Abstract
Background
There are few studies focused on carbapenem-resistant Klebsiella pneumoniae (CRKP) bloodstream infection (BSI). The aim of this study is to identify the prevalence and risk factors for infection and mortality of CRKP BSI.
Methods
Susceptibility of Klebsiella pneumoniae (KP) isolated from blood samples and the proportion of CRKP were recorded annually. One hundred sixty-four patients with CRKP and 328 with carbapenem-susceptible Klebsiella pneumoniae (CSKP) BSI were categorized as the case group and control group to identify risk factors for CRKP infection and mortality by univariable analysis and multivariable logistic-regression analysis.
Results
The proportion and mortality of CRKP BSI increased significantly, with the percentage of KP in BSI increasing from 7 to 12% from 2014 to 2019 with a concomitant resistance to meropenem increasing from 16.7 to 41.8%. Compared with CSKP group, patients in CRKP group had longer hospitalization time before bacteremia (median 14 vs 4, P < 0.001) and longer total hospitalization time (median 31 vs 19, P < 0.001). The proportion of admission to ICU was higher (70.7% vs 17.7%, P < 0.001), and APACHE II score was higher (median 12 vs 8, P < 0.001). The mortality in CRKP group was 43.9% (72/164), while 14.9% (49/328) in CSKP group (p < 0.001). KP detection in other sites(P = 0.036, OR 1.964), blood purification(P = 0.018, OR 3.326), bronchoscopy(P = 0.011, OR 5.423), surgery (P = 0.001, OR 3.084), carbapenem use(P = 0.001, OR 3.395), tigecycline use(P = 0.006, OR 4.595) were independent risk factors for CRKP BSI. Previous hospitalization (P = 0.048, OR 2.755), long hospitalization (P = 0.003, OR 1.035), bone marrow puncture (P = 0.037, OR3.856), use of β-lactamase inhibitor (P = 0.005, OR 3.890) were independent risk factors for mortality in CRKP BSI.
Conclusion
The prevalence and mortality of CRKP BSI are still increasing. Timely treatment of KP infection in other site, strengthening the hospital infection control of blood purification, bronchoscopy and surgery, control the use of carbapenem and tigecycline, may help to prevent CRKP BSI. More preventative hospital resources are needed for severely ill patients with prolonged hospitalizations and intensive care.
Keywords: Carbapenem resistant Klebsiella pneumoniae (CRKP), Bloodstream infection, Prevalence, Mortality, Risk factors
Introduction
Carbapenems are the most effective and reliable β-lactams for the treatment of severe infections caused by multidrug resistant Enterobacteriaceae [1, 2]. In the past 10 years, carbapenems have been regarded as the last line of defense in the treatment of drug-resistant gram-negative bacterial infections. However, with the extensive use of carbapenems, many bacteria resistant to carbapenems have appeared, carbapenem resistant Klebsiella pneumoniae (CRKP) is one of which.
CRKP was first discovered in North Carolina in 1996, and now it has become the most common type of carbapenem resistant Enterobacteriaceae (CRE) in the United States [3]. At the same time, CRKP is also prevalent in Israel [4], Europe [5] and some South American countries [6, 7]. Carbapenem resistance among Klebsiella pneumoniae (KP) in the United States was as high as 12% of all isolates in 2009–2010 [8], while it was less than 1% in 2000 [3]. In Europe, It was 7.2% in 2017, Greece was the highest, 64.7% [9].
According to the data of China Antimicrobial Surveillance Network (CHINET) in 2018 [10], the proportion of KP in CRE strains is 73.5% and the resistance rates of KP to imipenem and meropenem were 26.3 and 25% respectively. The top five provinces with the highest prevalence of CRKP in China were Henan Province (61.8%), Shanxi Province (58.3%), Beijing City (55.7%), Zhejiang Province (53.3%) and Hebei Province (38%). However, CHINET only included data of KP from all parts of the body, the data of bloodstream infection (BSI) was not available.
Many studies have shown that CRKP significantly prolongs hospital stays and increases mortality compared to carbapenem-susceptible Klebsiella pneumoniae (CSKP) [11, 12]. Among the infections caused by CRKP in many sites, BSI is the most important type of infection with a high mortality rate [13]. A case-control study showed that the mortality rate of CRKP BSI was as high as 71.9%, which is much higher than the 21.9% of CRKP infection in other sites [14]. At present, there are many studies on the risk factors of CRKP infection, but most of them do not distinguish the infection sites. In this study, we will focus on BSI to analyze prevalence, mortality and risk factors for CRKP infection and mortality.
Materials and methods
Study design
Susceptibility of KP isolated from blood samples and the proportion of CRKP were recorded annually. To identify the risk factors for CRKP infections, we conducted a retrospective case-control study at the second hospital of Hebei Medical University, which is a grade III, class A university affiliated hospital in Shijiazhuang, Hebei province in North China with 2800 beds. All the adult inpatients (age ≥ 18 years) with positive blood cultures of KP, both CRKP and CSKP, who met diagnostic criteria of BSI according to the CDC / NHSN standard [15], were selected from the medical records in the hospital’s computerized microbiology laboratory database, dated between January 1, 2014 and June 30, 2019. The first positive sample of each patient was analyzed in the study. The mortality of KP, CRKP and CSKP BSI in each year and the total mortality in 5 years were calculated and analyzed. The prevalence of CRKP means the proportion of CRKP in KP strains.
Antimicrobial susceptibility testing
BacT / Alert3D automatic blood culture instrument is used for blood culture. Strain identification was performed with the Vitek2-compact automated microbiology system. Antimicrobial susceptibilities were determined by the VITEK system or the disk diffusion method. The results were interpreted according to the criteria recommended by the Clinical Laboratory Standards Institute (CLSIM100-S28), in which the US FDA standard was adopted for tigecycline test. CRKP was defined as an isolate with ertapenem (MICs ≥2 μg/ml), or imipenem and/or meropenem (MICs ≥4 μg/ml). The KP isolates susceptible to ertapenem, imipenem, and meropenem were considered as CSKP [16]. Escherichia coli ATCC 25922, and Pseudomonas aeruginosa ATCC 27853 were used as internal quality control.
Data collection
The following data was collected from medical records of each patient: ① Demographic characteristics: gender, age, length of stay, etc.; ② Comorbidities and underlying diseases: cardiovascular, pulmonary, renal disease, diabetes mellitus, solid tumor, hematological malignancy and immunocompromised; ③ Disease severity: acute physiology and chronic health status scoring system II (APACHE II)score; ④ Hospitalization and treatment before KP were isolated: admission to intensive care unit (ICU), previous hospitalization (≤3 months), special treatment (Corticosteroids, radiotherapy / chemotherapy), previous surgery (within 1 month) and recent invasive procedures (nasogastric or urinary catheter, central venous catheter (CVC), peripheral arterial catheter, blood purification (including continuous renal replacement therapy (CRRT), bedside hemofiltration, hemodialysis, plasmapheresis), tracheal cannula, tracheotomy, non-invasive mechanical ventilation, bone marrow puncture, bronchoscopy, parenteral nutrition, etc. a total of 17 items), use of antibiotics. ⑤ KP detection in other sites and death during the current admission.
Statistical analysis
SPSS version 22.0 was used to perform all the statistical analysis. Continuous variables were described as mean ± standard deviation (SD), which was compared using the Student’s t-test, if their distributions were not normal, they were described as median (quartiles) and were compared with the Mann-Whitney U test. Categorical variables were compared with a Pearson’s Chi-square test or a Chi-square test with a continuity correction if the frequency is < 5. Variables with P<0.05 in the univariable analysis above were included in multivariable logistic regression model. We calculated the odds ratios (ORs) and the 95% confidence intervals (CIs) for each variable. In multivariable logistic regression, the risk factors whose P<0.05 and OR > 1 were considered as independent risk factors. We used Hosmer-Lemeshow test to test the goodness of fit for logistic regression model and the logistic model is reliable when P>0.05 in this test.
Results
Trends in prevalence and mortality of CRKP BSI over the past 5 years
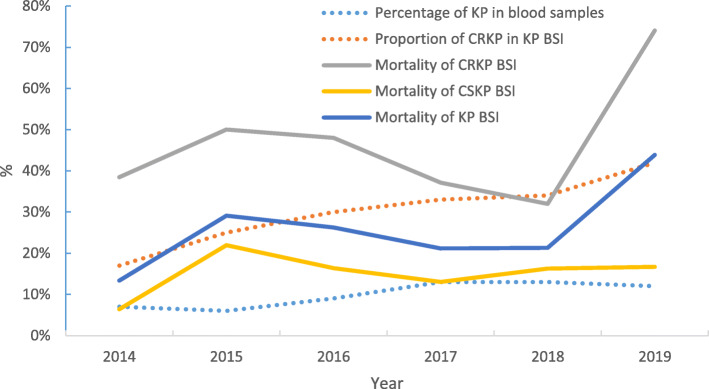
A total of 5545 strains were cultured and identified in the blood samples in our hospital, including 551 KP strains. The percentage of KP in blood sample from 2014 to the first half of 2019 were 7, 6, 9, 13, 13 and 12% respectively, which rose fastest from 2015 to 2017, then tended to be stable from 2017 to the first half of 2019. The percentage of CRKP in KP BSI increased annually from 17% in 2014 to 42% in the first half of 2019, as shown in Fig. 1.
Fig. 1.
Trends in prevalence and mortality of CRKP BSI over the past 5 years. The prevalence of KP BSI and the proportion of CRKP has been increasing year by year and the mortality of KP BSI also increased. The mortality of CRKP BSI is higher than that of CSKP. KP: Klebsiella pneumoniae; CRKP: Carbapenem-resistant KP; CSKP: Carbapenem-susceptible KP; BSI: bloodstream infection
Over the past 5 years, the drug resistance rate of KP detected in blood samples to all drugs except tigecycline has been increasing, especially to carbapenems (details are shown in Table 1).
Table 1.
Susceptibility of Klebsiella pneumoniae to antimicrobial agents from 2014 to 2019
| Antimicrobial agents | 2014(n = 72) | 2015(n = 59) | 2016(n = 88) | 2017(n = 115) | 2018(n = 150) | 2019(n = 67) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R(%) | S(%) | R(%) | S(%) | R(%) | S(%) | R(%) | S(%) | R(%) | S(%) | R(%) | S(%) | |
| Ampicillin | 84.7 | 2.8 | 83.1 | 0 | 84.1 | 0 | 93 | 0 | 94.4 | 0 | 95.5 | 0 |
| Ampicillin / sulbactam | 43.1 | 50 | 61 | 35.6 | 46.6 | 52.3 | 64.3 | 34.8 | 65.3 | 29.3 | 63.6 | 33.3 |
| Piperacillin | 40.3 | 54.2 | 61 | 35.6 | 45.3 | 52.3 | 61.1 | 34.5 | 66 | 31.3 | 67.2 | 32.8 |
| Piperacillin / tazobactam | 22.2 | 75 | 30.5 | 66.1 | 30.2 | 68.6 | 37.2 | 59.3 | 35.6 | 60.4 | 43.3 | 55.2 |
| Cefuroxime | 40.3 | 54.2 | 59.3 | 40.7 | 45.5 | 52.3 | 62.6 | 36.5 | 64.7 | 32 | 67.2 | 31.3 |
| Cefatriaxone | 40.3 | 59.7 | 59.3 | 40.7 | 44.3 | 55.7 | 62.6 | 37.4 | 64.7 | 35.3 | 65.7 | 34.3 |
| Ceftazidime | 29.2 | 70.8 | 39 | 59.3 | 35.2 | 64.8 | 50.4 | 46.1 | 45.3 | 50.7 | 52.2 | 46.3 |
| Cefepime | 25 | 73.6 | 28.8 | 62.7 | 35.2 | 63.6 | 50.4 | 47.8 | 42 | 53.3 | 51.5 | 47 |
| Cefotetan | 18.1 | 79.2 | 23.7 | 76.3 | 21.6 | 78.4 | 31.3 | 66.1 | 28.7 | 70 | 38.8 | 59.7 |
| Cefoperazone / sulbactam | 27.6 | 69 | 22.2 | 61.1 | 24.7 | 71.4 | 38.5 | 51.9 | 32.6 | 57.2 | 45.2 | 51.6 |
| Aztreonam | 31.9 | 68.1 | 37.3 | 61 | 38.6 | 61.4 | 56.5 | 43.5 | 51.3 | 48.7 | 61.2 | 38.8 |
| Levofloxacin | 28.1 | 71.9 | 33.9 | 59.3 | 33 | 67 | 50.4 | 49.6 | 38.7 | 57.3 | 55.2 | 43.3 |
| Ciprofloxacin | 29.2 | 68.1 | 37.3 | 59.3 | 35.2 | 62.5 | 53 | 43.5 | 46.7 | 50.7 | 56.7 | 43.3 |
| Gentamicin | 34.7 | 34.7 | 42.4 | 55.9 | 30.7 | 68.2 | 44.3 | 55.7 | 42 | 55.3 | 44.8 | 50.7 |
| Tobramycin | 13.9 | 72.2 | 25.4 | 52.5 | 30.7 | 61.4 | 41.7 | 46.1 | 24 | 54.7 | 38.8 | 53.7 |
| Imipenem | 22.2 | 76.4 | 25.4 | 71.2 | 29.5 | 69.3 | 34.8 | 64.3 | 34 | 65.3 | 43.3 | 53.7 |
| Meropenem | 16.7 | 83.3 | 25.4 | 74.6 | 30.2 | 68.6 | 33.6 | 65.5 | 34.2 | 65.8 | 41.8 | 58.2 |
| Amikacin | 8.3 | 91.7 | 16.9 | 81.4 | 14 | 86 | 25.2 | 73.9 | 12.8 | 87.2 | 28.4 | 71.6 |
| SMZ-TMP | 36.1 | 63.9 | 45.8 | 54.2 | 36.4 | 63.6 | 52.2 | 47.8 | 47.7 | 52.3 | 40.3 | 59.7 |
| Tigecycline | 0 | 65.9 | 0 | 67 | ||||||||
R Resistance, S susceptible
Four hundred ninety-two patients who met the criteria for BSI included 164 (33.3%) CRKP cases and 328 (66.7%) CSKP cases, among which 121 patients (24.6%) died during the current admission. The mortality in CRKP group was 43.9% (72/164), while 14.9% (49/328) in CSKP group. The difference was statistically significant (P<0.001). The mortality of KP BSI has increased from 14% in 2014 to 44% in the first half of 2019. The mortality of CRKP BSI and CSKP BSI increased from 38 and 6% in 2014 to 74 and 17% in the first half of 2019, respectively. Details were shown in Fig. 1.
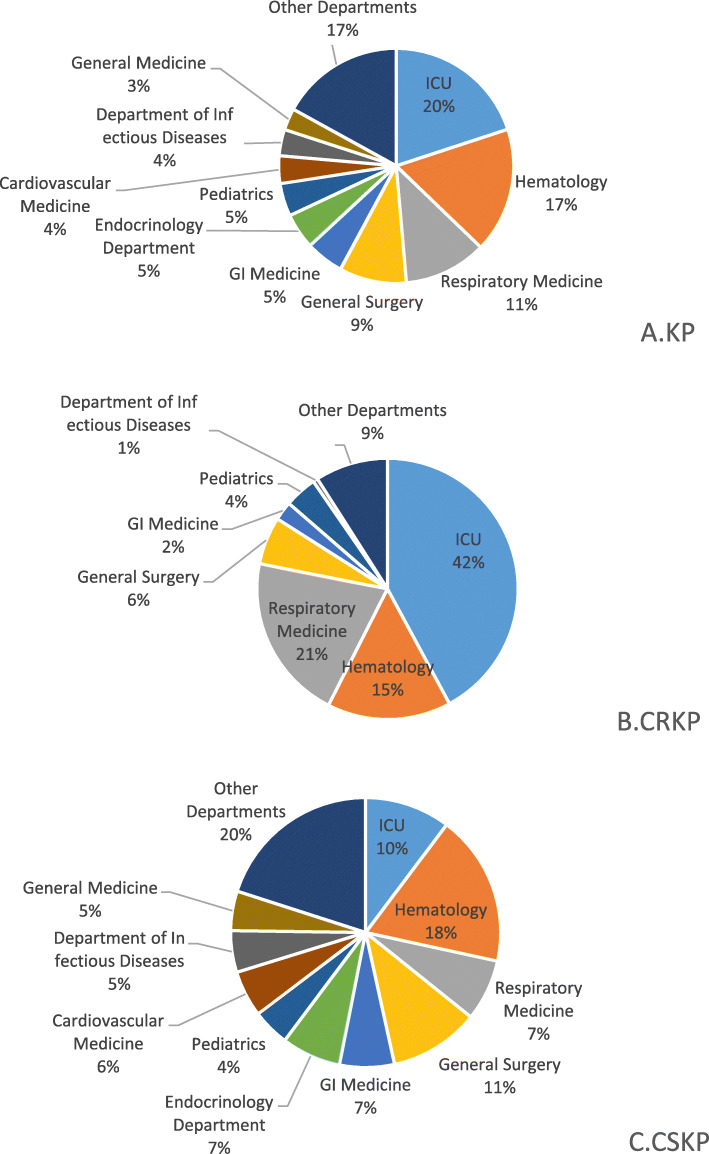
KP BSI was mainly distributed in Intensive Care Unit (ICU), Hematology Department, Respiratory Medicine and General Surgery Department (20, 17, 11 and 9% respectively, as shown in Fig. 2a). Nearly half of the CRKP was distributed in ICU (42%), followed by Respiratory Medicine (21%) and Hematology Department (15%). Details were shown in Fig. 2b. In 2014, 83% of the KP detected in blood from ICU was CRKP, which decreased and stabilized between 50 to 63% in the following years. The proportion of CRKP in KP rose to 42% in the first half of 2019 from 8% in 2014 in Hematology Department. Hematology Department and ICU account for 40% of CRKP BSI in 2014, which rose to 64% in the first half of 2019. CSKP was mainly detected from Hematology, General surgery and ICU (18, 11 and 10% respectively). Details were shown in Fig. 2c.
Fig. 2.
Distribution of KP, CRKP and CSKP in various Departments in the last 5 years. a, b and c showed the distribution of KP, CRKP and CSKP in various departments in the last 5 years, respectively. Half of the CRKP was distributed in ICU, while the distribution of CSKP in each department was relatively discrete. KP: Klebsiella pneumoniae; CRKP: Carbapenem-resistant KP; CSKP: Carbapenem-susceptible KP; ICU: Intensive Care Unit
Risk factors for CRKP BSI
Compared with CSKP group, patients in CRKP group had longer hospitalization time before bacteremia (median 14 vs 4, P < 0.001) and longer total hospitalization time (median 31 vs 19, P < 0.001). The proportion of admission to ICU was higher (70.7% vs 17.7%, P < 0.001), and APACHE II score was higher (median 12 vs 8, P < 0.001). In terms of underlying diseases, the proportion of hypertension, chronic obstructive pulmonary disease (COPD) and kidney disease in CRKP group was higher (43.9% vs 34.1, 8.5% vs 2.7, 13.4% vs 6.1%, P < 0.05), and that of diabetes was higher in CSKP group (20.7% vs 37.8%, P < 0.001).
In addition to blood samples, KP could be detected in samples from other sites in 186(37.8%) patients. The proportion of KP detection in other sites in CRKP group was higher than that in CSKP group (65.9% VS 23.8%, P < 0.001). In addition to blood samples, KP could be detected in one site in 63 (38.4%) patients in CRKP group, while 69(21%) in CSKP group. And KP could be detected in two or more other sites in 45 (27.4%) patients in CRKP group, while 9(2.7%) in CSKP group. Details were shown in Table 2.
Table 2.
KP detection in samples from other sites
| KP detection in samples from other sites | KP (n = 492) |
CRKP (n = 164) |
CSKP (n = 328) |
|---|---|---|---|
| KP detection in samples from other sites | 186(37.8%) | 108(65.9%) | 78(23.8%) |
| 1 site | 132 | 63 | 69 |
| Sputum | 75 | 49 | 26 |
| Urine | 15 | 1 | 14 |
| Drainage fluid of liver abscess | 13 | 0 | 13 |
| Pleural/peritoneal effusion | 11 | 8 | 3 |
| Skin and soft tissue | 6 | 3 | 3 |
| Bile | 6 | 1 | 5 |
| Cerebrospinal fluid | 2 | 0 | 2 |
| Catheter tip | 2 | 1 | 1 |
| Stool | 2 | 0 | 2 |
| 2 sites | 45 | 36 | 9 |
| Sputum+ Pleural/peritoneal effusion | 18 | 16 | 2 |
| Sputum+ Urine | 9 | 8 | 1 |
| Sputum+ Catheter tip | 6 | 2 | 4 |
| Sputum+ Skin and soft tissue | 4 | 4 | 0 |
| Sputum+ Cerebrospinal fluid | 2 | 2 | 0 |
| Sputum+ Drainage fluid of liver abscess | 2 | 0 | 2 |
| Sputum+ Pleural/peritoneal effusion | 2 | 2 | 0 |
| Sputum+ Stool | 1 | 1 | 0 |
| Stool+ Skin and soft tissue | 1 | 1 | 0 |
| 3 sites | 7 | 7 | 0 |
| Sputum+ Urine+ Pleural/peritoneal effusion | 4 | 4 | 0 |
| Sputum+ Skin and soft tissue+ Catheter tip | 1 | 1 | 0 |
| Sputum+ Urine+ Bile | 1 | 1 | 0 |
| Sputum+ Urine+ Catheter tip | 1 | 1 | 0 |
| 4 sites | 2 | 2 | 0 |
| Sputum+ Skin and soft tissue + Catheter tip + Pleural/peritoneal effusion | 2 | 2 | 0 |
KP Klebsiella pneumoniae, CRKP Carbapenem-resistant KP, CSKP Carbapenem-susceptible KP
In the total 492 patients, in addition to blood samples, KP could be detected in samples from other sites in 186(37.8%) patients, among which, 108(65.9%) were in CRKP group, which was significantly higher than 78(23.8%) in CSKP group (P < 0.001); In addition to blood samples, KP could be detected in one site in 63 (38.4%) patients in CRKP group, while 69(21%) in CSKP group; KP could be detected in two or more other sites in 45 (27.4%) patients in CRKP group, while 9(2.7%) in CSKP group
Univariate analysis showed that enema, indwelling nasogastric catheter, urinary catheter, CVC, peripheral arterial catheter, blood purification, tracheal cannula, tracheotomy, non-invasive ventilation, bronchoscopy, sputum aspiration, thoracentesis, abdominocentesis, lumbar puncture, previous surgery, enteral and parenteral nutrition, previous use of any antibiotics, carbapenems, glycopeptides, quinolones, β-lactamase inhibitors, linezolid and tigecycline were associated with CRKP BSI. Details were shown in Table 3.
Table 3.
Comparison of clinical characteristics between CRKP group and CSKP group
| Items | CRKP (n = 164) |
CSKP (n = 328) |
Z /χ2 | P value |
|---|---|---|---|---|
| Male | 110(67.1%) | 195(59.5%) | 2.696 | 0.101 |
| Age (years) | 56(29) | 59(19) | −0.103 | 0.918 |
| Previous hospitalization | 121(73.8%) | 214(65.2%) | 3.667 | 0.056 |
| Hospital stay (days) | 31(30) | 19(19) | −5.973 | <0.001 |
| Hospital stay before bacteremia (days) | 14(20) | 4(12) | −8.556 | <0.001 |
| APACHE II score | 12(6) | 8(5) | −9.025 | <0.001 |
| ICU admission | 116(70.7%) | 58(17.7%) | 134.604 | <0.001 |
| Use of systemic steroids | 46(28%) | 68(20.7%) | 3.288 | 0.070 |
| Chemotherapy/Radiotherapy | 30(18.3%) | 72(22%) | 0.890 | 0.345 |
| Use of immunosuppressant | 34(20.7%) | 79(24.1%) | 0.695 | 0.404 |
| Hypertension | 72(43.9%) | 112(34.1%) | 4.445 | 0.035 |
| Coronary heart disease | 37(22.6%) | 67(20.4%) | 0.299 | 0.585 |
| Diabetes mellitus | 34(20.7%) | 124(37.8%) | 14.619 | <0.001 |
| COPD | 14(8.5%) | 9(2.7%) | 8.233 | 0.004 |
| Renal diseases | 22(13.4%) | 20(6.1%) | 7.497 | 0.006 |
| Autoimmune diseases | 7(4.3%) | 10(3.0%) | 0.487 | 0.485 |
| Solid tumor | 19(11.6%) | 45(13.7%) | 0.440 | 0.507 |
| Hematologic malignancy | 24(14.6%) | 55(16.8%) | 0.369 | 0.543 |
| Enema | 14(8.5%) | 9(2.7%) | 8.233 | 0.004 |
| Nasogastric catheter | 117(71.3%) | 76(23.2%) | 106.419 | <0.001 |
| Urinary catheter | 126(76.8%) | 95(29%) | 101.245 | <0.001 |
| CVC | 142(86.6%) | 126(38.4%) | 102.298 | <0.001 |
| Peripheral arterial catheter | 34(20.7%) | 17(5.2%) | 28.499 | <0.001 |
| Blood purification | 36(22.0%) | 11(3.4%) | 43.766 | <0.001 |
| Tracheal cannula | 94(57.3%) | 39(11.9%) | 114.383 | <0.001 |
| Tracheostomy | 35(21.3%) | 21(6.4%) | 24.191 | <0.001 |
| Non-invasive ventilation | 22(13.4%) | 15(4.6%) | 12.215 | <0.001 |
| Gastroscopy | 6(3.7%) | 6(1.8%) | 0.865 | 0.352 |
| Colonoscopy | 2(1.2%) | 1(0.3%) | 0.377 | 0.539 |
| Bronchoscopy | 35(21.3%) | 4(1.2%) | 60.654 | <0.001 |
| Sputum suction | 102(62.2%) | 48(14.6%) | 116.699 | <0.001 |
| Thoracentesis | 37(22.6%) | 9(2.7%) | 50.660 | <0.001 |
| Abdominocentesis | 19(11.6%) | 5(1.5%) | 23.851 | <0.001 |
| Bone marrow puncture | 26(15.9%) | 56(17.1%) | 0.117 | 0.732 |
| Lumbar puncture | 24(14.6%) | 24(7.3%) | 6.649 | 0.01 |
| Previous surgery | 87(53.0%) | 82(25.0%) | 38.144 | <0.001 |
| Abdominal surgery | 50(30.5%) | 88(26.8%) | 0.725 | 0.394 |
| Enteral nutrition | 80(48.8%) | 44(13.4%) | 72.541 | <0.001 |
|
Parenteral nutrition Previous use of antibiotics |
95(57.9%) | 73(22.3%) | 61.866 | <0.001 |
| Any antibiotics | 158(96.3%) | 229(69.8%) | 45.822 | <0.001 |
| Carbapenems | 96(58.5%) | 44(13.4%) | 109.342 | <0.001 |
| Glycopeptides | 61(37.2%) | 24(7.3%) | 68.293 | <0.001 |
| Quinolones | 80(48.8%) | 85(25.9%) | 25.646 | <0.001 |
| 3rd/4th generation cephalosporins | 30(18.3%) | 47(14.3%) | 1.301 | 0.254 |
| 1st/2nd generation cephalosporins | 23(14.0%) | 42(12.8%) | 0.142 | 0.706 |
| Penicillins | 6(3.7%) | 21(6.4%) | 1.587 | 0.208 |
| β-lactamase inhibitor | 103(62.8%) | 104(31.7%) | 43.383 | <0.001 |
| Aminoglycosides | 7(4.3%) | 4(1.2%) | 3.359 | 0.067 |
| Linezolid | 19(11.7%) | 5(1.5%) | 24.044 | <0.001 |
| Tigecycline | 38(23.2%) | 10(3%) | 50.280 | <0.001 |
| Daptomycin | 3(1.8%) | 0(0.0%) | 3.396 | 0.065 |
| Nitroimidazoles | 8(4.9%) | 13(4.0%) | 0.224 | 0.636 |
|
KP detection in other sites Outcome |
108(65.9%) | 78(23.8%) | 82.311 | <0.001 |
| Death | 72(43.9%) | 49(14.9%) | 49.456 | <0.001 |
KP Klebsiella pneumoniae, CRKP Carbapenem-resistant KP, CSKP Carbapenem-susceptible KP, APACHE Acute Physiology and Chronic Health Evaluation, ICU Intensive Care Unit, COPD chronic obstructive pulmonary disease, CVC central venous catheter
Multivariable logistic regression showed that KP detection in other sites (P = 0.036, OR 1.964), blood purification (P = 0.018, OR 3.326), bronchoscopy (P = 0.011, OR 5.423), previous surgery (P = 0.001, OR 3.084), use of carbapenems (P = 0.001, or 3.395), use of tigecycline (P = 0.006, OR 4.595) were independent risk factors for CRKP BSI. Details were shown in Table 4. Hosmer-Lemeshow test showed P = 0.177, suggesting that the logistic regression model was reliable.
Table 4.
Multivariate logistic regression analysis of risk factors for CRKP BSI
| Items | P | OR | 95%CI |
|---|---|---|---|
| KP detection in other sites | 0.036 | 1.964 | (1.044, 3.695) |
| Blood purification | 0.018 | 3.326 | (1.224, 9.033) |
| Bronchoscopy | 0.011 | 5.423 | (1.483, 19.830) |
| Previous surgery | 0.001 | 3.084 | (1.572, 6.050) |
| Use of Carbapenems | 0.001 | 3.395 | (1.616, 7.134) |
| Use of Tigecycline | 0.006 | 4.595 | (1.557, 13.566) |
KP Klebsiella pneumoniae, CRKP Carbapenem-resistant KP, BSI bloodstream infections, OR Odds ratio, CI Confidence interval
Risk factors of mortality of KP, CRKP and CSKP BSI
The results of univariate analysis of risk factors of mortality in KP, CRKP and CSKP were shown in Table 5. Variables with statistical difference between the death group and survival group in the univariable analysis were included in multivariable logistic regression model.
Table 5.
Univariate analysis of risk factors associated with death in KP, CRKP and CSKP BSI
| Items | KP BSI | CRKP BSI | CSKP BSI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Death (n = 121) |
Survival (n = 371) |
P | Death (n = 72) |
Survival (n = 92) |
P | Death (n = 49) |
Survival (n = 279) |
P | |
| Male | 72(59.5%) | 233(62.0%) | 0.506 | 44(61.1%) | 66(71.7%) | 0.151 | 28(57.1%) | 167(59.9%) | 0.721 |
| Age (years) | 63(23) | 56(24) | <0.001 | 69(21) | 48(29) | <0.001 | 62(17) | 59(20) | 0.268 |
| previous hospitalization | 98(81.0%) | 237(63.9%) | <0.001 | 60(83.3%) | 61(66.3%) | 0.014 | 38(77.6%) | 176(63.1%) | 0.05 |
| Hospital stay (days) | 23(24) | 23(23) | 0.579 | 28(27) | 36(33) | 0.038 | 16(26) | 19(18) | 0.072 |
|
Hospital stay before bacteremia (days) bacteremia (days) |
13(26) | 5(14) | <0.001 | 14(28) | 14(14) | 0.041 | 6(24) | 3(12) | 0.056 |
| APACHE II SCORE | 13(7) | 8(5) | <0.001 | 14(8) | 12(5) | <0.001 | 12(8) | 8(4) | <0.001 |
| ICU admission | 67(55.4%) | 107(28.8%) | <0.001 | 53(73.6%) | 63(68.5%) | 0.473 | 14(28.6%) | 44(15.8%) | 0.03 |
| Use of systemic steroids | 37(30.6%) | 77(20.8%) | 0.026 | 23(31.9%) | 23(25.0%) | 0.326 | 14(28.6%) | 54(19.4%) | 0.142 |
| Chemotherapy/Radiotherapy | 31(25.6%) | 71(20.7%) | 0.127 | 17(23.6%) | 13(14.1%) | 0.119 | 14(28.6%) | 58(20.8%) | 0.225 |
| Use of immunosuppressant | 33(27.3%) | 80(21.6%) | 0.195 | 17(23.6%) | 17(18.5%) | 0.421 | 16(32.7%) | 63(22.6%) | 0.128 |
| Hypertension | 57(47.1%) | 127(34.2%) | 0.011 | 37(51.4%) | 35(38.0%) | 0.087 | 20(40.8%) | 92(33.0%) | 0.286 |
| Coronary heart disease | 33(27.3%) | 71(19.1%) | 0.057 | 25(34.7%) | 12(13.0%) | 0.001 | 8(16.3%) | 59(21.1%) | 0.44 |
| Diabetes mellitus | 36(29.8%) | 122(32.9%) | 0.522 | 21(29.2%) | 13(14.1%) | 0.018 | 15(30.6%) | 109(39.1%) | 0.26 |
| COPD | 11(9.1%) | 12(3.2%) | 0.008 | 8(11.1%) | 6(6.5%) | 0.297 | 3(6.1%) | 6(2.2%) | 0.273 |
| Renal diseases | 18(14.9%) | 24(6.5%) | 0.004 | 12(16.7%) | 10(10.9%) | 0.280 | 6(12.2%) | 14(5.0%) | 0.104 |
| Autoimmune diseases | 6(5.0%) | 11(3.0%) | 0.297 | 4(5.6%) | 3(3.3%) | 0.740 | 2(4.1%) | 8(2.9%) | 0.996 |
| Solid tumor | 27(22.3%) | 37(10.0%) | <0.001 | 10(13.9%) | 9(9.8%) | 0.415 | 17(34.7%) | 28(10.0%) | <0.001 |
| Hematologic malignancy | 21(17.4%) | 58(15.6%) | 0.654 | 14(19.4%) | 10(10.9%) | 0.123 | 7(14.3%) | 48(17.2%) | 0.614 |
| Enema | 6(5.0%) | 17(4.6%) | 0.865 | 6(8.3%) | 8(8.7%) | 0.934 | 0 | 9(3.2%) | 0.432 |
| Nasogastric catheter | 70(57.9%) | 123(33.2%) | <0.001 | 52(72.2%) | 65(70.7%) | 0.825 | 18(36.7%) | 58(20.8%) | 0.015 |
| Urinary catheter | 74(61.2%) | 147(39.6%) | <0.001 | 54(75.0%) | 72(78.3%) | 0.623 | 20(40.8%) | 75(26.7%) | 0.047 |
| CVC | 95(78.5%) | 173(46.6%) | <0.001 | 65(90.3%) | 77(83.7%) | 0.220 | 30(61.2%) | 96(34.4%) | <0.001 |
| Peripheral arterial catheter | 16(13.2%) | 35(9.4%) | 0.235 | 12(16.7%) | 22(23.9%) | 0.256 | 4(8.2%) | 13(4.7%) | 0.502 |
| Blood purification | 19(15.7%) | 28(7.5%) | 0.008 | 16(22.2%) | 20(21.7%) | 0.941 | 3(6.1%) | 8(2.9%) | 0.461 |
| Tracheal cannula | 51(42.1%) | 82(22.1%) | <0.001 | 42(58.3%) | 52(56.5%) | 0.816 | 9(18.4%) | 30(10.8%) | 0.129 |
| Tracheostomy | 21(17.4%) | 35(9.4%) | 0.017 | 16(22.2%) | 19(20.7%) | 0.808 | 5(10.2%) | 16(5.7%) | 0.239 |
| Non-invasive ventilation | 23(19.0%) | 14(3.8%) | <0.001 | 14(19.4%) | 8(8.7%) | 0.045 | 9(18.4%) | 6(2.2%) | <0.001 |
| Gastroscopy | 3(2.5%) | 9(2.4%) | 1.000 | 2(2.8%) | 4(4.3%) | 0.910 | 1(2.0%) | 5(1.8%) | 1.000 |
| Colonoscopy | 1(0.8%) | 2(0.5%) | 1.000 | 1(1.4%) | 1(1.1%) | 1.000 | 0 | 1(0.4%) | 1.000 |
| Bronchoscopy | 20(16.5%) | 19(5.1%) | <0.001 | 20(27.8%) | 15(16.3%) | 0.075 | 0 | 4(1.4%) | 0.890 |
| Sputum suction | 58(47.9%) | 92(24.8%) | <0.001 | 46(63.9%) | 56(60.9%) | 0.692 | 12(24.5%) | 36(12.9%) | 0.034 |
| Thoracentesis | 19(15.7%) | 27(7.3%) | 0.006 | 17(23.6%) | 20(21.7%) | 0.776 | 2(4.1%)) | 7(2.5%) | 0.883 |
| Abdominocentesis | 11(9.1%) | 13(3.5%) | 0.013 | 10(13.9%) | 9(9.8%) | 0.415 | 1 (2.0%) | 4(1.4%) | 1.000 |
| Bone marrow puncture | 28(23.1%) | 54(14.6%) | 0.028 | 17(23.6%) | 9(9.8%) | 0.016 | 11(22.4%) | 45(16.1%) | 0.278 |
| Lumbar puncture | 11(9.1%) | 37(10.0%) | 0.776 | 9(12.5%) | 15(16.3%) | 0.494 | 2(4.1%) | 22(7.9%) | 0.519 |
| Previous surgery | 45(37.2%) | 124(33.4%) | 0.449 | 33(45.8%) | 54(58.7%) | 0.101 | 12(24.5%) | 70(25.1%) | 0.929 |
| Abdominal surgery | 36(29.8%) | 102(27.5%) | 0.631 | 19(26.4%) | 31(33.7%) | 0.313 | 17(34.7%) | 71(25.4%) | 0.178 |
| Enteral nutrition | 47(38.8%) | 77(20.8%) | <0.001 | 38(52.8%) | 42(45.7%) | 0.365 | 9(18.4%) | 35(12.5%) | 0.270 |
|
Parenteral nutrition Previous use of antibiotics |
61(50.4%) | 107(28.8%) | <0.001 | 39(54.2%) | 56(60.9%) | 0.388 | 22(44.9%) | 51(18.3%) | <0.001 |
| Any antibiotics | 106(87.6%) | 281(75.7%) | 0.006 | 71(98.6%) | 87(94.6%) | 0.171 | 35(71.4%) | 194(69.5%) | 0.790 |
| Carbapenems | 65(53.7%) | 75(20.2%) | <0.001 | 51(70.8%) | 45(48.9%) | 0.005 | 14(28.6%) | 30(10.8%) | 0.001 |
| Glycopeptides | 38(31.4%) | 47(12.7%) | <0.001 | 30(41.7%) | 31(33.7%) | 0.295 | 8(16.3%) | 16(5.7%) | 0.02 |
| Quinolones | 52(43.0%) | 113(30.5%) | 0.011 | 41(56.9%) | 39(42.4%) | 0.064 | 11(22.4%) | 74(26.5%) | 0.548 |
| 3rd/4th generation cephalosporins | 22(18.2%) | 55(14.8%) | 0.377 | 17(23.6%) | 13(14.1%) | 0.119 | 5(10.2%) | 42(15.1%) | 0.372 |
| 1st/2nd generation cephalosporins | 14(11.6%) | 51(13.7%) | 0.539 | 8(11.1%) | 15(16.3%) | 0.342 | 6(12.2%) | 36(12.9%) | 0.899 |
| Penicillins | 5(4.1%) | 22(5.9%) | 0.451 | 2(2.8%) | 4(4.3%) | 0.910 | 3(6.1%) | 18(6.5%) | 1.000 |
| β-lactamase inhibitor | 71(58.7%) | 136(36.7%) | <0.001 | 54(75.0%) | 49(53.3%) | 0.004 | 17(34.7%) | 87(31.2%) | 0.626 |
| Aminoglycosides | 8(6.6%) | 3(0.8%) | 0.001 | 6(8.3%) | 1(1.1%) | 0.059 | 2(4.1%) | 1(0.7%) | 0.108 |
| Linezolid | 14(11.6%) | 10(2.7%) | <0.001 | 13(18.1%) | 6(6.6%) | 0.024 | 1(2.0%) | 4(1.4%) | 0.557 |
| Tigecycline | 26(21.5%) | 22(5.9%) | <0.001 | 20(27.8%) | 18(19.6%) | 0.216 | 6(12.2%) | 4(1.4%) | <0.001 |
| Daptomycin | 2(1.7%) | 1(0.3%) | 0.305 | 2(2.8%) | 1(1.1%) | 0.830 | 0 | 0 | – |
| Nitroimidazoles | 7(5.8%) | 14(3.8%) | 0.342 | 6(8.3%) | 2(2.2%) | 0.146 | 1(2.0%) | 12(4.3%) | 0.726 |
| KP detection in other sites | 68(56.2%) | 118(31.8%) | <0.001 | 52(72.2%) | 56(60.9%) | 0.128 | 16(32.7%) | 62(22.2%) | 0.114 |
| CRKP | 72(59.5%) | 92(24.8%) | <0.001 | ||||||
KP Klebsiella pneumoniae, CRKP Carbapenem-resistant KP, CSKP Carbapenem-susceptible KP, BSI bloodstream infections, APACHE Acute Physiology and Chronic Health Evaluation, ICU Intensive Care Unit, COPD chronic obstructive pulmonary disease, CVC central venous catheter
Multivariate analysis showed that previous hospitalization(P = 0.008, OR 2.484), solid tumor(P<0.001, OR 4.960), non-invasive ventilation(P = 0.007, OR 4.227) and use of β-lactamase inhibitor(P = 0.046, OR 2.007) were independent risk factors for death in KP BSI. And previous hospitalization(P = 0.048, OR 2.755), long hospitalization (P = 0.003, OR 1.035), bone marrow puncture (P = 0.037, OR 3.856), use of β-lactamase inhibitor (P = 0.005, OR 3.890) were independent risk factors for mortality in CRKP BSI. Solid tumor (P<0.001, OR 7.068) and non-invasive ventilation (P = 0.016, OR 9.778) were independent risk factors for mortality of CSKP BSI. Details were shown in Table 6. The P value of Hosmer-Lemeshow tests was 0.885, 0.468 and 0.518, respectively, suggesting that the logistic regression models were sufficiently reliable.
Table 6.
Multivariate logistic regression analysis of risk factors for mortality of KP, CRKP and CSKP BSI
| Items | P | OR | 95%CI | |
|---|---|---|---|---|
| KP BSI | Previous hospitalization | 0.008 | 2.484 | (1.269, 4.864) |
| Solid tumor | <0.001 | 4.960 | (2.148, 11.454) | |
| Non-invasive ventilation | 0.007 | 4.227 | (1.477, 12.098) | |
| Use β-lactamase inhibitor | 0.046 | 2.007 | (1.012, 3.978) | |
| CRKP BSI | Previous hospitalization | 0.048 | 2.755 | (1.009, 7.541) |
| Hospital stay (days) | 0.003 | 1.035 | (1.011, 1.058) | |
| Bone marrow puncture | 0.037 | 3.856 | (1.082, 13.741) | |
| Use β-lactamase inhibitor | 0.005 | 3.890 | (1.494, 10.127) | |
| CSKP BSI | Solid tumor | <0.001 | 7.068 | (2.683, 18.616) |
| Non-invasive ventilation | 0.016 | 9.778 | (1.529, 62.515) |
KP Klebsiella pneumoniae, CRKP Carbapenem-resistant KP; CSKP Carbapenem-susceptible KP, BSI bloodstream infection, OR Odds ratio, CI Confidence interval
Discussion
This study was focused on CRKP BSI. One of the major findings of our study was the increasing prevalence of KP and CRKP infections, both as a percentage of KP BSI/year and as absolute number. The possible reasons are as follows. In our hospital, the proportion of KP in samples from all sites of the body ranked second in 2014 and 2015(14.0 and 14.2%, respectively), which rose to the first place in 2016–2019(16.1,16.7 and 18.6%, respectively). This may increase its proportion in BSI. Over the past 5 years, the proportion of Staphylococcus hominis detected in blood samples was relatively high in 2014 and 2015(22 and 21%), ranking first, then it continued to decline from 2016 to 2019(15, 13 and 11%, respectively). It has been reported that staphylococcus hominis is the main pathogen of blood culture contamination [17]. With the increasing standardization of the blood specimen collection process, the isolation rate of Staphylococcus hominis decreased and the proportion of KP BSI increased. In addition, the distribution of CRKP was more and more concentrated in ICU and Hematology Department, where the development of CRKP is much easier, according to the risk factors for CRKP infection based on a meta-analysis [18]. Meanwhile, we found that under the same infection-control measures, the carbapenem resistance among Acinetobacter Baumannii and methicillin resistance among Staphylococcus aureus isolated from blood samples both decreased while CRKP increased. Combined with his concentrated distribution, we speculate that CRKP BSIs were mostly from endogenous infection. Furthermore, the increasing prevalence of KP and CRKP maybe be related to its special outer membrane proteins, which likely contribute to the integrity and selective impermeability of the cell membrane and also strengthen KP against anionic detergents and certain antibiotics [19].
The proportion of admission to ICU and APACHE II score was higher in CRKP group. Among patients admitted to ICU, 99% (115/116) cases in CRKP group and 86% (50/58) in CSKP group were detected after the admission but not prior to ICU, determining that the CRKP BSI was diagnosed during ICU treatment. So CRKP may not be a factor in ICU admission. Instead, ICU may be the causative factor in CRKP.
At present, the researches about CRKP infection were mostly carried out in ICU patients, which also showed that ICU is a severe area of CRKP infection. ICU has been described as a factory for creating, disseminating, and amplifying antimicrobial resistance [20]. In many previous studies, admission to ICU was regarded as a risk factor for CRKP infection [11, 21–25],though most of these studies that came to this conclusion did not focus on BSI. But ICU admission was not an independent risk factor in the multivariate analysis. This may be due to the presence of other confounding factors. ICU admission itself is just a medical behavior, which does not directly lead to the high prevalence of CRKP. The high prevalence of CRKP in ICU maybe related to the presence of multiple infections in patients, the heavy use of invasive procedures, and the frequent application of high-grade antimicrobials.
In addition to ICU, KP and CRKP BSI were frequent in the hematology and respiratory departments. Most of the inpatients in the Hematology Department are hematologic malignancies. Underlying hematological malignancies, intensive chemotherapy, neutropenia, gastrointestinal mucositis and prolonged hospitalization are all conditions favoring CRKP spread and infections, especially bacteremia [26]. Respiratory tract infection is the most common infection among all infections, followed by urinary tract infection [27, 28]. Antibiotics are widely used in Respiratory Medicine, and even optimal antibiotic use often leads to the development of resistance [29]. Life support, such as tracheal intubation, sputum suction, tracheoscopy and other invasive procedures in critical patients often lead to the damage of airway mucosa, then the bacteria can pass through the barrier into blood flow, which can increase the probability of BSI.
Compared with CSKP group, patients in CRKP group had longer hospitalization time before bacteremia and longer total hospitalization time. It is well known that long hospitalization is a risk factor for the colonization of antibiotic resistant bacteria [30]. Approximately 10% of hospitalizations are complicated by a healthcare-associated infection, and up to 75% of these are due to organisms resistant to first-line antimicrobial therapy [31]. Drug resistant bacteria infection and multi-site infection make conditions more complex and treatment more difficult, which eventually leads to prolonged hospitalization.
The proportion of KP detected in multiple sites in the CRKP group was significantly higher than that in CSKP group. KP detection in other sites was an independent risk factor for CRKP BSI. The concentration of antibiotics in some parts of the body is low, which is insufficient to kill bacteria. Not only that, the inappropriate use of antibiotics may lead to the drug-resistant subpopulations which are present prior to initiation of antimicrobial treatment that may further amplify the resistance [32]. And then the resistant strains of organisms that may become predominant [33]. CRKP in some sites is difficult to be eradicated and eventually spread to the whole body, which will inevitably increase the risk of CRKP BSI. Therefore, for KP BSI with mixed infection in multiple sites, we recommend the antibiotics combination therapy with sufficient tissue penetration and effective concentration to reduce the occurrence of CRKP.
Blood purification, including continuous renal replacement therapy (CRRT), bedside hemofiltration, hemodialysis, plasmapheresis and so on, has not been analyzed in the previous studies. In 2009, a population-based surveillance showed that dialysis patients are more prone to KP bacteremia [34]. In this study, most patients who received blood purification treatment had renal failure, severe acute pancreatitis and shock. They are vulnerable to be infected. And in order to do blood purification, it is requisite to insert the venous catheter first, which belongs to invasive procedures. This may also increase the risk of CRKP infection.
This study suggested that bronchoscopy was an independent risk factor for CRKP BSI. As far as we know, this is the first study to analyze bronchoscopy as a risk factor for CRKP infection. Endoscopy has been considered as a risk factor for CRE transmission for the first time since 2012 [35]. Bronchoscopy is widely used for a variety of diagnostic or therapeutic purposes. Nasopharynx, as one of the common colonization sites of KP [36], is the only way to operate bronchoscopy. This operation may cause mucosal damage in nasopharynx and increase the chance of CRKP entering blood. Furthermore, bronchoscopy may cause the spread of CRKP if it is not well disinfected [35, 37]. But the number of cases in our study is relatively small, which may lead to bias, and further studies are needed to confirm this finding.
Previous surgery (within 1 month) was an independent risk factor for CRKP BSI. Earlier studies have shown that previous surgery was a risk factor for CRKP infection/colonization [21]. Da Silva Kesia Esther et al. suggested that there was a close relationship between surgery and CRKP [38]. Surgery can cause man-made trauma to the human body, increase the chance of bacterial infection, and these patients generally stay in hospital for a longer time, which will also increase the risk of CRKP infection.
The prevalence of drug-resistant bacteria is usually related to the selective pressure caused by the use of antibiotics which changes the patient’s microbiome, causing CRKP to become dominant [25, 26, 33]. Moreover, CRKP can be transmitted through drug-resistant plasmids and it will cause a wider range of CRKP infection [39]. The application of carbapenems can induce the production of acquired Klebsiella pneumoniae carbapenemase (KPC) [40], which is one of the main mechanisms of CRKP resistance.
Previous use of tigecycline was an independent risk factor for CRKP BSI. Tigecycline is a glycine antibiotic with activity against many Gram-positive and Gram-negative pathogens [41]. Since the drug resistance rate of bacteria is increasing year by year, tigecycline has become a new choice for severe drug-resistant bacterial infection. The patients exposed to tigecycline included in this study were mostly patients with hematologic malignancies and multiple severe infections caused by more than one kind of drug-resistant bacteria. As a new last line of defense antibiotic, tigecycline was rarely included in risk factor analysis of CRKP infection. However, tigecycline use may cause nausea and vomiting [42], which change the patients microbiome, resulting in CRKP dominance.
It is worth mentioning that as far as we know, this is the first study to consider enemas, one kind of medical procedures, as a potential risk factor, and there was significant difference between the two groups indeed. The gastrointestinal tract is one of the sites of KP colonization [19]. Patients received an enema mostly because of constipation or the preoperative preparation of gastrointestinal surgery. Enemas can cause damage to intestinal mucosa and change the intestinal microbiome. However, the results of this study didn’t not show that an enema was an independent risk factor for CRKP BSI. The number of cases was relatively small and this was a retrospective study, large sample data and prospective study are needed to further determine the relationship between enema and CRKP BSI.
In this study, the mortality of KP BSI was 24.6%, which was lower than 67.6% reported abroad [43]. The mortality of CRKP BSI was 43.9%, which was higher than 14.9% of CSKP BSI. The mortality rates of CRKP infection reported in North America, South America, Europe and Asia were 33.24, 46.71, 50.06 and 44.82% respectively [13]. The mortality rate of CRKP group in our hospital was similar to that in Asia. In the past 5 years, increase in the mortality of KP infections from 14 to 44% as the percentage of resistance increases, implies that resistance is a primary factor in patient mortality, but this was not supported by multivariate analysis. There are also previous studies suggesting that CRKP infection was not a risk factor for the death of KP infection [44, 45]. This finding was supported in the literature review where CSKP were infections from hypervirulent Klebsiella pneumonia (HVKP), which are sensitive to most of the antibiotics except for the natural resistance to ampicillin [46, 47]. But because of high virulence and high pathogenicity, they can also increase the mortality. HVKP has aroused widespread attention. Currently, it has been reported that the mortality of HVKP BSI was 29.2% [48].
Of the patients who died from CSKP, 38.8% (19/49) was the mortality directly related to the infection, others died from liver failure, kidney failure, cardiovascular and cerebrovascular diseases and malignancies. Solid tumor and non-invasive ventilation were independent risk factors for mortality of CSKP BSI. Of the patients who died from CRKP, 61.1% (44/72) directly related to the infection, which was much higher than that of CSKP(P = 0.016), others died from severe pancreatitis, hemorrhagic shock, malignancy, organ failure and cerebrovascular disease. The proportion of CSKP in KP BSI was relatively high, and only a small minority died directly from infection, and the death was mainly related to the underlying disease. But once CRKP infection occurs, if it is not well controlled, patients would die from infection.
The long hospitalization and previous hospitalization both reflect the complex conditions and poor therapeutic effects. Bone marrow puncture, as one kind of invasive procedures, is often used in the diagnosis and treatment of patients with hematological diseases. The prognosis of solid tumor patients is generally poor. Most patients are in the middle and late stage when tumor is diagnosed, and the therapeutic effect is hardly satisfactory. Some patients refuse the necessary invasive operations such as tracheal cannula or tracheotomy due to traditional concepts or economic reasons, which may lead to delay of illness or even death.
So, what can we do to reverse this trend? Based on the risk factors for CRKP infection and death, we call for: 1.Screening and determining the microbiome of previous hospitalized patients; 2 Increased resources and more stringent protocols for terminal cleaning of rooms, especially in the ICU, and cleaning of equipment (for example bronchoscopes); 3. Increased attention to and training of physicians regarding antibiotic stewardship; 4. Possible implementing checklists, similar to those of central line-associated bloodstream infection (CLABSI), for procedures such as bone marrow punctures which are performed in the patient’s rooms.
Conclusion
The prevalence and mortality of CRKP BSI are increasing year by year, suggesting that we need to strengthen the monitoring of the spatiotemporal evolution of CRKP BSI. KP detection in other sites, blood purification, bronchoscopy, previous surgery, use of carbapenems, use of tigecycline were independent risk factors for CRKP BSI. Timely and effective treatment of KP infection in other sites, strengthening the hospital infection control of blood purification, bronchoscopy and recent surgery, enhancing the management of carbapenem and tigecycline and promoting their reasonable application may help prevent and control CRKP BSI. Previous hospitalization, long hospitalization, bone marrow puncture and use of β-lactamase inhibitor were independent risk factors for death in CRKP BSI. To reverse the Upward trend of mortality in KP even CRKP BSI, we appeal to hospitals to:1. Screen and determine the microbiome of previous hospitalized patients; 2. Increase resources and more stringent protocols for terminal cleaning of rooms, especially in the ICU, and cleaning of equipment (for example bronchoscopes); 3. Increase attention to and training of physicians regarding antibiotic stewardship;4. Implement possible checklists for procedures such as bone marrow punctures which are performed in the patient’s rooms.
Innovation and limitations
Our study focused on KP BSI, analyzed the change of morbidity and mortality of KP and CRKP BSI in our hospital in the past 5 years. We analyzed the department distribution of KP and CRKP for the first time, and included enema, blood purification, bronchoscopy and previous use of tigecycline as possible risk factors in the analysis for the first time. Our study had several limitations: first, this study was a retrospective study; second, this study was a single center study; third, this study only focused on CRKP BSI, lack of analysis and comparison of CRKP infections in other sites and infections caused by other bacteria, even viruses and fungi during this hospitalization; fourth, we used the patients infected with susceptible organisms as controls might generate a selection bias, which may overestimate the effect of carbapenem exposure [49].
Acknowledgements
Not applicable.
Abbreviations
- KP
Klebsiella pneumoniae
- CRKP
carbapenem-resistant Klebsiella pneumoniae
- CSKP
carbapenem-susceptive Klebsiella pneumoniae
- CRE
carbapenem resistant Enterobacteriaceae
- CHINET
China Antimicrobial Surveillance Network
- BSI
bloodstream infection
- KPC
Klebsiella pneumoniae carbapenemase
- CDC
Centers for Disease Control and Prevention
- NHSN
National Healthcare Safety Network
- CLSI
Clinical Laboratory Standards Institute
- FDA
Food and Drug Administration
- APACHE
Acute Physiology and Chronic Health Evaluation
- ICU
Intensive Care Unit
- COPD
chronic obstructive pulmonary disease
- CVC
central venous catheter
- OR
odds ratios
- CI
confidence intervals
- CRRT
continuous renal replacement therapy
- HVKP
Hypervirulent Klebsiella pneumoniae
- CLABSI
Central line-associated bloodstream infection
Authors’ contributions
YL and JL designed the work, acquired data, interpreted the data, drafted the work, modified the submitted version. HT and JH acquired data, drafted the work, and modified the submitted version. NS designed the work, interpreted the data, modified the submitted version. YZ and YC interpreted the data, modified the submitted version. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The retrospective study was approved by the Ethics Committee of the Second Hospital of Hebei Medical University. Informed consent was waived because this was a retrospectively study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuanyuan Li, Jihong Li, Tong Hu and Jia Hu contributed equally to this work.
Contributor Information
Jihong Li, Email: 952224925@qq.com.
Ning Song, Email: songning1972@hotmail.com.
References
- 1.Gupta N, Limbago BM, Patel JB, et al. Carbapenem resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011;53(1):60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 2.Vardakas KZ, Tansarli GS, Rafailidis PI, et al. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother. 2012;67(12):2793–2803. doi: 10.1093/jac/dks301. [DOI] [PubMed] [Google Scholar]
- 3.Lledo W, Hernandez M, Lopez E, Molinari OL, Soto RQ, Hernandez E, Santiago N, Flores M, Vazquez GJ, Robledo IE, Garca-Rivera E, Cortes A, Ramos M, Srinivasan A, Stine N, Bell M, Anderson K, Kitchel B, Wong B, Rasheed JK. Guidance for Control of Infections with Carbapenem-Resistant or Carbapenemase-Producing Enterobacteriaceae in Acute Care Facilities. Morbidity Mortality Weekly Report. 2009;58(10):256–260. [PubMed] [Google Scholar]
- 4.Navon-Venezia S, Leavitt A, Schwaber MJ, Rasheed JK, Srinivasan A, Patel JB, Carmeli Y. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother. 2009;53:818–820. doi: 10.1128/AAC.00987-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Centre for Disease Prevention and Control (ECDC). 2012. Antimicrobial resistance surveillance in Europe 2011. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Available at http://ecdc.europa.eu/en/publications/_layouts/forms/Publication_DispForm.aspx? List=4f55ad51-4aed-4d32-b960-af70113dbb90&ID=719 (Accessed 15 Mar 2020).
- 6.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9:228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 7.Pavez M, Mamizuka EM, Lincopan N. Early dissemination of KPC-2-producing Klebsiella pneumoniae strains in Brazil. Antimicrob Agents Chemother. 2009;53:2702. doi: 10.1128/AAC.00089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 9.European Centre for Disease Prevention and Control (ECDC). 2018. Surveillance of antimicrobial resistance in Europe 2017. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Available at https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2017(Accessed 26 Sept 2019).
- 10.Hu F, Yan G, Yang Y, et al. Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38:2275–2281. doi: 10.1007/s10096-019-03673-1. [DOI] [PubMed] [Google Scholar]
- 11.Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother. 2008;52:1028–1033. doi: 10.1128/AAC.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debby BD, Ganor O, Yasmin M, David L, Nathan K, Ilana T, Dalit S, Smollan G, Galia R. Epidemiology of carbapenem resistant Klebsiella pneumoniae colonization in an intensive care unit. Eur J Clin Microbiol Infect Dis. 2012;31:1811–1817. doi: 10.1007/s10096-011-1506-5. [DOI] [PubMed] [Google Scholar]
- 13.Xu L, Sun X, Ma X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob. 2017;16(1):18. doi: 10.1186/s12941-017-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borer A, Saidel-Odes L, Riesenberg K, Eskira S, Peled N, Nativ R, Schlaeffer F, Sherf M. Attributable mortalityrate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol. 2009;30:972–976. doi: 10.1086/605922. [DOI] [PubMed] [Google Scholar]
- 15.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR. Clinical practice guideline for the use ofantimicrobial agents in neutropenic patients with cancer: 2010 update bythe infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 16.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Shi D, Li X, et al. Detection of Staphylococcus aureus and its antiseptic gene in bloodstream infection. Chin J Disinfection. 2017;34(9):823–826. [Google Scholar]
- 18.Jihong Li; Yuanyuan Li; Ning Song; Yuan Chen. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection: a meta-analysis. J Global Antimicrobial Resistance. 2019 (DOI: 10.1016/j.jgar.2019.09.006). [DOI] [PubMed]
- 19.Paczosa Michelle K, Joan M. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol Mol Biol Rev. 2016;80:629–661. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu Y, Ping Y, Li L, Xu H, Yan X, Dai H. A retrospective study of risk factors for carbapenem-resistant Klebsiella pneumoniae acquisition among ICU patients. J Infect Dev Ctries. 2016;10(3):208–213. doi: 10.3855/jidc.6697. [DOI] [PubMed] [Google Scholar]
- 21.Kofiteridis DP, Valachis A, Dimoupoulou D, Maraki S, Mantadakis E, Mantadakis E, Samonis G. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection/colonization: a case control study. J Infect Chemother. 2014;20:293–297. doi: 10.1016/j.jiac.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Rueda VG, Tobón JJZ. Risk factors for infection with carbapenem-resistant Klebsiella pneumoniae: a case-case-control study. Colombia Medica (Cali, Colombia) 2014;45(2):54–60. doi: 10.25100/cm.v45i2.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills JP, Talati NJ, Alby K, Han JH. The epidemiology of Carbapenem-resistant Klebsiella pneumoniae colonization and infection among long-term acute care hospital residents. Infect Control Hosp Epidemiol. 2016;37(1):55–60. doi: 10.1017/ice.2015.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussein K, Sprecher H, Mashiach T, Oren I, Kassis I, Finkelstein R. Carbapenem resistance among Klebsiella pneumoniae isolates: risk factors, molecular characteristics, and susceptibility patterns. Infect Control Hosp Epidemiol. 2009;30(7):666–671. doi: 10.1086/598244. [DOI] [PubMed] [Google Scholar]
- 25.Bleumin D, Cohen MJ, Moranne O, Esnault VLM, Benenson S, Paltiel O, Tzukert K, Levi IM-Y, Ben-Dov IZ, Levi R, Bloch A, Haviv YS. Carbapenem-resistant Klebsiella pneumoniae is associated with poor outcome in hemodialysis patients. J Infection. 2012;65(4):318–325. doi: 10.1016/j.jinf.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Micozzi A, Gentile G, Minotti C, Cartoni C, Capria S, Ballar D, Santilli S, Pacetti E, Grammatico S, Bucaneve G, Fo R. Carbapenem-resistant Klebsiella pneumoniae in high-risk haematological patients: factors favouring spread, risk factors and outcome of carbapenem-resistant Klebsiella pneumoniae bacteremias. BMC Infectious Dis. 2017;17(1):203. doi: 10.1186/s12879-017-2297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanini S, Jarvis WR, Nicastri E, Privitera G, Gesu G, Marchetti F, Giuliani L, Piselli P, Puro V, Nisii C, Ippolito G. Healthcare-Associated Infection in Italy: Annual Point-Prevalence Surveys, 2002–2004. Infection Control Hospital Epidemiol. 2009;30(7):659–665. doi: 10.1086/597596. [DOI] [PubMed] [Google Scholar]
- 28.Youning Liu, Bin Cao, Hui Wang, et al. Adult hospital acquired pneumonia: a multicenter study on microbiology and clinical characteristics of patients from 9 Chinese cities. Chin J Tubere Respir Dis, 2012, (10):739–746. (In Chinese). [PubMed]
- 29.Keith EB, Gaur Aditya H. The use and abuse of antibiotics and the development of antibiotic resistance. Adv. Exp. Med. Biol. 2010;659:73–82. doi: 10.1007/978-1-4419-0981-7_6. [DOI] [PubMed] [Google Scholar]
- 30.Cohen Matan J, Olga A, David R, et al. Acquisition of multidrug-resistant organisms among hospital patients hospitalized in beds adjacent to critically ill patients. Infect Control Hosp Epidemiol. 2006;27:675–681. doi: 10.1086/505919. [DOI] [PubMed] [Google Scholar]
- 31.Lautenbach E, Perencevich EN. Addressing the emergence and impact of multidrug-resistant gram-negative organisms: a critical focus for the next decade. Infect Control Hosp Epidemiol. 2014;35:333–335. doi: 10.1086/675592. [DOI] [PubMed] [Google Scholar]
- 32.Drlica, Karl( drlica@phri.org);Xilin Zhao. Mutant selection window hypothesis updated. Clin Infectious Dis. 2007, 44(5):681–688. [DOI] [PubMed]
- 33.McGowan JE., Jr Antimicrobial resistance in hospital organisms and its relation to antibiotic use. Rev Infect Dis. 1983;5:1033–1048. doi: 10.1093/clinids/5.6.1033. [DOI] [PubMed] [Google Scholar]
- 34.Meatherall BL, Gregson D, Ross T, Pitout JDD, Laupland KB. Incidence, risk factors, and outcomes of Klebsiella pneumoniae bacteremia. Am J Med. 2009;122(9):866–873. doi: 10.1016/j.amjmed.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 35.Mehta AC, Muscarella LF. Bronchoscope-Related "Superbug" Infections. Chest. 2020;157(2):454–469. doi: 10.1016/j.chest.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Dao TT, Liebenthal D, Tran TK, Ngoc Thi Vu B, Ngoc Thi Nguyen D, Thi Tran HK, Thi Nguyen CK, Thi Vu HL, Fox A, Horby P, Van Nguyen K, Wertheim HFL. Klebsiella pneumoniae oropharyngeal carriage in rural and urban Vietnam and the effect of alcohol consumption. PLoS One. 2014;9:e91999. doi: 10.1371/journal.pone.0091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galdys AL, Marsh JW, Delgado E, Pasculle AW, Pacey M, Ayres AM, Metzger A, Harrison LH, Muto CA. Bronchoscope-associated clusters of multidrug-resistant Pseudomonas aeruginosa and carbapenem-resistant Klebsiella pneumoniae. Infection Control Hospital Epidemiol. 2019;40(1):40–46. doi: 10.1017/ice.2018.263. [DOI] [PubMed] [Google Scholar]
- 38.Da Silva Kesia Esther, Maciel Wirlaine Glauce, Sacchi Flávia Patussi Correia et al. Risk factors for KPC-producing Klebsiella pneumoniae: watch out for surgery. J. Med. Microbiol., 2016, 65: 547–553. [DOI] [PubMed]
- 39.Cross SN, Potter JA, Aldo P, et al. Viral infection sensitizes human fetal membranes to bacterial lipopolysaccharide by MERTK inhibition and inflammasome activation. J Immunol. 2017;199(8):2885–2895. doi: 10.4049/jimmunol.1700870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orsi GB, Bencardino A, Vena A, Carattoli A, Venditti C, Falcone M, Giordano A, Venditti M. Patient risk factors for outer membrane permeability and KPC-producing carbapenem-resistant Klebsiella pneumoniae isolation: results of a double case-control study. Infection. 2013;41(1):61–67. doi: 10.1007/s15010-012-0354-2. [DOI] [PubMed] [Google Scholar]
- 41.Stein GE, Babinchak T. Tigecycline: an update. Diagn Microbiol Infect Dis. 2013;75(4):331–336. doi: 10.1016/j.diagmicrobio.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Wyeth Pharmaceuticals Inc. Tygacil® [tigecycline] for injection for intravenous use. Full prescribing information. http://labeling.pfizer.com/showlabeling.aspx?id=491. Accessed 15 Mar 2020.
- 43.Delle Rose D, Sordillo P, Gini S, Cerva C, Boros S, Rezza G, Meledandri M, Gallo MT, Prignano G, Caccese R, et al. Microbiologic characteristics and predictors of mortality in bloodstream infections in intensive care unit patients: a 1-year, large, prospective surveillance study in 5 Italian hospitals. Am J Infect Control. 2015;43(11):1178–1183. doi: 10.1016/j.ajic.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 44.Luci C, Valle MMD, Itacy S, et al. A hospital-based matched case-control study to identify clinical outcome and risk factors associated with carbapenem-resistant Klebsiella pneumoniae infection. BMC Infect. Dis. 2013;13:80. doi: 10.1186/1471-2334-13-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vardakas KZ, Matthaiou DK, Falagas ME, Antypa E, Koteli A, Antoniadou E. Characteristics, risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae infections in the intensive care unit. The Journal of infection. 2015;70(6):592–599. doi: 10.1016/j.jinf.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Wang XL, Xie YZ, Li G, et al. Whole-genome-sequencing characterization of bloodstream infection-causing hypervirulent Klebsiella pneumoniae of capsular serotype K2 and ST374[J] Virulence. 2018;9(1):510–521. doi: 10.1080/21505594.2017.1421894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Compain F, Babosan A, Brisse S, et al. Multiplex PCR for detection of seven virulence factors and K1/K2 capsular serotypes of Klebsiella pneumonia. J Clin Microbiol. 2014;52(12):4377–4380. doi: 10.1128/JCM.02316-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hiroki N, Koichi Y, Arata S, Waki I, Kazushi Y, Wataru S, Naoko Y, Makoto N, Kiyotaka N, Ken-Ichi O, Taishi T, Mamiko N, Yoshihiro T, Yasuhiko T, Yukihiro K, Taichi S, Hiroshi K. Clinical characteristics of bacteremia caused by hypermucoviscous Klebsiella pneumoniae at a tertiary hospital. Diagnostic Microbiol Infect Dis. 2019;95(1):84–88. [DOI] [PubMed]
- 49.Harris AD, Karchmer TB, Carmeli Y, Samore MH. Methodological Principles of Case-Control Studies That Analyzed Risk Factors for Antibiotic Resistance: A Systematic Review. Clin Infect Dis. 2001;32(7):1055–61. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.