Comparative transcriptome analyses reveal a major contribution of regulatory divergence in conserved genes during the response of Pentapetalae plants to the fungal pathogen Sclerotinia sclerotiorum.
Abstract
Quantitative disease resistance (QDR) is a conserved form of plant immunity that limits infections caused by a broad range of pathogens. QDR has a complex genetic determinism. The extent to which molecular components of the QDR response vary across plant species remains elusive. The fungal pathogen Sclerotinia sclerotiorum, causal agent of white mold diseases on hundreds of plant species, triggers QDR in host populations. To document the diversity of local responses to S. sclerotiorum at the molecular level, we analyzed the complete transcriptomes of six species spanning the Pentapetalae (Phaseolus vulgaris, Ricinus communis, Arabidopsis [Arabidopsis thaliana], Helianthus annuus, Solanum lycopersicum, and Beta vulgaris) inoculated with the same strain of S. sclerotiorum. About one-third of plant transcriptomes responded locally to S. sclerotiorum, including a high proportion of broadly conserved genes showing frequent regulatory divergence at the interspecific level. Evolutionary inferences suggested a trend toward the acquisition of gene induction relatively recently in several lineages. Focusing on a group of ABCG transporters, we propose that exaptation by regulatory divergence contributed to the evolution of QDR. This evolutionary scenario has implications for understanding the QDR spectrum and durability. Our work provides resources for functional studies of gene regulation and QDR molecular mechanisms across the Pentapetalae.
INTRODUCTION
The plant immune system includes multiple molecular mechanisms for pathogen detection and defense. Gene-for-gene resistance involves single dominant resistance genes often belonging to the nucleotide binding domain and leucine-rich repeat (NLR) family of proteins (Dodds and Rathjen, 2010). This form of resistance typically protects plants against a few genotypes of biotrophic pathogens, which keep host cells alive during infection. There are however no dominant resistance genes described to function against many necrotrophic pathogens (which actively kill host cells during infection), such as the fungus Sclerotinia sclerotiorum (Guyon et al., 2014; Mbengue et al., 2016). S. sclerotiorum is the causal agent of white and stem mold diseases on a broad range of dicot plants (Bolton et al., 2006). The host range of S. sclerotiorum covers all major groups of the Pentapetalae, a subclade of the core eudicots including Rosidae, Asteridae, and Caryophyllaceae (Moore et al., 2010; Navaud et al., 2018). This fungus notably causes severe damage on oil crops such as soybean (Glycine max) and rapeseed (Brassica napus) when conditions are favorable (Derbyshire and Denton-Giles, 2016). Plants typically exhibit quantitative disease resistance (QDR) against S. sclerotiorum, a form of immunity resulting in a full continuum of disease symptom severity in plant populations (Roux et al., 2014). The distribution of disease symptoms on plants challenged by S. sclerotiorum follows an approximately Gaussian distribution in populations of sunflower (Helianthus annuus, Asterales; Fusari et al., 2012), soybean (Fabales; Bastien et al., 2014), rapeseed (Brassicales; Wu et al., 2013), and thale cress, Arabidopsis (Arabidopsis thaliana, Brassicales; Perchepied et al., 2010), for instance. QDR is a complex type of resistance involving genes of very diverse families (Corwin and Kliebenstein, 2017). Because of the functional diversity and complexity of QDR, the genetic bases and evolution of QDR remain largely unexplored.
How many genes contribute to the QDR response to a given pathogen is an unresolved question of fundamental and practical interest (Corwin and Kliebenstein, 2017). This information is indeed critical to understand the evolutionary dynamics of plant pathogens with a broad host range and to optimize breeding for disease resistance in crops. Genome-wide association (GWA) mapping in Arabidopsis natural populations and the analysis of fungal small RNA putative targets identified five QDR genes against S. sclerotiorum (Badet et al., 2017, 2019; Derbyshire et al., 2019; Barbacci et al., 2020). The inactivation of these genes caused 16 to 36% variation in the QDR phenotype each, in line with previous quantitative trait loci analyses for QDR in crops (Micic et al., 2004; Poland et al., 2011; Bonhomme et al., 2014). Together with other approaches (Perchepied et al., 2010; Stotz et al., 2011; Mbengue et al., 2016), these studies bring to a few dozen the number of known Arabidopsis genes contributing to QDR against S. sclerotiorum. Global transcriptomic analyses revealed that the number of genes differentially expressed upon S. sclerotiorum challenge adds up to 4703 in Arabidopsis and 3513 in tomato (Solanum lycopersicum, Solanales; Badet et al., 2017), suggesting that the number of QDR genes may exceed by far the current validated list. In agreement, GWA mapping for resistance against four distinct Botrytis cinerea strains in 110 Arabidopsis accessions identified 3504 genes as candidates for controlling QDR (Corwin et al., 2016). Extending the analysis to several parameters describing the disease lesion (size, shape, color) increased the number of candidate QDR genes to 7940 in the Arabidopsis–B. cinerea interaction (Fordyce et al., 2018).
These findings provide molecular foundations for an infinitesimal model (or polygenic model) of plant QDR. The infinitesimal model of quantitative genetics postulates that continuous variation in a trait is often controlled by environmental factors and a large number of loci, each making a very small (infinitesimal) contribution to the phenotype (Fisher, 1919; Nelson et al., 2013). The existence of very large numbers of causal genetic variants is proposed to explain the small proportion of phenotypic variance resulting from variants identified in GWA studies, a problem known as missing heritability (Manolio et al., 2009; Barton et al., 2017). Because the infinitesimal model notably assumes linkage equilibrium and additive phenotypic effect of loci, the genetic architecture of disease resistance probably involves an additional layer of complexity (Barton et al., 2017; Turelli, 2017). In a recent variant of the infinitesimal model coined the omnigenic model, Boyle et al. (2017) suggest that any gene differentially regulated in infected tissues has a non-null impact on the disease outcome. Following this view, a modest number of core genes would directly and strongly affect QDR and could each be modulated by numerous small effect genes as a result of their interactions in global gene networks (Boyle et al., 2017; Liu et al., 2019). Although the most efficient way to move forward in understanding the genetics of complex traits is debatable, the omnigenic model emphasizes the relevance of large-scale transcriptomics for studying diseases (Boyle et al., 2017; Wray et al., 2018).
A global knowledge of gene regulation is also key to study complex traits from an evolutionary point of view according to the cis-regulatory hypothesis. This theory predicts that because cis-regulatory mutations are likely to have reduced pleiotropic effects, they should lead to phenotypic evolution more frequently than mutations in coding regions (Stern and Orgogozo, 2008, 2009). A literature survey covering multicellular plants and animals provided support for the cis-regulatory hypothesis over long time scales (beyond the species level; Stern and Orgogozo, 2008). In the harlequin ladybird beetle (Harmonia axyridis), allelic variation in the cis-regulatory region of the transcription factor gene pannier is responsible for more than 200 distinct patterns of red and black on the elytra (the hardened forewing), providing a striking example of phenotypic variation caused by cis-regulatory variants at the intraspecific level (Ando et al., 2018; Gautier et al., 2018). In our previous work, we showed that the ARPC4 gene encoding an actin-related protein complex member controls the dynamics of the actin filament network with an impact on QDR against S. sclerotiorum (Badet et al., 2019). Two major alleles of ARPC4 were detected in Arabidopsis populations, associated with contrasted levels of QDR. Remarkably, the two ARPC4 alleles encoded identical proteins, but they differed markedly in their induction upon S. sclerotiorum inoculation. The Arp2/3 complex involving ARPC4 is conserved in all eukaryotes (Deeks and Hussey, 2005), indicating that transcriptional regulation of conserved pleiotropic genes can underlie variation in plant QDR phenotypes. Similarly, the POQR gene showed conserved expression pattern and function in QDR upon S. sclerotiorum challenge in Arabidopsis and tomato (Badet et al., 2017). These results suggest that some QDR determinants are conserved between distantly related plants and that gene expression polymorphisms could be drivers of QDR evolution.
S. sclerotiorum has one of the broadest host ranges among plant pathogens (Derbyshire et al., 2017; Navaud et al., 2018), providing a rare opportunity to analyze the diversity and long-term evolution of transcriptional programs in the interaction of various plants with a single fungal pathogen species. To document how diverse the local plant responses to S. sclerotiorum are at the molecular level, we analyzed the complete transcriptomes of six plant species spanning the Pentapetalae group inoculated with the same strain of S. sclerotiorum. Differentially expressed genes (DEGs) were enriched with core ortholog genes (conserved in all six species) but depleted from lineage-specific genes. Among core orthologs, patterns of gene expression varied significantly across species and deviated significantly from a null model of expression heritability. Only 4.34% of core orthogoups contained upregulated genes from all six species, including a group of ATP Binding Cassette type G (ABCG) transporters. Functional analysis of AtABCG40 supports a scenario of exaptation in which ancestral stress response genes gained a function in local disease resistance to S. sclerotiorum recently in plant evolution.
RESULTS
Common Features of S. sclerotiorum–Responsive Gene Pools across Distant Species
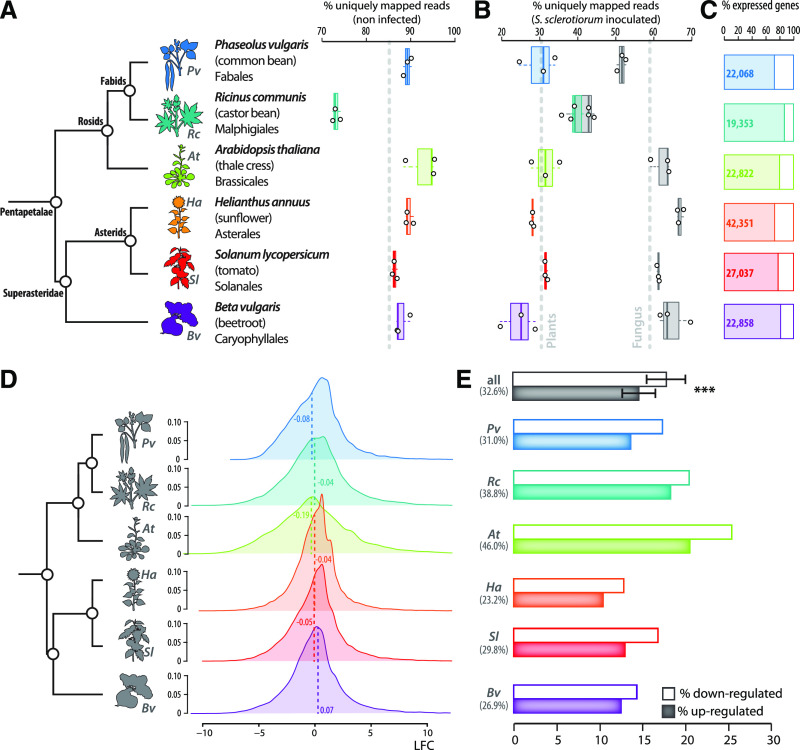
To document the transcriptional changes induced locally by S. sclerotiorum infection in taxonomically diverse eudicot plant species, we performed RNA sequencing (RNA-seq) of healthy and inoculated leaves from six plant species, chosen to cover a broad diversity in the Pentapetalae group of angiosperms (Chanderbali et al., 2017; Zeng et al., 2017). Common bean (Phaseolus vulgaris, Fabales) and castor bean (Ricinus communis, Malpighiales) were chosen as representatives of the fabid group of the rosids clade and Arabidopsis (Brassicales) as a representative of the malvid group of the rosids clade. Sunflower (Asterales) and tomato (Solanales) were chosen as representatives of the asterids clade and sugar beet/beetroot (Beta vulgaris, Caryophyllales) as a representative of basal Superasteridae lineages (Figure 1A; Supplemental Data Set 1.1; Zeng et al., 2017). A reference genome is available for all of these species, to which we aligned RNA-seq reads to quantify plant gene expression. In noninoculated plants, a minimum of 90% of the total sequence reads were mapped to the corresponding plant reference genome, except for R. communis in which only 74.26% of sequence reads mapped to annotated transcripts.
Figure 1.
Overview of Genes Responsive Locally to S. sclerotiorum Inoculation in Six Plant Species of the Pentapetalae Clade.
(A) Proportion of RNA-seq reads from noninfected samples mapped to a unique position in plant genomes (% of all reads). Results from samples collected in three independent biological replicates are shown; gray dotted line shows average for all samples.
(B) Proportion of RNA-seq reads from infected samples mapped to a unique position in plant genomes (colors) and S. sclerotiorum genome (gray, % of all reads). Results from samples collected in three independent biological replicates are shown; gray dotted lines show the average for all samples.
(C) Proportion of plant genes expressed in all samples, both in healthy and infected plants. Labels indicate number of expressed genes (% of complete annotated transcriptomes).
(D) Distribution of gene expression log2 fold change (LFC) in infected compared to healthy plants (% of transcripts). Dotted lines and labels indicate median LFC.
(E) Proportion of DEGs in each species (colors) and overall (gray, % of transcripts). Plain bars correspond to the percentage of of upregulated genes, empty bars to the percentage of downregulated genes, and labels indicate the total number of DEGs per species. Error bars show sd. Boxplots show first and third quartiles (box), median (thick line), and the most dispersed values within 1.5 times the interquartile range (whiskers).
With an aim to study comparable stages of infection on all six plant species, we harvested the edge of disease lesions before they reached 25 mm in diameter (Supplemental Methods; Badet et al., 2017; Peyraud et al., 2019). As expected, due to the presence of sequence reads corresponding to S. sclerotiorum RNAs, an average 32.5% of sequence reads mapped to their corresponding plant genome in infected samples (Figure 1B; Supplemental Data Set 1.2). To estimate the degree of fungal colonization in these samples, we mapped RNA-seq reads to the S. sclerotiorum reference genome (Derbyshire et al., 2017). Our results suggest that S. sclerotiorum colonization was consistent across replicates and across species, with an average 58.25% ± 4.83% of reads aligned to the fungal genome (Figure 1B).
The number of genes expressed both in noninoculated and infected leaves ranged from 19,353 (R. communis) to 42,351 (H. annuus), reaching a total 156,497 genes across the six plant species (Figure 1C; Table 1; Supplemental Data Set 1.3). The proportion of expressed genes was remarkably consistent across all six plant species, corresponding to 78.48% ± 2.47% of complete transcriptomes (Figure 1C).
Table 1. General Transcriptome Statistics for the Six Species Analyzed in This Work.
| Statistics | Rosids | Superasterids | |||||
|---|---|---|---|---|---|---|---|
| P. vulgaris | R. communis | A. thaliana | H. annuus | S. lycopersicum | B. vulgaris | All | |
| Expressed genes | 22,068 | 19,353 | 22,822 | 42,351 | 27,045 | 22,858 | 156,497 |
| DEG total (%) | 6,843 (31.01) | 7,510 (38.81) | 10,496 (45.99) | 9,844 (23.24) | 8,069 (29.84) | 6,148 (26.90) | 48,910 |
| Upregulated genes | 3,012 | 3,545 | 4,692 | 4,402 | 3,513 | 2,867 | 22,031 |
| Downregulated genes | 3,831 | 3,965 | 5,804 | 5,442 | 4,556 | 3,281 | 26,879 |
| Up/down ratio | 0.79 | 0.89 | 0.81 | 0.81 | 0.77 | 0.87 | 0.82 |
| Mean reads per DEG (healthy) | 2,834.65 | 3,210.32 | 3,314.90 | 2,485.06 | 3,445.84 | 3,771.52 | 3,183.08 |
| Mean reads per DEG (inoculated) | 1,535.95 | 2,372.43 | 1,640.26 | 924.75 | 1,675.03 | 1,438.26 | 1,584.93 |
| Core orthologs total; [DEGs] | 10,307; [3,611] | 8,919; [3,604] | 10,551; [5,358] | 13,906; [3,825] | 10,206; [3,444] | 8,949; [2,879] | 62,838; [22,721] |
| Other orthologs total; [DEGs] | 5,693; [1,795] | 5,837; [2,327] | 5,224; [2,427] | 8,057; [2,168] | 5,668; [1,910] | 4,446; [1,357] | 34,925; [11,984] |
| Lineage-specific total; [DEGs] | 6,068; [1,437] | 4,597; [1,579] | 7,047; [2,711] | 20,388; [3,851] | 11,171; [2,715] | 9,463; [1,912] | 58,734; [14,205] |
| % of expressed genes with GO; Pfam | 59.36; 85.75 | 69.21; 89.45 | 74.13; 89.66 | 47.08; 76.12 | 48.47; 83.86 | 59.69; 80.49 | 73.96; 83.08 |
| No. with ≥3 occurrences GO; Pfam | 3,791; 4,284 | 2,410; 4,128 | 3,792; 4,862 | 3,059; 5,913 | 2,600; 4,969 | 2,381; 4,353 | 6,171; 13,100 |
| % of core genes with GO; Pfam | 88.36; 90.60 | 87.79; 96.56 | 97.71; 97.05 | 86.51; 96.89 | 80.73; 96.46 | 86.90; 96.56 | 87.99; 95.72 |
| No. of GOs enriched in up; downregulated genes | 156; 344 | 31; 28 | 54; 67 | 57; 56 | 55; 45 | 25; 42 | 262; 411 |
To determine the number of plant genes misregulated upon S. sclerotiorum infection in each genome, we calculated log2 fold change (LFC) of expression in healthy and inoculated plant samples. We used LFCs shrinkage by the empirical Bayes approach implemented in DESeq2 to avoid bias in genes with low read coverage (Supplemental Methods; Love et al., 2014). Genomic median for LFC ranged from –0.19 in Arabidopsis to 0.07 in B. vulgaris (Figure 1D; Supplemental Data Sets 1.4 to 1.9). The distribution of LFC values was very similar between genomes, with a stronger dispersion from the median in rosid species. We considered as DEGs showing a log2 fold change higher than 1.5 and a P value lower than 0.01. We detected 6148 DEGs in B. vulgaris, 6843 in P. vulgaris, 7510 in R. communis, 8069 in S. lycopersicum, 9844 in H. annuus, and 10,496 in Arabidopsis (Table 1; Supplemental Data Set 1.3). Although there was a 2.7-fold variation in total number of genes per genome (from 22,336 in R. communis to 58,138 in H. annuus), the number of DEGs varied by 1.7-fold only. DEGs represented an average 32.63% ± 4.17% of expressed genes (Figure 1E). The lowest proportion of DEGs corresponded to species from the Superasteridae clade (average 26.7% ± 1.65%), whereas species from the rosids clade showed 38.6% ± 3.75% of DEGs on average. In all species, the proportion of downregulated genes exceeded that of upregulated genes (Student’s t test, P value = 0.0013), with an upregulated/downregulated ratio of 0.82 ± 0.03 (Figure 1E; Table 1). On average, expressed genes included 17.9% ± 2.27% downregulated DEGs, 14.7% ± 1.93% upregulated DEGs, and 67.4% ± 4.17% genes not differentially expressed. These results highlight a number of common global features of S. sclerotiorum–responsive plant transcriptomes across distant plant lineages.
S. sclerotiorum–Responsive Genes Are Enriched with Core Orthologs
To compare local transcriptional responses to S. sclerotiorum in the six selected plant species, we performed sequence-based clustering of all predicted proteins from the six species using OrthoMCL (Li et al., 2003). This identified 14,983 orthogroups shared between two or more species and containing from 2 to 110 expressed genes, for a total of 97,763 genes (Figure 2A; Table 1; Supplemental Data Set 1.10). Of these genes, 62,838 genes (40.2%) classified into 7918 orthogroups we designated as core Pentapetalae that contained genes from all six plant species and could represent ancestral gene groups. There were 58,726 expressed genes (37.5%) unique to one species and not assigned to orthogroups, classified as lineage specific (see Methods). The 7065 other ortholog groups, shared between two to five species, included a total of 34,925 genes (22.3%; Figure 2A; Supplemental Data Set 1.11). The proportion of core Pentapetalae genes represented 41.5% ± 2.9% per species; other ortholog genes represented 23.1% ± 2.1% per species (Figure 2B; Table 1; Supplemental Data Set 1.12). The proportion of lineage-specific genes was more variable, ranging from 23.8% in R. communis to 48.1% in H. annuus (average 35.6% ± 4.8%).
Figure 2.
Distribution of Genes Differentially Expressed upon S. sclerotiorum Inoculation (DEGs) According to Gene Conservation.
(A) Distribution of orthologous gene groups across species. Groups including genes from all six species are designated as core Pentapetalae, groups including genes from two to five species are designated as other orthologs, and genes not included in multi-species ortholog groups are designated as lineage specific. The number of gene group is indicated in bold, number of genes is in italics, and the proportion (% of all expressed genes) is indicated between square brackets.
(B) Proportion of core Pentapetalae (Penta.) genes, lineage-specific (spe.) genes, and other ortholog (ortho.) genes in each plant genome (% of expressed genes).
(C) Relative proportion of DEGs belonging to core Pentapetalae groups (dark blue), other ortholog groups (green), and lineage-specific genes (yellow). Branches of the phylogenetic tree in inset are color coded according to corresponding gene groups. Results are shown for downregulated genes only (Down), upregulated genes only (Up), or both together (All). Filled dots show proportions for Superasteridae species; open dots are for rosid species. Letters define groups of significance as determined by pairwise Student t tests (P value < 0.01).
(D) Relative proportion of DEGs, either downregulated (open circles) or upregulated (filled circles), in rosids and Superasteridae species. Genes belonging to core Pentapetalae groups (dark blue); other ortholog groups (green) and lineage-specific genes (yellow) are shown separately. Letters define groups of significance as determined by pairwise Student t tests (P value < 0.01). Boxplots show first and third quartiles (box), median (thick line), and the most dispersed values within 1.5 times the interquartile range (whiskers). At, Arabidopsis; Bv, B. vulgaris; Ha, H. annuus; Pv, P. vulgaris; Rc, R. communis; Sl, S. lycopersicum.
To test for the relative contribution of conserved genes in the plant transcriptional responses to S. sclerotiorum, we calculated for each species the proportion of DEGs that were core Pentapetalae, other ortholog, and lineage-specific genes (Figure 2C). On average, 46.7% ± 2.6% of DEGs were core Pentapetalae genes, 24.7% ± 1.7% were other ortholog genes, and 28.6% ± 3.6% were lineage-specific genes. The percentage core Pentapetalae in DEGs was significantly higher than that of other ortholog genes (Student’s t test, P value = 4.2e−08) and that of lineage-specific genes (P value = 2.3e−06). The proportion of upregulated and downregulated genes was similar among the other ortholog and lineage-specific classes. It showed a slight difference among core Pentapetalae genes in which 43.15% ± 1.7% were upregulated and 49.63% ± 3.6% were downregulated (P value = 0.058). The percentage of DEGs being lineage specific showed the strongest variation between species, especially regarding downregulated genes that included 17.6% of lineage-specific genes in P. vulgaris and up to 42.1% in H. annuus.
To determine whether the prevalence of core Pentapetalae genes in DEGs was conserved at different phylogenetic levels, we analyzed separately the distribution of DEGs in the rosid and Superasteridae clades (Figure 2D; Supplemental Data Set 1.13). In both clades, DEGs were significantly more abundant in core Pentapetalae genes than in any other class (P value < 5.5e−10 for rosids, P value < 0.005 for Superasteridae). In the rosids, other orthologs and lineage-specific genes contributed to a similarly low proportion of DEGs (on average 26.9 and 22.9%, respectively; P value = 0.14). By contrast, in the Superasteridae, DEGs were significantly less abundant among other orthologs than among lineage-specific genes (on average 22.7 and 34.7%, respectively; P value = 7.0e−05). As a complementary analysis, we used chi-squared tests to show that core Pentapetalae genes were significantly enriched with DEGs in all six species, between 1.041-fold (P value = 1.74e−04) in R. communis and 1.196-fold (P value = 3.11e−46) in B. vulgaris (Table 2; Supplemental Data Set 1.13). Other ortholog genes were significantly enriched with DEGs in superasterids species only, between 1.129-fold (P value = 6.91e−12) in S. lycopersicum and 1.157-fold (P value = 3.97e−17) in H. annuus. Conversely, lineage-specific genes were depleted with DEGs in all six species, between 0.751-fold (P value = 5.46e−81) in B. vulgaris and 0.885 (P value = 9.79e−12) in R. communis.
Table 2. Distribution of DEGs among core Orthologs, Other Orthologs, and Lineage-Specific Genes.
| Species | % of DEGs in core Genes | % of DEGs in Other Ortho Genes | % of DEGs in Lineage-Specific Genes | DEG Enrichment Fold in core Genes | Chi-Squared P Value | DEG Enrichment Fold in Other Ortho Genes | Chi-Squared P Value | DEG Enrichment Fold in Lineage-Specific Genes | Chi-Squared P Value |
|---|---|---|---|---|---|---|---|---|---|
| P. vulgaris | 52.77 | 26.23 | 21.00 | 1.13 | 8.40e–33 | 1.02 | 2.32e+00 | 0.76 | 1.20e–46 |
| R. communis | 47.99 | 30.99 | 21.03 | 1.04 | 1.74e–04 | 1.03 | 3.38e–01 | 0.89 | 9.79e–12 |
| Arabidopsis | 51.05 | 23.12 | 25.83 | 1.10 | 2.06e–40 | 1.01 | 3.14e+00 | 0.84 | 1.78e–51 |
| H. annuus | 38.86 | 22.02 | 39.12 | 1.18 | 7.60e–47 | 1.16 | 3.97e–17 | 0.81 | 5.95e–92 |
| S. lycopersicum | 42.68 | 23.67 | 33.65 | 1.13 | 6.07e–27 | 1.13 | 6.91e–12 | 0.81 | 1.64e–61 |
| B. vulgaris | 46.83 | 22.07 | 31.10 | 1.20 | 3.11e–46 | 1.13 | 9.81e–09 | 0.75 | 5.46e–81 |
| All | 1.16 | 9.53e–257 | 1.10 | 1.20e–43 | 0.77 | 0.00e+00 |
Significance of enrichment (fold >1) or depletion (fold <1) was assessed using a chi-squared test followed by a Bonferroni correction for multiple testing.
We conclude that the plant transcriptome is enriched with core Pentapetalae genes during local responses to S. sclerotiorum. Because of a relatively high proportion of lineage-specific genes differentially regulated in species from the Superasteridae clade, this trend is less pronounced in the Superasteridae than in rosids. Comparing the transcriptome of resistant and susceptible genotypes at the species level, together with further functional assays, will be required to determine which of these responsive genes contribute to the QDR phenotype.
Accumulation of S. sclerotiorum–Induced Genes during the Evolution of the Pentapetalae
To document global patterns of evolution in the repertoire of S. sclerotiorum–responsive genes across plants, we inferred gene gains and losses along the Pentapetalae phylogeny. Following a simple parsimony hypothesis, we considered a gain along a branch of the tree when genes were present in all derived clades (among ortholog groups or lineage specific), but not in three or more paraphyletic clades (Figure 3A; Supplemental Data Sets 1.14 and 1.15). Reciprocally, we considered a loss along a branch when genes were present in all paraphyletic clades but absent from all derived clades. This identified a total of 5131 loss events associated with 10,143 DEGs and 10,630 gain events associated with 16,046 DEGs.
Figure 3.
Relative Increase in Upregulated Genes Inferred from the Evolutionary History of S. sclerotiorum–Responsive Transcriptomes.
(A) Principles of a parsimony approach to infer gene gains and losses in the evolutionary history of the Pentapetalae. In these hypothetical examples, a gene of interest is present in lineages with a green tick and absent in lineages with a red cross. We inferred presence along branches shown in green and absence along branches shown as dotted gray lines.
(B) Inferred transcriptome evolution along the phylogeny of the Pentapetalae. The size of gray circles indicates the relative total number of genes gained (solid circle, left) and lost (dotted circles, right) along each branch. The size of colored circles indicates the relative DEGs gained (solid circle, left) and lost (dotted circles, right) along each branch. The color scale represents the ratio of up/downregulated genes for gained and lost DEGs.
(C) Estimated relative number of genes gained and lost per Mya along intermediate and terminal branches of the Pentapetalae phylogeny (% of Pentapetalae Core set). Estimates are given for whole genomes and DEG pools. Significance of the difference between gains and losses was assess by a paired t test.
(D) Ratio between the number of genes upregulated and downregulated among DEGs gained and lost along intermediate and terminal branches of the Pentapetalae phylogeny. Significance of the difference between gains and losses was assess by a paired t test. Boxplots show first and third quartiles (box), median (thick line), and the most dispersed values within 1.5 times the interquartile range (whiskers).
Considering core Pentapetalae genes as the ancestral state, we calculated the change in gene content along each branch of the tree relative to the ancestral state (Figure 3B). There were 3148 orthogroups (including 4083 DEGs) that could not be associated unambiguously to one branch of the tree, and they were excluded from this analysis. We used estimated dates of divergence in the Pentapetalae phylogeny (Figure 3B; Zeng et al., 2017) to calculate the proportion of genes gained and lost per million year (Mya) along branches of the tree. Except the two most ancestral branches (Pentapetalae to Superasteridae and Pentapetalae to rosids), all branches showed higher rates of gene gain than gene losses. Along intermediate and terminal branches of the tree, whole genome losses were 0.95% ± 0.53%/Mya and whole genome gains were 0.35% ± 0.21%/Mya (paired t test, P value = 0.012; Figure 3C; Supplemental Data Set 1.15). For DEGs, losses were 0.12% ± 0.06%/Mya and gains were 0.25% ± 0.12% (P value = 0.009), indicating that S. sclerotiorum–responsive gene pools were less variable than whole genomes and that all lineages accumulated DEGs since ∼120 Mya.
Next, we calculated the ratio between the number of genes upregulated and downregulated upon S. sclerotiorum inoculation among orthogroups containing genes gained and lost along intermediate and terminal branches of the phylogeny (Figures 3A and 3D; Supplemental Data Set 1.15). The up/down ratio was ∼1.08 ± 0.20 in gained DEGs but only ∼0.80 ± 0.12 in lost DEGs (paired t test, P value = 0.0098), suggesting a trend to favor the retention of upregulated genes rather than downregulated genes. In agreement, the up/down ratio was 0.72 in Core Pentapetalae genes and ∼0.82 ± 0.02, on average, in the six plant species. These analyses suggest the existence of mechanisms leading to the progressive accumulation of genes upregulated upon S. sclerotiorum inoculation during the evolution of Pentapetalae plants.
Local Transcriptomic Responses to S. sclerotiorum Involve Core and Lineage-Specific Functions
To document the functional diversity of genes differentially expressed upon S. sclerotiorum inoculation, we collected Gene Ontology (GO) and Pfam domains annotations for the six plant species. Between 47.1% (H. annuus) and 74.1% (Arabidopsis) of expressed genes were annotated with GO, and between 76.1% (H. annuus) and 89.7% (Arabidopsis) of expressed genes were annotated with at least one Pfam domain per species (Table 1; Supplemental Data Sets 1.16 to 1.18; Supplemental Files 1 and 2). These annotations included between 2381 (B. vulgaris) and 3792 (Arabidopsis) distinct GOs and between 4128 (R. communis) and 5913 (H. annuus) distinct Pfams represented at least three times (Table 1). To highlight gene functions associated with the local response to S. sclerotiorum, we analyzed GO and Pfam annotation enrichment with upregulated and downregulated genes from each species. We mapped GOs to enriched Pfam domains to summarize annotations as GOs. We found 262 GOs significantly enriched with upregulated genes (chi-squared adjusted P value < 0.01) in at least one species, including 103 Biological Process ontologies, 28 Cellular Component ontologies, and 131 Molecular Function ontologies (Figure 4A; Supplemental Data Sets 1.19 and 1.20). Six GOs (4.65%) were enriched with upregulated genes in all species, including protein phosphorylation and kinase activities (GO:0006468, GO:0004674, GO:0004672). Two hundred and four GOs were enriched with upregulated genes in a single species, revealing the prevalence of abscisic acid signaling (GO:0009738) in R. communis and jasmonic acid signaling (GO:0009753, GO:0016629) in Arabidopsis and P. vulgaris. These GOs also point toward lineage-prevalent defense mechanisms, such as the biosynthesis of terpenes (GO:0010333) and inhibition of endopeptidases (GO:0004866) in S. lycopersicum, flavonoid glucuronidation (GO:0052696) in H. annuus, and vitamin B6 metabolism (GO:0042816) and ceramide biosynthesis (GO:0046513) in P. vulgaris. Fifty-two GOs were enriched with upregulated genes in two to five species, including defense response (GO: 0,006,952), response to wounding (GO:0009611), exocytosis (GO:0006887), response to chitin (GO:0010200), toxin catabolic process (GO:0009407), and flavonoid biosynthesis (GO:0009813). We found 411 GOs significantly enriched with downregulated genes (chi-squared adjusted P value < 0.01) in at least one species, including 187 Biological Process ontologies, 57 Cellular Component ontologies, and 167 Molecular Function ontologies (Figure 4B; Supplemental Data Set 1.19). Fifteen GOs were enriched with downregulated genes in all species, mostly related to the photosynthesis process (GO:0015979, GO:009654, GO:009522, GO:009507, GO:0016168). Three hundred forty GOs were enriched with downregulated genes in a single species, such as response to auxin (GO:0009733) in B. vulgaris and amine metabolism (GO:0009038) in H. annuus. GOs enriched with downregulated genes also included several GOs related to RNA processing (GO:0009451, GO:0001510), the regulation of protein translation (GO:0006400, GO:0006417), and DNA maintenance (GO:0004519, GO:0006298, GO:0006260, GO:0006508) in several species. Thirty-one GOs were enriched both with upregulated and downregulated genes, leading to a total 642 GOs enriched with DEGs.
Figure 4.
Distribution of DEGs According to GOs.
(A) and (B) Distribution of GOs enriched with upregulated genes (A) and downregulated genes (B) across species. Selected ontologies are labeled on the figure, colored according to the species in which they are enriched with DEGs. Labels are italicized for ontologies enriched both with upregulated genes and with downregulated genes.
(C) Network of Biological Process GOs covering all GOBPs including expressed genes in at least one species. GOs are represented by circles sized according to the number of expressed genes per GO. GOs significantly enriched in DEGs in a chi-squared test (adjusted P value [adj. p-val] < 0.01 after Bonferroni correction for multiple testing) are colored according to their enrichment score. Ontologies discussed in the main text are labeled. At, Arabidopsis; Bv, B. vulgaris; Ha, H. annuus; Pv, P. vulgaris; Rc, R. communis; Sl, S. lycopersicum.
To visualize hierarchical relationships between GOs enriched with DEGs, we constructed a network of Biological Process ontologies in which nodes are color coded according to enrichment with upregulated or downregulated genes (Figure 4C). Biological Process ontologies harboring an excess of upregulated over downregulated genes notably included response to toxin (up/down ratio 2.19), response to chitin (up/down ratio 5.80), response to wounding (up/down ratio 2.14), and cell surface receptor signaling (up/down ratio 1.19; Figure 4C). Ontologies with an excess of upregulated genes tended to cluster in defined sectors of the GO network related to secondary metabolism and detoxification on one side and signaling and response to stress on the other side (Figure 4C). Biological Process ontologies harboring an excess of downregulated over upregulated genes included response to light stimulus (up/down ratio 0.25), starch biosynthetic process (up/down ratio 0.13), and RNA methylation (up/down ratio 0.03). DEG-enriched ontologies spanned a broad range of the GO network, highlighting the diversity of processes involved in the response to S. sclerotiorum.
To study the relationship between conservation and regulation among major gene functions responsive to S. sclerotiorum infection, we classified GOs according to their content in core genes and their content in upregulated genes, focusing on the 282 Biological Process GOs enriched in DEGs. To this end, we determined the ratio between the number of core Pentapetalae genes and the number of lineage-specific DEGs as well as the ratio between the number of upregulated and downregulated DEGs for all GOs (Supplemental Data Set 1.19). For instance, ontology toxin catabolic process (GO:0009407) was identified in 71 upregulated genes, 12 downregulated genes, and 51 genes not differentially regulated (Figure 5A, up/down ratio 5.92), including 23 core Pentapetalae DEGs and 21 lineage-specific genes (core/lineage specific ratio 1.09). Ontology chlorophyll biosynthesis process (GO:0015995) was identified in 79 downregulated genes, 11 upregulated genes, and 48 genes not differentially regulated across six species (Figure 5B, up/down ratio 0.14), including 76 core Pentapetalae DEGs and 9 lineage-specific DEGs (core/lineage specific ratio 15.2). Fifty-one GOs did not contain upregulated or core genes and were excluded from the analysis. We observed a correlation between the content in core Pentapetalae genes and the content in downregulated genes (Spearman’s ρ = 0.325, P = 4.41e-07), suggesting a high degree of conservation or convergence in gene functions downregulated upon S. sclerotiorum infection (Figure 5C). A number of ontologies were exceptions to this trend, harboring a high content in core genes and high content in upregulated genes, such as callose deposition (GO:0052542), chorismate biosynthesis (GO:0009423), ceramide biosynthesis (GO:0046513), hypersensitive response (GO:0009626), and systemic acquired resistance (GO:0009862). Gene families lacking annotation, not covered by this analysis, may also contribute to the repertoire of genes responsive to S. sclerotiorum.
Figure 5.
Distribution of GOs According to Their Content in Genes Upregulated upon S. sclerotiorum Inoculation and Their Content in Core Genes.
(A) and (B) Number of genes annotated with GO:0009407 toxin catabolism (A) and GO:0015995 chlorophyll biosynthesis (B) that are upregulated (yellow), downregulated (blue), or not differentially expressed (white) upon S. sclerotiorum inoculation in each of the six plant species.
(C) Distribution of GOBPs significantly enriched with DEGs according to the ratio of the number of core Pentapetalae genes over the number of lineage-specific genes per annotation (x axis) and the ratio of the number of upregulated genes over the number of downregulated genes per annotation (y axis). Dotted lines indicate median values for all annotations; gray areas represent the interval containing the top 10% to top 90% annotations. Circles are sized according to the number of expressed genes per annotation and colored according their enrichment with DEGs. ABA, abscisic acid; At, Arabidopsis; Bv, B. vulgaris; Ha, H. annuus; Pv, P. vulgaris; Rc, R. communis; Sl, S. lycopersicum.
Transcriptional Response of Core Genes to S. sclerotiorum Varies Significantly between Species
We found only a few GOs enriched with DEGs in all species (Figures 4A and 4B). This could be due to (1) GOs enriched with DEGs being weakly conserved across species or to (2) conserved GOs harboring variable content in DEGs across species. To test for the second alternative, we analyzed the distribution of DEGs among genes, ontologies, and Pfam domains conserved in all six species. Genes, GOs, and Pfams retrieved in a maximum of five species were excluded from the following analyses. The core Pentapetalae class includes 62,838 genes conserved in all six species and distributed in 7918 orthogroups of 6 to 132 genes. Among those, 9520 genes (15.2%) were upregulated upon S. sclerotiorum infection, distributed between 3659 orthogroups (46.2%). This included 159 orthogroups (2.01%) containing upregulated genes from all six plant species (Figure 6A; Supplemental Data Sets 1.11 and 1.21). There were 13,201 core Pentapetalae genes downregulated (21.0%), distributed in 4962 orthogroups (62.7%), including 282 orthogroups (3.56%) containing downregulated genes from all six plant species. Overall, 1039 core Pentapetalae groups did not contain DEGs (13.1%), 438 contained DEGs in all six plant species (5.53%), and 6441 contained DEGs in one to five plant species (81.3%; Figure 6A; Supplemental Figures 1 and 2), indicating that a majority of conserved gene groups showed divergent regulation upon S. sclerotiorum inoculation in distinct plant species.
Figure 6.
Diversity of Gene Functions Differentially Regulated upon S. sclerotiorum Inoculation at the Interspecific Level.
(A) to (C) Distribution of core Pentapetalae (Penta.) groups (A), conserved GOs (B), and conserved Pfam domains (C) according to the number of plant species in which genes are upregulated (red), downregulated (blue), or both (gray). For instance, 2.00% of the core Pentapetalae groups contain genes upregulated in all six species. The proportion of groups containing DEGs from six species are labeled. The sum of groups containing DEGs from one to five species is labeled to illustrate how frequent interspecific divergence in gene expression is.
(D) Network of Biological Process GOs represented by circles sized according to the number of DEGs per GO and colored according to the number of species in which genes are upregulated for each GO. Ontologies discussed in the main text are labeled.
(E) Distribution of GOs’ level in the GO hierarchy according to the number of species in which genes are upregulated. Median values are shown by a black diamond and labeled. Significance of the difference to the six-species distribution was assessed by Student’s t test, with P values shown on top the plot.
(F) and (G) Pairwise correlations for the content of GOs in core Pentapetalae genes (F) or in DEGs (G) between the different plant species. Circles are sized and colored according to Spearman’s ρ. The average ρ and sd for all pairwise comparisons are indicated.
(H) Overlap between the list of top 100 GOs enriched in DEGs in each of the six species. Values shown in gray are common to two to five species.
(I) Number of GOs in the top 100 enriched in DEGs (red) or depleted in DEGs (gray) that are shared between all six species, between two and five species, or unique to one species. Boxplots show first and third quartiles (box), median (thick line), and the most dispersed values within 1.5 times the interquartile range (whiskers). At, Arabidopsis; Bv, B. vulgaris; Ha, H. annuus; Pv, P. vulgaris; Rc, R. communis; Sl, S. lycopersicum.
To test whether this observation holds true for conserved gene functions, we conducted a comparative analysis of GOs and Pfam domains expressed in all six species (corresponding to 3536 GOs and 5919 Pfam domains; Figures 6B and 6C; Supplemental Data Sets 1.22 and 1.23). We found that 71.5% of GOs and 64.5% of Pfam domains were associated with DEGs from one to five plant species. In agreement with frequent species-specific regulatory patterns for conserved genes, although detected in all six plant species, only 23% of GOs and 33.6% of Pfam domains were associated with DEGs in all six plant species (Figures 6B and 6C).
To characterize species-specific regulation across GOs, we color coded GO networks according to the number of plant species in which they are associated with upregulated genes (zero to six; Figure 6D). Ontologies associated with upregulated genes in five or six species where highly connected at the center of the network. Exceptions included the ontologies ATP transport, negative regulation of apoptosis, vacuolar acidification, lipid homeostasis, Cys biosynthesis from Ser, removal of superoxide radicals, and negative regulation of endopeptidase that were terminal nodes of the network associated with upregulated genes in five or six species. This prompted us to test for a relationship between association with upregulated genes in multiple species and the level of GOs in the ontology hierarchy. We found that the number of species in which GOs associate with upregulated genes decreased with ranks in the GO hierarchy, from an average rank 12.96 for GOs associated with upregulated genes in all six plant species to an average rank 17.66 for GOs associated with upregulated genes in a single species (Student’s t test, P value = 3.7e−09; Figure 6E; Supplemental Data Set 1.22). This highlights significant variations in genes and biological functions regulated locally upon S. sclerotiorum inoculation across diverse plant species.
To evaluate further the divergence in biological functions regulated upon S. sclerotiorum inoculation across six plant species, we analyzed 893 GOs represented in all six species and including at least one DEG and one core Pentapetalae gene in each species. As a reference, we calculated pairwise correlations for GOs content in core Pentapetalae genes between plant species. We found an average Spearman’s ρ of 0.601 ± 0.078 (Figure 6F; Supplemental Data Set 1.24). We then calculated pairwise correlations for GOs content in DEGs between plant species and found an average Spearman’s ρ of 0.370 ± 0.064 (Figure 6G; Supplemental Data Set 1.24). Next, we analyzed the top 100 GOs enriched in DEGs in each plant species. In total, the selection included 410 GOs for the six species, and indicated a limited overlap in individual top 100 lists. Indeed, only seven GOs related to the downregulation of photosynthesis belonged to the top 100 enriched in DEGs in all six species (Figure 6H; Supplemental Data Set 1.25). Forty-four (S. lycopsersicum) to 64 (P. vulgaris) GOs were part of the top 100 enriched in DEGs in a single species, including for instance structural constituent of cytoskeleton in Arabidopsis, carotenoid biosynthesis in B. vulgaris, flavonoid biosynthesis in H. annuus, and glucosylceramidase activity in P. vulgaris. For comparison purposes, we analyzed top 100 GOs depleted in DEGs in each plant species (Figure 6I; Supplemental Data Sets 1.25 and 1.26). Eleven GOs were highly depleted in DEGs in all six species; between 14 (H. annuus) and 38 (Arabidopsis and P. vulgaris) GOs were highly depleted in GOs in a single species. This supports the view that the repertoire of core GOs enriched with DEGs is rather diverse at the interspecific level.
Patterns of Core Genes Expression upon S. sclerotiorum Inoculation Deviate Significantly from a Null Model of Heritability
Of 3659 core Pentapetalae orthogroups including upregulated genes, only 159 (4.3%) included upregulated genes from all six species analyzed (Supplemental Data Set 1.21), suggesting a high degree of regulatory divergence at the interspecific level. Since genes from a given core Pentapetalae orthogroup most likely derive from a common ancestor, we considered the null hypothesis that their expression pattern is completely inherited, leading to similar expression patterns within orthogroups. We compared observed distributions of gene expression upon S. sclerotiorum inoculation to modeled distributions to test this hypothesis and estimate the degree of regulatory variation within core Pentapetalae genes.
First, we analyzed the distribution of the number of species harboring upregulated genes in each core Pentapetalae orthogroup. For this, we shuffled 100 times core Pentapetalae genes among orthogroups. Under the null hypothesis of complete inheritance, upregulation is inherited in six species. We conducted our bootstrap analysis by constraining identical expression (upregulated or not) in six species within an ortholog group (inheritance [h] = 6) to test this hypothesis. We next considered relaxed degrees of expression pattern inheritance (h = 5 to 2), down to a complete random assignment of expression patterns (h = 1; Figure 7A; Supplemental Data Set 1.27; Supplemental File 3). We used the sum of squared residuals (SSR) to estimate the deviation between observed and simulated distributions. The lowest SSR value (38.0) is obtained when gene expression is constrained as identical in two species only (h = 2). A similar analysis on the distribution of downregulated genes also identified h = 2 as the best approximation to observed distribution. This suggests that constraints on core genes upregulation are weak, allowing switches between upregulated and not upregulated states in four of six species on average.
Figure 7.
Analysis of Interspecific Regulatory Variation within Core Pentapetalae Gene Groups.
(A) Simulated (gray) and observed (red) distributions of the number of upregulated genes in each core Pentapetalae group. Similar expression (upregulated or not) has been constrained in h = 6 to 1 species (no constraint). The deviation between observed and simulated distribution was assessed using the SSR. The lowest SSR value corresponded to h = 2 species, suggesting that constraints to maintain similar gene expression across species are weak.
(B) Distribution of the CEI across 7918 core ortholog gene groups. The CEI is determined using gene expression clustering within each orthogroups and increases with the relative size of the largest expression cluster. Counts in the bottom quarter (CEI ≤ 0.33) and top quarter (CEI ≥ 0.67) of the range are labeled; red dotted line shows data set average.
(C) and (D) Detailed analysis of orthogroup no. 56 with low CEI (C) and orthogroup no. 1067 with high CEI (D) value. The dotplots show log2 fold change (LFC) of expression upon S. sclerotiorum inoculation for genes in each orthogroup. Arrows points toward the position of orthogroups no. 56 and no. 1067 in the CEI distribution. Pie charts show the relative size of expression clusters (as % of orthogroup size), with the largest expression cluster labeled.
Second, we clustered genes based on their LFC using the silhouette method (Charrad et al., 2014) within each orthogroup, and we determined the size of the largest expression cluster relative to the size of each orthogroup (Supplemental Data Set 1; Supplemental File 4). Under the null hypothesis of complete inheritance, gene expression patterns should be highly consistent within each Pentapetalae core orthogroup, and clusters should tend to cover complete orthogroups. By contrast, under the hypothesis that genes forming an orthogroup are fully independent (expression patterns are randomly distributed), expression clusters would correspond to one single gene. To determine the extent to which genes expression departs from independence in each core Pentapetalae orthogroup, we computed a consistency of gene expression index (CEI) corresponding to the difference between the relative size of the largest expression cluster observed and the relative size of the largest theoretical expression cluster under gene independence (Figure 7B; Supplemental Data Set 1.28). For instance, orthogroup no. 56 containing 50 genes (theoretical expression cluster size under gene independence was 1/50) included 17 expression clusters, the largest of which contained 6 genes (12% of the orthogroup; CEI = 0.12 – 0.02 = 0.1; Figure 7C). In our data set, CEI ranged from 0.0602 (92.8% of genes showed independent expression pattern) to 0.8824 (94.1% of genes showed consistent expression pattern). The CEI average was 0.46, meaning that, on average, about one-half of the genes composing a core orthogroup had consistent patterns of expression (Figure 7B). There were only 831 orthogroups (10.5%) with CEI ≥ 0.67 (largest expression cluster including at least 67.42% of genes in the orthogroup). One of them is orthogroup no. 1067 including 13 genes distributed into two expression clusters, the largest containing 10 genes (CEI = 0.692; Figure 7D). There were 2500 orthogroups (31.6%) with CEI ≤ 0.33 (largest expression cluster including <34.1% of genes in the orthogroup). We conclude that core orthogroups with weakly consistent expression patterns are abundant, suggesting that expression consistency is weakly constrained in many orthogroups. Our bootstrap and clustering approaches both lead to the conclusion that the expression of core genes upon S. sclerotiorum inoculation is only weakly heritable at the interspecific level.
Evidence for Exaptation into QDR in a Group of ABCG/PDR Transporters
To illustrate regulatory divergence in response to S. sclerotiorum at the interspecific level, we focused on the orthogroup no. 4. This group is remarkable for including upregulated genes in each of the six plant species, a property restricted to 2.01% of the core Pentapetalae class. Gene expression LFC ranges from –6.2 (XM_015728348.1) to 12.7 (PHASIBEAM10F011181T1) in orthogroup no. 4, ranking it 14/15,685 for intragroup LFC variation. Orthogroup no. 4 contains 96 expressed genes encoding class G ATP binding cassette (ABC) transporters (pleotropic drug resistance [PDR] class), among which 28 are upregulated and 14 are downregulated upon S. sclerotiorum inoculation (Figure 8A; Supplemental Data Set 1.29; Supplemental File 5). A phylogenetic analysis of orthogroup no. 4 reveals six monophyletic clades each containing genes from all six plant species, suggesting the divergence of these clades early in land plants, in agreement with a previous report (Hwang et al., 2016). Upregulated genes were restricted to one species in clades 1 and 3, three species in clade 2, and four species in clade 4. Clade 5 did not include upregulated genes, while clade 6 contained upregulated genes from each of the six plant species, including Arabidopsis ABCG40/PDR12 (AT1G15520, LFC 12.48; Figure 8A).
Figure 8.
Extreme Regulatory Diversity and Evidence for Exaptation of an Ancestral Function into QDR in a Group of ABCG Transporters.
(A) Phylogenetic relationship of 96 ABCG transporters from six Pentapetalae species covering orthogroup no. 4. The tree obtained by a maximum likelihood analysis is shown, with the number of substitutions per site used as branch length and branch support determined by an approximate likelihood-ratio test (red labels). Terminal nodes are color coded according to plant species. The circular bar plot shows gene expression log2 fold change (LFC) upon inoculation by S. sclerotiorum, in blue when significantly downregulated, in red when significantly upregulated, and in gray otherwise. Six major clades are delineated by shaded sectors, with the list of species including upregulated indicated by icons for each clade. Arabidopsis genes are labeled in green with their ABCG and PDR identifiers. Inset shows reverse cumulative distribution of orthogroups according to the delta LFC between most induced and most downregulated genes per group. Red line indicates position of orthogroup no. 4.
(B) Expression LFC for 14 Arabidopsis ABCG genes upon 12 stress treatments. The size and color of bubbles relates to LFC; bubbles are outlined when genes are significantly upregulated or downregulated. Adjusted P values obtained by DESeq-2 were Bonferroni corrected for multiple testing.
(C) Altered ABCG40 gene expression in Arabidopsis abcg40 insertion mutant lines. Top diagram represents ABCG40 gene structure with exons as blue boxes; red arrows indicate the position of T-DNA insertions in each line. Boxplots show ABCG40 gene expression in the healthy wild-type (Col-0) and mutant plants obtained from three or four plants per genotype measured twice. Expression is given relative to AT2G28390 housekeeping gene expression.
(D) Representative pictures of leaves infected by S. sclerotiorum carrying GFP shown under UV illumination at 24 h postinoculation. Bar = 2 mm.
(E) Measurements of leaf areas colonized by S. sclerotiorum 24 h postinoculation. The rlp30-1 mutant was used as a susceptibility control (Zhang et al., 2013). Significance of the difference to the wild type were assessed by Student’s t tests with Bonferroni correction for multiple testing, with P values shown in green. Boxplots show first and third quartiles (box), median (thick line), and the most dispersed values within 1.5 times the interquartile range (whiskers).
Because the six plant lineages analyzed here diverged before the emergence of the Sclerotinia genus (Navaud et al., 2018), we hypothesized that the ABCG clade induced in all species was exapted into QDR (Gould and Vrba, 1982). In this evolutionary scenario, an ancestor of ABCG clade 6 would have been responsive to signals of the ancestral environment, in which the Sclerotinia genus had not emerged yet, and underwent regulatory and functional changes associated with enhanced QDR against S. sclerotiorum. Because of the extensive resources available for this plant, we focused on Arabidopsis clade 6 gene ABCG40 to test this exaptation scenario. For this, we first analyzed the expression of Arabidopsis ABCG genes from orthogroup no. 4 in 12 stress conditions reported in the literature, including challenge by the fungal pathogens S. sclerotiorum, B. cinerea, Alternaria brassicicola, Verticillium dahlia, and Colletotrichum incanum. The other treatment surveyed covered inoculation by the fungal endophyte Colletotrichum tofeldiae, inoculation by avirulent (carrying the AvrRPS4 avirulence gene) and virulent (DC3000 cor-) strains of the bacterial pathogen Pseudomonas syringae pv tomato (Pst), inoculation by the nematode Heterodera schachtii, inoculation by the cabbage leaf curl virus, and upon heat and salt stress (Figure 8B; Supplemental Data Set 1.30). Six ABCG genes were significantly induced by two or more stresses. Salt treatment, inoculation by C. tofeldiae endophytic fungus, the virulent Pst DC3000 cor- strain, H. schachtii nematodes, and cabbage leaf curl virus did not significantly induce any the ABCG genes tested. The ABCG40/PDR12 gene was significantly induced by the broadest range of treatments (6/12), including fungal pathogens S. sclerotiorum, B. cinerea (LFC 8.08), A. brassicicola (LFC 4.15), Verticillium dahliae (LFC 1.58), avirulent Pst (LFC 9.04), and heat (LFC 8.16).
Second, we analyzed the disease resistance phenotype of four T-DNA mutant lines altered in the AtABCG40 gene. To characterize mutant lines at the transcript level, we first assessed AtABCG40 expression in healthy plants (Figure 8C; Supplemental Data Set 1.31). The abcg40-2 line (SALK_005635; Campbell et al., 2003) was strongly impaired in ABCG40 expression (<4% of the wild-type expression, P value = 1.4e−04), while ABCG40 was only weakly reduced in abcg40-3 (SAIL_885_E09) and abcg40-4 (SALK_148565; ∼62 and ∼54% of the wild-type, respectively) lines, and abcg40-5 (SALK_013945) showed an increased accumulation of ABCG40 transcripts (∼165% of the wild type). We inoculated leaves of these plants with a GFP-expressing strain of S. sclerotiorum to measure the area colonized by the fungus 24 h after inoculation, using the rlp30-1 mutant (Zhang et al., 2013) as a susceptible control (Figure 8D). A strong genotype effect with no significant experiment replicate effect was detected on lesion size (ANOVA P value = 9.81e–06 and 0.05, respectively). Average area colonized by S. sclerotiorum was 85.0 ± 9.7 mm2 in Arabidopsis ecotype Columbia (Col-0) and 123.7 ± 16.9 mm2 in rlp30-1 (Student’s t test adjusted P value = 9.9e–05; Figure 8E; Supplemental Data Set 1.32). Decrease in ABCG40 expression was associated with an increase in susceptibility in abcg40-3 (108.1 ± 13.0 mm2 colonized, adjusted P value = 0.02), and abcg40-2 (130.5 ± 17.4 mm2 colonized, adjusted P value = 2.5e–05). The abcg40-4 and abcg40-5 mutants were weakly, but not significantly, impaired in resistance to S. sclerotiorum (106.5 ± 10.1 and 98.4 ± 9.7 mm2 colonized, respectively; adjusted P value > 0.1), suggesting that ABCG40 transcripts may be functional in these lines. Overall, these results demonstrate a role for AtABCG40 in disease resistance to S. sclerotiorum and suggest that AtABCG40 was exapted into QDR from an ancestral stress response function through reinforcement of its transcription upon fungal pathogen challenge.
DISCUSSION
Our phylotranscriptomics analyses of local responses to S. sclerotiorum in plants from six Pentapetalae orders provide an unprecedented insight into the broad diversity of transcriptional reprograming upon challenge with a single fungal pathogen strain. Comparative transcriptomics studies of plant interactions with eukaryotic pathogens typically focused on gene reprogramming in closely related plant cultivars (Zhao et al., 2009) and species (Powell et al., 2017) and on the transcriptome of several parasite strains (Cooke et al., 2012; Palma-Guerrero et al., 2016; Zhang et al., 2017) or species (Hacquard et al., 2016) infecting a given plant genotype. The remarkably wide range of plants that S. sclerotiorum can infect allowed us to document molecular responses to this fungus in host species that diverged more than 140 Mya.
An Estimate of the Pan-Genomic Diversity of S. sclerotiorum–Responsive Genes
Recent sequencing efforts defined the complement of NLR genes across Arabidopsis accessions (Van de Weyer et al., 2019). Here, we provide an estimate of the QDR complement responding to S. sclerotiorum locally in the Pentapetalae. Together, we identified 48,910 genes differentially expressed upon S. sclerotiorum across six species, enriched in 642 GOs. Despite their evolutionary distance and strong variation in genome size, all species analyzed in this work upregulated ∼15% of their genome and downregulated ∼18% of their genome upon infection, suggesting the existence of a conserved optimal pool of S. sclerotiorum–responsive transcripts. On average, we found that ∼47% of genes differentially expressed in a species upon S. sclerotiorum inoculation were conserved in the five others (core QDR orthogroups), while ∼38% were specific to this species. Similarly, the core NLR orthogroups, conserved in >50 of 65 accessions, represent 53% of NLR genes (Van de Weyer et al., 2019). This suggests that a significant fraction of S. sclerotiorum–responsive pan-transcriptome is likely to be functional in multiple plant species, as shown for instance for the POQR putative peptidase in Arabidopsis and tomato (Badet et al., 2017). In this context, functional analyses of QDR genes in model species may offer relatively straightforward perspectives for improving QDR in crops.
Experimental evidence for a role in QDR is only available for a limited number of DEGs. Nevertheless, a diverse set of Arabidopsis DEGs were shown to function in resistance against S. sclerotiorum (Table 3). According to the omnigenic model (Boyle et al., 2017), any DEG in our pan-transcriptome is expected to impact the QDR phenotype. Our work therefore suggests that ∼32.4% of plant genes could have a non-null contribution to QDR against S. sclerotiorum. Because network connectivity is an important determinant of gene essentiality and conservation, it is tempting to speculate that core genes in the omnigenic model, that directly and strongly affect disease, would likely be among the 43% DEGs broadly conserved across species. Species-specific DEGs would modulate core gene activity according to genetic backgrounds, pathogen genotypes, and other environmental variables and may underlie the frequent variation in core gene regulation that we observed. Our enrichment analyses are consistent with a set of genes that could directly control QDR through their protective or antifungal activity (e.g., terpene and flavonoid biosynthesis genes, endopeptidase inhibitors, toxin catabolism genes), the activity of which could be modulated by numerous transcriptional and posttranscriptional regulators (e.g., WRKY DNA binding, ERF/AP2-type transcription factor, and kinase domain proteins). We identified several DEGs encoding proteins with domains of unknown function (DUF2870, DUF969, DUF3403) representing promising candidates for novel defense proteins. Studies with B. cinerea in Arabidopsis and tomato indicated that genes associated with QDR varied according to the fungal strain being inoculated (Corwin et al., 2016; Soltis et al., 2019), suggesting that the pan-genomic repertoire of QDR-associated transcripts may depend on pathogen genotypes and species and may exceed current estimates.
Table 3. A Selection of Arabidopsis Genes Involved in Disease Resistance to S. sclerotiorum and Differentially Expressed in This Study.
| TAIRa Gene ID | Gene Name | LFC (This Work) | Reference |
|---|---|---|---|
| At1g15520 | ABCG40 | 12.48 | This work |
| At3g26830 | PAD3 | 9.97 | (Stotz et al., 2011) |
| At1g20380 | POQR | 7.15 | (Badet et al., 2017) |
| At3g05360 | RLP30 | 4.46 | (Zhang et al., 2013) |
| At4g33430 | BAK1 | 2.88 | (Zhang et al., 2013) |
| At5g45900 | ATG7 | 2.54 | (Kabbage et al., 2013) |
| At1g64280 | NPR1 | 1.25 | (Guo and Stotz, 2007) |
| At3g48990 | AAE3 | 1.12 | (Foster et al., 2012) |
| At4g26080 | ABI1 | 1.03 | (Perchepied et al., 2010) |
| At4g14147 | ARPC4 | 1.02 | (Badet et al., 2019) |
| At2g39940 | COI1 | −1.23 | (Guo and Stotz, 2007) |
| At1g34210 | SERK2 | −2.25 | (Derbyshire et al., 2019) |
| At5g03280 | EIN2 | −2.89 | (Guo and Stotz, 2007) |
| At3g47450 | NOA1 | −3.22 | (Perchepied et al., 2010) |
| At1g0855 | NPQ1 | −3.95 | (Zhou et al., 2015) |
| At2g32680 | RLP23 | −5.41 | (Albert et al., 2015) |
| At5g61420 | MYB28 | −6.84 | (Stotz et al., 2011) |
TAIR, The Arabidopsis Information Resource.
Molecular Bases of Regulatory Variation and QDR Phenotype Evolution
Although 47% of DEGs belonged to core orthogroups conserved in six plant species, only 2.01% of core orthogroups contained genes upregulated in six species (and 3.56% genes downregulated in six species), highlighting frequent regulatory variation within orthogroups. In our previous work, we identified allelic variants of the ARPC4 gene differing in their promoter region, induction upon pathogen challenge, and associated with resistance to S. sclerotiorum (Badet et al., 2019). These findings are in line with a prominent role of regulatory variation in the evolution of QDR. It is also consistent with the alteration of host gene transcription being a major function of small RNA secreted by several fungal pathogens (Weiberg et al., 2013; Derbyshire et al., 2019). Species-specific gene expression patterns may result from directional selection on gene regulation or specific environmental influences (Romero et al., 2012). Recent progress in genomics allowed disentangling genetic and environmental factors in a number of cases, pointing toward several molecular mechanisms that may be responsible for expression polymorphisms in QDR genes. Molecular mechanisms underlying the evolution of gene expression include variations in epigenetic marks, gene copy number variation, and cis- and trans-regulatory variation.
Epigenetic marks such as DNA methylation control Arabidopsis stress-responsive gene expression upon challenge by bacterial and fungal pathogens (Yu et al., 2013; Le et al., 2014). Histone acetylation, methylation, and ubiquitination and the activity of chromatin remodeling complexes are other epigenetic mechanisms involved in the control of plant defense genes transcription (Ramirez-Prado et al., 2018). Transcription is also controlled by the effect of cis-regulatory elements and trans-acting factors that may drive phenotypic evolution. For instance, the homeobox transcription factor RCO underwent regulatory changes, gene duplication, and loss, leading to leaf shape diversity among Brassicaceae species (Vlad et al., 2014). This cis-regulatory variation results from the emergence of novel enhancer sequences (Long et al., 2016) or from genome shuffling recruiting new genes to transcriptionally active regions. In both cases, whole-genome duplications and transposable elements (TEs) can play prominent roles. Insertion of TE acting as an enhancer of the teosinte branched (tb1) transcription factor gene was a key molecular event in the acquisition of apical dominance during maize (Zea mays) domestication (Studer et al., 2011). In some Arabidopsis accessions, insertion of a TE-derived enhancer mediated WRKY33 binding to the promoter of the CYP82C2 gene, making it inducible upon bacterial pathogen inoculation, activating biosynthesis of the antimicrobial 4-hydroxyindole-3-carbonylnitrile metabolite (Barco et al., 2019). A similar mechanism could have driven the emergence of an S. sclerotiorum–inducible allele of ARPC4 associated with enhanced QDR in Arabidopsis (Badet et al., 2019). The pangenomic repertoire of QDR transcripts conserved across species but differentially regulated upon S. sclerotiorum inoculation provides an original resource for the identification of new transcription enhancer motifs. Since transcription factors often regulate multiple target genes, while cis-regulatory motifs act rather locally in the genome, cis-regulatory regions are considered less pleiotropic and therefore more likely to contribute to phenotypic evolution (Wray, 2007). Detailed promoter sequence analyses and cross-species functional validations will be required to estimate the relative contribution of cis- and trans-regulatory divergence in the evolution of QDR. The central role of gene expression in QDR phenotypes suggests that engineering the expression or copy number of endogenous QDR genes, rather than introgressing new genes or alleles, could be an efficient strategy to control diseases in the field.
An Exaptation Scenario for the Recruitment of Genes into QDR
Under the Red Queen hypothesis, plant immunity genes represent true adaptations, evolved under selection pressure associated with specific pathogen populations (Van Valen, 1973). In agreement, signatures of diversifying selection were detected in Arabidopsis and Solanum NLR genes (Mondragón-Palomino et al., 2017; Stam et al., 2019). In natural populations of Arabidopsis, polymorphism at the GS-ELONG locus controlling the structure of aliphatic glucosinolate defense compounds was associated with the presence of specific aphid species in the environment (Züst et al., 2012). In selection experiments, aphid species drove selection for contrasted glucosinolate profiles in five plant generations, demonstrating that herbivores drive the rapid evolution of some plant defense genes. Alternatively, plant genes could have emerged prior to exposure to pathogens to serve nonimmune functions, before being exapted into defense with the rise of pathogen populations. The glucosinolate 3-hydroxypropylglucosinolate produced by Brassicaceae plants has a role in defense and inhibits root growth in diverse dicot species (Malinovsky et al., 2017). Responses to 3-hydroxypropylglucosinolate involve the target of rapamycin pathway conserved across eukaryotes, suggesting that the target of rapamycin pathway was exapted into glucosinolate response early in plant evolution. Similarly, Arabidopsis CYP82C2 gene originated from the duplication the iron-stress response gene CYP82C4 and was exapted into defense through cis-regulatory variation (Barco et al., 2019).
We identified 159 orthogroups featuring genes upregulated by S. sclerotiorum in all six plant lineages, which diverged long before the emergence of the Sclerotinia genus (Navaud et al., 2018). For these genes, responsiveness to S. sclerotiorum likely evolved convergently in multiple plant lineages, through exaptation of ancient stress responses, or a combination of both processes. Here, we provide evidence for exaptation of the ABCG40/PDR12 transporter into QDR against S. sclerotiorum. The indirect pathway for abscisic acid synthesis probably evolved with the colonization of land (Hauser et al., 2011), similar to the ABCG/PDR class of transporters (Hwang et al., 2016). It is therefore probable that the abscisic acid importer function of ABCG40/PDR12 (Kang et al., 2010) evolved with the colonization of land, ∼450 Mya, to control drought tolerance, seed germination, and lateral root formation (Campbell et al., 2003; Kang et al., 2010). Consistent with an ancestral role in acclimation to abiotic stress, AtABCG40 was induced upon heat stress and downregulated by salt stress (Suzuki et al., 2016). AtABCG40 is also induced upon inoculation by the fungal pathogens A. brassicicola, Fusarium oxysporum, and more strongly and more rapidly by S. sclerotiorum (Campbell et al., 2003). In line with suppression of abscisic acid synthesis leading to susceptibility to S. sclerotiorum (Perchepied et al., 2010; Zhou et al., 2015; Mbengue et al., 2016), mutants defective in AtABCG40 were more susceptible to S. sclerotiorum than the wild type. Exaptation of AtABCG40 into QDR against S. sclerotiorum associates with strong transcriptional activation of the gene upon challenge with S. sclerotiorum, through a mechanism that remains to be elucidated.
Broad Spectrum and Durability in the Context of Exaptation into Resistance
The evolution of QDR genes through exaptation of ancestral stress responses and developmental pathways could be related to QDR often being relatively broad spectrum and durable (Poland et al., 2009; Roux et al., 2014; Corwin and Kliebenstein, 2017). Signals created by pathogen attack on plants are either molecular signals that can be specific to the pathogen genotype such as pathogen effectors (Guyon et al., 2014; Guo et al., 2018), or abiotic signals. Abiotic signals generated by pathogens during host colonization include altered pH (Xu et al., 2015; Fernandes et al., 2017), water status (Xin et al., 2016; Aung et al., 2018), and physical strain (Wilson and Talbot, 2009). Since similar abiotic signals may be generated by diverse pathogens, as for alkalinization by Pseudomonas phytotoxins (Bender et al., 1999) and by Fusarium RALF-like peptides (Masachis et al., 2016), plant responses mediating acclimation to these signals are likely to be exapted into QDR and to function against a broad spectrum of pathogens. A rapid transcriptional reprogramming was proposed to be critical for broad-spectrum efficiency of plant immune responses, supporting a central role of cis-regulatory divergence in adaptation to pathogens (Mine et al., 2018). In pathogens were single effector molecules are major determinants of the disease outcome, single amino acid substitution, gene presence/absence, and expression polymorphism are frequent mechanisms associated with evasion of recognition by the plant immune system (Raffaele and Kamoun, 2012). Abiotic signals are not directly encoded by pathogen genomes; therefore, evasion of the corresponding plant responses may require very complex genetic alterations to pathogen genomes.
Upon escape from adaptive conflict, genes with exapted function may retain their ancestral functions (Conant and Wolfe, 2008). In some animal lineages, structural proteins forming the ocular lens retained their ancestral argininosuccinate lyase enzymatic activity (Piatigorsky et al., 1988). A class of fungal dicarboxylic acid transporters were shown to have retained their ancestral activity and gained the ability to transport tricarboxylic acids after horizontal transfer to oomycetes (Savory et al., 2018). Plant enzymes in the dihydroflavonol-4-reductase and the SABATH methyltransferase families evolved novel substrate specificities during the diversification of dicots while retaining weak ancestral activity (Des Marais and Rausher, 2008; Huang et al., 2012). Because of such pleiotropic activity of some QDR genes, the suppression of their activity by pathogens may have adverse effects on availability of plant-derived nutrients or lead to pathogen detection and therefore increase the durability of disease resistance. The reconstruction of immune gene networks may facilitate the systematic investigation of pleiotropy in the QDR gene pool and elucidate how genomic backgrounds affect the activity of disease resistance genes (Mine et al., 2014; Peyraud et al., 2017; Smakowska-Luzan et al., 2018; Wu et al., 2018).
METHODS
Plant Materials and Sclerotinia sclerotiorum Infection
Arabidopsis (Arabidopsis thaliana) Col-0 genotype obtained from the Nottingham Arabidopsis Stock Centre (accession number N1093), Solanum lycopersicum cv Ailsa Craig, Helianthus annuus cv XRQ, Ricinus communis cv Hale (accession number PI 642000), and Beta vulgaris subsp vulgaris (accession number PI 355961) obtained from the USDA–ARS Germplasm Resources Information Network, and Phaseolus vulgaris (accession number G19833) obtained from the International Center for Tropical Agriculture were used for inoculations. Plants were grown in Jiffy pots under controlled conditions at 23°C, with a 9-h light period at intensity of 120 μmol/m2/s under Osram OSLON SSL 80 light-emitting diodes. Five-week-old plants were inoculated on fully developed leaves by 0.5-cm-wide plugs of potato dextrose agar (Fluka) colonized by S. sclerotiorum strain 1980 (ATCC18683; Supplemental Methods). Plugs of agar containing the fungal pathogen were placed on the adaxial surface of leaves, and plants were incubated in trays closed with plastic wrap to maintain 80% relative humidity and placed in a Percival AR-41L3 chamber under the same day/light conditions as for plant growth.
Tissue Sampling, RNA Extraction, and Sequencing
RNA-seq reads from Arabidopsis and S. lycopersicum were obtained from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database accession GSE106811 (Badet et al., 2017). For all infected plant species, the edge of 25-mm-wide developed necrotic lesions (Peyraud et al., 2019) was isolated with a scalpel blade from leaves placed on a glass slide cooled down by liquid nitrogen and immediately frozen in liquid nitrogen. Samples were harvested before lesions reached 25 mm in width, at various times after inoculation, owing to the different susceptibility level of each species. All inoculations were performed 5 h after lights were turned on; samples were harvested at 24 h postinoculation for H. annuus, between 47 and 50 h postinoculation for Arabidopsis, P. vulgaris, R. communis, and S. lycopersicum, and 72 h postinoculation for B. vulgaris. Samples from noninoculated plants were harvested simultaneously to serve as controls and exclude biases due to synchrony of the plants’ circadian clock. To determine the extent to which circadian synchrony varies across species in our data set, we analyzed normalized read counts corresponding to 10 genes of the circadian clock (LHY, TOC1, LUX, RVE8, PRR9, PRR3, PRR5, ZTL, GI, and ELF3) in the six species, in inoculated and healthy plants (Supplemental Methods). In a principal component analysis, the first two dimensions summarized 86.8% of the total inertia. All species variables appeared very well correlated, excepted for Arabidopsis, which differed slightly in a higher TOC1 expression. Correlations between inoculated and healthy samples were significant for all species (ρ ≥ 0.66; P value ≤ 0.04). Material obtained from leaves of three plants were pooled for each sample; all samples were collected in triplicates. Samples were ground with glass beads (2.5 mm) in a Retschmill apparatus (24 Hz for 2 × 1 min) before RNA extraction using NucleoSpin RNA extraction kits (Macherey-Nagel) following the manufacturer’s instructions. A 30-min DNase treatment (TURBO DNase, Ambion) was applied following manufacturer’s instructions to eliminate genomic DNA. The quality and concentration of RNA preparations were assessed with an Agilent 2100 Bioanalyzer and the RNA 6000 Nano kit. For H. annuus, P. vulgaris, R. communis, and B. vulgaris samples, mRNA sequencing was outsourced to Fasteris SA to produce Illumina paired reads (2 × 125 bp) using a HiSeq 2500 instrument. Three independent biological samples were sequenced per condition. The corresponding raw RNA-seq reads data were deposited in NCBI’s GEO under accession number GSE138039.
Mapping of Sequence Reads and Analysis of DEGs
Reads were mapped to reference genomes listed in Supplemental Data Set 1.1 using the RNA-seq analysis tool of the CLC Genomics Workbench 11.0.1 software (Qiagen). The following mapping parameters were used: mismatch cost 2, insertion cost 3, deletion cost 3, length fraction 0.8, similarity fraction 0.8, both strands mapping, and 10 hits maximum per read, with expression value given as total counts. All reads were mapped independently to S. sclerotiorum genome version 2 (Derbyshire et al., 2017) to estimate the fraction of fungal reads per sample. Number of uniquely mapped reads per transcript were exported in tab delimited text format. Differential gene expression analysis was conducted with the DESeq2 Bioconductor package version 1.8.2 (Love et al., 2014) in R 3.4.0 with total uniquely mapped read count per gene as input. Differential expression was calculated using expression in uninfected plants as a reference with ∼replicates + treatment as the design formula. For interspecific comparisons, LFCs were quantile normalized using the preprocessCore package in R. Genes with LFC ≥ 1.5 and less than or equal to –1.5 and a Benjamini–Hochberg adjusted P value < 0.01 in DESeq2 Wald test were considered significant for differential expression. For the analysis of AtPDR genes expression in multiple stress conditions, total read count per gene were retrieved from the NCBI GEO repository (accession numbers GSE66290, GSE83478, GSE104590, GSE70094, GSE116269, GSE90075, GSE72548, GSE56922, and GSE72806), and differential gene expression analysis performed in DESeq2 as described above.
Orthologous Gene Groups Analyses
To define groups of orthologous genes, the complete proteomes of the six species were used in a Markov clustering analysis through OrthoMCL version 1.4 via the Family Companion server (Cottret et al., 2018). Parameters were set to pi_cutoff = 0, pv_cutoff = 0.00001, pmatch_cutoff = 80, and inflation = 1.5. Genes not expressed in our RNA-seq data (read count = 0) were then discarded from orthogroups. Orthogroups including at least one expressed gene from each of the six plant species were designated as core Pentapetalae groups/genes. Orthogroups including one or more expressed gene from two to five plant species were designated as other orthologous groups/genes. Orthogroups including expressed genes from one single species together with expressed genes not included in orthogroups were designated as lineage specific. The largest 100 orthogroups were manually inspected for consistency in protein sequence, GO, and Pfam annotation. R scripts used for the generation of figures in this manuscript are provided in Supplemental File 6.
Gene Annotation and Enrichment Analyses
GO annotations for Arabidopsis and S. lycopersicum were obtained from the Ensembl Plant database release 37 (Kersey et al., 2018). GO annotations were mapped onto complete plant proteomes of H. annuus, P. vulgaris, B. vulgaris, and R. communis using the Blast2GO 5.2.5 (Conesa et al., 2005). Pfam domain annotations were obtained using the hmmscan function of the HMMER version 3.1b2 software against the Pfam31.0 database (Schaeffer et al., 2017). For enrichment analyses, the number of DEGs and genes not differentially expressed was summed across the six species for each GO and Pfam. Several occurrences of a same Pfam domain within a gene were counted once. GOs were mapped onto Pfam domains using the PFAM2GO file version 2019/12/14 provided by Interpro. Counts were used in a chi-squared test performed with R 3.5.0, and P values were adjusted with Bonferroni correction for multiple testing. Enrichment fold was defined as the ratio between proportion of DEGs in genes harboring a given GO or Pfam over the overall proportion of DEGs among expressed genes in each species. Annotations harboring enrichment fold > 1 and adjusted P value < 0.01 were considered significantly enriched in DEGs. Annotations harboring enrichment fold <1 and adjusted P value < 0.01 were considered significantly depleted in DEGs. GO network rendering was performed using the BiNGO plugin in Cytoscape 3.6.1. To visualize enrichment across all species for the complete GO network, the following parameters were used: statistical test—, significance level: 1.00, categories to be visualized: All categories, reference set: Use whole annotation as reference set, select ontology file: GO_Full, select organism/annotation: custom. As a custom annotation file, a list of 143,807 GO annotations from DEGs of the six species, including 6962 distinct GO terms, was provided (Supplemental File 7). Using the ‘Import table from file’ option, an attribute table containing DEG enrichment score (–log of adjusted P value from chi-squared test), up/down ratio, and number of species with upregulated genes for each of 6962 GO (Supplemental File 8) was mapped onto the full GO network. Rank in the GO hierarchy were determined using the GOMFANCESTOR, GOCCANCESTOR, and GOBPANCESTOR functions from the GO.db package version 3.8 from BiocManager in R 3.5.0. Violin plot rendering was performed using the easyGgplot2 package in R 3.4.0.
Bootstrapping and Expression Consistency Analyses
Bootstrapping and consistency analysis was performed using homemade scripts in R 3.5.0, provided in Supplemental Files 3 and 4. To simulate distributions of upregulated genes among core orthogroups, a total of 9520 upregulated gene states and 53,318 not upregulated gene states were shuffled among 7918 core orthogroups containing at least one DEG. Orthogroups in this simulation had the same number of genes from each species as experimentally determined core orthogroups. For each orthogroup, a minimal gene count is determined, corresponding to the number of genes for the species with the least genes in the group. Gene states are drawn first for minimal gene counts in each orthogroup, with h species assigned the same draw. The remaining gene states are drawn randomly for each species in each orthogroup. The percentage of orthogroups featuring zero to six species with at least one upregulated gene state was calculated of 100 bootstrap replicates. The procedure was repeated using 13,201 downregulated gene states and 49,637 not downregulated gene states. Residual sum of squares was calculated using the ssq function of the hydroGOF package in R 3.4.0. The CEI was computed on the basis of the number of clusters of core ortholog genes with similar expression. The number of clusters was computed by silhouette analysis (Rousseeuw, 1987) performed by the factoextra R library (Charrad et al., 2014). The index of conservation expression was then computed as the difference between the proportion of genes in the largest cluster and the proportion associated to one gene of the cluster. By construction, an index of conservation of expression equal to 0 indicates a group composed by genes with independent level of expression. An index tending toward 1 indicates a unique pattern of genes expression.
Molecular and Phenotypic Characterization of abcg40 Mutants
Arabidopsis T-DNA mutant lines SALK_005635 (abcg40-2), SAIL_885_E09 (abcg40-3), SALK_148565 (abcg40-4), and SALK_013945 (abcg40-5) were obtained from the Nottingham Arabidopsis Stock Centre. Plants were grown as described above. Homozygous T-DNA insertions were verified by PCR performed with primers given in Supplemental Data Set 1.33. The accumulation of ABCG40 transcripts in abcg40 mutants was assessed by reverse transcription quantitative PCR performed on a LightCycler480 device (Roche) following the manufacturer’s recommendation. RNA extraction, cDNA synthesis, and quantitative PCR reactions were performed as described in Badet et al. (2015) using primers given in Supplemental Data Set 1.33 and the At2g28390 gene as a housekeeping reference. A strain of S. sclerotiorum 1980 expressing GFP fused to the endogenous OAH1 gene (Badet et al., 2017) was inoculated on 4-week-old plants as described above. Lesions were imaged 24 h later with a Zeiss Axio Zoom V16 microscope under bright light and fluorescent illumination. Lesion areas were determined using the ImageJ 1.51k software. For this, fully developed lesions that have not reached the leaves borders were manually set as regions of interest. The area of the agar plug used for inoculation served as reference to normalize lesion measurements across images. Statistical analyses of disease phenotypes were performed in R via type III two-way ANOVA for unbalanced designs, and significance of the difference between genotype was assessed using Student’s t test with Bonferroni correction for multiple testing since no experiment replicate effect was detected.
Accession Numbers
Sequencing data generated in this work has been deposited in the NCBI’s GEO and are accessible through GEO series accession number GSE138039.
SUPPLEMENTAL DATA
Supplemental Figure 1. Species distribution of genes upregulated upon inoculation by S. sclerotiorum according to their Pfam domain annotation.
Supplemental Figure 2. Species distribution of genes downregulated upon inoculation by S. sclerotiorum according to their Pfam domain annotation.
Supplemental Methods. Supplemental materials and methods.
Supplemental Data Set 1. Includes Supplemental Data Sets 1.1 to 1.33.
Supplemental Data Set 1.1. List of reference genomes and annotations used for mapping RNA-seq reads and calling gene expression. (Related to Figure 1A)
Supplemental Data Set 1.2. Summary of read mappings for the 36 sequenced libraries. (Related to Figure 1B)
Supplemental Data Set 1.3. Summary of gene expression analysis per species. (Related to Figure 1C)
Supplemental Data Set 1.4. Log fold change, differential expression analysis and orthogroup assignment for Arabidopsis thaliana genes. (Related to Figure 1D)
Supplemental Data Set 1.5. Log fold change, differential expression analysis and orthogroup assignment for Phaseolus vulgaris genes. (Related to Figure 1D)
Supplemental Data Set 1.6. Log fold change, differential expression analysis and orthogroup assignment for Beta vulgaris genes. (Related to Figure 1D)
Supplemental Data Set 1.7. Log fold change, differential expression analysis and orthogroup assignment for Ricinus communis genes. (Related to Figure 1D)
Supplemental Data Set 1.8. Log fold change, differential expression analysis and orthogroup assignment for Helianthus annuus genes. (Related to Figure 1D)
Supplemental Data Set 1.9. Log fold change, differential expression analysis and orthogroup assignment for Solanum lycopersicum genes. (Related to Figure 1D)
Supplemental Data Set 1.10. List of genes assigned to orthogroups. (Related to Figure 2A)
Supplemental Data Set 1.11. Number of species, of expressed genes and DEGs per orthogoup. (Related to Figure 2A)
Supplemental Data Set 1.12. Distribution of orthogroups per species. (Related to Figure 2B)
Supplemental Data Set 1.13. Complete statistical analysis for enrichment in DEGs among core, ortho and lineage-specific genes. (Related to Figure 2D and Table 1)
Supplemental Data Set 1.14. Parsimonious inference of orthogoups evolution. (Related to Figure 3A)
Supplemental Data Set 1.15. Analysis of rates of gene loss and gain over evolution. (Related to Figures 3B, 3C, and 3D)
Supplemental Data Set 1.16. Summary of gene ontology and Pfam mappings per species. (Related to Figure 4A)
Supplemental Data Set 1.17. Gene ontology mapping with chi-squared test for DEG enrichment. (Related to Figure 4A)
Supplemental Data Set 1.18. Pfam mapping with chi-squared test for DEG enrichment. (Related to Figure 4A)
Supplemental Data Set 1.19. (List of gene ontologies (Gos) and Pfams enriched with upregulated and downregulated genes identified using a chi-squared test on counts for each species (as presented in main text). (Related to Figures 4 and 5)
Supplemental Data Set 1.20. Summary table for GOs enriched in DEGs. (Related to Figure 5C)
Supplemental Data Set 1.21. Species distribution for orthogroups expressed in all 6 species. (Related to Figure 6A)
Supplemental Data Set 1.22. Species distribution for gene ontologies expressed in all 6 species. (Related to Figures 6B and 6E)
Supplemental Data Set 1.23. Species distribution for Pfams expressed in all 6 species. (Related to Figure 6C)
Supplemental Data Set 1.24. Enrichment coefficients in core genes and DEGs for 893 GO and species correlation analysis. (Related to Figures 6F and 6G)
Supplemental Data Set 1.25. Top100 GO enriched in DEGs in each species. (Related to Figures 6H and 6I)
Supplemental Data Set 1.26. Top100 GO depleted in DEGs in each species. (Related to Figure 6I)
Supplemental Data Set 1.27. Bootstrap analysis of the % of orthogroups including up/downreg. Genes per number of species. (Related to Figure 7A)
Supplemental Data Set 1.28. Analysis of gene expression consistency within orthogroups. (Related to Figure 7B)
Supplemental Data Set 1.29. Expression of members of the ABCG/PDR orthogroup no. 4 upon inoculation by S. sclerotiorum. (Related to Figure 8A)
Supplemental Data Set 1.30. Expression of Arabidopsis PDR genes in 12 stress conditions. (Related to Figure 8B)
Supplemental Data Set 1.31. Assessment of AtPDR12 gene expression in pdr12 mutants by RT-qPCR. (Related to Figure 8C)
Supplemental Data Set 1.32. Disease lesion area measured on Arabidopsis pdr12 mutants after S. sclerotiorum inoculation. (Related to Figure 8E)
Supplemental Data Set 1.33. List of oligonucleotide primers used in this work. (Related to Methods)
Supplemental File 1. Complete list of Gene ontology annotations used in this work. (Related to Figure 4)
Supplemental File 2. Complete list of Pfam annotations used in this work. (Related to Figure 4)
Supplemental File 3. Input data and homemade R script used for the bootstrap analysis of upregulated/downregulated genes distribution. (Related to Figure 7A)
Supplemental File 4. Homemade R script used for the analysis of LFC consistency within orthogroups. (Related to Figure 7B)
Supplemental File 5. (related to Figure 8A) Maximum likelihood tree of phylogenetic relationship between 96 ABCG/PDR genes in Newick format.
Supplemental File 6. R code used for the generation of figures and statistical analyses. (Related to Figures 1 to 8)
Supplemental File 7. List of GO annotations used as a reference for the construction of the full GO network. (Related to Methods)
Supplemental File 8. Attribute table used to visualize properties of GO annotation on the full GO network. (Related to Methods)
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Sébastien Carrère, Ludovic Legrand, and the BioInformatique, Biodiversité, Représentation et Intégration des Connaissances computational facilities for access and assistance with bioinformatics tools. This work was supported by European Research Council (grant ERC-StG 336808 Project VariWhim to S.R.) and the French Laboratory of Excellence Project TULIP (grants ANR-10-LABX-41 and ANR-11-IDEX-0002-02).
AUTHOR CONTRIBUTIONS
J.S., M.M., and S.R. conceived and designed the experiments. J.S., M.M., M.B., and A.D. performed the experiments. J.S., M.D., A.B., and S.R. analyzed the data. J.S. and S.R. wrote the article.
Footnotes
Articles can be viewed without a subscription.
References
- Albert I., et al. (2015). An RLP23-SOBIR1-BAK1 complex mediates NLP-triggered immunity. Nat. Plants 1: 15140. [DOI] [PubMed] [Google Scholar]
- Ando T., et al. (2018). Repeated inversions within a pannier intron drive diversification of intraspecific colour patterns of ladybird beetles. Nat. Commun. 9: 3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung K., Jiang Y., He S.Y.(2018). The role of water in plant-microbe interactions. Plant J. 93: 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badet T., Léger O., Barascud M., Voisin D., Sadon P., Vincent R., Le Ru A., Balagué C., Roby D., Raffaele S.(2019). Expression polymorphism at the ARPC4 locus links the actin cytoskeleton with quantitative disease resistance to Sclerotinia sclerotiorum in Arabidopsis thaliana. New Phytol. 222: 480–496. [DOI] [PubMed] [Google Scholar]
- Badet T., Peyraud R., Raffaele S.(2015). Common protein sequence signatures associate with Sclerotinia borealis lifestyle and secretion in fungal pathogens of the Sclerotiniaceae. Front Plant Sci 6: 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badet T., Voisin D., Mbengue M., Barascud M., Sucher J., Sadon P., Balagué C., Roby D., Raffaele S.(2017). Parallel evolution of the POQR prolyl oligo peptidase gene conferring plant quantitative disease resistance. PLoS Genet. 13: e1007143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacci A., Navaud O., Mbengue M., Barascud M., Godiard L., Khafif M., Lacaze A., Raffaele S.(2020). Rapid identification of an Arabidopsis NLR gene as a candidate conferring susceptibility to Sclerotinia sclerotiorum using time-resolved automated phenotyping. Plant J. Available at: https://onlinelibrary.wiley.com/doi/full/10.1111/tpj.14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco B., Kim Y., Clay N.K.(2019). Expansion of a core regulon by transposable elements promotes Arabidopsis chemical diversity and pathogen defense. Nat. Commun. 10: 3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton N.H., Etheridge A.M., Véber A.(2017). The infinitesimal model: Definition, derivation, and implications. Theor. Popul. Biol. 118: 50–73. [DOI] [PubMed] [Google Scholar]
- Bastien M., Sonah H., Belzile F.(2014). Genome wide association mapping of resistance in soybean with a genotyping-by-sequencing approach. Plant Genome 7: 1–13. [Google Scholar]
- Bender C.L., Alarcón-Chaidez F., Gross D.C.(1999). Pseudomonas syringae phytotoxins: Mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol. Mol. Biol. Rev. 63: 266–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton M.D., Thomma B.P.H.J., Nelson B.D.(2006). Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 7: 1–16. [DOI] [PubMed] [Google Scholar]
- Bonhomme M., et al. (2014). High-density genome-wide association mapping implicates an F-box encoding gene in Medicago truncatula resistance to Aphanomyces euteiches. New Phytol. 201: 1328–1342. [DOI] [PubMed] [Google Scholar]
- Boyle E.A., Li Y.I., Pritchard J.K.(2017). An expanded view of complex traits: From polygenic to omnigenic. Cell 169: 1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E.J., Schenk P.M., Kazan K., Penninckx I.A.M.A., Anderson J.P., Maclean D.J., Cammue B.P.A., Ebert P.R., Manners J.M., Plant T.(2003). Pathogen-responsive expression of a putative ATP-binding cassette transporter gene conferring resistance to the diterpenoid sclareol is regulated by multiple defense signaling pathways in Arabidopsis. Plant Physiol 1: 1272–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanderbali A.S., Berger B.A., Howarth D.G., Soltis D.E., Soltis P.S.(2017). Evolution of floral diversity: Genomics, genes and gamma. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372: 20150509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrad M., Ghazzali N., Boiteau V., Niknafs A.(2014). NbClust: An R package for determining the relevant number of clusters in a data set. J. Stat. Softw. 61: 1–36. [Google Scholar]
- Conant G.C., Wolfe K.H.(2008). Turning a hobby into a job: How duplicated genes find new functions. Nat. Rev. Genet. 9: 938–950. [DOI] [PubMed] [Google Scholar]
- Conesa A., Götz S., García-Gómez J.M., Terol J., Talón M., Robles M.(2005). Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21: 3674–3676. [DOI] [PubMed] [Google Scholar]
- Cooke D.E.L., et al. (2012). Genome analyses of an aggressive and invasive lineage of the Irish potato famine pathogen. PLoS Pathog. 8: e1002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin J.A., Copeland D., Feusier J., Subedy A., Eshbaugh R., Palmer C., Maloof J., Kliebenstein D.J.(2016). The quantitative basis of the Arabidopsis innate immune system to endemic pathogens depends on pathogen genetics. PLoS Genet. 12: e1005789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin J.A., Kliebenstein D.J.(2017). Quantitative resistance: More than just perception of a pathogen. Plant Cell 29: 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottret L., Briand M., Rancurel C., Carrere S.(2018). Family-companion: Analyse, visualise, browse, query and share your homology clusters. bioRxiv 266742 Available at: 10.1101/266742. [DOI] [Google Scholar]
- Deeks M.J., Hussey P.J.(2005). Arp2/3 and SCAR: Plants move to the fore. Nat. Rev. Mol. Cell Biol. 6: 954–964. [DOI] [PubMed] [Google Scholar]
- Derbyshire M.C., Denton-Giles M.(2016). The control of sclerotinia stem rot on oilseed rape (Brassica napus): Current practices and future opportunities. Plant Pathol. 65: 859–877. [Google Scholar]
- Derbyshire M., et al. (2017). The complete genome sequence of the phytopathogenic fungus Sclerotinia sclerotiorum reveals insights into the genome architecture of broad host range pathogens. Genome Biol. Evol. 9: 593–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire M., Mbengue M., Barascud M., Navaud O., Raffaele S.(2019). Small RNAs from the plant pathogenic fungus Sclerotinia sclerotiorum highlight host candidate genes associated with quantitative disease resistance. Mol. Plant Pathol. 20: 1279–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Marais D.L., Rausher M.D.(2008). Escape from adaptive conflict after duplication in an anthocyanin pathway gene. Nature 454: 762–765. [DOI] [PubMed] [Google Scholar]
- Dodds P.N., Rathjen J.P.(2010). Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 11: 539–548. [DOI] [PubMed] [Google Scholar]
- Fernandes T.R., Segorbe D., Prusky D., Di Pietro A.(2017). How alkalinization drives fungal pathogenicity. PLoS Pathog. 13: e1006621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R.A.(1919). The correlation between relatives on the supposition of Mendelian inheritance. Trans. R. Soc. Edinb. 52: 399–433. [Google Scholar]
- Fordyce R.F., Soltis N.E., Caseys C., Gwinner R., Corwin J.A., Atwell S., Copeland D., Feusier J., Subedy A., Eshbaugh R., Kliebenstein D.J.(2018). Digital imaging combined with genome-wide association mapping links loci to plant-pathogen interaction traits. Plant Physiol. 178: 1406–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J., Kim H.U., Nakata P.A., Browse J.(2012). A previously unknown oxalyl-CoA synthetase is important for oxalate catabolism in Arabidopsis. Plant Cell 24: 1217–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusari C.M., et al. (2012). Association mapping in sunflower for sclerotinia head rot resistance. BMC Plant Biol. 12: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier M., et al. (2018). The genomic basis of color pattern polymorphism in the harlequin ladybird. Curr. Biol. 28: 3296–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S.J., Vrba E.S.(1982). Exaptation—A missing term in the science of form. Paleobiology 8: 4–15. [Google Scholar]
- Guo L., Cesari S., de Guillen K., Chalvon V., Mammri L., Ma M., Meusnier I., Bonnot F., Padilla A., Peng Y.-L., Liu J., Kroj T.(2018). Specific recognition of two MAX effectors by integrated HMA domains in plant immune receptors involves distinct binding surfaces. Proc. Natl. Acad. Sci. USA 115: 11637–11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Stotz H.U.(2007). Defense against Sclerotinia sclerotiorum in Arabidopsis is dependent on jasmonic acid, salicylic acid, and ethylene signaling. Mol. Plant Microbe Interact. 20: 1384–1395. [DOI] [PubMed] [Google Scholar]
- Guyon K., Balagué C., Roby D., Raffaele S.(2014). Secretome analysis reveals effector candidates associated with broad host range necrotrophy in the fungal plant pathogen Sclerotinia sclerotiorum. BMC Genomics 15: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacquard S., et al. (2016). Survival trade-offs in plant roots during colonization by closely related beneficial and pathogenic fungi. Nat. Commun. 7: 11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser F., Waadt R., Schroeder J.I.(2011). Evolution of abscisic acid synthesis and signaling mechanisms. Curr. Biol. 21: R346–R355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R., Hippauf F., Rohrbeck D., Haustein M., Wenke K., Feike J., Sorrelle N., Piechulla B., Barkman T.J.(2012). Enzyme functional evolution through improved catalysis of ancestrally nonpreferred substrates. Proc. Natl. Acad. Sci. USA 109: 2966–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.-U., et al. (2016). Plant ABC transporters enable many unique aspects of a terrestrial plant’s lifestyle. Mol. Plant 9: 338–355. [DOI] [PubMed] [Google Scholar]
- Kabbage M., Williams B., Dickman M.B.(2013). Cell death control: The interplay of apoptosis and autophagy in the pathogenicity of Sclerotinia sclerotiorum. PLoS Pathog. 9: e1003287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Hwang J.-U., Lee M., Kim Y.-Y., Assmann S.M., Martinoia E., Lee Y.(2010). PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc. Natl. Acad. Sci. USA 107: 2355–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersey P.J., et al. (2018). Ensembl Genomes 2018: An integrated omics infrastructure for non-vertebrate species. Nucleic Acids Res. 46 (D1): D802–D808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T.N., Schumann U., Smith N.A., Tiwari S., Au P.C.K., Zhu Q.H., Taylor J.M., Kazan K., Llewellyn D.J., Zhang R., Dennis E.S., Wang M.B.(2014). DNA demethylases target promoter transposable elements to positively regulate stress responsive genes in Arabidopsis. Genome Biol. 15: 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Stoeckert C.J. Jr., Roos D.S.(2003). OrthoMCL: Identification of ortholog groups for eukaryotic genomes. Genome Res. 13: 2178–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Li Y.I., Pritchard J.K.(2019). Trans effects on gene expression can drive omnigenic inheritance. Cell 177: 1022–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H.K., Prescott S.L., Wysocka J.(2016). Ever-changing landscapes: Transcriptional enhancers in development and evolution. Cell 167: 1170–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S.(2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinovsky F.G., Thomsen M.F., Nintemann S.J., Jagd L.M., Bourgine B., Burow M., Kliebenstein D.J.(2017). An evolutionarily young defense metabolite influences the root growth of plants via the ancient TOR signaling pathway. eLife 6: e29353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio T.A., et al. (2009). Finding the missing heritability of complex diseases. Nature 461: 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masachis S., Segorbe D., Turrà D., Leon-Ruiz M., Fürst U., El Ghalid M., Leonard G., López-Berges M.S., Richards T.A., Felix G., Di Pietro A.(2016). A fungal pathogen secretes plant alkalinizing peptides to increase infection. Nat. Microbiol. 1: 16043. [DOI] [PubMed] [Google Scholar]
- Mbengue M., Navaud O., Peyraud R., Barascud M., Badet T., Vincent R., Barbacci A., Raffaele S.(2016). Emerging trends in molecular interactions between plants and the broad host range fungal pathogens Botrytis cinerea and Sclerotinia sclerotiorum. Front Plant Sci 7: 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micic Z., Hahn V., Bauer E., Schön C.C., Knapp S.J., Tang S., Melchinger A.E.(2004). QTL mapping of Sclerotinia midstalk-rot resistance in sunflower. Theor. Appl. Genet. 109: 1474–1484. [DOI] [PubMed] [Google Scholar]
- Mine A., Sato M., Tsuda K.(2014). Toward a systems understanding of plant-microbe interactions. Front Plant Sci 5: 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mine A., Seyfferth C., Kracher B., Berens M.L., Becker D., Tsuda K.(2018). The defense phytohormone signaling network enables rapid, high-amplitude transcriptional reprogramming during effector-triggered immunity. Plant Cell 30: 1199–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondragón-Palomino M., Stam R., John-Arputharaj A., Dresselhaus T.(2017). Diversification of defensins and NLRs in Arabidopsis species by different evolutionary mechanisms. BMC Evol. Biol. 17: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M.J., Soltis P.S., Bell C.D., Burleigh J.G., Soltis D.E.(2010). Phylogenetic analysis of 83 plastid genes further resolves the early diversification of eudicots. Proc. Natl. Acad. Sci. USA 107: 4623–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaud O., Barbacci A., Taylor A., Clarkson J.P., Raffaele S.(2018). Shifts in diversification rates and host jump frequencies shaped the diversity of host range among Sclerotiniaceae fungal plant pathogens. Mol. Ecol. 27: 1309–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R.M., Pettersson M.E., Carlborg Ö.(2013). A century after Fisher: Time for a new paradigm in quantitative genetics. Trends Genet. 29: 669–676. [DOI] [PubMed] [Google Scholar]
- Palma-Guerrero J., Torriani S.F.F., Zala M., Carter D., Courbot M., Rudd J.J., McDonald B.A., Croll D.(2016). Comparative transcriptomic analyses of Zymoseptoria tritici strains show complex lifestyle transitions and intraspecific variability in transcription profiles. Mol. Plant Pathol. 17: 845–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchepied L., Balagué C., Riou C., Claudel-Renard C., Rivière N., Grezes-Besset B., Roby D.(2010). Nitric oxide participates in the complex interplay of defense-related signaling pathways controlling disease resistance to Sclerotinia sclerotiorum in Arabidopsis thaliana. Mol. Plant Microbe Interact. 23: 846–860. [DOI] [PubMed] [Google Scholar]
- Peyraud R., Dubiella U., Barbacci A., Genin S., Raffaele S., Roby D.(2017). Advances on plant-pathogen interactions from molecular toward systems biology perspectives. Plant J. 90: 720–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyraud R., Mbengue M., Barbacci A., Raffaele S.(2019). Intercellular cooperation in a fungal plant pathogen facilitates host colonization. Proc. Natl. Acad. Sci. USA 116: 3193–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatigorsky J., O’Brien W.E., Norman B.L., Kalumuck K., Wistow G.J., Borras T., Nickerson J.M., Wawrousek E.F.(1988). Gene sharing by delta-crystallin and argininosuccinate lyase. Proc. Natl. Acad. Sci. USA 85: 3479–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland J.A., Balint-Kurti P.J., Wisser R.J., Pratt R.C., Nelson R.J.(2009). Shades of gray: The world of quantitative disease resistance. Trends Plant Sci. 14: 21–29. [DOI] [PubMed] [Google Scholar]
- Poland J.A., Bradbury P.J., Buckler E.S., Nelson R.J.(2011). Genome-wide nested association mapping of quantitative resistance to northern leaf blight in maize. Proc. Natl. Acad. Sci. USA 108: 6893–6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J.J., Carere J., Sablok G., Fitzgerald T.L., Stiller J., Colgrave M.L., Gardiner D.M., Manners J.M., Vogel J.P., Henry R.J., Kazan K.(2017). Transcriptome analysis of Brachypodium during fungal pathogen infection reveals both shared and distinct defense responses with wheat. Sci. Rep. 7: 17212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaele S., Kamoun S.(2012). Genome evolution in filamentous plant pathogens: Why bigger can be better. Nat. Rev. Microbiol. 10: 417–430. [DOI] [PubMed] [Google Scholar]
- Ramirez-Prado J.S., Abulfaraj A.A., Rayapuram N., Benhamed M., Hirt H.(2018). Plant immunity: From signaling to epigenetic control of defense. Trends Plant Sci. 23: 833–844. [DOI] [PubMed] [Google Scholar]
- Romero I.G., Ruvinsky I., Gilad Y.(2012). Comparative studies of gene expression and the evolution of gene regulation. Nat. Rev. Genet. 13: 505–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseeuw P.J.(1987). Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 20: 53–65. [Google Scholar]
- Roux F., Voisin D., Badet T., Balagué C., Barlet X., Huard-Chauveau C., Roby D., Raffaele S.(2014). Resistance to phytopathogens e tutti quanti: Placing plant quantitative disease resistance on the map. Mol. Plant Pathol. 15: 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savory F.R., Milner D.S., Miles D.C., Richards T.A.(2018). Ancestral function and diversification of a horizontally acquired oomycete carboxylic acid transporter. Mol. Biol. Evol. 35: 1887–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer R.D., Liao Y., Cheng H., Grishin N.V.(2017). ECOD: New developments in the evolutionary classification of domains. Nucleic Acids Res. 45 (D1): D296–D302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smakowska-Luzan E., et al. (2018). An extracellular network of Arabidopsis leucine-rich repeat receptor kinases. Nature 553: 342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis N.E., Atwell S., Shi G., Fordyce R., Gwinner R., Gao D., Shafi A., Kliebenstein D.J.(2019). Interactions of tomato and Botrytis cinerea genetic diversity: Parsing the contributions of host differentiation, domestication, and pathogen variation. Plant Cell 31: 502–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam R., Silva-Arias G.A., Tellier A.(2019). Subsets of NLR genes show differential signatures of adaptation during colonization of new habitats. New Phytol. 224: 367–379. [DOI] [PubMed] [Google Scholar]
- Stern D.L., Orgogozo V.(2008). The loci of evolution: How predictable is genetic evolution? Evolution 62: 2155–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D.L., Orgogozo V.(2009). Is genetic evolution predictable? Science 323: 746–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz H.U., Sawada Y., Shimada Y., Hirai M.Y., Sasaki E., Krischke M., Brown P.D., Saito K., Kamiya Y.(2011). Role of camalexin, indole glucosinolates, and side chain modification of glucosinolate-derived isothiocyanates in defense of Arabidopsis against Sclerotinia sclerotiorum. Plant J. 67: 81–93. [DOI] [PubMed] [Google Scholar]
- Studer A., Zhao Q., Ross-Ibarra J., Doebley J.(2011). Identification of a functional transposon insertion in the maize domestication gene tb1. Nat. Genet. 43: 1160–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., et al. (2016). ABA is required for plant acclimation to a combination of salt and heat stress. PLoS One 11: e0147625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli M.(2017). Commentary: Fisher’s infinitesimal model: A story for the ages. Theor. Popul. Biol. 118: 46–49. [DOI] [PubMed] [Google Scholar]
- Van Valen L.(1973). A new evolutionary law. Evol. Theory 1: 1–30. [Google Scholar]
- Vlad D., et al. (2014). Leaf shape evolution through duplication, regulatory diversification and loss of a homeobox gene. Science 343: 780–783. [DOI] [PubMed] [Google Scholar]
- Weiberg A., Wang M., Lin F.-M.M., Zhao H., Zhang Z., Kaloshian I., Huang H.D., Jin H.(2013). Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 342: 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Weyer A.-L., Monteiro F., Furzer O.J., Nishimura M.T., Cevik V., Witek K., Jones J.D.G., Dangl J.L., Weigel D., Bemm F.(2019). A species-wide inventory of NLR genes and alleles in Arabidopsis thaliana. Cell 178: 1260–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R.A., Talbot N.J.(2009). Under pressure: Investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 7: 185–195. [DOI] [PubMed] [Google Scholar]
- Wray G.A.(2007). The evolutionary significance of cis-regulatory mutations. Nat. Rev. Genet. 8: 206–216. [DOI] [PubMed] [Google Scholar]
- Wray N.R., Wijmenga C., Sullivan P.F., Yang J., Visscher P.M.(2018). Common disease is more complex than implied by the core gene omnigenic model. Cell 173: 1573–1580. [DOI] [PubMed] [Google Scholar]
- Wu C.-H., Derevnina L., Kamoun S.(2018). Receptor networks underpin plant immunity. Science 360: 1300–1301. [DOI] [PubMed] [Google Scholar]
- Wu J., Cai G., Tu J., Li L., Liu S., Luo X., Zhou L., Fan C., Zhou Y.(2013). Identification of QTLs for resistance to sclerotinia stem rot and BnaC.IGMT5.a as a candidate gene of the major resistant QTL SRC6 in Brassica napus. PLoS One 8: e67740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X.-F., Nomura K., Aung K., Velásquez A.C., Yao J., Boutrot F., Chang J.H., Zipfel C., He S.Y.(2016). Bacteria establish an aqueous living space in plants crucial for virulence. Nature 539: 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Xiang M., White D., Chen W.(2015). pH dependency of sclerotial development and pathogenicity revealed by using genetically defined oxalate-minus mutants of Sclerotinia sclerotiorum. Environ. Microbiol. 17: 2896–2909. [DOI] [PubMed] [Google Scholar]
- Yu A., Lepère G., Jay F., Wang J., Bapaume L., Wang Y., Abraham A.L., Penterman J., Fischer R.L., Voinnet O., Navarro L.(2013). Dynamics and biological relevance of DNA demethylation in Arabidopsis antibacterial defense. Proc. Natl. Acad. Sci. USA 110: 2389–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L., Zhang N., Zhang Q., Endress P.K., Huang J., Ma H., Zhuang G.(2017). Resolution of deep eudicot phylogeny and their temporal diversification using nuclear genes from transcriptomic and genomic datasets. New Phytol. 214: 1338–1354. [DOI] [PubMed] [Google Scholar]
- Zhang W., Corwin J.A., Copeland D., Feusier J., Eshbaugh R., Chen F., Atwell S., Kliebenstein D.J.(2017). Plastic transcriptomes stabilize immunity to pathogen diversity: The jasmonic acid and salicylic acid networks within the Arabidopsis/Botrytis pathosystem. Plant Cell 29: 2727–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Fraiture M., Kolb D., Löffelhardt B., Desaki Y., Boutrot F.F.G., Tör M., Zipfel C., Gust A.A., Brunner F.(2013). Arabidopsis receptor-like protein30 and receptor-like kinase suppressor of BIR1-1/EVERSHED mediate innate immunity to necrotrophic fungi. Plant Cell 25: 4227–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Buchwaldt L., Rimmer S.R., Sharpe A., McGregor L., Bekkaoui D., Hegedus D.(2009). Patterns of differential gene expression in Brassica napus cultivars infected with Sclerotinia sclerotiorum. Mol. Plant Pathol. 10: 635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Zeng L., Liu J., Xing D.(2015). Manipulation of the xanthophyll cycle increases plant susceptibility to Sclerotinia sclerotiorum. PLoS Pathog. 11: e1004878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Züst T., Heichinger C., Grossniklaus U., Harrington R., Kliebenstein D.J., Turnbull L.A.(2012). Natural enemies drive geographic variation in plant defenses. Science 338: 116–119. [DOI] [PubMed] [Google Scholar]