Abstract
Current treatments for tumors expressing epidermal growth factor receptor (EGFR) include anti-EGFR monoclonal antibodies, often used in conjunction with the standard chemotherapy, radiation therapy, or other EGFR inhibitors. While monoclonal antibody treatment is efficacious in many patients, drawbacks include its high cost of treatment and side effects associated with multiple drug infusions. As an alternative to monoclonal antibody treatments, we have focused on peptide-based vaccination to trigger natural anti-tumor antibodies. Here, we demonstrate that peptides based on a region of the EGFR extracellular domain IV break immune tolerance to EGFR and elicit anti-tumor immunity. Mice immunized with isoforms of EGFR peptide p580–598 generated anti-EGFR antibody and T-cell responses. Iso-aspartyl (iso-Asp)-modified EGFR p580 immune sera inhibit in vitro growth of EGFR overexpressing human A431 tumor cells, as well as promote antibody-dependent cell-mediated cytotoxicity (ADCC). Antibodies induced by Asp and iso-Asp p580 bound homologous regions of the EGFR family members HER2 and HER3. EGFR p580 immune sera also inhibited the growth of the human tumor cell line MDA-MB-453 that expresses HER2 but not EGFR. Asp and iso-Asp EGFR p580 induced antibodies were also able to inhibit the in vivo growth of EGFR-expressing tumors. These data demonstrate that EGFR peptides from a region of the EGFR extracellular domain IV promote anti-tumor immunity, tumor cell killing, and antibodies that are cross reactive with ErbB family members.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2218-9) contains supplementary material, which is available to authorized users.
Keywords: EGFR, HER2, HER3, Peptide, Vaccine
Introduction
EGFR (ErbB1) is a 170 kDa cell surface glycoprotein that is overexpressed or mutated in a variety of solid tumors and is associated with more aggressive tumor growth and a poorer prognosis [1–5]. It is a member of the ErbB family of receptor tyrosine kinases and forms homodimers and heterodimers with other members of the ErbB family (HER2/ErbB2, HER3/ErbB3, and HER4/ErbB4) that result in downstream signaling [6].
Current therapies for EGFR overexpressing tumors include several groups of anti-EGFR drugs, among which are monoclonal antibodies directed against the extracellular ligand-binding domain of the receptor. These anti-EGFR antibodies act mechanistically in a variety of ways, both directly by blocking ligand binding, inhibiting receptor dimerization and activation, and indirectly through antibody-dependent cell cytotoxicity (ADCC) [7]. A number of therapeutic anti-EGFR monoclonal antibodies exist, although the most widely recognized, FDA approved antibody for advanced colorectal cancer is cetuximab (Erbitux®). Cetuximab has been used as a single therapy, with chemotherapy, and in combination with other EGFR-targeted therapies, such as small-molecule inhibitors of tyrosine kinase activity [8]. Although monoclonal antibodies represent a major advance in cancer treatment, there remain drawbacks and failures to this therapeutic approach. Monoclonal antibody therapy provides no long lasting immunity; therefore, tumor recurrence and metastasis may arise without any immunological surveillance in place. In addition, this therapy is complicated by the high cost of treatment and the side effects associated with multiple infusions of the drug [9]. Thus, the concepts of tumor-directed vaccinations have reemerged as an attractive therapeutic strategy [10]. Effective vaccination would provide long lasting immunity with immune surveillance in place should a tumor recur. Recent studies have explored the use of peptides in cancer vaccines [11–14] as well as their use as adjuvant therapy [15, 16] and as a prophylactic in at risk populations [17].
One challenge to tumor vaccines has been that many tumor antigens are normal, non-mutated self-proteins that are not recognized by the immune system, making it difficult to design tumor peptides that would elicit a strong anti-tumor immune response. To overcome the lack of immune recognition, our laboratory has exploited key concepts from the field of autoimmunity to promote anti-tumor responses. Most notably, our laboratory has demonstrated that the introduction of a naturally arising posttranslational modification, specifically the conversion of an aspartic acid residue into an iso-aspartic acid (iso-Asp) residue, often breaks immune tolerance to self-peptides [18, 19] (Supplementary Fig. 1).
In the present studies, we exploited our knowledge of iso-Asp biochemistry in attempts to develop immunogenic EGFR peptides. We report here that we are able to generate anti-EGFR immunity in mice using a 19 amino acid iso-Asp modified peptide representing a region of the EGFR extracellular domain IV. Immunized mice generated anti-EGFR peptide antibodies that recognize native human EGFR inhibit tumor growth and promote tumor cell killing. These antibodies also recognized homologous regions of other ErbB family members, in particular HER2 and HER3, and mediated growth inhibition of tumor cells expressing HER2. Thus, this immunization strategy has the potential to be developed into a therapy targeting multiple proteins of the ErbB family overexpressed on the same tumor.
Materials and methods
Peptides and recombinant proteins
The W.M. Keck Foundation Biotechnology Resource Laboratory at Yale University synthesized all peptides. Six different murine (mu) and human (hu) EGFR peptides were synthesized as both the Asp and iso-Asp isoforms. The peptide sequences are as follows (numbering based on the human EGFR precursor protein amino acid sequence; iso-Asp designated in bold): mu/hu p566–584, AMNITCTGRGPDNCIQCAH; mu/hu p572–590, TGRGPDNCIQCAHYIDGPH; mu/hu p580–598, IQCAHYIDGPHCVKTCPAG; mu p596–615, PAGIMGENNTLVWKYADANN; hu p596–615, PAGVMGENNTLVWKYADAGH; and mu p605–623, TLVWKYADANNVCHLCHAN. The sequences for the human HER2, HER3, and HER4 peptides were: hu HER2 p585–603, VACAHYKDPPFCVARCPSG; hu HER3 p574–592, AQCAHFRDGPHCVSSCPHG and hu HER4 p578–596, TKCSHFKDGPNCVEKCPDG. Peptides were reconstituted in water and stored at −20 °C. The extracellular domain of HER2 was a kind gift of Dr. Daniel Leahy, John Hopkins University School of Medicine, and was expressed and purified as previously described [20]. Recombinant human HER3 extracellular domain was purchased from R&D Systems.
Cell lines
The human epidermoid carcinoma cell line A431, which overexpresses EGFR, was obtained from ATCC (CRL-1555). A431 cells were maintained in high glucose DMEM (Gibco) containing 10% FBS. The HER2 expressing human mammary gland carcinoma cell line MDA-MB-453 (ATCC HTB-131), which does not express EGFR, was maintained in DMEM/Hams F12 (Gibco) containing 10% FBS. TUBO-EGFR cells (a kind gift of Dr. Yang-Xin Fu, Univ. of Chicago) are an HER2/neu-dependent TUBO cell line originally derived from Balb/c mice and transfected with human EGFR [21]. These cells were maintained in DMEM containing 10% FBS, 2 mmol/l l-glutamine, 0.1 mmol/l MEM nonessential amino acids, 100 U/ml penicillin, and 10 µg/ml streptomycin [21].
Antibodies
Cetuximab (anti-human EGFR monoclonal antibody) and trastuzumab (anti-human HER2 monoclonal antibody) were kind gifts of Drs. David Rimm and Michael DiGiovanna, respectively (Yale University School of Medicine). Rituximab (human anti-CD20 monoclonal antibody) was obtained from the Smilow Cancer Center Pharmacy, New Haven, CT.
Animals and immunization
Female Balb/c or female C57BL/6 mice, 6–8 weeks old, were obtained from the National Cancer Institute and housed at the Yale University School of Medicine Animal Facility. All protocols were consistent with accepted guidelines of the National Institutes of Health for the care and use of laboratory animals as well as approved by the Yale University Institutional Animal Care and Use Committee. For the generation of antibody, three-to-five Balb/c or C57BL/6 mice per group were immunized intraperitoneally and subcutaneously in one footpad with 100 µg of immunizing peptide emulsified 1:1 in complete Freund’s adjuvant (CFA). Twenty-one days later, mice were boosted intraperitoneally and subcutaneously in the other footpad with 100 µg of immunizing peptide emulsified in incomplete Freund’s adjuvant (IFA). Sera were collected via retro-orbital bleed at day 28 for antibody analyses. For T-cell assays, mice were immunized by the same route of administration with 100 µg of peptide emulsified 1:1 in CFA. Fourteen days later mice, mice were boosted by the same route of administration with 100 µg of the immunizing peptide emulsified 1:1 in IFA. Twenty-one day post-immunization, mice were euthanized, draining lymph nodes were excised, and single-cell suspensions prepared for experimental use as described below.
T-cell immunoassays
Single-cell suspensions were prepared from the popliteal, inguinal, and periaortic lymph nodes of immunized C57BL/6 mice. Cells were plated in triplicate in 96-well flat bottom plates at 5 × 105 cells per well in which serially diluted peptides had been added. Cells were incubated at 37 °C, 5% CO2 for 3 days, at which time they were pulsed with 1 µCi 3H-thymidine. Cultures were incubated an additional 18 h and harvested onto filter membranes and 3H-thymidine incorporation was quantified (Betaplate, Wallac). Cells stimulated with purified protein derivative (PPD) from M. tuberculosis H37 RA (Difco Laboratories) served as a positive control for cell proliferation.
ELISA
Serum antibodies were assessed by the standard ELISA as previously described [19]. Peptides (50 µg/ml) or proteins (1 µg) were dissolved in carbonate coating buffer and plated in microtiter plates. Serum dilutions (1:100) from immune and pre-immune animals were incubated for 2 h in peptide or protein-coated plates.
Flow cytometry
A431 and MDA-MB-453 cells (105 /sample) were stained with a 1:100 dilution of serum in PBS with 0.1% BSA and 0.05% sodium azide for 1 h at 4 °C. Cells were washed and incubated with a 1:100 dilution of either goat anti-mouse IgG FITC (Sigma, F0257) or goat anti-mouse IgG AlexaFluor 488 (Invitrogen) for 1 h at 4 °C. Some samples were further stained using anti-FITC AlexaFluor 488 (Invitrogen). Cells were washed and fixed in PBS + 1% paraformaldehyde. Cells were analyzed on a FACSCalibur (BD Biosciences) with the FlowJo software (Tree Star). Controls included unstained cells and cells stained with secondary antibody alone. A431 cells stained with cetuximab (5 µg/ml) and MDA-MB-453 cells stained with trastuzumab (5 µg/ml) were used as positive controls for EGFR and HER2 staining, respectively.
Western blotting
Epidermal growth factor (EGF, Cell Signaling Technology) stimulation of A431 cells in the presence of immune and pre-immune EGFR sera and subsequent western blotting was performed as described by Meira and coworkers [22]. Antibodies used were against: phospho-EGFR (Tyr1045) and GAPDH (all antibodies from Cell Signaling Technology). Cetuximab was used as positive control.
Tumor cell growth inhibition
The ability of immune sera to inhibit the growth of the EGFR overexpressing A431 cell line was measured using a 3H-thymidine assay. Briefly, A431 cells were grown to log phase in DMEM + 10% FBS and plated at 4000 cells/well in a 96-well flat bottom plates. Cells were allowed to adhere overnight prior to the addition of 1:25 dilutions of heat-inactivated (56 °C, 30 min) immune or pre-immune sera in DMEM + 1% FBS. All samples were run in triplicate. Cells were incubated for 48 h at 37 °C 5% CO2, at which time 1 µCi of 3H-thymidine (Perkin Elmer) was added to each well. Cells were further incubated for 18 h then harvested and thymidine incorporation counted by a beta plate reader. Cetuximab (10 µg/ml) served as the positive control, while rituximab (10 µg/ml), another humanized IgG1 antibody, served as an isotype control. Percent growth inhibition was calculated as [(pre-immune serum–immune serum)/pre-immune serum] × 100.
Growth inhibition of MDA-MB-453 cells by EGFR immune sera was done as described above for the growth inhibition of A431 cells, with the exception that cells were plated at 1000 cells/well and trastuzumab, rituximab, and cetuximab (each at 40 µg/ml) served as controls.
ADCC assays
All experimental sera were heat-inactivated (56 °C, 30 min) prior to use. A431 or MDA-MB-453 cells were incubated with a 1:25 dilution of heat-inactivated sera or control antibody (20 µg/ml) 30 min at 37 °C in 96-well U bottom plates. Freshly isolated human peripheral blood mononuclear cells (PBMC) from healthy donors were incubated with antibody coated target cells at a 50:1 effector to target ratio for 4 h at 37 °C. Fifty microliters of each cell culture supernatant was then assayed for lactate dehydrogenase released from dead cells using the CytoTox96 Non-radioactive Cytotoxicity Assay (Promega) per manufacturer’s instructions. Percent cytotoxicity was calculated as [(experimental–effector spontaneous–target spontaneous)/(target maximum–target spontaneous)] × 100.
In vivo tumor assays
Female Balb/c mice were immunized with either Asp or iso-Asp EGFR p580 as described above for the generation of antibody. TUBO-EGFR cells (5 × 105) were injected subcutaneously on the right flank of mice. Tumor volume was calculated by measuring three orthogonal axes (a, b, and c) with digital calipers, with the tumor volume = abc/2 [21].
Statistical analysis
Results are expressed as means ± SEM and p values calculated using the Mann–Whitney test or the Student t test (Prism, GraphPad Software). Results were considered significant if p < 0.05.
Results
Anti-EGFR antibodies are elicited by immunization with EGFR peptide p580 isoforms
Sites chosen for peptide-based induction of immunity were based on extracellular domain sequences near the transmembrane region of ErbB family proteins with sequence homology between EGFR, HER2, and HER3. HER2 is a member of the ErbB family and is often co-expressed with EGFR and also overexpressed in certain cancers. We aligned the amino acid sequences of the HER2 and EGFR proteins and identified a corresponding 20 amino acid residue region that spans EGFR amino acids 596–615 (p596). We synthesized four additional overlapping 19- or 20-mer peptides derived from the murine and/or human EGFR p566–623 amino acid sequence. The peptide sequences are identical between humans and mice with the exception of p596 and p605, which have murine and human sequences that differ by three amino acids. Mice were immunized with the Asp or iso-Asp modified forms of each peptide to increase the possibility of overcoming host immune tolerance to murine EGFR. Antisera were collected and examined by ELISA for antibodies against the panel of EGFR peptides. All mice developed antibodies with binding activity to the immunizing peptide. Peptide p580 isoforms elicited strong antibody responses with cross-reactive binding to multiple peptides (Fig. 1a, b). Immunization with Asp p580 (IQCAHYIDGPHCVKTCPAG) generated antibodies against Asp and iso-Asp p580, as well as Asp and iso-Asp p572 and Asp p596 (Fig. 1a). A similar pattern was seen upon immunization with iso-Asp p580 in which sera bound to both isoforms of p572 and Asp 596 (Fig. 1b).
Fig. 1.
Immunization with EGFR p580 peptide isoforms generates peptide-specific antibody and T-cell responses. Individual serum samples from C57BL/6 mice were tested by ELISA for binding to flanking EGFR peptide sequences. Pre-immune sera had < 0.1 O.D. units of antigen binding. Results are representative of four mice per immunization group and the experiment was repeated twice. a Antibody binding of Asp EGFR p580 immune sera to EGFR peptides. b Antibody binding of iso-Asp EGFR p580 immune sera to EGFR peptides. c Proliferation of T cells from EGFR p580 immunized mice in response to Asp and iso-Asp isoforms of EGFR p580. d T cells from C57BL/6 mice immunized with either Asp or iso-Asp p580 were re-stimulated with 10 µg Asp and iso-Asp p580. Data represent experimental cpm with background cpm subtracted. Cells stimulated with PPD (positive control) ranged from 11,000–49,000 cpm. Results represent the mean ± SEM of triplicate wells
Peptide immunization elicits T cells that recognize both iso-aspartyl and aspartyl isoforms of peptide p580
Since immunization with both p580 isoforms induced antibody that cross-reacted to other EGFR peptides, we tested the ability of this immunization to stimulate CD4 T-cell responses. In conventional proliferation assays, C57BL/6 mice were immunized with either the Asp or iso-Asp isoforms of p580 in Freund’s adjuvant. After 21 days, lymph node cells were isolated and re-stimulated for 72 h in vitro with peptide. Immunization with the p580 peptide resulted in a strong T-cell responses to both Asp and iso-Asp p580, with a more robust response in those mice immunized with iso-Asp p580 (Fig. 1c). More importantly for inducing reactivity with native EGFR, T cells from mice immunized with the iso-Asp modified p580 isoform also recognize Asp p580 (Fig. 1d), demonstrating cross reactivity of the T-cell receptor for both isoforms of the EGFR peptide.
Sera from EGFR p580 immune mice bind human EGFR
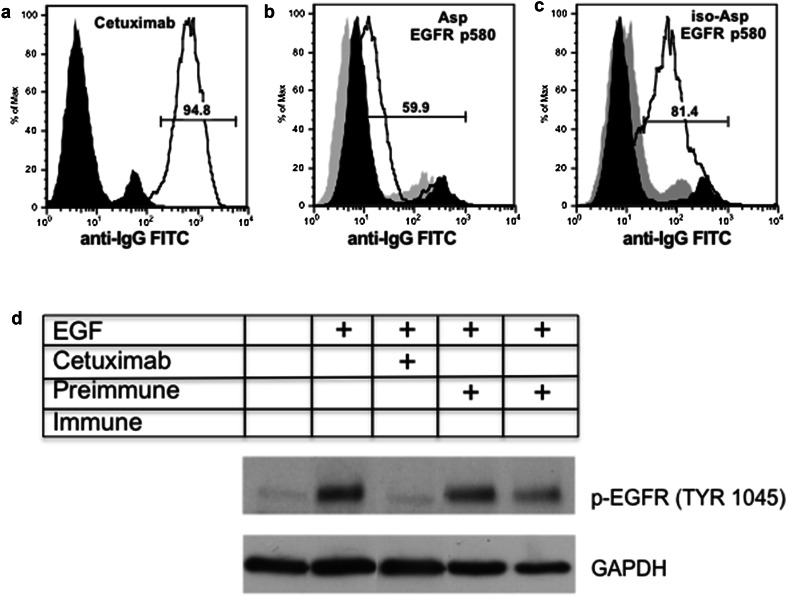
We next determined if the antibodies generated during immunization with p580 isoforms bound to intact human EGFR on cells. For these studies, A431 cells, a human EGFR overexpressing tumor cell line, were stained with peptide-induced antisera and analyzed by flow cytometry. As a control for staining, we stained A431 cells with cetuximab. A431 cells express EGFR on their surface and stain with cetuximab (Fig. 2a). Asp p580 immune sera stained A431 cells above that seen with pre-immune sera (Fig. 2b). However, staining with iso-Asp p580 immune sera revealed greater than a tenfold shift in staining intensity (Fig. 2c). These results demonstrate that the iso-Asp form of EGFR p580 isoform elicits an antibody response that recognizes human EGFR on tumor cells.
Fig. 2.
Sera from mice immunized with mouse iso-Asp p580 bind and engage human EGFR on A431 cells. Sera from Balb/c mice immunized with either Asp or iso-Asp p580 were incubated with A431 cells and stained for FACS analysis. Black histogram = conjugate control. Gray histogram = pre-immune sera. Solid line histogram = positive control antibody or immune sera. Gates represent percent positive cells staining with positive control antibody or immune sera. a A431 cells stained with the anti-EGFR antibody cetuximab (positive control) or secondary antibody alone (conjugate control). b Asp EGFR p580 immune serum binding of A431 cells (Asp EGFR p580 MFI = 12.6; pre-immune serum MFI = 5.83). c iso-Asp EGFR p580 immune serum binding of A431 cells (iso-Asp EGFR p580 MFI = 59.9; pre-immune serum MFI = 9.56). Results are representative of four mice per immunization. d EGFR p580 immune serum inhibits EGF induced phosphorylation of EGFR as assessed by Western blotting. GAPDH illustrates protein loading. The table denotes control (cetixumab), EGF, pre-immune or immune serum added to the A431 cultures and assessed for p-EGFR (Tyr 1045)
In addition, we tested whether or not the antibodies generated by EGFR p580 immunization were able to alter or inhibit EGFR signaling. A431 cells preincubated with cetuximab, pre-immune serum, or EGFR p580 immune sera were stimulated with epidermal growth factor (EFG). The amount of phosphorylated EGFR was assessed by Western blot. As shown in Fig. 2d, EGFR phosphorylation was significantly inhibited by cetuximab in the presence of EGF (positive control). Similarly, significant inhibition of p-EGFR (Tyr 1045) was observed with EGFR p580 immune serum compared to pre-immune serum. Overall, data from Fig. 2 demonstrates that anti-EGFR p580 antibodies do engage EGFR on human tumor cells and inhibit phosphorylation signaling of EGFR (Tyr 1045).
Anti-EGFR p580 antibodies bind HER2 and HER3
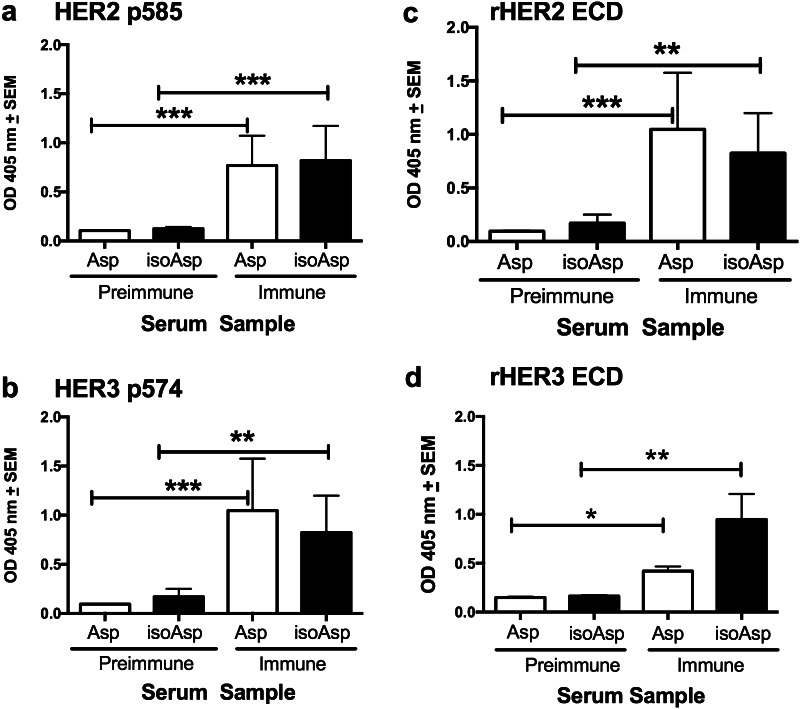
EGFR is a member of the ErbB family of tyrosine kinases, which also includes HER2, HER3, and HER4. HER2 is overexpressed in a subset of breast cancers and is itself a target of the therapeutic antibody trastuzumab. Examination of the amino acid sequences of EGFR and HER2, HER3, and HER4 extracellular domains revealed significant homology between these four proteins in the domain IV region represented by EGFR p580 (Supplementary Table 1; Supplementary Fig. 2). Thus, we tested anti-p580 immune serum for the ability to bind homologous peptides of human HER2, HER3, and HER4. Immune sera from both Asp and iso-Asp p580 bound HER2 p585 by ELISA (Fig. 3a) as well as HER3 p574 (Fig. 3b). In contrast, neither the Asp nor iso-Asp EGFR p580 antiserum bound the homologous HER4 p578 peptide (data not shown).
Fig. 3.
Anti-EGFR p580 antibodies bind homologous HER2 and HER3 peptides and proteins. Sera from Balb/c mice immunized with Asp p580 or iso-Asp p580 were tested by ELISA for antibody binding. a Immune sera binding to HER2 p585 peptide. b Immune sera binding to HER3 p574 peptide. c Immune sera binding to recombinant HER2 extracellular domain. d Immune sera binding to recombinant HER3 extracellular domain. Results represent nine-to-ten mice per group. *p < 0.05; **p < 0.01; ***p < 0.001
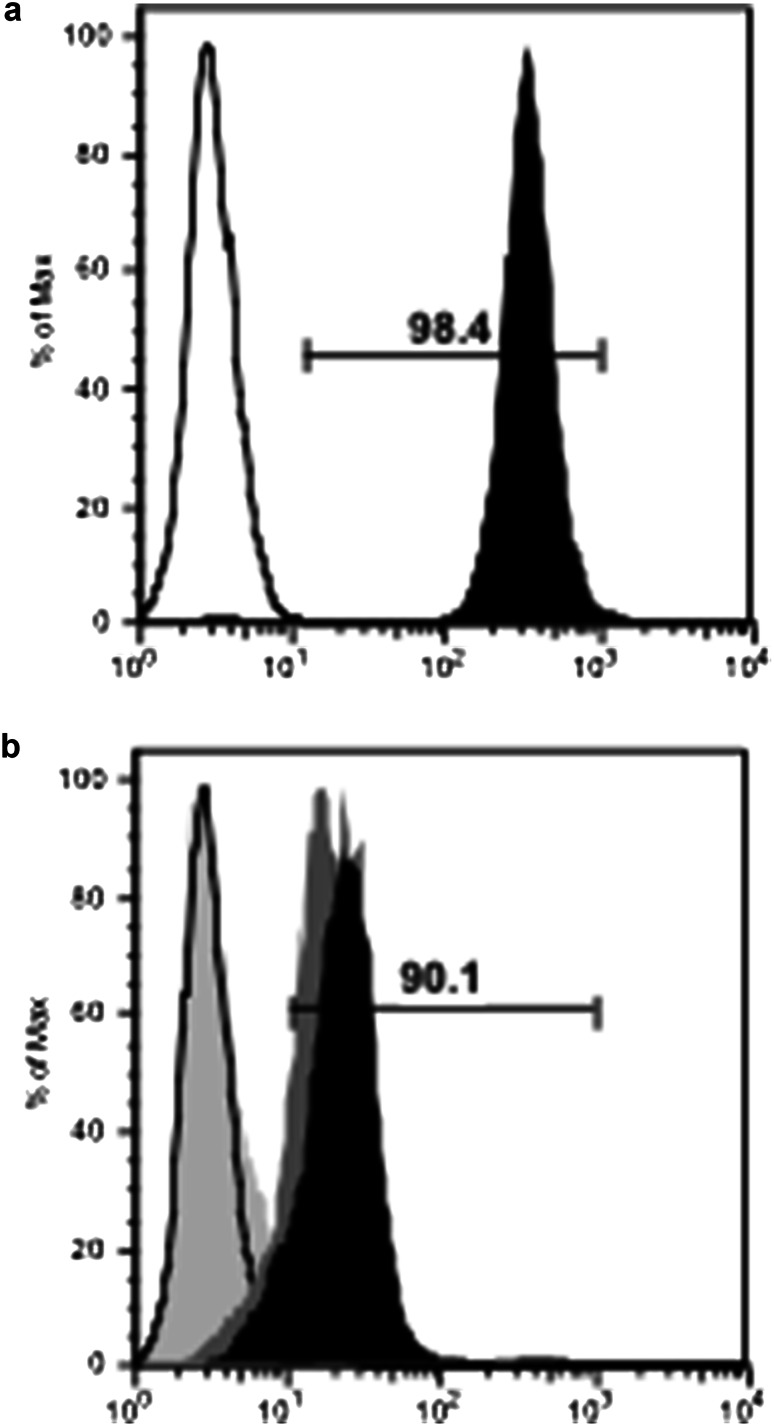
We examined the ability of EGFR p580 antiserum to bind the extracellular domain of the HER2 protein. Asp and iso-Asp EGFR p580 antiserum bound the recombinant extracellular domain of HER2 by ELISA (Fig. 3c), while sera from pre-immune mice did not. In a similar manner, Asp and iso-Asp EGFR p580 immune serum bound the HER3 extracellular domain (Fig. 3d). EGFR p580 antisera also bound HER2 on living human tumor cells (Fig. 4). Flow cytometry was used to assess antibody binding to the cell line MDA-MB-453, which expresses HER2 mRNA, but lacks EGFR mRNA expression as determined by RT-PCR [23]. As expected, the anti-HER2 antibody trastuzumab bound to MDA-MB-453 cells demonstrating HER2 expression (Fig. 4a), while the anti-EGFR antibody cetuximab did not (Fig. 4b). Both Asp and iso-Asp EGFR p580 immune serum showed affinity for MDA-MB-453 cells by flow cytometry (Fig. 4b).
Fig. 4.
Sera from anti-EGFR peptide immune mice bind HER2-positive, EGFR-negative human MDA-MB-453 cells. Sera from Balb/c mice immunized with either Asp or iso-Asp p580 were incubated with MDA-MB-453 cells (HER2 positive, EGFR negative) and stained for FACS analysis. a MDA-MB-453 cells stained with the anti-HER2 antibody trastuzumab (positive control, black histogram). Conjugate control staining is represented by a black line. Gate represents percent positive staining by trastuzumab. b Asp (dark gray, MFI = 16.4) and iso-Asp (black, MFI = 23.3) EGFR p580 immune serum binding of MDA-MB-453 cells. Cetuximab (light gray) served as a control to confirm that cells are EGFR negative. Conjugate control staining is represented by a black line. Gate represents percent positive cells staining with iso-Asp EGFR p580 serum. Percent positive staining with Asp EGFR p80 was 82.1% (gate not shown). Results are representative of four mice per immunization group
Anti-tumor antibodies elicited with EGFR p580 inhibit tumor cell growth and promote ADCC
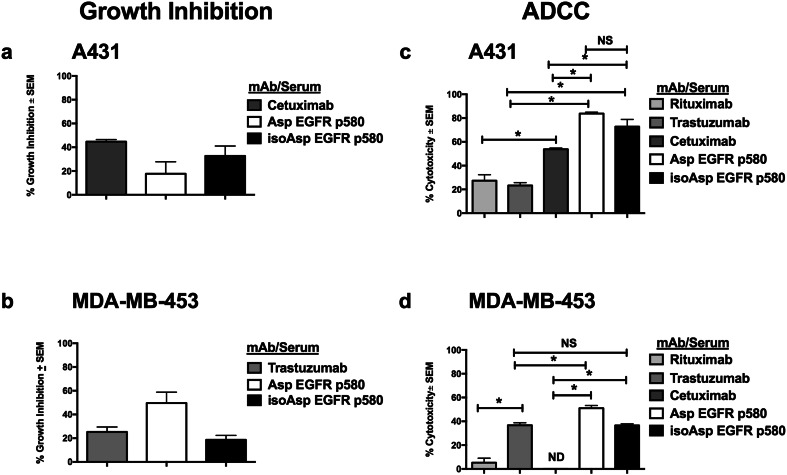
The EGFR peptide antibodies induced by immunizing mice were evaluated for inhibition of human tumor cell growth with methods previously used to characterize cetuximab [7, 24]. We examined tumor cell growth inhibition by incubating A431 cells with EGFR peptide immune sera for 48 h prior to the addition of 3H-thymidine. Cetuximab inhibited A431 cell growth by 42%. Asp p580 immune serum and iso-Asp p580 immune serum inhibited A431 growth at 1:25 dilutions (19 and 33%, respectively) (Fig. 5a).
Fig. 5.
Sera from anti-EGFR peptide immune mice inhibit the growth and promote the killing of both EGFR and HER2 expressing cells. Cells were incubated with cetuximab (for A431) or trastuzumab (for MDA-MB-453) or with 1:25 dilutions of sera from either Asp or iso-Asp p580 immunized Balb/c mice. Cell proliferation was measured by 3H-thymidine incorporation, and growth inhibition calculated, as described in “Materials and methods”. a Growth inhibition of A431 cells by EGFR p580 immune sera. b Growth inhibition of MDA-MB-453 cells by EGFR p580 immune sera. For ADCC assays, cells were incubated with pre-immune or immune serum followed by incubation with human PBMC. ADCC and % cytotoxicity were calculated as described in “Materials and methods”. c ADCC of EGFR p580 immune sera against A431 cells. d ADCC of EGFR p580 immune serum against MDA-MB-453 cells. Rituximab served as a negative control for both cell lines. Trastuzumab and cetuximab served as positive controls for A431 and MDA-MB-453 cells, respectively. **p < 0.05; ***p < 0.001; NS, not significant; ND, inhibition not detected. Results are representative of three independent experiments
We also found that the EGFR p580 antiserum inhibited the growth of MDA-MB-453 cells. Similar to the growth inhibition assays using A431 cells, MDA-MB-453 cells were incubated with EGFR peptide antisera, as well as control monoclonal antibodies. Trastuzumab (Fig. 5b) inhibited MDA-MB-453 cell growth by 27.5%, while Asp EGFR p580 antisera inhibited tumor cell growth by 50% and iso-Asp EGFR p580 inhibited tumor cell growth by 19% (Fig. 5b).
We also examined the ability of the immune sera to induce ADCC, one mechanism by which therapeutic monoclonal antibodies are assumed to function [25]. For this analysis, A431 cells were incubated with immune sera then incubated with human PBMC. As illustrated, the EGFR-specific monoclonal antibody cetuximab and antisera generated to either EGFR p580 isoform exhibited significant killing of A431 cells (Fig. 5c). In a similar manner, anti-EGFR p580 polyclonal sera significantly induced ADCC killing of HER2 expressing MDA-MB-453 human tumor cells as compared to the negative control antibodies rituximab and cetuximab (Fig. 5d). As a positive control, trastuzumab triggered significant ADCC toward MDA-MB-453 cells as compared to rituximab, while cetuximab failed to induce MDA-MB-453 cell killing (Fig. 5d).
EGFR immunization inhibits tumor growth in vivo
While immunization with the EGFR peptides elicited that antibodies are capable of inhibiting tumor growth and mediating ADCC in vitro, we wanted to know if immunization would be able to inhibit tumor growth in vivo. For these studies, we used the Balb/c-derived TUBO cell line expressing human EGFR (TUBO-EGFR) [21]. Asp or iso-Asp EGFR p580-immunized Balb/c mice were injected subcutaneously with TUBO-EGFR cells and their tumor size measured over a period of 18 days. Mice immunized with either Asp or iso-Asp EGFR p580 had significantly smaller tumors as compared mice injected with adjuvant alone (PBS-CFA) on days 11, 13, and 18 (Fig. 6).
Fig. 6.
Tumor growth is inhibited in EGFR p580 immunized Balb/c mice. Asp or iso-Asp EGFR p580 immunized mice were injected subcutaneously with TUBO-EGFR cells. PBS-CFA-injected mice served as controls. Tumors were measured and tumor volume calculated as described in “Materials and methods” on days 11, 13, and 18 post-injection. *p < 0.05; **p < 0.01; NS, not significant as determined by the Student t test. Results represent ten mice per group
Discussion
One of the most successful advancements in cancer immunotherapy has been the development of monoclonal antibodies as immunotherapies against specific cancers [25]. Among the best established of these is the anti-HER2 monoclonal antibody trastuzumab (Herceptin®), approved for the treatment of HER2-positive breast cancer. Another member of the ErbB family, EGFR, is overexpressed in more than 30% of human cancers, including non-small lung cell carcinoma and colorectal cancer. Although there are monoclonal antibodies available for the treatment of EGFR-positive cancers (cetuximab and panitumumab), these treatments often provide only transitory effects and are cost-prohibitive to many within a global patient population [9].
Specific, active immunotherapy with vaccines targeting tumor-associated antigens may reduce the need for prolonged administration of chemotherapy and therapeutic antibodies [26, 27]. Cancer vaccines can potentially reduce side effects and provide immunologic memory to respond to tumor recurrence and metastases. Herein, we describe a novel peptide vaccine candidate that can target multiple members of the ErbB receptor family. As reported elsewhere, immunization with modified tumor peptides to break immune tolerance to tumor antigens remains an attractive therapeutic strategy [18, 19]. We examined the immunogenicity and anti-tumor properties of aspartyl and iso-aspartyl peptide isoforms derived from EGFR extracellular domain IV.
We initially designed five sets of EGFR peptides, each set having both the Asp and iso-Asp isoform of the peptide. These peptides are derived from a membrane-proximal region of the EGFR extracellular domain that is distinct from the epitope recognized by cetuximab (Supplemental Fig. 2) and panitumumab [7]. Mice immunized with these peptides all developed antibodies to the immunizing peptide. However, several peptides were unique in that they elicited antibodies that not only bound the immunizing peptides, but also bound to other ErbB family peptides with overlapping sequences. This was especially notable with the Asp and iso-Asp forms of the EGFR p580 sequence, which is identical in human and murine EGFR. These peptide immunizations circumvented immune self-tolerance to EGFR. They elicited antibodies that cross-reacted with human EGFR and induced ADCC cell lysis and growth inhibition of human tumor cells in vitro. Moreover, no signs of aberrant tissue autoimmunity were observed in immunized mice over 10 months of observation.
In addition to eliciting antibody responses, EGFR p580 induced robust T-cell responses. T cells from mice immunized with the iso-Asp isoform of p580 proliferated to a greater extent when exposed to the immunizing iso-Asp peptide as compared to T cells from mice immunized with the Asp isoform. The increased response of iso-Asp p580 reactive T cells to iso-Asp p580 may be a result of the iso-Asp residue altering how the peptide is processed (i.e., at a faster rate) or an alteration in the binding of the peptide to MHC class II, scenarios described for peptides with posttranslational modifications [28, 29].
Our data demonstrate that antibodies elicited by immunization with p580 recognized intact EGFR molecules present on the surface of living human tumor cells, and is able to inhibit EGFR signaling. While the inhibition of EGFR phosphorylation was not as profound as that seen with cetuximab, this may be due to the use of serum which contains growth factors that would stimulate some background signaling not seen using a purified monoclonal antibody. As predicted by EGFR extracellular domain crystal structures [30], the p580 sequence is surface exposed on the native EGFR protein and accessible for anti-peptide antibodies (Supplementary Fig. 2). We also found that iso-Asp EGFR p580 antibody bound to EGFR-expressing A431 tumor cells nearly tenfold better than its Asp-isoform counterpart.
The ability of antibodies to inhibit tumor cell growth and trigger ADCC has been described in the analyses of other anti-EGFR antibodies [7, 25]. Interestingly, both Asp and iso-Asp EGFR p580 antisera inhibited A431 growth when tested at 1:25 dilutions. Similar to reported mechanisms for monoclonal antibody therapies, polyclonal antibodies elicited by EGFR p580 exhibited significant ADCC for both EGFR+ and EGFR−/HER2+ tumor targets, emphasizing the cross-binding properties of the immune response. Perhaps most important of all, is the fact that the antibodies elicited by EGFR immunization are able to inhibit the growth of EGFR-expressing tumors in vivo (Fig. 6). The rapid growth of this transplantable tumor precludes long-term studies of vaccination efficacy.
Patients with a therapeutic response to anti-EGFR monoclonal antibodies as a first line of therapy often develop resistance to the antibody and subsequent tumor progression [31, 32]. Acquired tumor resistance to cetuximab is the result of a number of mechanisms including mutations, amplifications, and altered regulation of oncogenes and receptors [33–36]. Resistance also can arise when other ErbB family members (HER2, HER3, and HER4) compensate for the signaling loss of EGFR, triggering tumor outgrowth [37, 38]. ErbB family members form heterodimers with each other, thus forming several signaling pathways that compensate for loss of EGFR. HER2 and/or HER3 are often co-expressed with EGFR in colorectal and breast cancers [3, 39, 40]. Thus, targeting two or more members of the ErbB family simultaneously, as our vaccine does, is a strategy to overcome antibody resistance. In pancreatic carcinoma xenograft models, targeting EGFR and HER2 simultaneously with cetuximab and trastuzumab was a more effective treatment than a combination of trastuzumab and erlotinib (an EGFR tyrosine kinase inhibitor) or even lapatinib alone (an irreversible dual EGFR/HER2 tyrosine kinase inhibitor) [41]. These results were attributed to disrupted EGFR/HER2 heterodimerization, receptor down-regulation, and/or decreased Akt phosphorylation.
While our data demonstrate the efficacy of the EGFR p580 vaccine as a stand-alone treatment, it has the potential to be used in combination with other immunotherapies. Multiple therapeutic combinations have been tried with success in recent years to enhance the benefit of cetuximab treatment [42–44]. Studies have shown that the combination of cancer vaccines with immune checkpoint inhibitors [12, 14] or radiation [45] can result in tumor regression or delay tumor progression and promote survival, respectively. Our data clearly show that the ability of EGFR immunization to inhibit tumor growth, and combined with other immunotherapies, has the potential for synergistic tumor inhibition. It also has the added benefit of targeting several ErbB family members thus mimicking the effect of multiple therapeutic monoclonal antibody combinations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Debby Beck for her assistance with the animal studies and Dr. Alan Sutherland for figure preparation. The work presented here is motivated by the remarkable spirit and memories of Mr. Anthony Collins and his dog Walter.
Abbreviations
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- Akt
Protein kinase B
- Asp
Aspartyl
- CFA
Complete Freund’s adjuvant
- EGF
Epidermal growth factor
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- HER3
Human epidermal growth factor receptor 3
- HER4
Human epidermal growth factor receptor 4
- hu
Human
- IFA
Incomplete Freund’s adjuvant
- iso-Asp
Iso-aspartyl
- ms
Mouse
- µCi
Micro Curie
Author contributions
MJM, RAK, and HAD conceived and designed the experiments. RAK and NB designed the immunizing peptides. HAD, RAB, SMT, and RJG performed the experiments. HAD and MJM analyzed the data. HAD wrote the manuscript.
Funding
This study was supported by the National Institutes of Health Grants CA128165 and AI48120 to M.J. Mamula and by generous support from Ms. Alva Greenberg through the M.J. and Caral G. Lebworth Foundation.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the Yale University Institutional Animal Care and Use Committee.
Animal source
Animals used in this study were purchased from the National Cancer Institute.
Cell line authentication
A431 and MDA-MB-453 cell lines were authenticated by ATCC for viability, growth, and morphology. Cell lines were stored according to the supplier’s instructions and were used over a period of no more than 5 month post-thaw. TUBO cells tested Mycoplasma negative by PCR prior to use in animal studies (Molecular Diagnostic Laboratory, Section of Comparative Medicine, Yale School of Medicine).
References
- 1.Fischer-Colbrie J, Witt A, Heinzl H, Speiser P, Czerwenka K, Sevelda P, Zeillinger R. EGFR and steroid receptors in ovarian carcinoma: comparison with prognostic parameters and outcome of patients. Anticancer Res. 1997;17:613–619. [PubMed] [Google Scholar]
- 2.Magne N, Pivot X, Bensadoun RJ, et al. The relationship of epidermal growth factor receptor levels to the prognosis of unresectable pharyngeal cancer patients treated by chemo-radiotherapy. Eur J Cancer. 2001;37:2169–2177. doi: 10.1016/S0959-8049(01)00280-5. [DOI] [PubMed] [Google Scholar]
- 3.Beji A, Horst D, Engel J, Kirchner T, Ullrich A. Toward the prognostic significance and therapeutic potential of HER3 receptor tyrosine kinase in human colon cancer. Clin Cancer Res. 2012;18:956–968. doi: 10.1158/1078-0432.CCR-11-1186. [DOI] [PubMed] [Google Scholar]
- 4.Milella M, Nuzzo C, Bria E, et al. EGFR molecular profiling in advanced NSCLC: a prospective phase II study in molecularly/clinically selected patients pretreated with chemotherapy. J Thorac Oncol. 2012;7:672–680. doi: 10.1097/JTO.0b013e31824a8bde. [DOI] [PubMed] [Google Scholar]
- 5.da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49–69. doi: 10.1146/annurev-pathol-011110-130206. [DOI] [PubMed] [Google Scholar]
- 6.Roskoski R., Jr ErbB/HER protein-tyrosine kinases: Structures and small molecule inhibitors. Pharmacol Res. 2014;87C:42–59. doi: 10.1016/j.phrs.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Peipp M, Dechant M, Valerius T. Effector mechanisms of therapeutic antibodies against ErbB receptors. Curr Opin Immunol. 2008;20:436–443. doi: 10.1016/j.coi.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Vecchione L, Jacobs B, Normanno N, Ciardiello F, Tejpar S. EGFR-targeted therapy. Exp Cell Res. 2011;317:2765–2771. doi: 10.1016/j.yexcr.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Fojo T, Grady C. How much is life worth: cetuximab, non-small cell lung cancer, and the $440 billion question. J Natl Cancer Inst. 2009;101:1044–1048. doi: 10.1093/jnci/djp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss EM, Wunderlich R, Ebel N, et al. Selected anti-tumor vaccines merit a place in multimodal tumor therapies. Front Oncol. 2012;2:132. doi: 10.3389/fonc.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson DO, Byrd K, Vreeland TJ, et al. Interim analysis of a phase I/IIa trial assessing E39 + GM-CSF, a folate binding protein vaccine, to prevent recurrence in ovarian and endometrial cancer patients. Oncotarget. 2017;8:15912–15923. doi: 10.18632/oncotarget.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahin U, Derhovanessian E, Miller M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 14.Strauss J, Madan RA, Gulley JL. Considerations for the combination of anticancer vaccines and immune checkpoint inhibitors. Exp Opin Biol Ther. 2016;16:895–901. doi: 10.1517/14712598.2016.1170805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clifton GT, Peoples GE, Mittendorf EA. The development and use of the E75 (HER2 369–377) peptide vaccine. Fut Oncol. 2016;12:1321–1329. doi: 10.2217/fon-2015-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clifton GT, Litton JK, Arrington K, Ponniah S, Ibrahim NK, Gall V, Alatrash G, Peoples GE, Mittendorf EA. Results of a phase Ib trial of combination immunotherapy with a CD8 + T Cell eliciting vaccine and trastuzumab in breast cancer patients. Ann Surg Oncol. 2017;24:2161–2167. doi: 10.1245/s10434-017-5844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bautz DJ, Sherpa AT, Threadgill DW. Prophylactic vaccination targeting ERBB3 decreases polyp burden in a mouse model of human colorectal cancer. Oncoimmunology. 2017;6:e1255395. doi: 10.1080/2162402X.2016.1255395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mamula MJ, Gee RJ, Elliot JI, Sette A, Southwood S, Jones P, Blier PR. Isoaspartyl post-translational modification triggers autoimmune responses to self-proteins. J Biol Chem. 1999;274:22321–22327. doi: 10.1074/jbc.274.32.22321. [DOI] [PubMed] [Google Scholar]
- 19.Doyle HA, Zhou J, Wolff MJ, Harvey BP, Roman RM, Gee RJ, Koski RA, Mamula MJ. Isoaspartyl posttranslational modification triggers anti-tumor T and B lymphocyte immunity. J Biol Chem. 2006;281:32676–32683. doi: 10.1074/jbc.M604847200. [DOI] [PubMed] [Google Scholar]
- 20.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr, Leahy DJ. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 21.Yang X, Zhang X, Mortenson ED, Radkevich-Brown O, Wang Y, Fu YX. Cetuximab-mediated tumor regression depends on innate and adaptive immune responses. Mol Ther. 2013;21:91–100. doi: 10.1038/mt.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meira DD, Nobrega I, de Almeida VH, Mororo JS, Cardoso AM, Silva RL, Albano RM, Ferreira CG. Different antiproliferative effects of matuzumab and cetuximab in A431 cells are associated with persistent activity of the MAPK pathway. Eur J Cancer. 2009;45:1265–1273. doi: 10.1016/j.ejca.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Kao J, Salari K, Bocanegra M, et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One. 2009;4:e6146. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riemer AB, Kurz H, Klinger M, Scheiner O, Zielinski CC, Jensen-Jarolim E. Vaccination with cetuximab mimotopes and biological properties of induced anti-epidermal growth factor receptor antibodies. J Natl Cancer Inst. 2005;97:1663–1670. doi: 10.1093/jnci/dji373. [DOI] [PubMed] [Google Scholar]
- 25.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 26.Pol J, Bloy N, Buque A, et al. Trial Watch: Peptide-based anticancer vaccines. Oncoimmunology. 2015;4:e974411. doi: 10.4161/2162402X.2014.974411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiedermann U, Davis AB, Zielinski CC. Vaccination for the prevention and treatment of breast cancer with special focus on Her-2/neu peptide vaccines. Br Cancer Res Treat. 2013;138:1–12. doi: 10.1007/s10549-013-2410-8. [DOI] [PubMed] [Google Scholar]
- 28.Pritzker LB, Joshi S, Gowan JJ, Harauz G, Moscarello MA. Deimination of myelin basic protein. 1. Effect of deimination of arginyl residues of myelin basic protein on its structure and susceptibility to digestion by cathepsin D. Biochem. 2000;39:5374–5381. doi: 10.1021/bi9925569. [DOI] [PubMed] [Google Scholar]
- 29.Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, Cairns E. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J Immunol. 2003;171:538–541. doi: 10.4049/jimmunol.171.2.538. [DOI] [PubMed] [Google Scholar]
- 30.Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–311. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 32.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 33.Kruser TJ, Wheeler DL. Mechanisms of resistance to HER family targeting antibodies. Exp Cell Res. 2010;316:1083–1100. doi: 10.1016/j.yexcr.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Montagut C, Dalmases A, Bellosillo B, et al. Identification of a mutation in the extracellular domain of the epidermal growth factor receptor conferring cetuximab resistance in colorectal cancer. Nat Med. 2012;18:221–223. doi: 10.1038/nm.2609. [DOI] [PubMed] [Google Scholar]
- 35.Brand TM, Iida M, Wheeler DL. Molecular mechanisms of resistance to the EGFR monoclonal antibody cetuximab. Cancer Biol Ther. 2011;11:777–792. doi: 10.4161/cbt.11.9.15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Emburgh BO, Sartore-Bianchi A, Di Nicolantonio F, Siena S, Bardelli A. Acquired resistance to EGFR-targeted therapies in colorectal cancer. Mol Oncol. 2014;8:184–194. doi: 10.1016/j.molonc.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wheeler DL, Huang S, Kruser TJ, Nechrebecki MM, Armstrong EA, Benavente S, Gondi V, Hsu KT, Harari PM. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–3956. doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yonesaka K, Zejnullahu K, Okamoto I, et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med. 2011;3:99ra86. doi: 10.1126/scitranslmed.3002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kavuri SM, Jain N, Galimi F, et al. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov. 2015;5:832–841. doi: 10.1158/2159-8290.CD-14-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takegawa N, Yonesaka K, Sakai K, et al. HER2 genomic amplification in circulating tumor DNA from patients with cetuximab-resistant colorectal cancer. Oncotarget. 2016;7:3453–3460. doi: 10.18632/oncotarget.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larbouret C, Gaborit N, Chardes T, et al. In pancreatic carcinoma, dual EGFR/HER2 targeting with cetuximab/trastuzumab is more effective than treatment with trastuzumab/erlotinib or lapatinib alone: implication of receptors’ down-regulation and dimers’ disruption. Neoplasia. 2012;14:121–130. doi: 10.1593/neo.111602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramalingam S, Forster J, Naret C, Evans T, Sulecki M, Lu H, Teegarden P, Weber MR, Belani CP. Dual inhibition of the epidermal growth factor receptor with cetuximab, an IgG1 monoclonal antibody, and gefitinib, a tyrosine kinase inhibitor, in patients with refractory non-small cell lung cancer (NSCLC): a phase I study. J Thorac Oncol. 2008;3:258–264. doi: 10.1097/JTO.0b013e3181653d1b. [DOI] [PubMed] [Google Scholar]
- 43.Regales L, Gong Y, Shen R, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest. 2009;119:3000–3010. doi: 10.1172/JCI38746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertotti A, Papp E, Jones S, et al. The genomic landscape of response to EGFR blockade in colorectal cancer. Nature. 2015;526:263–267. doi: 10.1038/nature14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mason NJ, Gnanandarajah JS, Engiles JB, Gray F, Laughlin D, Gaurnier-Hausser A, Wallecha A, Huebner M, Paterson Y. Immunotherapy with a HER2-targeting listeria induces HER2-specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine osteosarcoma. Clin Cancer Res. 2016;22:4380–4390. doi: 10.1158/1078-0432.CCR-16-0088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.