Abstract
Objectives:
Neuroinflammation, implicated in epilepsy, can be imaged in humans with positron emission tomography (PET) ligands for translocator protein 18 kDa (TSPO). Previous studies in patients with temporal lobe epilepsy and mesial temporal sclerosis found increased [11C]PBR28 uptake ipsilateral to seizure foci. Neocortical foci present more difficult localization problems and more variable underlying pathology.
Methods:
We studied 11 patients with neocortical seizure foci using [11C]PBR28 or [11C]N,N-diethyl-2-(4-methoxyphenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidine-3-acetamide (DPA) 713, and 31 healthy volunteers. Seizure foci were identified with structural magnetic resonance imaging (MRI) and ictal video–electroencephalography (EEG) monitoring. Six patients had surgical resections; five had focal cortical dysplasia type 2A or B and one microdysgenesis. Brain regions were delineated using FreeSurfer and T1-weighted MRI. We measured brain radioligand uptake (standardized uptake values [SUVs]) in ipsilateral and contralateral regions, to compare calculated asymmetry indices [AIs; 200% *(ipsilateral − contralateral)/(ipsilateral + contralateral)] between epilepsy patients and controls, as well as absolute [11C]PBR28 binding as the ratio of distribution volume to free fraction (VT/fP) in 9 patients (5 high affinity and 4 medium affinity binders) and 11 age-matched volunteers (5 high-affinity and 6 medium affinity) who had metabolite-corrected arterial input functions measured.
Results:
Nine of 11 patients had AIs exceeding control mean 95% confidence intervals in at least one region consistent with the seizure focus. Three of the nine had normal MRI. There was a nonsignificant trend for patients to have higher binding than volunteers both ipsilateral and contralateral to the focus in the group that had absolute binding measured.
Significance:
Our study demonstrates the presence of focal and distributed inflammation in neocortical epilepsy. There may be a role for TSPO PET for evaluation of patients with suspected neocortical seizure foci, particularly when other imaging modalities are unrevealing. However, a complex method, inherent variability, and increased binding in regions outside seizure foci will limit applicability.
Keywords: focal epilepsy, inflammation, neocortical epilepsy, PET
1 |. INTRODUCTION
Patients with neocortical epilepsy resistant to antiepileptic drug treatment often face greater difficulties in seizure focus localization and treatment planning than patients with mesial temporal lobe epilepsy, particularly when magnetic resonance imaging (MRI) does not show a structural lesion.1,2 Sixty percent to 70% of patients become seizure-free after temporal lobectomy, compared to about 45% for patients with extratemporal foci, and as low as 30%-35% for nonlesional patients in some series.3 In series of frontal lobe resections, only 25%-30% were free of all seizures.4 Although additional imaging procedures such as positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) may help identity seizure foci and predict surgical outcome, presurgical evaluation for patients with suspected neocortical foci continues to present challenges.5
Evidence from both animal models and human studies suggests that inflammatory processes play a role in epilepsy.6 Animal models of epilepsy implicating inflammatory mechanisms are particularly interesting because they are associated with pharmacoresistance.7 Inflammatory pathways including interleukin-1 receptor/toll-like receptor signaling, cyclooxygenase-2, tumor necrosis factor-α, complement signaling, and chemokines have been implicated.8 Up-regulation of inflammatory intermediates, as well as expression of activated microglia and reactive astrocytes, have been reported in lesions such as focal cortical dysplasia and developmental tumors, often found in patients with neocortical epilepsy.9–11 Activation of High Mobility Group Box 1 (HMGB1)/toll-like receptor four axis initiates inflammation in several clinical and experimental epileptogenic processes.12
Translocator protein 18 kDa (TSPO) is a marker of neuroinflammation, overexpressed on activated microglia and reactive astrocytes. Several PET ligands can be used to measure TSPO.13 A number of studies in animal models have shown increased seizure-associated receptor binding. Limbic TSPO expression in Wistar rats measured with 1[18F]PBR111 PET was significantly increased, peaking 2 weeks after kainic acid– induced status epilepticus.14 Binding was correlated with activated microglia, cell loss, and seizures. In the mouse kainic acid epilepsy model, micro-PET with [18F]DPA714 showed increased TSPO expression mainly in microglia at day 7 and in reactive astrocytes at day 14 in conjunction with development of hippocampal sclerosis.15 In the lithium-pilocarpine status epilepticus model, increased TSPO binding was seen with ([11C]PK11195 PET, correlated with microglial and astroglial activation as well as neuronal loss.16,17 Translocator protein measured with [18F]PBR111 PET was highly overexpressed 2 weeks post–status epilepticus in limbic structures, returning toward baseline by 4 weeks post–status epilepticus induced by repeated low-dose kainic acid.18 During monitoring over 12 weeks, binding correlated with severity of depression like and sensorimotor-related deficits during chronic epilepsy. PET imaging data at 2 weeks after status predicted spontaneous seizure frequency in animals developing epilepsy.17 Similar results were found using [18F]GE-180 PET: correlational analysis identified brain regions, particularly amygdala, within which TSPO-binding increases could predict epileptogenesis.19
A small number of human imaging studies support the hypothesis that epilepsy is associated with increased TSPO binding, suggesting the presence of inflammation. We showed that patients with mesial temporal lobe epilepsy have increased binding with PET ligands [11C]PBR28 and [11C]DPA-713.20,21 One patient with focal cortical dysplasia had increased binding measured with [11C]PK11195.22 We now report results in 11 patients with neocortical epileptogenic zones.
2 |. METHODS
2.1 |. Patients
We studied 11 patients (5 men and 6 women; mean age 32 years) referred to the NIH Clinical Epilepsy Section for evaluation of drug-resistant epilepsy (Table 1) and 31 healthy controls (11 women; mean age 38 years). Subjects were screened for the TPSO-binding polymorphism by in vitro leukocyte assay, and low affinity (LL) binders were excluded.23 Six patients were high (HH) and five mixed (HL) binders. No screened patients needed to be excluded for LL binding. About 5% of controls were excluded. All patients had seizure foci outside of the mesial temporal lobe based on MRI, ictal video-electroencephalography recording (video-EEG), and in some cases FDG-PET scan. FDG-PET showed focal hypometabolism in only 2 of 11 patients. Structural MRI showed clear focal abnormalities in five patients. Patient 2 had an MRI initially read as normal but reassessed after the [11C]PBR PET scan. Patient 7’s MRI scan was read initially as normal but subsequently found to show probable cortical dysplasia independently of other data. Only Patient 4 did not have confirmation of the focus localization by either pathology, subdural ictal EEG, or both. In this case, only surface ictal video-EEG, showing a left frontotemporal focus, was available. MRI showed an enlarged right temporal horn of uncertain significance. Seizures were characterized by hypermotor activity and jerking of the right arm. The patient declined further evaluation.
TABLE 1.
Patient characteristics
| Patient no. | Age/sex | Age of Onset | Epileptogenic zone | MRI | FDG-PET | Pathology |
|---|---|---|---|---|---|---|
| 1 | 54/F | 22 | Right posterior temporal | Right posterior temporal lesion | Right temporal hypometabolism | DNET + FCD 2A |
| 2 | 41/F | 8 | Right orbito-frontal | Read as normal. Post-[11C]PBR28 PET review: Right frontal blurring of gray-white matter junction | Normal | FCD 2B |
| 3 | 25/M | 17 | Left inferior-posterior temporal | Normal | Normal | No surgery |
| 4 | 35/M | 2 months | Left frontotemporal | Enlarged right temporal horn | Normal | No surgery |
| 5 | 25/F | 23 | Right centroparietal | Normal | Not done | No surgery |
| 6 | 22/F | 6 | Left frontal | Left frontal hypointensity | Normal | No surgery |
| 7 | 20/F | 3 | Left frontal | Left frontal FCD | Normal | FCD 2A |
| 8 | 26/M | 15 | Left posterior superior temporal | Normal | Normal | No surgery |
| 9 | 28/M | 9 | Right posterior parietal | Normal | Not done | FCD 2A |
| 10 | 44/M | 32 | Right frontopolar | Normal | Right temporal hypometabolism | Microdysgenesis |
| 11 | 22/F | 21 | Left parietal | Bilateral motor cortex thinning | Normal | FCD 2A |
Abbreviations: DNET, dysembryoplastic neuroepithelial tumor; FCD, focal cortical dysplasia; MRI, magneitc resonance imaging; PBR, peripheral benzodiazepine receptor; PET, positron emission tomography.
Six patients had surgery; five are seizure-free. Patients 3 and 5 declined surgery because their seizure foci overlapped with functionally important regions. TSPO PET results were not used as part of clinical evaluations. The study was approved by the NIH Combined Neurosciences Institutional Review Board. All subjects signed written consent.
2.2 |. PET imaging
The methods have been described previously.20,21 After an attenuation correction transmission scan, a bolus injection of [11C]PBR28 (n = 10) or [11C]DPA-713 (n = 1) was administered and dynamic PET images acquired for 90 minutes on an Advance scanner (GE Healthcare). Data were compared to 31 healthy volunteers. Metabolite-corrected arterial input functions were obtained during the [11C]PBR28 scans of 9 epilepsy patients (5 high affinity and 4 mixed affinity binders) and 11 age-matched healthy volunteers (5 high-affinity and 6 medium affinity). [11C]PBR28 plasma-free fraction (fP) was measured by ultrafiltration.
2.3 |. Image analysis
We automatically segmented 41 brain regions in each hemisphereusing FreeSurfer 5.1.1 (http://surfer.nmr.mgh.harvard.edu) after reslicing T1-weighted MR images to 1 mm isovoxel space, correcting for inhomogeneity, skull-stripping, and segmenting into gray and white matter. The mask was applied to PET images coregistered to T1-weighted MR image resliced into isovoxel space. We combined individual temporal cortical regions into one larger region for our template to reduce the number of comparisons and to minimize the noise from calculating binding in very small regions. The larger temporal region was used for asymmetry index (AI) but not absolute distribution volume analysis. The template covered the whole brain surface.
2.4 |. Measurement of tracer distribution
[11C]PBR28 uptake was measured as standardized uptake values (SUVs), which correct for body weight and injected activity, from 40 to 90 minutes after injection. TSPO AIs were calculated from SUVs [200% * (ipsilateral − contralateral)/(ipsilateral + contralateral)]. Because patients had seizure foci in varied locations, we compared AIs in brain regions consistent with the seizure focus for each patient to the 95% side-to-side AI confidence intervals measured in healthy controls (N = 31) as estimates of physiologic asymmetry in each region.
We measured absolute [11C]PBR28 distribution volume corrected for plasma-free fraction (VT/fP), calculated with a two-tissue compartment model, in 9 patients and 11 healthy volunteers who had arterial sampling. Regions were selected to match the distribution of seizure foci: superior frontal cortex, inferior frontal cortex, medial and lateral orbitofrontal cortices, superior parietal cortex, inferior parietal cortex, insula, temporal pole, and temporal cortex. We did not perform quantitative analysis in all 88 FreeSurfer regions.
2.5 |. Statistical analysis
We tested the hypothesis of higher TSPO expression in epilepsy patients than controls by comparing (a) tracer uptake asymmetry to volunteer 95% confidence intervals and (b) absolute [11C]PBR28 binding, or ratio of distribution volume to free fraction (VT/fP), using repeated measures analysis of variance (rmANOVA), with group (patient or control), focus (contralateral or ipsilateral to the seizure focus; this was omitted in testing the hemispheres separately), and region as fixed factors and subject as a random factor. Differences were considered significant at P < 0.05.
3 |. RESULTS
Consistent with our hypothesis and previous results in mesial temporal foci that epilepsy patients would have increased TSPO ipsilateral to neocortical seizure foci, 9 of 11 patients had AIs exceeding mean 95% confidence intervals for 31 controls in the anatomic region of interest consistent with the seizure focus (Table 2). Four of the patients had either normal or nonlocalizing MRI scans. One patient’s MRI originally read as normal was reevaluated after [11C]PBR28 PET (Figure 1). In three cases, visual analysis showed increased signal coinciding with MRI lesions. We found more wide-spread binding increases as well, including ipsilateral subcortical as well as cortical regions. Several patients also showed scattered relative decreases, but not in the region of the seizure focus.
TABLE 2.
TSPO positron emission tomography (PET) imaging results
| Patient | ROI | Asymmetry index | Control mean | 95% CI |
|---|---|---|---|---|
| 1 | Temporal cortex | 5.9 | −0.62 | −1.4 to 0.14 |
| 2 | Medial orbitofrontal cortex | 9.4 | −0.87 | −2.2 to 0.48 |
| 3 | Temporal cortex | −1.0 | −0.62 | −1.4 to 0.14 |
| 4 | Precentral gyrus | 3.28 | −0.18 | −1.4 to 1 |
| 5 | Superior parietal cortex | 2.57 | −0.09 | −0.96 to 0.78 |
| 6 | Paracentral gyrus | 1.72 | 0.18 | −1.3 to −1.7 |
| 7 | Medial orbitofrontal cortex | 7.05 | −0.87 | −2.2 to 0.48 |
| 8 | Temporal cortex | 0.05 | −0.62 | −1.4 to 0.14 |
| 9 | Inferior parietal cortex | 1.02 | −0.46 | −1.3 to 0.41 |
| 10 | Frontal pole | 24.4 | 0.39 | −3.5 to 4.3 |
| 11 | Left parietal | 3.31 | −0.27 | −1.70 to 1.20 |
FIGURE 1.
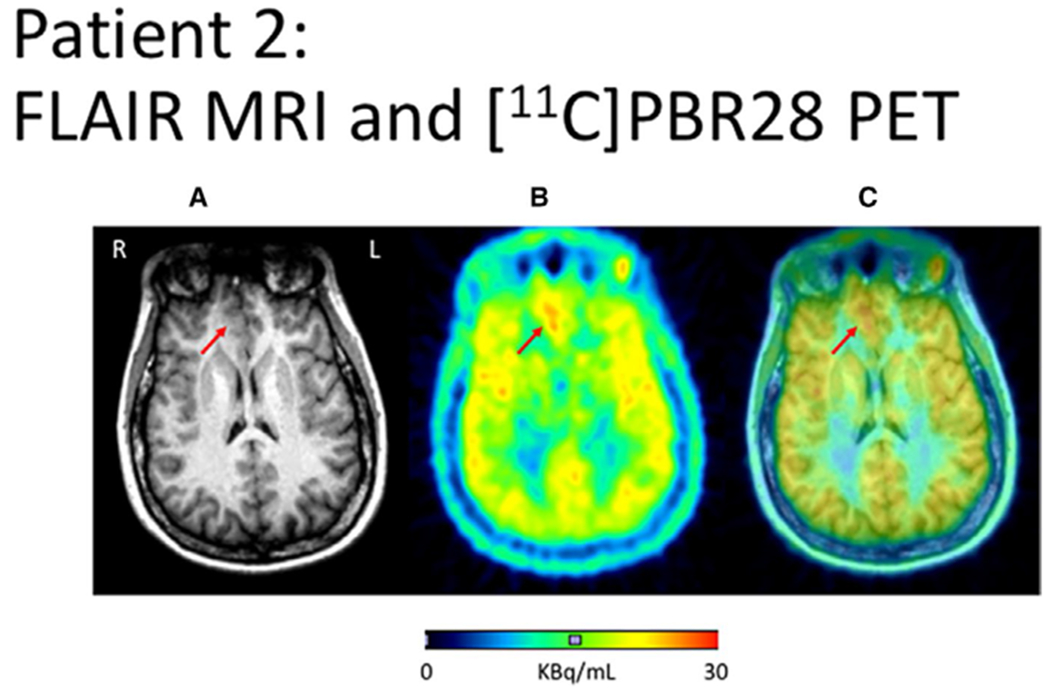
Focal Cortical dysplasia 2B, right frontal seizure onset. A, Fluid-attenuated inversion recovery (FLAIR)–weighted magnetic resonance imaging (MRI) showing right frontal lesion. B, [11C]PBR28 positron emission tomography (PET) scan averaged over (averaged 40-90 min after injection) showing Increased uptake in the region of the MRI signal increase. C, Fused image
In the quantitative binding analysis, there was a nonsignificant trend for absolute [11C]PBR28 binding (VT/fP) to be higher in patients than controls: mean VT/fP Ipsilateral to the focus (left for controls) was 100.0 ± 36.0 mL/cm3 for patients, and 85.7 ± 24.1 mL/cm3 for controls. Contralateral (right for controls) was 100.3 ± 35.2 mL/cm3 for patients, and 85.9 ± 24.1 mL/cm3 for controls. Group effect Ipsilateral to the focus was F = 2.0, P = 0.17; contralateral F = 0.99, P = 0.33 (overall main effect of group: F = 1.4, P = 0.25; Figure 2). Due to the relatively high standard deviation for the absolute values in the controls with arterial lines, only two of the nine patients had AI exceeding the control 95% confidence intervals. We did not find a relation between patient age, time since last seizure, or seizure frequency and AI. There were weak nonsignificant trends for HH patients to have higher AI for both SUV and quantitative binding measurements.
FIGURE 2.
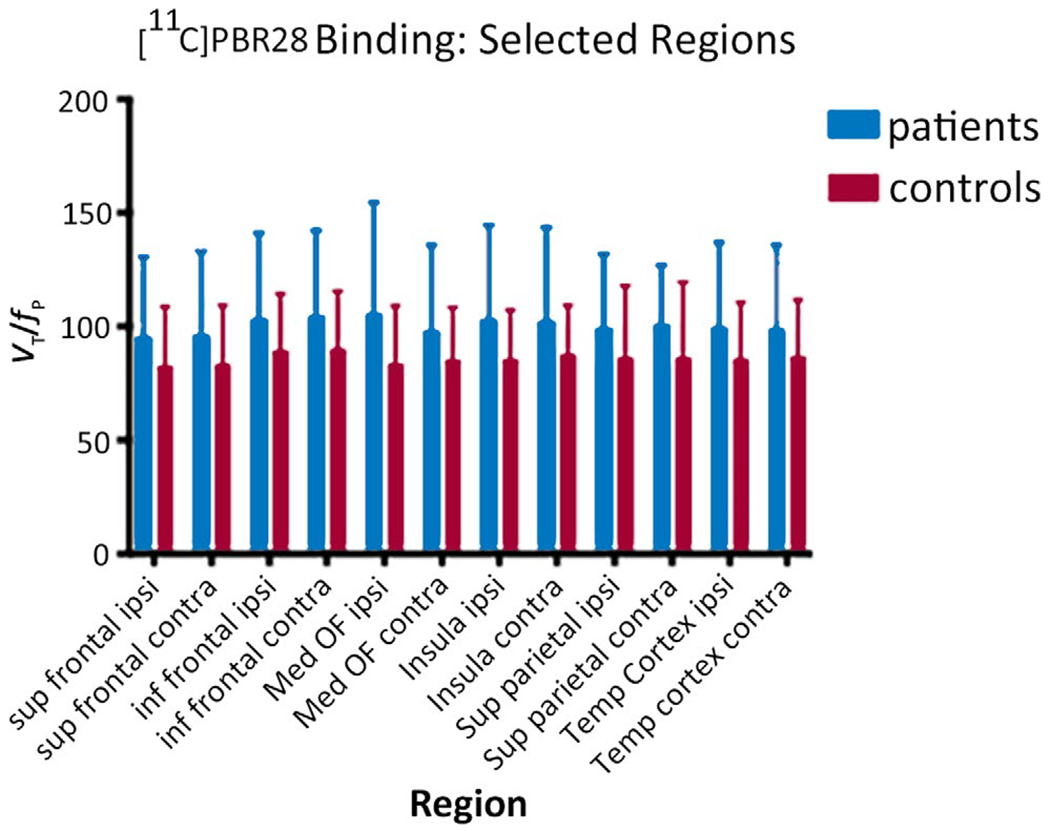
Comparison of [11C]PBR binding in patients and healthy volunteers shows increased binding in patients both ipsilateral and contralateral to the seizure focus. For healthy volunteers, “ipsilateral” was left. The regions were selected in advance as being most likely to contain seizure foci across a disparate group. The differences shown in the figure are not significant. Sup, superior; Inf, inferior; Ipsi, ipsilateral; Contra, contralateral; Med, medial; OF, orbitofrontal; Temp, temporal
4 |. DISCUSSION
Our study shows that patients with neocortical foci may show increased TSPO binding in ictal-onset regions, suggesting the presence of inflammation. In addition, we found a trend toward increased binding in patients both ipsilateral and contralateral to the seizure focus. Cortical dysplasia (both types 2A and 2B) was found in five of six patients who had surgery, consistent with previous reports of increased inflammatory markers in pathologic specimens from patients with these lesions.9–11
TSPO PET, in our small series, supplemented focus localization by structural MR, [18F]FDG-PET, and ictal video-EEG monitoring. In one case, a clear focus of increased [11C]PBR28 uptake prompted re-review of an MRI that was initially read as normal but did in fact show a corresponding structural abnormality. However, the binding found beyond seizure foci suggests that the method may have limited specificity.
Imaging studies may provide markers for the role of neuroinflammation in epilepsy and epileptogenesis.24 Previous studies have shown increased TSPO binding in several epilepsy syndromes. Two patients with Rasmussen’s encephalitis imaged with [11C]PK11195 had focal and diffuse binding increases in the affected hemisphere, but three with hippocampal sclerosis were not different from healthy controls.25 A 5-year-old with drug-resistant epilepsy due to encephalitis of unknown origin had diffuse increased temporooccipital [11C]PK11195 binding consistent with EEG seizure onset; pathology showed “microglial activation” and gliosis.26 [11C]PK11195 binding was increased in a region of focal glucose hypometabolism in a patient with focal cortical dysplasia,21 and was greater when a scan was performed 36 hours after a seizure than during a seizure-free period in another for whom pathology was not available.27
In studies using the ligands [[11C]PBR28 and [11C]DPA-713, with higher specific binding than [11C]-PK11195, we found increased binding compared to controls both contralateral and ipsilateral to mesial temporal seizure foci, although ipsilateral binding was greater than contralateral.20,21 Patients with mesial temporal sclerosis had greater ipsilateral-contralateral differences than those with nonspecific pathologic findings.
The lack of significant group TSPO absolute binding measurement differences between patients and controls in this study is in contrast to significant increases in our previous series of patients with mesial temporal lobe epilepsy. The contrast probably is due to variability in focus localization and patient heterogeneity, and also to a relatively smaller sample size. The analysis regions are relatively larger in comparison to the patients’ extratemporal seizure foci and structural lesions (if present), than in the case of our previous study of patients with mesial temporal epilepsy, for which the measurements were obtained from more discrete structures such as the hippocampus. This may have affected the results. We used FreeSurfer and predetermined regions to avoid bias that would be introduced by drawing regions around MRI lesions or presumed EEG foci. Statistical parametric mapping (Wellcome Department of Imaging Neuroscience) offers an alternative analytic approach.
Bilateral inflammation, found in our previous study and suggested by our current results, may also mask group differences when using asymmetry as a measure of TSPO over-expression.21 It has been reported in animal models as well as human studies. In the intrahippocampal kainic acid status epilepticus model, bilateral increases in TSPO binding measured with [18F]GE180 PET were found both ipsilateral and contralateral to the seizure focus.28
Our study has several limitations. The limited patient number and large number of regions in the template limits the statistical power of the study. One patient did not have either pathological or ictal subdural EEG confirmation of focus localization. The relatively wide variability in absolute binding values complicates the interpretation of the results. In addition, using relatively large FreeSurfer regions may have obscured findings in relatively small seizure foci. On the other hand, it avoids the bias that might be introduced by drawing individualized regions, and also facilitates analyzing regional data across studies. Only two patients had absolute binding AIs exceeding the control mean. We did not find a relation between seizure recency or frequency and AI, which might have been detected by repeat scanning in the same patients.27 We were not able to study the pathological specimens with specific inflammatory markers; Patient 11 only had [11C]DPA-713 data available. We decided to include her because there are only limited data on the relation of TSPO binding to structural lesions in patients with epilepsy. We could compare her data to healthy control [11C]DPA-713 scans obtained in our previous study, where we found no differences between [11C]DPA and [11C]PBR AI.21
There was a nonsignificant trend for patients to be younger than controls. Although a linear age-related increase in overall TSPO binding measured with both [11C]PK1119529 and [11C]PBR2830 has been reported, the regional pattern of tracer activity remained constant. Thus, patients and controls in our study would not be expected to differ in baseline tracer distribution or side-to-side asymmetries.
Our study demonstrates the presence of inflammation in neocortical focal epilepsy. However, the complexity of the method, inherent variability, and presence of increased binding in regions outside seizure foci will limit applicability of the technique. TSPO PET may be helpful in the evaluation of patients with suspected neocortical epilepsy when other imaging modalities are unrevealing, drawing attention to the potential presence of unsuspected lesions.
Key Points.
Nine of 11 patients with neocortical seizure foci studied with positron emission tomography (PET) ligands for the translocator protein 18 kDa (TSPO) receptor had increased binding in seizure foci
Three of the nine patients had normal magnetic resonance imaging (MRI)
Six of the 9 had surgery; pathology was focal cortical dysplasia 2A or 2B, and a dysembryoplastic neuroepithelial tumor
Inflammation is present in neocortical focal epilepsy. TSPO PET may aid evaluation of patients with suspected neocortical seizure foci
ACKNOWLEDGMENTS
Dr Robert B Innis provided helpful suggestions and support. Gina Norato provided statistical advice.
Funding information
Supported by the Divisions of Intramural Research of the National Institutes of Mental Health and of Neurological Diseases and Stroke.
Footnotes
DISCLOSURE
None of the authors has anything to disclose. All the authors performed the work reported as part of their official US Government duties. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Jobst BC, Cascino GD. Resective epilepsy surgery for drug-resistant focal epilepsy: a review. JAMA. 2015;313:285–93. [DOI] [PubMed] [Google Scholar]
- 2.Englot DJ, Breshears JD, Sun PP, Chang EF, Auguste KI. Seizure outcomes after resective surgery for extra-temporal lobe epilepsy in pediatric patients. J Neurosurg Pediatr. 2013;12:126–33. [DOI] [PubMed] [Google Scholar]
- 3.Téllez-Zenteno JF, Hernández Ronquillo L, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2010;89:310–8. [DOI] [PubMed] [Google Scholar]
- 4.Lazow SP, Thadani VM, Gilbert KL, Morse RP, Bujarski KA, Kulandaivel K, et al. Outcome of frontal lobe epilepsy surgery. Epilepsia. 2012;53:1746–55. [DOI] [PubMed] [Google Scholar]
- 5.Wong CH, Bleasel A, Wen L, Eberl S, Byth K, Fulham M, et al. Relationship between preoperative hypometabolism and surgical outcome in neocortical epilepsy surgery. Epilepsia. 2012;53:1333–40. [DOI] [PubMed] [Google Scholar]
- 6.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker-Haliski ML, Löscher W, White HS, Galanopoulou AS. Neuroinflammation in epileptogenesis: insights and translational perspectives from new models of epilepsy. Epilepsia. 2017;58(Suppl 3):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Vliet EA, Aronica E, Vezzani A, Ravizza T. Neuroinflammatory pathways as treatment targets and biomarker candidates in epilepsy: emerging evidence from preclinical and clinical studies. Neuropathol Appl Neurobiol. 2018;44:91–111. [DOI] [PubMed] [Google Scholar]
- 9.Ravizza T, Boer K, Redeker S, Spliet WG, van Rijen PC, Troost D, et al. The IL-1β system in epilepsy-associated malformations of cortical development. Neurobiol Dis. 2006;24:128–43. [DOI] [PubMed] [Google Scholar]
- 10.Iyer A, Zurolo E, Spliet WGM, van Rijen PC, Baayen JC, Gorter JA, et al. Evaluation of the innate and adaptive immunity in type I and type II focal cortical dysplasias. Epilepsia. 2010;51:1763–73. [DOI] [PubMed] [Google Scholar]
- 11.Zurolo E, Iyer A, Maroso M, Carbonell C, Anink JJ, Ravizza T, et al. Activation of toll-like receptor, RAGE and HMGB1 signaling in malformations of cortical development. Brain. 2011;134:1015–32. [DOI] [PubMed] [Google Scholar]
- 12.Ravizza T, Terrone G, Salamone A, Frigerio F, Balosso S, Antoine DJ, et al. High Mobility Group Box 1 is a novel pathogenic factor and a mechanistic biomarker for epilepsy. Brain Behav Immun. 2018;72:14–21. [DOI] [PubMed] [Google Scholar]
- 13.Cumming P, Burgher B, Patkar O, Breakspear M, Vasdev N, Thomas P, et al. Sifting through the surfeit of neuroinflammation tracers. J Cereb Blood Flow Metab. 2018;38:204–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amhaoul H, Hamaide J, Bertoglio D, Reichel SN, Verhaeghe J, Geerts E, et al. Brain inflammation in a chronic epilepsy model: evolving pattern of the translocator protein during epileptogenesis. Neurobiol Dis. 2015;82:526–39. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen DL, Wimberley C, Truillet C, Jego B, Caillé F, Pottier G, et al. Longitudinal positron emission tomography imaging of glial cell activation in a mouse model of mesial temporal lobe epilepsy: toward identification of optimal treatment windows. Epilepsia. 2018;59:1234–44. [DOI] [PubMed] [Google Scholar]
- 16.Brackhan M, Bascuñana P, Postema JM, Ross TL, Bengel FM, Bankstahl M, et al. Serial quantitative TSPO-targeted PET reveals peak microglial activation up to 2 weeks after an epileptogenic brain insult. J Nucl Med. 2016;57:1302–8. [DOI] [PubMed] [Google Scholar]
- 17.Yankam Njiwa J, Costes N, Bouillot C, Bouvard S, Fieux S, Becker G, et al. Quantitative longitudinal imaging of activated microglia as a marker of inflammation in the pilocarpine rat model of epilepsy using [11C]-(R)-PK11195 PET and MRI. J Cereb Blood Flow Metab. 2017;37:1251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertoglio D, Verhaeghe J, Santermans E, Amhaoul H, Jonckers E, Wyffels L, et al. Non-invasive PET imaging of brain inflammation at disease onset predicts spontaneous recurrent seizures and reflects comorbidities. Brain Behav Immun. 2017;61:69–79. [DOI] [PubMed] [Google Scholar]
- 19.Russmann V, Brendel M, Mille E, Helm-Vicidomini A, Beck R, Günther L, et al. Identification of brain regions predicting epileptogenesis by serial [18F]GE-180 positron emission tomography imaging of neuroinflammation in a rat model of temporal lobe epilepsy. Neuroimage Clin. 2017;15:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirvonen J, Kreisl W, Fujita M, Dustin I, Khan O, Appel S, et al. Increased in vivo expression of an inflammatory marker in temporal lobe epilepsy. J Nucl Med. 2012;53:234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gershen LP, Zanotti-Fregonara P, Dustin IM, Liow J-S, Hirvonen J, Kreisl WC, et al. Neuroinflammation in temporal lobe epilepsy measured using PET imaging of translocator protein. JAMA Neurol. 2015;72:882–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler T, Ichise M, Teich AF, Gerard E, Osborne J, French J, et al. Imaging inflammation in a patient with epilepsy due to focal cortical dysplasia. J Neuroimaging. 2013;23:129–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owen DRJ, Gunn RN, Rabiner EA, Bennacef I, Fujita M, Kreisl WC, et al. Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J Nucl Med. 2011;52(1):24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koepp MJ, Årstad E, Bankstahl JP, Dedeurwaerdere S, Friedman A, Potschka H, et al. Neuroinflammation imaging markers for epileptogenesis. Epilepsia. 2017;58(Suppl 3):11–9. [DOI] [PubMed] [Google Scholar]
- 25.Banati RB, Goerres GW, Myers R, Gunn RN, Turkheimer FE, Kreutzberg GW, et al. [11C](R)-PK11195 positron emission tomography imaging of activated microglia in vivo in Rasmussen’s encephalitis. Neurology. 1999;53:2199–203. [DOI] [PubMed] [Google Scholar]
- 26.Kumar A, Chugani HT, Luat A, Asano E, Sood S. Epilepsy surgery in a case of encephalitis: use of 11 C-PK11195 positron emission tomography. Pediatr Neurol. 2008;38:439–42. [DOI] [PubMed] [Google Scholar]
- 27.Butler T, Li Y, Tsui W, Friedman D, Maoz A, Wang X, et al. Transient and chronic seizure-induced inflammation in human focal epilepsy. Epilepsia. 2016;57:e191–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brackhan M, Bascunana P, Ross TL, Bengel FM, Bankstahl JP, Bankstahl M. [18F]GE180 positron emission tomographic imaging indicates a potential double-hit insult in the intrahippocampal kainite mouse model of temporal lobe epilepsy. Epilepsia. 2018;59:617–26. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A, Muzik O, Shandal V, Chugani D, Chakraborty P, Chugani HT. Evaluation of age-related changes in translocator protein (TSPO) in human brain using 11C-[R]-PK11195 PET. J Neuroinflammation. 2012;9:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul S, Gallagher E, Liow J-S, Mabins S, Henry K, Zoghbi SS, et al. Building a database for brain 18 kDa translocator protein imaged using [11C]PBR28 in healthy subjects. J Cereb Blood Flow Metab. 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


