Abstract
Background
The etiology of idiopathic pulmonary fibrosis (IPF) is unknown. Because it shares genetic, histopathologic, and radiographic features with the fibrosing interstitial lung disease seen in rheumatoid arthritis (RA), the goal of this study was to investigate RA-related autoantibodies in IPF.
Methods
The study included patients with IPF from two separate cohorts at National Jewish Health and Brigham Women’s Hospital (n = 181), general population control subjects (n = 160), and control subjects with disease (n = 86 [40 with RA-usual interstitial pneumonia and 46 with hypersensitivity pneumonitis]). Serum was tested for RA-associated antibodies (including IgG and IgA) to citrullinated protein antigens (ACPA). Lung tissue in 11 patients with IPF was examined for ectopic lymphoid aggregates.
Results
An increased prevalence of ACPA positivity was found in two separate IPF cohorts. In particular, positivity for IgA-ACPA was increased in these two IPF cohorts compared with general population control subjects (21.3% and 24.8% vs 5.6%; P < .01). Patients with IPF were more likely to be IgA-ACPA-positive than IgG-ACPA-positive (23.2% vs 8.3%; P < .01), whereas patients with RA were more likely to be IgG-ACPA-positive than IgA-ACPA-positive (72.5% vs 52.5%; P = .04). There was a strong correlation between IgA-ACPA level and the number of ectopic lymphoid aggregates on lung histologic examination in IPF (r = 0.72; P = .01).
Conclusions
In this study, IgA-ACPA was elevated in patients with IPF and correlated with lymphoid aggregates in the lung, supporting the theory that IgA-ACPA may play a role in lung disease pathogenesis in a subset of individuals with IPF. Future studies are needed to determine whether this subset of ACPA-positive patients with IPF is distinct from patients with IPF but without antibodies.
Key Words: immunology (lung), interstitial lung disease, rheumatoid arthritis
Abbreviations: ACPA, antibodies to citrullinated protein antigens; BWH, Brigham and Women’s Hospital; Dlco, diffusing capacity of the lung for carbon monoxide; ELISA, enzyme-linked immunosorbent assay; HP, hypersensitivity pneumonitis; HRCT, high-resolution CT; hs-CRP, high-sensitivity C-reactive protein; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; LTRC, Lung Tissue Research Consortium; NJH, National Jewish Health; RA, rheumatoid arthritis; UIP, usual interstitial pneumonia
Idiopathic pulmonary fibrosis (IPF) is a form of interstitial lung disease (ILD) defined by a usual interstitial pneumonia (UIP) pattern of parenchymal fibrosis with no identifiable cause.1 Although IPF is one of the most common forms of ILD, investigators have not identified the etiology.
Another form of ILD that commonly involves a UIP pattern of lung fibrosis is rheumatoid arthritis (RA)-associated ILD. In addition to the histopathologic similarities between IPF and RA-associated UIP (RA-UIP), IPF and RA-UIP share the risk factors of smoking and older age,2,3 both have a generally poor prognosis,4 and both have been associated with a gain-of-function promoter variant for the gene encoding mucin 5B (MUC5B)5,6 and mutations in telomere maintenance genes.7,8 These shared features raise the question of whether IPF and RA-UIP could have common pathways of disease development.9
A key autoantibody system associated with RA-UIP involves antibodies to citrullinated protein antigens (ACPA).10,11 Citrullination is the posttranslational modification of peptidyl-arginine to peptidyl-citrulline. Citrullinated proteins are the antigen targets of ACPA, and these proteins are increased in the lung of smokers, patients with RA-UIP, and patients with IPF compared with healthy control subjects.12, 13, 14 ACPA are touted to be highly specific for RA,15 and they can play a direct role in joint disease pathogenesis.16 In addition, ACPA levels are associated with lung disease severity in individuals with RA, suggesting their possible role in lung disease pathogenesis.17,18
Interestingly, up to 25% of patients with IPF have elevated levels of circulating autoantibodies; this scenario suggests a role for a dysregulated adaptive immune response in a subset of individuals with IPF.19, 20, 21, 22 In previous studies, investigators observed ACPA in the serum or BAL fluid from patients with IPF or RA-associated ILD23,24; however, in those studies, subjects were tested only for IgG reactivity to ACPA. Because IgA is the predominant antibody isotype of the lung, and IgA-ACPA is more strongly associated with smoking,25 we hypothesized that the prevalence of circulating IgA-ACPA would be greater in patients with IPF compared with control subjects. We aimed to measure IgA-ACPA and IgG-ACPA in patients with IPF, healthy control subjects, and control subjects with disease.
Patients and Methods
Ethical Considerations
All sample obtainment and study procedures were approved by the Institutional Review Boards for human studies at each institution (National Jewish Health [NJH] HS#2584; Brigham and Women’s Hospital [BWH] #2012P000840).
Study Subjects
Patients with IPF, RA-UIP, and hypersensitivity pneumonitis (HP) and population control subjects were included from established serum biorepositories, including the NJH serum biobank, the David E. Herlihy Patient Research Registry of BWH, and Bonfils Blood Center (Fig 1). All patients with lung disease (in the IPF, RA-UIP, and HP cohorts) were seen at NJH or BWH by pulmonologists specializing in ILD, and all high-resolution CT (HRCT) scans were read by expert thoracic radiologists at the respective institutions. The NJH biobank is part of the Integrated Bioinformation and Specimen Center at NJH; it is an ongoing, institutionally sponsored program that collects and stores clinical data and samples from patients evaluated at NJH. The BWH Herlihy registry is a biobank associated with the BWH Center for Chest Diseases; it contains key clinical data and serum samples from patients with ILD, including those with pulmonary fibrosis. Bonfils Blood Center is a Colorado-based blood donation center through which serum samples from random blood donors in the general population can be obtained.
Figure 1.
Study subject cohorts. The figure depicts the different cohorts in the study, which include patients with IPF, RA-UIP, and HP from the NJH Serum Biobank, patients with IPF from BWH David E. Herlihy Patient Research Registry, population control subjects from the Bonfils Blood Center, and patients with IPF from the LTRC. BWH = Brigham and Women’s Hospital; HP = hypersensitivity pneumonitis; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; NJH = National Jewish Health; LTRC = Lung Tissue Research Consortium; RA = rheumatoid arthritis; UIP = usual interstitial pneumonia.
NJH IPF
Eighty-eight patients with a clinical diagnosis of IPF seen between December 2000 and February 2015 who had serum specimens available through the NJH serum biobank were considered. All subjects underwent a standardized medical chart review of clinical and serologic data, pattern on HRCT imaging, and, when available, histopathologic pattern to confirm compliance with 2018 American Thoracic Society guidelines for the diagnosis of IPF.1 Eight subjects were excluded based on chart review, leaving 80 subjects in the final cohort. All had a joint examination performed by a pulmonologist or rheumatologist within 3 months of serum collection that confirmed the absence of synovitis. Approximately one-quarter (21 of 80 [26.3%]) of subjects were taking immunosuppressing medications, including prednisone, mycophenolate mofetil, or azathioprine, at the time of serum testing.
BWH IPF
We included 101 subjects from BWH as an IPF validation cohort. All had serum collected between July 2009 and April 2018. All underwent a standardized medical chart review to confirm compliance with the 2018 American Thoracic Society guidelines for diagnosing IPF, and all had a joint examination by a board-certified rheumatologist within 3 months of serum collection that showed no evidence on physical examination of synovitis. Ninety-two subjects had data available regarding medication usage at the time of serum collection, and 18 of 92 (19.6%) were taking immunosuppressing medications, including prednisone, mycophenolate mofetil, or azathioprine, at the time of serum collection.
General Population Control subjects
We obtained 160 serum samples from people who had donated blood through the Bonfils Blood Center. The only clinical information available for these subjects was age and sex. History of RA or lung disease was unknown.
RA-UIP
We included 40 patients with RA-UIP seen between April 2002 and November 2012 who had stored serum specimens available through the NJH serum biobank. All subjects underwent a chart review to confirm the diagnoses of RA and RA-UIP. RA was diagnosed by a board-certified rheumatologist, and RA-UIP was diagnosed by a pulmonologist with expertise in ILD. All HRCT scans were independently reviewed by two expert thoracic radiologists (J. H. C. and S. B. H.), and disagreements were resolved through consensus. For the remainder of this article, unless otherwise stated, RA-UIP refers to the subgroup with either UIP or probable UIP on HRCT imaging. Our group has reported on this cohort in a previous article.26
Hypersensitivity Pneumonitis
We included 46 patients with HP seen between August 2001 and January 2015 who had stored serum specimens available through the NJH serum biobank. HP was diagnosed by a pulmonologist with expertise in ILD and following discussion at a multidisciplinary conference when the diagnosis was in question.
ACPA Testing
All serum samples underwent enzyme-linked immunosorbent assay (ELISA) testing in the University of Colorado Division of Rheumatology Clinical Research Laboratory for isotype-specific IgA-ACPA and IgG-ACPA. For IgG-ACPA, the commercial assay cyclic citrullinated peptide-3 (IgG, Inova Diagnostics, Inc.) was used, and results in ELISA units were established by using the assay-provided standard curve. For IgA-ACPA, a cyclic citrullinated peptide-3 ELISA plate was used with an IgA secondary conjugate that detects total IgA and was provided by Inova Diagnostics, Inc. (for research only). For IgA-ACPA, an in-house standard was generated from pooled RA patient serum that underwent serial dilutions to generate a standard curve. For both IgA-ACPA and IgG-ACPA, a cutoff for positivity was set at a level above the 98th percentile in a separate group of 126 non-RA healthy control subjects.
All ACPA testing was performed in duplicate, and the mean level in ELISA units was used for analyses. For IgA-ACPA testing, serum underwent serial dilutions to determine the antibody level if initial results were above the upper limit of assay detection. Because our validation cohort (BWH IPF) was tested at a later time point than other cohorts, and to account for any assay lot-to-lot variability, the 126 non-RA healthy control subjects were retested at the time of BWH IPF sample testing; the same approach (98th percentile) was used to establish cutoff levels for positivity.
C-Reactive Protein Testing
Serum from all NJH IPF subjects was tested for high-sensitivity C-reactive protein (hs-CRP) using nephelometry (Dade-Behring) with results reported in milligrams per liter.
Pulmonary Function Tests
We included the results of pulmonary function tests obtained as part of routine clinical care if they were collected within 6 months of serum collection. All NJH IPF subjects, 71 of 101 (70%) BWH patients with IPF, 46 of 56 (82%) patients with RA-UIP, and 43 of 46 (93%) patients with HP had pulmonary function test results available.
Lung Tissue Research Consortium
To evaluate histologic features of lung tissue associated with IPF and ACPA, we included 11 patients with IPF who had stored paraffin-embedded lung tissue and serum specimens available from the Lung Tissue Research Consortium (LTRC). Tissue and serum were collected contemporaneously in these subjects. The LTRC is a National Heart, Lung, and Blood Institute-sponsored program from which serum and lung tissue samples are obtained and stored from individuals undergoing planned surgical lung biopsy for clinical purposes. In addition, clinical data and CT imaging of the chest were available for all subjects to re-confirm the diagnosis of IPF.
Hematoxylin and eosin-stained lung biopsy slides were reviewed by a single pathologist (C. C.) blinded to the subjects’ clinical and antibody status. All subjects were confirmed to have a UIP pattern on pathologic evaluation. One of 11 subjects had evidence of organizing pneumonia; none had evidence of nonspecific interstitial pneumonia or findings to suggest acute exacerbation of IPF. Immunohistochemical staining for B cells (CD20, L26 MA5-13141; Thermo Fisher Scientific), T cells (CD3, SP7 RM9107; Thermo Fisher Scientific), and plasma cells (CD138, MI15 MS-1793; Thermo Fisher Scientific) was also performed to confirm the presence of ectopic lymphoid tissue as either lymphoid aggregates and/or germinal centers. Lymphoid aggregates were defined as clusters of > 100 lymphocytes without spacing. Germinal centers were defined as well-organized lymphoid aggregates composed of activated B cells, follicular dendritic cells, and tingible body macrophages, surrounded by a loosely defined T-cell zone, lymphatics, and high endothelial venules.
Statistical Analyses
Demographic characteristics, baseline characteristics, and ACPA positivity (yes/no) were compared between groups by using χ2 or Fisher exact testing, as appropriate. Within-group comparisons of ACPA positivity (eg, IgA vs IgG among patients with IPF) were performed by using the McNemar test. Logistic regression was used to identify variables associated with ACPA positivity while accounting for covariates with available data from the control population; these included age and sex (smoking history is not collected for this group of control subjects). Because of non-normal distribution, the Wilcoxon rank sum test was used to determine whether median levels of ACPA or hs-CRP differed significantly between groups. Associations between ACPA positivity and pathologically identified ectopic lymphoid aggregates or germinal centers were examined by using the Fisher exact test and the Spearman correlation coefficients. The Kaplan-Meier method was used to display survival curves, and the log-rank test was used to compare survival curves for the NJH IPF cohort stratified according to IgA-ACPA positivity. We also used Cox proportional hazards to examine IgA-ACPA positivity as a predictor of time of death while controlling for potentially influential variables, including smoking status and disease severity. Analyses were performed by using SPSS version 24 (IBM SPSS Statistics, IBM Corporation).
Results
Subject Characteristics
Subject characteristics are listed in Table 1. Patients with IPF were older and more often male than subjects in the comparator groups. Baseline FVC and diffusing capacity of the lung for carbon monoxide (Dlco) percentages were not significantly different between ILD groups.
Table 1.
Baseline Subject Characteristics
| Characteristic | NJH IPF (n = 80) | BWH IPF (n = 101) | Control Subjects (n = 160) | RA-UIP (n = 40) | HP (n = 46) | P Valuea |
|---|---|---|---|---|---|---|
| Age, y | 68 ± 7 | 68 ± 8 | 56 ± 11 | 64 ± 10 | 61 ± 10 | < .01 |
| Female sex | 12 (15) | 29 (29)b | 127 (79) | 20 (50) | 22 (48) | < .01 |
| Ever smoker | 57 (71) | 57 (59)c | … | 24 (62)c | 25 (56)c | .25 |
| FVC, % predicted | 67 ± 15 | 64 ± 17d | … | 72 ± 18d | 67 ± 19d | .17 |
| Dlco, % predicted | 43 ± 15 | 43 ± 18e | … | 46 ± 15e | 49 ± 15 | .35 |
Data are presented as mean ± SD or No. (%). BWH = Brigham Women’s Hospital; Dlco = diffusing capacity of the lung for carbon monoxide; HP = hypersensitivity pneumonitis; IPF = idiopathic pulmonary fibrosis; NJH = National Jewish Health; RA-UIP = rheumatoid arthritis-associated usual interstitial pneumonia.
P values compare mean levels (analysis of variance) or percent positive (χ2) between all groups.
Two subjects had missing data for sex.
Four BWH patients with IPF, one patient with HP, and one patient with RA-UIP had missing smoking history.
FVC results missing in 31 BWH patients with IPF, five patients with RA-UIP, and one patient with HP.
Dlco results missing in 52 BWH patients with IPF and four patients with RA-UIP.
IgA-ACPA and IgG-ACPA in IPF Compared With Population Control Subjects
In the NJH IPF cohort, IgA-ACPA positivity was more prevalent than in the general population control subjects (21.3% vs 5.6%; P < .01), and there was a trend toward greater prevalence of IgG-ACPA positivity in patients with IPF compared with general population control subjects (7.5% vs 1.9%; P = .06) (Table 2). In addition, median IgA-ACPA and IgG-ACPA levels were higher in NJH patients with IPF compared with general population control subjects (both, P < .01). As in the primary cohort, in the BWH IPF validation cohort, IgA-ACPA positivity was more prevalent in patients with IPF compared with general population control subjects (24.8% vs 5.6%; P < .01). In the BWH IPF group, IgG-ACPA positivity was also more prevalent in patients with IPF than in general population control subjects (8.9% vs 1.9%; P = .02). Using logistic regression and including all patients with IPF (NJH and BWH combined) or the general population control subjects (who only had clinical data for age and sex), both IgA-ACPA and IgG-ACPA positivity were associated with IPF after adjusting for age and sex (for IgA-ACPA, OR = 3.3 [95% CI, 1.3-8.4]; for IgG-ACPA, OR = 5.8 [95% CI, 1.1-31.5]).
Table 2.
ACPA Positivity in IPF and Control Cohorts
| Variable | Control Subjects (n = 160) | NJH IPF (n = 80) | BWH IPF (n = 101) | RA-UIP (n = 40) | HP (n = 46) |
|---|---|---|---|---|---|
| IgA-ACPA (+) | 9 (6) | 17 (21)a | 25 (25)a | 21 (53)a | 4 (9) |
| IgG-ACPA (+) | 3 (2) | 6 (8) | 9 (9)b | 29 (73)a | 5 (11)b |
| IgA-ACPA (+) only | 8 (5) | 13 (16)a | 23 (23)a | 2 (5) | 1 (2) |
| IgG-ACPA (+) only | 2 (1) | 2 (3) | 5 (5) | 10 (25)a | 2 (4) |
| IgA-ACPA and IgG-ACPA (+) | 1 (1) | 4 (5)b | 3 (3) | 19 (48)a | 3 (7)b |
Data are presented as No. (%). ACPA = antibodies to citrullinated protein antigens. See Table 1 legend for expansion of other abbreviations.
P < .01 comparing each cohort vs population control subjects by using χ2/Fisher exact testing as appropriate.
P < .05 comparing each cohort vs population control subjects using χ2/Fisher exact testing as appropriate.
In the NJH and BWH patients with IPF, there was no significant difference in age, sex, ever smoking, percent predicted FVC, or percent predicted Dlco between ACPA-positive and ACPA-negative subjects (Table 3). Among the 59 NJH patients with IPF and 74 BWH patients with IPF not taking immunosuppressing drugs, as in the overall cohorts, IgA-ACPA and IgG-ACPA positivity was significantly more prevalent in patients with IPF than in the general population control subjects (26.3% vs 5.6% [P < .01] and 6.0% vs 1.9% [P < .05], respectively). In addition, in the subgroup of 23 NJH IPF and 40 BWH IPF never smokers, IgA-ACPA and IgG-ACPA positivity was also more prevalent than in the general population control subjects (23.8% vs 5.6% [P < .01] and 9.5% vs 1.9% [P < .01]).
Table 3.
Subject Characteristics Stratified According to ACPA Positivity in IPF Subjects
| Variable | IgA-ACPA Positive (n = 42) | IgA-ACPA Negative (n = 139) | P Valuea | IgG-ACPA Positive (n = 15) | IgG-ACPA Negative (n = 166) | P Valuea |
|---|---|---|---|---|---|---|
| Age, y | 69 ± 7 | 68 ± 7 | .20 | 66 ± 6 | 68 ± 7 | .32 |
| Female sexb | 7 (17) | 34 (25) | .29 | 2 (13) | 39 (24) | .53 |
| Ever smoker | 27 (64) | 87 (64) | .99 | 9 (60) | 105 (65) | .71 |
| FVC, % predictedb | 66 ± 15 | 67 ± 16 | .95 | 63 ± 17 | 66 ± 16 | .59 |
| Dlco, % predictedb | 41 ± 17 | 44 ± 16 | .30 | 39 ± 14 | 43 ± 17 | .47 |
Data are presented as mean ± SD or No. (%). See Table 1 and 2 legends for expansion of abbreviations.
P values compare ACPA-positive vs ACPA-negative patients with IPF by using the Student t test, χ2 test, or Fisher exact test as appropriate.
BWH patients with IPF missing sex (n = 2), FVC results (n = 31), and Dlco results (n = 52).
ACPA Positivity in IPF Compared With RA-UIP and HP
The proportion of patients with IgA-ACPA or IgG-ACPA positivity was significantly higher in RA-UIP compared with NJH IPF (52.5% vs 21.3% [P < .01] and 72.5% vs 7.5% [P < .01], respectively) (Table 2). The same was true when patients with RA-UIP were compared vs BWH patients with IPF (all, P < .01). Patients with RA-UIP were more likely to be positive for IgG-ACPA than for IgA-ACPA (72.5% vs 52.5%; P = .04), whereas patients with IPF were more likely to be positive for IgA-ACPA than for IgG-ACPA (in NJH and BWH IPF combined, 23.2% vs 8.3%; P < .01). Compared with population control subjects, patients with IPF were more likely to be positive for an isolated IgA-ACPA (P < .01), whereas patients with RA-UIP were more often dual antibody positive (for IgA-ACPA and IgG-ACPA; P < .01) than positive for only one isoform.
In addition, there was a higher prevalence of IgA-ACPA positivity in patients with IPF compared with patients with HP (23.2% vs 8.7%; P = .04), but there was no difference between these two groups in IgG-ACPA positivity (8.3% vs 10.9%; P = .57). Patients with HP also had a higher prevalence of IgG-ACPA positivity compared with that of the general population control subjects (10.9% vs 1.9%; P = .02), but there was no difference between those groups in IgA-ACPA positivity (8.7% vs 5.6%; P = .49) (Table 2).
ACPA and hs-CRP in NJH Patients With IPF
There was no significant difference in hs-CRP levels between ACPA-positive and ACPA-negative patients with IPF (for IgA-ACPA, P = .72; for IgG-ACPA, P = .74). There was also no significant correlation between levels of hs-CRP and IgA-ACPA (r = 0.05; P = .68) or IgG-ACPA (r = –0.06; P = .62).
ACPA and Ectopic Lymphoid Aggregates in IPF
Characteristics of LTRC patients with IPF are listed in Table 4. Compared with NJH IPF, the LTRC patients with IPF were younger, more often female, had a lower percent predicted Dlco, and had a higher prevalence of IgA-ACPA positivity. In LTRC patients with IPF, there was a strong correlation between IgA-ACPA level and the number of ectopic lymphoid aggregates in their surgical biopsy specimens (r = 0.72; P = .01). There was a similar but nonsignificant trend in the correlation between IgG-ACPA level and the number of lymphoid aggregates (r = 0.54; P = .09). Representative images are presented in Figure 2.
Table 4.
Subject Characteristics and ACPA Positivity in LTRC Patients With IPF Compared With NJH Patients With IPF
| Characteristic | LTRC IPF (n = 11) | NJH IPF (n = 80) | P Valuea |
|---|---|---|---|
| Age, y | 63 ± 9 | 68 ± 7 | .01 |
| Female sex | 5 (46) | 12 (15) | .03 |
| Ever smoker | 10 (91) | 57 (71) | .28 |
| IgA-ACPA (+) | 7 (64) | 17 (21) | < .01 |
| IgG-ACPA (+) | 1 (9) | 6 (8) | 1.0 |
| FVC, % predicted | 70 ± 24 | 68 ± 15 | .74 |
| Dlco, % predicted | 28 ± 5 | 43 ± 16 | < .01 |
Figure 2.
Histologic images from LTRC patients with IPF. The figure depicts representative images of ectopic lymphoid aggregates from patients with IPF. Panels A and B include routine hematoxylin and eosin staining, and panels C and D include anti-CD138 (plasma cell) immunohistochemical staining. See Figure 1 legend for expansion of abbreviations.
Survival
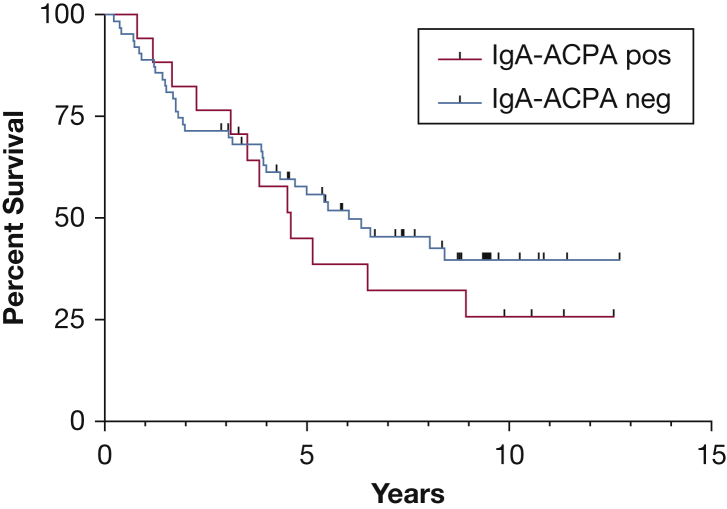
Survival data were available for the NJH IPF cohort. IgA-ACPA-negative patients with IPF had a median survival that was numerically 2 years longer than IgA-ACPA-positive patients with IPF, although this outcome did not reach statistical significance (6.35 years [CI, 4.0-] vs 4.60 years [CI, 2.3-8.9]; P = .3) (Fig 3). In a multivariate model that included ACPA-IgA (positive/negative), GAP (Gender, Age, Physiology) stage (stage III reference group), and smoking status, IgA-ACPA positivity was associated with an increased risk of death during follow-up (hazard ratio, 1.82; 95% CI, 0.94-3.52; P = .07).
Figure 3.
Survival in the NJH IPF cohort stratified according to IgA-antibodies to citrullinated protein antigens (ACPA) status. Kaplan-Meier curve comparing survival between NJH patients with IPF who are IgA-ACPA-positive and IgA-ACPA-negative. Median survival of 6.3 vs 4.6 years (P = .3). In a multivariate model that included ACPA-IgA (+/–), GAP stage (stage III reference group), and smoking status, IgA-ACPA positivity was associated with an increased risk of death during follow-up (hazard ratio, 1.82; 95% CI, 0.94-3.52; P = .07). See Figure 1 legend for expansion of other abbreviations.
Discussion
We tested serum samples and found ACPA, particularly IgA-ACPA, in a substantial number of patients with IPF. The prevalence of ACPA positivity was higher than in population control subjects in the primary cohort and in a validation cohort from a different institution. We also found that higher levels of IgA-ACPA were associated with increased numbers of ectopic lymphoid aggregates in lung tissue of patients with IPF and a trend toward a worse survival. To our knowledge, this study is the first to show that ACPA positivity in IPF is common and, when present, ACPA are more likely to be IgA than IgG.
The finding that ACPA in IPF were predominately IgA suggests a mucosal origin of these antibodies. This finding, combined with the results from the histologic analysis (ectopic lymphoid aggregates in the lung correlated with IgA-ACPA levels), further suggests that these antibodies are likely generated in the lung in IPF. We did not test for lung generation of ACPA in this study. However, data from patients with RA as well as individuals at increased risk of developing RA have revealed this to be the case.24,27,28 In addition, protein citrullination is also known to occur in the IPF lung, presumably as a response to inhaled irritants or injurious agents (eg, cigarette smoke), and it seems likely that antibodies directed against these citrullinated proteins (ACPA) are generated locally.12 Additional studies are needed to confirm the lung generation of ACPA in IPF and to determine whether patients with IPF have unique or shared citrullinated protein targets of ACPA compared with patients with RA.
We found no association between hs-CRP and ACPA in IPF. This outcome suggests that ACPA are not simply associated with general inflammation. Although mucosal IgA antibodies are often nonspecific and T-cell independent, it is of note that citrullinated peptide-specific IgA-ACPA has been identified in the lung of patients with RA-ILD, consistent with an antigen-specific immune response.24
Furthermore, it is well established that systemic ACPA elevations, both IgA and IgG, can precede the onset of joint inflammation in RA during a preclinical period of systemic autoimmunity (ie, prior to development of synovitis), including in some patients who present with ILD and later develop classifiable RA.15,29,30 Compared with IgA-ACPA, IgG-ACPA is more strongly associated with predicting RA-related joint disease.31 This finding is in line with our results: patients with RA-UIP were most likely to have IgG-ACPA, and patients with IPF were most likely to have IgA-ACPA. We speculate that IgA-ACPA is associated with lung disease (and may even be involved in the pathogenesis of RA-related ILD), but the development of joint inflammation and transition to classifiable RA may require evolution of the immune response from isolated IgA-ACPA to include IgG-ACPA. Interestingly, specific peptides (eg, citrullinated vimentin) have been identified as shared antigens, present in both the bronchial and synovial tissue of patients with RA.32 As such, we hypothesize that ACPA could be generated first against citrullinated proteins in the lung and later target similar citrullinated proteins present in the joint.
In patients with IPF, the implications of IgA-ACPA positivity are not entirely clear. In a survival analysis, we found that patients with IPF who were IgA-ACPA-positive had a median survival that was 2 years less that those who were IgA-ACPA-negative. Although this finding did not reach statistical significance, it raises important questions that require additional investigation. Could ACPA directly contribute to ongoing inflammation and injury in the lung? They have been shown to stimulate cytokine production through the formation of immune complexes,33 as well as activate complement34 and osteoclasts.16 Or is formation of ACPA simply an immunologic response in patients with more severe lung injury? Could it define a unique phenotypic subset of IPF that could benefit from different treatment approaches? Or does ACPA positivity represent a preclinical state in the spectrum of RA pathogenesis? Regardless, ACPA positivity poses a diagnostic and treatment dilemma for both rheumatologists and pulmonologists.35
It is also of interest that previously published data found that ectopic lymphoid tissue can display dysregulated immune cells and aberrant expression of chemokines and co-stimulatory or survival factors (eg, CXCL13) that may promote selection of autoreactive B cells.36,37 Development of ectopic lymphoid aggregates in the lung in patients with IPF may similarly promote expansion of autoreactive B cells. Rangel-Moreno et al38 reported an association between ectopic lymphoid aggregates in the lung and ACPA in the serum and BAL fluid in patients with RA-ILD. In these patients, the investigators also noted the presence of lung plasma cells generating antibodies to citrullinated fibrinogen and speculated that this local immune response contributed to ACPA generation and lung disease.
Analyzing samples from multiple cohorts, including a validation cohort from another center, and the novel focus on IgA-ACPA are strengths of the current study. However, there are several caveats. Because NJH and BWH are tertiary referral centers for patients with advanced lung disease, the patients with IPF in these cohorts may not accurately reflect patients in general, and larger community-based studies are needed to understand when in the course of IPF ACPA generation occurs. In addition, the LTRC patients with IPF were younger and more often female, which is potentially a reflection of who is more likely to undergo a lung biopsy in the clinical evaluation needed to confirm a clinical diagnosis of IPF. We did not know the smoking status of our control population and could not exclude the presence of lung disease in this cohort. Finally, although we evaluated several demographic factors that could have influenced ACPA positivity, larger prospective studies are needed to fully evaluate the influence of environmental factors, medications, and genetics on the development of ACPA in IPF.
Conclusions
IgA-ACPA positivity is present in a substantial portion of individuals with IPF, suggesting that a mucosal-based immune response in the lung to citrullinated proteins could be a novel pathway of disease pathogenesis in IPF. Future studies are needed to determine whether ACPA is associated with disease prognosis and to identify mechanisms of ACPA generation, which could lead to novel treatment targets.
Acknowledgments
Author contributions: J. J. Solomon is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article. Each author contributed to the study as follows: substantial contributions to the conception or design of the work, J. J. Solomon, K. D. D., J. J. Swigris, and M. K. D.; acquisition of data, J. J. Solomon, S. M., L. B. K., J. H. C., S. B. H., I. O. R., P. F. D., T. J. D., S. P., A. J. E., A. V., I. A., E. R. F. P., K. K. B., M. M., D. H., C. C., K. D. D., J. J. Swigris, and M. K. D.; and analysis or interpretation of data, J. J. Solomon, S. M., L. B. K., A. I. M., I. A., K. K. B., C. C., K. D. D., J. J. Swigris, and M. K. D. All authors drafted the work or revising it critically for intellectual content, and all authors agree to be accountable for all aspects of the work.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: M. M. is an employee of Inova Diagnostics. K. D. D. has received research support for unrelated biomarker studies of at-risk populations from Janssen R&D; and consulting support in preclinical RA from Inova, BMS, and Janssen R&D. None declared (J. J. Solomon, S. M., L. B. K., J. H. C., S. B. H., I. O. R., P. F. D., T. J. D., S. P., A. J. E., A. V., A. I. M., I. A., E. R. F. P., K. K. B., D. H., C. C., J. J. Swigris, M. K. D.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The contents are the authors’ sole responsibility and do not necessarily represent official National Institutes of Health views.
Footnotes
FUNDING/SUPPORT: This work was supported by the National Institutes of Health [Grants AR066712 (M. K. D.) and AI103023 (K. D. D.)] and the Rheumatology Research Foundation [Scientist Development Award (L. B. K.) and Resident Research Preceptorship (S. M.)].
References
- 1.Raghu G., Remy-Jardin M., Myers J.L., American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y., Li H., Wu N., Dong X., Zheng Y. Retrospective study of the clinical characteristics and risk factors of rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol. 2017;36(4):817–823. doi: 10.1007/s10067-017-3561-5. [DOI] [PubMed] [Google Scholar]
- 3.Ley B., Collard H.R. Epidemiology of idiopathic pulmonary fibrosis. Clin Epidemiol. 2013;5:483–492. doi: 10.2147/CLEP.S54815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim E.J., Elicker B.M., Maldonado F. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. 2010;35(6):1322–1328. doi: 10.1183/09031936.00092309. [DOI] [PubMed] [Google Scholar]
- 5.Juge P.A., Lee J.S., Ebstein E. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med. 2018;379(23):2209–2219. doi: 10.1056/NEJMoa1801562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seibold M.A., Wise A.L., Speer M.C. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364(16):1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juge P.A., Borie R., Kannengiesser C. Shared genetic predisposition in rheumatoid arthritis-interstitial lung disease and familial pulmonary fibrosis. Eur Respir J. 2017;49(5) doi: 10.1183/13993003.02314-2016. pii: 1602314. [DOI] [PubMed] [Google Scholar]
- 8.Armanios M.Y., Chen J.J., Cogan J.D. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356(13):1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 9.Paulin F., Doyle T.J., Fletcher E.A., Ascherman D.P., Rosas I.O. Rheumatoid arthritis-associated interstitial lung disease and idiopathic pulmonary fibrosis: shared mechanistic and phenotypic traits suggest overlapping disease mechanisms. Rev Invest Clin. 2015;67(5):280–286. [PMC free article] [PubMed] [Google Scholar]
- 10.Doyle T.J., Patel A.S., Hatabu H. Detection of rheumatoid arthritis-interstitial lung disease is enhanced by serum biomarkers. Am J Respir Crit Care Med. 2015;191(12):1403–1412. doi: 10.1164/rccm.201411-1950OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly C.A., Saravanan V., Nisar M., British Rheumatoid Interstitial Lung (BRILL) Network Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics—a large multicentre UK study. Rheumatology (Oxford) 2014;53(9):1676–1682. doi: 10.1093/rheumatology/keu165. [DOI] [PubMed] [Google Scholar]
- 12.Samara K.D., Trachalaki A., Tsitoura E. Upregulation of citrullination pathway: from autoimmune to idiopathic lung fibrosis. Respir Res. 2017;18(1):218. doi: 10.1186/s12931-017-0692-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makrygiannakis D., Hermansson M., Ulfgren A.K. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis. 2008;67(10):1488–1492. doi: 10.1136/ard.2007.075192. [DOI] [PubMed] [Google Scholar]
- 14.Bongartz T., Cantaert T., Atkins S.R. Citrullination in extra-articular manifestations of rheumatoid arthritis. Rheumatology (Oxford) 2007;46(1):70–75. doi: 10.1093/rheumatology/kel202. [DOI] [PubMed] [Google Scholar]
- 15.Demoruelle M.K., Parish M.C., Derber L.A. Performance of anti-cyclic citrullinated peptide assays differs in subjects at increased risk of rheumatoid arthritis and subjects with established disease. Arthritis Rheum. 2013;65(9):2243–2252. doi: 10.1002/art.38017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harre U., Georgess D., Bang H. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest. 2012;122(5):1791–1802. doi: 10.1172/JCI60975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giles J.T., Danoff S.K., Sokolove J. Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann Rheum Dis. 2014;73(8):1487–1494. doi: 10.1136/annrheumdis-2012-203160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rocha-Munoz A.D., Ponce-Guarneros M., Gamez-Nava J.I. Anti-cyclic citrullinated peptide antibodies and severity of interstitial lung disease in women with rheumatoid arthritis. J Immunol Res. 2015;2015:151626. doi: 10.1155/2015/151626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahluwalia N., Shea B.S., Tager A.M. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am J Respir Crit Care Med. 2014;190(8):867–878. doi: 10.1164/rccm.201403-0509PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li F.J., Surolia R., Li H. Autoimmunity to vimentin is associated with outcomes of patients with idiopathic pulmonary fibrosis. J Immunol. 2017;199(5):1596–1605. doi: 10.4049/jimmunol.1700473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahloon R.A., Xue J., Bhargava A. Patients with idiopathic pulmonary fibrosis with antibodies to heat shock protein 70 have poor prognoses. Am J Respir Crit Care Med. 2013;187(7):768–775. doi: 10.1164/rccm.201203-0506OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoyne G.F., Elliott H., Mutsaers S.E., Prele C.M. Idiopathic pulmonary fibrosis and a role for autoimmunity. Immunol Cell Biol. 2017;95(7):577–583. doi: 10.1038/icb.2017.22. [DOI] [PubMed] [Google Scholar]
- 23.Lee J.S., Kim E.J., Lynch K.L. Prevalence and clinical significance of circulating autoantibodies in idiopathic pulmonary fibrosis. Respir Med. 2013;107(2):249–255. doi: 10.1016/j.rmed.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harlow L., Gochuico B.R., Rosas I.O. Anti-citrullinated heat shock protein 90 antibodies identified in bronchoalveolar lavage fluid are a marker of lung-specific immune responses. Clin Immunol. 2014;155(1):60–70. doi: 10.1016/j.clim.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svard A., Skogh T., Alfredsson L. Associations with smoking and shared epitope differ between IgA- and IgG-class antibodies to cyclic citrullinated peptides in early rheumatoid arthritis. Arthritis Rheumatol. 2015;67(8):2032–2037. doi: 10.1002/art.39170. [DOI] [PubMed] [Google Scholar]
- 26.Solomon J.J., Chung J.H., Cosgrove G.P. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. 2016;47(2):588–596. doi: 10.1183/13993003.00357-2015. [DOI] [PubMed] [Google Scholar]
- 27.Demoruelle M.K., Harrall K.K., Ho L. Anti-citrullinated protein antibodies are associated with neutrophil extracellular traps in the sputum in relatives of rheumatoid arthritis patients. Arthritis Rheumatol. 2017;69(6):1165–1175. doi: 10.1002/art.40066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynisdottir G., Olsen H., Joshua V. Signs of immune activation and local inflammation are present in the bronchial tissue of patients with untreated early rheumatoid arthritis. Ann Rheum Dis. 2016;75(9):1722–1727. doi: 10.1136/annrheumdis-2015-208216. [DOI] [PubMed] [Google Scholar]
- 29.Rantapaa-Dahlqvist S., de Jong B.A., Berglin E. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48(10):2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 30.Fischer A., Solomon J.J., du Bois R.M. Lung disease with anti-CCP antibodies but not rheumatoid arthritis or connective tissue disease. Respir Med. 2012;106(7):1040–1047. doi: 10.1016/j.rmed.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kokkonen H., Mullazehi M., Berglin E. Antibodies of IgG, IgA and IgM isotypes against cyclic citrullinated peptide precede the development of rheumatoid arthritis. Arthritis Res Ther. 2011;13(1):R13. doi: 10.1186/ar3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ytterberg A.J., Joshua V., Reynisdottir G. Shared immunological targets in the lungs and joints of patients with rheumatoid arthritis: identification and validation. Ann Rheum Dis. 2015;74(9):1772–1777. doi: 10.1136/annrheumdis-2013-204912. [DOI] [PubMed] [Google Scholar]
- 33.Law S.C., Street S., Yu C.H. T-cell autoreactivity to citrullinated autoantigenic peptides in rheumatoid arthritis patients carrying HLA-DRB1 shared epitope alleles. Arthritis Res Ther. 2012;14(3):R118. doi: 10.1186/ar3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trouw L.A., Haisma E.M., Levarht E.W. Anti-cyclic citrullinated peptide antibodies from rheumatoid arthritis patients activate complement via both the classical and alternative pathways. Arthritis Rheum. 2009;60(7):1923–1931. doi: 10.1002/art.24622. [DOI] [PubMed] [Google Scholar]
- 35.Ferri C., Manfredi A., Sebastiani M. Interstitial pneumonia with autoimmune features and undifferentiated connective tissue disease: our interdisciplinary rheumatology-pneumology experience, and review of the literature. Autoimmunity Reviews. 2016;15(1):61–70. doi: 10.1016/j.autrev.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Vinuesa C.G., Sanz I., Cook M.C. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009;9(12):845–857. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 37.Klimatcheva E., Pandina T., Reilly C. CXCL13 antibody for the treatment of autoimmune disorders. BMC Immunol. 2015;16:6. doi: 10.1186/s12865-015-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rangel-Moreno J., Hartson L., Navarro C., Gaxiola M., Selman M., Randall T.D. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest. 2006;116(12):3183–3194. doi: 10.1172/JCI28756. [DOI] [PMC free article] [PubMed] [Google Scholar]