Abstract
Background
Sepsis causes acute kidney injury (AKI) in critically ill patients. Roflumilast, a phosphodiesterases-4 (PDE4) inhibitor, has been shown to be therapeutically effective in sepsis-induced organ injury. However, the function of roflumilast in sepsis-induced AKI is not clearly understood. The present study aimed to explore the protective effect of roflumilast on sepsis-induced AKI in mice.
Material/Methods
A sepsis model was established by cecal ligation and puncture surgery. Roflumilast (1 mg/kg and 3 mg/kg) was used once daily for 7 consecutive days for treatment. Kidney tissues were pathologically examined by hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) staining. The levels of kidney injury markers including blood urea nitrogen (BUN), creatinine (Cre), kidney injury molecule-1 (KIM-1), and neutrophil gelatinase-associated lipocalin (NGAL), and inflammatory cytokines including interleukin (IL)-6, tumor necrosis factor (TNF)-α, and IL-1β were detected by their corresponding test kits. The protein expression was measured using western blot and cell apoptosis of kidney tissue was determined by TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) assay.
Results
Roflumilast was demonstrated to alleviate sepsis-induced AKI by reducing histopathological changes and decreasing the levels of kidney injury markers in a concentration-dependent way. The production of TNF-α, IL-6, and IL-1β was significantly suppressed by roflumilast. Besides, roflumilast inhibited the activation of NLRP3 (nucleotide-binding domain (NOD)-like receptor protein 3) and NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells). Additionally, roflumilast inhibited cell apoptosis and changes in expression of apoptosis related proteins induced by sepsis. Finally, high concentration of roflumilast (3 mg/kg) did not have an adverse effect on liver, heart, lung, or spleen.
Conclusions
Our study indicated that roflumilast could ameliorate AKI induced by sepsis through restraining inflammatory response and apoptosis of the kidney, providing a molecular basis for a novel medical treatment of septic AKI.
MeSH Keywords: Acute Kidney Injury, Apoptosis, Inflammation, Phosphodiesterase 4 Inhibitors, Sepsis
Background
Sepsis is a severe infectious disease triggered by viral, bacterial, or fungal infection and induces an inflammatory response in the host, resulting in septic shock and multiple organ failure [1]. The kidney is one of the major organs that is commonly afflicted during sepsis, and acute kidney injury (AKI) has become a common severe complication of sepsis [2]. The in-hospital mortality rate is up to 50% in sepsis-induced AKI, and survivors have an increased risk of developing chronic kidney disease [3]. A large amount of evidence has reported on the long-range and short-range risk of death in sepsis-induced AKI [4]. Despite improvements in supportive care, such as management of nosocomial infections and utilization of blood products, no singular effective therapy for AKI has been confirmed. Early detection of kidney injury coupled with appropriate supportive care and preventive care remains the mainstream of therapy.
Roflumilast is an orally administrated selective phosphodiesterases-4 (PDE4) inhibitor that is specific to cyclic adenosine 3′,5′-monophosphate (cAMP) degradation, and has been approved by Food and Drug Administration (FDA) to reduce the risk of exacerbations in patients with chronic obstructive pulmonary disease (COPD) associated with chronic bronchitis and a history of COPD exacerbations [5–7]. Roflumilast has been demonstrated to regulate airway inflammation by inhibiting inflammatory activity [8]. Previous studies have reported that roflumilast could alleviate sepsis-induced liver damage and lung injury [9,10], however, whether it is also therapeutically effective in sepsis-induced kidney injury remain unclear. Hence, we aimed to investigate this potential therapeutic option.
In this study, we examined the protective role of roflumilast on kidney dysfunction, inflammatory response, and apoptosis in sepsis-induced kidney injury. Additionally, we explored the molecular mechanism underlying the protective role of roflumilast to identify a potential therapeutic drug for sepsis-induced kidney injury.
Material and Methods
Animals and experimental design
In vivo experiments were conducted on adult female BALB/c mice (6–8 weeks, 35–40 g weight). The mice were purchased from the Experimental Animal Center of Wuhan University (Wuhan, China) and housed in the animal house at 22±2°C with 12 hours light/dark cycles; the mice had free access to food and water. The cecal ligation and puncture surgery (CLP) was performed to trigger sepsis-induced kidney injury as previously described [11]. The sham mice underwent the same procedure without cecal ligation and perforation. The animals were allocated to 6 groups including: 1) Control group, without any treatment; 2) ROF (3 mg/kg) group, administered roflumilast 3 mg/kg only; 3) Sham group; 4) CLP group; 5) CLP+ROF (1 mg/kg group), administrated with roflumilast at 1 mg/kg once daily for 7 consecutive days before CLP surgery; 6) CLP+ROF (3 mg/kg) group. All animal experiments were performed in accordance with the Care and Use of Laboratory Animals established by the US National Institutes of Health, as approved by Shanghai Baoshan Hospital of Integrated Traditional Chinese and Western Medicine.
Western blot
The total protein in kidney tissues were extracted using radioimmunoprecipitation assay (RIPA) buffer containing proteinase inhibitor; the concentration was detected using bicinchoninic acid (BCA) protein assay kit. The same amount of protein was subjected to 12% SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and transferred onto PVDF (polyvinylidene fluoride) membranes. The membranes were incubated in 5% non-fat milk for 2 hours at room temperature, and incubated with the primary antibodies against TNF-α, IL-1β, IL-6, NLRP3, NF-κB p65, Bcl-2, Bax, cleaved caspase-3, cleaved caspase-9, caspase-3, caspase-9, and GAPDH overnight at 4°C. The membranes were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 1 hour at room temperature. The protein bands were detected using the electrochemiluminescence (ECL) western blotting analysis system (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Histopathological analysis
The tissue samples including kidney, heart, liver, spleen, and lung were collected and fixed in 4% paraformaldehyde, and embedded in paraffin, respectively. The tissue sections of 4 μm thickness were stained with hematoxylin and eosin (H&E). In addition, the kidney sections were stained with periodic acid-Schiff (PAS). The sections were then observed under an optical microscope (Olympus BX53, Tokyo, Japan).
Measurement of renal function
The serum and urine of mice in each group was collected for analysis. The serum levels of blood urea nitrogen (BUN), creatinine (Cre), urine levels of kidney injury molecule-1 (KIM-1), and neutrophil gelatinase-associated lipocalin (NGAL) were detected using commercially available assay kits in accordance with the respective manufacturer’s protocols.
Enzyme-like immunosorbent assay (ELISA)
The concentrations of IL-6, TNF-α, and IL-1β in serum were measured using an enzyme-like immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
TUNEL assay
A terminal transferase dUTP nick-end labeling (TUNEL) assay with the in situ Apoptosis Detection Kit (KeyGen Biotech) was used to evaluate apoptosis. Briefly, the tissue sections were dewaxed by xylene and ethanol, followed by washing with phosphate-buffered saline (PBS), and incubation with 20 μg/mL proteinase K (Abcam) for 25 minutes at 37°C. Then, the sections were incubated with the TUNEL reaction mixture for 60 minutes at 3°C. The apoptotic cells were observed and photographed by fluorescence microscopy.
Data analysis
Data are presented as mean ± standard deviation (SD). The experiments in vitro were performed 3 times. The statistical package for the SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, USA) was used in the statistical analysis. ANOVA following by Tukey’s multiple comparison test was performed to compare the differenced among groups. P<0.05 was regarded as statistically significant.
Results
Roflumilast alleviated sepsis-induced kidney injury
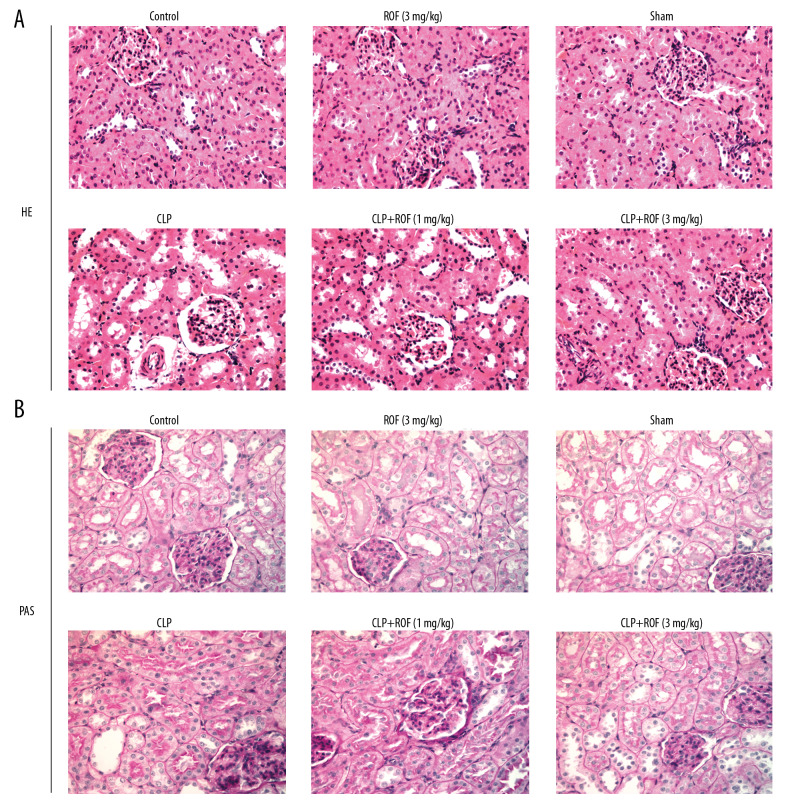
The kidney tissues were collected and stained with H&E and PAS to observe the histopathological changes. As shown in Figure 1A and 1B, the structure of glomerulus was clear and complete, and extracellular matrix was distributed normally, and the kidney tubules were tightly packed in the Control group, ROF (3 mg/kg) group, and Sham group. But in the CLP group, mice developed severe histological kidney injuries as glomerular basement membranes markedly thickened companied with glomerular hypertrophy, and the kidney tubules exhibited diffuse expansion. The kidney structures were improved in CLP+ROF group and more obviously in CLP+ROF (3 mg/kg) group, compared with those in the CLP group.
Figure 1.
Roflumilast alleviated sepsis-induced kidney injury. The kidney tissues were collected and stained with hematoxylin-eosin (H&E) (A) and periodic acid-Schiff (PAS) (B) to observe the histopathological changes. ROF – roflumilast; CLP – cecal ligation and puncture surgery.
Roflumilast alleviated sepsis-induced kidney dysfunction
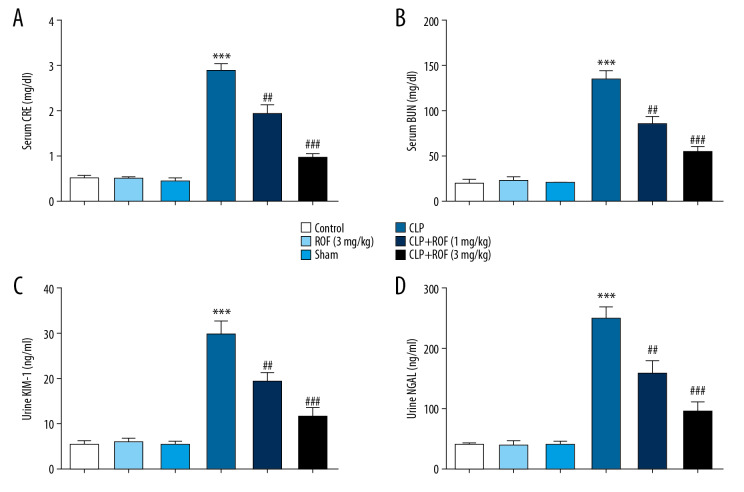
To examine whether roflumilast reduced sepsis-induced AKI, the functional parameters were examined. Sepsis-induced mice developed kidney dysfunction as reflected by a significant increase of Cre, BUN, KIM1, and NGAL. The levels of Cre, BUN, KIM1, and NGAL in roflumilast treated-mice following CLP surgery were significantly decreased compared to those in mice following CLP surgery only (Figure 2A–2D). These data suggested that kidney function following CLP surgery was relatively preserved by the treatment of roflumilast, especially at 3 mg/kg.
Figure 2.
Roflumilast alleviated sepsis-induced kidney dysfunction. The serum levels of creatinine (Cre) (A), blood urea nitrogen (BUN) (B), urine levels of kidney injury molecule-1 (KIM-1) (C), and neutrophil gelatinase-associated lipocalin (NGAL) (D) were detected using commercially available assay kits. *** P<0.001 versus Sham; ## P<0.01, ### P<0.001, versus CLP. ROF – roflumilast; CLP – cecal ligation and puncture surgery.
Roflumilast decreased sepsis-induced inflammatory response
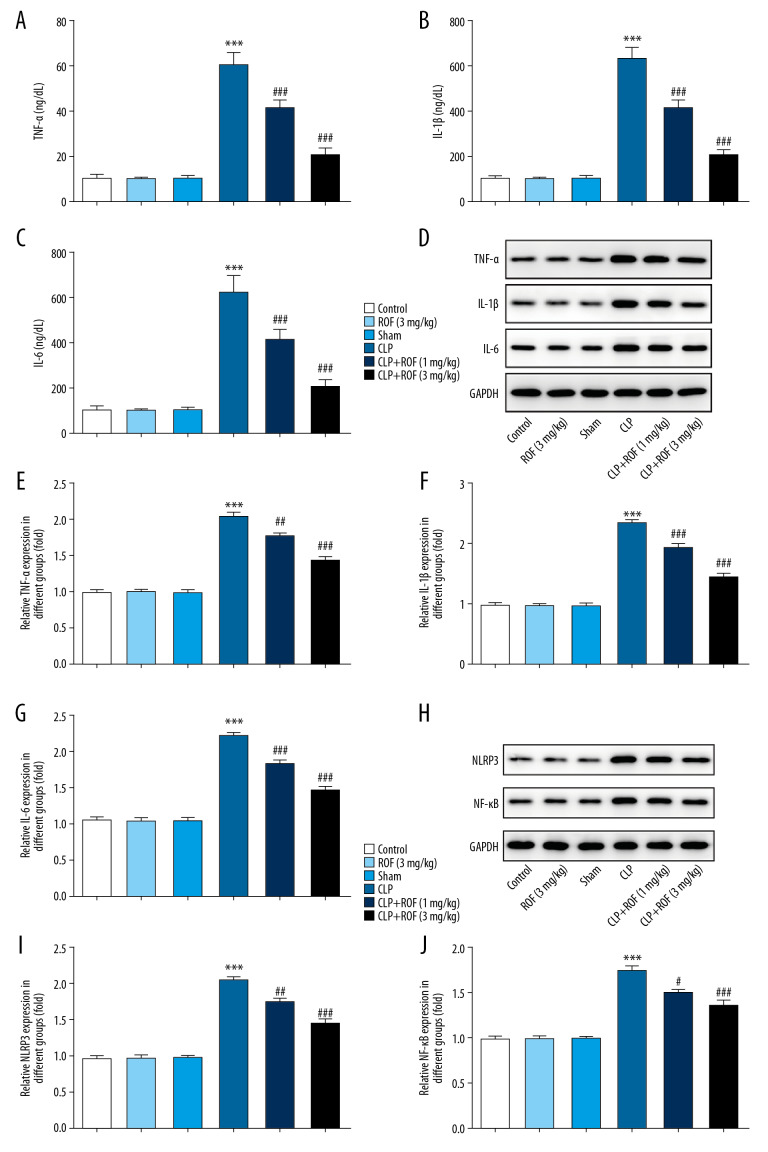
Next, we explored whether roflumilast suppresses the expression of pro-inflammatory cytokines involved in sepsis. The results of ELISA assay and western blot assay in Figure 3A–3G revealed that the expressions of IL-1β, TNF-α, and IL-6 were substantially increased in the kidney of mice that received CLP surgery compared to the other groups. Whereas, roflumilast significantly reduced the expressions of IL-1β, TNF-α, and IL-6 dose-dependently in the kidney of mice that received CLP surgery. These results suggested that roflumilast inhibited inflammatory cytokines levels in kidney of sepsis-induced AKI.
Figure 3.
Roflumilast decreased sepsis-induced inflammatory response. The concentrations of IL-1β, IL-6, and TNF-α in serum were determined by ELISA kits (A–C). The protein expression of IL-1β, IL-6, and TNF-α in kidney tissue was determined using western blot (D–G). The protein expression of NLRP3 and NF-κB was determined using western blot (H–J) *** P<0.001 versus Sham; ## P<0.01, ### P<0.001 versus CLP. ROF – roflumilast; CLP – cecal ligation and puncture surgery; IL – interleukin; TNF – tumor necrosis factor; ELISA – enzyme-like immunosorbent assay; NLRP3 – nucleotide-binding domain (NOD)-like receptor protein 3; NF-κB – nuclear factor kappa-light-chain-enhancer of activated B cells.
NLRP3 and NF-κB are considered as important mediator of inflammatory process. Thus, we examined whether roflumilast also regulated the activity of NLRP3 and NF-κB. As shown in Figure 3H–3J, we revealed an increased expression of NLRP3 and NF-κB in CLP group, whereas the expressions of NLRP3 and NF-κB were significantly decreased in mice following the treatment of roflumilast compared to the mice with CLP surgery only.
Roflumilast reduced sepsis-induced cell apoptosis
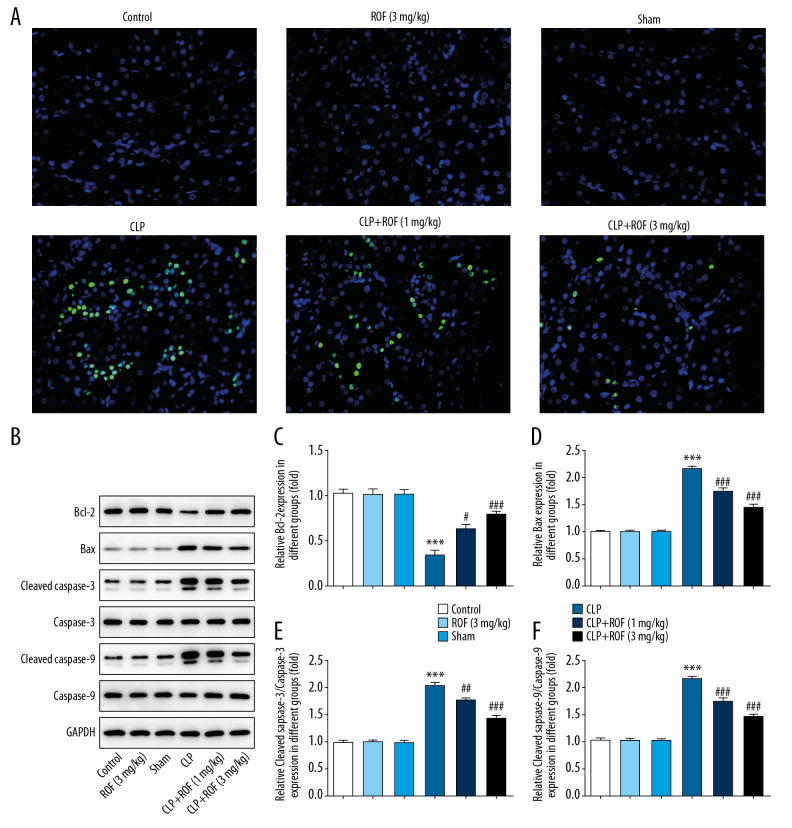
Then, we detected whether roflumilast attenuated sepsis-induced kidney cell apoptosis. As shown in Figure 4A–4F, the CLP group showed the most apoptotic cells in the kidney tissue samples, whereas the apoptotic cells were markedly reduced in the kidney tissue samples of roflumilast-treated mice, especially at 3 mg/kg. In addition, we examined the expression of apoptosis-related proteins in the kidney tissue in each group. By western blot assay, we revealed that Bcl-2 was decreased, and Bax, cleaved caspase-3, and cleaved caspase-9 were increased in the CLP group. By contrast, treatment of roflumilast significantly increased the expression of Bcl-2, and decreased the expressions of Bax, cleaved caspase-3, and cleaved caspase-9. These findings suggested that roflumilast prevented kidney cell apoptosis through increasing Bcl-2 and inhibiting Bax, cleaved caspase-3, and cleaved caspase-9 in response to CLP surgery.
Figure 4.
Roflumilast reduced sepsis-induced cell apoptosis. Kidney cell apoptosis in mice were detected using TUNEL assay (A). The protein expressions of Bcl-2, Bax, cleaved caspase-3, caspase-3, cleaved caspase-9, and caspase-9 were detected using western blot assay (B–F). *** P<0.001 versus Sham; ## P<0.01, ### P<0.001 versus CLP. ROF – roflumilast; CLP – cecal ligation and puncture surgery; TUNEL – terminal transferase dUTP nick-end labeling.
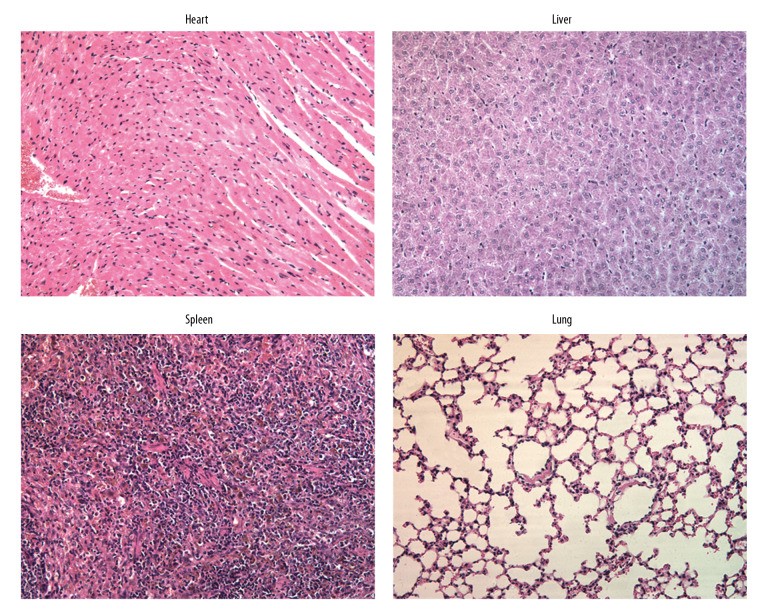
Roflumilast had no adverse effect on other organs
To understand whether roflumilast at a high concentration had any adverse effect on other organs, H&E staining was performed to detect the histopathology of heart, liver, spleen, and lung under the treatment of roflumilast (3 mg/kg) in mice. The heart, liver, spleen, and lung tissues were collected from mice that were treated with roflumilast (3 mg/kg) only and stained with H&E. The results in Figure 5 revealed that the structure of heart, liver, spleen, and lung were normal without any damage. These results suggested that roflumilast, as used in this study, had no adverse effect on organs in mice.
Figure 5.
Roflumilast had no adverse effect on other organs. The heart, liver, spleen, and lung tissues were collected from mice that were treated with roflumilast (3 mg/kg) only and stained with hematoxylin and eosin (H&E) for observation.
Discussion
In our study, we revealed the protective ability of roflumilast in sepsis-induced AKI, using a realistic model of human sepsis. This was, to the best of our knowledge, the first study that investigated the influence of roflumilast on histopathological injury and kidney dysfunction in sepsis-induced AKI in mice, as well as investigated the underlying mechanism of roflumilast on inflammatory response and cell apoptosis during sepsis-induced AKI. Our results clearly revealed that roflumilast significantly alleviated sepsis-induced histopathological damage and improved kidney function. Furthermore, roflumilast was found to retard the release of pro-inflammatory cytokines and inhibit cell apoptosis, showing its anti-inflammatory activity and anti-apoptotic activity in sepsis-induced AKI. Additionally, high concentration of roflumilast was demonstrated to be nontoxic and have no adverse effect to the organs in vivo. Thus, roflumilast may be potential drug candidates for curing sepsis-induced AKI.
KIM1 and NGAL expression are the main characteristic of early AKI, and their monitoring has significance guiding the prognosis of AKI. KIM1, comprising an extracellular portion and cytoplasmic portion, is expressed at very low levels in the normal kidney. Once the kidney is injured, the extracellular portion could cleave and enter tubule lumens, and then could be detected in the urine [12]. NGAL has been recognized as a hallmark of renal tubular injury [13]. NGAL has a high sensitivity and a high negative predictive value for detection of AKI in sepsis patients in serum and urine, which can be used as diagnostic and predictive biomarkers for AKI in critically ill patients with sepsis [14,15]. Our experimental results showed that roflumilast could reduce the expression of KIM1 and NGAL, which were elevated in sepsis-induced AKI, indicating that roflumilast had a protective role on kidney function. The pathological changes of sepsis-induced AKI involved in the kidney are considered to be closely related to the course of the disease, and the histological results in our study demonstrated this.
A previous study revealed that roflumilast suppressed the release of pro-inflammatory cytokines including IL-6 and TNF-α in mice suffering from sepsis, and reduced the bacterial load, as well as an improved survival of mice, suggesting that roflumilast lead to a better outcome for septic mice [10]. In addition, roflumilast has also been shown to prevent inflammation of multiple organs involved in sepsis, such as liver and lung [9,10]. In the present study, the results revealed that roflumilast obviously decreased the concentration of and IL-1β, IL-6, and TNF-α both in serum and kidney tissue, indicating that roflumilast also prevented inflammation of kidney tissue in septic mice. As sepsis is a systemic inflammatory disease, roflumilast might exert its protective function through suppressing inflammatory response of multiple organs that including liver, lung, and kidney. It is known that activation of NLRP3 inflammasome could result in the generation of series of pro-inflammatory cytokines, such as cleaved caspase-1, IL-1β, IL-6, IL-18, and TNF-α [16]. Rolipram, another type of PDE4 inhibitor, has been demonstrated to inhibit NLRP3 inflammasome and cleaved caspase-1 in lipopolysaccharides-induced inflammation in salivary glands [17]. Here, roflumilast was also demonstrated to inhibit NLRP3 inflammasome which was elevated in sepsis-induced AKI. Therefore, roflumilast play a suppressive role on NLRP3 inflammasome to mediate the release of inflammatory cytokines. Moreover, NF-κB is pivotal in inflammatory and immune response. The activation of NF-κB was observed in CLP-induced septic mice, and the inhibition of it might be an effective way to discover drugs that have a protective effect on kidney injury [18]. Roflumilast has been reported to suppress inflammation by inhibiting NF-κB signaling, thus protecting against lung injury, renal damage, and diabetic bladder dysfunction [19–21]. In our study, results also showed a significant suppressive effect of roflumilast on NF-κB, indicating that roflumilast might suppress inflammation through inhibiting NF-κB.
Apoptosis, a type of programmed cell death, plays a pivotal role in the injury and development of multicellular organisms in different tissues [22]. A combined action that include inflammation and apoptosis may take major responsibility for AKI caused by sepsis [23,24]. Here, we found that apoptotic cells were increased in the CLP group, whereas this condition was reversed in mice receiving roflumilast treatment. Besides, roflumilast treatment significantly increased the expression of Bcl-2, an indicator of anti-apoptosis, which was decreased in septic mice. On the contrary, roflumilast showed an opposite function on cleaved caspase-3, cleaved caspase-9, and Bax, indicators of apoptosis, which were elevated in septic mice, suggesting that roflumilast could protect mice against kidney injury partly through downregulating cell apoptosis.
However, some limitations existed in our study. We focused on the potential effect of roflumilast in sepsis-induced AKI, so as to provide a basis for clinical therapy. In our opinion, a stable and effective plasma concentration is important for an effective treatment in vivo. Treatment with roflumilast prior to CLP induction could make the effective plasma concentration stable and reach a threshold value. Once CLP surgery induced sepsis resulted in acute kidney injury, the stable plasma concentration exerted an effect immediately, and we could directly observe the effect of roflumilast. Therefore, roflumilast was begun 7 days prior to CLP in our study. However, in a real-life scenario, it is not possible to take the drug prior to sepsis, which was not taken into account in this study. Thus, more basic studies are needed to prove that roflumilast can have a potential role once sepsis is diagnosed. We plan to investigate the therapeutic effect of roflumilast after CLP in our further investigations to strengthen our understanding of the benefit of roflumilast in sepsis-induced AKI.
Conclusions
To conclude, our study indicated that roflumilast could ameliorate AKI induced by sepsis through restraining inflammatory response and apoptosis of the kidney, which might be associated with its suppressive effect on NLRP3/NF-κB. According to such evidence, in sepsis-induced AKI treatment, roflumilast has a potential application.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–74. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei S, Gao Y, Dai X, et al. SIRT1-mediated HMGB1 deacetylation suppresses sepsis-associated acute kidney injury. Am J Physiol Renal Physiol. 2019;316(1):F20–31. doi: 10.1152/ajprenal.00119.2018. [DOI] [PubMed] [Google Scholar]
- 3.Martensson J, Bellomo R. Sepsis-induced acute kidney injury. Crit Care Clin. 2015;31(4):649–60. doi: 10.1016/j.ccc.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Murugan R, Karajala-Subramanyam V, Lee M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77(6):527–35. doi: 10.1038/ki.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calverley PM, Rabe KF, Goehring UM, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: Two randomised clinical trials. Lancet. 2009;374(9691):685–94. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]
- 6.Martinez FJ, Rabe KF, Sethi S, et al. Effect of roflumilast and inhaled corticosteroid/long-acting beta2-agonist on chronic obstructive pulmonary disease exacerbations (RE(2)SPOND). A randomized clinical trial. Am J Respir Crit Care Med. 2016;194(5):559–67. doi: 10.1164/rccm.201607-1349OC. [DOI] [PubMed] [Google Scholar]
- 7.Kim SW, Kim JH, Park CK, et al. Effect of roflumilast on airway remodelling in a murine model of chronic asthma. Clin Exp Allergy. 2016;46(5):754–63. doi: 10.1111/cea.12670. [DOI] [PubMed] [Google Scholar]
- 8.Kawamatawong T. Roles of roflumilast, a selective phosphodiesterase 4 inhibitor, in airway diseases. J Thorac Dis. 2017;9(4):1144–54. doi: 10.21037/jtd.2017.03.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang X, Hu LF, Ma XJ, et al. Influence of roflumilast on sepsis mice through the JAK/STAT signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(3):1335–41. doi: 10.26355/eurrev_201902_17028. [DOI] [PubMed] [Google Scholar]
- 10.Feng H, Chen J, Wang H, et al. Roflumilast reverses polymicrobial sepsis-induced liver damage by inhibiting inflammation in mice. Lab Invest. 2017;97(9):1008–19. doi: 10.1038/labinvest.2017.59. [DOI] [PubMed] [Google Scholar]
- 11.Wilson RL, Selvaraju V, Lakshmanan R, et al. Thioredoxin-1 attenuates sepsis-induced cardiomyopathy after cecal ligation and puncture in mice. J Surg Res. 2017;220:68–78. doi: 10.1016/j.jss.2017.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin C, Wang N. Kidney injury molecule-1 in kidney disease. Ren Fail. 2016;38(10):1567–73. doi: 10.1080/0886022X.2016.1193816. [DOI] [PubMed] [Google Scholar]
- 13.Abassi Z, Sagi O, Armaly Z, et al. [Neutrophil gelatinase-associated lipocalin (NAGL): a novel biomarker for acute kidney injury]. Harefuah. 2011;150(2):111–6. [in Hebrew] [PubMed] [Google Scholar]
- 14.Kim S, Kim HJ, Ahn HS, et al. Is plasma neutrophil gelatinase-associated lipocalin a predictive biomarker for acute kidney injury in sepsis patients? A systematic review and meta-analysis. J Crit Care. 2016;33:213–23. doi: 10.1016/j.jcrc.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Dai X, Zeng Z, Fu C, et al. Diagnostic value of neutrophil gelatinase-associated lipocalin, cystatin C, and soluble triggering receptor expressed on myeloid cells-1 in critically ill patients with sepsis-associated acute kidney injury. Crit Care. 2015;19:223. doi: 10.1186/s13054-015-0941-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao ST, Cao F, Chen JL, Chen W, et al. NLRP3 is required for complement-mediated caspase-1 and IL-1beta activation in ICH. J Mol Neurosci. 2017;61(3):385–95. doi: 10.1007/s12031-016-0874-9. [DOI] [PubMed] [Google Scholar]
- 17.Lee DU, Shin DM, Hong JH. The regulatory role of rolipram on inflammatory mediators and cholinergic/adrenergic stimulation-induced signals in isolated primary mouse submandibular gland cells. Mediators Inflamm. 2016;2016 doi: 10.1155/2016/3745961. 3745961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu C, Li P, Qi D, et al. Osthole protects sepsis-induced acute kidney injury via down-regulating NF-kappaB signal pathway. Oncotarget. 2017;8(3):4796–813. doi: 10.18632/oncotarget.13592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng CK, Huang KL, Wu CP, et al. Phosphodiesterase-4 inhibitor roflumilast attenuates pulmonary air emboli-induced lung injury. J Surg Res. 2019;241:24–30. doi: 10.1016/j.jss.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 20.Ansari MN, Aloliet RI, Ganaie MA, et al. Roflumilast, a phosphodiesterase 4 inhibitor, attenuates cadmium-induced renal toxicity via modulation of NF-kappaB activation and induction of NQO1 in rats. Hum Exp Toxicol. 2019;38(5):588–97. doi: 10.1177/0960327119829521. [DOI] [PubMed] [Google Scholar]
- 21.Ding H, Zhang P, Li N, et al. The phosphodiesterase type 4 inhibitor roflumilast suppresses inflammation to improve diabetic bladder dysfunction rats. Int Urol Nephrol. 2019;51(2):253–60. doi: 10.1007/s11255-018-2038-z. [DOI] [PubMed] [Google Scholar]
- 22.Liu W, Dong M, Bo L, et al. Epigallocatechin-3-gallate suppresses alveolar epithelial cell apoptosis in seawater aspiration-induced acute lung injury via inhibiting STAT1-caspase-3/p21 associated pathway. Mol Med Rep. 2016;13(1):829–36. doi: 10.3892/mmr.2015.4617. [DOI] [PubMed] [Google Scholar]
- 23.Zhao H, Liu Z, Shen H, et al. Glycyrrhizic acid pretreatment prevents sepsis-induced acute kidney injury via suppressing inflammation, apoptosis and oxidative stress. Eur J Pharmacol. 2016;781:92–99. doi: 10.1016/j.ejphar.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Lin Z, Jin J, Shan X. Fish oils protects against cecal ligation and puncture induced septic acute kidney injury via the regulation of inflammation, oxidative stress and apoptosis. Int J Mol Med. 2019;44(5):1771–80. doi: 10.3892/ijmm.2019.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]