite treatment implies more severe or suboptimal treatment of the disease. One study found that FEV1 reversibility was associated with worse asthma control in a large group of patients attending for outpatient assessment.25 The patients in that study were younger, with more preserved spirometry, when compared with the current cohort. Our findings again confirm the association between BDR assessed by spirometry and poor symptom control in a group of patients with more severe asthma. The ongoing presence of BDR may be another treatable trait in asthma and may be a possible indication for escalation of antiinflammatory or long-acting bronchodilator treatments.
The current results also indicate that reactance measured by FOT is a clinically important parameter when assessing BDR in asthma. Normative data for FOT have been previously published along with suggested cutoffs for BDR.18 However, these cutoffs have not been studied in patients with airway disease. Specifically, the most clinically relevant FOT parameter (or combination thereof) is uncertain in the BDR assessment of patients. The subjective nature of patient-reported outcomes, despite the use of validated questionnaires, would suggest that it is highly unlikely that a single physiologic parameter can account for a significant proportion of patient symptoms. The results from this study indicate that resistance alone relates poorly to symptom control. In comparison, the results of BDR assessment by reactance parameters were more sensitive than those derived by spirometry, identifying 17% more subjects with poor asthma control and supporting previous findings.26 As often observed, the improved sensitivity of BDR assessed by reactance parameters was associated with reduced specificity compared with spirometry. Since the identification of patients with poor asthma control is a clinical priority in the management of asthma, the tradeoff of improved sensitivity with a higher false positive rate is reasonable and may be offset by the combined use of FOT, spirometry, and clinical assessment.
Improvement in lung function after the administration of an inhaled bronchodilator may potentially be due to several mechanisms including a reduction in bronchomotor tone and greater airway caliber, less small-airway closure, enhanced clearance of mucus, and an improvement in the extent of ventilation distribution.27 Although BDR assessed by spirometry or FOT (reactance) were similarly associated with poor asthma control, only BDR assessed by reactance related to the degree of asthma control and identified additional patients with poor control. Reactance measured by FOT is affected by a number of complex interactions in the lungs, including the properties of the airways as well as communicating lung volume and ventilation heterogeneity28,29; the latter are a reflection of the distribution of parallel lung units available to the oscillatory signal.29 The BDR in reactance may therefore reflect the opening up of small airways and their associated lung units to the oscillatory signal, and it is these changes to the small airways that may be specifically related to the degree of asthma control. Clearly, the spirometric BDR also reflects these mechanistic changes to the extent that they affect flow in the more central larger airways, and as such has traditionally been the “gold standard” in routine clinical practice. The peripheral airways, on the other hand, contribute very little to bulk flow measured at the mouth, partly due to the significantly larger cumulative cross-sectional area when compared with the central airways. As a consequence, spirometry is less sensitive in detecting more peripheral airway changes that may be reflected only by parameters such as reactance,30 and this potentially accounts for the stronger association seen with reactance parameters and asthma control.
The current study has some limitations. Subjects were attending a tertiary airway clinic, and thus may represent an asthma cohort with more impaired lung function, greater symptom burden, and more difficult-to-control asthma than those treated in the community setting. Nevertheless, the proportion of subjects with BDR was similar to that described in the published literature. Further studies are required to elucidate if these findings translate to patients with milder disease, those not receiving ICS, or patients with asthma who currently smoke. The limited exclusion criteria may also broaden clinical applicability. In particular, subjects with asthma and a smoking history were not excluded, nor did they present as data outliers. The relationship between BDR and symptoms, even though most subjects were already receiving ICS, is of clinical relevance and highlights the importance of this phenomenon when reviewing patients with asthma in the clinic. This study presents a relatively small sample size; however, the cohort was well characterized and encompassed a range of asthma severity and symptom burden. To the authors’ knowledge this study presents the largest adult cohort to report on BDR assessed by FOT in combination with symptom scores in a real-world population. As such, these results are clinically relevant and may improve outcomes especially in a difficult-to-treat patient cohort with significant symptom burden.
Conclusions
We have established that the published BDR cutoffs for FOT, using normative data, are useful in the assessment of asthma and relate to asthma symptom control. More importantly, the results of BDR assessment based on FOT reactance parameters were more sensitive than those of spirometry in detecting poor asthma control. Our results support the use of BDR assessed by FOT to complement spirometry in the clinical management of asthma.
Acknowledgments
Author contributions: A. M. C. takes responsibility for the content of the manuscript, including the data and analysis. A. M. C., C. S. F., and G. G. K. were involved in study conception and design and A. M. C., L. M. S., and C. S. F. were involved in data acquisition. All authors were involved in interpretation of data, and A. M. C., L. M. S., and C. S. F. in drafting of the manuscript. All authors contributed to critical revision of important intellectual content and final approval of the version to be published.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: Disclosures reported by the authors are unrelated to the submitted work and do not constitute any conflict of interest. C. S. F. reports honoraria from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Mundipharma unrelated to the submitted work, and serves on advisory boards for AstraZeneca, GlaxoSmithKline, and Sanofi Genzyme. M. J. P. reports honoraria from AstraZeneca, GlaxoSmithKline, and Mundipharma unrelated to the submitted work, and serves on advisory boards for AstraZeneca and GlaxoSmithKline. C. T. reports membership with the ERS Task Force for Technical Standards for the Forced Oscillation Technique, and intellectual property arrangements (nonfinancial) with Thorasys and Restech. G. G. K. reports grants from the National Asthma Foundation, National Health and Medical Research Council, Boehringer Ingelheim, GlaxoSmithKline, Menarini, and Mundipharma; fees for consultancy paid to the Woolcock Institute and used for funding the research group from AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Novartis, and Mundipharma; personal travel support from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Menarini, Mundipharma, and Novartis; co-chairmanship of the ERS Task Force for Technical Standards for the Forced Oscillation Technique; and intellectual property arrangements (nonfinancial) with Thorasys and Restech. None declared (A. M. C., L. M. S.).
Footnotes
FUNDING/SUPPORT: A. M. C. is supported by a National Health and Medical Research Council (NHMRC) Postgraduate Research Scholarship.
References
- 1.Wells K.E., Cajigal S., Peterson E.L. Assessing differences in inhaled corticosteroid response by self-reported race-ethnicity and genetic ancestry among asthmatic subjects. J Allergy Clin Immunol. 2016;137(5):1364–1369. doi: 10.1016/j.jaci.2015.12.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J.-K., Jung J.-Y., Kim H., Eom S.-Y., Hahn Y.-S. Combined use of fractional exhaled nitric oxide and bronchodilator response in predicting future loss of asthma control among children with atopic asthma. Respirology. 2017;22(3):466–472. doi: 10.1111/resp.12934. [DOI] [PubMed] [Google Scholar]
- 3.Szefler S.J., Martin R.J., King T.S. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol. 2002;109(3):410–418. doi: 10.1067/mai.2002.122635. [DOI] [PubMed] [Google Scholar]
- 4.Heffler E., Crimi C., Campisi R. Bronchodilator response as a marker of poor asthma control. Respir Med. 2016;112:45–50. doi: 10.1016/j.rmed.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Busse W.W., Holgate S.T., Wenzel S.W. Biomarker profiles in asthma with high vs low airway reversibility and poor disease control. Chest. 2015;148(6):1489–1496. doi: 10.1378/chest.14-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pongracic J.A., Krouse R.Z., Babineau D.C. Distinguishing characteristics of difficult-to-control asthma in inner-city children and adolescents. J Allergy Clin Immunol. 2016;138(4):1030–1041. doi: 10.1016/j.jaci.2016.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulrik C.S., Frederiksen J. Mortality and markers of risk of asthma death among 1,075 outpatients with asthma. Chest. 1995;108(1):10–15. doi: 10.1378/chest.108.1.10. [DOI] [PubMed] [Google Scholar]
- 8.Global Initiative for Asthma Global Strategy for Asthma Management and Prevention, 2018. www.ginasthma.org
- 9.Aburuz S., McElnay J., Gamble J., Millership J., Heaney L. Relationship between lung function and asthma symptoms in patients with difficult to control asthma. J Asthma. 2005;42(10):859–864. doi: 10.1080/02770900500371187. [DOI] [PubMed] [Google Scholar]
- 10.Yaegashi M., Yalamanchili V.A., Kaza V., Weedon J., Heurich A.E., Akerman M.J. The utility of the forced oscillation technique in assessing bronchodilator responsiveness in patients with asthma. Respir Med. 2007;101(5):995–1000. doi: 10.1016/j.rmed.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Komarow H.D., Skinner J., Young M. A study of the use of impulse oscillometry in the evaluation of children with asthma: analysis of lung parameters, order effect, and utility compared with spirometry. Pediatr Pulmonol. 2012;47(1):18–26. doi: 10.1002/ppul.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marotta A., Klinnert M.D., Price M.R., Larsen G.L., Liu A.H. Impulse oscillometry provides an effective measure of lung dysfunction in 4-year-old children at risk for persistent asthma. J Allergy Clin Immunol. 2003;112(2):317–322. doi: 10.1067/mai.2003.1627. [DOI] [PubMed] [Google Scholar]
- 13.Song T.W., Kim K.W., Kim E.S., Park J.W., Sohn M.H., Kim K.E. Utility of impulse oscillometry in young children with asthma. Pediatr Allergy Immunol. 2008;19(8):763–768. doi: 10.1111/j.1399-3038.2008.00734.x. [DOI] [PubMed] [Google Scholar]
- 14.Zerah F., Lorino A.M., Lorino H., Harf A., Macquin-Mavier I. Forced oscillation technique vs spirometry to assess bronchodilatation in patients with asthma and COPD. Chest. 1995;108(1):41–47. doi: 10.1378/chest.108.1.41. [DOI] [PubMed] [Google Scholar]
- 15.Young H.M., Guo F., Eddy R.L., Maksym G., Parraga G. Oscillometry and pulmonary MRI measurements of ventilation heterogeneity in obstructive lung disease: relationship to quality of life and disease control. J Appl Physiol. 2018;125(1):73–85. doi: 10.1152/japplphysiol.01031.2017. [DOI] [PubMed] [Google Scholar]
- 16.Delacourt C., Lorino H., Herve-Guillot M., Reinert P., Harf A., Housset B. Use of the forced oscillation technique to assess airway obstruction and reversibility in children. Am J Respir Crit Care Med. 2000;161(3 Pt 1):730–736. doi: 10.1164/ajrccm.161.3.9904081. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen K.G., Bisgaard H. Discriminative capacity of bronchodilator response measured with three different lung function techniques in asthmatic and healthy children aged 2 to 5 years. Am J Respir Crit Care Med. 2001;164(4):554–559. doi: 10.1164/ajrccm.164.4.2006119. [DOI] [PubMed] [Google Scholar]
- 18.Oostveen E., Boda K., van der Grinten C.P.M. Respiratory impedance in healthy subjects: baseline values and bronchodilator response. Eur Respir J. 2013;42(6):1513–1523. doi: 10.1183/09031936.00126212. [DOI] [PubMed] [Google Scholar]
- 19.Oostveen E., MacLeod D., Lorino H. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22(6):1026–1041. doi: 10.1183/09031936.03.00089403. [DOI] [PubMed] [Google Scholar]
- 20.Miller M.R., Hankinson J., Brusasco V. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 21.Quanjer P.H., Stanojevic S., Cole T.J. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pellegrino R., Viegi G., Brusasco V. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 23.Nathan R.A., Sorkness C.A., Kosinski M. Development of the Asthma Control Test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Schatz M., Sorkness C.A., Li J.T. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117(3):549–556. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Ferrer Galvan M., Javier Alvarez Gutierrez F., Romero Falcon A., Romero Romero B., Saez A., Medina Gallardo J.F. Is the bronchodilator test a useful tool to measure asthma control? Respir Med. 2017;126:26–31. doi: 10.1016/j.rmed.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Kelly V.J., Sands S.A., Harris R.S. Respiratory system reactance is an independent determinant of asthma control. J Appl Physiol. 2013;115(9):1360–1369. doi: 10.1152/japplphysiol.00093.2013. [DOI] [PubMed] [Google Scholar]
- 27.Verbanck S., Schuermans D., Noppen M., Van Muylem A., Paiva M., Vincken W. Evidence of acinar airway involvement in asthma. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1545–1550. doi: 10.1164/ajrccm.159.5.9809017. [DOI] [PubMed] [Google Scholar]
- 28.Bates J.H., Irvin C.G., Farre R., Hantos Z. Oscillation mechanics of the respiratory system. Compr Physiol. 2011;1(3):1233–1272. doi: 10.1002/cphy.c100058. [DOI] [PubMed] [Google Scholar]
- 29.Milne S., Jetmalani K., Chapman D.G. Respiratory system reactance reflects communicating lung volume in chronic obstructive pulmonary disease. J Appl Physiol. 2019;126(5):1223–1231. doi: 10.1152/japplphysiol.00503.2018. [DOI] [PubMed] [Google Scholar]
- 30.Wagner E.M., Bleecker E.R., Permutt S., Liu M.C. Direct assessment of small airways reactivity in human subjects. Am J Respir Crit Care Med. 1998;157(2):447–452. doi: 10.1164/ajrccm.157.2.9611043. [DOI] [PubMed] [Google Scholar]