The activity of the blue light photoreceptor phot1 for inducing phototropic responses under variable light intensities is modulated via the inhibitory and light-inducible protein RPT2.
Abstract
The Arabidopsis (Arabidopsis thaliana) blue light photoreceptor phototropin1 (phot1) is a blue light–activated Ser/Thr protein kinase that mediates various light responses, including phototropism. The function of phot1 in hypocotyl phototropism is dependent on the light induction of ROOT PHOTOTROPISM2 (RPT2) proteins within a broad range of blue light intensities. It is not yet known however how RPT2 contributes to the photosensory adaptation of phot1 to high intensity blue light and the phototropic responses under bright light conditions. We show that RPT2 suppresses the activity of phot1 and demonstrate that RPT2 binds to the PHOT1 light, oxygen or voltage sensing1 (LOV1) domain that is required for its high photosensitivity. Our biochemical analyses revealed that RPT2 inhibits autophosphorylation of phot1, suggesting that it suppresses the photosensitivity and/or kinase activity of phot1 through the inhibition of LOV1 function. We found that RPT2 proteins are degraded via a ubiquitin-proteasome pathway when phot1 is inactive and are stabilized under blue light in a phot1-dependent manner. We propose that RPT2 is a molecular rheostat that maintains a moderate activation level of phot1 under any light intensity conditions.
INTRODUCTION
Plant life is strongly dependent on light and in order to effectively adapt to various light conditions, Arabidopsis (Arabidopsis thaliana) requires several kinds of photoreceptors: phytochromes, which are red/far-red-light photoreceptors; cryptochromes; phototropins; the light, oxygen or voltage sensing (LOV)/F-box/Kelch-domain proteins (ZTL, FKF1, and LKP2), which are blue light photoreceptors; and a UV photoreceptor, UVR8 (de Wit et al., 2016). In the case of the phytochrome and cryptochrome families, phyA and cry2 are highly expressed in Arabidopsis seedlings under dark conditions and become unstable under bright light conditions, thus contributing strongly to the responses to weak, but not bright, light conditions (Clough and Vierstra, 1997; Lin et al., 1998; Sharrock and Clack, 2002; Casal et al., 2014). By contrast, phyB and cry1 are stable and function as major photoreceptors under bright light conditions (Lin et al., 1998; Li et al., 2011). Plants therefore use different photoreceptor families and members of these families to recognize light quality and quantity in order to adapt to their various light environments.
In the phototropin family, the highly photosensitive photoreceptor phot1 and the less photosensitive photoreceptor phot2 function redundantly in a fluence rate–dependent manner (Sakai et al., 2001). For members of this family, we use the nomenclature suggested by Briggs et al. (2001), in which the wild-type genes are designated PHOT1 and PHOT2, the apoproteins lacking the chromophores are designated PHOT1 and PHOT2, and the photoreactive holoproteins containing the functional chromophores are designated phot1 and phot2. The phototropins have LOV1 and LOV2 domains in their N-terminal portions and a Ser/Thr kinase domain belonging to the cAMP-dependent protein kinase A, cGMP-dependent protein kinase G, and phospholipid-dependent protein kinase C VIII kinase domain in their C-terminal half (Christie, 2007; Rademacher and Offringa., 2012). The phototropins localize on the inner surface of the plasma membrane, show autophosphorylation activities under blue light conditions, and mediate blue light responses such as phototropism, chloroplast photorelocation, and stomatal opening (Christie et al., 1998; Kagawa et al., 2001; Kinoshita et al., 2001; Sakai et al., 2001; Sakamoto and Briggs, 2002). Each LOV domain contains flavin mononucleotide (FMN) as a blue light–absorbing chromophore (Christie et al., 1998; Sakai et al., 2001) and transiently forms a cysteinyl adduct with a blue light–excited FMN (Christie, 2007). This cysteinyl adduct undergoes thermal decay and LOV domains thus show dark reversion (Okajima, 2016). The photochemical reaction mediated through the LOV2 domain is indispensable for phototropin function and leads to their conformational change and activation as a Ser/Thr kinase (Christie et al., 2002; Christie, 2007). The lifetime of the cysteinyl-FMN adduct of phot2 LOV2 is 10-fold greater than that of phot1, which appears to be one of the reasons why phot2 does not function under low intensity blue light conditions (Okajima et al., 2012). By contrast, phot1 is a unique photoreceptor that mediates the phototropic responses in etiolated hypocotyls over a broad dynamic range of blue light intensities between 10−5 and 102 µmol m–2 s–1 (Sakai et al., 2001; Haga et al., 2015). The photochemical reaction at the LOV1 of phot1 is unnecessary for the phototropic response (Christie et al., 2002), but this domain itself is necessary for the induction of the phototropic responses under low intensity blue light conditions (Sullivan et al., 2008). The phot1 LOV1 domain thus appears to play an important role in the control of phot1 photosensitivity, but the precise underlying molecular functions are yet unrevealed.
The ROOT PHOTOTROPISM2 (RPT2) protein is a signal transducer in Arabidopsis phototropism (Sakai et al., 2000). It localizes on the plasma membrane and forms a complex with phot1 in vivo (Inada et al., 2004). RPT2 expression is suppressed in etiolated seedlings and upregulated by red and/or blue light irradiation (Sakai et al., 2000; Tsuchida-Mayama et al., 2010). The phytochromes and cryptochromes are necessary for the induction of RPT2 transcription (Tsuchida-Mayama et al., 2010). Rpt2 loss-of-function mutants exhibit increased responses to very low intensity blue light at 10−5 µmol m–2 s–1 and decreased responses to blue light at 10−3 µmol m–2 s–1 or more during hypocotyl phototropism (Haga et al., 2015). By contrast, the expression of RPT2 prior to a phototropic stimulation in etiolated wild-type seedlings accelerates continuous light-induced phototropism (Haga et al., 2015). These findings have suggested that the light induction of RPT2 expression reduces the photosensitivity of phot1, which is required for the photosensory adaptation of phot1 and the phototropic responses under bright light conditions (Haga et al., 2015).
In this study, we tested the hypothesis that RPT2 controls the photosensitivity of phot1 through its LOV1 domain. A yeast two-hybrid assay indicated that RPT2 binds to the PHOT1 LOV1 domain, and immunoblotting using Phos-tag SDS-polyacrylamide gels indicated that a rpt2 mutation enhances the autophosphorylation of phot1 and that the RPT2 overexpression suppresses this autophosphorylation. These data indicated that RPT2 controls the autophosphorylation activity of phot1 through the LOV1 domain. We further showed that RPT2 expression is upregulated not only by phytochromes and cryptochromes but also by phototropins. Based on our current results, we propose that RPT2 acts as a molecular rheostat that maintains a moderate activation of phot1 under any light intensity conditions.
RESULTS
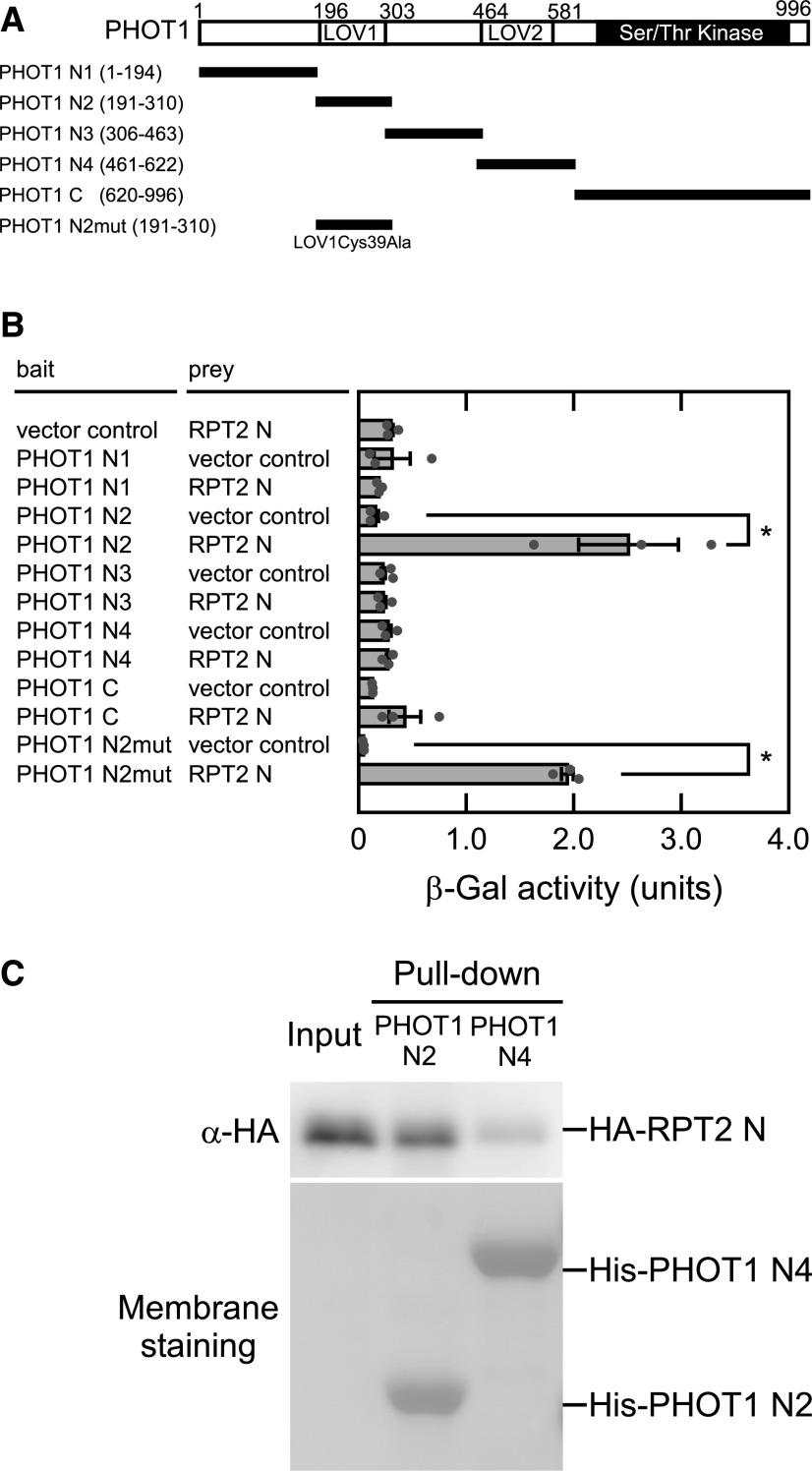
RPT2 Binds to the LOV1 Domains of Phot1
Our previous study demonstrated that the N-terminal half of RPT2 (RPT2 N), including the broad complex, tramtrack and bric-à-brac/Pox virus and zinc finger (BTB/POZ) protein–protein interaction domain, binds to the N-terminal half of PHOT1 that includes two LOV domains (Inada et al., 2004). We divided the N-terminal half of PHOT1 into four fragments and examined RPT2 N binding to these fragments or its kinase domain (PHOT1 C) using a yeast two-hybrid assay (Figure 1A; Inada et al., 2004). As shown in Figure 1B, RPT2 N bound only to the LOV1 domain (PHOT1 N2). We confirmed its binding using an in vitro pull-down assay. The hemagglutinin-tagged (HA-) RPT2 N proteins specifically interacted with the His/ProS2-tagged (His-) PHOT1 N2 proteins on metal affinity resins in contrast with His-PHOT1 N4 harboring the LOV2 domain (Figure 1C). These results suggest that RPT2 affects phot1 photosensitivity via the LOV1 domain.
Figure 1.
Interaction between PHOT1 and RPT2.
(A) PHOT1 structure. The Ser/Thr protein kinase and LOV domains are denoted by the solid and dotted blocks, respectively. The amino acid residues contained in each construct are indicated in parentheses.
(B) Yeast two-hybrid assay of the PHOT1–RPT2 interaction. β-Galactosidase assays were performed for the bait/prey combinations indicated on the left. The data on the right show mean values ± se (n = 3 experiments). Dots on the bar graphs represent the results of individual experiments. The asterisks denote statistically significant differences compared with the vector control (two-tailed Student’s t test, P < 0.05). See the Supplemental File for details of all statistical analyses.
(C) In vitro pull-down assay to verify the interaction between the LOV domains of PHOT1 and the N-terminal half of RPT2. HA-tagged proteins representing the N-terminal half of RPT2 (HA-RPT2 N) were incubated with the His-tagged LOV1 or LOV2 domains of PHOT1 for the His-tag pull-down assay and detected by immunoblotting using anti-HA antibodies. HA-RPT2 N proteins without pull-down were also electrophoresed as a control (input). The PVDF membrane was stained using a Pierce reversible protein staining kit.
Christie et al. (2002) reported previously that by using the LOV1Cys39Ala mutant, the LOV1 domain of phot1 is photochemically active but that its photochemical reaction is unessential for phot1 function. Our current yeast two-hybrid assay data showed that the LOV1 domain harboring a Cys39Ala mutation (PHOT1 N2mut) also binds to RPT2 N as well as PHOT1 N2 (Figure 1B). Furthermore, the phot1 phot2 transgenic seedlings expressing PHOT1 with the LOV1Cys39Ala mutation showed a shortened time lag to the induction of phototropic responses by a red light pretreatment (Supplemental Figure 1), which is dependent on RPT2 (Haga et al., 2015). Our previous study had already indicated that RPT2 can form a complex with phot1 in vivo under both conditions of darkness and blue light (Inada et al., 2004). These results suggest that RPT2 binding and function are unaffected by the photochemical reaction of the phot1 LOV1 domain and the phosphorylation state of phot1.
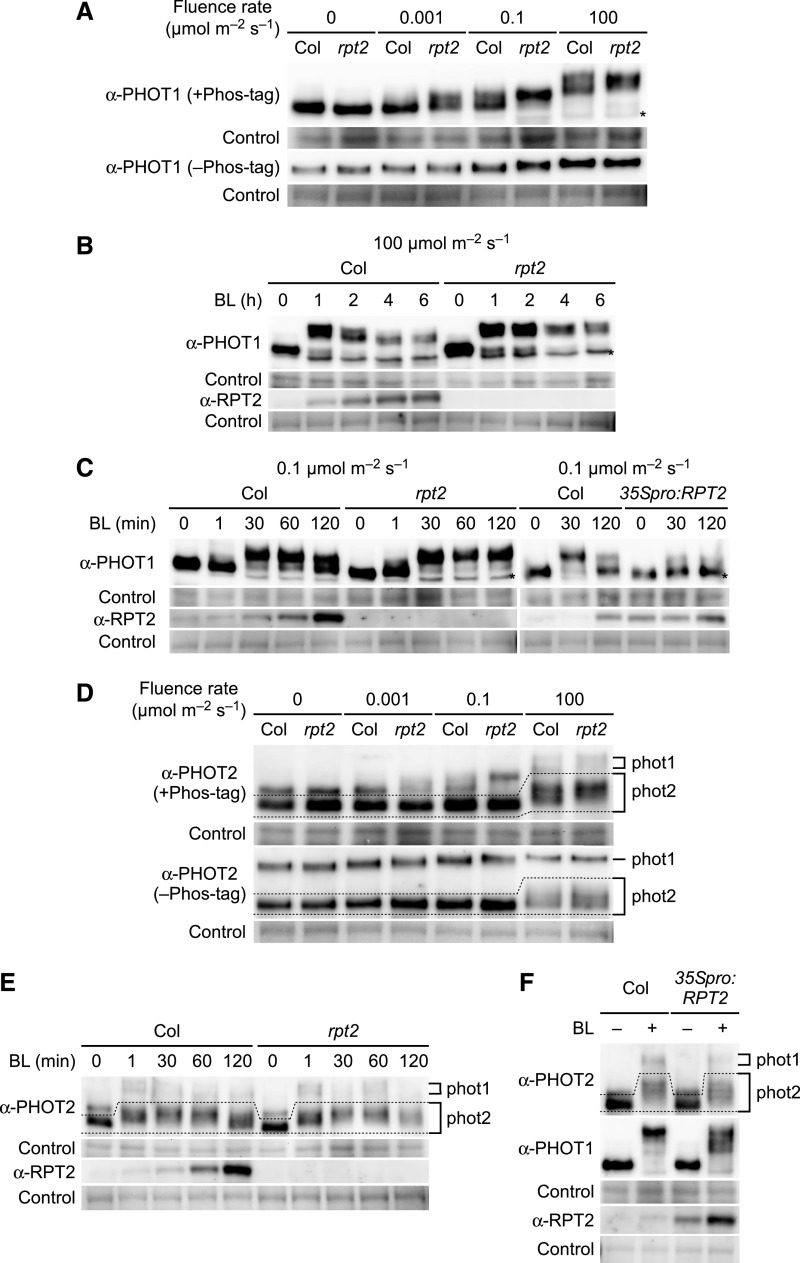
RPT2 Suppresses Autophosphorylation by Phot1
Our previous genetic study suggested that RPT2 reduces the photosensitivity of phot1, which is required for the phototropic responses under bright light conditions (Haga et al., 2015). This indicated that RPT2 may suppress the activity of phot1. To test this possibility, we examined the autophosphorylation pattern of phot1 in both the wild-type Arabidopsis seedlings and the rpt2 mutants grown on the surface of vertically oriented agar medium. For this experiment, we conducted immunoblotting analysis using a Phos-tag acrylamide gel (Kinoshita and Kinoshita-Kikuta, 2011) in which the migration of phosphorylated proteins is specifically retarded by the Phos-tag. We observed autophosphorylation patterns of phot1 in response to blue light irradiation for 2 h at 0.001, 0.1, and 100 µmol m–2 s–1 (Figure 2A). When the wild-type seedlings were irradiated, the mobilities of PHOT1 proteins were much lower if the fluence rates were higher (Figure 2A). These mobility shifts were more clearly observed using a Phos-tag acrylamide gel (+Phos-tag) compared with a normal SDS-polyacrylamide gel (–Phos-tag; Figure 2A). This result suggested that the autophosphorylation activity of phot1 increases as the fluence rates increase.
Figure 2.
RPT2 Suppresses the Autophosphorylation of phot1.
(A) Immunoblot analysis of PHOT1 in the wild type (Col) and rpt2 mutant Arabidopsis. Two-day-old etiolated seedlings were irradiated for 2 h with unilateral blue light at the indicated fluence rates. Total proteins (20 µg) extracted from the seedlings were separated on 6% SDS-PAGE gels with 2 µM Phos-tag (+Phos-tag), followed by immunoblotting with anti-PHOT1 antibodies. Total protein (10 µg) was separated on 6% SDS-PAGE gels without Phos-tag (–Phos-tag) for comparison. The asterisk indicates a nonspecific band. The PVDF membranes were stained with Pierce reversible protein staining kit as a loading control.
(B) and (C) Time-course analysis of phot1 autophosphorylation. Two-day-old etiolated seedlings were irradiated with unilateral blue light (BL) at 100 µmol m–2 s–1 in (B) or at 0.1 µmol m–2 s–1 in (C) for the indicated times. Other details were as described in (A).
(D) Immunoblotting analysis of PHOT2 in the wild type (Col) and rpt2 mutant. Immunoblotting was performed using an anti-PHOT2 antibody. Other details were as in (A).
(E) Time-course analysis of phot2 autophosphorylation. Two-day-old etiolated seedlings were irradiated with unilateral blue light (BL) at 100 µmol m–2 s–1 for the indicated times. Immunoblotting was performed with anti-PHOT2 and anti-RPT2 antibodies. Other details are as described in (A).
(F) Autophosphorylation of phot1 and phot2 in the rpt2 mutants transformed with a 35Spro:RPT2 gene (35Spro:RPT2). Two-day-old etiolated seedlings were irradiated with unilateral blue light (BL) at 100 µmol m–2 s–1 for 30 min. Immunoblotting was performed with anti-PHOT1, anti-PHOT2, and anti-RPT2 antibodies. Other details were as described in (A).
We next investigated the autophosphorylation pattern of phot1 during blue light irradiation. When we monitored the phosphorylation status of the PHOT1 protein under blue light conditions at 100 µmol m–2 s–1 (Figure 2B), mobility shifts of this protein were detectable at 1 h, but further irradiation suppressed phot1 autophosphorylation in parallel with the accumulation of RPT2 proteins. Under blue light conditions at 0.1 µmol m–2 s–1, mobility shifts in the PHOT1 proteins were marginally detectable at 1 min and became saturated at 30 min, but they were suppressed at 60 and 120 min after the onset of irradiation in the wild-type etiolated seedlings (Figure 2C).
The Arabidopsis wild-type hypocotyls show a delayed phototropic response when grown along the surface of vertically oriented agar medium and a quick response when grown on the agar medium without touching the agar medium (Haga and Sakai, 2012; Sullivan et al., 2019). We examined the autophosphorylation of phot1 in the wild-type Arabidopsis seedlings grown on agar medium in 0.2-mL tubes (Haga and Sakai, 2012). They showed similar phot1 autophosphorylation patterns to those in the seedlings grown on vertically oriented agar medium (Supplemental Figure 2), suggesting that the phot1 autophosphorylation activity in seedlings is unaffected by the friction or the moisture between the agar surface and the shoots. We therefore used the seedlings grown on the surface of vertically oriented agar medium in later analyses.
In the –Phos-tag gel, the rpt2 mutation did not produce any obvious effect on the mobility shifts of the PHOT1 proteins (Figure 2A), as previously reported by Inada et al. (2004) and Haga et al. (2015). However, in the +Phos-tag gel, the rpt2 mutation appeared to cause a pronounced mobility shift in the PHOT1 protein under blue light conditions at 0.001, 0.1, and 100 µmol m–2 s–1 (Figure 2A). The mobility shift of PHOT1 with the rpt2 mutation disappeared when the extracted proteins were treated with alkaline phosphatase (Supplemental Figure 3), indicating that this shift reflects differences in phosphorylation state of PHOT1. Interestingly, the rprt2 mutants did not exhibit any attenuation of phot1 autophosphorylation at 2, 4, and 6 h (Figure 2B) or at 60 and 120 min (Figure 2C) after the onset of blue light irradiation of 100 and 0.1 µmol m–2 s–1, respectively. By contrast, a constitutive RPT2 expression line (35Spro:RPT2; Haga et al., 2015) showed an attenuation of phot1 autophosphorylation at 30 min after the onset of the blue light irradiation (Figure 2C). These results suggested that RPT2 proteins can suppress the autophosphorylation or enhance the dephosphorylation of phot1 and that a loss-of-function mutation in RPT2 leads to a continuous hyperactivation of phot1 in seedlings.
The effect of RPT2 on the autophosphorylation of phot2 was also examined using an anti-PHOT2 antibody, which recognizes both PHOT1 (∼120 kD) and PHOT2 (∼110 kD; Supplemental Figure 4). When the wild-type seedlings were irradiated with blue light at 0.001, 0.1, and 100 µmol m–2 s–1, mobility shifts in the PHOT2 proteins were detectable only at 100 µmol m–2 s–1 (Figure 2D, dotted area). Under blue light conditions of 100 µmol m–2 s–1, the mobility shifts of the PHOT2 proteins were marginally detectable at 1 min, saturated at 30 min, and attenuated at 120 min after the onset of the irradiation in the wild-type etiolated seedlings (Figure 2E). Those autophosphorylation patterns were also detected in the rpt2 mutants and the 35Spro:RPT2 transgenic lines (Figures 2E and 2F). Although green fluorescent protein (GFP)–fused phot2 formed a complex with RPT2 in vivo (Supplemental Figures 5A and 5B) and RPT2 N showed binding activity to the PHOT2 LOV1 domain in yeast (Supplemental Figure 5C), the results of our phenotypic analysis suggested that RPT2 has no significant impact on the autophosphorylation of phot2. This finding was consistent with the finding of a previous study that the rpt2 mutation has no effect on phot2-dependent phototropic responses (Inada et al., 2004).
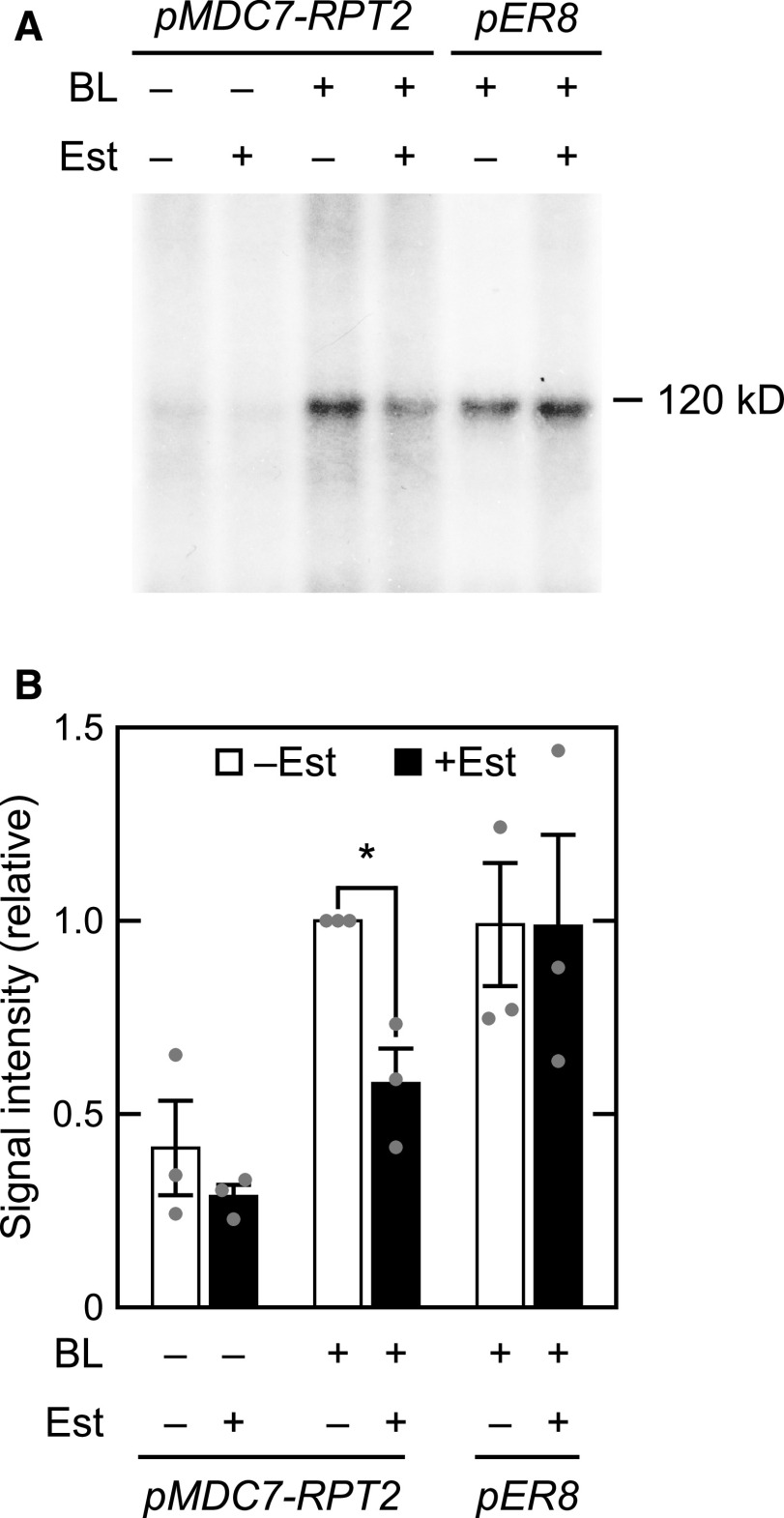
We next examined the inhibitory effects of RPT2 on the autophosphorylation activity of phot1 using an in vitro phosphorylation assay (Figure 3). This assay was performed with microsomal proteins extracted from rpt2 mutants transformed with a pMDC7-RPT2 construct, in which the expression of RPT2 is inducible by estradiol (Est) treatment (Supplemental Figure 6; Zuo et al., 2000). The autophosphorylation of phot1 was detected as a radiolabeled 120-kD protein in the microsomal fraction, as described previously (Liscum and Briggs, 1995). As expected, blue light irradiation caused the phosphorylation of these 120-kD proteins in the microsomal fractions of the pMDC7-RPT2 seedlings and pER8 vector control line (Figure 3A). By contrast, Est treatments suppressed this phot1 phosphorylation in the pMDC7-RPT2 seedlings, but not in the pER8 vector control line (Figure 3). Our immunoblotting analysis confirmed that PHOT1 protein expression was comparable among all of the microsomal fractions and that the RPT2 proteins were expressed only in the microsomal fraction of Est-treated pMDC7-RPT2 seedlings (Supplemental Figure 6B). These results suggested that Est-induced RPT2 proteins suppress the in vitro autophosphorylation of phot1.
Figure 3.
In Vitro Blue Light–Induced Phosphorylation of a 120-kD Protein in Microsomal Membranes.
Two-day-old etiolated rpt2 mutant seedlings transformed with a pMDC7-RPT2 construct (pMDC7-RPT2) or the wild-type seedlings transformed with a pER8 vector control (pER8) were grown on agar medium without (–) or with (+) 10 µM Est under darkness. Microsomal proteins were then extracted.
(A) In vitro blue light (BL)–induced phosphorylation of a 120-kD protein in microsomal membranes. Microsomal proteins were irradiated without (BL –) or with (BL +) blue light at 10 µmol m–2 s–1 for 16 min in the presence of [γ-32P]ATP. The reacted samples were resolved on 6% SDS-PAGE gels and autoradiographed.
(B) Relative quantity of phosphorylated 120-kD proteins. Values were calculated relative to the data from Est-untreated, blue light (BL)–irradiated pMDC7-RPT2 seedlings. The data shown are mean values ± se (n = 3 experiments). Dots on the bar graphs represent the results of individual experiments. An asterisk indicates a statistically significant difference (two-tailed Student’s t test, P < 0.05; Supplemental File 1).
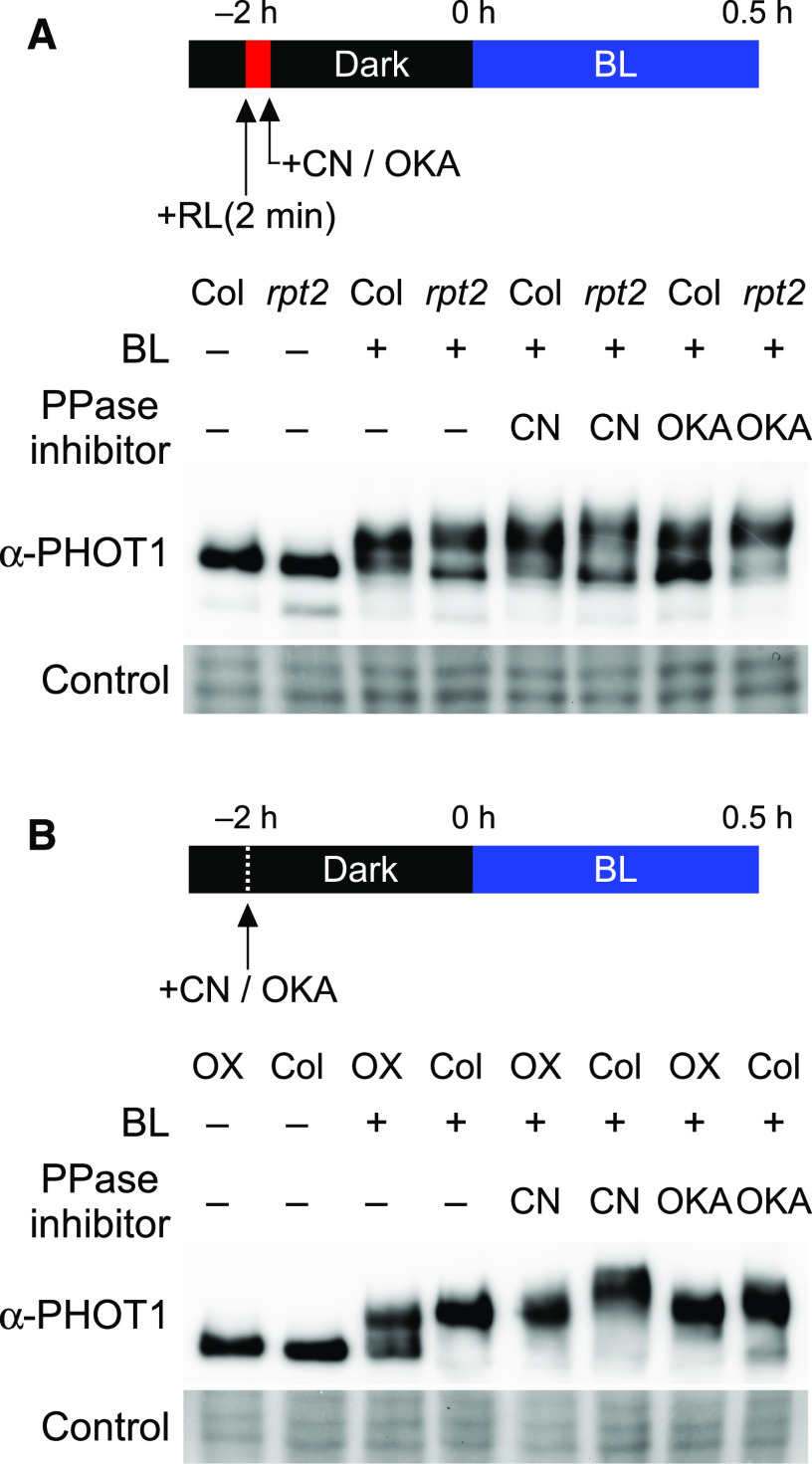
Our present analyses suggested that RPT2 proteins either suppress phot1 autophosphorylation or enhance its dephosphorylation. If a loss-of-function mutation in RPT2 leads to an enhanced phosphorylation of phot1 due to a block in its dephosphorylation and RPT2 overexpression enhances its dephosphorylation, treatments with protein phosphatase inhibitors seemed to impair the effects of a loss-of-function mutation and overexpression of RPT2. We therefore next examined the effects of protein phosphatase inhibitors in the rpt2 mutants and the 35Spro:RPT2 transgenic lines by immunoblotting analysis using a Phos-tag acrylamide gel. First, we examined the effects of the protein phosphatase inhibitors cantharidin (CN) or okadaic acid (OKA) on the dephosphorylation of phot1. Autophosphorylated PHOT1 proteins in the wild-type seedlings with a pulse irradiation of blue light for 2 min at 100 µmol m–2 s–1 were dephosphorylated with a subsequent dark incubation for 14 min (Supplemental Figure 7). When the seedlings were treated with CN or OKA, the mobility of the PHOT1 protein was slightly less in Phos-tag SDS-PAGE (Supplemental Figure 7). These results indicated that CN and OKA have some inhibitory effects on phot1 dephosphorylation.
We next examined the effects of these inhibitors in the rpt2 mutants. When the wild-type seedlings were irradiated with blue light for 0.5 h in the CN- or OKA-containing medium with a red light pretreatment (for the induction of RPT2; Haga et al. 2015), the PHOT1 proteins showed a hypermobility shift in contrast to the untreated seedlings (Figure 4A). The phosphorylation of phot1 was enhanced in the rpt2 mutants in comparison with those in the wild-type seedlings independently of the treatments of CN or OKA (Figure 4A). The effects of CN and OKA in the 35Spro:RPT2 transgenic line were also examined (Figure 4B). Red light pretreatment was not done here to ensure that RPT2 expression was not induced in the wild-type seedlings. Constitutive expression of RPT2 suppressed the phosphorylation of phot1 in the 35Spro:RPT2 transgenic lines without being affected by the CN and OKA treatments (Figure 4B). These results suggested that RPT2 suppresses the autophosphorylation activity of phot1, but does not enhance the dephosphorylation of phot1.
Figure 4.
Effects of Protein Phosphatase Inhibitors on the Phosphorylation Status of phot1.
The aerial parts of 2-d-old etiolated seedlings were incubated under darkness for 2 h in liquid medium with a protein phosphatase inhibitor (PPase inhibitor), 30 µM CN or 1 µM OKA, and subsequently treated with blue light (BL) at 0.1 µmol m–2 s–1 for 0.5 h. Total proteins (15 µg, see [A]; 20 µg, see [B]) extracted from the seedlings were separated on 6% SDS-PAGE gels with 2 µM Phos-tag, followed by immunoblotting with anti-PHOT1 antibodies. The PVDF membranes were stained using a Pierce reversible protein staining kit as a loading control.
(A) Effects of protein phosphatase inhibitors (PPase inhibitors) on the aerial parts of the wild-type Col seedlings and rpt2 mutants. Red light pretreatments were conducted to induce RPT2 expression before exposure to the PPase inhibitor.
(B) Effects of protein phosphatase inhibitors (PPase inhibitors) on the aerial parts of the wild-type Col seedlings and the 35Spro:RPT2 lines (OX).
RPT2 Is Induced by Blue Light Irradiation Posttranscriptionally
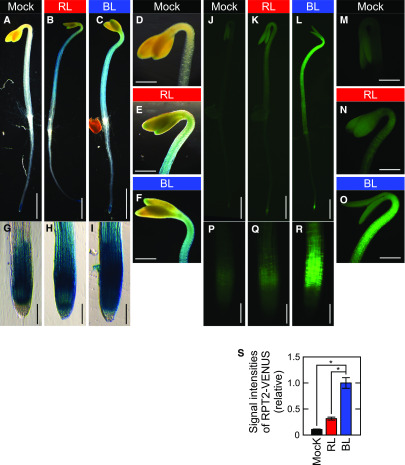
Our current results suggested that the induction of RPT2 expression by light irradiation is an important mechanism for controlling both photosensitivity and the autophosphorylation activity of phot1. We thus investigated the light inducibility of RPT2 expression in more detail. We first observed the RPT2 expression patterns using transgenic plants carrying the RPT2pro:GUS gene and the RPT2pro:RPT2-VENUS gene. β-Glucuronidase (GUS) staining was detected in a root tip under darkness in the 2-d-old etiolated seedlings carrying the RPT2pro:GUS gene (Figures 5A, 5D, and 5G). Both red light and blue light irradiation enhanced its expression in whole seedlings, most notably the hypocotyls, hooks, and root tips including the elongation zone, in a similar manner (Figures 5B, 5C, 5E, 5F, 5H, and 5I). When the expression patterns of the RPT2pro:RPT2-VENUS gene in the rpt2 mutants were analyzed, we noticed a clear induction of RPT2-VENUS proteins in the aerial part of seedlings (Figures 5L and 5S), especially the elongation zones of the hypocotyls (Figure 5O), and the elongation zone of roots (Figure 5R), but only under blue light irradiation and not red light irradiation (Figures 5K, 5N, and 5Q). These results indicated that both red light and blue light irradiation can activate the RPT2 promoter but that only blue light irradiation can induce the RPT2-VENUS proteins effectively.
Figure 5.
Expression Patterns of RPT2pro:GUS and RPT2pro:RPT2-VENUS.
Two-day-old etiolated seedlings were not irradiated (Mock: see [A], [D], [G], [J], [M], and [P]) or irradiated with either red light (RL: see [B], [E], [H], [K], [N], and [Q]) or blue light (BL: see [C], [F], [I], [L], [O], and [R]) at 10 µmol m–2 s–1 for 4 h. Bar in (A) to (C) and (J) to (L) = 1.0 mm; bar in (D) to (F) and (M) to (O) = 400 µm; bar in (G) to (I) and (P) to (R) = 100 µm.
(A) to (I) GUS staining patterns of the wild-type seedlings transformed with RPT2pro:GUS.
(J) to (R) VENUS fluorescent images of the rpt2 mutants transformed with RPT2pro:RPT2-VENUS.
(S) Signal intensities of RPT2-VENUS fluorescence. Fluorescent signals were measured in the upper region of the hypocotyls and calculated relative to the value from BL-irradiated seedlings. The data shown are the mean values ± se from nine seedlings. Asterisks indicate statistically significant differences compared with mock seedlings (two-tailed Student’s t test, P < 0.05; Supplemental File).
Accumulation of RPT2 Proteins Is Enhanced by Photoropin Activation
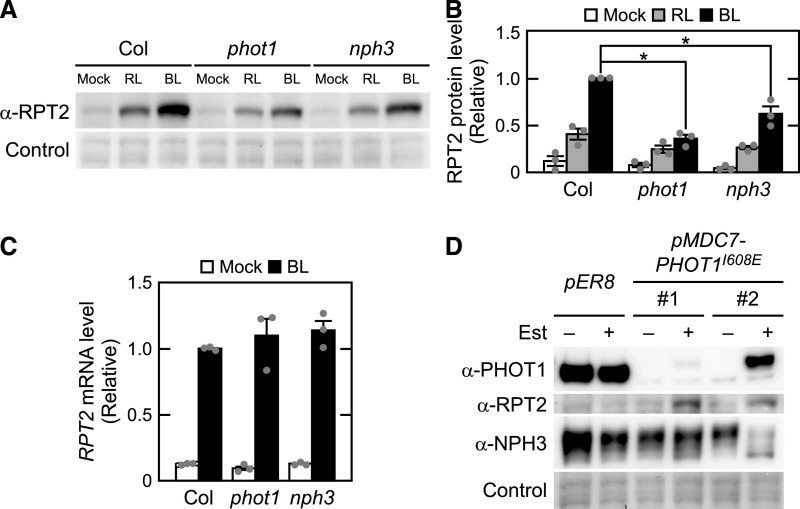
The RPT2-VENUS fluorescent signal was detectable in the elongation zones of hypocotyls and roots (Figures 5O and 5R), in which the strong expression of phot1 has been reported previously (Sakamoto and Briggs, 2002). Thus, we hypothesized that RPT2 proteins are unstable under red light conditions and that phot1 stabilizes them under blue light conditions. We tested this using immunoblotting analysis. When the wild-type seedlings were irradiated for 6 h with red or blue light at 10 µmol m–2 s–1, RPT2 protein expression was induced in both cases, but blue light irradiation was much more effective (Figures 6A and 6B). By contrast, the accumulation of RPT2 proteins under blue light conditions was attenuated in the phot1 mutants, which was statistically significant (Figures 6A and 6B). The mutation of the NPH3 gene, which is required for the phot1 signaling during phototropism (Motchoulski and Liscum, 1999), also caused a decrease of RPT2 accumulation under blue light conditions (Figures 6A and 6B). Neither phot1 nor nph3 mutations affected the red light–induced accumulation of RPT2 (Figures 6A and 6B). RT-qPCR analysis confirmed that the blue light induction of RPT2 transcripts was not affected by mutations of phot1 and nph3 (Figure 6C). These results suggested that phot1 and its associated protein NPH3 contribute to the accumulation of RPT2 proteins under blue light conditions in a posttranscriptional manner.
Figure 6.
Posttranscriptional Regulation of RPT2 Expression by Phototropins.
(A) Immunoblot analysis of RPT2 proteins in the wild-type (Col), phot1, and nph3 seedlings. Two-day-old etiolated seedlings were either not irradiated (mock) or irradiated for 6 h with either red light (RL) or blue light (BL) at 10 µmol m–2 s–1. Total proteins (10 µg) extracted from the seedlings were resolved on 10% SDS-PAGE gels, followed by immunoblotting with anti-RPT2 antibodies. The PVDF membranes were stained using a Pierce reversible protein staining kit as a loading control.
(B) Statistical analysis of the data in (A). The values were normalized with a loading control and then calculated relative to the data from the blue light–irradiated wild-type seedlings. The data shown are mean values ± se (n = 3 experiments). Dots on the bar graphs represent the results of individual experiments. Asterisks indicate statistically significant differences (two-tailed Student’s t test, P < 0.05; Supplemental File).
(C) RT-qPCR analysis of RPT2 in the wild-type (Col), phot1, and nph3 seedlings. Two-day-old etiolated seedlings were irradiated with blue light (BL) at 10 µmol m–2 s–1 for 6 h or not irradiated. Triplicate PCR reactions were performed in each case, and three independent biological independent samples were used for each gene. The values were normalized using an internal control (18S rRNA) and then calculated relative to values from blue light–irradiated wild-type seedlings. The data shown are the mean values ± se (Supplemental File). Dots on the bar graphs represent the results of individual experiments.
(D) Immunoblot analysis of PHOT1, RPT2, and NPH3 in the phot1 mutants transformed with a pMDC7-PHOT1I608E construct (pMDC7-PHOT1I608E: two independent lines, #1 and #2) and the pER8 vector control line. Two-day-old etiolated seedlings were grown on agar medium with (+) or without (–) 10 µM Est. Microsomal proteins (7.5 µg) extracted from the seedlings were separated on 7.5% SDS-PAGE gels followed by immunoblotting with anti-PHOT1, anti-RPT2, and anti-NPH3 antibodies. The PVDF membranes were stained using a Pierce reversible protein staining kit as a loading control.
We next examined whether the sole activation of the phot1 photoreceptor is sufficient for the accumulation of RPT2 proteins using phot1 mutants transformed with a pMDC7-PHOT1I608E construct, in which constitutively active PHOT1 proteins (PHOT1I608E; Harper et al., 2004) are inducible by Est treatment. In the etiolated seedlings of the pER8 vector control line, Est treatment had no effect on the RPT2 protein levels or the phosphorylation status of the NPH3 protein (Figure 6D). By contrast, in the phot1 mutants transformed with a pMDC7-PHOT1I608E construct (no. 1 and no. 2), Est exposure caused the accumulation of RPT2 protein in the absence of blue light irradiation. This suggested that the accumulation of RPT2 can be caused by phot1 activation alone, even in darkness.
The mobility shift of the NPH3 protein, which reflects a phot1-induced dephosphorylation (Pedmale and Liscum, 2007; Tsuchida-Mayama et al., 2008), was also observed in the no. 2 transgenic line (Figure 6D), suggesting that PHOT1I608E expression can cause NPH3 dephosphorylation under darkness. A previous study reported that the expression of PHOT1R472H, which encodes another constitutively active variant of PHOT1, was not sufficient to promote NPH3 dephosphorylation under darkness (Petersen et al., 2017). The discrepancies between the prior result and our current observations may be due to differences in the natures of PHOT1I608E and PHOT1R472H and/or the transgene expression levels.
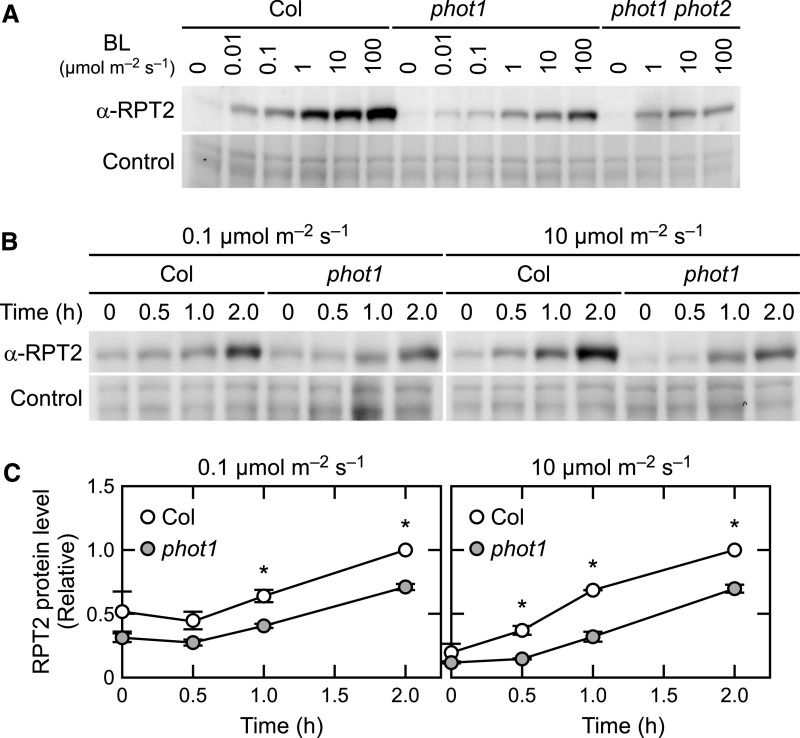
We next analyzed the fluence rate and time dependence of RPT2 protein accumulation. When the wild-type seedlings were irradiated with blue light at 0.01 to 100 µmol m–2 s–1 for 6 h, the RPT2 protein levels were increased as the fluence rates of blue light increased (Figure 7A). This induction seemed to be caused by both transcriptional regulation of phytochrome and cryptochrome (Tsuchida-Mayama et al., 2010) and posttranscriptional regulation of phot1. In the phot1 mutants, a weakened induction of the RPT2 protein was observed at all fluence rates examined (Figure 7A), suggesting that phot1 contributes to RPT2 accumulation, at least between 0.01 to 100 µmol m–2 s–1. In the phot1 phot2 double mutants, the blue light induction of RPT2 at 100 µmol m–2 s–1 was obviously lower than that in the phot1 single mutant (Figure 7A), suggesting that phot2 also contributes to the accumulation of RPT2 proteins at 100 µmol m–2 s–1. When the seedlings were irradiated with blue light at 0.1 or 10 µmol m–2 s–1 for various times, the induction of RPT2 proteins was detectable at least after 1 h of the onset of irradiation in both wild-type and phot1 seedlings but at a higher level in the wild type (Figures 7B and 7C). This observation suggested that both the transcriptional induction of RPT2 by phytochromes and cryptochromes (Tsuchida-Mayama et al., 2010) and the posttranscriptional induction of phot1 contribute to an early induction of RPT2 under both fluence rate conditions.
Figure 7.
Fluence Rate and Time Dependencies of RPT2 Induction.
(A) Fluence rate dependency of RPT2 induction in the wild type (Col), phot1, and phot1 phot2 double mutants. Two-day-old etiolated seedlings were irradiated for 6 h with blue light (BL) at the indicated fluence rates. Total proteins (10 µg) extracted from the seedlings were resolved on 10% SDS-PAGE gels followed by immunoblotting with anti-RPT2 antibodies. The PVDF membranes were stained using a Pierce reversible protein staining kit as a loading control.
(B) Time-course analysis of RPT2 induction in the wild-type (Col) and phot1 seedlings. Two-day-old etiolated seedlings were irradiated with blue light at 0.1 µmol m–2 s–1 (left) or 10 µmol m–2 s–1 (right) for the indicated periods. Other details were as described in (A).
(C) Statistical analysis of the data generated in (B). The values were normalized using a loading control and then calculated relative to the values from the wild-type seedlings irradiated for 2 h. The data shown are mean values ± se (n = 3). Dots on the bar graphs represent the results of individual experiments. Asterisks indicate a statistically significant difference (two-tailed Student’s t test, P < 0.05; Supplemental File).
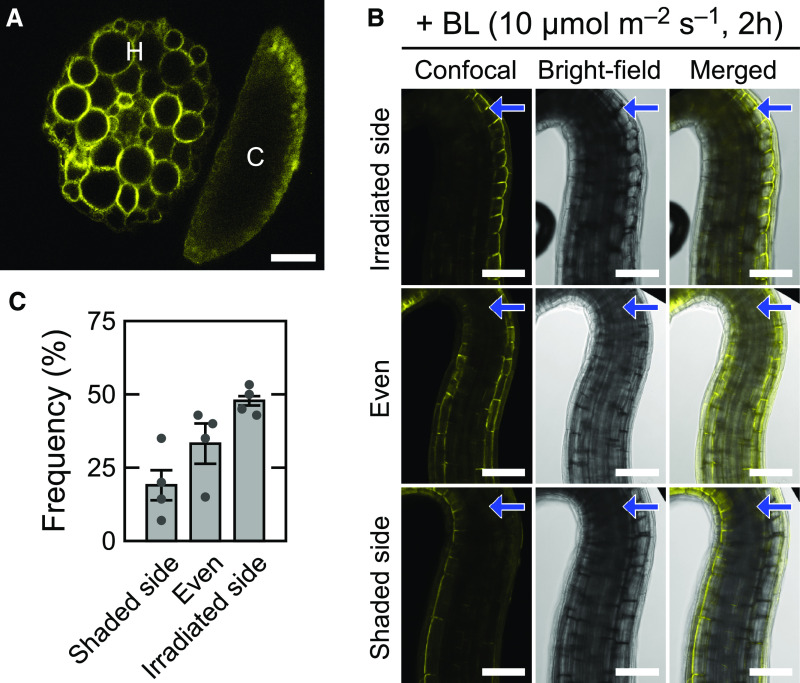
Previous study has indicated that phot1 is localized to the plasma membrane region in the epidermal cells and the cortical cells of both root and hypocotyl of Arabidopsis etiolated seedlings (Sakamoto and Briggs, 2002). When the rpt2 transgenic seedlings expressing RPT2-VENUS were irradiated with blue light from above, a fluorescent image of a transverse section of upper hypocotyls showed that the RPT2-VENUS proteins were localized to the plasma membrane region in all tissues of hypocotyls and were strongly expressed in the cortex (Figure 8A). These expression and subcellular localization patterns were similar with those of phot1 (Sakamoto and Briggs, 2002).
Figure 8.
Distribution Patterns of the RPT2-VENUS Proteins in Etiolated Hypocotyls.
Two-day-old etiolated seedlings of rpt2 mutants transformed with RPT2pro:RPT2-VENUS were irradiated for 6 h with blue light (BL) at 10 µmol m–2 s–1 from above (A) or for 2 h from the unilateral side (B).
(A) A typical distribution pattern of RPT2-VENUS in the hypocotyl cross section. C, cotyledon; H, hypocotyl. Bar = 50 µm.
(B) and (C) Distribution patterns of RPT2-VENUS in the upper region of the hypocotyls. Distribution patterns were classified into three types (irradiated side, even, and shaded side). Representative confocal (left), bright-field (middle), and merged images (right) are shown in (B). Arrows indicate the direction of blue light (BL). The frequencies of each expression pattern are shown in (C). The data shown are the mean values ± se of four repeated experiments. Dots on the bar graphs represent the results of individual experiments. Seven to 20 seedlings were analyzed in each experiment. Bars = 100 µm.
Suzuki et al. (2019) has reported that unilateral irradiation of blue light induces the asymmetric distribution of phosphorylated Zm-phot1 in coleoptiles of Zea mays in response to the gradient of blue light intensity in these organs. When the seedlings were unilaterally irradiated, the RPT2-VENUS proteins were often expressed more strongly on the irradiated side of the hypocotyls than on the shaded side (∼47% of seedlings; Figures 8B and 8C). By contrast, there were also seedlings showing symmetric expression patterns (∼33%) or a strong expression of RPT2-VENUS on the shaded side (∼19%). As the light induction of endogenous RPT2 proteins was already detectable at 1 h after the onset of blue light irradiation at 10 µmol m–2 s–1 in the immunoblotting analysis (Figure 7A), their distribution patterns at 1 h could also be observed. The RPT2-VENUS fluorescent signal, however, was barely detectable (data not shown). One hour of irradiation seemed to be too short for the expression, maturation, and accumulation of RPT2-VENUS proteins to detect its fluorescence. Therefore, we could not draw any conclusions regarding the asymmetric induction of RPT2 in hypocotyls irradiated by unilateral blue light in our present study.
RPT2 Proteins Are Degraded through the Ubiquitin-Proteasome Pathway
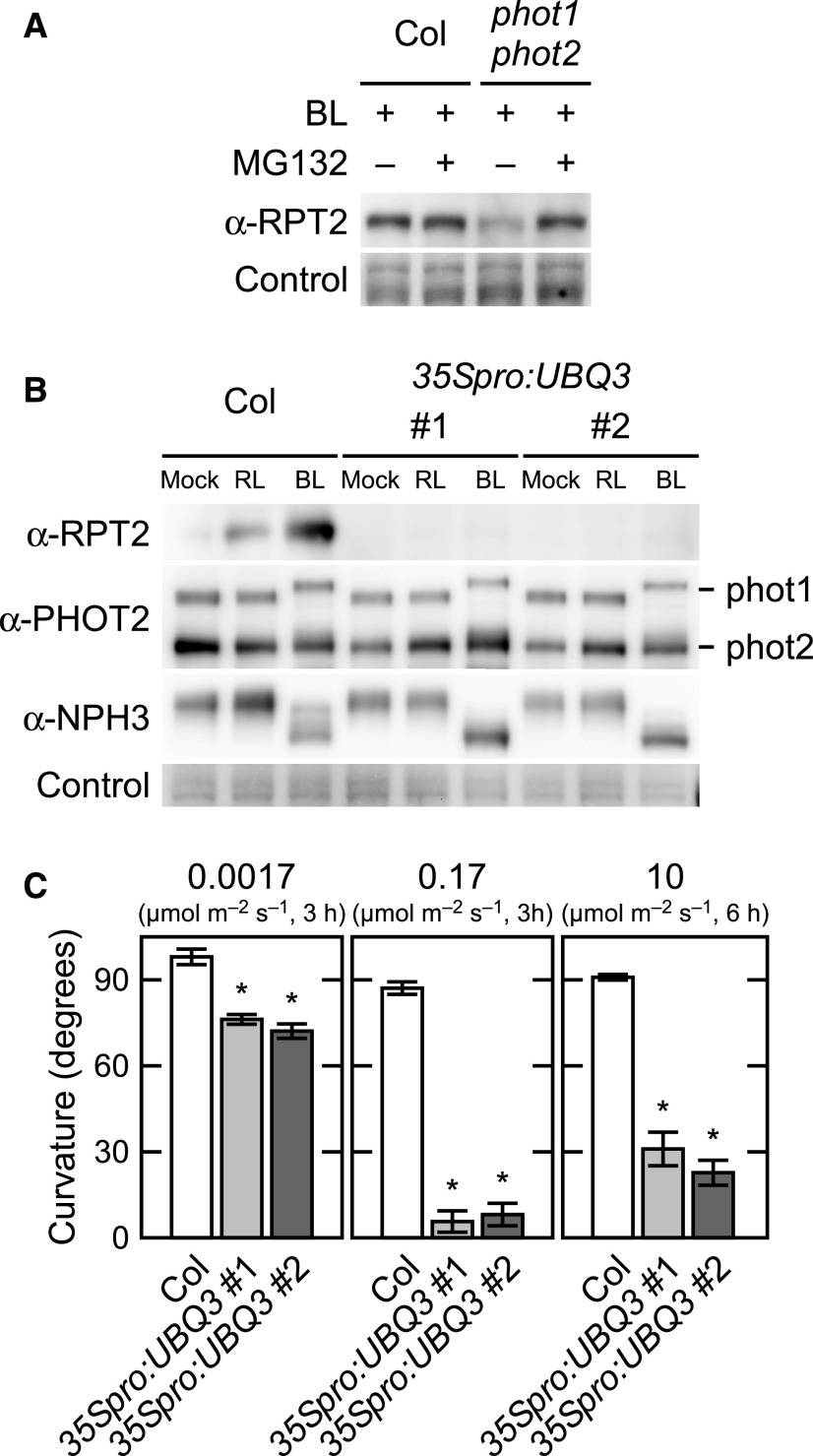
We examined whether the posttranscriptional induction of RPT2 protein by phot1 is through inhibition of the ubiquitin-proteasome pathway. When red light–grown seedlings of the wild type were treated with the proteasome inhibitor MG132, RPT2 accumulated with or without blue light irradiation (Supplemental Figure 8). When etiolated seedlings were irradiated with blue light at 10 µmol m–2 s–1 for 3 h, the wild-type seedlings showed the accumulation of RPT2 protein with or without MG132 (Figure 9A) treatment. By contrast, the phot1 phot2 double mutants required MG132 treatment for the accumulation of RPT2 protein even under blue light conditions (Figure 9A). These results suggested that RPT2 is degraded in a proteasome-dependent manner and is stabilized through the activation of phototropins.
Figure 9.
Destabilization of RPT2 via a Ubiquitin-Proteasome Dependent Pathway.
(A) Effect of the proteasome inhibitor MG132 on RPT2 expression. Two-day-old etiolated seedlings of the wild type (Col) and the phot1 phot2 double mutant were transferred into liquid medium with (+) or without (–) 50 µM MG132, kept in the dark for 1 h, and subsequently irradiated with blue light (BL) at 10 µmol m–2 s–1 for 3 h (+) or not irradiated (–). Total proteins (10 µg) extracted from the seedlings were separated on 7.5% SDS-PAGE gels followed by immunoblotting with anti-RPT2 antibodies. The PVDF membranes were stained using a Pierce reversible protein staining kit as a loading control.
(B) Immunoblot analysis of RPT2, PHOT1, PHOT2, and NPH3 in the wild-type (Col) seedlings and wild-type seedlings transformed with 35Spro:UBQ3 (35Spro:UBQ3: two independent lines; #1 and #2). Two-day-old etiolated seedlings were either not irradiated or irradiated with red light (RL) or blue light (BL) at 10 µmol m–2 s–1 for 6 h. Total proteins (10 µg) extracted from the seedlings were then separated on 10% SDS-PAGE gels followed by immunoblotting with anti-RPT2, anti-PHOT2, and anti-NPH3 antibodies. The PVDF membranes were stained using a Pierce reversible protein staining kit as a loading control.
(C) Hypocotyl phototropism in the wild-type (Col) seedlings and 35Spro:UBQ3 transgenic lines. Two-day-old etiolated seedlings were irradiated with unilateral blue light at 0.0017 or 0.17 µmol m–2 s–1 for 3 h or at 10 µmol m–2 s–1 for 6 h. The data shown are the mean values ± se for hypocotyl curvatures of 14 to 24 seedlings. Asterisks indicate a statistically significant difference (two-tailed Student’s t test, P < 0.05; Supplemental File).
To detect polyubiquitinated RPT2 under red light irradiation, we attempted to immunoprecipitate it from extracts of UBIQUITIN3 (UBQ3) overexpression lines (35Spro:UBQ3). Although this was not successful, we unexpectedly found a significant decrease of RPT2 protein in the 35Spro:UBQ3 transgenic lines under both red light and blue light conditions (Figure 9B). Those transgenic lines showed the high expression of RPT2 transcripts (Supplemental Figure 9), suggesting that UBQ3 expression leads to the decrease of RPT2 protein in a posttranscriptional manner. Correspondingly, these transgenic lines showed an abnormality in hypocotyl phototropism; that is, their phototropic curvatures were moderate under weak intensity blue light conditions and decreased as the fluence rate increased (Figure 9C), in a manner comparable with that of the rpt2 mutants (Haga et al., 2015). By contrast, UBQ3 overexpression had no effect on the expression patterns of PHOT1, PHOT2, or NPH3 (Figure 9B) or on the growth of seedlings (Supplemental Figures 10A and 10B). These results suggested that the RPT2 proteins are degraded through the ubiquitin-proteasome pathway and that the activation of phot1 and phot2 may negatively regulate the polyubiquitination of RPT2 proteins and/or its degradation by proteasomes.
DISCUSSION
Our current results demonstrate that RPT2 suppresses the autophosphorylation of phot1 under blue light conditions. Previous studies have indicated that the phot1 LOV1 domain is necessary for the induction of the phototropic responses under low intensity blue light conditions (Sullivan et al., 2008) and that the light induction of RPT2 expression suppresses phot1 photosensitivity, which is required for the photosensory adaptation of phot1 to high intensity blue light (Haga et al., 2015). These prior results and our current data suggest that RPT2 negatively regulates the autophosphorylation of phot1 through the LOV1 domain, which is probably required for a formation of a suitable gradient of phot1 activity between the irradiated side and the shaded side in accordance with the high intensity unilateral blue light. Hence, this is the photosensory adaptation mechanism of phot1 in the phototropism of Arabidopsis etiolated hypocotyls. The expression level of RPT2 proteins is increased in response to an increase of blue light intensity (Figure 7A), indicating that RPT2 functions as a molecular rheostat that maintains a moderate activation level of phot1 in etiolated hypocotyls of Arabidopsis seedlings under any light intensity conditions.
The molecular mechanisms by which RPT2 functions in the suppression of phot1 activity remain to be elucidated. Some prior studies have suggested that the LOV1 domain mediates the dimerization of phot1, which thereby enhances the autophosphorylation of this blue light photoreceptor (Nakasako et al., 2004; Xue et al., 2018). Thus, RPT2 may inhibit the LOV1-mediated dimerization of phot1 to suppress its kinase activity. Previous studies had also suggested that LOV1 suppresses the decay of the cysteinyl-FMN adduct of LOV2 and enhances the Ser/Thr kinase activity of the phototropins (Kaiserli et al., 2009; Okajima et al., 2012; Okajima, 2016). RPT2 may enhance the decay of the cysteinyl-FMN adduct of phot1 LOV2 through the binding to LOV1 and thus suppress the Ser/Thr kinase activity of phot1. Although our current results suggest that RPT2 suppresses the autophosphorylation activity of phot1, the possibility of enhancement of phot1 dephosphorylation by RPT2 still cannot be excluded. RPT2 might act as a scaffold of protein phosphatases to dephosphorylate phot1. We also need to examine whether a defect of RPT2 binding to the LOV1 domain indeed affects the autophosphorylation of phot1. Further studies are warranted to elucidate the mechanisms underlying the function of the LOV1 domain and RPT2 in phototropin photoactivation in more detail.
The posttranscriptional regulation of RPT2 forms a negative feedback loop of phot1 activation. This regulation appears to ensure the formation of a gradient of phot1 signaling activity between the irradiated and shaded sides of plant organs under a broad range of blue light intensity. This gradient then seems to induce light-induced differential growth including not only phototropic responses but also leaf flattening and cotyledon/leaf positioning (Sakai et al., 2000; Harada et al., 2013). Previous studies have reported that unilateral blue light irradiation results in differential NPH3 aggregate formation in response to phot1 activity across the etiolated hypocotyl, which suppresses and fine-tunes NPH3 activity (Sullivan et al., 2019), and that the light-induced RPT2 proteins suppress the dephosphorylation and aggregation of NPH3 proteins in the etiolated seedlings (Haga et al., 2015). Our current study findings strongly suggest that RPT2 indirectly suppresses their dephosphorylation and aggregation through the suppression of phot1 activity. By contrast, our current results also indicate that NPH3 partially contributes to the stabilization of RPT2 proteins under blue light conditions (Figure 6B). Thus, the aggregation of NPH3 proteins might decrease the stabilization of RPT2 proteins at the irradiated side and fine-tune the phot1 activity at both the irradiated and the shaded side. Adjustments both of the light induction of PRT2 and of the plasma membrane localization of NPH3 probably contribute to the fine-tuning of phot1 signaling across hypocotyls and to an induction of phototropic responses under various light conditions in the etiolated seedlings of Arabidopsis. By contrast, RPT2 is not required for the phototropic responses in de-etiolated hypocotyls of Arabidopsis seedlings (Sullivan et al., 2019). Other unknown factors and/or mechanisms may suppress the excessive activation of phot1 in green seedlings.
Our current observations indicate that RPT2 is degraded by the ubiquitin-proteasome pathway and that phot1 activation suppresses this degradation. The mechanism by which RPT2 is ubiquitinated or stabilized under blue light conditions has been a question of some importance. NPH3 is an essential signal transducer during phototropism and shows binding activity toward RPT2 and possesses ubiquitin E3 ligase activity with Cullin3 (Motchoulski and Liscum, 1999; Inada et al., 2004; Roberts et al., 2011). Although we speculated that NPH3 may ubiquitinate RPT2 and promote its degradation under red light conditions, our immunoblotting analysis revealed that NPH3 contributes to its accumulation under blue light conditions (Figure 6A). As RPT2 belongs to the same protein family as NPH3 and also has a BTB/POZ domain that often interacts with Cullin3 (Genschik et al., 2013), it may be ubiquitinated on its own, and its binding to the active forms of the phototropins may suppress its polyubiquitination and degradation. The issue of whether phot1 controls a ubiquitin-proteasome pathway also remains to be resolved.
RPT2 belongs to the NPH3/RPT2-like (NRL) gene family (Sakai, 2005), and other NRL members might also have a similar function to RPT2. RPT2 did not show an obvious effect on the suppression of phot2 activity, although it can bind to its LOV1 domain and form a complex with phot2 in vivo. However, for example, NRL PROTEIN FOR CHLOROPLASTMOVEMENT1 (NCH1) functions in the chloroplast accumulation response in parallel with RPT2 (Suetsugu et al., 2016). Suetsugu et al. (2016) revealed that nch1 mutants show an enhancement of the phot2-induced avoidance response. One of the hypotheses from this is that NCH1 suppresses the photosensitivity and/or photoactivation of phot2 with RPT2. Thus, the relationships between the NRL members and phototropins in various plants should be re-examined in future studies.
METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 (Col) was used as the wild-type control. Mutant seeds of rpt2-2 (Col background) and nph3-102 (Salk_110039; Col background) were obtained as previously described by Inada et al. (2004) and Tsuchida-Mayama et al. (2008). The phot1-Salk146058 (Col background) and phot2-Salk142275 mutants (Col background) were obtained from the Arabidopsis Biological Resource Center (Alonso et al., 2003) and crossed to obtain a phot1 phot2 double mutant. The rpt2-2 mutants transformed with 35Spro:RPT2 or RPT2pro:RPT2-VENUS were prepared as previously described Tsuchida-Mayama et al. (2010) and Haga et al. (2015). Transgenic Col lines harboring RPT2pro:GUS were also prepared as previously described by Inada et al. (2004). The phot1 phot2 mutants transformed with 35Spro:PHOT1LOV1Cys39Ala were kindly provided by John Christie (University of Glasgow).
The pMDC7-RPT2 transgenic lines were generated as follows. The RPT2 coding region was subcloned into the entry vector pENTR/D-TOPO (Invitrogen) and shuttled via an LR clonase reaction (Invitrogen) into the Est-inducible vector pMDC7 (Curtis and Grossniklaus, 2003), kindly provided by Nam-Hai Chua (Rockefeller University, New York). The pMDC7-RPT2 plasmid was used for the transformation of Agrobacterium tumefaciens (strain C58C1) that was then used for subsequent transformations of rpt2-2 mutants. Several independent rpt2 transgenic lines showed phototropism complementation following Est treatment (Supplemental Figure 6A). pER8 is an original vector of pMDC7 that lacks the Gateway cassette (Zuo et al., 2000) and was used for the transformation of A. tumefaciens that was then used for subsequent transformations of the Col wild type for use as a vector control line.
The pMDC7-PHOT1I608E transgenic lines were generated as follows. The PHOT1I608E mutated cDNA was generated from PHOT1 cDNA by PCR-based site-directed mutagenesis using PHOT1 gene-specific primers including 5′-ACTGCAGTTTTTTCACCAGGTCTTCTCCCTC-3′ and 5′-ACTGCAGTGAATGAAGATGAAGCGGTTCGAGAACT-3′ (underlined bases denote the Glu codon at position 608; double underlines indicate the PstI sites for subsequent subcloning), subcloned into the entry vector pDONR222 (Invitrogen), and shuttled into pMDC7 via an LR clonase reaction. The pMDC7-PHOT1I608E plasmid was used for the transformation of A. tumefaciens that was then used for subsequent transformations of phot1-Salk146058 mutants.
The 35Spro:UBQ3 transgenic lines were generated as follows. The UBQ3 coding region was amplified from Col genome DNA by PCR using UBQ3 gene-specific primers (Supplemental Table 1), subcloned into the entry vector pENTR/D-TOPO, and shuttled via an LR clonase reaction into the cauliflower mosaic virus 35S promoter–containing binary vector pH35GS, kindly provided by Taku Demura (Nara Institute of Science and Technology, Nara, Japan). The resulting pH35GS-UBQ3 plasmid was used for the transformation of A. tumefaciens that was then used for subsequent transformations of the wild-type Col plants.
For experiments, the seeds were surface sterilized in 10% (v/v) bleach for 15 min with gentle rotation, rinsed five times with autoclaved deionized water, and then plated in Petri dishes with half-strength Okada and Shimura medium containing 1.5% (w/v) agar, as previously described by Ohgishi et al. (2004). Seeds were kept at 4°C for 3 d and then exposed to red light for 6 h to induce uniform germination. After germination was induced, the Petri dishes were positioned vertically to let the seedlings grow on the surface of the agar at 21 to 22°C under dark conditions. Blue light and red light irradiations were performed under various conditions with light-emitting diodes (470 ± 30 nm, LED-B; Eyela for blue light, 660 ± 20 nm, LED-R; Eyela for red light), as described in the figure legends.
Yeast Two-Hybrid Analysis
The GAL4 DNA binding domain vector pGBDKT7-GWRFC was constructed by insertion of the Gateway reading frame cassette RfcC (Invitrogen) into the Klenow-Fragment–treated NdeI-SalI sites of pGBKT7 (pGBDKT7, Clontech; http://www.clontech.com). PCR was used to generate the coding sequences of PHOT1 and PHOT2 with gene-specific primers (Supplemental Table 1). A DNA fragment for PHOT1 N2mut was separately generated by PCR using N2 FW and N2mut RV and N2mut FW and N2 RV primers and then combined by PCR using N2 FW and N2 RV primers. These amplified products were subcloned into the entry vector pENTR/D-TOPO and shuttled into the pGBDKT7-GWRFC plasmid. The GAL4 transcription-activating domain vector pGADT7 and its derivative pGADT7-RPT2 N were prepared as previously described by Inada et al. (2004). Pairwise combinations of vectors were cotransformed into the yeast strain Y187 (Clontech) and plated onto the same selective medium. Quantitative β-galactosidase assays were performed in liquid cultures of yeast using o-nitrophenyl-β-d-galactopyranoside (Ausubel et al., 2001). One unit of β-galactosidase activity was defined as the amount of enzyme required to convert 1 µmol of o-nitrophenyl-β-d-galactopyranoside to o-nitrophenol and d-Gal in 1 min at 30°C. Three independent experiments were performed to calculate the average and se.
In Vitro Pull-Down Assay
To prepare PHOT1 LOV1 and LOV2 proteins, DNA fragments for PHOT1 N2 and N4 in pENTR/D-TOPO (Invitrogen) were transferred into the pCold ProS2 plasmid (TaKaRa) harboring a Gateway reading flame cassette (Invitrogen). These constructs were introduced into Escherichia coli strain BL21 (DE3) pLysS (Novagen), and His-ProS–tagged PHOT1 N2 and N4 proteins (His-PHOT1N2 and His-PHOT1N4) were prepared from the transformed lines in accordance with the manufacturer’s protocol. HA-tagged RPT2 N proteins were prepared with in vitro transcription and translation of pGADT7-RPT2 N using the TNT Quick Coupled Transcription/Translation systems (Promega).
Each purified protein preparation of His-PHOT1 N2 and N4 was incubated with TALON Magnetic Beads (TaKaRa) at 4°C for 30 min and further incubated at 4°C for 30 min with in vitro transcription and translation reactant containing RPT2 N. The beads were then collected on the magnetic rack and washed five times with washing buffer (sodium phosphate buffer, pH 7.0, 150 mM NaCl, and 0.2% [v/v] Triton X-100). The proteins were then released from the beads into 50 μL of 1× SDS gel loading buffer and resolved by SDS-PAGE.
Immunoblotting Analysis
For immunoblotting of Phos-tag SDS-PAGE gels, total proteins were extracted from ∼100 etiolated seedlings in a buffer containing 50 mM Tris-MES, pH 7.5, 300 mM Suc, 150 mM NaCl, 10 mM potassium acetate, 0.2% (v/v) Triton X-100, and a protease inhibitor mixture (Complete Mini EDTA-free; Roche Diagnostics). The extracts were then centrifuged at 10,000g at 4°C for 10 min to remove cell debris, and the supernatants were collected and mixed with a half-volume of 3× SDS gel loading buffer and boiled at 95°C for 15 min. The samples were separated with 6% (w/v) SDS-PAGE gels containing 2 µM Phos-tag (FUJIFILM Wako Pure Chemical) in accordance with the previously described Zn2+-Phos-tag SDS-PAGE method (Kinoshita and Kinoshita-Kikuta, 2011). Following electrophoresis, the gels were washed twice with methanol-free transfer buffer (25 mM Tris and 192 mM Gly) with 10 mM EDTA for 10 min and then once with methanol-free transfer buffer without EDTA for 10 min. The separated total proteins were then blotted onto a polyvinylidene difluoride (PVDF) membrane (GE Healthcare) using a wet tank blotting system at a constant voltage of 350 mA for 4 h. Later immunoblotting steps were performed as previously described by Inada et al. (2004). For alkaline phosphatase treatment, microsomal pellets were obtained as previously described by Inada et al. (2004) and resuspended in the 1× NEBuffer 3 (NEB) with 0.5% (v/v) Triton X-100 and protease inhibitor mixture (Complete Mini EDTA-free; Roche Diagnostics). Microsomal proteins (30 µg) were then treated with 30 units of calf intestinal alkaline phosphatase (TaKaRa) at 37°C for 2 h. The reaction was stopped adding 3× SDS gel loading buffer.
For immunoblotting of SDS-PAGE gels without Phos-tag, total proteins were extracted, separated on 6, 7.5, or 10% (w/v) SDS-PAGE gels, and blotted onto PVDF membranes as previously described by Inada et al. (2004). Anti-PHOT2 antiserum was produced in rabbit using 10× His-tagged PHOT2 products incorporating residues 294 to 474 as the antigen. Other antibodies are as follows: horseradish peroxidase (HRP)–conjugated anti-HA (catalog no. 12013819001, Roche Diagnostics; 1:8000 dilution), anti-PHOT1 (catalog no. KR095, TransGenic; 1:4000 dilution), anti-RPT2 (Inada et al., 2004), anti-NPH3 (Tsuchida-Mayama et al., 2008), anti-GFP (catalog no. 598, MBL Life Science; 1:1000 dilution), and HRP-conjugated anti-rabbit IgG (catalog no. NA934-1ML, GE Healthcare; 1:50,000 dilution). HRP activity was detected with the super-signal west femto maximum sensitivity substrate (Thermo Fisher Scientific) and the Image Quant LAS4000 Mini device (GE Healthcare). As a loading control, the PVDF membranes were stained using the Pierce reversible protein staining kit (Thermo Fisher Scientific). The results were confirmed using independent samples. For the statistical analysis, the signal intensities of the protein bands were quantified with Fiji software (Schindelin et al., 2012).
In Vitro Phosphorylation Assay
In vitro phosphorylation assays of a 120-kD protein in microsomal membranes were performed as previously described by Sakai et al. (2000), with some modifications. Briefly, microsomal membrane pellets were obtained from ∼650 2-d-old etiolated transgenic seedlings of pMDC7-PRT2 and pER8 that were grown on half-strength Okada and Shimura agar medium with or without 10 µM Est. The pellets were resuspended in 30 μL of phosphorylation buffer (50 mM Tris-MES, pH 7.5, 5 mM MgSO4, 150 mM NaCl, 1 mM EGTA, 1 mM DTT, 0.5% (v/v) Triton X-100, and a protease inhibitor mixture [Complete Mini EDTA-free; Roche Diagnostics]) by pipetting. All manipulations were performed at 4°C under a dim red safelight.
Twenty micrograms of microsomal extract was diluted to a final volume of 9 μL in phosphorylation buffer, and [γ-32P]ATP was added to a final concentration of 200 µM (specific activity, 2.5 Ci/mmol). The membrane extracts were then incubated for 2 min at 30°C and irradiated with blue light at 10 μmol m–2 s–1 for 16 min. Dark control samples were mock irradiated. After the irradiations, the samples were mixed with an equal volume of 2× SDS gel loading buffer to stop the reaction. Ten micrograms of each sample was then electrophoresed on a 6% (w/v) SDS-PAGE gel. Gels were dried and then autoradiographed by exposure to x-ray film. The images of these films were recorded with a scanner (ES8500; Epson) and the signal intensities of the 120-kD protein were quantified with Fiji software (Schindelin et al., 2012). The experiments were performed three times using independent samples.
Treatments of Protein Phosphatase Inhibitors
CN and OKA (FUJIFILM Wako Pure Chemical) were prepared as 50 and 1 mM stock solutions, respectively, in DMSO. The treatments with these chemicals were performed as previously described by Sullivan et al. (2019), with some modifications. Briefly, the aerial portions of the hypocotyls were prepared from ∼100 2-d-old etiolated seedlings with a blade under safe green light conditions. Red light irradiation at 10 µmol m–2 s–1 for 2 min was performed prior to preparation of the segment. The segments were then dipped in half strength Okada and Shimura medium containing 30 µM CN and 1 µM OKA or an equivalent volume of DMSO and vacuum infiltrated for 15 min. After a subsequent incubation under darkness for 105 min at 60 rpm, the segments were irradiated with blue light at 0.1 µmol m–2 s–1 for 30 min, immediately harvested with forceps, and frozen with liquid nitrogen.
GUS Histochemical Analysis
Transgenic seedlings carrying the RPT2pro:GUS gene were stained with 5-bromo-4-chloro-3-indolyl-β-glucuronide as described previously (Nagashima et al., 2008). Seedling images were obtained with an MZ-16FA/DFC500 digital stereomicroscope (Leica; http://www.leica-microsystems.com).
VENUS Imaging
VENUS fluorescence was visualized with an MZ-16FA/DFC500 digital stereomicroscope with the YFP filter (Leica). Confocal fluorescence images were recorded as previously described by Haga et al. (2015). The VENUS signal intensity was quantified with Fiji software (Schindelin et al., 2012). To prepare hypocotyl cross sections, the rpt2 mutant transformed with a RPT2pro:RPT2-VENUS gene were irradiated with blue light at 10 µmol m–2 s–1 for 6 h, subsequently mounted in 2% (w/v) agarose, and hand sectioned with a blade.
Transcriptional Analysis by RT-qPCR
Total RNA was extracted using a RNeasy kit (QIAGEN). RT-qPCR was performed using a PCR system (StepOne; Applied Biosystems) and the SYBR Green dye–based RT-qPCR kit (Luna One-Step RT-qPCR Kit; NEB) in accordance with the manufacturer’s protocol. Triplicate PCR reactions were performed in each case and three biological independent samples were used for each gene. The value was calculated relative to that of the sample mock irradiated after normalization by the 18S rRNA expression level. The primers used are listed in Supplemental Table 2.
Measurement of Phototropic Curvature
Phototropic curvatures of hypocotyls were measured on agar medium in 0.2-mL tubes using the advanced method previously described by Haga and Sakai (2012) and Haga and Kimura (2019).
Accession Numbers
The sequence data for this article can be found in the Arabidopsis Genome Initiative or the EMBL/GenBank data libraries under the following accession numbers: RPT2 (AT2G30520), PHOT1 (AT3G45780), NPH3 (AT5G64330), PHOT2 (AT5G58140), and UBQ3 (AT5G03240). Germplasm used included nph3-102 (Salk_110039), phot1 (Salk_146058), and phot2 (Salk_142275).
Supplemental Data
Supplemental Figure 1. Time-course analysis of continuous light-induced phototropism in the phot1 phot2 double mutants transformed with 35Spro:PHOT1LOV1Cys39Ala.
Supplemental Figure 2. Time-course analysis of phot1 autophosphorylation in tube-grown seedlings.
Supplemental Figure 3. The effect of phosphatase on the rpt2-induced mobility shift of PHOT1 during Phos-tag SDS-PAGE.
Supplemental Figure 4. Evaluation of the anti-PHOT2 antibody.
Supplemental Figure 5. Binding activity of phot2 to RPT2.
Supplemental Figure 6. Characterization of the rpt2 mutants transformed with a pMDC7-RPT2 construct.
Supplemental Figure 7. Suppression of phot1 dephosphorylation by protein phosphatase inhibitors.
Supplemental Figure 8. Effect of the proteasome inhibitor MG132 on RPT2 protein expression in wild-type seedlings under red light conditions.
Supplemental Figure 9. mRNA expression levels of RPT2 and NPH3 in the 35Spro:UBQ3 transgenic lines.
Supplemental Figure 10. Phenotypes of the 35Spro:UBQ3 transgenic lines.
Supplemental Table 1. Gene-specific primers used for construction.
Supplemental Table 2. Gene-specific primers used for RT-qPCR.
Supplemental File. Generation of the 35Spro:GFP-PHOT2 transgenic line.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank the Arabidopsis Biological Resource Center for providing the phot1 (Salk_146058) and phot2 (Salk_142275) seeds. We also thank John Christie (University of Glasgow, Glasgow, United Kingdom) for kindly providing the seeds of the 35Spro:PHOT1LOV1C39A transgenic line, Nam-Hai Chua (Rockefeller University, New York) for generously donating the pMDC7 vector, and Masatoshi Yamaguchi (Saitama University, Saitama, Japan) and Taku Demura (Nara Institute of Science and Technology, Ikoma, Japan) for generously supplying the pH35GS binary vectors. This work was supported by the Japan Society for the Promotion of Science (KAKENHI 22570058, 25120710, 16H01231, 17H03694, and 18K19329 to T.S.).
AUTHOR CONTRIBUTIONS
T.K., T.T.-M., and T.S. designed and conducted most of the research and analyzed the data. H.I., K.O., and K.I. designed and performed the phot1 in vitro phosphorylation assay. T.K. and T.S. wrote the article.
References
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. [DOI] [PubMed] [Google Scholar]
- Ausubel F.M., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A., Struhl K.(2001). Current Protocols in Molecular Biology. (New York: John Wiley & Sons; ). [Google Scholar]
- Briggs W.R., et al. (2001). The phototropin family of photoreceptors. Plant Cell 13: 993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal J.J., Candia A.N., Sellaro R.(2014). Light perception and signalling by phytochrome A. J. Exp. Bot. 65: 2835–2845. [DOI] [PubMed] [Google Scholar]
- Christie J.M.(2007). Phototropin blue-light receptors. Annu. Rev. Plant Biol. 58: 21–45. [DOI] [PubMed] [Google Scholar]
- Christie J.M., Reymond P., Powell G.K., Bernasconi P., Raibekas A.A., Liscum E., Briggs W.R.(1998). Arabidopsis NPH1: A flavoprotein with the properties of a photoreceptor for phototropism. Science 282: 1698–1701. [DOI] [PubMed] [Google Scholar]
- Christie J.M., Swartz T.E., Bogomolni R.A., Briggs W.R.(2002). Phototropin LOV domains exhibit distinct roles in regulating photoreceptor function. Plant J. 32: 205–219. [DOI] [PubMed] [Google Scholar]
- Clough R.C., Vierstra R.D.(1997). Phytochrome degradation. Plant Cell Environ. 20: 713–721. [Google Scholar]
- Curtis M.D., Grossniklaus U.(2003). A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133: 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit M., Galvão V.C., Fankhauser C.(2016). Light-mediated hormonal regulation of plant growth and development. Annu. Rev. Plant Biol. 67: 513–537. [DOI] [PubMed] [Google Scholar]
- Genschik P., Sumara I., Lechner E.(2013). The emerging family of CULLIN3-RING ubiquitin ligases (CRL3s): Cellular functions and disease implications. EMBO J. 32: 2307–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga K., Kimura T.(2019). Physiological characterization of phototropism in Arabidopsis seedlings In Phototropism: Methods and Protocols, Methods in Molecular Biology, Yamamoto K.T., ed, Volume. Vol. 1924 (New York: Human Press; ), pp. 3–17. [DOI] [PubMed] [Google Scholar]
- Haga K., Sakai T.(2012). PIN auxin efflux carriers are necessary for pulse-induced but not continuous light-induced phototropism in Arabidopsis. Plant Physiol. 160: 763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga K., Tsuchida-Mayama T., Yamada M., Sakai T.(2015). Arabidopsis ROOT PHOTOTROPISM2 contributes to the adaptation to high-intensity light in phototropic responses. Plant Cell 27: 1098–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A., Takemiya A., Inoue S., Sakai T., Shimazaki K.(2013). Role of RPT2 in leaf positioning and flattening and a possible inhibition of phot2 signaling by phot1. Plant Cell Physiol. 54: 36–47. [DOI] [PubMed] [Google Scholar]
- Harper S.M., Christie J.M., Gardner K.H.(2004). Disruption of the LOV-Jalpha helix interaction activates phototropin kinase activity. Biochemistry 43: 16184–16192. [DOI] [PubMed] [Google Scholar]
- Inada S., Ohgishi M., Mayama T., Okada K., Sakai T.(2004). RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with phototropin 1 in Arabidopsis thaliana. Plant Cell 16: 887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Sakai T., Suetsugu N., Oikawa K., Ishiguro S., Kato T., Tabata S., Okada K., Wada M.(2001). Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high-light avoidance response. Science 291: 2138–2141. [DOI] [PubMed] [Google Scholar]
- Kaiserli E., Sullivan S., Jones M.A., Feeney K.A., Christie J.M.(2009). Domain swapping to assess the mechanistic basis of Arabidopsis phototropin 1 receptor kinase activation and endocytosis by blue light. Plant Cell 21: 3226–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita E., Kinoshita-Kikuta E.(2011). Improved Phos-tag SDS-PAGE under neutral pH conditions for advanced protein phosphorylation profiling. Proteomics 11: 319–323. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Doi M., Suetsugu N., Kagawa T., Wada M., Shimazaki K.(2001). Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656–660. [DOI] [PubMed] [Google Scholar]
- Li J., Li G., Wang H., Deng X.Q.(2011). Phytochrome signaling mechanism In The Arabidopsis Book, Volume 9, p. e0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Yang H., Guo H., Mockler T., Chen J., Cashmore A.R.(1998). Enhancement of blue-light sensitivity of Arabidopsis seedlings by a blue light receptor cryptochrome 2. Proc. Natl. Acad. Sci. USA 95: 2686–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E., Briggs W.R.(1995). Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell 7: 473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motchoulski A., Liscum E.(1999). Arabidopsis NPH3: A NPH1 photoreceptor-interacting protein essential for phototropism. Science 286: 961–964. [DOI] [PubMed] [Google Scholar]
- Nagashima A., et al. (2008). Phytochromes and cryptochromes regulate the differential growth of Arabidopsis hypocotyls in both a PGP19-dependent and a PGP19-independent manner. Plant J. 53: 516–529. [DOI] [PubMed] [Google Scholar]
- Nakasako M., Iwata T., Matsuoka D., Tokutomi S.(2004). Light-induced structural changes of LOV domain-containing polypeptides from Arabidopsis phototropin 1 and 2 studied by small-angle X-ray scattering. Biochemistry 43: 14881–14890. [DOI] [PubMed] [Google Scholar]
- Ohgishi M., Saji K., Okada K., Sakai T.(2004). Functional analysis of each blue light receptor, cry1, cry2, phot1, and phot2, by using combinatorial multiple mutants in Arabidopsis. Proc. Natl. Acad. Sci. USA 101: 2223–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima K.(2016). Molecular mechanism of phototropin light signaling. J. Plant Res. 129: 149–157. [DOI] [PubMed] [Google Scholar]
- Okajima K., Kashojiya S., Tokutomi S.(2012). Photosensitivity of kinase activation by blue light involves the lifetime of a cysteinyl-flavin adduct intermediate, S390, in the photoreaction cycle of the LOV2 domain in phototropin, a plant blue light receptor. J. Biol. Chem. 287: 40972–40981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedmale U.V., Liscum E.(2007). Regulation of phototropic signaling in Arabidopsis via phosphorylation state changes in the phototropin 1-interacting protein NPH3. J. Biol. Chem. 282: 19992–20001. [DOI] [PubMed] [Google Scholar]
- Petersen J., Inoue S.I., Kelly S.M., Sullivan S., Kinoshita T., Christie J.M.(2017). Functional characterization of a constitutively active kinase variant of Arabidopsis phototropin 1. J. Biol. Chem. 292: 13843–13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher E.H., Offringa R.(2012). Evolutionary adaptations of plant AGC kinases: From light signaling to cell polarity regulation. Front Plant Sci 3: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D., Pedmale U.V., Morrow J., Sachdev S., Lechner E., Tang X., Zheng N., Hannink M., Genschik P., Liscum E.(2011). Modulation of phototropic responsiveness in Arabidopsis through ubiquitination of phototropin 1 by the CUL3-Ring E3 ubiquitin ligase CRL3(NPH3). Plant Cell 23: 3627–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T.(2005). NPH3 and RPT2: s+Signal transducers in phototropin signaling pathways In Light Sensing in Plants, Wada M., Shimazaki K., and Iino M., eds (Tokyo: Springer-Verlag; ), pp. 179–184. [Google Scholar]
- Sakai T., Kagawa T., Kasahara M., Swartz T.E., Christie J.M., Briggs W.R., Wada M., Okada K.(2001). Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc. Natl. Acad. Sci. USA 98: 6969–6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Wada T., Ishiguro S., Okada K.(2000). RPT2. A signal transducer of the phototropic response in Arabidopsis. Plant Cell 12: 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K., Briggs W.R.(2002). Cellular and subcellular localization of phototropin 1. Plant Cell 14: 1723–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., et al. (2012). Fiji: An open-source platform for biological-image analysis. Nat. Methods 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock R.A., Clack T.(2002). Patterns of expression and normalized levels of the five Arabidopsis phytochromes. Plant Physiol. 130: 442–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S., Kharshiing E., Laird J., Sakai T., Christie J.M.(2019). Deetiolation enhances phototropism by modulating NON-PHOTOTROPIC HYPOCOTYL3 phosphorylation status. Plant Physiol. 180: 1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S., Thomson C.E., Lamont D.J., Jones M.A., Christie J.M.(2008). In vivo phosphorylation site mapping and functional characterization of Arabidopsis phototropin 1. Mol. Plant 1: 178–194. [DOI] [PubMed] [Google Scholar]
- Suetsugu N., Takemiya A., Kong S.G., Higa T., Komatsu A., Shimazaki K., Kohchi T., Wada M.(2016). RPT2/NCH1 subfamily of NPH3-like proteins is essential for the chloroplast accumulation response in land plants. Proc. Natl. Acad. Sci. USA 113: 10424–10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Koshiba T., Fujita C., Yamauchi Y., Kimura T., Isobe T., Sakai T., Taoka M., Okamoto T.(2019). Low-fluence blue light-induced phosphorylation of Zmphot1 mediates the first positive phototropism. J. Exp. Bot. 70: 5929–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida-Mayama T., Nakano M., Uehara Y., Sano M., Fujisawa N., Okada K., Sakai T.(2008). Mapping of the phosphorylation sites on the phototropic signal transducer, NPH3. Plant Sci. 174: 626–633. [Google Scholar]
- Tsuchida-Mayama T., Sakai T., Hanada A., Uehara Y., Asami T., Yamaguchi S.(2010). Role of the phytochrome and cryptochrome signaling pathways in hypocotyl phototropism. Plant J. 62: 653–662. [DOI] [PubMed] [Google Scholar]
- Xue Y., et al. (2018). Arabidopsis blue-light receptor phototropin 1 undergoes blue-light-induced activation in membrane microdomains. Mol. Plant 11: 846–859. [DOI] [PubMed] [Google Scholar]
- Zuo J., Niu Q.W., Chua N.H.(2000). Technical advance: An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 24: 265–273. [DOI] [PubMed] [Google Scholar]