ABSTRACT
Chronic inflammation is a common denominator linking a wide range of health conditions, including tissue response to radiation exposure. This pilot study investigates whether inflammatory cytokines—interleukins IL-6, −8, −10, monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor α (TNFα)—can be used as early biomarkers of radiation-induced adverse health effects in occupationally exposed individuals. The study included 33 workers externally exposed to gamma radiation from the nuclear industry with cumulated doses from 0.11 to 190 mSv and 42 non-exposed controls of comparable age and socio-economic status. IL-6, IL-8, MCP-1, TNFα and IL-10 were analyzed by enzyme-linked assay (ELISA) in blood plasma samples. Total antioxidant status (TAS) of blood plasma was determined by a colorimetric assay. The radiation-exposed and control groups measured significantly different levels of MCP-1, TNFα and IL-10. Seventy-five percent of radiation workers had either high MCP-1 levels or low IL-10 levels and 30% had all three cytokines dysregulated. Approximately 50% of workers showed upregulated antioxidant status, which appeared to compensate the pro-inflammatory cytokine shift in these individuals. In contrast, only 2% of the control subjects were found to have three dysregulated cytokines, and all of them measured within the normal TAS range. The present study may represent an important step towards the establishment of a reliable set of biomarkers for health-risk estimation in population cohorts exposed to low radiation doses.
Keywords: low-dose radiation, chronic radiation exposure, occupational exposure, NOD-like receptor protein 4, nuclear workers, inflammatory cytokines, biomarkers
INTRODUCTION
It is a well-established fact that ionizing radiation (IR) increases the risk of cancer and non-cancer diseases in exposed individuals. However, while at high and moderate doses the excess risk of radiation-induced adverse health effects follows a linear dose–response dependency, the magnitude of health risks at low doses (<100 mSv) remains elusive [1]. More than 1 million workers have been employed in the nuclear industry since the 1940s, with almost 60% of them having accumulated during their employment a dose of <10 mSv and 80% a dose of <50 mSv [2]. A retrospective cohort study of 308 297 nuclear workers from the UK, France and the USA (The International Nuclear Workers Study, INWORKS), has demonstrated a positive association between occupational radiation exposure and excess risk from cancer and cardiovascular mortality [3–5]. Similarly to cancer disease, risk of cardiovascular disease (CVD) was assessed to approximate that for A-bomb survivors (0.18 Sv−1 for all circulatory diseases [6]). However, the borderline significance and big discrepancy between excess relative risk (ERR) estimates in the three countries (from 0.026 Sv−1 for the USA to 0.31 Sv−1 for the French cohort) underscores the uncertainties associated with low-dose radiation epidemiology and the necessity to find sensitive and reliable biomarkers for evaluation of radiation-induced health effects.
Chronic inflammation is a common denominator linking multiple pathologies, including cancer, atherosclerosis and neurodegenerative diseases. It results from failure to resolve acute inflammation and is characterized by persisting high concentrations of specific molecules, such as proinflammatory cytokines and chemokines, adhesion molecules, oxidants and prostaglandins [7]. A number of in vitro studies in primary or immortalized cultured cells have shown that inflammatory reactions are part of the cellular response to ionizing radiation [8–10], which has led to the idea that markers of chronic inflammation may represent a promising class of biomarkers in low-dose radiation molecular epidemiology. Indeed, persistent inflammatory response has been demonstrated in sera of uranium miners with cumulated doses of >20 mSv/year, where 9 out of 28 investigated cytokines proved to be upregulated [11]. Among those were tumor necrosis factor α (TNFα), interleukins IL-6 and IL-10, which were also shown to be upregulated in the blood plasma of 442 A-bomb survivors more than 50 years after the exposure [12]. Multivariate statistical analysis of inflammatory factors in this particular A-bomb survivor group was used to identify two independent sets of inflammatory factors, correlating with radiation dose and age, one of which included IL-6 and CRP (C-reactive protein) and was dependent on ROS (reactive oxygen species) [12]. The authors concluded that radiation, alongside natural ageing, maintains chronic inflammation by more than one pathway, and each pathway is characterized by the dysregulation of a specific combination of inflammatory factors.
Notably, the above-mentioned molecular epidemiological studies emphasize the close association between inflammatory markers and redox-status responses. Inflammatory mediators and ROS are implicated independently in chronic inflammation but are involved in complex interplay within shared pathways [13]. As signaling molecules, ROS activate stress-response pathways, which then leads to a cascade production of more ROS, i.e. ROS-induced ROS generation. Studies in in vitro irradiated cell cultures reveal that it is these feed-forward loops, rather than the initial oxidant insult by radiation, that cause persisting oxidative stress when the generation of ROS exceeds the antioxidant capacity of the cell [14]. The transcription factor NF-kB (nuclear factor kappa-light-chain enhancer of activated B cells) appears to be the point where oxidative and inflammatory pathways converge. Multiple inflammatory cytokines are under NF-kB regulation, while NF-kB itself can be activated by both ROS and inflammatory cytokines, such as TNFα, the evidence for which comes from a variety of models, including human tumor cells or genetically modified cells [15, 16]. One mechanism of radiation-induced inflammatory response is the NADPH (nicotinamide adenine dinucleotide phosphate-oxidase) pathway, which upon activation is a potent intracellular source of ROS. In rat brain microvascular endothelial cells, pharmacological and genetic inhibition of NADPH has been shown to block radiation-mediated upregulation of intracellular ROS, activation of NF-kB and upregulation of adhesion molecules [17].
Here we aim to evaluate the suitability of six factors—five inflammatory cytokines and chemokines: TNFα, IL-6, IL-10, IL-8 and MCP-1 (monocyte chemoattractant protein-1), and total antioxidant status (TAS) of blood plasma—as biomarkers to be used in molecular epidemiological studies on low-dose radiation-induced inflammatory response.
MATERIALS AND METHODS
Study design and recruitment of participants
The voluntary participants in this study fall within two groups: a group of 33 male radiation workers, employees of the strictly controlled zone of the “Kozloduy” Nuclear Power Plant (NPP), who throughout the period of their employment have been exposed externally to mainly gamma radiation; a control group of 42 male individuals with various professions (medical personnel, airway dispatchers, police officers, laboratory technicians), who according to their dosimetry data, for the period of their employment have not absorbed a detectable radiation dose, i.e. cumulative radiation dose of 0 mSv.
The “Kozloduy” NPP operates a policy of employing in the strictly controlled zone only healthy male workers, without history of cancer diseases, major cardiac events, chronic respiratory diseases, epilepsy, obesity, etc. None of the workers had ever undergone radiotherapy or chemotherapy. Therefore, in order to avoid a healthy workers’ effect, similar exclusion criteria were applied to the control group. On the other hand, individuals with milder chronic conditions, such as hypertension (who have not been diagnosed with atherosclerosis), high blood sugar (without insulin dependency) and high cholesterol, are allowed to work in the strictly controlled zone and were therefore included in the current study (Table 1).
Table 1.
Characteristics of the participants included in the control (non-exposed) and occupationally exposed groups
| Control group (n = 42) | Exposed group (n = 33) | P value | |
|---|---|---|---|
| Median absorbed dose (interquartile range), mSv | 0 | 47.73 (19.5–104.6) | |
| Median age (interquartile range), years | 47.5 (39–53) | 46 (41–48) | |
| Years of occupational exposure (interquartile range) | 0 | 20 (16–25) | |
| Subjects with different smoking habits, n (%) | |||
| Non-smokers | 15 (45.4) | 19 (45.2) | |
| Light smokers | 11 (33.3) | 13 (30.9) | |
| Heavy smokers, >10 cigarettes/day | 7 (21.2) | 10 (23.8) | |
| Subjects with hypertensiona, n (%) | 14 (33) | 8 (24) | |
| Subjects with high cholesterola, n (%) | 2 (5) | 3 (9) | |
| Subjects with high blood sugara, n (%) | 1 (2) | 1 (3) | |
| Cytokines, pg/ml | |||
| Interleukin-6 | 4.9 (2.7–9.7) | 4.2 (2.1–7.1) | 0.457 |
| Interleukin-8 | 5.9 (4.1–10.8) | 4.5 (2.9–6.6) | −0.068 |
| Interleukin-10 | 8.3 (6.5–10.3) | BDLb (BDL–4.6)* | <0.001 |
| Tumor necrosis factor-α | BDLb (BDL–5.5) | 6.4 (BDL–16.9)* | 0.014 |
| Monocyte chemoattractant protein-1 | 54 (30–193) | 267 (194–313)* | <0.001 |
| Total antioxidant status, mmol/l | 1.73 (1.62–1.92) | 2.22 (2.12–2.35)* | <0.001 |
*
p < 0.05 compared with the control group; values are medians with interquartile range. aHypertension is defined as blood pressure of 140/100 mmHg measured on more than one occasion; high blood sugar indicates fasting hyperglycemia and is defined as blood sugar of 130 mg/dL after 8 h of fasting (measured on the day of the last routine medical examination); high cholesterol is defined as total cholesterol level of 240 mg/dL at the time of the last routine medical examination. bBDL, below detection limit. BDL values were replaced with a constant determined by dividing the limit of detection value by 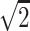 . Thus the replacement constants are equal to 1.42 pg/ml for IL-10, IL-6 and IL-8, to 2.8 pg/ml for TNFα and to 4.96 pg/ml for MCP-1.
. Thus the replacement constants are equal to 1.42 pg/ml for IL-10, IL-6 and IL-8, to 2.8 pg/ml for TNFα and to 4.96 pg/ml for MCP-1.
Results from clinical blood tests were also examined and only subjects with cell counts within the clinical reference norms were selected, in order to ensure that variations in inflammatory cytokine levels are not the result of temporarily elevated inflammatory status resulting from trivial diseases, such as colds or influenza. In addition, the medical records included information about symptoms of chronic psychological stress (frequent headaches, shortness of breath, anxiety, etc.), as well as the presence of chronic joint pain, unhealthy body-weight and allergies. Caution was taken to not include in the study subjects whose immune response and inflammatory profile is likely to be affected by such conditions. And finally, diagnostic medical procedures, which might result in significant radiation exposure, such as multiple X-rays or CT scans, were also considered reasons for exclusion from either the exposed or control group.
All participants in the study are ethnic Bulgarians and comprise a fairly homogenous group by the three main variables of socio-economic status: employment (full-time, 5-day working week), income (average or slightly-above-average for the Bulgarian working population) and education. A small discrepancy between the exposed and control groups exists by the variable education—all NPP workers have College or University degrees, while most controls have University degrees, and a small number (10%) do not have post-high school education. Statistical analysis indicates that the discrepancies in the education profile do not contribute to differences in the analyzed inflammatory markers (data not shown), therefore they are not discussed further. Data on alcohol consumption and smoking habits were collected by questionnaires. All participants described themselves as social drinkers and indicated their alcohol consumption habits as mild/moderate. According to smoking habits, the participants fall within three categories: non-smokers, light smokers (<10 cigarettes per day) and heavy smokers (>10 cigarettes per day) (Table 1). The study was carried out within the framework of regular bilateral contracts between “the National Center of Radiobiology and Radiation Protection” and several other establishments (the “Kozloduy” NPP, as well as several hospitals, universities and airports) concerning health monitoring of employees. As part of these agreements, medical records for all employees are compiled through clinical examinations and tests, as well as questionnaires. During routine annual clinical tests, volunteers for the current study agreed to provide an additional one-time blood sample. Radiation dosimetry data was obtained from personal dosimeters and cumulative radiation doses were calculated by summing up the records for the whole period of employment.
Informed consent was obtained from each participant. The study protocol conforms to the ethical guidelines of the Declaration of Helsinki (2008) of the World Medical Association and has been approved by the Institution’s Ethics Committee.
Plasma samples
Blood samples were taken in the morning, after a minimum of 8 h fasting and 17–19 h after the last radiation exposure for the radiation workers. Blood drawn by venipuncture (5–6 mL) and collected in tubes with EDTA (Vakutainer, Benton Dickinson, Oxford, UK) was delivered to the laboratory and stored at 4°C for up to 24 h before processing. Blood samples were centrifuged at 1000 x g for 30 min for plasma isolation and plasma samples were stored at −80°C for up to 3 months before cytokine levels were evaluated. The samples from the control and exposed subjects were handled concurrently and all assays were run on coded samples.
Measurements of cytokines and chemokines in blood plasma samples
Concentrations of TNFα, IL-6, IL-8, IL-10 and MCP-1 in blood plasma samples were evaluated based on conventional sandwich enzyme-linked immunosorbent assay (ELISA) by using Human Ready-Set-Go kits (eBiosience, San Diego, USA). Wash buffer, sample diluent, substrate solution and stop solution were also supplied by eBiosience. Ninety-six-well plates (Corning Costar 9018, Sigma-Aldrich GmbH, Munich, Germany) included with the ELISA kits were pre-coated with capture antibody overnight at 4°C. Pre-coating and all subsequent procedures were performed by strictly following the manufacturer’s instructions. Optical density was measured at 450 nm and 550 nm (SpectraMax M5 microplate reader, MDS Analytical technologies Inc., CA, USA) and the 550 nm values were subtracted from the 450 nm values in order to correct for optical imperfections.
All plasma samples were added in duplicates and the mean of the two measurements was used for further analyses. Variability between the duplicates was <20% of the value. Standards were run on every plate and standard curves were fitted to a four-parameter sigmoidal model (GraphPad Prism 6 software). When values measured in plasma samples were statistically different from the blank control (sample diluent), but below the limit of detection indicated by the manufacturer (2 pg/ml for IL-10, IL-6 and IL-8; 4 pg/ml for TNFα and 7 pg/ml for MCP-1), they were replaced with a constant obtained by dividing the limit of detection value by the square root of 2 (LOD/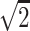 ). Comparison of different approaches to handling values below the detection limit has demonstrated that substituting with LOD/
). Comparison of different approaches to handling values below the detection limit has demonstrated that substituting with LOD/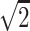 performs similarly to extrapolation methods at low detection limits and does not affect the results from statistical analyses [18, 19]. Thus the replacement constant is equal to 1.42 pg/ml for IL-10, IL-6 and IL-8; 2.8 pg/ml for TNFα and 4.96 pg/ml for MCP-1.
performs similarly to extrapolation methods at low detection limits and does not affect the results from statistical analyses [18, 19]. Thus the replacement constant is equal to 1.42 pg/ml for IL-10, IL-6 and IL-8; 2.8 pg/ml for TNFα and 4.96 pg/ml for MCP-1.
Evaluation of total antioxidant status of plasma
TAS of blood plasma was determined based on a colorimetric reaction by using a commercially available TAS kit supplied by Randox (Randox Laboratories, Crumlin, County Antrim, UK). Following the manufacturer’s instructions, the substrate 2,2-azinodi-(3-ethylbenzthiazoline sulphonate) (ABTS) was incubated with peroxidase and hydrogen peroxide (H2O2) in 96-well plates and the concentration of the resulting ABTS+ ions was determined by measuring absorption at 600 nm (SpectraMax M5 microplate reader). Plasma samples were then added to the wells and after a 4-min incubation the plate was measured again. The suppression of ABTS+ production and the subsequent decrease in color intensity is proportional to the concentration of antioxidants in the plasma sample. All samples were run in triplicates and the mean value from the three measurements was used for further analysis. The variability between the triplicates was >30% of the value.
Statistical analysis
First-pass data exploration, multivariate analyses and calculation of correction coefficients, as well as pivoting and organizing into subgroups, was performed with data visualization and statistics software Wizard for Macintosh. Data was then exported to GraphPad Prism 6 software, which was used for further statistical analysis and creation of graphs. Following the Shapiro-Wilk normality test it was confirmed that all data sets follow non-normal distributions. Groups and subgroups were compared by the non-parametric Kruskal-Wallis and Mann-Witney statistical tests and all values are presented as median with interquartile range. Prevalence of dysregulated cytokines was analyzed by Chi-Square correlation test. In all cases P < 0.05 was considered statistically significant.
RESULTS
Main characteristics of the control and exposed groups
The participants in this study comprise a group of 33 male employees from the “Kozloduy” NPP and a control group of 42 male individuals who had not been occupationally exposed to radiation. The main characteristics of the control and exposed groups, such as cumulative radiation dose, age, period of employment at the NPP, smoking habits, chronic conditions, and all parameters determined through measurements (cytokine levels and total antioxidant status) are presented in Table 1.
Association between occupational radiation exposure and cytokines in blood plasma
Our experimental data showed significantly higher plasma levels of the pro-inflammatory MCP-1 and TNFα and lower levels of the anti-inflammatory IL-10 in the group of radiation workers as compared with the control group; no differences were observed for IL-8 and IL-6 (Table 1, Fig. 1A). In the case of MCP-1 (Fig. 1A) the median value was almost five times higher in the exposed group in comparison to the controls, and the 75-percentile was almost twice as high.
Fig. 1.

Association of cytokine levels with occupational exposure to radiation. Levels of MCP-1, TNFα, IL-10, IL-6 and IL-8 in the blood plasma of exposed subjects and controls. (A) For non-parametric comparison by Mann-Whitney, the control and exposed groups are represented as dot plots with a longer line indicating the median and a range indicating the 1st and 3rd quartile. (B) For univariate (interrupted line) and multivariate (uninterrupted line) analyses, all subjects are presented in continuous dose–response plots, where P-values indicate the significance of the dose–cytokine association corrected for age, smoking, hypertension, high cholesterol and blood sugar.
The univariate linear regression analysis (Fig. 1B, interrupted lines) showed a positive correlation between cumulated radiation dose and cytokine levels in blood plasma, which became statistically significant for doses >10 mSv for MCP-1 and >25 mSv for IL-10 and TNFα. Multivariate linear regression analysis was performed to account for the effects of traditional risk factors for heart disease. For our purposes, the variables hypertension, cholesterol and blood sugar were treated as categorical data (normal or above clinical reference) and the variable smoking was treated as categorical with three categories: non-smoker, light smoker (<10 cigarettes per day) and heavy smoker (>10 cigarettes per day). After obtaining regression equations with coefficients for the variable “Dose, mSv” adjusted for age, smoking habits, high blood sugar, high cholesterol and hypertension, the slope of the dose–response dependency remained practically unchanged (Fig. 1B, uninterrupted line and Supplementary Table 1). In summary, the linear regression models predicted cytokine changes with coefficient mean estimates and confidence intervals for radiation exposure as follows: for MCP-1 levels—increase of 1.2 [0.4; 2.0] pg.ml−1.mSv−1; for TNFα levels—increase of 0.1 [0.01; 0.1] pg.ml−1.mSv−1; for IL-10 levels—decrease of −0.05 [−0.02; −0.8] pg.ml−1.mSv−1. The broad confidence intervals of the coefficients obviously limit the predictive power of the model. However, given the small scale of the study, it was judged that a linear model is the most appropriate in order to avoid false discoveries.
Fig. 1.

Continued.
Examining the incidence of dysregulated cytokine levels may represent an important addition to the analysis. However, reference levels for cytokines in the healthy population have not been established, and it is a common approach to take a certain percentile of the control group as a reference level [20, 21]. It was considered for the sake of this study that “normal” TNFα and MCP-1 levels are those falling within the 75-percentile of the control group, i.e. ≥5.5 pg/ml TNFα and ≥193 pg/ml MCP-1 were considered high. For IL-10 “normal” levels were those above the 25-percentile of the control group, i.e. ≤ 6.5 pg/ml was considered low. Thus by definition a quarter of the controls had high MCP-1 or/and TNFα levels and a quarter of them had low IL-10 levels. In contrast, in the exposed group the incidence of upregulated pro-inflammatory cytokines was 76% for MCP-1 and 55% for TNFα, and the incidence of downregulated IL-10 was also 76% (Table 2).
Table 2.
Correlation between radiation exposure and cytokine dysregulation
| Subjects beyond reference range | Control, n (%) | Exposed, n (%) | P-value |
|---|---|---|---|
| Tumor necrosis factor-α | 10 (24) | 18 (55) | 0.0085 |
| Monocyte chemoattractant protein-1 | 10 (24) | 25 (76) | <0.0001 |
| Interleukin-10 | 10 (24) | 25 (76) | <0.0001 |
| Interleukin-6 | 10 (24) | 5 (15) | 0.4 |
| Interleukin-8 | 10 (24) | 2 (6) | 0.06 |
n, Number of participants. Values beyond the reference range: exceeding the 75-percentile of the control group for MCP-1 (≥193 pg/ml), TNFα (≥5.5 pg/ml), IL-6 (≥9.65 pg/ml), IL-8 (≥10.77 pg/ml); and below the 25-percentile for IL-10 (≤ 6.5 pg/ml). The incidence of dysregulated cytokines was analyzed by Fisher’s exact test of independence.
Cytokine levels interdependencies
Cytokines such as TNFα and IL-6 can induce or suppress the expression of other cytokines, thus regulating the inflammatory response [22–24]. Since our data showed that TNFα was increased in radiation workers, it appeared plausible that other cytokines may be affected via the TNFα pathway. The correlation between different cytokines was initially examined by the non-parametric Spearman correlation test (data not shown). The results suggested that among controls there was a significant interdependency between TNFα, MCP-1 and IL-6, but this interdependency was not present in the exposed group. However, Spearman correlation coefficients should be treated with caution in small and middle-sized data sets. Therefore, the preferred approach was to divide the control and exposed groups into subgroups according to their TNFα levels (Fig. 2A–C). The cut-off value was set at 5.5 pg/ml, and the “low TNFα” and “high TNFα” groups were compared by their MCP-1, IL-6, IL-8 and IL-10 levels. As explained in the previous section, cut-off values were chosen to correspond to the 75-percentile for the control group. In agreement with the results from the Spearman correlation test, MCP-1 and IL-6 levels did not depend on TNFα in the exposed group, while the control “high TNFα” subgroup also possessed higher MCP-1 and IL-6 levels (Fig. 2A and B). Also, in agreement with the Spearman correlation test, no significant interdependencies were found between IL-10 and other cytokines in either the exposed or control groups (Fig. 2C and D).
Fig. 2.

Interdependencies between plasma levels of TNFα, MCP-1, IL-10 and IL-6. MCP-1 (A), IL-6 (B) and IL-10 (C) in control and exposed groups divided into subgroups on the basis of their TNFα levels with a cut-off at 5.5 pg/ml§. MCP-1 (D) in subgroups formed on the basis of IL-10 levels (cut off value of 6.5 pg/ml§). Each subgroup is represented by a dot plot with a longer line indicating the median and a range indicating the 1st and 3rd quartiles. Analysis was performed by using the Kruskal-Wallis statistical test. §Cut-off values were chosen to correspond to the 75-percentile of the control group, the rational for which is explained in the last segment of the Results section.*P < 0.05; **P< 0.01; ***P< 0.001; ****P< 0.0001.
Correlation between cytokine levels and TAS
Over the years, our group has compiled data on TAS and we have determined for the asymptomatic Bulgarian population the reference range of 1.39–2.22 mmol/l [25]. Recently, we have reported on the enhanced TAS of blood plasma from radiation workers and its correlation to higher levels of the cardiac remodeling biomarker ST2 [25]. Here, TAS levels higher than the upper reference limit of 2.22 mmol/l were measured in 17 (52%) out of the 33 exposed persons. In contrast, all of the TAS values measured in 21 randomly selected non-exposed individuals were within the normal range (<2.22 mmol/l) (Figure 3A, Table 1). Thus, there was a significant difference between the antioxidant status of controls and radiation workers (P< 0.001). Similarly to cytokine levels, multivariate analysis revealed that radiation exposure was the only explanatory variable that had a statistically significant positive association with TAS levels. The lower limit of the dose–response significance was 10 mSv, and the slope was described with a coefficient of 0.003 [0.0003; 0.04] (Fig. 3A).
Fig. 3.

Association of total antioxidant status (TAS) with occupational exposure to radiation; correlation between TAS and plasma cytokine levels. TAS in the blood plasma of exposed subjects and their controls. (A) Mann-Whitney comparison and linear regression (univariate analysis, interrupted line and multivariate analysis, uninterrupted line, P-value representing the significance of the radiation dose, TAS association corrected for age, smoking, hypertension, high cholesterol and blood sugar). IL-10 (B) and MCP-1 (C) levels in control and exposed subgroups formed on the basis of their TAS levels with a cut-off at 2.23 mmol/l (reference range for the healthy Bulgarian population). Each group or subgroup is represented as a dot plot with a longer line indicating the median and a range indicating the 1st and 3rd quartile. Analysis was performed by using the Kruskal-Wallis statistical test. *P < 0.05; **P < 0.01.
Two subgroups of radiation workers were formed according to their TAS levels: a “low TAS” subgroup (TAS < 2.23 mmol/l) and a “high TAS” subgroup (TAS ≥ 2.23 mmol/l) (Fig. 3B and C). A strong correlation was found between TAS and MCP-1 where the median MCP-1 level in the “low TAS” subgroup exceeded almost 1.5-fold that in the “high TAS” subgroup (Figure 3C). In contrast, no statistically significant difference existed between the exposed TAS subgroups in terms of their IL-10 levels (Fig. 3B). Notably, the levels of both MCP-1 (Fig. 3C) and IL-10 (Fig. 3B) in the “high TAS” subgroups were comparable to those of the non-exposed group, and only the subgroup of radiation workers with TAS within the reference range differed significantly from the control.
Association between cytokine levels and hypertension
Hypertension was identified in 8 subjects from the exposed group and in 14 subjects from the control group, representing 24% and 33%, respectively (Table 1). IL-10 was the only factor for which a significant difference between hypertension patients and subjects with normal blood pressure was found (Fig. 4). Surprisingly, hypertension patients expressed significantly more of this anti-inflammatory cytokine. Among radiation workers, 4 out of the 8 hypertension subjects had low IL-10 levels, compared with 19 out of the 25 subjects with normal blood pressure, hinting at a possible compensatory mechanism. In conclusion, elevated MCP-1 and TNFα levels emerged as independent parameters in the occupationally exposed groups, as the difference between exposed and non-exposed subjects remained evident regardless of the presence or absence of hypertension. IL-10 levels, however, should be treated with caution in the presence of clinical symptoms.
Fig. 4.

Association of cytokine levels with blood pressure in occupationally exposed subjects and non-exposed controls. MCP-1 (A), TNFα (B) and IL-10 (C) levels in hypertensive and normotensive subjects. Participants belonging to the exposed group are denoted by filled symbols (filled squares: exposed normotensive; filled diamonds: exposed hypertensive), and control subjects are denoted by empty symbols (empty squares: non-exposed normotensive; empty diamonds: non-exposed hypertensive). Analysis was performed by the Mann-Whitney non-parametric test for comparison of two groups, and P < 0.05 was considered statistically significant.
DISCUSSION
This study examines the inflammatory cytokine profile of a group of radiation workers from the “Kozloduy” NPP, whose cumulated radiation doses over the period of their employment ranged from 0.1 to 190.5 mSv external, predominantly gamma radiation. In comparison to the non-exposed controls, radiation workers exhibited higher levels of the pro-inflammatory cytokines MCP-1 and TNFα and lower levels of the anti-inflammatory IL-10, as well as altered redox status. Recently, similar associations between occupational exposure to radiation and altered levels of inflammatory cytokines and redox parameters have been reported in occupationally exposed health care workers [26, 27]. Here, the median MCP-1 level in the exposed group exceeded five times that of the control group, while the median IL-10 level was found to be more than four times lower than that of the controls.
Multivariate linear regression analysis suggested that MCP-1, TNFα and TAS increase with radiation dose at doses >10 mSv for MCP-1 and TAS, and >25 mSv for TNFα, while the anti-inflammatory IL-10 decreases with dose at doses >25 mSv. The linear-regression model should be considered a rough approximation and is not expected to have high accuracy of prediction due to the interpersonal variability of cytokine levels, resulting in broad confidence intervals for the slope. Despite all these limitations of the analysis, the strongly significant association between radiation dose and the above-mentioned inflammatory factors is an indication of their potential validity as markers for low-dose radiation exposure.
TNFα and MCP-1 levels determined for the control group were in agreement with published data for the healthy population, namely a typical range of 0.5–5 pg/ml for TNFα and 10–500 pg/ml for MCP-1 [28]. However, clinical reference values for cytokines have not been determined, since cytokine levels in the healthy population depend on ethnic, socio-economic and other factors [29]. In addition, short-term increase in cytokine secretion may be triggered by stress, physical activities and many other factors, thus an asymptomatic group of people may be expected to contain a significant number of individuals with elevated cytokine levels at any time of testing [30, 31]. We considered here as “normal” the MCP-1 and TNFα levels within the non-exposed group’s 75-percentile, and for IL-10 the values higher than the 25-percentile of the control group. Thus, by definition, each cytokine was dysregulated in 25% of control subjects. In contrast, 75% and 50% of exposed subjects had “high” levels of MCP-1 and TNFα respectively, and 75% had “low” levels of the anti-inflammatory IL-10. Ten radiation workers, representing 30% of the group, had all three cytokines dysregulated, versus only one person (2%) in the control group. These results indicate a significant shift towards a more pro-inflammatory profile in the group of nuclear workers in comparison to the non-exposed controls. It is worth noting, however, that there was a big variation in cytokine levels among the exposed subjects, especially for TNFα where 50% appear to not show an exposure-related response. We hypothesize that individual radiosensitivity may play an important role in cytokine regulation after radiation exposure.
MCP-1 is directly involved in the recruitment of monocytes to the vascular wall, thus contributing to the formation and progression of atherosclerotic lesions, as well as to destabilization of the plaque and adverse remodeling following myocardial infarction [32]. In the presence of clinical symptoms, high MCP-1 values have been clearly linked to adverse clinical outcome [33]. As a sub-clinical and pre-clinical marker, MCP-1 has also been proven applicable, as it has been linked to other markers of vascular dysfunction, as well as to traditional risk factors for CVD, such as age, smoking and hypertension [34, 35]. Besides CVD, MCP-1 is believed to be an important player in various neuroinflammatory diseases, such as multiple sclerosis, stroke or Alzheimer’s disease by regulating the infiltration of immune cells in the central nervous system [36].
In contrast to MCP-1, IL-10 has been shown to suppress inflammatory activation of monocytes and macrophages by transcriptional and post-transcriptional inhibition of pro-inflammatory cytokines, thus contributing to the down-regulation of the inflammatory response and to protection against atherosclerotic disease [37]. The importance of IL-10 has been demonstrated in patients with acute coronary syndrome where IL-10 levels below 2.5 pg/ml were associated with higher risk of myocardial infarction during a 6 month follow up [38]. In addition, IL-10 plays an important role in neurodegenerative diseases such as Parkinson’s disease, multiple sclerosis and osteoarthritis [39]. The role of IL-10 as a pre-clinical marker is not well established.
TNFα can induce multiple stress signaling pathways and amplify the inflammatory response, leading to the upregulation of various adhesion molecules and inflammatory cytokines. Increased TNFα levels are implicated in a wide variety of chronic inflammatory diseases, including atherosclerosis and heart failure, as well as Alzheimer’s disease and obesity. TNFα is also closely associated with traditional cardiovascular risk factors and with frailty and disability in the elderly [40]. Even though in the current study both TNFα and MCP-1 were increased in radiation workers, there seemed to be a very weak correlation between the two markers, i.e. MCP-1 levels did not differ significantly between the “high TNFα” and “low TNFα” exposed subgroups. Indeed, depending on the activated stress-response pathways, expression of cytokines such as IL-6, IL-8 and MCP-1 may increase without the accompanying significant elevation of TNFα levels. In human monocytes and macrophages it has been shown that low-level inflammatory insults may lead to the activation of the ERK (extracellular signal-regulated kinases) and p38 MAPK (mitogen-activated protein kinases) pathways but fail to activate the JNK (c-Jun N-terminal kinases) pathway, which is essential for significant TNFα expression [41].
The total antioxidant activity of blood plasma was significantly upregulated in the group exposed to radiation: 50% of subjects had TAS higher than the reference level of 2.22 mmol/l, previously determined as the upper reference limit for the Bulgarian population [25], in contrast to non-exposed controls whose TAS levels were within the reference limits. Upregulated TAS corresponded to MCP-1 and IL-10 levels comparable to those in the control group. In fact, significantly different MCP-1 and IL-10 levels between the exposed and control groups were found only in the lower TAS subgroup of radiation workers. Previous studies on inflammatory status and oxidative stress in cardiac syndrome patients have proven the association of low antioxidant capacity with high MCP-1 levels and unfavorable prognosis [42]. It is also a well-established fact that inflammation is closely linked to cellular redox imbalance, and oxidative stress is present at all stages of the inflammatory response [43].
Recently, we have reported that among a similar group of radiation workers from the same nuclear facility, the incidence of high ST2 levels was increased in comparison to non-exposed controls. ST2 is an early clinical marker for heart failure, with a determined reference level of 35 ng/ml, beyond which it is considered that adverse cardiac remodeling is in progress [44–46]. In the “Kozloduy” NPP ST2 group, similarly to the current study, we found upregulated TAS in about 50% of exposed subjects [25]. However, in contrast to MCP-1 levels reported here, the radiation workers subgroup with high TAS did not differ from the low TAS subgroup by ST2 levels (data not shown). This observation implies that antioxidant capacity upregulation may not be sufficient for cardioprotection in the long-term. Activation of antioxidant pathways may suppress inflammation but fail to resolve it, especially if the external inflammatory stimuli are not removed, i.e. high TAS, low MCP-1 subjects may not be at lower risk from CVD. In the current exposed cohort, there was no correlation between MCP-1 and ST2 levels (data not shown), supporting the notion that these factors play roles in different stages of the inflammatory response, and may be differently affected by chronic oxidative stress.
MCP-1 and TNFα did not differ between hypertension and normal blood pressure subgroups, supporting the idea for the applicability of these cytokines as independent inflammatory biomarkers in the general population. Interestingly, persons with hypertension had higher levels of IL-10 with borderline significance. Six out of eight hypertension radiation workers (75%) had IL-10 levels above the detection level versus only 28% of the normal blood pressure workers. This observation is in agreement with a recent report that CVD progression may be accompanied by an overall increase of cytokines, including some anti-inflammatory such as IL-10 [47]. It remains, however, unclear whether this represents a defense mechanism in hypertension patients.
In conclusion, the current study reports a high degree of inflammatory status dysregulation in a group of individuals occupationally exposed to gamma radiation. Radiation workers exhibited a pro-inflammatory shift in their cytokine profile or an upregulation of antioxidant activity. While in subjects with high antioxidant capacity the pro-inflammatory shift appeared to be suppressed, the clinical consequences of this compensation remain unclear. The pre-clinical markers analyzed here can be considered an intermediate in the pathway of radiation-induced health effects. While this study addressed the exposure-to-cytokine relationship, the second part of the causal pathway—the cytokine-to-effect relationship—can only be established with a follow-up of the examined individuals over a period of 5 or more years, in order to evaluate the predictive power of cytokines as biomarkers for radiation risk.
Supplementary Material
ACKNOWLEDGMENTS
The authors are thankful to Dr. I. Guleva for her assistance in collecting blood samples and interpretation of clinical outcomes.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
FUNDING
This work was financed by the budget of the Bulgarian National Center for Radiobiology and Radiation Protection.
REFERENCES
- [1]. Kamiya K, Ozasa K, Akiba S et al. Long-term effects of radiation exposure on health. Lancet 2015;386:469–78. [DOI] [PubMed] [Google Scholar]
- [2]. Council N.R Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington, DC: The National Academies Press, 2006, 189–94. [PubMed] [Google Scholar]
- [3]. Hamra GB, Richardson DB, Cardis E et al. Cohort profile: The international nuclear workers study (INWORKS). Int J Epidemiol 2016;45:693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Richardson DB, Cardis E, Daniels RD et al. Risk of cancer from occupational exposure to ionising radiation: Retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS). BMJ 2015;351:h5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Gillies M, Richardson DB, Cardis E et al. Mortality from circulatory diseases and other non-Cancer outcomes among nuclear Workers in France, the United Kingdom and the United States (INWORKS). Radiat Res 2017;188:276–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Shimizu Y, Imano H, Ohira T et al. Gamma-Glutamyltranspeptidase and incident stroke among Japanese men and women: The circulatory risk in communities study (CIRCS). Stroke 2010;41:385–8. [DOI] [PubMed] [Google Scholar]
- [7]. Lawrence T, Gilroy DW. Chronic inflammation: A failure of resolution? Int J Exp Pathol 2007;88:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Dieriks B, De Vos WH, Derradji H et al. Medium-mediated DNA repair response after ionizing radiation is correlated with the increase of specific cytokines in human fibroblasts. Mutat Res 2010;687:40–8. [DOI] [PubMed] [Google Scholar]
- [9]. El-Saghire H, Thierens H, Monsieurs P et al. Gene set enrichment analysis highlights different gene expression profiles in whole blood samples X-irradiated with low and high doses. Int J Radiat Biol 2013;89:628–38. [DOI] [PubMed] [Google Scholar]
- [10]. Petit-Frere C, Capulas E, Lyon DA et al. Apoptosis and cytokine release induced by ionizing or ultraviolet B radiation in primary and immortalized human keratinocytes. Carcinogenesis 2000;21:1087–95. [PubMed] [Google Scholar]
- [11]. Li K, Chen Y, Li X et al. Alteration of cytokine profiles in uranium miners exposed to long-term low dose ionizing radiation. Scientific World Journal 2014;2014:216408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Hayashi T, Morishita Y, Khattree R et al. Evaluation of systemic markers of inflammation in atomic-bomb survivors with special reference to radiation and age effects. FASEB J 2012;26:4765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Khaper N, Bryan S, Dhingra S et al. Targeting the vicious inflammation-oxidative stress cycle for the management of heart failure. Antioxid Redox Signal 2010;13:1033–49. [DOI] [PubMed] [Google Scholar]
- [14]. Spitz DR, Azzam EI, Li JJ et al. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: A unifying concept in stress response biology. Cancer Metastasis Rev 2004;23:311–22. [DOI] [PubMed] [Google Scholar]
- [15]. Senftleben U, Cao Y, Xiao G et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 2001;293:1495–9. [DOI] [PubMed] [Google Scholar]
- [16]. Bubici C, Papa S, Pham CG et al. The NF-kappaB-mediated control of ROS and JNK signaling. Histol Histopathol 2006;21:69–80. [DOI] [PubMed] [Google Scholar]
- [17]. Collins-Underwood JR, Zhao W, Sharpe JG et al. NADPH oxidase mediates radiation-induced oxidative stress in rat brain microvascular endothelial cells. Free Radic Biol Med 2008;45:929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Croghan C, Egeghy P. Methods of dealing with values below the limit of detection using SAS. Presented at Southeastern SAS User Group FL: St. Petersburg, 2003. [Google Scholar]
- [19]. The Interstate Technology & Regulatory Council Groundwater Statistics and Monitoring Compliance, Statistical Tools for the Project Life Cycle 2013. https://www.itrcweb.org/gsmc-1/Content/Resources/GSMCPDF.pdf (7 June 2019, date last accessed).
- [20]. Selvarajah S, Todd I, Tighe PJ et al. Multiple circulating cytokines are Coelevated in chronic obstructive pulmonary disease. Mediators of Inflammation 2016;2016:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Seys SF, Scheers H, Van Den Brande P et al. Cluster analysis of sputum cytokine-high profiles reveals diversity in T(h)2-high asthma patients. Respir Res 2017;18:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Hayden MS, Ghosh S. Regulation of NF-kappaB by TNF family cytokines. Semin Immunol 2014;26:253–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol 2015;16:448–57. [DOI] [PubMed] [Google Scholar]
- [24]. Scheller J, Chalaris A, Schmidt-Arras D et al. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813;2011:878–88. [DOI] [PubMed] [Google Scholar]
- [25]. Katsarska O, Zaharieva E, Aneva N et al. The soluble receptor ST2 is positively associated with occupational exposure to radiation. Int J Radiat Biol 2016;92:87–93. [DOI] [PubMed] [Google Scholar]
- [26]. Ahmad IM, Abdalla MY, Moore TA et al. Healthcare workers occupationally exposed to ionizing radiation exhibit altered levels of inflammatory cytokines and redox parameters. Antioxidants 2019;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Heidari S, Taheri M, Ravan AP et al. Assessment of some immunological and hematological factors among radiation workers. J Biol Today's World 2016;5:113–9. [Google Scholar]
- [28]. Ueland T, Gullestad L, Nymo SH et al. Inflammatory cytokines as biomarkers in heart failure. Clin Chim Acta 2015;443:71–7. [DOI] [PubMed] [Google Scholar]
- [29]. Stowe RP, Peek MK, Cutchin MP et al. Plasma cytokine levels in a population-based study: Relation to age and ethnicity. J Gerontol A Biol Sci Med Sci 2010;65:429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Peake JM, Suzuki K, Hordern M et al. Plasma cytokine changes in relation to exercise intensity and muscle damage. Eur J Appl Physiol 2005;95:514–21. [DOI] [PubMed] [Google Scholar]
- [31]. Picotte M, Campbell CG, Thorland WG. Day-to-day variation in plasma interleukin-6 concentrations in older adults. Cytokine 2009;47:162–5. [DOI] [PubMed] [Google Scholar]
- [32]. Gonzalez-Quesada C, Frangogiannis NG. Monocyte chemoattractant protein-1/CCL2 as a biomarker in acute coronary syndromes. Curr Atheroscler Rep 2009;11:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. De Lemos JA, Morrow DA, Sabatine MS et al. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation 2003;107:690–5. [DOI] [PubMed] [Google Scholar]
- [34]. Deo R, Khera A, Mcguire DK et al. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Am Coll Cardiol 2004;44:1812–8. [DOI] [PubMed] [Google Scholar]
- [35]. Martinovic I, Abegunewardene N, Seul M et al. Elevated monocyte chemoattractant protein-1 serum levels in patients at risk for coronary artery disease. Circ J 2005;69:1484–9. [DOI] [PubMed] [Google Scholar]
- [36]. Conductier G, Blondeau N, Guyon A et al. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J Neuroimmunol 2010;224:93–100. [DOI] [PubMed] [Google Scholar]
- [37]. De Waal Malefyt R, Abrams J, Bennett B et al. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J Exp Med 1991;174:1209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Heeschen C, Dimmeler S, Hamm CW et al. Serum level of the antiinflammatory cytokine interleukin-10 is an important prognostic determinant in patients with acute coronary syndromes. Circulation 2003;107:2109–14. [DOI] [PubMed] [Google Scholar]
- [39]. Kwilasz AJ, Grace PM, Serbedzija P et al. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology 2015;96:55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. De Gonzalo-Calvo D, Neitzert K, Fernandez M et al. Differential inflammatory responses in aging and disease: TNF-alpha and IL-6 as possible biomarkers. Free Radic Biol Med 2010;49:733–7. [DOI] [PubMed] [Google Scholar]
- [41]. Patrone JB, Bish SE, Stein DC. TNF-alpha-independent IL-8 expression: Alterations in bacterial challenge dose cause differential human monocytic cytokine response. J Immunol 2006;177:1314–22. [DOI] [PubMed] [Google Scholar]
- [42]. On YK, Park R, Hyon MS et al. Are low total serum antioxidant status and elevated levels of C-reactive protein and monocyte chemotactic protein-1 associated with cardiac syndrome X? Circ J 2005;69:1212–7. [DOI] [PubMed] [Google Scholar]
- [43]. Mittal M, Siddiqui MR, Tran K et al. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 2014;20:1126–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Rehman SU, Mueller T, Januzzi JL Jr. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol 2008;52:1458–65. [DOI] [PubMed] [Google Scholar]
- [45]. Shimpo M, Morrow DA, Weinberg EO et al. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation 2004;109:2186–90. [DOI] [PubMed] [Google Scholar]
- [46]. Weir RA, Miller AM, Murphy GE et al. Serum soluble ST2: A potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J Am Coll Cardiol 2010;55:243–50. [DOI] [PubMed] [Google Scholar]
- [47]. Yilmaz MI, Solak Y, Saglam M et al. The relationship between IL-10 levels and cardiovascular events in patients with CKD. Clin J Am Soc Nephrol 2014;9:1207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


