Supplemental Digital Content is available in the text
Keywords: Children, COVID-19, Fecal shedding
ABSTRACT
Objective:
To investigate differences in viral shedding in respiratory and fecal samples from children with COVID-19.
Methods:
We searched PubMed, SCOPUS, Embase and Web of Science databases to identify pediatric studies comparing the pattern of fecal and respiratory shedding of SARS-CoV-2 RNA. Summary estimates were calculated using random-effects models.
Results:
Four studies reporting data from 36 children were included. A higher proportion of children had viral shedding in stools after 14 days of symptoms onset compared to respiratory samples (RR= 3.2, 95%CI 1.2 to 8.9, I2 = 51%). Viral RNA shedding was longer in fecal samples with a mean difference of approximately 9 days (Mean Difference = 8.6, 95%CI 1.7 to 15.4, I2 = 77%) compared with respiratory samples.
Conclusion:
SARS-CoV-2 shedding seems to be present in feces for a longer time than in the respiratory tract of children. Although fecal SARS-CoV-2 presence in feces do not confirm its transmissibility, the high and fast spread of the COVID-19 disease worldwide indicate other transmission routes are also plausible.
INTRODUCTION
In late December 2019, the Novel Coronavirus Disease 19 (COVID-19) emerged in Wuhan, Hubei Province, China, and rapidly spread to other countries around the world. Although COVID-19 is predominantly a respiratory disease mostly transmitted through respiratory droplets and skin to mucosae contact, other routes have been proposed, including fecal-oral transmission (1).
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) uses the angiotensin- converting enzyme 2 (ACE2) receptor to invade host cells in the lungs and other tissues leading to enhanced release of cytokines and a hyperinflammatory state with high morbidity and mortality (2–4). Although children are susceptible to infection, most infections are asymptomatic or have mild clinical presentations, raising the possibility that children could be an imperceptible source of infection (5).
Recently reports suggest that SARS-CoV-2 RNA remains detectable in the stools of children, even after tests of samples from their respiratory tracts have become negative, suggesting persistence of fecal viral shedding after symptom resolution (6). We systematically investigated the differences in viral shedding patterns of respiratory and fecal samples from children with COVID-19.
METHODS
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (7). Searches for studies were performed from January 1, 2020 up to April 19, 2020 in the peer-reviewed (PubMed, Embase, Web of Science, and Scopus) and grey literature, including Google Scholar and preprint publications in the bioRxiv and medRxiv repositories. The reference lists of all eligible studies were also scanned to identify additional studies for inclusion. The search terms used were: “COVID-19”, “SARS-CoV-2”, “coronavirus”, “pediatrics”, “pediatric”, “children”, “neonates”, “newborns”, “shedding”, and “viral load”.
The following elements were used to define eligibility criteria: (1) population: children with COVID-19; (2) comparison groups: respiratory versus fecal samples; (3) outcomes: viral shedding of SARS-CoV-2 RNA detection by RT-PCT beyond 14 days of symptoms onset; (4) study type: observational studies. Duration of viral shedding in respiratory swabs and stool samples was also extracted and defined as the number of days from symptoms onset to the day when the last specimen in which SARS-CoV-2 was detected. We excluded publications with potential overlapping reports based on data collection and setting and studies in which data extraction was not possible. In situations of studies with potential overlapping data, we selected the study with the most complete information.
Reports were screened in two stages: screening of titles and abstracts followed by the retrieval and screening of full-text articles. Two authors extracted data from all reports and crosschecked them for accuracy. The risk of bias assessment was conducted using the Joanna Briggs Critical Appraisal Tools for Case Series (8). A random-effects meta-analysis was used to pool the individual measures from the included studies, and results were expressed as the risk ratio (RR) (for persistence of viral shedding) and the mean difference (MD) (for the duration of viral shedding) with 95% confidence intervals (CI). Statistical heterogeneity was quantified by the I2 index. Analyses were conducted using the Review Manager 5.3 (Cochrane IMS, Copenhagen, Denmark).
RESULTS
After screening 212 titles and abstracts, five full-text articles were assessed for eligibility. Of these, one case series of three patients was excluded. Four studies (6,9–11) reporting data from 36 patients (15 boys and 21 girls) were included. All studies had clear objectives and eligibility criteria and recruited subjects from the same population. However, some of the included children remained hospitalized at the time the studies reported the data, and whether participants were recruited consecutively is unclear, possibly introducing bias.
The mean ages ranged from 56 to 91 months. In all studies, children had mild symptoms of COVID-19: 58.3% (21/36) presented with fever, 46.7% (14/30) with cough, and 10% (3/10) with diarrhea. No child had dyspnea, needed respiratory support or Intensive Care Unit (ICU) care, and none died. On admission, SARS-CoV-2 RNA was detected in 94.4% (34/36) and 68.8% (22/32) of respiratory and fecal samples by RT-PCR, respectively (see Table in Supplemental Digital Content, http://links.lww.com/MPG/B855). After 14 days, children were more likely to continue shedding SARS-CoV-2 RNA in fecal samples compared to respiratory swabs (RR = 3.2, 95%CI 1.2 to 8.9, I2 = 51%) (Figure 1). The duration of SARS- CoV-2 RNA shedding was longer in fecal than respiratory samples with a mean difference of 8.6 days (95%CI 1.7 to 15.4, I2 = 77%).
FIGURE 1.
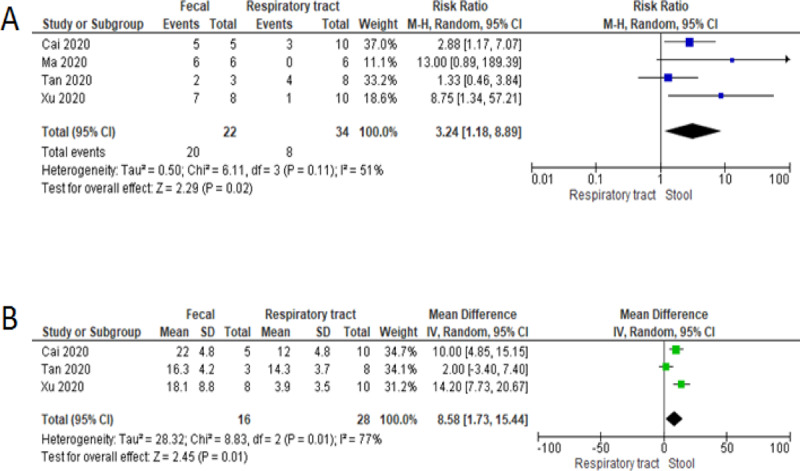
Forest plot showing the risk of persistent viral shedding in fecal samples compared to respiratory tract swabs (A) and mean difference in duration of shedding between samples (B).
DISCUSSION
This meta-analysis confirms that SARS-CoV-2 RNA is present in feces of a high proportion of children with COVID-19 and that viral RNA shedding occurs for a longer time than in the respiratory tract, even after RT-PCR tests of the respiratory tract have become negative.
The COVID-19 pandemic disproportionately impacts adults compared with children. Adults have an increased risk of developing severe COVID-19, including the acute respiratory distress syndrome, which can result in death, especially among older individuals with comorbidities (3,4). In contrast, children are more likely to have asymptomatic infections and to experience mild symptoms (5). Children who require ambulatory care or hospitalization seem to have more gastrointestinal symptoms than adults, including vomiting and diarrhea (12). These gastrointestinal manifestations thus pose important challenges to clinicians at the time of diagnosis, and testing stools of children with suspected SARS-CoV-2 infections who have negative RT-PCR tests from respiratory samples could aid to confirm infections, especially among children presenting late to the clinics.
During the SARS-CoV-1 outbreak in 2003, fecal shedding was observed in 86% of infected individuals during the first 6-14 days of illness and could persist for >30 days from illness onset (13). Similarly, the Middle East Respiratory Syndrome-CoV (MERS-CoV) was detected in the stools of 15% of patients for up to 24 days after illness onset (14). In this study, SARS-CoV-2 RNA was detected more frequently in respiratory samples at admission, but fecal samples remained RNA-positive well after respiratory samples had become RT-PCR-negative.
Although it is uncertain at this moment whether these are live virus or just RNA fragments, this finding could raise a concern on the isolation policy for the COVID-19 patients, particularly during the recovery phase.
Although our focus was on SARS-CoV-2 shedding in fecal samples of children, similar persistent viral RNA shedding is reported in adults (15,16). A retrospective cohort of COVID-19 in China identified SARS-CoV-2 RNA in the stools of 59% of patients, with a similarly longer duration of shedding in stools than in the respiratory samples and peak RNA loads that occurred later in stools than in respiratory samples (17).
Our findings should be interpreted with caution. This is a preliminary review of case series on fecal SARS-CoV-2 transmission, with findings based on hospitalized children who typically have a high viral load. Fecal RNA shedding from asymptomatic children who did not require medical care is still unknown. Limited evidence exists on whether SARS-CoV-2 RNA shedding from stools is infectious and whether fecal viral shedding plays a role in the dissemination of infection.
In summary, as SARS-CoV-2 RNA shedding seems to be present in feces for a longer time than in respiratory samples. Although fecal SARS-CoV-2 presence in feces does not confirm its transmissibility, the rapid and prolific spread of the COVID-19 disease worldwide indicates other transmission routes are also plausible. More evidence of SARS- CoV-2 RNA in stools is necessary to determine its longer term patterns of fecal excretion and whether asymptomatic and mildly ill individuals have persistence of fecal viral shedding.
Footnotes
Conflict of interest: None declared.
Financial source: This study was not funded.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal's Web site (www.jpgn.org).
REFERENCES
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; doi:10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal– Oral Transmission. Gastroenterology 2020; 158 (6):1518–1519. doi:10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martins-Filho PR, Tavares CSS, Santos VS. Factors associated with mortality in patients with COVID-19. A quantitative evidence synthesis of clinical and laboratory data. Eur J Intern Med 2020; doi:10.1016/j.ejim.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; doi:10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelvin AA, Halperin S. COVID-19 in children: the link in the transmission chain. Lancet Infect Dis 2020; doi:10.1016/S1473-3099(20)30236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Y, Li X, Zhu B, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med 2020; 26 (4):502–505. doi:10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6 (7):e1000097.doi:10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moola S, Munn Z, Tufanaru C. Aromatari E, Munn Z, et al. Systematic review of etiology and risk. The Joanna Briggs Institute, Joanna Briggs Institute Reviewer's Manual. Adelaide, Australia:2017. [Google Scholar]
- 9.Cai J, Xu J, Lin D, et al. A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis 2020; doi:10.1093/cid/ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma X, Su L, Zhang Y, Zhang X, Gai Z, Zhang Z. Do children need a longer time to shed SARS-CoV-2 in stool than adults? J Microbiol Immunol Infect 2020; doi:10.1016/j.jmii.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan Y-P, Tan B-Y, Pan J, Wu J, Zeng S-Z, Wei H-Y. Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J Clin Virol 2020; 127:104353.doi:10.1016/j.jcv.2020.104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmermann P, Curtis N. Coronavirus Infections in Children Including COVID-19. Pediatr Infect Dis J 2020; 39 (5):355–368. doi:10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan KH, Poon LLLM, Cheng VCC, et al. Detection of SARS Coronavirus in Patients with Suspected SARS. Emerg Infect Dis 2004; 10 (2):294–299. doi:10.3201/eid1002.030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corman VM, Albarrak AM, Omrani AS, et al. Viral Shedding and Antibody Response in 37 Patients With Middle East Respiratory Syndrome Coronavirus Infection. Clin Infect Dis 2015; civ951.doi:10.1093/cid/civ951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung KS, Hung IF, Chan PP, et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples from the Hong Kong Cohort and Systematic Review and Meta-analysis. Gastroenterology 2020; doi:10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han C, Duan C, Zhang S, et al. Digestive Symptoms in COVID-19 Patients With Mild Disease Severity. Am J Gastroenterol 2020; 1.doi:10.14309/ajg.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ 2020; 369:m1443.doi:10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]


