Abstract
Background
Nasopharyngeal carcinoma (NPC) is a common head and neck cancer epidemic in southern China and southeast Asia. LeiGongTeng has been widely used for the treatment of cancers. The purpose of this study was to determine the pharmacological mechanism of action of LeiGongTeng in the treatment of NPC using a network pharmacological approach.
Material/Methods
The traditional Chinese medicine systems pharmacology (TCMSP) database was used to identify active ingredients and associated target proteins for LeiGongTeng. Cytoscape was utilized to create a drug-disease network and topology analysis was conducted to analyze the degree of each ingredient. The Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) online tool was applied for the construction and analysis of the protein-protein interaction (PPI) network, while Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment and Gene Ontology (GO) functional analyses were utilized to determine drug-disease common genes.
Results
22 active ingredients including kaempferol, nobiletin, and beta-sitosterol, and 30 drug-disease common genes including VEGFA, CASP3, ESR1, and RELA were identified. GO analysis indicated that 94 biological processes, including RNA polymerase II, apoptotic process, response to drug, cell adhesion, and response to hypoxia, were found to be associated with NPC. The KEGG enrichment analysis showed that 58 pathways, including the PI3K-Akt signaling pathway, microRNAs in cancer, tumor necrosis factor (TNF) signaling pathway and pathways in cancer were found to be associated with NPC.
Conclusions
LeiGongTeng exerts its therapeutic effect through various biological processes and signaling pathways since it acts on several target genes. Systematic pharmacology can be used to predict the underlying function of LeiGongTeng and its mechanism of action in NPC.
MeSH Keywords: Medicine, Chinese Traditional; Nasopharyngeal Neoplasms; Pharmacologic Actions
Background
Nasopharyngeal carcinoma (NPC), a type of head and neck cancer, is an epithelial carcinoma arising from the nasopharyngeal mucosal lining [1]. It has a characteristic specific geographic distribution and racial prevalence [2]. The incidence of NPC is 50 cases per 100 000 people per year in southern China and southeast Asia [3]. Distant metastasis has resulted in the failure of treatment for these patients [4]. Due to its anatomical location, radiotherapy and chemotherapy have been considered standard treatment for patients with NPC [5]. However, side effects, such as radiation-related skin reaction, vomiting, and leukopenia may be particularly concerning for NPC patients [6]. Therefore, more effective and safe strategies for the treatment of NPC are still needed.
In Asia, traditional Chinese medicine (TCM) has been used frequently in cancer treatment [7]. TCM has the advantage of producing a reliable therapeutic efficacy, while inducing fewer adverse effects, and has drawn much attention in western countries during recent years [8]. LeiGongTeng is also known as Tripterygium wilfordii or Tripterygii Radix. Anti-cancer activity of Tripterygii Radix against many kinds of cancers has been illustrated [9–11]. Triptolide is a bio-active component isolated from Tripterygii Radix [12], previous studies reported that triptolide induce Epstein-Barr virus (EBV) nuclear antigen 1 (EBNA1) degradation and stimulate NPC cells apoptosis via mitochondria apoptotic pathway [13], triptolide in combined with cisplatin (DDP) showed a synergistic effect against DDP-resistant in NPC cells [14], moreover, triptolide in combination with ionizing radiation exhibits synergistic effects of anti-cancer and anti-angiogenesis in NPC cells [15]. The complex active compounds of TCM are difficult to clarify, while previous and potential pharmacological mechanisms of Tripterygii Radix in NPC have not been fully elucidated. Network pharmacology is an advanced method based on chemoinformatics, bioinformatics, network biology, and pharmacology [16]. Using a network-based approach, the active ingredients, fundamental molecular mechanisms, and pathways of TCM used for treatment can be systematically identified. In the study by He et al., 33 constituents of the Compound Kushen Injection (CKI) were found to be associated with anti-cancer activity, while 113 targeted proteins, 129 biological processes and 93 related pathways were identified [17]. The report by Yang et al. showed that 146 related proteins of 82 bioactive compounds in Wei Pi Xiao (WPX) decoction may explain the mechanism of activity involved in gastric precancerous lesion treatment, and 21 signaling pathways and 26 key biological processes were identified [18].
The purpose of this study was to analyze molecular mechanisms involved in the treatment effect and the active ingredients of Tripterygii Radix using the network pharmacology approach. The targets and active ingredients of Tripterygii Radix were identified and used to determine those that were common with Tripterygii Radix for the treatment of NPC. Then, construction of the protein-protein interaction (PPI) network, as well as Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) term enrichment analyses were conducted to identify related biological processes and signaling pathways.
Material and Methods
Identification of active compounds
Major compounds of Tripterygii Radix were derived using a database of Chinese herbal medicines that contains information on herbal entries, drug-disease networks and drug-target networks, and the traditional Chinese medicine systems pharmacology (TCMSP) database and analysis platform (http://lsp.nwu.edu.cn/tcmsp.php) [19]. The screening cutoff standards used were drug-likeness (DL) of ≥0.18 and oral bioavailability (OB) of ≥30%, and the compounds that satisfied these criteria were regarded as candidate compounds. The genes corresponding to the targets were obtained from the UniProt database.
Identification of disease target genes
Information on NPC-associated target genes was obtained from the GeneCards database (http://www.genecards.org/) and the Online Mendelian Inheritance in Man database (OMIM, http://www.ncbi.nlm.nih.gov/omim). The GeneCards database is a website that integrates genetic, proteomic, transcriptomic, and genomic information [20]. OMIM was created by McKusick in the early 1960s; it contains an authoritative and comprehensive compendium of human genetic phenotypes and genes [21]. The VennDiagram package of R software was utilized to obtain common target genes for drugs and diseases.
Construction of the “drug-disease” network
In order to comprehensively explore the pharmacological mechanisms involved, the Cytoscape (http://www.cytoscape.org/) visualization software 3.6.1 was used to create the drug-disease network [22]. Targets and compounds were input into Cytoscape and the drug-disease interaction network was established. Furthermore, we used the plug-in, NetworkAnalysizer, to calculate the degree of each compound. Degree is a critical parameter of the topology structure that is used to assess the importance of a compound.
Construction of the PPI network
The Search Tool for the Retrieval of Interacting Genes/Proteins (STRING, https://string-db.org/) is an online database of known and predicted protein-protein interactions [23]. The interactions included indirect (functional) and direct (physical) interactions. The network nodes are proteins and the edges show their associations. To further explore protein interactions systematically, we input common target genes for drugs into STRING to obtain relevant information on protein interactions. Then, this network was exported, and we carried out further statistical analysis of the protein interactions using Cytoscape 3.6.1 software. We used the plugin, Cytohubba, on hub genes from the PPI network in Cytoscape with a degree of >16.
GO analysis and KEGG pathway enrichment analysis
The GO Consortium database provides and limits the functions of genes. Biological processes related to various biological phenomena can be effectively identified through this method [24]. KEGG is a database developed by the University of Tokyo and Kyoto University, Japan, which is applied to screen functional and metabolic pathways [25]. The database for annotation, visualization, and integrated discovery (DAVID) was used to conduct the KEGG enrichment pathway and GO analyses [26]. DAVID is an online database, which can be used for enrichment analysis to show highly associated GO terms and KEGG pathways. We used the ClusterProfiler package of R software to visualize these results.
Results
Screening of active ingredients and target genes
The targets and active ingredients of Tripterygii Radix were predicted using the TCMSP database. We identified 144 compounds of Tripterygium wilfordii in total. Using the cutoff criteria of DL ≥0.18 and OB ≥30%, 51 active ingredients were identified (Table 1). Furthermore, we entered the target name and chose “human” as the species in the UniProt database. In total, 62 genes corresponding the potential targets of these 51 ingredients were identified.
Table 1.
The active ingredients of Tripterygii Radix.
| Mol ID | Molecule name | OB (%) | DL | Caco-2 | Molecular formula |
|---|---|---|---|---|---|
| MOL000211 | Mairin | 55.38 | 0.78 | 0.73 | C30H48O3 |
| MOL000296 | Hederagenin | 36.91 | 0.75 | 1.32 | C30H48O4 |
| MOL000358 | Beta-sitosterol | 36.91 | 0.75 | 1.32 | C29H50O |
| MOL000422 | Kaempferol | 41.88 | 0.24 | 0.26 | C15H10O6 |
| MOL000449 | Stigmasterol | 43.83 | 0.76 | 1.44 | C29H48O |
| MOL002058 | Medioresinol | 57.2 | 0.62 | 0.49 | C21H24O7 |
| MOL003184 | Neotriptophenolide | 45.42 | 0.53 | 0.85 | C21H26O4 |
| MOL003185 | Triptonoterpenol | 48.84 | 0.38 | 0.47 | C21H30O4 |
| MOL003187 | Triptolide | 51.29 | 0.68 | 0.25 | C20H24O6 |
| MOL003196 | Tryptophenolide | 48.5 | 0.44 | 1.11 | C20H24O3 |
| MOL003199 | 5,8-Dihydroxy-7-(4-hydroxy-5-methyl-coumarin-3)-coumarin | 61.85 | 0.54 | 0.02 | C19H12O7 |
| MOL003217 | Isoxanthohumol | 56.81 | 0.39 | 0.76 | C21H22O5 |
| MOL003229 | Triptinin B | 34.73 | 0.32 | 0.84 | C20H26O3 |
| MOL003231 | Triptoditerpenic acid B | 40.02 | 0.36 | 0.97 | C21H28O3 |
| MOL003245 | Triptonoditerpenic acid | 42.56 | 0.39 | 0.81 | C21H28O4 |
| MOL003248 | Triptonoterpene | 48.57 | 0.28 | 1.22 | C20H28O2 |
| MOL003266 | 21-Hydroxy-30-norhopan-22-one | 34.11 | 0.77 | 0.9 | C29H48O2 |
| MOL003280 | Triptonolide | 49.51 | 0.49 | 0.72 | C20H22O4 |
| MOL003283 | Isolariciresinol | 66.51 | 0.39 | −0.2 | C20H24O6 |
| MOL005828 | Nobiletin | 61.67 | 0.52 | 1.05 | C21H22O8 |
| MOL007535 | 5alpha-Stigmastane-3,6-dione | 33.12 | 0.79 | 0.9 | C29H48O2 |
| MOL009386 | 3,3′-bis-(3,4-dihydro-4-hydroxy-6-methoxy)-2H-1-benzopyran | 52.11 | 0.54 | 0.14 | C20H22O6 |
OB – oral bioavailability; DL – drug-likeness.
Screening for disease-related genes
We searched the GeneCards and OMIM databases using the keyword “nasopharyngeal carcinoma”, to obtain 1866 NPC related targets. Overlapping targets between the NPC-related target genes and the target genes of active ingredients were screened for. As a result, we identified a total of 30 overlapping genes between 1866 NPC related target genes and 62 target genes of active ingredients, as shown in Figure 1. After integrating and deleting duplicated genes, 22 active ingredients were identified.
Figure 1.
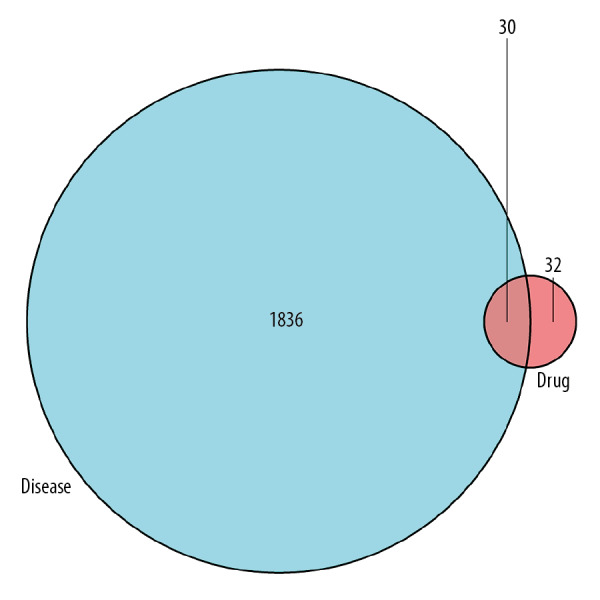
Venn diagram of disease related genes and drug targeted genes.
Drug-compound-target-disease network
We input 22 active ingredients and 30 “drug-disease” target genes into Cytoscape 3.6.1 software to construct a visualized drug-disease network. The network contained 54 nodes and 136 edges, as shown in Figure 2. A network analysis was conducted by assessing heterogeneity and centralization, which were found to be 1.131 and 0.491, respectively. Moreover, this network contained certain compounds with multiple targets, including high-degree compounds, such as MOL000422 (kaempferol, degree=19), MOL005828 (nobiletin, degree=12) and MOL000358 (beta-sitosterol, degree=9), as shown in Table 2.
Figure 2.
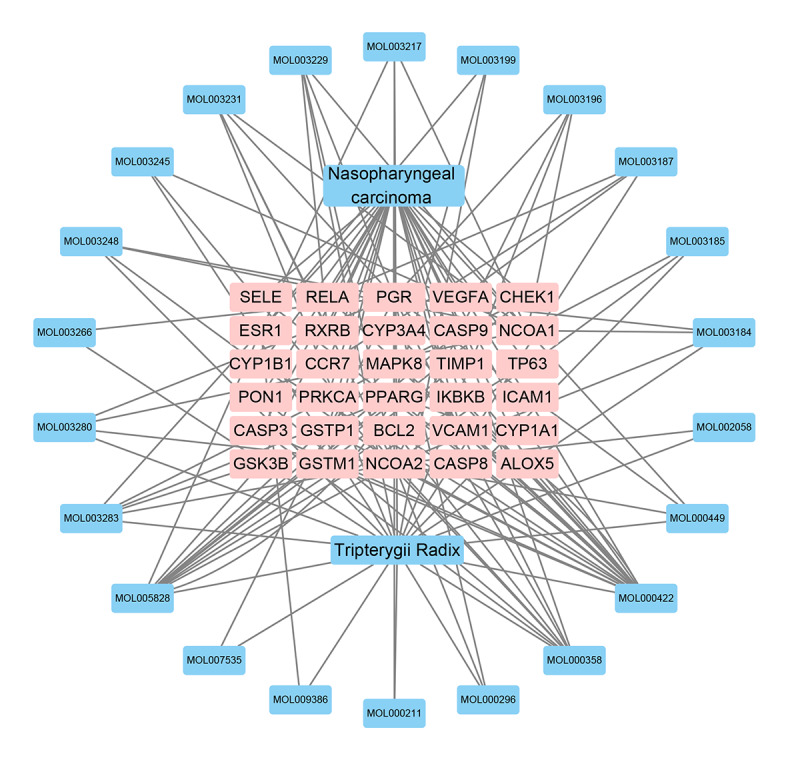
Drug-disease network analyses map.
Table 2.
Node degree of the drug–disease target network.
| Name | Type | Degree | Name | Type | Degree |
|---|---|---|---|---|---|
| Nasopharyngeal carcinoma | Disease | 30 | MAPK8 | Gene | 3 |
| Tripterygii Radix | Drug | 22 | CASP3 | Gene | 3 |
| MOL000422 | Mol | 19 | CASP9 | Gene | 3 |
| NCOA2 | Gene | 17 | RXRB | Gene | 3 |
| PGR | Gene | 14 | RELA | Gene | 3 |
| MOL005828 | Mol | 12 | MOL009386 | Mol | 2 |
| NCOA1 | Gene | 11 | MOL007535 | Mol | 2 |
| MOL000358 | Mol | 9 | MOL002058 | Mol | 2 |
| PPARG | Gene | 7 | MOL000211 | Mol | 2 |
| MOL003283 | Mol | 6 | MOL003266 | Mol | 2 |
| ESR1 | Gene | 6 | TIMP1 | Gene | 2 |
| MOL003231 | Mol | 5 | TP63 | Gene | 2 |
| MOL003229 | Mol | 5 | GSTM1 | Gene | 2 |
| MOL000449 | Mol | 4 | GSTP1 | Gene | 2 |
| MOL003280 | Mol | 4 | ALOX5 | Gene | 2 |
| MOL003248 | Mol | 4 | CYP1B1 | Gene | 2 |
| MOL003217 | Mol | 4 | VCAM1 | Gene | 2 |
| MOL003196 | Mol | 4 | SELE | Gene | 2 |
| MOL003187 | Mol | 4 | ICAM1 | Gene | 2 |
| MOL003184 | Mol | 4 | CYP1A1 | Gene | 2 |
| BCL2 | Gene | 4 | CYP3A4 | Gene | 2 |
| MOL003245 | Mol | 3 | IKBKB | Gene | 2 |
| MOL003199 | Mol | 3 | PON1 | Gene | 2 |
| MOL003185 | Mol | 3 | PRKCA | Gene | 2 |
| MOL000296 | Mol | 3 | CASP8 | Gene | 2 |
| CHEK1 | Gene | 3 | CCR7 | Gene | 2 |
| GSK3B | Gene | 3 | VEGFA | Gene | 2 |
PPI network analysis
We constructed the PPI network (Figure 3) using the STRING database; 139 edges and 30 nodes were identified in the “drug-disease network” as shown in Figure 2. In order to better understand these genes based on the PPI network, we visualized the network using Cytoscape software and the plug-in Cytohubba to screen out hub genes with a degree of >16, which included estrogen receptor 1 (ESR1), vascular endothelial growth factor A (VEGFA), caspase 3 (CASP3) and RELA proto-oncogene, NF-κB subunit (RELA). Then, the top 4 hub genes with the highest degrees were identified (Figure 4).
Figure 3.
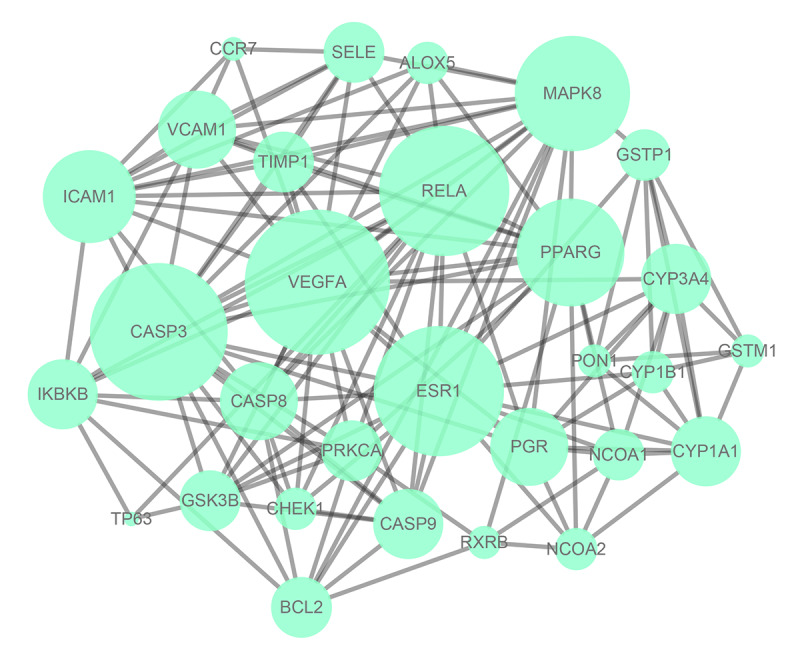
Protein target interaction network (PPI) of common target genes (the larger the size, the greater degree of the node).
Figure 4.
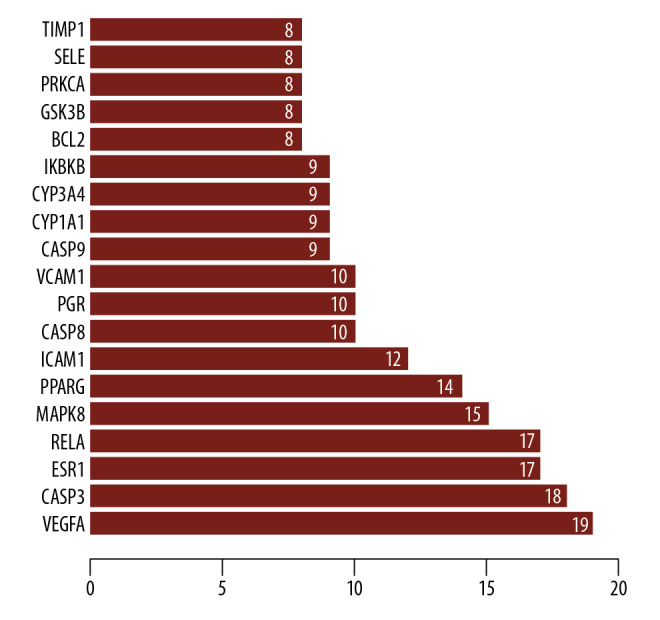
Protein interaction relationship histogram of common target genes.
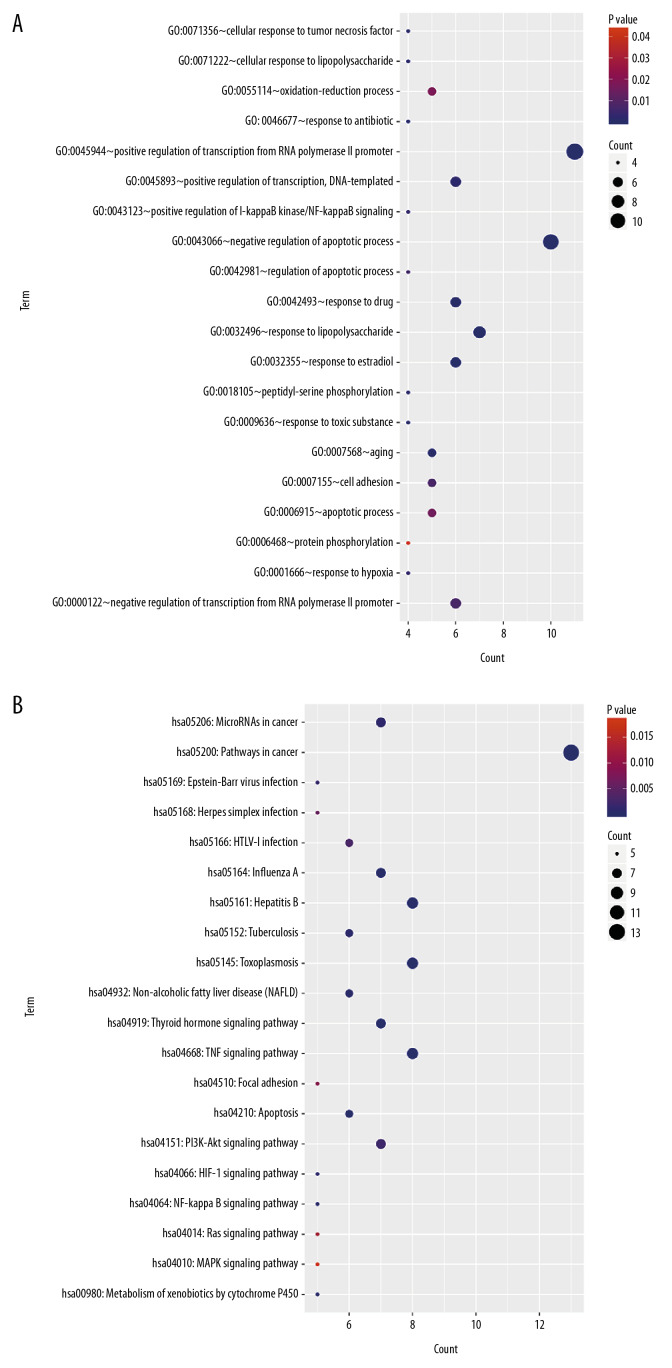
Analyses of GO function and KEGG pathway enrichment
In order to deeply explore the functional role of the “drug-disease” target genes and key pathways involved in the use of Tripterygii Radix for the treatment of NPC, KEGG pathway enrichment and GO functional analyses were conducted (Figure 5). The results of the signaling pathway analysis indicated that these genes were mainly associated with pathways, including the PI3K-Akt signaling pathway, microRNAs in cancer, tumor necrosis factor (TNF) signaling pathway, and pathways in cancer, while the GO analysis indicated that these genes were significantly enriched in biological processes (BP), including DNA-templated, positive regulation of transcription, apoptotic process, interfering with transcription from RNA polymerase II promoter, response to drug, cell adhesion, and response to hypoxia.
Figure 5.
Enrichment analysis. (A) Enrichment analysis of Gene Ontology (GO) biological processes of common target genes. (B) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of common target genes.
Discussion
NPC was found to be associated with the Epstein-Barr virus (EBV), while non-keratinizing differentiated or undifferentiated carcinoma are predominant histological subtypes in endemic areas [27]. More than 70% of patients were diagnosed with locoregionally advanced disease at presentation [28]. According to the National Comprehensive Cancer Network (NCCN) guidelines, the combination of chemo-radiotherapy (CRT) is the standard treatment modality for locoregionally advanced NPC [29], although this treatment strategy may be promising, patients may suffer from a series of complications that significantly lower the quality of life. Additionally, considering the complex pathogenesis of NPC, single-target or single-drug treatment methods remain insufficient to exert a significant therapeutic effect. Therefore, it is essential to identify new therapeutic strategies and uncover its underlying molecular mechanism to provide new options for NPC patients.
Tripterygii Radix contains several active ingredients, which affect multiple targets and pathways of action, and it has been proven to be an inexpensive and effective method of treatment for many diseases. Triptonide, a component of Tripterygii Radix, exerts preventive potential against NPC [30]. Furthermore, Celastrol, which is also extracted from Tripterygii Radix, induces apoptosis of NPC cells through the ERK1/2 and p38 MAPK pathways [31]. Nevertheless, its pharmacological mechanisms of action in NPC treatment are still unclear. In the present study, the mechanism of action of Tripterygii Radix involved in the treatment of NPC was investigated using a network pharmacology approach. A total of 22 NPC related active compounds of Tripterygii Radix with an OB of ≥30% and a DL of ≥0.18 were selected using the TCMSP database, and the active compounds-target gene network was constructed. The results showed that the top 3 highest degree compounds were kaempferol, nobiletin, and beta-sitosterol. Kaempferol is a polyphenolic compound that has been described as a key element in inducing cancer cell apoptosis, as well as in suppressing angiogenesis and cancer cell growth [32]. Yoshida et al. showed that kaempferol could induce human colon cancer cell apoptosis by significantly upregulating TNF-related apoptosis-inducing ligand (TRAIL) receptors (DR5 and DR4) [33]. Nobiletin is a polymethoxy flavonoid, which was shown by Ma et al. to be able to significantly inhibit the growth of hepatic cancer cells via increasing the expressions of caspase-3 and Bax, while reducing the expressions of COX-2 and Bcl-2 [34]. Beta-sitosterol is a type of phytosterol, which was shown by Awad et al. to be able to significantly inhibit the growth of breast cancer cells by increasing caspase-8 activity [35].
The results of the PPI network analysis indicated that 4 hub genes, VEGFA, CASP3, ESR1, and RELA, were regulated by Tripterygii Radix in NPC. VEGFA is a significantly pro-angiogenic factor in cancer, which has been well studied during the past decades [36]. In NPC, Epstein-Bar virus induced VEGF was found to promote cancer metastasis through the recruitment and activation of macrophages [37]. High expression of VEGFA was found to be involved in the poor long-term survival of NPC patients [38]. CASP3 performs an essential function in executing cell apoptosis in tumor behaviors and is involved in both extrinsic and intrinsic cell death signaling pathways [39]. Chen et al. showed that genetic variation in CASP3 may contribute to increased risk of head and neck squamous cell carcinoma [40]. Abnormal expression of ESR1 often occurs in various of human epithelial cancers [41]. Marc et al. reported that hypermethylation ESR1 was associated with in EBV-positive NPC [42]. RelA (p65), a member of the NF-κB/Rel family, has been proven to be involved in drug resistance and migration of NPC [43,44].
In terms of GO enrichment, for our study we infer that Tripterygii Radix might exert its treatment effect by interfering with the positive regulation of response to drug, transcription, transcription of the RNA polymerase II promoter, apoptotic process, DNA-templated, cell adhesion, and response to hypoxia. RNA polymerase II is a type of RNAP enzymes, which is found in eukaryotic cell nucleus [45], and it has been linked to the growth of NPC cells [46]. Tan et al. showed that Tripterygii Radix could suppress the adhering of breast cancer cells by inducing the cleavage of focal adhesion kinase (FAK) [47]. Hypoxia-inducible factor-1α (HIF-1α), has been proven to be extensively linked with drug resistance, aggressive progression and tumor survival [48]. Tripterygii Radix may mediate this antitumor effect by reducing transcriptional activity and increasing the accumulation of hypoxia-inducible factor-1α [49].
The KEGG pathway enrichment analysis results showed an association with PI3K-Akt signaling pathway, microRNAs in cancer, TNF signaling pathway, and pathways in cancer. This is similar to that of previous studies on NPC molecular biology. For example, a number of studies have indicated the function of TNF-α in the inflammatory process related to carcinogenesis and the progression of cancer, while it also performs an essential function in enhancing angiogenesis and increasing the invasion and migration of tumor cells [50]. In the study by Yu et al., high expression of TNF-α was identified as an unfavorable prognostic indicator in NPC [51]. MiRNAs function as negative gene regulators and have been proven to inhibit critical cancer-related gene expressions and may be promising for the diagnosis and treatment of various cancers, including NPC [52]. The PI3K-Akt signaling pathway is closely associated with cancer proliferation, invasion, and metastasis and is one of the most common and significant signaling pathways for the progression of cancer [53]. Wang et al. showed that triptolide reduced the viability of NPC cells through the PI3K/Akt pathway [54]. Therefore, we proposed that the pharmacological effects of Tripterygii Radix in NPC may occur through these pathways.
This is the first study which included the systematical exploration of potential mechanisms of action of Tripterygii Radix in NPC. However, in vivo and in vitro experiments should be undertaken to validate the relationship between key genes and pathways of Tripterygii Radix for the treatment of NPC. Despite the limitations of this study, the results of this study provide new evidence and information to be used in subsequent theoretical and clinical research studies.
Conclusions
In conclusion, we utilized a systems pharmacology method by combining active ingredient screening, target prediction, PPI analysis, biological process, and KEGG pathway analyses to explore the fundamental molecular mechanism of action of Tripterygii Radix for its therapeutic effect in NPC. Some of these predicted targets and pathways are similar to the pharmacological effects reported in previous studies. The results of this analysis indicated that Tripterygii Radix acts on multiple targets and plays a therapeutic role in NPC through its action in multiple pathways.
Footnotes
Source of support: This study was supported by the Basic Ability Enhancement Project of Young Teachers in Guangxi Zhuang Autonomous Region (No. 2018KY0134), Guangxi Science and Technology Cooperation and Exchange Project (GKH 159905-2-11), Central Guided Local Science and Technology Development Project (GK ZY18076006), and Guangxi Science and Technology Program Project (GK AD17129013)
References
- 1.Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 2.Le QT, Colevas AD, O’Sullivan B, et al. Current treatment landscape of nasopharyngeal carcinoma and potential trials evaluating the value of immunotherapy. J Natl Cancer Inst. :2019. doi: 10.1093/jnci/djz044. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang LQ, Chen DP, Guo L, et al. Concurrent chemoradiotherapy with nedaplatin versus cisplatin in stage II-IVB nasopharyngeal carcinoma: An open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2018;19:461–73. doi: 10.1016/S1470-2045(18)30104-9. [DOI] [PubMed] [Google Scholar]
- 4.Lee AW, Ma BB, Ng WT, Chan AT. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33:3356–64. doi: 10.1200/JCO.2015.60.9347. [DOI] [PubMed] [Google Scholar]
- 5.Frikha M, Auperin A, Tao Y, et al. A randomized trial of induction docetaxel-cisplatin-5FU followed by concomitant cisplatin-RT versus concomitant cisplatin-RT in nasopharyngeal carcinoma (GORTEC 2006-02) Ann Oncol. 2018;29:731–36. doi: 10.1093/annonc/mdx770. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16:645–55. doi: 10.1016/S1470-2045(15)70126-9. [DOI] [PubMed] [Google Scholar]
- 7.Xu W, Towers AD, Li P, Collet JP. Traditional Chinese medicine in cancer care: Perspectives and experiences of patients and professionals in China. Eur J Cancer Care. 2006;15:397–403. doi: 10.1111/j.1365-2354.2006.00685.x. [DOI] [PubMed] [Google Scholar]
- 8.Qu Y, Zhang Z, Lu Y, et al. Network pharmacology reveals the molecular mechanism of Cuyuxunxi prescription in promoting wound healing in patients with anal fistula. Evid Based Complement Alternat Med. 2019;2019 doi: 10.1155/2019/3865121. 3865121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong KF, Yuan Y, Luk JM. Tripterygium wilfordii bioactive compounds as anticancer and anti-inflammatory agents. Clin Exp Pharmacol Physiol. 2012;39:311–20. doi: 10.1111/j.1440-1681.2011.05586.x. [DOI] [PubMed] [Google Scholar]
- 10.Law SK, Simmons MP, Techen N, et al. Molecular analyses of the Chinese herb Leigongteng (Tripterygium wilfordii Hook.f.) Phytochemistry. 2011;72:21–26. doi: 10.1016/j.phytochem.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Ma L, Zhou GB. The main anticancer bullets of the Chinese medicinal herb, thunder god vine. Molecules. 2011;16:5283–97. doi: 10.3390/molecules16065283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corson TW, Crews CM. Molecular understanding and modern application of traditional medicines: Triumphs and trials. Cell. 2007;130:769–74. doi: 10.1016/j.cell.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou H, Liu Y, Wang C, et al. Triptolide inhibits Epstein-Barr nuclear antigen 1 expression by increasing sensitivity of mitochondria apoptosis of nasopharyngeal carcinoma cells. J Exp Clin Cancer Res. 2018;37:192. doi: 10.1186/s13046-018-0865-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Zhang JJ, Sun YM, et al. Triptolide induces apoptosis and synergizes with cisplatin in cisplatin-resistant HNE1/DDP nasopharyngeal cancer cells. Folia Biol (Praha) 2015;61:195–202. doi: 10.14712/fb2015061050195. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Kang M, Zhang T, et al. Triptolide combined with radiotherapy for the treatment of nasopharyngeal carcinoma via NF-κB-related mechanism. Int J Mol Sci. 2016;17(12):2139. doi: 10.3390/ijms17122139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopkins AL. Network pharmacology: The next paradigm in drug discovery. Nat Chem Biol. 2008;4:682–90. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 17.He R, Ou S, Chen S, Ding S. Network pharmacology-based study on the molecular biological mechanism of action for Compound Kushen Injection in anti-cancer effect. Med Sci Monit. 2020;26:e918520. doi: 10.12659/MSM.918520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L, Liu W, Hu Z, et al. A systems pharmacology approach for identifying the multiple mechanisms of action of the Wei Pi Xiao decoction for the treatment of gastric precancerous lesions. Evid Based Complement Alternat Med. 2019;2019 doi: 10.1155/2019/1562707. 1562707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ru J, Li P, Wang J, et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J Cheminformatics. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safran M, Chalifa-Caspi V, Shmueli O, et al. Human Gene-Centric Databases at the Weizmann Institute of Science: GeneCards, UDB, CroW 21 and HORDE. Nucleic Acids Res. 2003;31:142–46. doi: 10.1093/nar/gkg050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amberger JS, Bocchini CA, Schiettecatte F, et al. OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015;43:D789–98. doi: 10.1093/nar/gku1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su G, Morris JH, Demchak B, Bader GD. Biological network exploration with Cytoscape 3. Curr Protoc Bioinformatics. 2014;47:8.13.1–24. doi: 10.1002/0471250953.bi0813s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szklarczyk D, Franceschini A, Wyder S, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–52. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashburner M, Ball CA, Blake JA, et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanehisa M, Sato Y, Kawashima M, et al. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44:D457–62. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 27.Fang W, Yang Y, Ma Y, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: Results from two single-arm, phase 1 trials. Lancet Oncol. 2018;19:1338–50. doi: 10.1016/S1470-2045(18)30495-9. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Chen L, Hu GQ, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019;381:1124–35. doi: 10.1056/NEJMoa1905287. [DOI] [PubMed] [Google Scholar]
- 29.Chan AT, Grégoire V, Lefebvre JL, et al. Nasopharyngeal cancer: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii83–85. doi: 10.1093/annonc/mds266. [DOI] [PubMed] [Google Scholar]
- 30.Wang SS, Lv Y, Xu XC, et al. Triptonide inhibits human nasopharyngeal carcinoma cell growth via disrupting Lnc-RNA THOR-IGF2BP1 signaling. Cancer Lett. 2019;443:13–24. doi: 10.1016/j.canlet.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh MJ, Wang CW, Lin JT, et al. Celastrol, a plant-derived triterpene, induces cisplatin-resistance nasopharyngeal carcinoma cancer cell apoptosis though ERK1/2 and p38 MAPK signaling pathway. Phytomedicine. 2019;58:152805. doi: 10.1016/j.phymed.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida T, Konishi M, Horinaka M, et al. Kaempferol sensitizes colon cancer cells to TRAIL-induced apoptosis. Biochem Biophys Res Commun. 2008;375:129–33. doi: 10.1016/j.bbrc.2008.07.131. [DOI] [PubMed] [Google Scholar]
- 33.Chen AY, Chen YC. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013;138:2099–107. doi: 10.1016/j.foodchem.2012.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma X, Jin S, Zhang Y, et al. Inhibitory effects of nobiletin on hepatocellular carcinoma in vitro and in vivo. Phytother Res. 2014;28:560–67. doi: 10.1002/ptr.5024. [DOI] [PubMed] [Google Scholar]
- 35.Awad AB, Chinnam M, Fink CS, Bradford PG. Beta-sitosterol activates Fas signaling in human breast cancer cells. Phytomedicine. 2007;14:747–54. doi: 10.1016/j.phymed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Ye Z, Wang F, Yan F, et al. Identification of candidate genes of nasopharyngeal carcinoma by bioinformatical analysis. Arch Oral Biol. 2019;106:104478. doi: 10.1016/j.archoralbio.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Huang D, Song SJ, Wu ZZ, et al. Epstein-Barr virus-induced VEGF and GM-CSF drive nasopharyngeal carcinoma metastasis via recruitment and activation of macrophages. Cancer Res. 2017;77:3591–604. doi: 10.1158/0008-5472.CAN-16-2706. [DOI] [PubMed] [Google Scholar]
- 38.Xueguan L, Xiaoshen W, Yongsheng Z, et al. Hypoxia inducible factor-1 alpha and vascular endothelial growth factor expression are associated with a poor prognosis in patients with nasopharyngeal carcinoma receiving radiotherapy with carbogen and nicotinamide. Clin Oncol (R Coll Radiol) 2008;20:606–12. doi: 10.1016/j.clon.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z, Yu X, Guo Y, et al. Genetic variant in CASP3 affects promoter activity and risk of esophageal squamous cell carcinoma. Cancer Sci. 2012;103:555–60. doi: 10.1111/j.1349-7006.2011.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen K, Zhao H, Hu Z, et al. CASP3 polymorphisms and risk of squamous cell carcinoma of the head and neck. Clin Cancer Res. 2008;14:6343–49. doi: 10.1158/1078-0432.CCR-08-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun H, Deng Q, Pan Y, et al. Association between estrogen receptor 1 (ESR1) genetic variations and cancer risk: A meta-analysis. J BUON. 2015;20:296–308. [PubMed] [Google Scholar]
- 42.Ooft ML, van Ipenburg J, van Loo R, et al. Molecular profile of nasopharyngeal carcinoma: analysing tumour suppressor gene promoter hypermethylation by multiplex ligation-dependent probe amplification. J Clin Pathol. 2018;71:351–59. doi: 10.1136/jclinpath-2017-204661. [DOI] [PubMed] [Google Scholar]
- 43.Lung HL, Kan R, Chau WY, et al. The anti-tumor function of the IKK inhibitor PS1145 and high levels of p65 and KLF4 are associated with the drug resistance in nasopharyngeal carcinoma cells. Sci Rep. 2019;9:12064. doi: 10.1038/s41598-019-48590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Deng L, Huang J, et al. High expression of Fibronectin 1 suppresses apoptosis through the NF-κB pathway and is associated with migration in nasopharyngeal carcinoma. Am J Transl Res. 2017;9:4502–11. [PMC free article] [PubMed] [Google Scholar]
- 45.Young RA. RNA polymerase II. Annu Rev Biochem. 1991;60:689–715. doi: 10.1146/annurev.bi.60.070191.003353. [DOI] [PubMed] [Google Scholar]
- 46.Takacs M, Segesdi J, Banati F, et al. The importance of epigenetic alterations in the development of Epstein-Barr virus-related lymphomas. Mediterr J Hematol Infect Dis. 2009;1:e2009012. doi: 10.4084/MJHID.2009.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan BJ, Tan BH, Chiu GN. Effect of triptolide on focal adhesion kinase and survival in MCF-7 breast cancer cells. Oncol Rep. 2011;26:1315–21. doi: 10.3892/or.2011.1406. [DOI] [PubMed] [Google Scholar]
- 48.Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008;15:678–85. doi: 10.1038/cdd.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou ZL, Luo ZG, Yu B, et al. Increased accumulation of hypoxia-inducible factor-1α with reduced transcriptional activity mediates the antitumor effect of triptolide. Mol Cancer. 2010;9:268. doi: 10.1186/1476-4598-9-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sethi G, Sung B, Aggarwal BB. TNF: A master switch for inflammation to cancer. Front Biosci. 2008;13:5094–107. doi: 10.2741/3066. [DOI] [PubMed] [Google Scholar]
- 51.Yu Y, Ke L, Xia WX, et al. Elevated Levels of TNF-α and decreased levels of CD68-positive macrophages in primary tumor tissues are unfavorable for the survival of patients with nasopharyngeal carcinoma. Technol Cancer Res Treat. 2019;18 doi: 10.1177/1533033819874807. 1078142455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Esquela-Kerscher A, Slack FJ. Oncomirs – microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 53.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell. 2003;4:257–62. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 54.Wang M, Chen B, Chai L. Triptolide suppresses the proliferation and induces the apoptosis of nasopharyngeal carcinoma cells via the PI3K/Akt pathway. Oncol Lett. 2019;17:1372–78. doi: 10.3892/ol.2018.9726. [DOI] [PMC free article] [PubMed] [Google Scholar]