Abstract
Background
There are concerns that starting nicotine replacement therapy (NRT) in the immediate perioperative period may negatively impact wound healing. We investigated the association of NRT with postoperative outcomes among smokers hospitalized for a surgical procedure.
Methods
This was a retrospective study in 552 hospitals of active smokers hospitalized between January 1, 2015 and December 31, 2016 for a major surgical procedure (Medicare Severity Diagnosis-Related Group expected length of stay, ≥ 2 days). We analyzed the association of receipt of NRT within 2 days of admission with a composite outcome of inpatient complications and with other outcomes. We developed a propensity score for receipt of NRT and examined differences in outcomes in a propensity-matched cohort.
Results
Of 147,506 active smokers, 25,651 (17.4%) were prescribed NRT within 2 days of admission. Patients treated with NRT were younger; less likely to be black or Hispanic; more likely to have Medicaid; and more likely to have a diagnosis of alcohol or other substance use disorder, or COPD, compared with those who were not treated. In the propensity-matched analysis, there was no association between receipt of NRT and in-hospital complications (OR, 0.99; 95% CI, 0.93-1.05), mortality (OR, 0.84; 95% CI, 0.68-1.04), all-cause 30-day readmissions (OR, 1.02; 95% CI, 0.97-1.07), or 30-day readmission for wound complications (OR, 0.96; 95% CI, 0.86-1.07).
Conclusions
This is the first large observational study of surgical patients to demonstrate that perioperative NRT is not associated with adverse outcomes after surgery. These results strengthen the evidence that NRT should be prescribed routinely in the perioperative period.
Key words: nicotine replacement, smoking, surgery
Abbreviations: ICD-9/ICD-10 CM, International Classification of Diseases, 9th and 10th Revisions, Clinical Modification; IQR, interquartile range; NRT, nicotine replacement therapy
Thirty percent of all patients undergoing elective surgery procedures smoke, with an estimated 10 million operations performed on smokers annually in the United States.1 Several investigations found that smoking within 1 year postsurgery is associated with an increased risk of postoperative complications, including general morbidity, wound complications, infection, pulmonary complications, and death.2, 3, 4 Clinical practice guidelines recommend that all patients who smoke should be advised to stop smoking during each clinical encounter,5 and the American College of Surgeons has developed extensive resources to implement smoking cessation programs in surgical practices. Although quitting before surgery is optimal, there is still benefit to quitting during the postoperative period.6, 7, 8, 9, 10
Two small single-center observational studies have reported that receipt of nicotine replacement therapy (NRT) is associated with increased mortality after coronary artery bypass surgery11 and in critically ill patients.12 In addition, there is concern that NRT may impede wound/bone healing, even though there is no evidence from human studies.13,14 As such, surgeons and hospital systems have not embraced the routine use of NRT perioperatively. Because little is known about the in-hospital use of pharmacotherapy to support perioperative cessation, and there are limited clinical data on the association between NRT and postoperative outcomes, we investigated the receipt of NRT among hospitalized smokers and its associations with outcomes in active smokers hospitalized for a surgical procedure in a large sample of US hospitals. We hypothesized that NRT would not be associated with higher risks in the immediate perioperative period.
Materials and Methods
Data Source, Setting, and Patients
We conducted a retrospective study using the Premier inpatient database between January 1, 2015 and December 31, 2016. Premier is an enhanced administrative database developed for measuring quality and health care use. Participating hospitals represent all regions of the United States and are primarily small to medium-sized nonteaching located mostly in urban areas. In addition to the information contained in the standard hospital discharge file, Premier contains a date-stamped log of all billed items, including medications dispensed.
We identified active adult smokers (≥ 18 years old) on the basis of the presence of International Classification of Diseases, 9th and 10th Revisions, Clinical Modification diagnosis codes for active smoking (ICD-9 CM code 305.1 and ICD-10 code F17.2x; Z72.0; O99.33); ICD-9 codes pertaining to active smoking status have previously been validated and found to have fair sensitivity, but high specificity.15 We included only patients who underwent major surgeries, defined as those surgeries with an estimated length of stay of 2 days or more based on the geometric mean surgery length of stay in the Medicare Severity-Diagnosis Related Group (MS-DRG). We hypothesized that providers are less likely to prescribe NRT for short stays. We restricted the analysis to patients who underwent surgeries during the first 2 days after admission because patients undergoing surgery after day 3 could have been more complicated patients who needed several days of hospitalization to stabilize before surgery; this restriction ensured a more homogeneous cohort. Because our exposure window included day 1 or 2, we also excluded patients who died or were discharged during the first 2 days of hospitalization. We examined outcomes after day 2.
Receipt of NRT
Exposure to NRT was assessed by screening daily pharmacy data for any charge for nicotine replacement, including nicotine patch, gum, lozenge, spray, and inhaler. To ensure that the NRT exposure preceded the study outcomes, we included only patients who were initiated on NRT during the first 2 days of hospitalization (early NRT). Patients who started NRT after day 2 (late NRT) were excluded to ensure that exposure preceded the outcomes. We did not study the use of varenicline because prescriptions were rare (617 patients received varenicline), or of bupropion because we were unable to differentiate between bupropion prescribed for depression vs tobacco dependence treatment.
Patient, Surgery, and Hospital Characteristics
We collected demographic data, comorbidities, and insurance status and classified surgeries as either urgent/emergency or elective and into 11 specialty-based categories (orthopedic, endocrinology, vascular, gastroenterology, obstetrics, gynecology, neurosurgery, thoracic, plastic, spinal, and urology). We classified comorbidities by Elixhauser comorbidity measure, using Healthcare Cost and Utilization Project Elixhauser comorbidity software version 3.7.2016.2.2017. Because the acuity of a patient’s condition at the time of admission could influence the decision to order NRT, we included diagnosis codes for organ failure at the time of admission (cardiovascular, hepatic, respiratory, hematologic, neurologic, and renal); early receipt of mechanical ventilation; and early receipt of vasopressors or transfusions (within 2 days of admission). In addition, we recorded hospital characteristics, such as size, teaching status, urban or rural population served, and census region.
Outcome Measures
Our main outcomes were (1) a composite outcome of postoperative complications, (2) inpatient mortality, and (3) all-cause 30-day readmissions. We used the American College of Surgeons National Surgical Quality Improvement Program classification for postoperative complications, which includes postoperative site infection, infection of the device, nonhealing wound, pneumonia, mechanical ventilation, pulmonary embolism, myocardial infarction, sepsis, septic shock, and other procedure-related complications when these diagnoses were not present at admission. Outcomes including mortality and mechanical ventilation were assessed on day 3 or later. To investigate the association of NRT with surgical site infection or delayed wound healing, we report separately on postoperative site infection, infection of the device, and nonhealing wound for the index hospitalization and for readmission within 30 days.
Statistical Analysis
We calculated descriptive statistics for patients and hospitals, using percentages for categorical variables and means or medians for continuous variables. We computed unadjusted hospital-specific rates of NRT use across hospitals with ≥ 10 eligible patients. We compared the characteristics of surgical patients who received NRT with those of patients who did not receive NRT, using absolute standardized differences, where a difference greater than 10% is considered meaningful.16 By a hierarchical generalized logistic regression with clustering of patients within hospitals, we identified factors independently associated with use of NRT during hospital days 1 and 2. We report the median OR from the model to characterize the hospital contribution to a patient’s chance of receiving NRT.
Our primary analysis was in a propensity-matched cohort. We developed a propensity score for early NRT, using all patient characteristics, comorbidities, surgical type, organ failure, and selected interaction terms. We then used a greedy matching algorithm17 and matched NRT-exposed with nonexposed patients without replacement, and we carried out a conditional logistic regression analysis, accounting for the match. Unadjusted (accounting for clustering), covariate-adjusted, and propensity score-adjusted models for each outcome were also evaluated in the full cohort. In addition, we used standardized mortality ratio weighting to obtain estimates of average treatment effect among treated patients. Adjusted OR with associated 95% CI for NRT were calculated. To explore the possibility of a difference in treatment effect by subgroups, we repeated the analysis with the addition of interaction terms with NRT prescription for age groups and then for surgical type. In a sensitivity analysis, we evaluated characteristics and outcomes of patients who underwent surgery after day 2 of hospitalization.
All analyses were performed with SAS version 9.4 (SAS Institute, Inc). The Institutional Review Board at the University of Massachusetts-Baystate Medical Center approved the study, which was not considered human subjects research because the data set does not contain any identifiable patient information.
Results
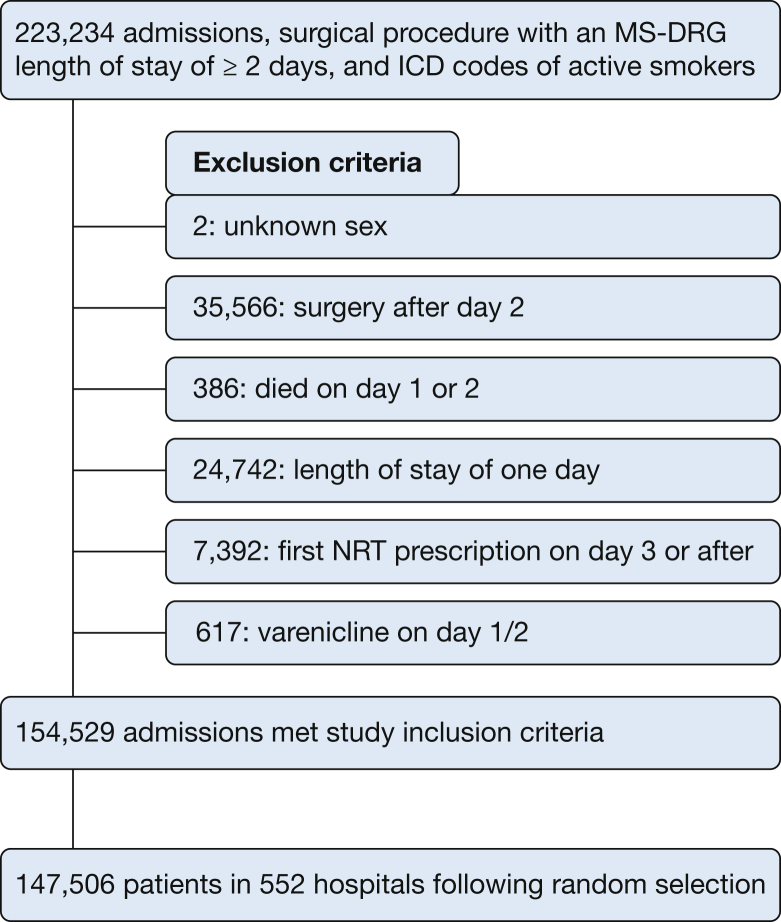
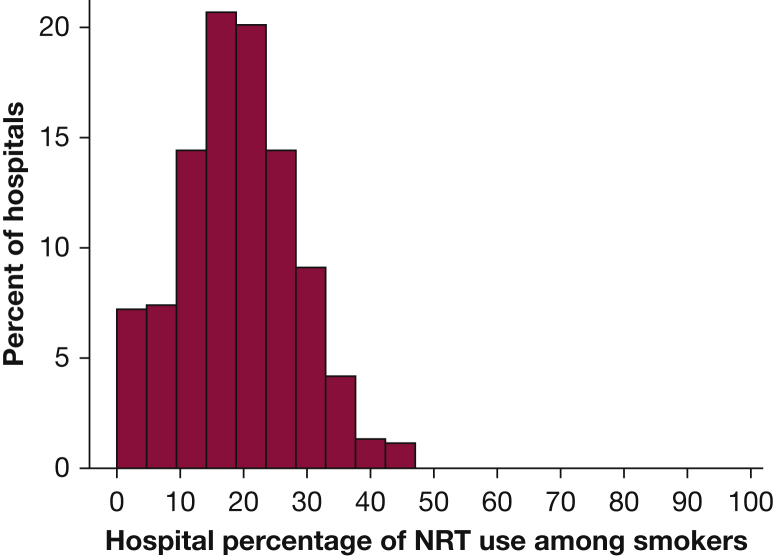
Of the 1,757,882 adult patients, 223,234 (12.7%) were identified as active preoperative smokers. After applying the exclusion criteria, 147,506 smokers at 552 hospitals were included (Fig 1). Of these, 25,651 (17.4%) were prescribed NRT within 2 days of admission. The most common therapy among NRT users was the nicotine patch (98.2%). The median hospital-level rate of NRT prescribing was 18.6% (interquartile range [IQR], 13.0%-24.9%), and only 1% of the hospitals prescribed NRT in more than 50% of their surgical patients coded as smokers (Fig 2). The inpatient composite complication rate was 9.1%, mortality was 0.9%, the median length of stay was 3 days (IQR, 3-6 days), and the all-cause 30-day readmission rate was 13.5%.
Figure 1.
Patient selection flow chart. ICD = International Classification of Diseases; MS-DRG = Medicare Severity-Diagnosis Related Group; NRT = nicotine replacement therapy.
Figure 2.
Variation among hospitals in use of pharmacotherapy for smoking cessation. See Figure 1 legend for expansion of abbreviation.
Factors Associated With NRT Prescription
On average, patients who received NRT were younger; less likely to be black or Hispanic; more likely to have Medicaid or to be uninsured; and more likely to have a diagnosis of alcohol or substance abuse disorder. Patients admitted to small and to nonteaching hospitals were more likely to be prescribed NRT (Table 1).
Table 1.
Characteristics of Patients Treated and Not Treated With Nicotine Replacement Therapy in the Full and Propensity-Matched Cohort
| Variable | Full Cohort |
Propensity-Matched Cohort |
|||
|---|---|---|---|---|---|
| No NRT | NRT 0/1/2a | SD (%)b | No NRT | NRT 0/1/2a | |
| Patients, No. (%) | 121,855 (82.6) | 25,651 (17.4) | 25,370 (50.0) | 25,370 (50.0) | |
| Age, median (IQR), y | 56 (42-65) | 55 (45-63) | 1.1 | 56 (44-63) | 55 (45-63) |
| Male sex, No. (%) | 59,187 (48.6) | 12,992 (50.7) | 12,751 (50.3) | 12,812 (50.5) | |
| Race, No. (%) | |||||
| White | 87,786 (72.0) | 20,782 (81.0) | 22.9 | 20,572 (81.1) | 20,538 (81.0) |
| Black | 17,453 (14.3) | 2,203 (8.6) | 2,236 (8.8) | 2,198 (8.7) | |
| Other or unknown | 10,987 (9.0) | 1,978 (7.7) | 1,893 (7.5) | 1,952 (7.7) | |
| Hispanic | 5,629 (4.6) | 688 (2.7) | 669 (2.6) | 682 (2.7) | |
| Insurance, No. (%) | |||||
| Medicare | 43,818 (36.0) | 9,137 (35.6) | 14.9 | 9,060 (35.7) | 9,068 (35.7) |
| Medicaid | 27,616 (22.7) | 6,931 (27.0) | 6,903 (27.2) | 6,815 (26.9) | |
| Private | 35,593 (29.2) | 6,350 (24.8) | 6,209 (24.5) | 6,316 (24.9) | |
| Uninsured | 7,023 (5.8) | 1,909 (7.4) | 1,891 (7.5) | 1,854 (7.3) | |
| Admission type, No. (%) | |||||
| Elective | 63,756 (52.3) | 11,540 (45.0) | 15.1 | 11,465 (45.2) | 11,486 (45.3) |
| Emergency/trauma | 57,220 (47.0) | 13,974 (54.5) | 13,783 (54.3) | 13,751 (54.2) | |
| Surgery classification, No. (%) | |||||
| Endocrine | 673 (0.6) | 147 (0.6) | 22.6 | 165 (0.7) | 140 (0.6) |
| GI | 28,740 (23.6) | 5,700 (22.2) | 5,731 (22.6) | 5,663 (22.3) | |
| Gynecologic | 6,237 (5.1) | 945 (3.7) | 959 (3.8) | 941 (3.7) | |
| Neurologic | 5,686 (4.7) | 1,170 (4.6) | 1,133 (4.5) | 1,151 (4.5) | |
| Obstetric | 6,028 (5.0) | 537 (2.1) | 439 (1.7) | 534 (2.1) | |
| Plastic | 4,348 (3.6) | 1,327 (5.2) | 1,299 (5.1) | 1,290 (5.1) | |
| Spinal | 9,567 (7.9) | 1,548 (6.0) | 1,548 (6.1) | 1,543 (6.1) | |
| Thoracic | 6,443 (5.3) | 1,797 (7.0) | 1,785 (7.0) | 1,758 (6.9) | |
| Urologic | 3,366 (2.8) | 603 (2.4) | 623 (2.5) | 600 (2.4) | |
| Orthopedic | 42,299 (34.7) | 10,056 (39.2) | 9,913 (39.1) | 9,945 (39.2) | |
| Vascular | 8,468 (7.0) | 1,821 (7.1) | 1,775 (7.0) | 1,805 (7.1) | |
| Comorbidities, No. (%) | |||||
| Hypertension | 62,285 (51.1) | 13,287 (51.8) | 1.4 | 13,110 (51.7) | 13,141 (51.8) |
| Chronic pulmonary disease | 34,051 (27.9) | 9,659 (37.7) | 20.8 | 9,553 (37.7) | 9,495 (37.4) |
| Psychoses/depression | 23,934 (19.6) | 6,911 (26.9) | 17.3 | 6,930 (27.3) | 6,779 (26.7) |
| Diabetes | 24,295 (19.9) | 4,899 (19.1) | 2.1 | 4,883 (19.2) | 4,866 (19.2) |
| Fluid and electrolyte disorders | 22,059 (18.1) | 5,616 (21.9) | 9.5 | 5,574 (22.0) | 5,468 (21.6) |
| Obesity | 21,961 (18.0) | 3,794 (14.8) | 8.7 | 3,952 (15.6) | 3,775 (14.9) |
| Chronic blood loss anemia | 19,362 (15.9) | 3,794 (14.8) | 3.1 | 3,778 (14.9) | 3,738 (14.7) |
| Drug abuse | 10,639 (8.7) | 3,447 (13.4) | 15.0 | 3,398 (13.4) | 3,334 (13.1) |
| Peripheral vascular disease | 11,057 (9.1) | 2,508 (9.8) | 2.4 | 2,507 (9.9) | 2,479 (9.8) |
| Alcohol abuse | 9,086 (7.5) | 3,634 (14.2) | 21.7 | 3,531 (13.9) | 3,486 (13.7) |
| Hypothyroidism | 9,920 (8.1) | 1,980 (7.7) | 1.6 | 2,017 (8.0) | 1,965 (7.8) |
| Paralysis/other neurologic diagnosis | 9,156 (7.5) | 2,275 (8.9) | 4.9 | 2,195 (8.7) | 2,240 (8.8) |
| Renal failure | 8,800 (7.2) | 1,395 (5.4) | 7.3 | 1,377 (5.4) | 1,387 (5.5) |
| Weight loss | 5,827 (4.8) | 1,477 (5.8) | 4.4 | 1,479 (5.8) | 1,437 (5.7) |
| Congestive heart failure | 5,716 (4.7) | 1,159 (4.5) | 0.8 | 1,146 (4.5) | 1,141 (4.5) |
| Liver disease | 5,021 (4.1) | 1,390 (5.4) | 6.1 | 1,387 (5.5) | 1,349 (5.3) |
| Gagne score, median (IQR) | 1 (0-3) | 1 (1-3) | 7.3 | 1 (1-3) | 1 (1-3) |
| Organ failure POA, No. (%) | |||||
| Respiratory | 2,273 (1.9) | 423 (1.6) | 1.7 | 443 (1.8) | 414 (1.6) |
| Cardiovascular shock | 3,290 (2.7) | 689 (2.7) | 0.1 | 714 (2.8) | 670 (2.6) |
| Renal | 5,852 (4.8) | 1,221 (4.8) | 0.2 | 1,215 (4.8) | 1,203 (4.7) |
| Hepatic | 604 (0.5) | 140 (0.5) | 0.7 | 128 (0.5) | 126 (0.5) |
| Acidosis | 2,852 (2.3) | 611 (2.4) | 0.3 | 600 (2.4) | 597 (2.4) |
| Neurologic | 2,101 (1.7) | 376 (1.5) | 2.1 | 356 (1.4) | 362 (1.4) |
| Hematologic | 4,090 (3.4) | 1,002 (3.9) | 2.9 | 992 (3.9) | 980 (3.9) |
IQR = interquartile range; NRT = nicotine replacement therapy; POA = present on admission; SD = significant difference.
NRT 0/1/2 = nicotine replacement therapy administered to patients before surgery (day 0) or postsurgery on hospital day 1 or 2.
Standard difference more than 10% is considered significant.
In multivariable logistic regression, several independent patient factors were associated with higher NRT use: younger age; Medicaid insurance (when compared with Medicare); chronic lung disease; psychiatric illness; and alcohol and drug abuse. Patients of black or Hispanic race/ethnicity were roughly 40% less likely to receive NRT than those of white race. Patients undergoing elective surgeries had a lower likelihood of receiving NRT than those undergoing urgent/emergency surgeries. There was also wide variation in the rates of NRT treatment between different surgical types. Specifically, compared with orthopedic patients, those undergoing thoracic surgery and those undergoing plastic surgery were 12% and 13%, respectively, more likely to be prescribed NRT while obstetrics and gynecology were 65% and 40% less likely (Table 2). The median OR was 1.8 (higher than any patient factors), indicating that the hospital where the patients were admitted had a large impact on their chance of receiving NRT.
Table 2.
Predictors for the Receipt of Nicotine Replacement Therapy
| Predictor | OR (95% CI) |
|---|---|
| Age, y | |
| 65+ | Referent |
| 45-64 | 1.65 (1.58-1.73) |
| 18-44 | 1.57 (1.48-1.67) |
| Sex | |
| Female | Referent |
| Male | 1.01 (0.98-1.04) |
| Race | |
| White | Referent |
| Other | 0.71 (0.66-0.76) |
| Hispanic | 0.57 (0.52-0.62) |
| Black | 0.54 (0.51-0.57) |
| Insurance | |
| Medicare | Referent |
| Medicaid | 1.13 (1.08-1.19) |
| Uninsured | 1.12 (1.05-1.20) |
| Private | 0.78 (0.75-0.82) |
| Admission type | |
| Emergency | Referent |
| Elective | 0.78 (0.76-0.81) |
| Surgery classification | |
| Orthopedic | Referent |
| Plastic | 1.13 (1.05-1.21) |
| Thoracic | 1.12 (1.05-1.19) |
| Endocrine | 1.04 (0.85-1.24) |
| Vascular | 0.99 (0.92-1.05) |
| Neurologic | 0.95 (0.88-1.01) |
| Urologic | 0.88 (0.80-0.96) |
| Spinal | 0.80 (0.75-0.85) |
| GI | 0.78 (0.75-0.81) |
| Gynecologic | 0.59 (0.54-0.64) |
| Obstetric | 0.35 (0.32-0.39) |
| Comorbidities | |
| Alcohol abuse | 1.73 (1.65-1.81) |
| Chronic pulmonary disease | 1.52 (1.47-1.57) |
| Drug abuse | 1.41 (1.35-1.48) |
| Psychoses/depression | 1.29 (1.25-1.34) |
| Fluid and electrolyte disorders | 1.13 (1.08-1.17) |
| Perivascular | 1.07 (1.01-1.12) |
| Weight loss | 1.07 (1.00-1.14) |
| Paralysis/other neurologic disorders | 1.04 (0.99-1.10) |
| Hypertension | 1.03 (1.00-1.07) |
| Liver disease | 1.01 (0.94-1.08) |
| Diabetes | 0.96 (0.93-1.00) |
| Chronic blood loss anemia/deficiency anemias | 0.92 (0.88-0.96) |
| Congestive heart failure | 0.91 (0.84-0.97) |
| Hypothyroidism | 0.90 (0.85-0.94) |
| Obesity | 0.80 (0.76-0.83) |
| Renal failure | 0.76 (0.71-0.81) |
| Organ failure present on admission | |
| Hematologic | 1.09 (0.98-1.20) |
| Renal | 1.00 (0.93-1.07) |
| Cardiovascular shock | 0.99 (0.90-1.08) |
| Hepatic | 0.97 (0.78-1.16) |
| Acidosis | 0.94 (0.85-1.04) |
| Respiratory | 0.88 (0.78-0.98) |
| Neurologic | 0.81 (0.71-0.90) |
Association Between NRT and Outcomes
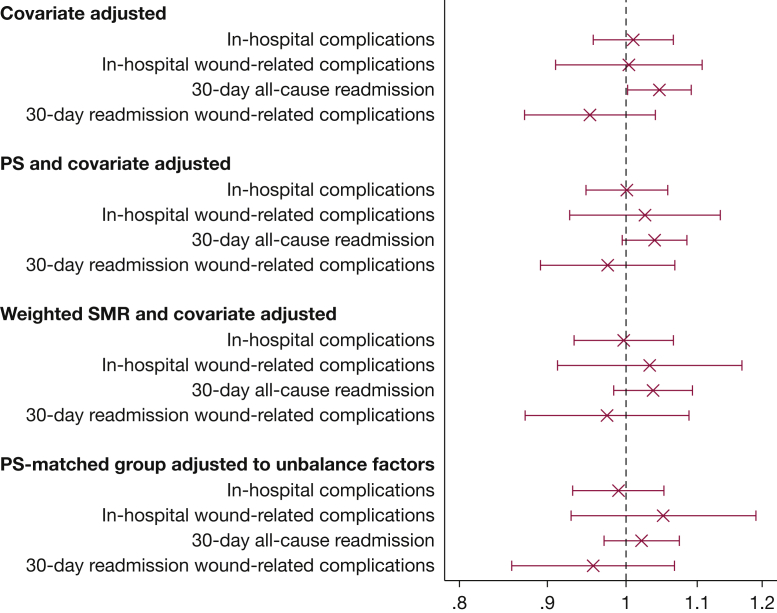
Compared with those who did not receive NRT, treated patients had lower mortality (0.64% vs 0.97%), higher all-cause readmission (14.2% vs 13.3%), and longer median hospital stay (3 days [IQR, 3-6 days] vs 4 days [IQR, 2-6 days]); in-hospital complications and readmissions for wound disruption or infections were not different between the groups. Overall, 25,370 of smokers receiving NRT (98.9%) were matched with patients who did not receive NRT with a similar propensity for treatment. The propensity model had a C-statistic of 0.73, and most variables were well balanced after matching. In the propensity score-matched analyses, with adjustment for unbalanced variables, no significant associations were found between NRT prescription and in-hospital complications (OR, 0.99; 95% CI, 0.93-1.05), mortality (OR, 0.84, 95% CI, 0.68-1.04), and all-cause readmissions (OR, 1.02; 95% CI, 0.97-1.07) (Fig 3). In addition, we found no associations between NRT and wound disruption or wound infections as inpatient complications or as causes for readmission. Results from covariate-adjusted, propensity score-adjusted, and standardized mortality ratio-weighted models were consistent with those from the propensity score-matched analysis. We found no evidence of treatment effect heterogeneity based on age or type of surgery.
Figure 3.
Association of receipt of nicotine replacement therapy with outcomes. PS = propensity score; SMR = standardized mortality ratio.
Sensitivity Analyses
Patient characteristics and unadjusted outcomes among patients with surgery after day 2 are presented in the online supplement. Of note, NRT prescription was slightly higher in this group, probably reflecting the longer stay; most of these patients (91%) underwent nonelective surgeries (e-Table 1). With the exception of mortality rate, which was slightly higher in patients not treated with NRT (but similar to the findings in the main cohort), the other outcomes were not significantly different between those who received NRT and those who did not (e-Table 2).
Discussion
In this large observational study of almost 150,000 active smokers undergoing a variety of surgical procedures at more than 500 hospitals in the United States, we found that fewer than one in five active smokers received NRT during hospitalization, and that receipt of NRT was not associated with adverse in-hospital outcomes. These results, in a “real-world,” nationally representative sample, using rigorous analytical methods, are significant because they reduce the uncertainty about the safety of NRT use in the immediate postoperative period, suggesting that tobacco treatment does not raise the risk of perioperative complications.
Only 17% of the smokers hospitalized for more than 2 days for a surgical procedure were prescribed NRT. This low rate of prescribing represents a missed opportunity for providing pharmacologic therapy for tobacco dependence as the hospitalization for the surgical procedure includes a period of forced cessation and a favorable environment for delivering smoking cessation interventions, one in which patients may have higher levels of motivation to quit. Our findings are consistent with the results of another large study, which assessed smoking pharmacotherapy practices in patients hospitalized with coronary artery disease, in whom 22.7% of patients were found to have received treatment.18 A notable finding of our study was the large variation in hospital rates of NRT prescribing, and in treatment rates among patients undergoing different surgical procedures, which may reflect underlying differences in hospital policies and in the culture and beliefs of surgical specialties.
A number of patient factors were independently associated with NRT prescribing: patients of black race or Hispanic ethnicity were less likely to be treated by NRT, while those with associated alcohol or drug abuse disorders as well as those who were uninsured or who had Medicaid insurance were more likely to receive NRT. Although previously reported, it is not clear what determines these patterns of prescribing. Our finding could reflect providers’ views regarding patients who will be more likely to have symptoms of perioperative withdrawal during hospitalization, or there may be differential rates of treatment refusal.
What factors explain the low rate of NRT prescribing observed in this study? There is still a concern among some surgeons regarding the safety of NRT in the perioperative period, although current data do not support this belief.8,9 Nicotine has the potential to trigger vasospasm and theoretically could influence wound healing. However, a 2012 systematic review found that although nicotine replacement drugs appear to attenuate inflammatory healing mechanisms, the effect is marginal and there is no evidence to suggest that NRT has a detrimental effect on postoperative outcomes of wound or tissue healing.2,19 Consistent with those results, we found that NRT was not associated with the risk of postoperative complications. Patients who received NRT did not have higher risk of inpatient mortality, readmission, or wound-related events. Our results are also consistent with the majority of observational and randomized trials, among patients with coronary artery disease, those with cardiac surgeries, or among the general population, which have demonstrated the safety of NRT.7,13,14,18,20,21
Our findings should be interpreted considering several limitations, which stem mostly from the observational nature of the study. First and foremost, NRT was not randomly assigned and, although we adjusted for multiple factors, there is a possibility of residual unmeasured confounding. Second, rates of wound infection most likely vary by surgery type and, although we did not specifically compare the rates of outcome by individual surgical type, we did not find an interaction between smoking and surgical type. Third, we used ICD-9 and ICD-10 codes that have high specificity but relatively low sensitivity for the identification of active smokers. This is probably why the prevalence of smoking in our cohort was lower (12.7%) compared with the 30% figure reported previously. Fourth, we were unable to determine whether the NRT given during the first two hospital days represented a continuation of outpatient therapy vs a new prescription in the hospital, or whether patients received a prescription of NRT at discharge. Similarly, our assessment of outcomes was limited to the 30 days after discharge; however, this is a standard time frame within which to assess surgical complications. Fifth, we did not have information on the duration and intensity of smoking or on other sources of nicotine exposure. Finally, our results represent clinical practices in 2016 and may not be fully representative of practice in 2019.
The findings from this study have important implications suggesting that it is safe for physicians to order NRT in the perioperative period to decrease the risk of craving and improve the hospital experience for smokers undergoing surgeries. It is hypothesized that initiation of NRT during a hospitalization may serve as a bridge for lifelong cessation.10 Surgical patients who smoke will also need specialized cessation services after hospitalization; a systematic review found that inpatient-initiated tobacco treatment needed to continue for more than 30 days after discharge to result in long-term abstinence.22,23 Ideally, the goal should be to complete smoking cessation before surgery.
In summary, in this large national sample we found that a minority of smokers hospitalized for a surgical procedure received NRT during their hospitalization and that receipt of NRT was not associated with adverse events. Although more high-quality studies would be welcome, this study offers compelling evidence from a real-world setting that NRT is not associated with in-hospital harm. Given that hospitalization is a teachable moment with high patient motivation to quit smoking, there appears to be a large opportunity to improve the care of hospitalized patients who undergo surgery, and policies should be developed and implemented to support the use of NRT in surgical patients.
Acknowledgments
Author contributions: M. S. S. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Each author meets the criteria for authorship credit set forth by the International Committee of Medical Journal Editors (ICMJE), as revised in 2013.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in data collection, management, analysis; study design, conduct, or interpretation of study findings; or the preparation, review, or approval of the manuscript submitted for publication.
Additional information: The e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: P. K. L. is supported by the National Heart, Lung, and Blood Institute [Grant K24HL132008].
Supplementary Data
References
- 1.Kamath A.S., Vaughan Sarrazin M., Vander Weg M.W., Cai X., Cullen J., Katz D.A. Hospital costs associated with smoking in veterans undergoing general surgery. J Am Coll Surg. 2012;214(6):901–908.e1. doi: 10.1016/j.jamcollsurg.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 2.Sørensen L.T. Wound healing and infection in surgery: the clinical impact of smoking and smoking cessation: a systematic review and meta-analysis. Arch Surg. 2012;147(4):373–383. doi: 10.1001/archsurg.2012.5. [DOI] [PubMed] [Google Scholar]
- 3.Grønkjær M., Eliasen M., Skov-Ettrup L.S. Preoperative smoking status and postoperative complications: a systematic review and meta-analysis. Ann Surg. 2014;259(1):52–71. doi: 10.1097/SLA.0b013e3182911913. [DOI] [PubMed] [Google Scholar]
- 4.Turan A., Mascha E.J., Roberman D. Smoking and perioperative outcomes. Anesthesiology. 2011;114(4):837–846. doi: 10.1097/ALN.0b013e318210f560. [DOI] [PubMed] [Google Scholar]
- 5.American College of Surgeons Strong for Surgery. https://www.facs.org/quality-programs/strong-for-surgery
- 6.Turan A., Koyuncu O., Egan C. Effect of various durations of smoking cessation on postoperative outcomes: a retrospective cohort analysis. Eur J Anaesthesiol. 2018;35(4):256–265. doi: 10.1097/EJA.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 7.Mills E., Eyawo O., Lockhart I., Kelly S., Wu P., Ebbert J.O. Smoking cessation reduces postoperative complications: a systematic review and meta-analysis. Am J Med. 2011;124(2):144–154.e8. doi: 10.1016/j.amjmed.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Sorensen L.T., Karlsmark T., Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238(1):1–5. doi: 10.1097/01.SLA.0000074980.39700.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warner D.O., Patten C.A., Ames S.C., Offord K.P., Schroeder D.R. Effect of nicotine replacement therapy on stress and smoking behavior in surgical patients. Anesthesiology. 2005;102(6):1138–1146. doi: 10.1097/00000542-200506000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Warner D.O. Perioperative abstinence from cigarettes: physiologic and clinical consequences. Anesthesiology. 2006;104(2):356–367. doi: 10.1097/00000542-200602000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Paciullo C.A., Short M.R., Steinke D.T., Jennings H.R. Impact of nicotine replacement therapy on postoperative mortality following coronary artery bypass graft surgery. Ann Pharmacother. 2009;43(7):1197–1202. doi: 10.1345/aph.1L423. [DOI] [PubMed] [Google Scholar]
- 12.Lee A.H., Afessa B. The association of nicotine replacement therapy with mortality in a medical intensive care unit. Crit Care Med. 2007;35(6):1517–1521. doi: 10.1097/01.CCM.0000266537.86437.38. [DOI] [PubMed] [Google Scholar]
- 13.Maa J. A second look at nicotine replacement therapy before surgical procedures. Mayo Clin Proc. 2015;90(11):1462–1464. doi: 10.1016/j.mayocp.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Nolan M.B., Warner D.O. Safety and efficacy of nicotine replacement therapy in the perioperative period: a narrative review. Mayo Clin Proc. 2015;90(11):1553–1561. doi: 10.1016/j.mayocp.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Wiley L.K., Shah A., Xu H., Bush W.S. ICD-9 tobacco use codes are effective identifiers of smoking status. J Am Med Inform Assoc. 2013;20(4):652–658. doi: 10.1136/amiajnl-2012-001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin P.C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228–1234. [Google Scholar]
- 17.Austin P.C. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33(6):1057–1069. doi: 10.1002/sim.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pack Q.R., Priya A., Lagu T.C. Short-term safety of nicotine replacement in smokers hospitalized with coronary heart disease. J Am Heart Assoc. 2018;7(18) doi: 10.1161/JAHA.118.009424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Møller A.M., Villebro N., Pedersen T., Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114–117. doi: 10.1016/S0140-6736(02)07369-5. [DOI] [PubMed] [Google Scholar]
- 20.Meine T.J., Patel M.R., Washam J.B., Pappas P.A., Jollis J.G. Safety and effectiveness of transdermal nicotine patch in smokers admitted with acute coronary syndromes. Am J Cardiol. 2005;95(8):976–978. doi: 10.1016/j.amjcard.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 21.Woolf K.J., Zabad M.N., Post J.M., McNitt S., Williams G.C., Bisognano J.D. Effect of nicotine replacement therapy on cardiovascular outcomes after acute coronary syndromes. Am J Cardiol. 2012;110(7):968–970. doi: 10.1016/j.amjcard.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 22.Rigotti N.A., Arnsten J.H., McKool K.M., Wood-Reid K.M., Singer D.E., Pasternak R.C. The use of nicotine-replacement therapy by hospitalized smokers. Am J Prev Med. 1999;17(4):255–259. doi: 10.1016/s0749-3797(99)00095-1. [DOI] [PubMed] [Google Scholar]
- 23.Rigotti N.A., Clair C., Munafò M.R., Stead L.F. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012;5:CD001837. doi: 10.1002/14651858.CD001837.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.