Abstract
Background
Each year, > 1.5 million Americans are diagnosed with an incidentally detected lung nodule. Practice guidelines attempt to balance the benefit of early detection of lung cancer with the risks of diagnostic testing, but adherence to guidelines is low. The goal of this study was to determine guideline adherence rates in the setting of a multidisciplinary nodule clinic and describe reasons for nonadherence as well as associated outcomes.
Methods
This cohort study included 3 years of follow-up of patients aged ≥ 35 years with an incidentally detected lung nodule evaluated in a multidisciplinary clinic that used the 2005 Fleischner Society Guidelines.
Results
Among 113 patients, 67% (95% CI, 58-76) were recommended a guideline-concordant nodule evaluation; 7.1% (95% CI, 3.1-13) and 26% (95% CI, 18-25) were recommended less or more intense evaluation, respectively. In contrast, 58% (95% CI, 48-67), 22% (95% CI, 18-25), and 23% (95% CI, 16-32) received a guideline-concordant, less intense, or more intense evaluation. The most common reason for recommending guideline-discordant care was concern for two different diagnoses that would each benefit from early detection and treatment. A majority of lung cancer diagnoses (88%) occurred in patients who received guideline-concordant care. There were no lung cancer cases in those who received less intense nodule care.
Conclusions
A multidisciplinary nodule clinic may serve as a system-level intervention to promote guideline-concordant care, while also providing a multidisciplinary basis by which to deviate from guidelines to address the needs of a heterogeneous patient population.
Key Words: clinical decision-making, guidelines, lung neoplasm, lung nodule
Abbreviations: FSG, Fleischner Society Guidelines; MDNC, multidisciplinary nodule clinic
Each year, > 1.5 million Americans are diagnosed with an incidentally detected lung nodule.1 Management requires balancing the benefits of early detection with the risks of diagnostic testing. Practice guidelines attempt to achieve this balance by recommending varying intensities of evaluation depending on nodule characteristics and an individual’s risk of lung cancer.2, 3, 4, 5, 6 Studies show that radiologists variably recommend guideline-adherent nodule evaluation7, 8, 9, 10 and that surprisingly few patients receive guideline-concordant care.2,11 The latter finding has prompted interest in system-level interventions that increase guideline adherence rates.2
One potential system-level intervention is to refer patients to a multidisciplinary nodule clinic (MDNC). To better understand the potential of such an intervention, we studied a cohort diagnosed with an incidentally detected pulmonary nodule and referred to an MDNC. The aims of this hypothesis-generating study were to determine: (1) the rate of guideline-concordant recommendations; (2) the rate of guideline-concordant care received (regardless of recommendations); (3) reasons for guideline-discordant recommendations and received care; and (4) associated outcomes.
Materials and Methods
Study Design and Study Population
We performed a cohort study (2010-2012) of adults with an incidentally detected lung nodule (≤ 30 mm) with at least 3 years of follow-up unless loss of follow-up was due to death. Study subjects were patients referred to the Seattle Cancer Care Alliance Lung Cancer Early-Detection and Prevention Clinic who consented to participate in a lung biorepository. Pulmonologists, thoracic surgeons, and chest radiologists reviewed cases together prior to the clinic evaluation by a pulmonologist. The lung biorepository prospectively gathered clinical information and biospecimens from consenting patients for a broad range of observational studies (Fred Hutchinson Cancer Research Center Institutional Review Board file no. 6663, protocol no. 2242).
Because the clinic used the 2005 Fleischner Society Guidelines (FSG)3 during the study period, exclusion criteria were individuals aged < 35 years, those with a current or recent cancer diagnosis within 5 years (except nonmelanoma skin cancer), and patients undergoing surveillance imaging with CT scans for a known lung nodule, cancer surveillance, a known underlying disease (eg, sarcoid), suspected nonlung malignancy, unexplained fever, or lung cancer screening. Consistent with a previous study,2 a 3-year follow-up period was selected to determine if surveillance persisted beyond 2 years.
Data Abstraction
Trained chart abstractors ascertained information on patient characteristics, lung cancer risk factors, and nodule characteristics from the electronic medical record. Two clinicians (F. C. V. and F. F.) abstracted information on the intensity of nodule evaluation and outcomes for a consensus-based determination of these variables. A third clinician (G.-S. C.) adjudicated cases as needed.
Intensity of Lung Nodule Evaluation
We assessed the intensity of nodule evaluation recommended by the MDNC relative to guideline recommendations. The first deviation from guideline recommendations determined the intensity of recommended nodule evaluation. Less intense recommendations were defined by no follow-up or a longer-than-recommended interval for reimaging. More intense recommendations were defined by a shorter-than-recommended interval for follow-up CT scan; use of advanced imaging or an invasive test when a CT scan or no follow-up is recommended by the guidelines; or surveillance beyond 2 years. Clinician abstractors assumed that absence of deviation from guidelines represented a guideline-concordant recommendation. Clinician abstractors recorded deviations from guidelines based on documentation provided by the pulmonologist who evaluated the patient during the clinic visit. When documented, clinician abstractors also recorded the reason why clinic recommendations deviated from guidelines.
We additionally evaluated the intensity of care that patients actually received (regardless of recommendations) relative to guidelines. Because there are many reasons why follow-up may not occur within the exact recommended interval, windows of ascertainment were defined. For recommended testing within the first 6 months of nodule diagnosis, a 2-month window was allowed for guideline-concordant evaluation (eg, 1 month prior to and following the recommended interval). A 6-month window was allowed for guideline-concordant evaluation (eg, 3 months prior to and following the recommended interval) for recommended testing 9 to 24 months from nodule diagnosis.
Diagnostic and Patient Outcomes
Pathologic and microbiologic findings from biopsies and/or surgical resections preferentially defined diagnostic outcomes. In the absence of pathologic confirmation of malignancy, clinician documentation of a cancer diagnosis along with a documented intent-to-treat (or reason why treatment was not indicated) were used to define a cancer diagnosis. Patients with 3 years of follow-up, who were alive at 3 years, and without documented clinical or pathologic evidence of malignancy were categorized as having a nodule that was “presumed benign.”
Other outcomes included imaging utilization, adverse events associated with invasive procedures, and 3-year overall survival. Research staff contacted patients and families without clinical documentation of vital status at 3 years.
Analysis
We determined 95% CIs for the frequencies of intensities of recommended and received nodule evaluation as well as diagnostic and patient outcomes. We did not pursue a time-to-event analysis primarily because we did not believe the assumption of noninformative censoring to be valid in this study of patients referred to a specialty clinic at an academic cancer center. STATA/SE version 14 (StataCorp) was used for all analyses.
Results
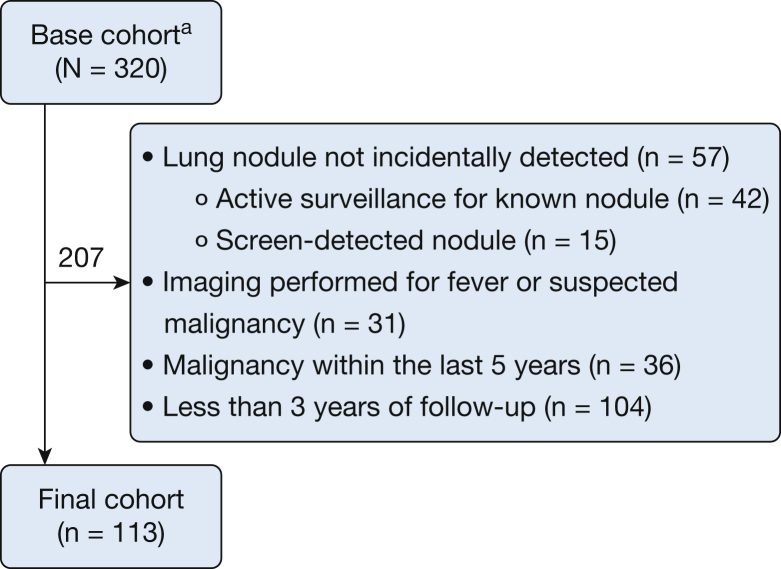
Of 320 patients referred to an MDNC, 113 were eligible for this study. The most common reason for exclusion was < 3 years of follow-up data (Fig 1). A majority of the 113 eligible patients were aged > 60 years, female, white, overweight, and a former or current smoker. In addition, a majority had more than one nodule. The dominant nodule had a median size of 8 mm and was most often solid and located in the upper lobes (Table 1).
Figure 1.
Cohort formation. aIndividuals referred to the Seattle Cancer Care Alliance Lung Cancer Early-Detection and Prevention Clinic between January 2010 and December 2012 who consented to participate in a lung nodule biorepository. Individuals excluded met one or more of the exclusion criteria.
Table 1.
Characteristics of the Study Population (N = 113)
| Characteristic | Value |
|---|---|
| Age, median (IQR), y | 61 (15) |
| Female sex | 66 (58%) |
| Race | |
| White | 97 (86%) |
| Black | 4 (3.5%) |
| Asian/Pacific Islander | 9 (8.0%) |
| Hispanic or Latino | 3 (2.7%) |
| BMI, median (IQR), kg/m2 | 28 (9) |
| No. of comorbidities | |
| 0 | 19 (17%) |
| 1 | 30 (27%) |
| 2 | 35 (31%) |
| ≥ 3 | 29 (25%) |
| Smoking status | |
| Never | 35 (31%) |
| Former | 51 (45%) |
| Current | 27 (24%) |
| Pack-years, median (IQR), ya | 27 (35) |
| Years since quit, median (IQR)b | 16 (20) |
| Personal history of extrathoracic malignancy | 10 (8.9%) |
| Personal history of thoracic radiation therapy | 4 (3.5%) |
| Family history of lung cancer | 25 (22%) |
| Previous imaging available | 38 (34%) |
| Indication for CT imagingc | |
| Chest radiographic abnormality | 35 (31%) |
| Cough | 26 (23%) |
| Dyspnea | 24 (21%) |
| Chest pain | 14 (12%) |
| Other | 45 (40%) |
| Nodule characteristicsd | |
| Size | |
| Median (IQR), mm | 8 (13) |
| ≤ 4 mm | 20 (18%) |
| > 4-6 mm | 22 (20%) |
| > 6-8 mm | 17 (15%) |
| > 8 mm | 54 (48%) |
| More than 1 nodule | 69 (61%) |
| Density | |
| Solid | 98 (88%) |
| Partially solid | 12 (11%) |
| Ground-glass opacity | 3 (2.7%) |
| Spiculated | 29 (26%) |
| Lobar location of nodule | |
| Upper lobes | 58 (51%) |
| Lower lobes | 47 (42%) |
| Spans multiple lobes | 1 (0.88%) |
| Not reported | 7 (6.2%) |
| Year | |
| 2010 | 39 (35%) |
| 2011 | 39 (35%) |
| 2012 | 35 (31%) |
| Median follow-up (IQR), ye | 4.4 (1.8) |
IQR = interquartile range.
Among 78 current or former smokers.
Among 51 former smokers.
Columns may not add to 100% because patients may have multiple conditions or indications.
Refers to the dominant nodule.
Follow-up < 3 years is due to death.
The MDNC recommended guideline-concordant evaluation in 76 patients (67%; 95% CI, 58-76). Recommendations were less intense than guidelines in eight patients (7%; 95% CI, 3.1-13) and more intense than guidelines in 29 patients (26%; 95% CI, 18-25). Sixty-five patients (58%; 95% CI, 48-67) received (regardless of what was recommended) guideline-concordant evaluations, whereas 22 (20%; 95% CI, 13-28) and 26 (23%; 95% CI, 16-32) patients received less and more intense evaluations, respectively. Table 2 shows the relation between recommended and received nodule evaluation, and Tables 3 and 4 summarize the reasons for deviations in recommended and received care. Of the patients who were recommended guideline-discordant care, the most common reason for deviating from guidelines was concern for more than one diagnosis, each of which would benefit from early detection and treatment. The most common reason why actual care received deviated from guideline-recommended care was an alternative recommendation by the MDNC or referring provider. Utilization of imaging was higher with increasing intensity of nodule evaluation. The rate of invasive procedures was highest among patients who received guideline-concordant care (Table 5).
Table 2.
Concordance of Recommended and Received Nodule Care Relative to Guideline Recommendations
| Intensity of Recommended Nodule Care | Intensity of Received Nodule Care |
||
|---|---|---|---|
| Less Intense | Guideline Concordant | More Intense | |
| Less intense | 7 | 1 | 0 |
| Guideline-concordant | 12 | 62 | 2 |
| More intense | 3 | 2 | 24 |
Boldface indicates the number of cases with concordance in intensity of nodule care recommended and received.
Table 3.
Reasons Why Recommended Nodule Evaluation Deviated From Guideline Recommendations
| Variable | Any Deviation From Guideline Recommendations (n = 37) | Less Intense Recommendation (n = 8) | More Intense Recommendation (n = 29) |
|---|---|---|---|
| Competing diagnoses | 15 | 0 | 15 |
| Different data or criteria used | 2 | 1 | 1 |
| Patient considered lower risk | 2 | 2 | 0 |
| Semi-solid nodule or other radiographic appearance | 6 | 3 | 3 |
| Pretransplantation evaluation | 2 | 0 | 2 |
| Could not be determineda | 10 | 2 | 8 |
Counts will not add up to total sample because some individuals will have multiple documented reasons for guideline nonadherent care.
Could not determine from the clinical documentation.
Table 4.
Reasons Why Received Nodule Evaluation Deviated From Guideline Recommendations
| Variable | Any Deviation From Guideline-Recommended Care (n = 48) | Less Intense Actual Care (n = 22) | More Intense Actual Care (n = 26) |
|---|---|---|---|
| Referring/outside provider preference | 2 | 0 | 2 |
| Multidisciplinary team recommendation | 32 | 8 | 24 |
| Insurance related | 0 | 0 | 0 |
| Short-term loss of follow-up | 3 | 3 | 0 |
| Could not be determineda | 11 | 11 | 0 |
Counts will not add up to total sample because some individuals will have multiple documented reasons for guideline non-adherent care.
We could not discern reason behind less intense care in patients who did not undergo recommended studies and were lost to follow-up from the multidisciplinary clinic but were known to be alive through other interactions with the health-care system.
Table 5.
Diagnostic Test Utilization Associated With the Intensity of Received Lung Nodule Evaluation
| Variable | Less Intense (n = 22) |
Guideline-Concordant (n = 65) |
More Intense (n = 26) |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % (95% CI) | No. of Patients | % (95% CI) | No. of Patients | % (95% CI) | |
| No. of imaging tests | ||||||
| 0 | 7 | 32 (15-55) | 18 | 28 (18-40) | 4 | 15 (5.5-36) |
| 1 | 10 | 46 (25-67) | 35 | 54 (41-66) | 2 | 7.7 (1.8-28) |
| 2 | 3 | 14 (4.05-37) | 5 | 7.7 (3.2-18) | 6 | 23 (10-44) |
| 3 | 1 | 4.6 (0.5-30) | 2 | 3.1 (0.7-12) | 9 | 35 (18-56) |
| ≥ 4 | 1 | 4.6 (0.5-30) | 5 | 7.7 (3.2-18) | 5 | 19 (7.7-40) |
| Any invasive procedure | 0 | 0 (0-15) | 37 | 57 (44-69) | 10a | 39 (21-59) |
Of the 10 patients with more intense nodule evaluation who underwent an invasive procedure, the testing that resulted in more intense evaluation included shorter-than-recommended interval for imaging (n = 6), > 2 years of surveillance (n = 1), and bronchoscopy (n = 3).
The prevalence of lung cancer, metastasis from a non-lung primary, and confirmed or presumed benign nodules was 29%, 1.2%, and 67%, respectively. A majority of lung cancer diagnoses were among the group who received guideline-concordant care. No subject who received less intense care had a lung cancer diagnosis following 3 years of follow-up, and four individuals who received more intense care were ultimately diagnosed with lung cancer. Most deaths (10 of 16) occurred following a cancer diagnosis. In a post hoc analysis, we determined that the frequency of any cancer using a cumulative incidence rate accounting for the competing risk of death resulted in a similar estimate (32%) as our primary analysis. Three-year survival rates were lowest for the group that received guideline-concordant care (Table 6). We conducted a post hoc survival analysis using Kaplan-Meier methods, and the 3-year survival rates were similar to our primary analysis across the less intense, as intense, and more intense groups (90%, 82%, and 92%).
Table 6.
Outcomes According to the Intensity of Received Lung Nodule Evaluation
| Outcome | Less Intense (n = 22) |
Guideline-Concordant (n = 65) |
More Intense (n = 26) |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % (95% CI) | No. of Patients | % (95% CI) | No. of Patients | % (95% CI) | |
| Diagnostic outcomes | ||||||
| Benign nodule | 21 | 95 (77-100) | 34 | 52 (40-65) | 21 | 81 (61-93) |
| Infection | 0 | 0 (0-15) | 4 | 6.2 (1.7-15) | 0 | 0 (0-13) |
| Inflammatory | 0 | 0 (0-15) | 3 | 4.6 (0.96-13) | 4 | 15 (4.4-35) |
| Clinically benigna | 9 | 41 (21-64) | 23 | 35 (24-48) | 15 | 58 (37-77) |
| Presumed benignb | 12 | 55 (32-76) | 4 | 6.2 (1.7-15) | 2 | 7.7 (0.95-25) |
| NSCLC | 0 | 0 (0-15) | 27 | 42 (29-54) | 4 | 15 (4.4-35) |
| Stage I/II | 0 | 0 (0-15) | 14 | 22 (12-33) | 4 | 15 (4.4-35) |
| Stage III/IV | 0 | 0 (0-15) | 13 | 20 (11-32) | 0 | 0 (0-13) |
| SCLC | 0 | 0 (0-15) | 2 | 3.1 (3.7-11) | 0 | 0 (0-13) |
| Limited stage | 0 | 0 (0-15) | 1 | 1.5 (0.04-8.3) | 0 | 0 (0-13) |
| Extensive stage | 0 | 0 (0-15) | 1 | 1.5 (0.04-8.3) | 0 | 0 (0-13) |
| Metastatic disease from non-lung primary | 0 | 0 (0-15) | 2 | 3.1 (0.4-11) | 0 | 0 (0-13) |
| Not reported (%)c | 1 | 4.5 (0.1-23) | 0 | 0 (0-5.5) | 1 | 3.9 (0.1-20) |
| Patient outcomes | ||||||
| Unplanned hospitalization | 0 | 0 (0-15) | 1 | 1.6 (0.04-8.5) | 2 | 7.7 (1.7-28) |
| Three-year overall survival (% alive) | 20 | 91 (71-99) | 53 | 82 (70-90) | 24 | 92 (75-99) |
NSCLC = non-small cell lung cancer; SCLC = small cell lung cancer.
Patients with clinician documentation of a benign nodule due to clinical stability or pathologic confirmation of a benign diagnosis were categorized as “clinically benign.”
Patients with 3 years of follow-up and without documented clinical or pathologic evidence of malignancy were categorized as having a nodule that was “presumed benign.”
Final diagnosis not reported in medical record, and patient died of other causes within 3 years.
Of the 10 individuals in the more intense group who underwent an invasive procedure, six had a benign diagnosis with four undergoing bronchoscopy, one underwent percutaneous lung biopsy, and another underwent mediastinoscopy. Of these six individuals, three were diagnosed with sarcoidosis, one was presumed to have benign disease after two nondiagnostic bronchoscopies and three years of follow-up, and the remaining two were found to have benign nodules, allowing the patients to proceed with transplantation. None of the 47 patients who underwent an invasive procedure experienced hemothorax, pneumothorax, respiratory failure requiring mechanical ventilation, or death (upper bound of a 97.5% CI, 7.5). Three patients had unplanned hospitalizations for hypoxia following invasive diagnostic procedures.
Discussion
Our investigation sought to understand care delivery by an MDNC in the context of published guidelines on the management of pulmonary nodules. An MDNC recommended guideline-concordant evaluations in 67% of patients, and 58% of patients received guideline-concordant care. The most common reason for deviating from guideline recommendations was to discern between two equally plausible conditions that would each benefit from early detection and treatment (eg, lung cancer and sarcoidosis). The most common reason for receiving guideline-discordant care was a provider recommendation.
To the best of our knowledge, this study is the first to describe the frequency of nodule care recommendations made by a clinician other than a radiologist. Previous studies report that radiologists variably recommend guideline-concordant care in 27% to 83% of patients.7, 8, 9, 10,12 These rates are not comparable to ours because providers in an MDNC have better access to information other than images (eg, lung cancer risk factors, symptom assessment). This information, along with an ability to have a real-time discussion between providers with expertise in imaging, diagnostic procedures, and management of benign and malignant pulmonary conditions, results in more granular patient assessment and personalized care than guidelines can offer. Supporting this assertion is the finding that the most common reason for recommending guideline-discordant care was to discern between two equally plausible conditions that would both benefit from early detection and treatment. These findings suggest that an MDNC may maximize guideline adherence while allowing for deviations that address the needs of a heterogeneous patient population.
Findings from other studies further motivate the hypothesis that implementation of an MDNC may promote guideline-concordant recommendations. Pulmonologists knowingly deviate from guidelines because of preferred nodule evaluation strategies arising from personal experience, perceptions of patient health status, anxiety about a cancer diagnosis, and fear of litigation.13 Primary care physicians generally do not know how to estimate cancer risk among individuals with a lung nodule and commonly rely on radiologist recommendations.2,14 Patients often do not understand the significance of a nodule, citing suboptimal communication on the part of their providers, resulting in patients taking a more passive role in their care.15, 16, 17 A multidisciplinary assessment may safeguard against the potential biases of any one provider.
Not all patients referred to an MDNC received guideline-concordant care. The rate of guideline-concordant nodule care in this study was higher than that in a multicenter observational study of patients with lung nodules recruited by pulmonologists to investigate plasma proteomic markers (58% vs 39%)18; the rate, however, was no different from that in patients cared for by Veterans Affairs providers (58% vs 55%).2 Cross-study comparisons are difficult because the patient populations were markedly different, as evident from the prevalence of lung cancer in the Veterans Affairs study,2 our investigation, and the multicenter community study18 (9% vs 25% vs 47%, respectively). As shown in the Veterans Affairs study and the current one, provider recommendations were an important factor associated with the receipt of guideline-discordant care. Patient autonomy and self-efficacy are unmeasured factors that may account for the difference between actual and recommended care. Other providers can undermine the clinic’s recommendations. The absence of structures and processes of care to ensure follow-up is another potential barrier to the clinic achieving its recommendations.
Importantly, the significance of adherence to guidelines remains uncertain. Evidence supporting guideline recommendations, based largely on uncontrolled studies of diagnostic accuracy or expert opinion, is low.4 To judge the quality of nodule care, a link between the intensity of evaluation and patient outcomes is necessary. Our study did not find a single case of late-stage lung cancer in patients who received less intense nodule evaluation. A more intense recommendation by the MDNC led to an earlier cancer diagnosis in three patients, although the significance of an earlier diagnosis in terms of stage shift cannot be known. A fourth patient who was recommended a more intense evaluation had imaging beyond the recommended follow-up period, leading to a lung cancer diagnosis that would have likely been missed. As expected, more intense nodule evaluation was associated with greater utilization of diagnostic tests; however, imaging rather than invasive procedures accounted for the majority of utilization in the more intense groups. Procedure-related adverse events were rare. Although this study was underpowered and not designed to test the relation between the intensity of evaluation and outcomes, two investigations currently underway should resolve this area of uncertainty.19,20
Our study has several limitations. Exclusion of patients with < 3 years of follow-up may have biased our estimates of recommended and received care, but this design was necessary to ascertain the intensity of evaluation and outcomes. Even some integrated health systems lose one-third of patients 3 years following enrollment.21 We compared the measured baseline characteristics of subjects with and without 3 years of complete follow-up (post hoc analysis) (Table 7) and found that patients with < 3 years of follow-up tended to have smaller nodules. Another source of bias arises from the patients who did not consent to participate in the biorepository. Because we did not have approval to collect their data, we do not know if they are different from the current study cohort. It is possible that we may have mischaracterized the intent of the MDNC recommendations to follow or deviate from the 2005 FSG because we relied on clinic documentation. There was insufficient documentation within the medical record to determine why the MDNC deviated from guidelines in 27% of individuals who were not recommended guideline-concordant care, and why 23% of patients received guideline-discordant care. The 11 patients who received guideline-discordant care for unknown reasons tended to have smaller nodules compared with those who received guideline-concordant care or whose reason for deviation from guidelines was known (post hoc analysis) (Table 8). Although it is unlikely that we mischaracterized the most common reasons for deviations, we may have underestimated others. In the absence of a gold standard, we have no way of knowing if the recommended deviations from guidelines were appropriate.
Table 7.
Characteristics of Patients With and Without ≥3 Years of Follow-up
| Variable | Study Cohort (n = 113) | <3 y Follow-upa (n = 62) | P Value |
|---|---|---|---|
| Age, median (IQR), y | 61 (15) | 57 (17) | .46 |
| Female sex | 66 (58%) | 37 (60%) | .87 |
| Race | .26 | ||
| White | 97 (86%) | 56 (90%) | |
| Black | 4 (3.5%) | 2 (3.2%) | |
| Asian/Pacific Islander | 9 (8.0%) | 1 (1.6%) | |
| Hispanic or Latino | 3 (2.7%) | 2 (3.2%) | |
| Native American | 0 (0%) | 1 (1.6%) | |
| Smoking status | .36 | ||
| Never | 35 (31%) | 15 (24%) | |
| Former | 51 (45%) | 35 (57%) | |
| Current | 27 (24%) | 12 (19%) | |
| Pack-years, median (IQR), yb | 27 (35) | 19 (26) | .17 |
| Years since quit, median (IQR), yc | 16 (20) | 20 (20) | .43 |
| Nodule characteristicsd | |||
| Size | |||
| Median (IQR), mm | 8 (13) | 5.9 (6.1) | .002 |
| Size category | .023 | ||
| ≤ 4 mm | 20 (18%) | 23 (37%) | |
| > 4-6 mm | 22 (20%) | 12 (19%) | |
| > 6-8 mm | 17 (15%) | 9 (15%) | |
| > 8 mm | 54 (48%) | 18 (29%) | |
| Density | .36 | ||
| Solid | 98 (87%) | 57 (92%) | |
| Partially-solid | 12 (11%) | 5 (8.1%) | |
| Ground-glass opacity | 3 (2.7%) | 0 (0%) |
See Table 1 legend for expansion of abbreviation.
Of the 104 subjects with less than 3 years of follow-up, 62 were included in the analysis. Of the 42 who were excluded, 23 did not have an incidentally-detected nodule, 7 had imaging performed for a fever, 15 had malignancy within the last 5 years, and 3 were younger than age 35 years.
Among 125 current or former smokers.
Among 86 former smokers.
Refers to the dominant nodule.
Table 8.
Characteristics of Patients Who Were Lost to Follow-up for Unknown Reasons Compared to Those Whose Reasons for Deviation Was Known or Received Guideline Concordant Care
| Variable | Patients Who Did not Deviate or the Reason for Deviation was Known (n = 102) | Patients Lost to Follow-up for Reasons that Could not be Determined (n = 11) | P Value |
|---|---|---|---|
| Age, median (IQR), y | 62 (14) | 56 (16) | .12 |
| Female sex | 60 (59%) | 6 (55%) | .78 |
| Race | .73 | ||
| White | 86 (84%) | 11 (100%) | |
| Black | 4 (3.9%) | 0 (0%) | |
| Asian/Pacific Islander | 8 (7.8%) | 0 (0%) | |
| Hispanic or Latino | 3 (2.9%) | 0 (0%) | |
| Native American | 1 (1.0%) | 0 (0%) | |
| Smoking status | .59 | ||
| Never | 32 (31%) | 3 (27%) | |
| Former | 47 (46%) | 4 (36%) | |
| Current | 23 (23%) | 4 (36%) | |
| Pack-years, median (IQR), ya | 29 (36) | 15 (45) | .74 |
| Years since quit, median (IQR), yb | 17 (20) | 21 (20) | .54 |
| Nodule characteristicsc | |||
| Size | |||
| Median (IQR), mm | 8 (14) | 8 (2) | .81 |
| Size category | .001 | ||
| ≤ 4 mm | 20 (20%) | 0 (0%) | |
| > 4-6 mm | 21 (21%) | 1 (9.1%) | |
| > 6-8 mm | 11 (11%) | 6 (55%) | |
| > 8 mm | 50 (49%) | 4 (36%) | |
| Density | .37 | ||
| Solid | 89 (87%) | 9 (82%) | |
| Partially-solid | 11 (11%) | 1 (9.1%) | |
| Ground-glass opacity | 2 (2.0%) | 1 (9.1%) |
See Table 1 legend for expansion of abbreviation.
Among 78 current or former smokers
Among 51 former smokers
Refers to the dominant nodule
Our findings may not be generalizable to the use of the 2017 FSG,22 but patient and provider biases, preferences, self-efficacy, knowledge, attitudes, and beliefs are unlikely to have changed over such a short time. In addition, our findings are not generalizable to the management of semi-solid lesions. Although the 2005 FSG3 state that longer follow-up may be needed for part-solid/ground-glass lesions, it provided no specific guidance. Finally, our findings may not be generalizable to other populations and care settings given that our study involved clinicians and patients from an academic cancer center. Important challenges associated with implementing an MDNC into routine practice may include the lack of requisite expertise (eg, chest radiology) at a given institution, limited provider bandwidth, and the lack of reimbursement for such activities.
Conclusions
An MDNC is a system-level intervention that may promote guideline adherence while also allowing for a multidisciplinary basis by which to deviate from guideline recommendations. Such clinics may be more likely to incorporate other system-level interventions believed to improve nodule management.23
Acknowledgments
Author contributions: F. F. takes responsibility for (is the guarantor of) the content of this manuscript, including data and analysis. All authors made substantial contributions to conception and design and interpretation of data; revised it critically and substantially for important intellectual content; have provided final approval of the version to be published; and have agree to be accountable for all aspect of the work in ensuring that the questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. F. C. V., D. K. M., G.-S. C., J. J. H., M. Z., and F. F. also made substantial contributions to acquisition of data and analysis.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: D. E. W. was a consultant for Olympus Respiratory America and GRAIL; the vice chair of the American Cancer Society National Lung Cancer Roundtable; an advisory board member for the GO2 Lung Cancer Foundation; and an expert advisor for the BMS Foundation. F. C. V. was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health [Grant T32DK070555]. None declared (D. K. M., G.-S. C., S. P., R. K., J. J. H., M. Z., F. F.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The content is solely the responsibility of the authors and does not represent the views of the National Institutes of Health.
Footnotes
FUNDING/SUPPORT: Dr Verdial was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number T32DK070555.
References
- 1.Gould M.K., Tang T., Liu I.L. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192(10):1208–1214. doi: 10.1164/rccm.201505-0990OC. [DOI] [PubMed] [Google Scholar]
- 2.Wiener R.S., Gould M.K., Slatore C.G., Fincke B.G., Schwartz L.M., Woloshin S. Resource use and guideline concordance in evaluation of pulmonary nodules for cancer: too much and too little care. JAMA Intern Med. 2014;174(6):871–880. doi: 10.1001/jamainternmed.2014.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacMahon H., Austin J.H., Gamsu G. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395–400. doi: 10.1148/radiol.2372041887. [DOI] [PubMed] [Google Scholar]
- 4.Gould M.K., Donington J., Lynch W.R. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e93S–e120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gould M.K., Fletcher J., Iannettoni M.D. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(suppl 3):108S–130S. doi: 10.1378/chest.07-1353. [DOI] [PubMed] [Google Scholar]
- 6.Naidich D.P., Bankier A.A., MacMahon H. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304–317. doi: 10.1148/radiol.12120628. [DOI] [PubMed] [Google Scholar]
- 7.Lacson R., Prevedello L.M., Andriole K.P. Factors associated with radiologists’ adherence to Fleischner Society Guidelines for management of pulmonary nodules. J Am Coll Radiol. 2012;9(7):468–473. doi: 10.1016/j.jacr.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberg R.L., Bankier A.A., Boiselle P.M. Compliance with Fleischner Society Guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology. 2010;255(1):218–224. doi: 10.1148/radiol.09091556. [DOI] [PubMed] [Google Scholar]
- 9.Eisenberg R.L., Fleischner Society Ways to improve radiologists’ adherence to Fleischner Society guidelines for management of pulmonary nodules. J Am Coll Radiol. 2013;10(6):439–441. doi: 10.1016/j.jacr.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Esmaili A., Munden R.F., Mohammed T.L. Small pulmonary nodule management: a survey of the members of the Society of Thoracic Radiology with comparison to the Fleischner Society guidelines. J Thorac Imaging. 2011;26(1):27–31. doi: 10.1097/RTI.0b013e3181d73a78. [DOI] [PubMed] [Google Scholar]
- 11.Tanner N.T., Aggarwal J., Gould M.K. Management of pulmonary nodules by community pulmonologists: a multicenter observational study. Chest. 2015;148(6):1405–1414. doi: 10.1378/chest.15-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masciocchi M., Wagner B., Lloyd B. Quality review: Fleischner criteria adherence by radiologists in a large community hospital. J Am Coll Radiol. 2012;9(5):336–339. doi: 10.1016/j.jacr.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 13.Wiener R.S., Slatore C.G., Gillespie C., Clark J.A. Pulmonologists’ reported use of guidelines and shared decision-making in evaluation of pulmonary nodules: a qualitative study. Chest. 2015;148(6):1415–1421. doi: 10.1378/chest.14-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golden S.E., Wiener R.S., Sullivan D., Ganzini L., Slatore C. Primary care providers and a system problem: a qualitative study of clinicians caring for patients with incidental pulmonary nodules. Chest. 2015;148(6):1422–1429. doi: 10.1378/chest.14-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiener R.S., Gould M.K., Woloshin S., Schwartz L.M., Clark J.A. What do you mean, a spot?: A qualitative analysis of patients’ reactions to discussions with their physicians about pulmonary nodules. Chest. 2013;143(3):672–677. doi: 10.1378/chest.12-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slatore C.G., Golden S.E., Ganzini L., Wiener R.S., Au D.H. Distress and patient-centered communication among veterans with incidental (not screen-detected) pulmonary nodules. A cohort study. Ann Am Thorac Soc. 2015;12(2):184–192. doi: 10.1513/AnnalsATS.201406-283OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slatore C.G., Sullivan D.R., Pappas M., Humphrey L.L. Patient-centered outcomes among lung cancer screening recipients with computed tomography: a systematic review. J Thorac Oncol. 2014;9(7):927–934. doi: 10.1097/JTO.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanner N.T., Porter A., Gould M.K., Li X.J., Vachani A., Silvestri G.A. Physician assessment of pretest probability of malignancy and adherence with guidelines for pulmonary nodule evaluation. Chest. 2017;152(2):263–270. doi: 10.1016/j.chest.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institutes of Health. The effectiveness, safety, and costs of guideline-concordant lung nodule care. https://projectreporter.nih.gov/project_info_description.cfm?aid=9830022&icde=48644936&ddparam=&ddvalue=&ddsub=&cr=1&csb=default&cs=ASC&pball=. Accessed February 5, 2020.
- 20.National Institutes of Health Clinical Center. The watch the spot trial. NCTR01CA207375. ClinicalTrials.gov. Bethesda, MD: National Institutes of Health; 2015. https://clinicaltrials.gov/ct2/show/NCT02623712. Updated October 28, 2019.
- 21.National Cancer Institute, Cancer Research Network Scientific & Data Resources. https://crn.cancer.gov/resources/
- 22.MacMahon H., Naidich D.P., Goo J.M. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology. 2017;284(1):228–243. doi: 10.1148/radiol.2017161659. [DOI] [PubMed] [Google Scholar]
- 23.Simmons J., Gould M.K., Iaccarino J., Slatore C.G., Wiener R.S. Systems-level resources for pulmonary nodule evaluation in the United States: a national survey. Am J Respir Crit Care Med. 2016;193(9):1063–1065. doi: 10.1164/rccm.201511-2163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]