ABSTRACT
Coronavirus disease 2019 (COVID-19) may lead to a severe inflammatory response referred to as a cytokine storm. We describe a case of severe COVID-19 infection in a recently diagnosed pediatric Crohn's disease patient successfully treated with Tumor Necrosis Factor-alpha (TNF-α) blockade. The patient presented with five days of fever, an erythematous maculopapular facial rash, and abdominal pain without respiratory symptoms. SARS-CoV-2 PCR was positive. Despite inpatient treatment for COVID-19 and a perianal abscess, the patient acutely decompensated, with worsening fever, tachycardia, fluid-refractory hypotension, elevation of liver enzymes, and transformation of the rash into purpura extending from the face to the trunk, upper and lower extremities, including the palmar and plantar surfaces of the hands and feet. Cytokine profile revealed rising levels of interleukin (IL)-6, IL-8, and TNF-α, higher than those described in either inflammatory bowel disease (IBD) or severe COVID-19 alone. The patient was treated with infliximab for TNF-α blockade to address both moderately to severely active Crohn's disease and multisystem inflammatory syndrome in children (MIS-C) temporally related to COVID-19. Within hours of infliximab treatment, fever, tachycardia and hypotension resolved. Cytokine profile improved with normalization of TNF-α, a decrease in IL-6, and IL-8 concentrations. This case supports a role for blockade of TNF-α in the treatment of COVID-19 inflammatory cascade. The role of anti-TNF agents in patients with MIS-C temporally related to COVID-19 requires further investigation.
Keywords: biologics, COVID-19, crohn's disease, disease, inflammatory bowel, infliximab, MIS-C
Abbreviations: COVID-19, Coronavirus Disease 2019, CRP, C-reactive protein, EBV, epstein barr virus, ESR, Erythrocyte sedimentation rate, HLH, Hemophagocytic lymphohistiocytosis, IBD, Inflammatory bowel disease, IL, Interleukin, IV, Intravenous, MR, Magnetic Resonance, MIS-C, Multisystem inflammatory syndrome in children, PCR, Polymerase chain reaction, SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2, TNF-α, Tumor necrosis factor-alpha
What Is Known/What Is New
What Is Known
Coronavirus disease 2019 (COVID-19) may lead to severe inflammatory response and cytokine storm.
More than 200 patients with multisystem inflammatory syndrome in children and adolescents (MIS-C) temporally related to COVID-19 infection have been reported.
Anti-Tumor necrosis factor-α therapy with infliximab is effective for the induction and maintenance of remission in pediatric Crohn's disease patients.
What Is New
Higher levels of pro-inflammatory cytokines can be seen in patients with inflammatory bowel disease and cytokine storm associated with COVID-19 infection than are reported in either inflammatory bowel disease or with COVID-19 alone.
Infliximab therapy can effectively treat both pediatric Crohn's disease and MIS-C temporally associated with COVID-19 infection.
INTRODUCTION
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, may lead to a severe inflammatory response or cytokine storm (1). Immune dysfunction in untreated Crohn's disease may augment the risk for a severe inflammatory response with COVID-19 (2). We describe a pediatric patient recently diagnosed with Crohn's disease who developed severe COVID-19 infection successfully treated with infliximab.
CASE
A 14 year old male with recently diagnosed small bowel, perianal Crohn's disease presented with 5 days of fevers and abdominal pain without respiratory symptoms. Physical exam was notable for tachycardia, an erythematous maculopapular facial rash, abdominal tenderness and a perianal lesion with purulent drainage. Initial laboratory tests revealed a C-reactive protein (CRP) of 79.8 mg/L (normal 0–5 mg/L), an erythrocyte sedimentation rate (ESR) of 64 mm/hr (normal 0–15 mm/hr) and hypoalbuminemia of 2.9 g/dL. SARS-CoV-2 PCR was positive. Magnetic resonance (MR) enterography revealed 28 cm of ileitis, a 2.3 cm perianal abscess and fistula. Chest x-ray was negative for an acute pulmonary process. Blood culture and stool PCR were negative. Treatment was initiated with intravenous (IV) piperacillin/tazobactam for his perianal abscess, hydroxychloroquine and azithromycin for SARS-CoV-2 infection, enoxaparin for prophylaxis of venous thromboembolism, and IV fluids.
On day 3, cytokine profile revealed elevated serum concentrations of interleukin (IL)-6 of 73.6 (normal 0–5) pg/mL, IL-8 of 43.1 (normal 0–5) pg/mL, and tumor necrosis factor-alpha (TNF-α) of 68.7 (normal 0–22) pg/mL. D-Dimer and ferritin (2.14 μg/mL fibrinogen equivalent units (FEU) and 920 ng/mL, respectively) were also elevated. Fevers persisted despite a 3 and 5 day course of azithromycin and hydroxychloroquine, respectively. Drainage of the perianal abscess occurred on day 6.
Elevated liver enzymes developed on day 7, with an aspartate aminotransferase (AST) of 145 U/L, alanine aminotransferase (ALT) of 98 U/L, gamma-glutamyl transferase of 282 U/L, alkaline phosphatase of 199 U/L, and normal total bilirubin. Fevers persisted (temperature maximum 39.3°C) with tachycardia and fluid refractory hypotension. The rash also became purpuric, with areas of confluence progressing initially from the face to the trunk, upper and lower extremities including the palmar and plantar surfaces of the hands and feet. Antibiotics were switched to IV ciprofloxacin and metronidazole without improvement. Computed Tomography (CT) scan of the chest, abdomen, and pelvis did not show any progression of perianal or ileal disease, nor pulmonary infiltrates consistent with COVID-19, but did reveal new findings of mediastinal lymphadenopathy and hepatosplenomegaly.
Infectious disease, rheumatology, dermatology, cardiology and hematology were consulted on day 7. Differential diagnosis at the time included a drug hypersensitivity reaction, immune-mediated vasculitis, viral infection, atypical Kawasaki disease and MIS-C. Repeat concentrations of IL-6, IL-8, and TNF-α were rising (Table 1). Multidisciplinary discussion of potential therapeutic options included remdesivir, an antiviral therapy, and tocilizumab, an IL-6 inhibitor, for the treatment of COVID-19, IV immune globulin for the treatment of an immune-mediated vasculitis, and infliximab, an anti-TNF-α therapy. Infliximab would address the management of Crohn's disease and potentially, the cytokine storm attributable to MIS-C associated with COVID-19. Given the possible dual role and rapid clinical deterioration, he received 10 milligrams/kilogram (mg/kg) of infliximab on day 8 without additional studies such as an echocardiogram or skin biopsy. Within hours, fever, tachycardia and hypotension resolved.
TABLE 1.
Serum cytokine profile trend over time in response to treatment with infliximab on hospitalization day 8
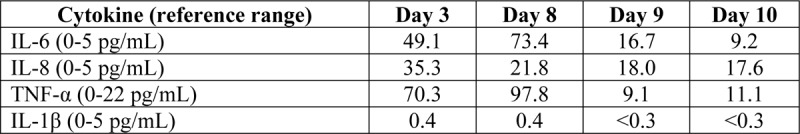
Cytokines improved with normalization of TNF-α, a decrease in IL-6, and IL-8. The progression of the rash was halted after infliximab treatment on hospitalization day 8 and significantly improved by discharge (Fig. 1). CRP normalized. He was given a second infusion of infliximab10 mg/kg prior to discharge, 5 days after the initial dose due to a high inflammatory burden and presence of active perianal disease. Prometheus infliximab concentration prior to the second infusion was > 34 ug/mL without antibodies. Follow up two weeks after discharge revealed complete resolution of clinical symptoms and normalization of all labs previously elevated.
FIGURE 1.
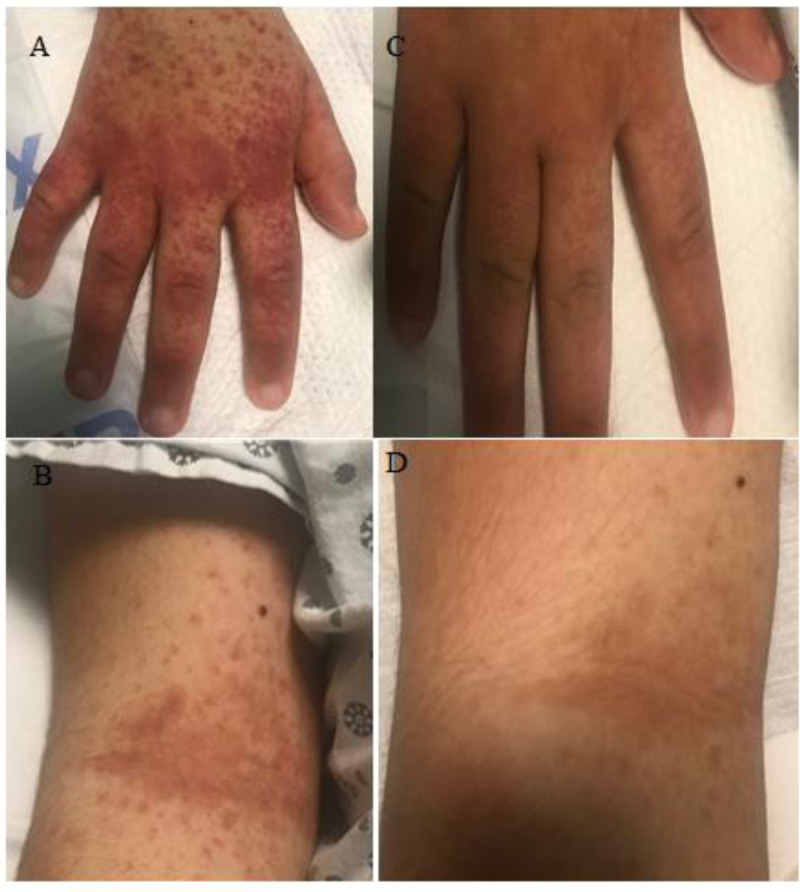
Purpuric rash on the dorsum of the hand and antecubital-fossa before and after treatment with infliximab on hospitalization day 8. Figure 1A and 1B represent the rash on the dorsum of the hand and antecubital fossa prior to infliximab treatment on hospitalization day 8. Figure 1C and 1D demonstrate the improvement in his rash on day 13 prior to discharge.
DISCUSSION
This is the first reported case of a patient with recently diagnosed Crohn's disease with suspected MIS-C temporally related to COVID-19 treated with infliximab to co-manage both entities. Despite surgical management of his active perianal disease and antibiotic therapy, the patient continued to worsen clinically. His clinical deterioration was unclear, as both IBD and MIS-C temporally related to COVID-19 could be implicated, with all other infectious studies negative.
Pro-inflammatory cytokines, TNF-α, IL-1β, and IL-6 are overproduced in IBD patients (3). Elevated levels of TNF-α, mean of 62.3 pg/ml, have been described in the intestinal mucosa of IBD patients (4). Serum concentration of TNF-α and IL-6 (median 10.5 pg/ml and 41.5 pg/ml respectively) are also associated with severe COVID-19 illness (5). At the peak of our patient's illness, serum concentrations of TNF-α (97.8 pg/ml) and IL-6 (73.4 pg/ml) were higher than is reported in either IBD or severe COVID-19. Inflammatory disease overlap possibly contributed to cytokine storm and MIS-C. Cytokine storm has been found to be a major cause of morbidity in patients with severe COVID-19 infection (1,5).
Treatment targeting these pro-inflammatory cytokines, such as IL-6 inhibition with tocilizumab, have shown evidence of clinical benefit in subsets of severe COVID-19 patients (6). TNF-α is also implicated in severe COVID-19 cases. Blockade of TNF-α is effective in the treatment of auto-inflammatory conditions in which several cytokines are elevated, suggesting anti-TNF-α therapy alone may inhibit a cytokine cascade (7). Currently, therapy targeting TNF-a has not been associated with negative outcomes in IBD patients with COVID-19 (8). This case supports a role for blockade of TNF-α in the treatment of COVID-19 inflammatory cascade. Whether this is specific for immune dysfunction in the setting of IBD or the non-IBD COVID-19 patient requires further investigation.
Overall, increased TNF-α levels are seen in cytokine storm due to COVID-19 and in active IBD. Treatment with infliximab is known to be effective for the treatment of children with Crohn's disease, and as evidenced by this case, was effectual in halting a systemic inflammatory response in our patient with COVID-19. The role of anti-TNF agents in patients with MIS-C temporally related to COVID-19 requires further investigation.
Footnotes
Contributors Statement Page: Dr. Michael T. Dolinger, Dr. Hannibal Person, Dr. Lauren Jarchin, Dr. Marla C. Dubinsky, and Dr. Joanne Lai conceptualized and designed this case report, drafted the initial manuscript, reviewed and revised the manuscript.
Dr. Rachel Smith and Dr. Nanci Pittman contributed to the care of the patient, critically reviewed the manuscript for important intellectual content and revised the manuscript.
Patient anonymity and informed consent: The patient and parents of the patient are aware of the intent to publish this case report and agree to it.
Conflicts of Interests and Sources of Funding: Dr. Marla C. Dubinsky: Consulting fees from Abbvie, Allergan, Amgen, Arena Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, Celgene, Ferring, Genentech, Gilead, Hoffmann-La Roche, Janssen, Pfizer, Prometheus Biosciences, Takeda, Target PharmaSolutions. Research funding from Abbvie, Janssen, Pfizer, Prometheus Biosciences, Takeda. The remaining authors have no financial relationships relevant to this article to disclose. No funding was secured for this study.
REFERENCES
- 1.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J infection 2020; 17:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubin DT, Abreu MT, Rai V, Siegel CA. International Organization for the Study of Inflammatory Bowel Disease. Management of Patients with Crohn's Disease and Ulcerative Colitis During the COVID-19 Pandemic: Results of an International Meeting. Gastroenterology 2020; Apr 6; Pii: S0016-5085(20)20465-0 doi. 10.1053/j.gastro.2020.04.002. [Google Scholar]
- 3.Singh UP, Singh NP, Murphy EA, Price RL, et al. Chemokine and cytokine levels in inflammatory bowel disease patients. Cytokine 2016; 77:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reimund JM, Wittersheim C, Dumont S, Muller CD, et al. Increased production of tumour necrosis factor-alpha interleukin 1-beta, and interleukin 6 by morphologically normal intestinal biopsies from patients with Crohn's disease. Gut 1996; 39 (5):684–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen G, Wu D, Guo W, Cao Y, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. Journal of Clinical Investigation 2020; 130 (5):2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michot JM, Albiges L, Chaput N, Saada V, et al. Tocilizumab, an anti-IL6 receptor antibody, to treat COVID-19 related respiratory failure: a case report. Annals of Oncology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldmann M, Maini RN, Woody JN, Holgate ST, et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet 2020; 395 (10234):1407–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF Antagonists, are Associated with Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results from an International Registry [published online ahead of print, 2020 May 18]. Gastroenterology 2020; 10.1053/j.gastro.2020.05.032. doi:10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]


