Abstract
Methamphetamine (METH) is a potent CNS stimulant that is widely used as a recreational drug. Due to its ability to increase bodily heat production and diminish heat loss due to peripheral vasoconstriction, METH is able to increase brain and body temperature. The hyperthermic effects of METH are potentiated when the drug is used under conditions of psycho-physiological activation and in warm ambient temperatures. In this short review, we present and discuss our data on the effects of METH on brain temperature and a number of neural parameters that characterize permeability of the blood-brain barrier (albumin immunoreactivity), glial activity (GFAP immunoreactivity), brain tissue water content, and structural abnormalities of brain cells. We demonstrate that the extent of these neural alterations strongly depends on METH-induced brain temperature elevation and they all dramatically increase following exposure to METH in warm (29°C) vs. standard (23°C) ambient temperatures. Based on these data we consider possible pathophysiological mechanisms underlying acute METH toxicity, suggesting the critical role of drug-induced brain hyperthermia, temperature-dependent leakage of the blood-brain barrier (BBB), and the development of vasogenic edema that could finally result in decompensation of vital functions and death.
Methamphetamine (METH) is a potent CNS stimulant that is widely used as a recreational drug. The current spike in the use of opioid drugs and the rise in lethality associated with these drugs shifted public attention away from METH and other related psychostimulants such as MDMA, which are still quite popular all over the world, especially with young users. While social and economic problems associated with drug abuse are well known, METH intake could result in serious health complications, including death following acute overdose. The typical symptoms of METH overdose in humans include arrhythmia, hyperventilation, hyperactivity, sweating, fever, muscular pain, and biphasic changes in arterial blood pressure (Rusyniak and Sprague, 2005; Prakash et al., 2017). Most of these symptoms reflect strong activation of the central nervous system (CNS) with subsequent hyperactivity of the sympathetic nervous system, which could be responsible for multiple autonomic effects of this drug. Among these effects, an extreme rise in body temperature (hyperthermia) is a prominent one, which could be responsible, at least in part, for the development of other pathological symptoms, including death.
It is generally believed that coma and lethality during METH overdose result primarily from heart attack or stroke. In this work, we aim to challenge this view and present several sets of data which suggest that brain hyperthermia, with subsequent leakage of the blood-brain barrier (BBB) and development of vasogenic edema, is the primary pathophysiological mechanism determining death during acute METH overdose. Although our data were obtained in rats, which have important differences from humans in temperature regulation (Gordon 1990, 2007), we believe that certain findings of our studies could be applicable for understanding the acute toxic effects of METH in human drug users.
We start our discussion with a short introduction that defines brain temperature as an important homeostatic parameter, which simultaneously depends on metabolic neural activity and affects all manifestations of neural activity and neural function. While traditionally viewed as a strictly regulated parameter, brain temperature fluctuates within relatively wide limits under physiological conditions and is affected by different psychoactive drugs. Second, we will consider the brain temperature effects of METH and show how these effects depend on activity state and environmental conditions. Third, we will show that acute METH intoxication results in dramatic changes in BBB permeability, activity of glial cells, and brain water homeostasis; these changes are tightly interrelated and their extent depends on drug-induced temperature elevation. To conclude, we discuss functional implications of our studies in terms of understanding the acute toxic effects of METH and related substances. The primary data regarding changes in brain parameters during METH intoxication were the result of a collaboration between the National Institute on Drug Abuse-Intramural Research Program, USA and Uppsala University, Sweden.
Brain temperature: Basic mechanisms of brain temperature homeostasis
While it is traditionally believed that brain temperature in healthy homeothermic organisms is stable and close to 37°C, relatively large fluctuations in brain temperature have been found to occur during different types of natural motivated behavior and following exposure to various environmental challenges (Abrams and Hammel, 1964; Baker et al., 1973; Delgado and Hanai, 1966; Fuller and Baker, 1983; Hayward and Baker, 1968; Kovalson, 1972; McElligott and Melzack, 1967; Serota and Gerard, 1938). By using miniature thermocouple sensors chronically implanted in different brain structures, we showed that hypothalamic temperature in awake, freely moving male rats can fall to ~35°C during deep sleep (Smirnov and Kiyatkin, 2008) and can phasically peak at ~39.5°C at the time of ejaculation during copulatory behavior (Kiyatkin and Mitchum, 2003). These lower and upper limits obviously define the range of “normal” brain temperature fluctuations (~4°C) within the entire physiological continuum.
Changes in brain temperature depend on two opposing forces. While metabolism-related intra-brain heat production tends to increase brain temperature, heat dissipation by cerebral blood flow to the rest of the body and then to the external environment tends to decrease it. Brain activity is highly energy consuming (Schmidt-Nielsen, 1997; Siesjo, 1978), and heat continuously generated by brain tissue is removed by cerebral circulation due to a temperature differential between arterial blood inflowing to the brain and brain tissue (Kiyatkin et al., 2002; Hayward and Baker, 1968). While it seems mechanistic, the cooling of an internal combustion engine appears to be a good analogy to brain temperature exchange. Similar to circulating coolant that continuously removes heat from a working engine, cool, oxygenated arterial blood removes heat from the brain via heat exchange and the now warmed venous blood returns to the heart to be cooled and oxygenated again in the lungs. Such an arrangement determines the critical role of cerebral blood flow in brain temperature homeostasis and the essential interdependence of temperature in the brain and the rest of the body. While physiological increases in brain temperature result from increased intra-brain heat production and diminished heat loss due to peripheral vasoconstriction, brain temperature can also rise when heat generated in brain tissue during “normal” metabolic activity cannot be properly dissipated to the body and then to the external environment. In this case, the extent of temperature increase is determined by the efficiency of heat outflow from the brain and the body, and this increase can exceed the physiological range (>39-40°C).
While brain temperature is at its lowest values during natural sleep and is increased to a different extent following exposure to arousing stimuli and during motivated behaviors, brain temperature can also be decreased below its physiological range by drugs that have direct inhibiting actions on the CNS (i.e., general anesthetics, alcohol, benzodiazepines, natural and synthetic cannabinoids; see Kiyatkin, 2018 for review). As shown previously (Kiyatkin and Brown, 2005), general anesthesia induced by sodium pentobarbital induces robust decreases in brain temperature that occur due to coordinated changes in two factors: a decrease in intra-brain heat production and increased heat loss resulting from sustained skin vasodilation. Since most psychoactive drugs affect metabolism as well as the state of peripheral and cerebral blood vessels, they should affect brain temperature, and brain temperature responses should depend on the organism’s ongoing state and environmental conditions. A drug at a certain dose could induce minimal temperature effects under one set of environmental conditions, in which adaptive mechanisms of heat loss are fully effective. However, the same drug at the same dose can induce pathological hyperthermia when used in different environmental conditions in which heat dissipation mechanisms are significantly impaired. Since peripheral vasodilatation and perspiration are powerful means for heat loss in humans, drug-induced impairment of these adaptive mechanisms could be a very important determinant of drug-induced increases in brain and body temperatures.
Brain hyperthermia induced by METH: State-dependency and environmental modulation
It is well known that METH induces dose-dependent body hyperthermia, which can reach clearly pathological levels following high dose exposure (Alberts and Sonsalla 1995; Miller and O’Callaghan, 2003; Sandoval et al. 2000; Seiden and Sabol, 1996). It has been also shown that METH-induced hyperthermia is greatly potentiated in warm environmental temperatures that diminish natural heat dissipation. This phenomenon could be important for human conditions because METH is often used by young adults under conditions of physical and emotional activation, often in a warm and humid environment. While METH-induced metabolic activation and enhanced heat production in the brain and body is an essential factor determining hyperthermia, METH also induces strong peripheral vasoconstriction (Lynch and House, 1992), thus diminishing heat dissipation from body surfaces and enhancing heat accumulation in the brain.
To assess how METH affects brain temperature homeostasis and evaluate how the brain temperature effects of this drug are modulated by activity state and environmental conditions, we examined temperature changes in the nucleus accumbens (NAc), hippocampus and temporal muscle induced in male rats by METH (1-9 mg/kg, subcutaneously) in quiet resting conditions at a normal laboratory temperature (23°C), during social interaction with a female, and at moderately warm ambient temperatures (29°C) (Brown et al. 2003). While most experiments in rats are typically conducted at standard laboratory temperatures (22-23°C), 29°C is not considered a truly hot temperature and it is within the range of normothermy or comfortable temperature in rats (Romanovsky et al. 2002), where basal metabolism is maintained at its lowest levels. While we initially assessed the temperature effects of METH at a wide range of doses and showed that drug-induced temperature elevations are dose-dependent, for most subsequent studies METH was administered subcutaneously at a 9 mg/kg dose. Although this drug dose exceeds the doses typically used by humans, this dose corresponds to only 1/6 of the LD50 in rats (49 mg/kg for ip injection; Davis et al. 1987; Yamamato 1963), and METH at this dose does not induce lethality under normal environmental conditions.
When tested at 29°C, NAc temperature increased rapidly in all animals, rising to clearly pathological values (>41-42°C) and resulting in death of 5 out of 6 animals within six hours post-injection. Lethality resulting from METH at doses much lower than the LD50 indicates that standard procedures for determining acute toxicity can be misleading, and that much smaller drug doses used under specific environmental conditions can induce life-threatening health complications, including death.
A powerful modulation of the temperature effects of METH by environmental conditions seen in rats may help to explain the exceptionally strong, sometimes fatal, responses of some individuals to amphetamine-like substances including METH and MDMA that occur under rave conditions. However, humans have much more efficient mechanisms of heat loss from body surfaces than do rats (Gordon 1990, 2007), thus making them more resistant to high environmental temperatures and thermogenic effects of psychomotor stimulants. In contrast to rats, humans have a well-developed ability to sweat and have a very high dynamic range of blood flow rates in the skin, thus allowing them to lose more metabolic heat than can be maximally produced in the body (Rowell 1983). These differences in the effector mechanisms of heat loss could explain weaker temperature increases induced by psychostimulants in monkeys (Banks et al 2007; Taffe et al. 2006; Von Huben et al. 2007) and humans (Freedman et al. 2005). Despite their high efficiency, the compensatory mechanisms of heat loss in humans could be greatly impaired under specific conditions, resulting in progressive heat accumulation in the organism. A simple bicycle exercise that induces a ~1°C brain temperature elevation under normal conditions produces much stronger hyperthermia (39.0-39.5°C) when the exercise is conducted in special water-impermeable clothing that prevents heat dissipation to the external environment (Nybo et al. 2002). Therefore, pathological brain hyperthermia induced by overdose of psychomotor stimulants under rave conditions results not only from excessive heat production due to drug-induced and psycho-physiological activation, but also from the impaired ability to dissipate metabolic heat due to powerful drug-induced peripheral vasoconstriction.
Changes in BBB permeability and brain water homeostasis during METH intoxication: Role of brain temperature
Considering the issue of neurotoxicity, it is usually assumed that METH and related drugs have direct toxic effects on neural cells, with relative selectivity towards specific cell groups, brain structures, and cellular organelles. In particular, it was shown that METH has damaging effects on fine dopamine axonal terminals in the striatum (Ricaurte et al. 1980; Riddle et al. 2006; Woolverton et al. 1989) and this damage results in multiple health complications associated with diminished dopamine transmission. Alterations in the activity and responsiveness of dopamine and other monoamine systems could be important factors in psycho-emotional and psychiatric disorders including acute METH psychosis and severe depression following long-term METH use (Kalant 2001). However, METH and other psychomotor stimulant drugs also induce strong metabolic activation and robust hyperthermia. Enhanced metabolism is tightly related to oxidative stress, which is caused by an accumulation of reactive oxygen species and an inability of an organism to detoxify them and repair the resulting damage. Disturbances in this normal redox state can cause toxic effects on brain cells through the production of peroxides and free radicals that damage all components of the cell including proteins, lipids, and DNA. Oxidative stress as a consequence of sustained metabolic activation is often viewed as a primary factor of METH-induced neurotoxicity (Cadet et al. 2007; De Vito and Wagner 1989; Stephans and Yamamoto 1994). Since brain cells are exceptionally temperature-sensitive, with structural abnormalities appearing at ~40°C, i.e., only three degrees above a normal baseline (Chen et al. 2003; Iwagami 1996; Oifa and Kleshchnev 1985; Lepock 2003; Sharma and Hoopes 2003; Yamamoto and Zhu 1998), high temperature per se could be an important contributor to acute neurotoxicity. Due to the temperature dependence of most physico-chemical processes governing neural activity, hyperthermia could also be a powerful factor that enhances the toxic effects of METH and its metabolites on brain cells.
In addition to the direct effects of high temperatures on brain cells and the potentiation of toxic effects of drug metabolites, brain hyperthermia appears to alter permeability of the BBB—an important barrier that maintains stability of the brain environment and protects neural cells from potentially dangerous ionic and chemical perturbations occurring in the body (Rapoport 1976; Zlokovic 2008). Leakage of the BBB has been previously documented during environmental warming (Cervos-Navarro et al. 1998; Sharma et al. 1992), intense physical exercise (Watson et al., 2005), various types of stress (Esposito et al. 2001; Ovadia et al. 2001; Sharma and Dey 1986), and morphine withdrawal (Sharma and Ali 2006). In our studies, we examined how acute METH intoxication (9 mg/kg) affects BBB permeability, the state of glial cells, brain ion and water homeostasis, and structural abnormalities of different types of brain cells (Kiyatkin et al., 2007; Sharma and Kiyatkin, 2008).
For these experiments, rats were surgically implanted with a chronic temperature sensor in the NAc. After several days of habituation to the recording environment, rats were subcutaneously injected with METH in two environments that differed in ambient temperature (22-23°C vs. 29°C) while brain temperature and locomotion were recorded. The brains were taken at different times after METH administration, when NAc temperature peaked or reached clearly pathological values (>41.5°C). The state of BBB permeability and edema were then determined by albumin immunoreactivity and the measuring of brain water content. Albumin is a relatively large plasma protein (molecular weight 59 kDa, molecular diameter 70 A) that is normally confined to the luminal side of endothelial cells and is not present in the brain under normal conditions. Thus, the appearance of albumin immunoreactivity in brain cells or neuropil indicates a breakdown of the BBB. Finally, brain slices were examined using light and electron microscopy to determine the extent and specifics of cellular abnormalities.
As shown in Figure 2, METH induced a robust increase in albumin immunoreactivity, suggesting BBB leakage. Compared to saline-treated controls, albumin immunoreactivity increased strongly in both METH groups and the changes were significantly larger when the drug was administered at 29° than 23°C (c). While rats in each condition showed significant increases in NAc temperature, the increases were significantly stronger when METH was administered at 29°C (a). Both METH groups also showed significant increases in brain water content and this increase was significantly larger in rats that received METH at 29°C (b). As shown in Figure 3A, albumin immunoreactivity was strongly dependent on brain temperature, with virtually no positive cells appearing at low basal temperatures and a progressive increase occurring at high temperatures. Tissue water content was directly related to brain temperature (B), suggesting a tight relationship between brain hyperthermia and edema.
Figure 2.
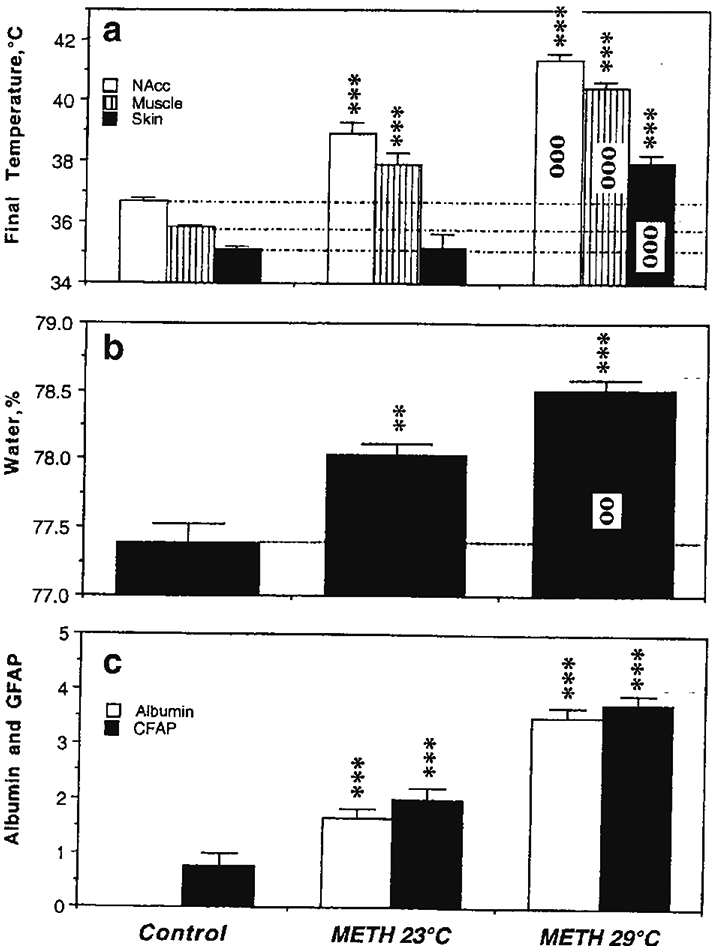
Mean (±SEM) values of temperature (a), brain tissue water content (b) and immunoreactivity for albumin and GFAP (c) in rats exposed to METH in standard (23°C) and warm (29°C) environmental temperatures. Asterisks show values significantly different from the control (intact rats that received saline injections), and circles indicate significant differences between 23 and 29°C. Original data were published in Kiyatkin et al., 2007.
Figure 3.
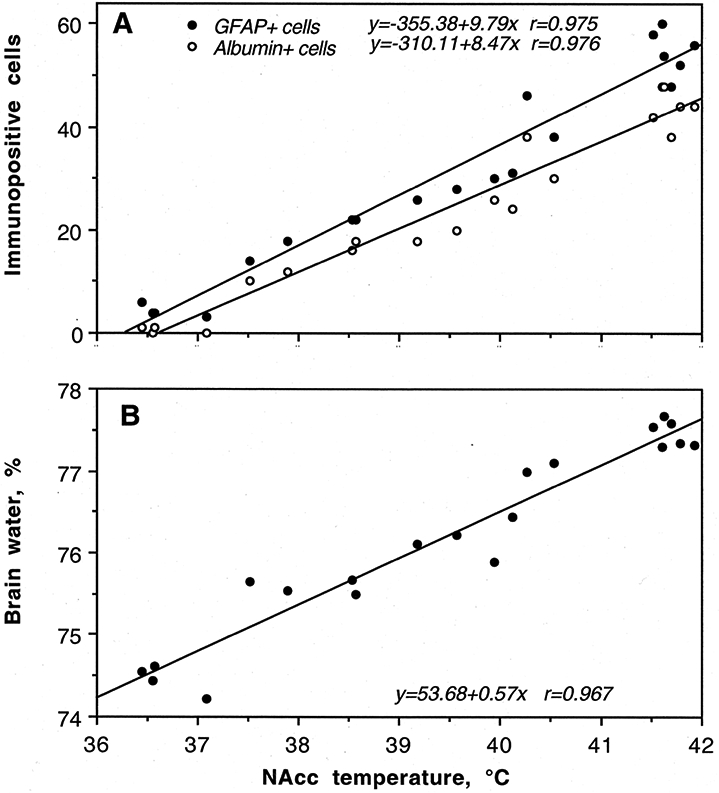
Correlative relationships between METH-induced changes in brain temperature and (A) albumin and GFAP immunoreactivity and (B) brain tissue water content. The changes in all three neural parameters were tightly correlated with brain temperature (r=coefficient of correlation). Original data were published in Kiyatkin et al., 2007.
Figure 4 shows leakage of serum albumin in the cerebral cortex in rats that were administered METH at 23°C (b) and 29° (c) ambient temperatures. In contrast to the control (a), in METH-treated rats albumin-positive cells were found within the neuropil (arrows) largely in neurons. In rats exposed to METH at 29°C, the intensity of staining was stronger than that in the METH-23°C group, with the frequent appearance of distorted neurons with perineuronal edema.
Figure 4.
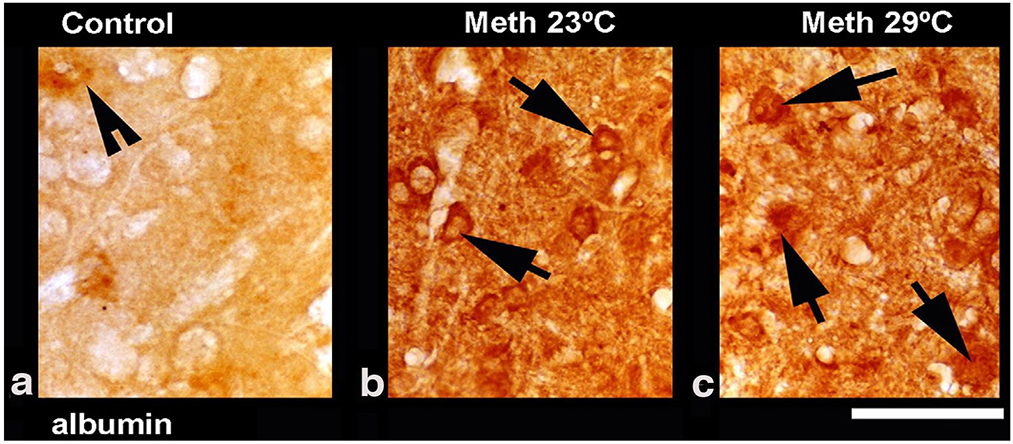
Leakage of serum albumin in the cerebral cortex in rats exposed to METH at 23° C (b) and 29°C (c) as compared to the saline-treated control (a). In METH-treated rats, albumin positive cells are seen within the neuropil (arrows) largely in the neurons (b and c). Distorted neurons with perineuronal edema were more frequent in rats that received METH at 29°C compared to 23°C. Data were modified after Sharma and Kiyatkin, 2010.
It appears that METH-induced leakage of the BBB and accumulation of brain tissue water are phenomena occurring in the brain as a whole. While METH increased albumin immunoreactivity and water tissue content in each of tested structures and the effect was significantly stronger when METH was administered at 29°C, the extent of changes differed among individual structures (Figure 5).
Figure 5.
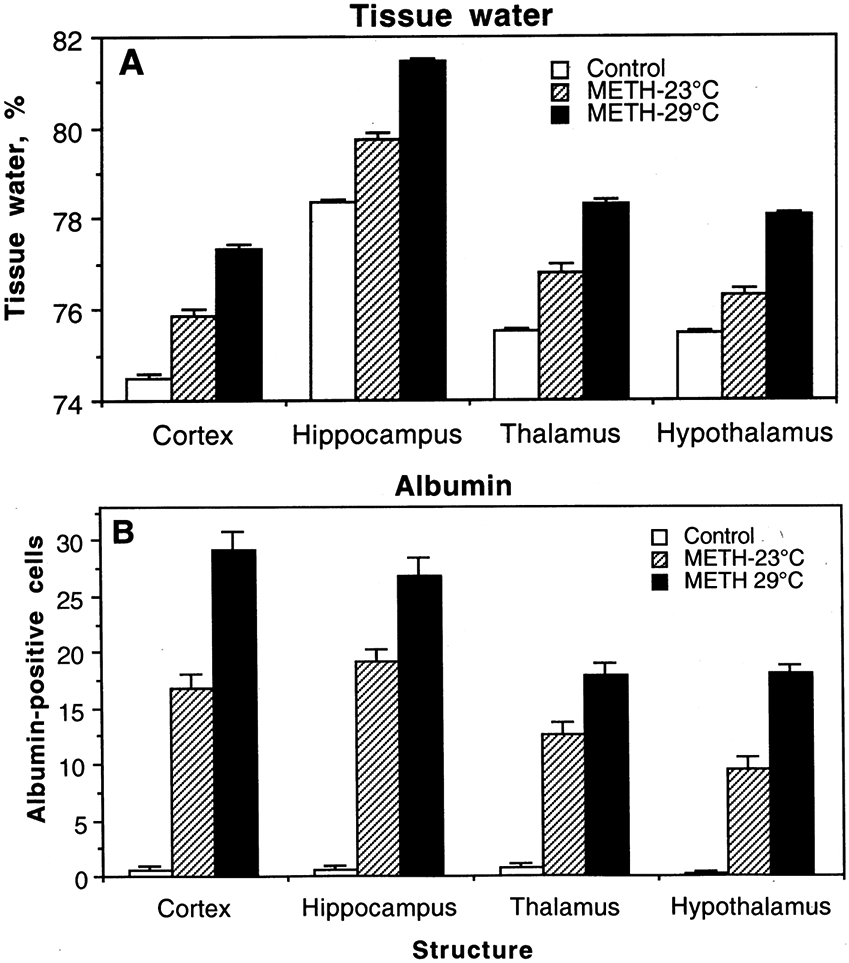
Changes in brain tissue water (A) and albumin immunoreactivity (B) in several brain structures induced by METH administered to freely-moving rats at 23°C and 29°C ambient temperatures.
Acute METH exposure also induced structural abnormalities of brain cells (Figure 5). The number of abnormal cells assessed in the cortex tightly correlated with brain and muscle temperatures (A), albumin leakage and glial activation (B), and tissue water accumulation (C). Morphological cell abnormalities were virtually absent in the saline-treated control, significantly increased in the METH-23°C group, and were further increased in the METH-29°C group. Correlations between all of these parameters were linear within the entire range of their fluctuations.
Figure 7 shows several examples of ultrastructural changes in the cortex (a, c, e) and thalamus (b, d, f) in rats exposed to METH at 23°C (c, d) and 29°C (e, f) as compared to saline-treated control (a, b). Degeneration of the neuronal nucleus and eccentric nucleolus (arrow) is clearly seen in METH treated rats; the magnitude and intensity of cellular and membrane degeneration is higher at 29°C compared to 23°C (a, c, e). In the thalamus, myelin vesiculation (d, f, arrows), expansion of the neuropil (*) and membrane disruption were quite common in the METH-treated group. The intensity of these changes were aggravated much more by METH treatment at 29°C (f) compared to the identical treatment at 23°C (d).
Figure 7.
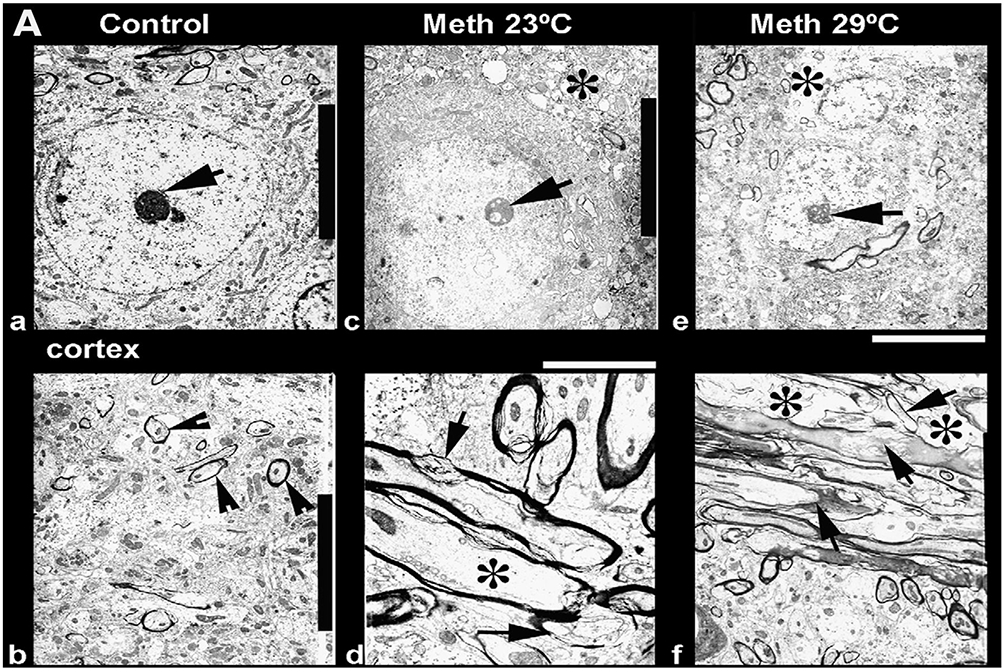
Ultrastructural changes in the cortex (a, c, e) and thalamus (b, d, f) after METH treatment at 23°C (c, d) and 29°C (e, f) as compared to the control (a, b). Degeneration of the neuronal nucleus and eccentric nucleolus (arrow) is clearly seen in METH-treated rats, and the magnitude and intensity of cellular and membrane degeneration was higher at 29°C as compared to 23°C (a, c, e). In the thalamus, myelin vesiculation (d, f, arrows), expansion of neuropil (*) and membrane disruption were quite common in the METH-treated group. The intensity of these changes was more aggravated by METH treatment at 29°C (f) as compared to identical treatment at 23°C (d). Data are modified from Sharma and Kiyatkin, 2010.
Therefore, these data suggest that acute METH intoxication results in robust breakdown of the BBB, development of edema, and structural abnormalities in brain cells. These effects are enhanced when the drug is administered in a moderately warm environment, and they tightly correlate with drug-induced brain temperature elevations. Finally, the neurotoxic effects of METH depend not only on direct drug action on brain cells and drug-induced metabolic activation, but they are also strongly modulated by the environmental conditions associated with the drug administration.
Conclusions and functional implications
In this work, we discussed the possible role of BBB leakage and subsequent changes in water homeostasis in acute METH toxicity and established the links between these changes and brain hyperthermia, possibly the most dangerous effect of this drug. While usually underappreciated, brain temperature is an important physiological parameter that reflects metabolic neural activity and affects all types of neural function. While the dose of an injected or ingested drug is usually viewed as the most important parameter for inducing pathological hyperthermia and overdose lethality, this review demonstrates that the temperature effects of drugs such as METH are strongly enhanced when they are used during physiological activation and in moderately warm environments. Social interaction, a procedure used in rats to model human psychophysiological activation, moderately enhanced intra-brain heat production and diminished heat dissipation due to peripheral vasoconstriction. The combination of these effects with the similar but more sustained effects induced by psychomotor stimulants results in a strong potentiation of drug-induced hyperthermic responses. A similar but stronger potentiation occurred when METH was administered in a warm environment where proper heat dissipation was important to maintain brain and body temperature homeostasis. Therefore, a certain drug used under adverse environmental conditions could induce unexpectedly strong effects and even death at doses that are usually viewed as “safe.” We also show that METH intoxication results in strong leakage of the BBB and accumulation of brain tissue water indicating vasogenic edema. These changes could be responsible for multiple abnormalities of different types of brain cells. All of the parameters indicating structural abnormalities of brain cells were strongly increased in rats that received METH at warm temperature vs. normal ambient temperature. A strong correlation was also found between changes in these parameters in individual rats of both groups. While robust temperature increases and the possible action of potentially toxic products of brain metabolism could definitely contribute to acute toxicity of METH, the strength of drug-induced changes in water content suggests that this change could be the leading factor determining development of coma and lethality. While rats differ from humans in their ability to regulate brain temperature and any animal experiment cannot fully mimic human conditions, we believe that our findings could add something new in terms of understanding the pathophysiological mechanisms underlying acute METH toxicity and lethality during METH overdose.
Figure 1.
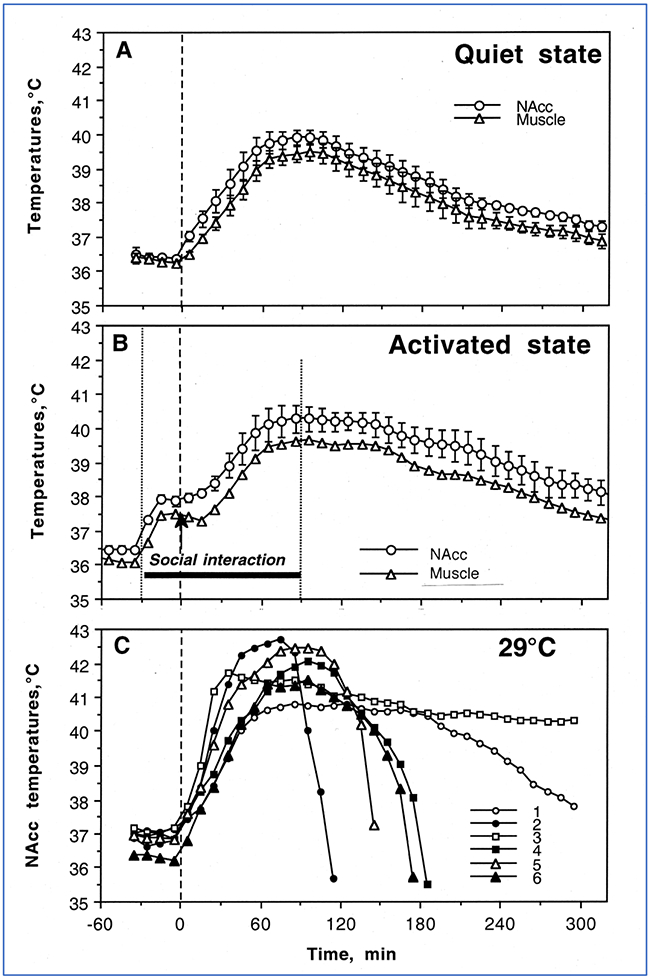
Mean (±SEM) changes in temperature recorded from the nucleus accumbens (NAc) and temporal muscle following sc administration of METH (9 mg/kg) in freely-moving rats in quiet resting conditions (A), during social interaction with another rat (B) and in warm ambient temperatures (C). Vertical hatched lines show the moment of drug injection. Additional vertical dotted lines in graph B show the time interval of social interaction. Original data were published in Brown et al., 2003.
Figure 6.
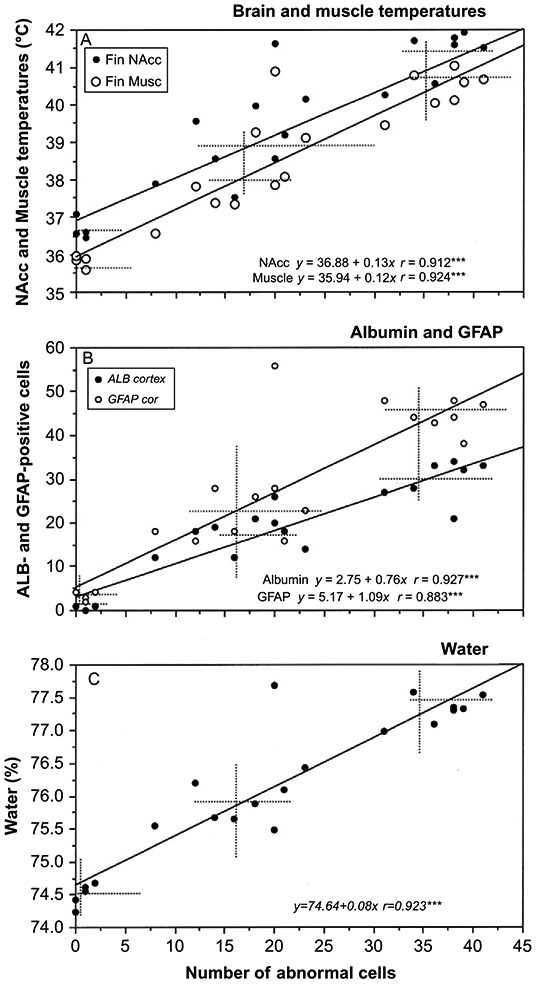
Correlative relationships between METH-induced structural abnormalities of brain cells (x-axis) and: (A) brain and muscle temperatures; (B) albumin and GFAP immunoreactivity; and (C) water content in brain tissue. The changes in all neural parameters tightly correlated with brain temperature (r=coefficient of correlation). Original data were published in Kiyatkin et al., 2007.
ACKNOWLEDGEMENTS
The study was supported by the Intramural Research Program of the NIH, NIDA.
References
- Abrams R, Hammel HT. Hypothalamic temperature in unanesthetized albino rats during feeding and sleeping. Am J Physiol 1964; 206:641–646. [DOI] [PubMed] [Google Scholar]
- Alberts DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J. Pharmacol. Exp. Ther 1995, 275:1104–1114. [PubMed] [Google Scholar]
- Baker MA, Frye FM, Millet VE. Origin of temperature changes evoked in the brain by sensory stimulation. Exp Neurol 1973; 38:502–519. [DOI] [PubMed] [Google Scholar]
- Banks ML, Sprague JE, Kisor DF, Czoty PW, Nichols DE, Nader MA. Ambient temperature effects on 3,4-methylenedioxymethamphetamine-induced theremodysregulation and pharmacokinetics in male monkeys. Drug Metab Dispos 2007; 35:1840–1845. [DOI] [PubMed] [Google Scholar]
- Brown PL, Wise RA, Kiyatkin EA. Brain hyperthermia is induced by methamphetamine and exacerbated by social interaction. J Neurosci 2003; 23:3924–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res 2007; 11:183–202. [DOI] [PubMed] [Google Scholar]
- Cervos-Navarro J; Sharma HS; Westman J; Bongcum-Rudloff E Glial cell reactions in the central nervous system following heat stress. Progr Brain Res 1998; 115:241–274. [DOI] [PubMed] [Google Scholar]
- Chen YZ, Xu RX. Huang QJ, Xu ZJ, Jiang XD, Cai YO. Effect of hyperthermia on tight junctions between endothelial cells of the blood-brain barrier model in vitro. Di Yi Jun Da Xue Xue Bao 2003; 23: 21–24. [PubMed] [Google Scholar]
- Davis WM, Hatoum HT, Walters IW. Toxicity of MDA (2.4-methylenedioxyamphetamine) considered for relevance to hazards of MDMA (Ecstasy) abuse. Alcohol Drug Res 1987; 7: 123–134. [PubMed] [Google Scholar]
- Delgado JMR, Hanai T. Intracerebral temperatures in free-moving cats. Am J Physiol 1966; 211:755–69. [DOI] [PubMed] [Google Scholar]
- De Vito MJ, Wagner GC. Methamphetamine-induced neuronal damage: a possible role for free radicals. Neuropharmacology 1989; 28:1145–1150. [DOI] [PubMed] [Google Scholar]
- Esposito P, Cheorghe D, Kendere K, Pang X, Connoly R, Jaconson S, Theodorides TC. Acute stress increases permeability of the blood-brain barrier through activation of brain must cells. Brain Res 2001; 888:117–127. [DOI] [PubMed] [Google Scholar]
- Fuller CA, Baker MA. Selective regulation of brain and body temperatures in the squirrel monkey. Am J Physiol 1983; 245:R293–7. [DOI] [PubMed] [Google Scholar]
- Gordon CJ. Thermal physiology of the laboratory rat. Physiol Behav 1990; 47:963–991. [DOI] [PubMed] [Google Scholar]
- Gordon CJ. Thermophysiological responses to hyperthermic drugs: extrapolating from rodents to human. Progr Brain Res 2007; 162:63–79. [DOI] [PubMed] [Google Scholar]
- Hayward JN, Baker MA. Role of cerebral blood flow in the regulation of brain temperature In the monkey. Am J Physiol 1968; 215:389–403. [DOI] [PubMed] [Google Scholar]
- Iwagami Y Changes in the ultrastructure of human cell related to certain biological responses under hyperthermic culture conditions. Human Cell 1996; 9:353–366. [PubMed] [Google Scholar]
- Kalant H The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. Can Med Ass J 2001; 165:917–28. [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA. Brain temperature: from physiology and pharmacology to neuropathology. Hand Clin Neurol 2019; 157:483–504. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL, Wise RA. 2002. Brain temperature fluctuation: a reflection of functional neural activation. Eur J Neurosci 2002; 16:164–68. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. Brain and body temperature homeostasis during sodium pentobarbital anesthesia with and without body warming in rats. Physiol Behav 2005; 84:563–570. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL, Sharma HS. Brain edema and breakdown of blood-brain barrier during methamphetamine intoxication: Critical role of brain temperature. Eur J Neurosci 2007; 26: 1242–1253. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Mitchum R. Fluctuations in brain temperatures during sexual behavior in male rats: An approach for evaluating neural activity underlying motivated behavior. Neuroscience 2003; 119:1169–1183. [DOI] [PubMed] [Google Scholar]
- Kovalson VM. Brain temperature variations during natural sleep and arousal in white rats. Physiol Behav 1972; 10:667–70. [DOI] [PubMed] [Google Scholar]
- Lepock JR. Cellular effects of hyperthermia: relevance to the minimum dose for thermal damage. Int J Hyperthermia 2003; 19:252–66. [DOI] [PubMed] [Google Scholar]
- Lynch J, House MA. Cardiovascular effects of methamphetamine. J Cardiovsc Nurs 1992; 6:12–18. [PubMed] [Google Scholar]
- McElligott JC, Melzack R. Localized thermal changes evoked in the brain by visual and auditory stimulation. Exp Neurol 1967; 17:293–312. [DOI] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. Elevated environmental temperature and methamphetamine neurotoxicity. Environ Res 2003; 92:48–53. [DOI] [PubMed] [Google Scholar]
- Nybo L, Secher MH, Nielson B. Inadequate heat release from the human brain during prolonged exercise with hyperthermia. J Physiol 2002; 545:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan JP. Quantitative features of reactive gliolis following toxicant-induced damage of the CNS. Ann NY Acad Sci 1993; 679:195–210. [DOI] [PubMed] [Google Scholar]
- Oifa AI, Kleshchnov VN. Ultrastructural analysis of the phenomenon of acute neuronal swelling. Zh Nevropato. Psikhiatr Im SS Korsakova 1985; 85:1016–1020. [PubMed] [Google Scholar]
- Ovadia H, Abramsky O, Feldman S, Weidenfeld J. Evaluation of the effects of stress on the blood-brain barrier: critical role of the brain perfusion time. Brain Res 2001; 905:21–25. [DOI] [PubMed] [Google Scholar]
- Prakash MD, Tanalakis K, Antonipillal J, Stojanovska L, Nurdall K, Apostolopoulos V. Methamphetamine: Effects on the brain, gut, and immune system. Pharmacol Res 2017; 120: 60–67. [DOI] [PubMed] [Google Scholar]
- Rapoport SI. Blood-brain barrier in Physiology and medicine, 1976. Raven Press, New York. [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res 1980; 193:153–163 [DOI] [PubMed] [Google Scholar]
- Riddle EL, Fleckenstein AE, Hanson GR. Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. The AAPS Journal 2006, 8(2) Article 48 (http://www.aapsj.org). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovsky AA, Ivanov AI, Shimansky YP. Ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol 2002; 92:2667–79. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Cardiovascular aspects of human thermoregulation. Circ Res 1983; 52:367–79. [DOI] [PubMed] [Google Scholar]
- Rusyniak DE, Sprague JE. Toxin-induced hyperthermic syndromes. Med Clin North Am 2005; 89: 1277–1296. [DOI] [PubMed] [Google Scholar]
- Sandoval V, Hanson GR, Fleckenstein AE. Methamphetamine decreases mouse striatal dopamine transport activity: roles of hyperthermia and dopamine. Eur J Pharmacol 2000; 409:265–271. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K Animal Physiology Adaptation and Environment. 5th Edition. Cambridge: Cambridge University Press, 1997. [Google Scholar]
- Seiden LS, Sabol KE. Methamphetamine and methylenedioxymethamphetamine neurotoxicity: possible mechanisms of cell destruction. NIDA Res Monogr 1996; 163:251–276. [PubMed] [Google Scholar]
- Serota HM, Gerard RM. Localized thermal changes in cat’s brain. J Neurophysiol 1938; 1:115–24. [Google Scholar]
- Siesjo B Brain Energy Metabolism. New York: Wiley, 1978. [Google Scholar]
- Sharma HS. Hyperthermia-induced brain edema: Current status and future perspectives. Indian J Med Res 2006; 123:629–652. [PubMed] [Google Scholar]
- Sharma HS. Ali SF Alterations in blood-brain barrier function by morphine and amphetamine. Ann NY Acad. Sci 2006, 1074:198–224. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Dey PK. Influence of long-term immobilization stress on regional blood-brain permeability, cerebral blood flow and 5-HT levels in conscious normotensive young rats. J Neurol Sci 1986; 72:61–76. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Hoopes PJ. Hyperthermia-induced pathophysiology of the central nervous system. Int J Hyperthermia 2003; 19:325–54. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Kiyatkin EA. Rapid morphological brain abnormalities during acute methamphetamine intoxication in the rat: An experimental study using light and electron microscopy. J Chem Neuroanat 2009; 37:18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma HS, Zimmer C, Westman J, Cervos-Navarro J. Acute systemic heat stress increases glial fibrillary acidic protein immunoreactivity in brain. An experimental study in the conscious normotensive young rats. Neuroscience 1992; 48:889–901. [DOI] [PubMed] [Google Scholar]
- Smirnov MS, Kiyatkin EA. Fluctuations in central and peripheral temperatures associated with feeding behavior in rats. Am J Physiol 2008; 295:R1414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BK. Methamphetamine-induced neurotoxicity: role for glutamate and dopamine influx. Synapse 1994; 17:203–209. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Lay CC, Von Huben SN, Davis SA, Crean RD, Katner SN. Hyperthermia induced by 3,4-methylenedioxymethamphetamine in unrestrained rhesus monkeys, Drug Alcohol Depend 2006; 20:276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Huben RD, Lay CC, Crean RD, Davis SA, Katner SN, Taffe MA. Impact of ambient temperature on hyperthermia induced by (+/−)3,4- methylenediomethamphetamine in rhesus macaques. Neuropsychopharmacology 2007; 32:673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P, Shirreffs SM; Maughan RJ. Blood-brain barrier integrity may be threatened by exercise in a warm environment. Am J Physiol 2005; 288:R1689–R1694. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Ricaurte GA, Forno L, Seiden LS. Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain Res 1989; 486:73–78. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Zhu W. The effect of methamphetamine on the production of free radicals and oxidative stress. J Pharmacol Exp Ther 1998; 287:107–114. [PubMed] [Google Scholar]
- Yamamoto H The central effects of xylopinine in mice. Jap J Pharmacol 1963; 13:230–239. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. (2008). The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008; 57:178–201. [DOI] [PubMed] [Google Scholar]


