Abstract
Background
Three biomarkers, soluble suppression of tumorigenicity 2 (ST2), galectin 3 (Gal3), and N-terminal brain natriuretic peptide prohormone (NT-proBNP), are approved for noninvasive risk assessment in left-sided heart failure, and small observational studies have shown their prognostic usefulness in heterogeneous pulmonary hypertension cohorts. We examined associations between these biomarkers and disease severity and survival in a large cohort of patients with pulmonary arterial hypertension (PAH) (ie, group 1 pulmonary hypertension). We hypothesized that additive use of biomarkers in combination would improve the prognostic value of survival models.
Methods
Biomarker measurements and clinical data were obtained from 2,017 adults with group 1 PAH. Associations among biomarker levels and clinical variables, including survival times, were examined with multivariable regression models. Likelihood ratio tests and the Akaike information criterion were used to compare survival models.
Results
Higher ST2 and NT-proBNP were associated with higher pulmonary pressures and vascular resistance and lower 6-min walk distance. Higher ST2 and NT-proBNP levels were associated with increased risk of death (hazard ratios: 2.79; 95% CI, 2.21-3.53; P < .001 and 1.84; 95% CI, 1.62-2.10; P < .001, respectively). The addition of ST2 to survival models composed of other predictors of survival, including NT-proBNP, significantly improved model fit and predictive capacity.
Conclusions
ST2 and NT-proBNP are strong, noninvasive prognostic biomarkers in PAH. Despite its prognostic value in left-sided heart failure, Gal3 was not predictive in PAH. Adding ST2 to survival models significantly improves model predictive capacity. Future studies are needed to develop multimarker assays that improve noninvasive risk stratification in PAH.
Key Words: biomarkers, heart failure, molecular biology, pulmonary hypertension
Abbreviations: 6MWD, 6-min walking distance; AIC, Akaike information criterion; Gal3, galectin 3; CTD-PAH, connective tissue disease-associated pulmonary arterial hypertension; HFpEF, heart failure with preserved ejection fraction; IPAH, idiopathic pulmonary arterial hypertension; mPAP, mean pulmonary arterial pressure; NT-proBNP, N-terminal brain natriuretic peptide prohormone; NYHA FC, New York Heart Association functional class; PAH, pulmonary arterial hypertension; PVR, pulmonary vascular resistance; RAP, right atrial pressure; REVEAL Registry, Registry to Evaluate Early and Long-Term PAH Disease Management; RHC, right-sided heart catheterization; RV, right ventricular; ST2, soluble suppression of tumorigenicity 2
Pulmonary arterial hypertension (PAH) is a debilitating disease that leads to right ventricular (RV) failure and death. Reliable markers of PAH severity and prognosis are crucial for deciding management strategies and timing escalations in care. Validated scoring systems for predicting survival, such as the REVEAL Registry risk score developed from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management, incorporate clinical variables obtained through a variety of testing procedures, including clinical assessment, echocardiography, pulmonary function, and invasive hemodynamic testing.1,2 Many testing procedures are invasive, cumbersome, or limited by subjectivity and lack of reproducibility. Circulating serum biomarkers obtained by venipuncture are increasingly investigated for their usefulness in predicting outcomes. One serum marker, brain natriuretic peptide (BNP), is a component of the REVEAL Registry risk score. By definition, biomarkers are objectively measured, reproducible, and indicative of pathogenic biologic processes.3 Circulating serum biomarkers are therefore appealing adjuncts and potential alternatives to other testing modalities currently used for mortality prediction in PAH.
Three serum biomarkers have been approved by the US Food and Drug Administration (FDA) for risk stratification in patients with left-sided heart failure: N-terminal brain natriuretic prohormone (NT-proBNP), a marker of cardiomyocyte stretch4,5; galectin 3 (Gal3), a mediator of inflammation and cardiac fibrosis6, 7, 8; and soluble suppression of tumorigenicity 2 (ST2), a shortened circulating IL-33 decoy receptor.9, 10, 11, 12 NT-proBNP has been associated with disease severity and survival in multiple subtypes of PAH.13, 14, 15 Significant correlations have been demonstrated between both Gal316 and ST217 and metrics of PAH severity, suggesting their usefulness as prognostic biomarkers. Yet only two studies have supported independent associations between Gal3 and ST2 and survival, and both involved small cohorts composed of patients with multiple different World Health Organization classifications of pulmonary hypertension.18,19 No study, to our knowledge, has examined associations between these markers of heart failure and survival in a large cohort composed only of patients with group 1 PAH. In this study, we sought to examine associations between ST2, Gal3, and NT-proBNP and disease severity and survival in a large multicenter cohort of group 1 patients. We hypothesized that each of these markers would be associated with survival, and that combining these biomarkers would improve prediction of survival in patients with PAH.
Materials and Methods
Analytic Cohort
The National Biological Sample and Data Repository for PAH (also known as the PAH Biobank) is a National Institutes of Health-funded repository of biologic samples and clinical data collected from 36 enrolling PAH centers across North America. Biorepository data collection was approved by the institutional review board at each participating center, and all patients gave informed consent at the time of enrollment. We requested clinical data and biologic samples from all patients with PAH, 21 years of age or older, and we received clinical data and serum for 2,032 patients. Of these, enzyme-linked immunosorbent assays (ELISAs) for ST2, Gal3, and NT-proBNP were successfully performed on 2,017 patients, which form the analytic cohort for this study. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Johns Hopkins University Institutional Review Board (NA_00069663; Baltimore, MD).
ELISA for NT-proBNP, Gal3, and ST2
A multiplex ELISA was developed to measure ST2, Gal3, and NT-proBNP simultaneously, using robotically spotted capture antibodies on the 96-well plate format (Meso Scale Discovery [MSD]). Capture antibody-spotted plates were blocked with PBS-T (5% bovine serum albumin/phosphate-buffered saline complemented with 0.05% Tween) and incubated at room temperature on an orbital shaker (500 rpm) for 60 min. Calibrators for NT-proBNP (C01XX-1; MSD), galectin 3 (840355; R&D Systems), and ST2 (840760; R&D Systems) were produced with commercially provided diluent (R51BB-3; MSD). The detection antibody cocktail for NT-proBNP (D21JK-1; MSD), galectin 3 (842759; R&D Systems), and ST2 (840354; R&D Systems) was prepared in commercially provided diluent (R51BA-5; MSD) and supplemented with streptavidin-SULFO-TAG (0.5 μg/mL) (R32AD-5; MSD). After incubation and washing, the plate was read with a SECTOR imager 2400 (MSD). Interassay reliability, as measured by percent coefficient of variation across 25 plates, was as follows: ST2, 2.3%; Gal3, 5.8%; and NT-proBNP, 3.0%.
Statistical Analysis
Continuous variables are presented as means with SDs and medians with interquartile ranges (IQRs). Unadjusted comparisons between groups were made with Student t-tests or Mann-Whitney tests, as appropriate. Analyses of biomarker relationships with disease severity were initially performed in subjects with serum obtained within 3 months of clinical variable determination to preserve a tight temporal relationship between measurements. Sensitivity analyses were performed in subjects with serum obtained within 6 and 12 months of clinical data collection and in the full cohort. Continuous clinical variables were examined with Spearman correlations and linear regressions adjusted for age and sex. Biomarkers had right-skewed distributions, and were log-transformed for regression. REVEAL Registry risk scores were calculated for all subjects at enrollment with cut points validated in the original REVEAL Registry 1.0 model, as well as with revised REVEAL Registry 2.0 cut points.1,2,20 On the basis of REVEAL Registry risk scores, subjects were classified into five REVEAL Registry risk categories as previously described20,21 to assess associations with survival. Full methodology for calculation of REVEAL Registry risk scores and classification of subjects into risk categories is available in e-Appendix 1, e-Figure 1.
Relationships between biomarker measurements at enrollment and survival were examined by Kaplan-Meier analysis, with each biomarker dichotomized at its median value, and by multivariable Cox proportional hazard modeling, with biomarkers entered as continuous variables. Thirty-three subjects lost to follow-up after enrollment were not included in time-to-event analyses. Survival model covariates were designated a priori and included age, sex, PAH subtype, PAH therapy drug class, New York Heart Association functional class (NYHA FC), 6-min walk distance (6MWD), and invasive hemodynamic measures. The proportional hazards assumption was examined for all covariates on the basis of Schoenfeld residuals.22 Candidate survival models (based on our a priori model and REVEAL Registry risk score parameters) were fit to survival data. Improvements in predictive capacity and model fit gained from adding biomarkers to base candidate models were judged by Akaike information criterion (AIC) and by comparing null vs extended models with likelihood ratio tests. Missing covariates were missing not at random, with sicker patients and patients with longer times from diagnosis to enrollment more likely to have missing data. Participants with missing covariates were excluded from initial multivariable analyses for complete case analyses. Sensitivity analyses were then performed by systematically excluding covariates with > 5% missingness from candidate models.
For biomarkers that improved the fit of base models, measurement cut points were designated on the basis of patterns in the data, in accordance with prior literature.20 Designated cut points were applied to recalculate REVEAL Registry risk scores to incorporate the new biomarker measurements. Subjects were reclassified into previously designated risk categories, using recalculated risk scores. Survival associations with reclassified risk categories were examined by Kaplan-Meier analyses and Cox proportional hazard models. Receiver operating characteristics and C-statistics were used to compare discrimination between risk scores modified to incorporate new biomarker data and REVEAL Registry 1.0 and 2.0 risk scores. Detailed methodology for the establishment of biomarker measurement thresholds, recalculation of risk scores, and reclassification of risk categories are described in e-Appendix 1, e-Figure 1.
A P value < .05 was considered statistically significant. All statistical analyses were performed with Stata (version 15.1; StataCorp).
Results
Patient Characteristics
Most patients in the cohort had idiopathic PAH (IPAH); the second largest disease subtype was connective tissue disease-associated PAH (CTD-PAH) (Table 1). Most patients in the cohort were white women in the sixth decade of life, with NYHA FC II/III symptoms. The median time from diagnosis to enrollment was 48 months (IQR, 48-96 months). Subjects had moderate to severe disease, with mean pulmonary arterial pressure (mPAP), 50 ± 15 mm Hg; pulmonary vascular resistance (PVR), 10 ± 6 Wood units; and cardiac index, 2.7 ± 1.2 L/min/m2. The majority of patients were treated with phosphodiesterase-5 inhibitor or endothelin receptor antagonist therapy after PAH diagnosis. The median survival time from enrollment was 41 months (IQR, 28-55 months).
Table 1.
Demographics and Clinical Characteristics of the Cohort at Enrollment
| Observations (No.) | Demographics/Clinical Characteristics | Overall (N = 2,017) | CTD-PAH (n = 623) | IPAH (n = 870) |
|---|---|---|---|---|
| Demographics | ||||
| 2,017 | Age, y | 55 (15) | 59 (14) | 55 (15) |
| 2,017 | Sex, female, No. (%) | 1,611 (80) | 565 (91) | 698 (80) |
| 2,017 | Race, white, No. (%) | 1,662 (82) | 564 (91) | 780 (90) |
| 1,448 | NYHA FC I/II/III/IV, No. (%III/IV) | 90/451/789/118 (63) | 24/140/266/34 (65) | 38/188/340/56 (64) |
| 1,023 | 6MWD, m | 347 (141) | 327 (160) | 351 (136) |
| 1,984 | Deaths during follow-up, No. (%) | 324 (16) | 125 (20) | 112 (13) |
| 2,017 | Etiology (FPAH/PVOD/PortoPulm/congenital/drug/HIV/other), No. | 81/8/111/171 /93/42/18 | … | … |
| 2,017 | REVEAL Registry 1.0 score | 7.3 (1.8) | 8.0 (1.6) | 6.7 (1.7) |
| 2,017 | REVEAL Registry 2.0 score | 7.3 (2.4) | 7.9 (2.1) | 6.5 (2.2) |
| Biomarker values | ||||
| 2,017 | NT-proBNP, median (P25-P75), pg/mL | 672 (217-2,164) | 907 (331-2,164) | 520 (183-1,621) |
| 2,017 | Gal3, median (P25-P75), pg/mL | 10,129 (6,925-14,073) | 10,444 (7,151-15,057) | 10,335 (7,010-14,270) |
| 2,017 | ST2, median (P25-P75), pg/mL | 5,593 (3,772-8,939) | 6,563 (4,398-11,249) | 5,005 (3,419-7,752) |
| Hemodynamics | ||||
| 1,972 | RAP, mm Hg | 9 (5) | 9 (5) | 9 (6) |
| 2,007 | mPAP, mm Hg | 50 (15) | 44 (11) | 51 (14) |
| 1,951 | PAWP, mm Hg | 10 (4) | 10 (4) | 10 (4) |
| 1,916 | PVR, Wood units | 10 (6) | 8 (5) | 10 (6) |
| 1,940 | Cardiac output, L/min | 4.7 (1.7) | 4.7 (1.6) | 4.6 (1.6) |
| 1,940 | Cardiac index, L/min/m2 | 2.7 (1.2) | 2.8 (0.9) | 2.6 (1.1) |
| 2,017 | Therapies, No. (%) | |||
| PDE5 inhibitor | 1,546 (77) | 470 (75) | 641 (74) | |
| ERA | 1,205 (60) | 370 (59) | 515 (59) | |
| IV/SC prostacyclin | 699 (35) | 161 (26) | 355 (41) | |
| CCB | 199 (10) | 51 (8) | 99 (11) |
All data are presented as mean (SD) unless otherwise specified. 6MWD = 6-min walk distance; CCB = calcium channel blocker; CTD-PAH = connective tissue disease-associated pulmonary arterial hypertension; ERA = endothelin receptor antagonist; FPAH = familial PAH; Gal3 = galectin 3; IPAH = idiopathic PAH; mPAP = mean pulmonary arterial pressure; NT-proBNP = N-terminal brain natriuretic peptide prohormone; NYHA FC = New York Heart Association functional class; P25, P75 = 25th and 75th percentiles; PAWP = pulmonary artery wedge pressure; PDE5 = phosphodiesterase-5; PortoPulm = portopulmonary hypertension; PVOD = pulmonary venoocclusive disease; PVR = pulmonary vascular resistance; RAP = right atrial pressure; REVEAL Registry = Registry to Evaluate Early and Long-Term PAH Disease Management; SC = subcutaneous; ST2 = soluble suppression of tumorigenicity 2.
Associations With Metrics of Disease Severity
ST2 and NT-proBNP weakly to modestly correlated with several hemodynamic measurements (right atrial pressure [RAP], mPAP, PVR). Gal3 weakly correlated with RAP and stroke volume and modestly correlated with RV stroke work. All three markers correlated with 6MWD (Table 2).
Table 2.
Correlations Between Biomarkers and Continuous Clinical Variables, Limited to Subjects With Biomarkers Obtained Within 3 Months of Clinical Tests: n = 191
| Variable | log NT-proBNP | log Gal3 | log ST2 |
|---|---|---|---|
| Demographics | |||
| Age, y | 0.39; < .001 | 0.32; < .001 | 0.12; .09 |
| 6MWD, m | –0.36; < .001 | –0.28; .006 | –0.31; .002 |
| Hemodynamics | |||
| RAP, mm Hg | 0.40; < .001 | 0.14; .05 | 0.37; < .001 |
| mPAP, mm Hg | 0.23; .001 | –0.05; .51 | 0.13; .07 |
| PAWP, mm Hg | 0.09; .22 | 0.09; .21 | 0.06; .41 |
| PVR, Wood units | 0.26; < .001 | –0.003; .97 | 0.18; .02 |
| Cardiac output, L/min | –0.20; .006 | –0.10; .19 | –0.11; .14 |
| Cardiac index, L/min/m2 | –0.22; .003 | –0.13; .09 | –0.14; .06 |
| Stroke volume, L | –0.21; .016 | –0.24; .008 | –0.15; .10 |
| PA compliance, mL/mm Hg | –0.26; .004 | –0.08; .37 | –0.09; .34 |
| Transpulmonary gradient, mm Hg | 0.23; .002 | –0.06; .43 | 0.12; .12 |
| Diastolic pulmonary gradient, mm Hg | 0.19; .01 | –0.03; .67 | 0.16; .03 |
| RV stroke work, mm Hg × L | –0.16; .07 | –0.32; < .01 | –0.14; .13 |
| RV stroke work index, g/m2/beat | –0.16; .06 | –0.33; < .01 | –0.16; .07 |
| RV power, mm Hg × L/min | –0.18; .05 | –0.05; .59 | –0.08; .38 |
All data are presented as follows: Spearman correlation coefficient; P value. PA = pulmonary arterial; RV = right ventricular. See Table 1 legend for expansion of other abbreviations.
When linear regressions were adjusted for age and sex, ST2 levels were significantly associated with RAP, mPAP, PVR, heart rate, and RV stroke work (Table 3, e-Tables 1-3). NT-proBNP levels were significantly associated with RAP, mPAP, PVR, cardiac output, stroke volume, and pulmonary arterial (PA) compliance. Gal3 levels were associated only with RAP and RV stroke work. All three biomarkers were significantly associated with 6MWD, with the largest effect seen for ST2. Among subjects with serum obtained within 3 months of right-sided heart catheterization (RHC), each log-unit higher ST2 was associated with a 44.8-m shorter 6MWD.
Table 3.
Age- and Sex-Adjusted Linear Regressions of Biomarkers and Continuous Clinical Variables, Limited to Subjects With Biomarkers Obtained Within 3 Months of Clinical Tests: n = 191
| Clinical Variable | log NT-proBNP | log Gal3 | log ST2 |
|---|---|---|---|
| RAP, mm Hg | 1.82 (1.25 to 2.38; < .001) | 1.53 (0.29 to 2.77; .01) | 2.58 (1.44 to 3.71; < .001) |
| mPAP, mm Hg | 2.69 (1.41 to 3.97; < .001) | 0.43 (–2.26 to 3.12; .75) | 2.54 (0.01 to 5.06; .049) |
| PAWP, mm Hg | 0.21 (–0.26 to 0.67; .40) | 0.53 (–0.40 to 1.46; .26) | –0.18 (–1.05 to 0.69; .69) |
| PVR, Wood units | 1.18 (0.64 to 1.72; < .001) | 0.50 (–0.64 to 1.64; .39) | 0.55 (0.18 to 0.92; .003) |
| Cardiac output, L/min | –0.19 (–0.36 to –0.02; .03) | –0.003 (–0.34 to 0.33; .98) | –0.27 (–0.59 to 0.04; .09) |
| Cardiac index, L/min/m2 | –0.12 (–0.22 to –0.03; .01) | –0.05 (–0.24 to 0.15; .63) | –0.17 (–0.36 to 0.02; .07) |
| Stroke volume, L | –0.004 (–0.007 to –0.001; .006) | –0.008 (–0.01 to –0.002; .01) | –0.006 (–0.011 to –0.001; .02) |
| PA compliance, mL/mm Hg | –0.17 (–0.26 to –0.08; < .001) | –0.10 (–0.32 to 0.12; .36) | –0.14 (–0.32 to 0.03; .11) |
| RV stroke work, mm Hg × L | –0.07 (–0.18 to 0.04; .21) | –0.32 (–0.005 to –0.07; .01) | –0.20 (–0.40 to 0.01; .05) |
| RV stroke work index, g/m2/beat | –0.001 (–0.002 to 0; .12) | –0.003 (–0.005 to –0.001; .004) | –0.002 (–0.003 to 0; .03) |
| RV power, mm Hg × L/min | –9.74 (–25.39 to 5.92; .22) | 7.44 (–23.2 to 38.09; .63) | –12.06 (–40.24 to 16.12; .40) |
| Heart rate, beats/min | 1.60 (–0.01 to 3.28; .06) | 6.25 (2.58 to 9.92; .001) | 3.88 (0.83 to 6.93; .01) |
| 6MWD, m | –22.43 (–39.85 to –5.02; .01) | –33.77 (–74.66 to 7.12; .10) | –44.76 (–78.03 to –11.50; .009) |
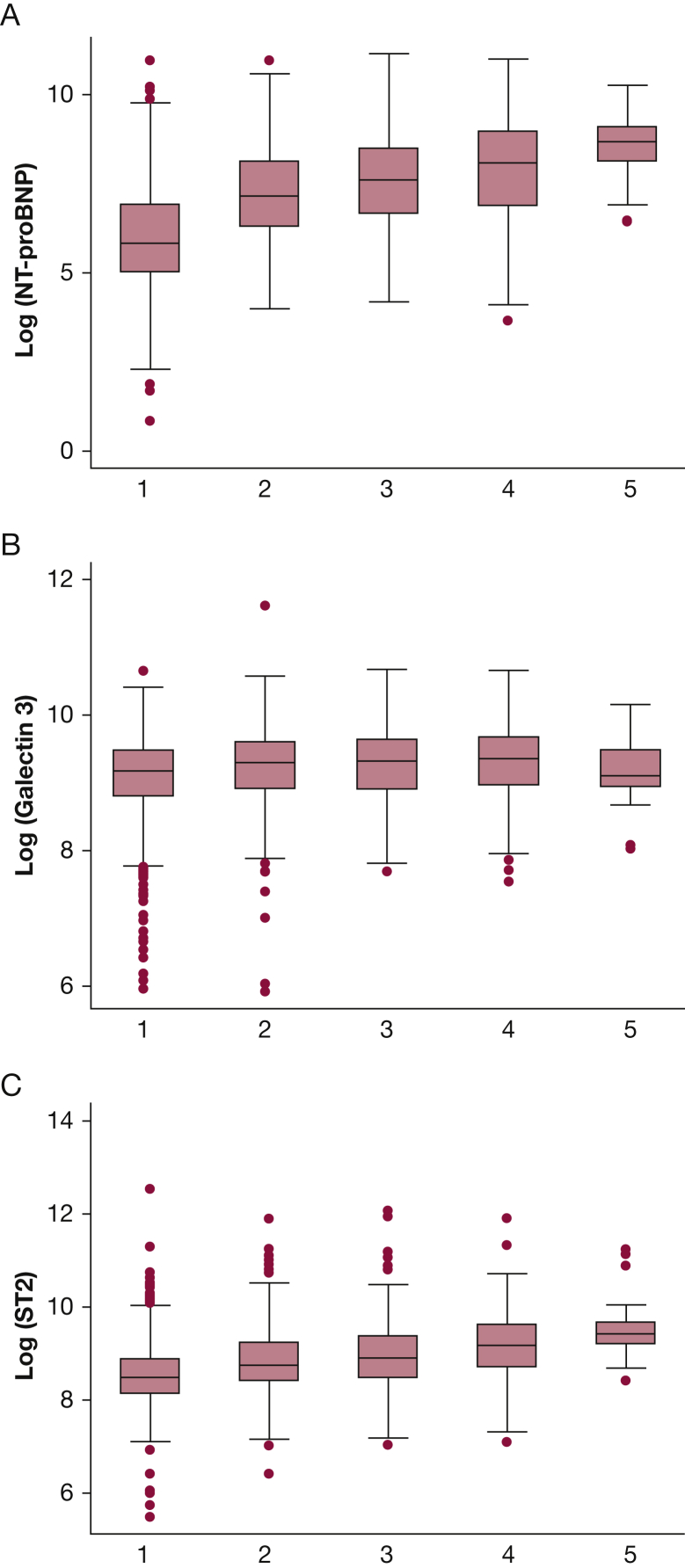
Calculation of a patient’s REVEAL Registry risk score enables assignment of the individual into one of five risk categories. When biomarker levels were examined by REVEAL Registry risk category, clear dose-response relationships were demonstrated with ST2 and NT-proBNP; however, no such relationship was seen with Gal3 (Fig 1).
Figure 1.
Comparison of median biomarker levels by REVEAL Registry risk category: A, NT-proBNP; B, Gal3; C, ST2. Gal3 = galectin 3; NT-proBNP = N-terminal brain natriuretic peptide prohormone; REVEAL Registry = Registry to Evaluate Early and Long-Term PAH Disease Management; ST2 = soluble suppression of tumorigenicity 2.
Associations With Survival
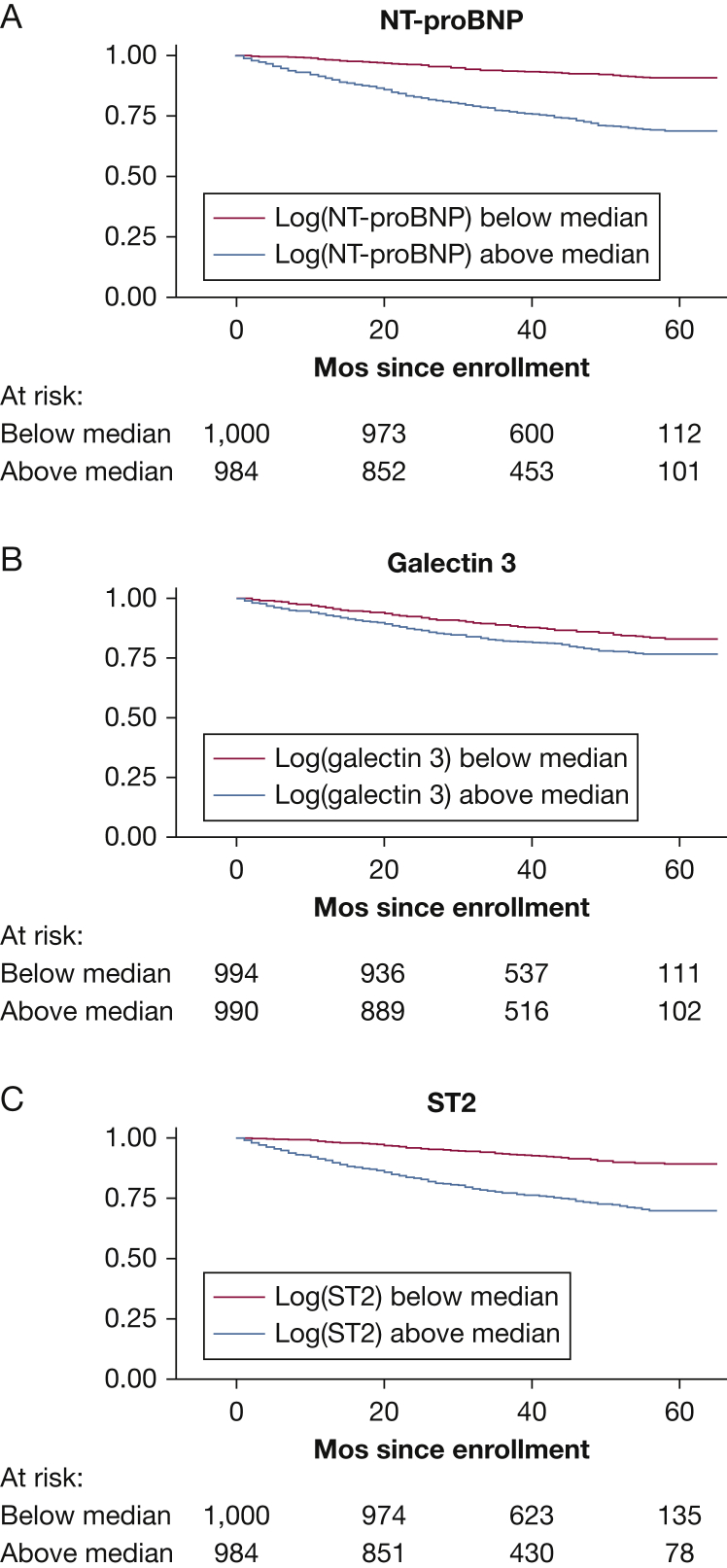
Kaplan-Meier curves demonstrated significantly shorter survival for subjects with higher ST2, Gal3, and NT-proBNP levels (each log-rank P < .001) (Fig 2). Each biomarker was significantly associated with mortality in univariable Cox proportional hazard modeling in the overall cohort. When survival analyses were repeated within the two largest PAH subgroups (IPAH and CTD-PAH), the significance of the hazard ratio (HR) for Gal3 was attenuated in the CTD-PAH subgroup (P = .06).
Figure 2.
Kaplan-Meier survival analysis of subjects with A, NT-proBNP; B, Gal3; and C, ST2 levels above vs below median values. Log-rank test P value is < .001 for each. See Figure 1 legend for expansion of abbreviations.
Multivariable models demonstrated significant associations between ST2 and NT-proBNP levels and survival in the overall cohort (Table 4). For each log-unit higher ST2, risk of mortality was nearly 300% higher (HR, 2.79; 95% CI, 2.21-3.53; P < .001). The association between Gal3 levels and mortality was not significant after adjustment for other clinical variables (HR, 1.12; 95% CI, 0.85-1.47; P = .44). For each log-unit higher NT-proBNP, risk of mortality was 84% higher (HR, 1.84; 95% CI, 1.62-2.10; P < .001). The magnitude of biomarker associations with survival persisted in sensitivity analyses performed to account for missing covariate data (e-Table 4). Survival analyses restricted to subjects enrolled within 12 months of a diagnostic RHC (e-Table 5) demonstrated 330% higher risk of mortality for each log-unit increase in ST2 (HR, 3.30; 95% CI, 1.95-5.58; P < .001) and 94% higher risk of mortality with each log-unit increase in NT-proBNP (HR, 1.94; 95% CI, 1.43-2.63; P < .001). There was no significant association between Gal3 levels and mortality (HR, 0.94; 95% CI, 0.56-1.58; P = .82).
Table 4.
Cox Multivariable Hazard Ratios for Mortalitya
| Cohort | log NT-proBNP | log Gal3 | log ST2 |
|---|---|---|---|
| Overall cohort | 1.84, 1.62-2.10; < .001 | 1.12, 0.85-1.47; .44 | 2.79, 2.21-3.53; < .001 |
| CTD-PAH subgroup | 1.74, 1.42-2.14; < .001 | 0.92, 0.59-1.44; .72 | 3.48, 2.30-5.26; < .001 |
| IPAH subgroup | 2.48, 1.90-3.23; < .001 | 1.33, 0.80-2.20; .27 | 3.92, 2.56-6.00; < .001 |
All data are presented as follows: hazard ratio, 95% CI; P value. See Table 1 legend for expansion of abbreviations.
Each model was adjusted for age, sex, subtype of PAH, PAH-specific therapy, NYHA FC, 6MWD, RAP, mPAP, cardiac index, and PVR.
When multivariable analyses were repeated in IPAH and CTD-PAH subgroups, associations with ST2 and NT-proBNP remained significant; however, no association existed between Gal3 and survival in either IPAH or CTD-PAH. In the IPAH subgroup, each log-unit higher ST2 was associated with 400% higher mortality (HR, 3.92; 95% CI, 2.56-6.00; P < .001) and each log-unit higher NT-proBNP was associated with 250% higher mortality (HR, 2.48; 95% CI, 1.90-3.23; P < .001). In the CTD-PAH subgroup, each log-unit higher ST2 was associated with 350% higher mortality (HR, 3.48; 95% CI, 2.30-5.26; P < .001) and each log-unit higher NT-proBNP was associated with 74% higher mortality (HR, 1.74; 95% CI, 1.42-2.14; P < .001).
The addition of ST2 to our a priori survival model and to a survival model composed of parameters included in the REVEAL Registry risk score resulted in significant improvements in prognostic value. Among five candidate survival models, the model with the best predictive capacity (as indicated by the lowest AIC) was a model composed of terms used in the REVEAL Registry risk score (including NT-proBNP, as well as PAH subtype, age, sex, NYHA FC, heart rate, systolic BP, RAP, PVR, and 6MWD) plus ST2. Candidate model parameters and AICs are shown in e-Table 6. Extended survival models, with ST2 added to other predictors (including NT-proBNP), improved model fit when comparisons were made with likelihood ratio tests (e-Table 7). Addition of Gal3 resulted in no further improvements in model predictive capacity.
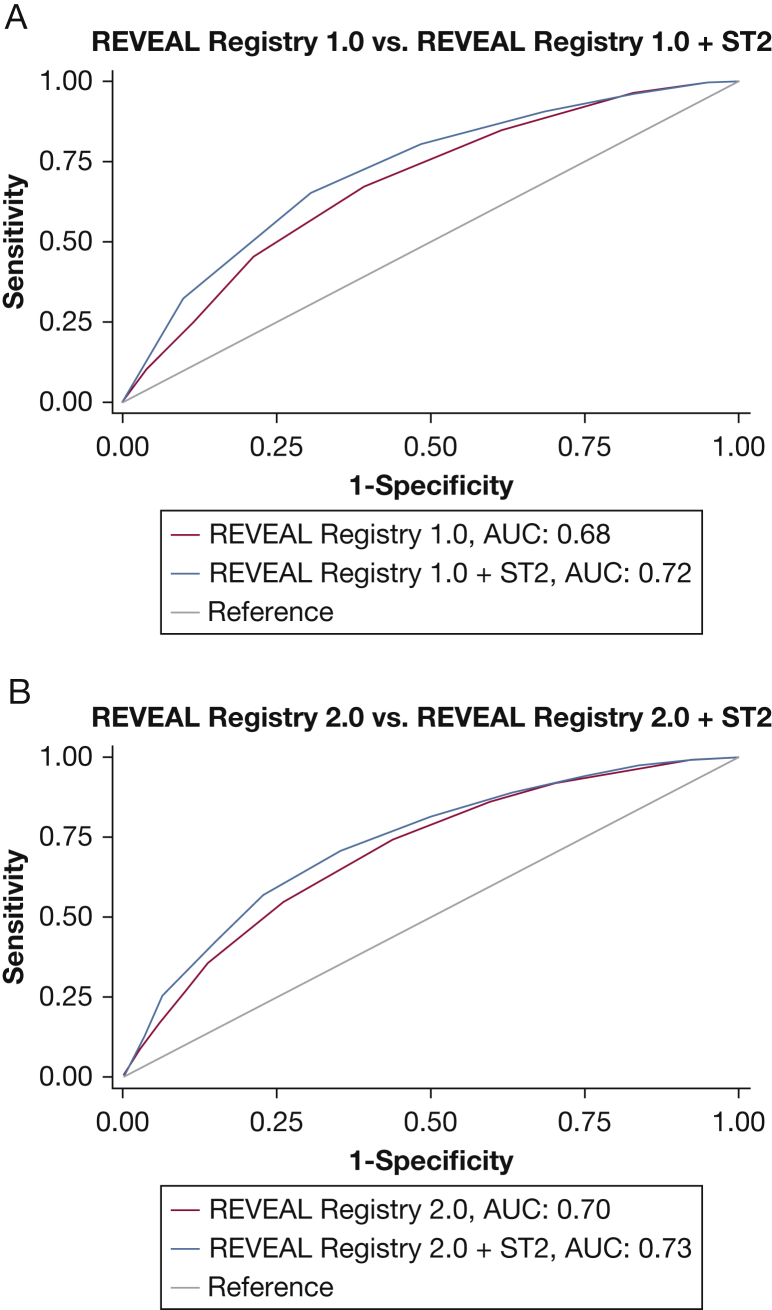
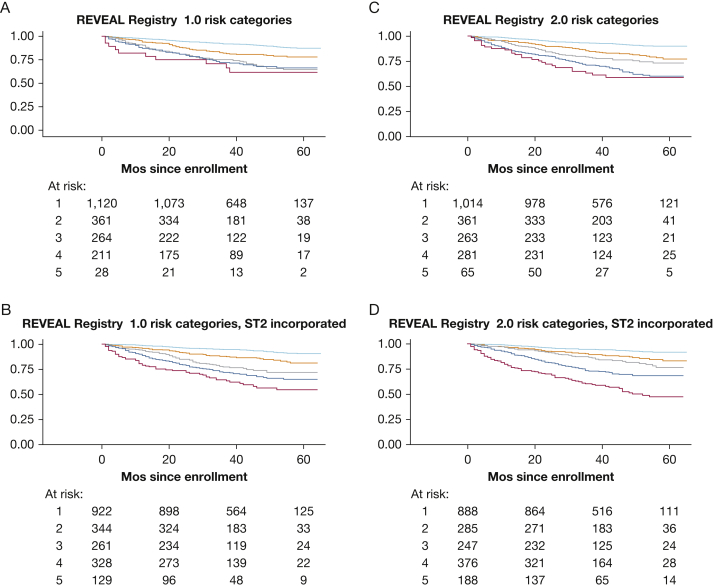
On the basis of the results of model comparisons, initial risk scores were recalculated for all subjects to incorporate ST2 measurements. Modifying both REVEAL Registry 1.0 and REVEAL Registry 2.0 risk scores to incorporate ST2 resulted in significantly improved discrimination of mortality. REVEAL Registry 2.0 plus ST2 achieved the best discrimination, with a C-statistic of 0.73 (Fig 3). When subjects who died during follow-up were reclassified into the previously described five risk categories after adding ST2 to REVEAL Registry 1.0, the percentage classified as risk category 4 or 5 based on enrollment data increased from 21% to 46% (reclassification by category shown in detail in e-Table 8). When subjects who died during follow-up were reclassified after adding ST2 to REVEAL Registy 2.0, the percentage classified as category 4 or 5 at enrollment increased from 37% to 58% (shown in detail in e-Table 9). Among subjects who did not die during follow-up, the percentage classified as risk category 4 or 5 increased from 9.01% to 19% when REVEAL Registry 1.0 was modified and from 13.40% to 22% when REVEAL Registry 2.0 was modified. On balance, reclassification resulted in improved discrimination of mortality and better separation of risk categories in Kaplan-Meier analysis (Fig 4).
Figure 3.
A, Receiver operating characteristic (ROC) curves comparing discrimination of mortality with REVEAL Registry risk score (in blue) to discrimination of mortality with points added to REVEAL Registry to incorporate ST2 (in red) (+1 has been added for ST2 measurements in the 4th quintile of ST2 and +2 has been added for 5th quintile of ST2) (area under the curve [AUC], 0.68 vs AUC, 0.72; P < .001), and B, ROC curves comparing discrimination of mortality with REVEAL Registry 2.0 risk score (in blue) to discrimination of mortality with points added to REVEAL Registry 2.0 to incorporate ST2 (in red), as previously described (AUC, 0.70 vs AUC, 0.73; P < .001). See Figure 1 legend for expansion of abbreviations.
Figure 4.
Kaplan-Meier curves for A, five risk categories classified according to REVEAL Registry risk score (χ2, 114.3; P < .001); B, five risk categories as reclassified after ST2 data incorporated into REVEAL Registry risk score (+1 has been added for 4th quintile of ST2 and +2 has been added for 5th quintile of ST2) (χ2, 189.5; P < .001); C, five risk categories classified according to REVEAL Registry 2.0 risk score (χ2, 150.8; P < .001); D, five risk categories as reclassified after ST2 data incorporated into REVEAL Registry 2.0 risk score, as previously described (χ2, 254.2; P < .001). For all plots, the green line depicts risk category 1; orange line, risk category 2; blue line, risk category 3; maroon line, risk category 4; gray line, risk category 5. See Figure 1 legend for expansion of abbreviations.
Discussion
To our knowledge, this is the largest single investigation of the FDA-approved biomarkers for risk stratification in left-sided heart failure (ST2, Gal3, and NT-proBNP) in PAH. Our results clearly demonstrate that ST2 is a robust predictor of mortality in PAH, and that addition of ST2 to survival models improves model fit and predictive capacity. Recalculation of the commonly used REVEAL Registry risk score to incorporate ST2 measurements results in improved discrimination of mortality in our cohort. Our results also validate previously demonstrated associations between NT-proBNP and survival in PAH. Contrary to previous findings, Gal3 was not a consistent predictor of mortality in our study.
Our study is, to our knowledge, the first to demonstrate that ST2 is significantly associated with both disease severity and survival in a large cohort composed solely of patients with group 1 PAH. Our finding that adding ST2 to survival models improves prediction aligns with a prospective study that found a multibiomarker score (inclusive of both NT-proBNP and ST2) had greater prognostic value than any one biomarker.19 These findings support an additive role for ST2 in risk stratification in PAH. There may be a mechanistic role for ST2 in PAH pathogenesis, as previous work has suggested that ST2 may serve as an endogenous inhibitor of IL-33, an IL-1 family member and regulator of inflammation.9
Our study confirms associations between natriuretic peptides and mortality in PAH, using NT-proBNP rather than the older and formerly more commonplace BNP, the full natriuretic peptide. Baseline and serial measurements of BNP, as well as the change in BNP over follow-up, have been associated with 5-year mortality in PAH.23 NT-proBNP has a greater range of values than BNP, which may enable recognition of more incremental changes. Further, NT-proBNP may be less falsely suppressed than BNP in obese patients.24 Our study demonstrates significant associations between NT-proBNP and mortality in the most common PAH subgroups, IPAH and CTD-PAH. Prior studies examining NT-proBNP in PAH subgroups have shown conflicting results: Fijalkowska et al13 (n = 55) found that NT-proBNP predicted mortality in overall PAH as well as in IPAH, whereas Mathai et al25 (n = 98) found no significant relationship between NT-proBNP and mortality in IPAH. These discrepant findings are likely due to the relatively small size of earlier cohorts, as the present study shows a strong, significant association between NT-proBNP levels and survival in both IPAH and CTD-PAH.
Our study shows no consistent association between Gal3 and survival in multivariable models. These findings contrast with the finding by Mazurek et al18 of a relationship between Gal3 and mortality in a smaller pulmonary hypertension cohort (n = 76), with a large proportion of patients with heart failure with preserved ejection fraction (HFpEF) (51%). The findings by Mazurek et al18 may have been driven by HFpEF physiology rather than PH. Indeed, Gal3 may be a better predictor of mortality and hospitalizations in HFpEF than in heart failure with reduced ejection fraction.26 Faludi et al27 showed that Gal3 independently predicts mortality in systemic sclerosis, regardless of the presence or absence of pulmonary vascular disease; thus, variations in Gal3 may not be specific to the pulmonary vasculature or the RV. In fact, Gal3 has been recognized as an independent predictor of all-cause mortality in the general population.8
This study offers several advantages over prior biomarker studies in PAH. The large sample size and large number of events within the cohort enabled extensive multivariable modeling and model comparisons. The study is further strengthened by its multicenter referral base. All institutions participating in the PAH Biobank are pulmonary hypertension referral centers, and therefore it is reasonable to expect that subjects included are classified correctly and that patients receive treatment according to the standard of care.
This study has several limitations. Although the multicenter registry allows for a large sample size, it relies on separate reports from multiple centers for data collection. Some covariates, notably 6MWD and NYHA FC, were missing for a large number of patients, and our initial multivariable survival analyses, in which subjects with missing data were omitted, may be biased as a result. However, results of sensitivity analyses in which covariates with significant missingness were excluded were generally consistent with complete case analyses. Some of the parameters included in the REVEAL Registry risk prediction tool (ie, echocardiographic information about the presence of pericardial effusion, diffusing capacity of the lung for carbon monoxide, hospitalizations over the last 6 months) were not available in this cohort. Notably, the REVEAL Registry risk score retains its predictive ability if at least seven of the 12 available risk parameters are available and included in the calculations,21 and thus this is unlikely to have affected our results. In real-world clinical practice, all 12 possible parameters are unlikely to be available at each time point that risk prediction is determined, and thus the scoring system is designed to be used with the data available. Pulmonary vasodilator therapies may affect circulating biomarker levels, as NT-proBNP has demonstrated responsiveness to PAH-specific therapies.28,29 This is a prevalent cohort, with the majority of patients receiving PAH-specific therapy at the time of biomarker assessment. However, we were able to adjust for the presence and class of PAH therapies in multivariable models to mitigate this limitation. For the majority of subjects, serum collection was not contemporaneous with assessments of other clinical variables such as 6MWD or RHC measurements. However, the large size of the cohort enabled meaningful analyses in a subset of subjects with biomarkers obtained within 3 months of other clinical measures of disease severity, and the strength and significance of associations were consistent in sensitivity analyses performed with larger sample sizes obtained by relaxing this 3-month time window. Finally, modifications of REVEAL Registry risk scores are performed here to illustrate the statistical added value of ST2 in a clinically meaningful way. Improvements in model accuracy gained by adding ST2 to survival models in our cohort have not been externally validated. The addition of ST2 to REVEAL Registry parameters will require validation in future cohorts before risk scores are modified in this way in real-world clinical practice.
There is mounting evidence in support of circulating biomarkers as objective and broadly accessible tools for noninvasively assessing disease severity and prognosis. This study shows that ST2, a commercially available biomarker approved for risk stratification in left-sided heart failure, correlates with disease severity and robustly predicts mortality in PAH, particularly when used in combination with NT-proBNP. Incorporating data about ST2 measurements may improve currently used point-in-time risk assessment tools such as the REVEAL Registry risk score. Future studies are needed to assess the additive effects of using multiple biomarkers in combination for risk stratification, and to develop noninvasive multimarker assays that outperform current risk calculators that rely on invasive and cumbersome testing procedures.
Acknowledgments
Author contributions: A. D. E. had full access to all of the data in the study and takes full responsibility for the integrity of the data and the content of the manuscript. C. E. S., R. L. D., P. M. H., L. J. M., E. D. A., and A. D. E. conceived of the study; J. Y., M. K. N., S. B., D. D. I., M. W. P., and W. C. N. contributed biologic specimens and conducted sample assays; C. E. S. and R. D. V. performed all statistical analyses; C. E. S. furnished a first draft of the manuscript; all authors revised the manuscript for important intellectual content and approved the final version.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix, e-Figure, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was supported by National Institutes of Health/National Heart, Lung, and Blood Institute awards R01HL135114 (A. D. E., J. Y., R. D., D. V., W. C. N., L. J. M., D. D. I., and E. D. A.), R24HL105333 (W. C. N., M. W. P., L. J. M., D. D. I., and E. D. A.), and T32HL007534 (C. E. S.). Serum/tissue samples were provided by the Pulmonary Hypertension Breakthrough Initiative (PHBI). Funding for the PHBI is provided under an NHLBI R24 grant [R24HL123767] and by the Cardiovascular Medical Research and Education Fund (CMREF). M. K. N. was supported by a Matthew and Michael Wojciechowski Pediatric Pulmonary Hypertension Proof-of-Concept Grant (Dr Robyn J. Barst Pediatric PH Research and Mentoring Fund Grant). The Johns Hopkins Pulmonary Hypertension program was supported by National Institutes of Health/National Heart, Lung, and Blood Institute awards P50 HL084946/R01 and HL114910 (P. M. H.). D. I. was supported by the Jayden DeLuca Foundation
Supplementary Data
References
- 1.Benza R.L., Gomberg-Maitland M., Miller D.P. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest. 2012;141(2):354–362. doi: 10.1378/chest.11-0676. [DOI] [PubMed] [Google Scholar]
- 2.Benza R.L., Miller D.P., Gomberg-Maitland M. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122(2):164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 3.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 4.Kirk V., Bay M., Parner J. N-terminal proBNP and mortality in hospitalised patients with heart failure and preserved vs. reduced systolic function: data from the prospective Copenhagen Hospital Heart Failure Study (CHHF) Eur J Heart Fail. 2004;6(3):335–341. doi: 10.1016/j.ejheart.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Gardner R.S., Ozalp F., Murday A.J., Robb S.D., McDonagh T.A. N-terminal pro-brain natriuretic peptide: a new gold standard in predicting mortality in patients with advanced heart failure. Eur Heart J. 2003;24(19):1735–1743. doi: 10.1016/j.ehj.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Ho J.E., Liu C., Lyass A. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60(14):1249–1256. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lok D.J., Van Der Meer P., de la Porte P.W. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol. 2010;99(5):323–328. doi: 10.1007/s00392-010-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imran T.F., Shin H.J., Mathenge N. Meta-analysis of the usefulness of plasma galectin-3 to predict the risk of mortality in patients with heart failure and in the general population. Am J Cardiol. 2017;119(1):57–64. doi: 10.1016/j.amjcard.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Shao D., Perros F., Caramori G. Nuclear IL-33 regulates soluble ST2 receptor and IL-6 expression in primary human arterial endothelial cells and is decreased in idiopathic pulmonary arterial hypertension. Biochem Biophys Res Commun. 2014;451(1):8–14. doi: 10.1016/j.bbrc.2014.06.111. [DOI] [PubMed] [Google Scholar]
- 10.Shah R.V., Chen-Tournoux A.A., Picard M.H., van Kimmenade R.R., Januzzi J.L. Serum levels of the interleukin-1 receptor family member ST2, cardiac structure and function, and long-term mortality in patients with acute dyspnea. Circ Heart Fail. 2009;2(4):311–319. doi: 10.1161/CIRCHEARTFAILURE.108.833707. [DOI] [PubMed] [Google Scholar]
- 11.Ky B., French B., McCloskey K. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4(2):180–187. doi: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghali R., Altara R., Louch W.E. IL-33 (interleukin 33)/sST2 axis in hypertension and heart failure. Hypertension. 2018;72(4):818–828. doi: 10.1161/HYPERTENSIONAHA.118.11157. [DOI] [PubMed] [Google Scholar]
- 13.Fijalkowska A., Kurzyna M., Torbicki A. Serum N-terminal brain natriuretic peptide as a prognostic parameter in patients with pulmonary hypertension. Chest. 2006;129(5):1313–1321. doi: 10.1378/chest.129.5.1313. [DOI] [PubMed] [Google Scholar]
- 14.Williams M.H., Handler C.E., Akram R. Role of N-terminal brain natriuretic peptide (N-TproBNP) in scleroderma-associated pulmonary arterial hypertension. Eur Heart J. 2006;27(12):1485–1494. doi: 10.1093/eurheartj/ehi891. [DOI] [PubMed] [Google Scholar]
- 15.Andreassen A.K., Wergeland R., Simonsen S., Geiran O., Guevara C., Ueland T. N-terminal pro-B-type natriuretic peptide as an indicator of disease severity in a heterogeneous group of patients with chronic precapillary pulmonary hypertension. Am J Cardiol. 2006;98(4):525–529. doi: 10.1016/j.amjcard.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 16.Fenster B.E., Lasalvia L., Schroeder J.D. Galectin-3 levels are associated with right ventricular functional and morphologic changes in pulmonary arterial hypertension. Heart Vessels. 2016;31(6):939–946. doi: 10.1007/s00380-015-0691-z. [DOI] [PubMed] [Google Scholar]
- 17.Carlomagno G., Messalli G., Melillo R.M. Serum soluble ST2 and interleukin-33 levels in patients with pulmonary arterial hypertension. Int J Cardiol. 2013;168(2):1545–1547. doi: 10.1016/j.ijcard.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Mazurek J.A., Horne B.D., Saeed W., Sardar M.R., Zolty R. Galectin-3 levels are elevated and predictive of mortality in pulmonary hypertension. Heart Lung Circ. 2017;26(11):1208–1215. doi: 10.1016/j.hlc.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Placido R., Cortez-Dias N., Robalo Martins S. Prognostic stratification in pulmonary hypertension: a multi-biomarker approach. Rev Port Cardiol. 2017;36(2):111–125. doi: 10.1016/j.repc.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Benza R.L., Gomberg-Maitland M., Elliott C.G. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest. 2019;156(2):323–337. doi: 10.1016/j.chest.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Benza R.L., Farber H.W., Frost A. REVEAL risk score in patients with chronic thromboembolic pulmonary hypertension receiving riociguat. J Heart Lung Transplant. 2018;37(7):836–843. doi: 10.1016/j.healun.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Hess K.R. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14(15):1707–1723. doi: 10.1002/sim.4780141510. [DOI] [PubMed] [Google Scholar]
- 23.Frantz R.P., Farber H.W., Badesch D.B. Baseline and serial brain natriuretic peptide level predicts 5-year overall survival in patients with pulmonary arterial hypertension: data from the REVEAL Registry. Chest. 2018;154(1):126–135. doi: 10.1016/j.chest.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krauser D.G., Lloyd-Jones D.M., Chae C.U. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. Am Heart J. 2005;149(4):744–750. doi: 10.1016/j.ahj.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Mathai S.C., Bueso M., Hummers L.K. Disproportionate elevation of N-terminal pro-brain natriuretic peptide in scleroderma-related pulmonary hypertension. Eur Respir J. 2010;35:95–104. doi: 10.1183/09031936.00074309. [DOI] [PubMed] [Google Scholar]
- 26.de Boer R.A., Lok D.J., Jaarsma T. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011;43(1):60–68. doi: 10.3109/07853890.2010.538080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faludi R., Nagy G., Tőkés-Füzesi M., Kovács K., Czirják L., Komócsi A. Galectin-3 is an independent predictor of survival in systemic sclerosis. Int J Cardiol. 2017;233:118–124. doi: 10.1016/j.ijcard.2016.12.140. [DOI] [PubMed] [Google Scholar]
- 28.Dimitroulas T., Giannakoulas G., Karvounis H. N-terminal probrain natriuretic peptide as a biochemical marker in the evaluation of bosentan treatment in systemic-sclerosis-related pulmonary arterial hypertension. Clin Rheumatol. 2008;27(5):655–658. doi: 10.1007/s10067-007-0828-2. [DOI] [PubMed] [Google Scholar]
- 29.Souza R., Jardim C., Martins B. Effect of bosentan treatment on surrogate markers in pulmonary arterial hypertension. Curr Med Res Opin. 2005;21(6):907–911. doi: 10.1185/030079905X46232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.